Abstract
Introduction
Lead exposure negatively affects cognitive functioning among children. However, there is limited evidence about whether exposure to lead in early life impairs later life cognitive functioning.
Methods
Participants in the prospective Wisconsin Longitudinal Study cohort (N = 8583) were linked to the 1940 Census, which was taken when they were young children. We estimated the effect of living near a lead mine in childhood on late life memory/attention and language/executive function in 2004 (mean age 64) and 2011 (mean age 71).
Results
Lead-exposed children had significantly steeper memory/attention decline between 2004 and 2011 and worse language/executive function at baseline in late life. These long-term effects of lead were not mediated through adolescent IQ or late life SES and health factors.
Discussion
Proximity to lead mining in childhood had long-term effects on late life memory/attention decline and language/executive function, reflecting a possible latent influence of lead exposure. More research is needed to understand behavioral and biological pathways underlying this relationship.
Keywords: Lead exposure, Adolescence, Late life, Cognition, Cohort study, Life course
Highlights
-
•
We linked the Wisconsin Longitudinal Study cohort to the 1940 U.S. Census.
-
•
Subjects who lived near a lead mine in 1940 had worse late life cognitive outcomes.
-
•
The effect of lead was not mediated by adolescent IQ, adult SES, or adult health.
-
•
This is the first prospective study of early lead exposure and late life cognition.
1. Introduction
The Centers for Disease Control and Prevention currently consider no amount of lead exposure to be safe for children due to its well-documented negative impacts on the developing brain (Bellinger, 2008; Caito et al., 2017). There is a strong inverse relationship between lead exposure and cognitive function in childhood, even at low levels of exposure (Lanphear et al., 2005; Needleman & Gatsonis, 1990). However, there is limited evidence about whether the negative effect of childhood lead exposure on cognitive functioning persists to late life.
There are multiple pathways through which childhood lead exposure (especially occurring before age 5) could harm later life cognition (Reuben, 2018). According to the “chains of risk” model, the effect of lead is mainly indirect, mediated by intervening cognitive, socio-economic, and health variables (Bellinger et al., 1992; Miranda et al., 2007; Muller et al., 2018; Navas-Acien et al., 2007; Schwartz, 1991; Vaziri, 2008). For instance, late life cognitive deficits among lead-exposed children may be explained by poor cognitive function during childhood or adolescence or by worse adult SES and/or cardiovascular health. By contrast, the “latency” model posits direct biological pathways through which childhood lead exposure could influence later life cognition. These include epigenetic changes that repress the expression of genes related to healthy cognitive aging and the remobilization of lead stored in the bones into the circulatory system late in life (Bolin et al., 2006; Dosunmu et al., 2012; Hu et al., 1998; Khalid & Abdollahi, 2019; Rabinowitz, 1991; Reuben, 2018). The latency model suggests that the effect of childhood lead exposure on cognitive functioning may be independent of any lead-induced cognitive deficits earlier in the life course.
Only two previous studies have prospectively measured the effect of childhood lead exposure on adult cognition (Mazumdar et al., 2011; Reuben et al., 2017). Among participants in a prospective study from Boston (N = 43), childhood blood levels were significantly and negatively associated with IQ at age 29 (Mazumdar et al., 2011). Additionally, the Dunedin Study (N = 565) showed that blood lead levels at age 11 were significantly and negatively associated with cognitive function at age 38 (Reuben et al., 2017). While these studies are valuable, they began in the 1970s and 1980s, and cohort members will not be old enough to investigate the associations between childhood lead exposure and late life cognition for several more decades. Novel data sources are needed to contribute empirical evidence to this question in a timelier manner. Investigating the long-term effect of childhood lead exposure on late life cognition is critically important, since American children born during the 1970s were exposed to historically unprecedented levels of lead via leaded gasoline and other sources (Egan et al., 2021). If lead-exposed children have worse cognition than their peers in late life, then these cohorts could experience excess risk of cognitive impairment as they age.
Cognitive functioning is a multifaceted construct that encompasses distinct domains, including attention, learning, memory, language, and executive function. These domains are supported by various underlying neural systems, which may be differentially susceptible to social and environmental influences in childhood (Hackman et al., 2010). Previous research has indicated that lead exposure affects distinct cognitive domains differently among children and adolescents (Ris et al., 2004; Tong et al., 1996). For example, Ris and colleagues showed that blood lead level measured at age 6 was significantly associated with academic skills (including reading, spelling, and arithmetic) and fine motor coordination, but not with memory or attention scores at ages 15–17. However, to date prospective studies linking childhood lead exposure to adult cognition have not investigated effects for separate cognitive domains. Furthermore, previous research has only examined effects of lead on cognition at a single point in adulthood, but not on the slope of age-related change in cognitive function. Current evidence suggests that both baseline functioning and rate of cognitive decline in late life are associated with outcomes such as Alzheimer's Disease (AD) and AD-related dementias (Marden et al., 2017; Zahodne et al., 2015). To fill these gaps in knowledge, our study investigates the long-term effect of childhood lead exposure on two distinct domains of cognitive function that were measured twice in an aging cohort.
We provide empirical, prospective evidence regarding the following questions: (1) Does childhood lead exposure negatively affect cognitive function in late life, net of potential confounders? (2) If lead exposure negatively affects late life cognitive function, is this mediated by intervening cognitive, SES, or health pathways?
2. Methods
2.1. Study population and design
Data for this study come from the Wisconsin Longitudinal Study (WLS). One-third of high school seniors in the state of Wisconsin in 1957 (N = 10,317) were randomly recruited into the WLS cohort. The cohort was initially surveyed in 1957 when they were in the 12th grade (ages 17–18). Since then, the cohort has completed 5 follow up surveys, with the most recent waves conducted in 2004 (mean age 64) and 2011 (mean age 71). Beginning in 1977, a sibling of each graduate was randomly selected to be added to the cohort (N = 8,733). Response rates in the WLS sample have been high; approximately 75% of the original cohort responded to the 2004 survey (Herd et al., 2014). The sample used in these analyses is includes both WLS graduates and siblings. Further details of this cohort have been described elsewhere (Herd et al., 2014).
The WLS has released a dataset in which the cohort has been linked to their household records in the 1940 census, which occurred when most cohort members were infants. Of the 19,050 cohort members (including both graduates and siblings), approximately 90% (N = 17,103) were located in the 1940 census. We use information from the census records to measure our exposure variable, described below.
2.2. Assessment of childhood lead exposure
The WLS does not contain direct measurements of blood lead levels in childhood. Instead, we operationalize lead exposure as living in a lead-mining town in 1940. Lead from mines contaminates surrounding air, soil, and water (Li et al., 2014). Young children who exhibit hand-to-mouth behavior are especially susceptible to lead-contaminated soil (Bello et al., 2016; Lanphear et al., 2002; Mielke & Reagan, 1998). Humans and animals living near a lead mine have higher blood lead levels on average than those who are not exposed to a lead mine (Berglund et al., 2010; Beyer et al., 2013; Choudhari et al., 2010; Dong et al., 2019). For example, one study showed that children living <2.5 km from a lead-zinc mine had blood lead levels that were 1.6 and 3.9 μg/dl higher for boys and girls, respectively, than children living at least 10 km away from the mine (Choudhari et al., 2010). Studies have shown that even these small differences in lead exposure among children can result in significant cognitive deficits (Bellinger, 2008; Lanphear et al., 2005). Our measure of exposure is based on the informed assumption that cohort members who lived near a lead mine as children experienced higher levels of environmental lead exposure than their peers, on average.
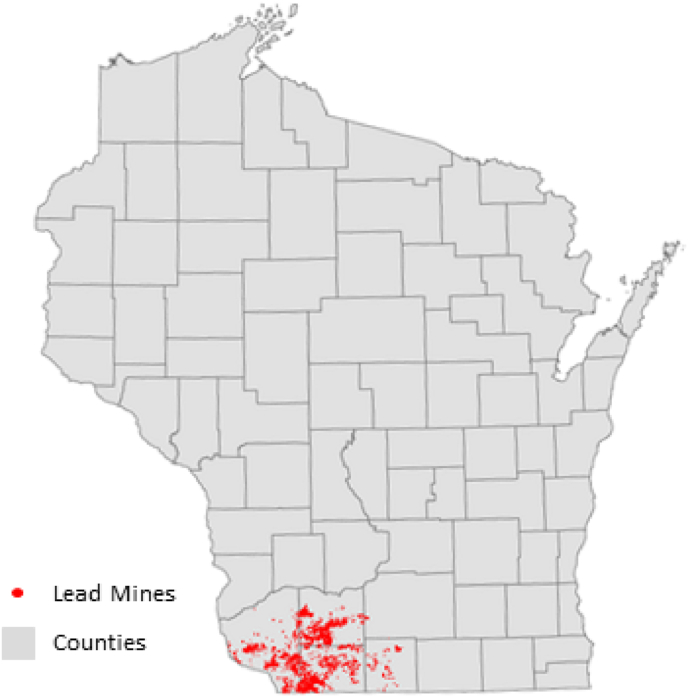
We identified children living near a lead mine using detailed spatial information from historical maps and the 1940 census. Digitized historical maps revealed the location of each lead mine that was in operation in Wisconsin in the early 1940s (Fig. 1). (Pepp et al., 2019) Lead production occurred heavily in the Southwest part of the state (Agnew & Heyl, 1946; Pepp et al., 2019). Using GIS, we calculated the shortest distance between the geographic center of each city, town, or village in the 1940 census with each historical lead mine. We defined lead-mining towns as those containing an historical lead mine within a 10-km radius of the town center. Using census data, we identified whether cohort members grew up in a lead-mining town using the name of the town they resided in during the 1940 enumeration. Of the 1322 unique towns that WLS respondents were living in during 1940, 89 contained a lead mine.
Fig. 1.
Location of lead mines in Wisconsin circa 1940.
Source: Digital Atlas of Historic Mining Activity in Southwestern Wisconsin (Pepp et al., 2019).
2.3. Assessment of late life cognitive outcomes
Late life cognition was measured in the two most recent follow-up surveys, occurring in 2004 (mean age 64) and 2011 (mean age 71). Multiple cognitive assessments were administered during these waves. Using a subscale of the Weschler Adult Intelligence Scale-Revised (WAIS-R), researchers asked participants to name similarities between six pairs of objects, such as an orange and a banana (Wechsler, 1981). Letter fluency was measured by asking participants to name as many words as they could beginning with the letter “L” or “F” in 1 min; similarly, category fluency was measured by asking participants to name all the animals or foods they could think of in 1 min (Tombaugh et al., 1999). Immediate and delayed word recall was measured by reading a list of 10 words and asking participants to repeat the words back immediately and several minutes later (Brandt et al., 1988). Finally, participants were asked to complete a digit ordering task by reordering a series of single digits from smallest to largest (Wechsler, 1997).
We conducted factor analyses using the six cognitive assessments. Results indicated a two-factor solution in which immediate word recall, delayed word recall, and digit ordering formed “memory/attention; ” and WAIS-R similarities, letter fluency, and category fluency formed “language/executive function” (see online supplement “Appendix A” for further details). Our factor analysis replicated results from previous studies of cognition in the WLS (Greenfield & Moorman, 2019; Moorman et al., 2018, 2019). Because these assessments used different metrics, we first transformed raw scores into the percentage of points earned out of the maximum possible for each test, then we averaged these scores within each domain (Cohen et al., 1999). Finally, we standardized the memory/attention and language/executive function scores in each wave by subtracting the baseline mean and dividing by the baseline standard deviation.
2.4. Confounders: childhood socio-economic and demographic factors
To account for potential confounding in the estimated effect of lead exposure on late life cognition, we controlled for childhood socio-economic and demographic factors measured in the 1940 census. These included parents' education (the highest of mother or father; coded as less than 12 years, 12 years, 13 or more years); logged parents’ wages; whether either parent reported non-wage income greater than $50; urban/rural status; whether the respondent lived on a farm; and whether the respondent resided in Southwest Wisconsin (including Columbia, Crawford, Dodge, Grant, Green, Iowa, Jefferson, Lafayette, Richland, Rock, and Sauk counties).
2.5. Mediator: adolescent cognition
We examined whether the effect of childhood lead exposure on late life cognitive function is mediated by adolescent cognition. Adolescent IQ was measured in 11th grade (age 16–17) using the Henmon-Nelson test, which assesses verbal and quantitative skills (Henmon & Nelson, 1954). This test is highly correlated with other IQ assessments: in one previous study, Henmon-Nelson scores were correlated at 0.83 with the full scale Wechsler Adult Intelligence Scale (WAIS) (Watson et al., 1981). Adolescent IQ scores were standardized to have a mean 0 and standard deviation 1.
2.6. Mediators: adult socio-economic and health factors
We adjusted for several mediating socio-economic and health mediating factors measured during adulthood. These include educational attainment (categorized as high school or less, some college, Bachelor's degree or higher) and net worth (sum of all financial assets minus outstanding debts, measured in quartiles), which were measured during the 1992 follow up; along with self-reported health (excellent/very good/good vs. fair/poor), self-reported hypertension, and self-reported heart disease, which were measured in 2004.
We also adjusted our statistical models for gender (male or female) and age at the 2004 survey. We did not adjust for race or ethnicity in any model because nearly all WLS respondents were non-Hispanic White, reflecting the composition of Wisconsin high school graduates in 1957 (Herd et al., 2014).
2.7. Sample restrictions and missing data imputation
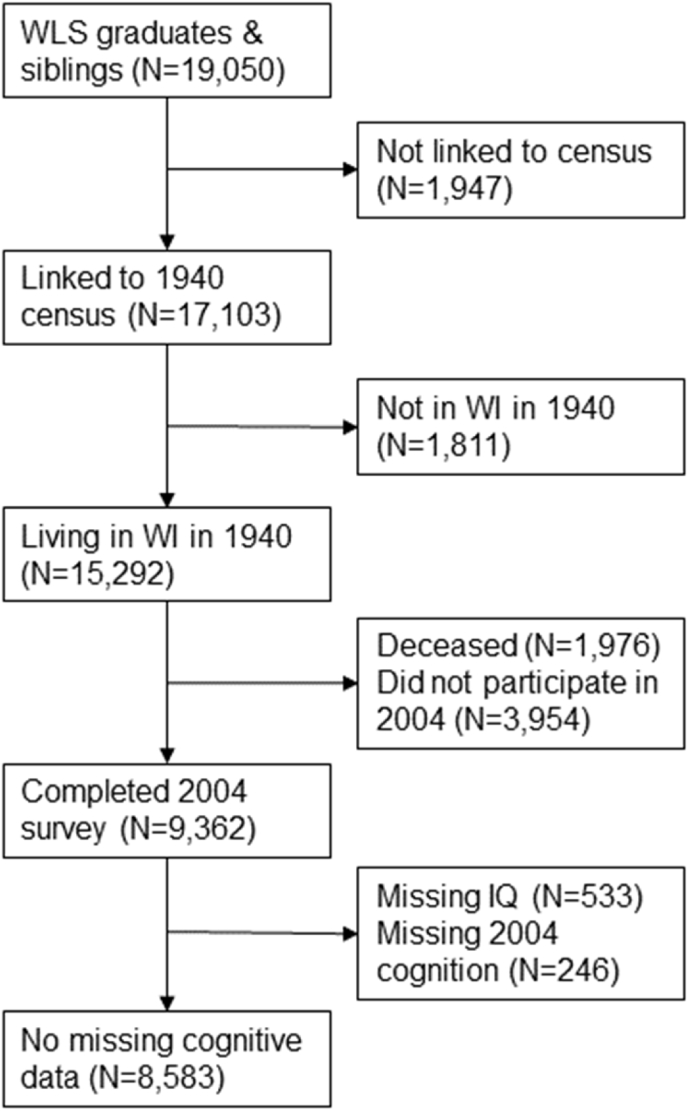
We excluded some WLS members from our analyses (Fig. 2). Of the 19,050 graduates and siblings, we excluded 1947 who were not linked to the 1940 census. We excluded an additional 1811 who were not living in Wisconsin in 1940. We further restricted the sample to those who completed the 2004 survey, thus excluding those who had died (N = 1976) or were lost to follow up (N = 3954). Finally, we excluded those who were missing data for adolescent IQ (N = 533) and those missing both memory/attention and language/executive function scores in 2004 (N = 246). Age and gender were significantly associated with exclusion due to missing IQ or late life cognitive scores. In order to make the sample representative of respondents who were excluded due to missing cognitive variables, we calculated and employed inverse probability of exclusion weights. The final analytic sample included 8583 participants.
Fig. 2.
Sample exclusion criteria.
Due to random sub-sampling for the late life cognitive tests, not every participant completed both memory/attention and language/executive function assessments in 2004. Because this selection was done randomly, the findings are not biased by the exclusion of those who did not take these tests. The regression models estimating effects on memory/attention rely on data from 6638 WLS participants, of whom 5088 participants completed memory/attention assessments again in 2011. The regression models estimating effects on language/executive function rely on data from 8238 participants, of whom 6215 completed language/executive function assessments again in 2011.
Some participants were missing values for covariates, including parents' education (N = 87), parents' wages (N = 432), parents’ non-wage income (N = 129), respondent education (N = 353), net worth (N = 946), self-rated health (N = 4), hypertension (N = 9), and heart disease (N = 10). To retain as many people as possible in the sample, and to reduce bias due to non-random missingness, we imputed missing values for these variables (Little & Rubin, 2002). We used the MICE function in Stata to impute 10 datasets, then averaged estimated coefficients across these datasets to produce our final results.
2.8. Statistical analysis
We first describe differences in the sample between those living in mining and non-mining towns. We tested for statistically significant differences using t-tests for continuous variables or chi squared for categorical variables.
To test whether childhood lead exposure affected late life memory/attention and language/executive function, we estimated multilevel linear regression models where up to two observations of each outcome were clustered within individuals. We included participants in the models who dropped out or died between the 2004 and 2011 surveys. Multilevel models can accommodate differing numbers of observations per cluster (Hox et al., 2010). We estimated coefficients for baseline (i.e., 2004) cognition and cognitive decline between 2004 and 2011. Associations with cognitive decline were estimated using interaction terms between each variable and a 2011 survey dummy variable. Baseline cognition was allowed to vary across individuals with a random intercept; however, we did not include a random age slope for cognitive decline because random slopes require at least three observations per individual (Hox et al., 2010). Because the exposure is measured at the town-level, we clustered the standard errors on unique towns.
We estimated a series of four nested models for each cognitive outcome. Model 1 included no covariates. In Model 2, we adjusted for childhood socio-economic and demographic factors. In Model 3, we adjusted for adolescent IQ. In Model 4, we further adjusted for respondents’ education, net worth, self-rated health, hypertension, and heart disease. To better understand the effect of lead exposure on baseline cognition and cognitive decline, we plotted marginal effects for each outcome from Model 4.
The sequence of our models is designed to address our two research questions specified above. Model 2 is intended to answer our first research question: Does lead effect late life cognition independently of socio-economic and demographic confounders? Models 3 and 4 are intended to answer our second research question: Is the effect of lead on late life cognition mediated through intervening cognitive, SES, or health pathways? We infer mediation from attenuation of the “lead-mining town” coefficient across models 2–4.
2.9. Sensitivity analysis
All the WLS respondents who were exposed to lead mining as children were living in Southwestern Wisconsin during the 1940 census. To check whether geographic clustering of our main exposure was driving our results, we estimated additional models restricted to individuals living in a Southwestern county as children.
3. Results
3.1. Descriptive statistics by childhood lead exposure
Descriptive statistics for all measures are presented in Table 1. The average age at baseline was 64.4. Approximately 54% of the sample (N = 4601) were female. There were several statistically significant differences between sample members who lived in a lead mining town as children (N = 324) and those who did not (N = 8259). On average, participants who grew up in a mining town had lower parental wages, although they were more likely to report non-wage income greater than $50. Those who lived in mining towns were also more likely to live in a rural area and to live on a farm as children. All of the lead-exposed respondents lived in Southwest Wisconsin as children, compared with just 15% of other respondents. There were no significant differences between groups in adolescent IQ. As adults, participants exposed to lead mining had lower levels of education and were less likely to report having heart disease. Living in a lead mining town was not associated with baseline (2004) memory/attention; however, those who grew up in a mining town had significantly lower memory/attention scores in 2011 and lower language/executive function in both waves.
Table 1.
Descriptive statistics by proximity to lead mine: WLS graduates and siblings.
| Obser-vations | Full Sample (N = 8583) | Stratified by Exposure |
|||
|---|---|---|---|---|---|
| No Lead Mine (N = 8259) | Lead Mine (N = 324) | p value | |||
| Demographics | |||||
| Baseline Age, M ± SD | 8583 | 64.4 ± 3.8 | 64.4 ± 3.8 | 64.0 ± 3.8 | 0.052 |
| Female, N(%) | 8583 | 4601 (54) | 4427 (54) | 174 (54) | 0.971 |
| Childhood Variables | |||||
| Live in lead mining town, N(%) | 8583 | 324 (4) | |||
| Parents' Education, N(%) | 8496 | 0.783 | |||
| <12 years | 4318 (51) | 4163 (51) | 155 (49) | ||
| High school graduate | 2604 (31) | 2503 (31) | 101 (32) | ||
| Some college or more | 1574 (19) | 1513 (19) | 61 (19) | ||
| Log(parents' wages), M ± SD | 8151 | 5.0 ± 3.2 | 5.0 ± 3.1 | 2.8 ± 3.3 | <0.001 |
| Parents >$50 of non-wage income, N(%) | 8454 | 3382 (40) | 3164 (39) | 218 (69) | <0.001 |
| Rural, N(%) | 8583 | 4355 (51) | 4055 (49) | 300 (93) | <0.001 |
| Farm, N(%) | 8583 | 2570 (30) | 2374 (29) | 196 (60) | <0.001 |
| Southwest WI, N(%) | 8583 | 1563 (18) | 1239 (15) | 324 (100) | <0.001 |
| Adolescent IQ Z-Score, M ± SD | 8583 | 0.0 ± 1.0 | 0.0 ± 1.0 | −0.1 ± 1.1 | 0.081 |
| Adult Variables | |||||
| Education, N(%) | 8230 | 0.007 | |||
| High school or less | 4571 (56) | 4376 (55) | 195 (63) | ||
| Some college | 1360 (16) | 1327 (17) | 33 (10) | ||
| Bachelor's degree | 2299 (28) | 2216 (28) | 83 (27) | ||
| Net Worth, N(%) | 7637 | 0.328 | |||
| Q1 (≤76,000) | 1924 (25) | 1837 (25) | 87 (29) | ||
| Q2 (76,001 to 148,000) | 1906 (25) | 1837 (25) | 69 (24) | ||
| Q3 (148,001 to 290,000) | 1903 (25) | 1834 (25) | 69 (24) | ||
| Q4 (≥290,001) | 1904 (25) | 1837 (25) | 67 (23) | ||
| Fair/Poor Health, N(%) | 8579 | 862 (10) | 831 (10) | 31 (10) | 0.770 |
| Hypertension, N(%) | 8574 | 4160 (49) | 4018 (49) | 142 (44) | 0.085 |
| Heart Disease, N(%) | 8573 | 1300 (15) | 1269 (15) | 31 (10) | 0.004 |
| Cognitive Variables | |||||
| 2004 Memory/Attention Z-Score, M ± SD | 6638 | 0.0 ± 1.0 | 0.0 ± 1.0 | 0.0 ± 1.1 | 0.456 |
| 2011 Memory/Attention Z-Score, M ± SD | 5088 | −0.3 ± 0.8 | −0.3 ± 0.8 | −0.5 ± 0.9 | 0.002 |
| 2004 Language/Executive Z-Score, M ± SD | 8238 | 0.0 ± 1.0 | 0.0 ± 1.0 | −0.2 ± 0.9 | <0.001 |
| 2011 Language/Executive Z-Score, M ± SD | 6215 | −0.2 ± 0.8 | −0.2 ± 0.8 | −0.4 ± 0.8 | <0.001 |
Note: p-values derived from chi-squared tests (for categorical variables) or t-tests (for continuous variables. “Observations” column indicates the number of sample respondents who were not missing data for each variable before imputation.
3.2. Association between childhood lead exposure and late life memory
Table 2 shows the estimated fixed effects from multilevel regression models of late life memory/attention. Coefficients in the “intercept” column reflect associations with baseline (2004) memory/attention, while coefficients in the “slope” column reflect associations with changes in memory/attention between waves—that is, between 2004 and 2011.
Table 2.
Regression of memory/attention score on proximity to lead mine: WLS graduates and siblings 2004–2011 (N = 6638).
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Intercept | Slope | Intercept | Slope | Intercept | Slope | Intercept | Slope | |
| Constant | −0.01 | −0.31*** | −0.27*** | −0.19*** | −0.24*** | −0.19*** | −0.35*** | −0.11 |
| (-0.06,0.05) | (-0.34,−0.28) | (-0.38, −0.15) |
(-0.29, −0.10) | (-0.34, −0.13) |
(-0.28, −0.10) | (-0.48, −0.22) |
(-0.21, 0.00) | |
| Lead mining town in childhood | −0.03 | −0.14 | 0.07 | −0.16* | 0.09 | −0.17* | 0.09 | −0.18* |
| (-0.18, 0.12) | (-0.28, 0.01) | (-0.08, 0.23) | (-0.32, 0.00) | (-0.07, 0.24) | (-0.33, −0.01) | (-0.06, 0.24) | (-0.34, −0.02) |
|
| Demographic variables | ||||||||
| Baseline age | −0.03*** | −0.01** | −0.03*** | −0.01 | −0.03*** | −0.01 | ||
| (-0.04, −0.02) |
(-0.02, 0.00) | (-0.04, −0.02) |
(-0.02, 0.00) | (-0.04, −0.02) |
(-0.02, 0.00) | |||
| Female | 0.44*** | −0.14*** | 0.43*** | −0.15*** | 0.46*** | −0.17*** | ||
| (0.37, 0.50) | (-0.20, −0.09) | (0.37, 0.49) | (-0.21, −0.09) | (0.40, 0.52) | (-0.23, −0.11) |
|||
| Childhood variables | ||||||||
| Parents' Education | ||||||||
| High school graduate | 0.13*** | −0.04 | 0.06* | −0.05 | 0.03 | −0.04 | ||
| (0.07, 0.18) | (-0.10, 0.02) | (0.01, 0.12) | (-0.11, 0.01) | (-0.03, 0.08) | (-0.10, 0.02) | |||
| College | 0.19*** | −0.02 | 0.08* | −0.04 | −0.01 | −0.01 | ||
| (0.11, 0.27) | (-0.12, 0.09) | (0.00, 0.15) | (-0.15, 0.06) | (-0.08, 0.06) | (-0.11, 0.10) | |||
| log(Parents' Wages) | 0.00 | −0.01 | 0.00 | −0.01 | 0.00 | −0.01 | ||
| (-0.01, 0.01) | (-0.02, 0.00) | (-0.01, 0.01) | (-0.02, 0.00) | (-0.01, 0.01) | (-0.02, 0.00) | |||
| Parents >$50 non-wage income | 0.03 | −0.06 | 0.01 | −0.06 | 0.00 | −0.06 | ||
| (-0.04, 0.10) | (-0.12, 0.01) | (-0.05, 0.08) | (-0.13, 0.01) | (-0.06, 0.07) | (-0.12, 0.01) | |||
| Rural | −0.04 | −0.07 | −0.01 | −0.06 | 0.00 | −0.07 | ||
| (-0.12, 0.04) | (-0.14, 0.00) | (-0.09, 0.06) | (-0.13, 0.01) | (-0.07, 0.07) | (-0.13, 0.00) | |||
| Farm | −0.10* | 0.04 | −0.09* | 0.04 | −0.08* | 0.03 | ||
| (-0.17, −0.02) |
(-0.04, 0.13) | (-0.16, −0.02) |
(-0.04, 0.12) | (-0.15, −0.01) |
(-0.05, 0.11) | |||
| Southwest WI | −0.10* | 0.06 | −0.09* | 0.06 | −0.09* | 0.06 | ||
| (-0.18, −0.02) |
(-0.01, 0.13) | (-0.17, −0.02) |
(-0.01, 0.13) | (-0.17, −0.02) |
(-0.01, 0.13) | |||
| Highschool IQ Z-Score | 0.22*** | 0.05*** | 0.17*** | 0.07*** | ||||
| (0.19, 0.25) | (0.03, 0.08) | (0.14, 0.20) | (0.04, 0.10) | |||||
| Late life variables | ||||||||
| Education | ||||||||
| Some College | 0.17*** | −0.11** | ||||||
| (0.11, 0.23) | (-0.18, −0.04) |
|||||||
| BA+ | 0.24*** | −0.10* | ||||||
| (0.17, 0.30) | (-0.18, −0.02) |
|||||||
| Net Worth | ||||||||
| Quartile 2 | 0.05 | 0.01 | ||||||
| (-0.04, 0.14) | (-0.08, 0.10) | |||||||
| Quartile 3 | 0.07 | −0.04 | ||||||
| (-0.03, 0.16) | (-0.12, 0.05) | |||||||
| Quartile 4 | 0.14** | −0.08* | ||||||
| (0.05, 0.22) | (-0.16, 0.00) | |||||||
| Fair/Poor Health | −0.23*** | 0.00 | ||||||
| (-0.31, −0.15) |
(-0.11, 0.11) | |||||||
| Hypertension | −0.02 | −0.01 | ||||||
| (-0.07, 0.02) | (-0.07, 0.04) | |||||||
| Heart Disease | −0.02 | −0.03 | ||||||
| (-0.10, 0.06) | (-0.11, 0.05) | |||||||
*p < 0.05; **p < 0.01; ***p < 0.001.
Standard errors are clustered at town level. Missing data for covariates were imputed.
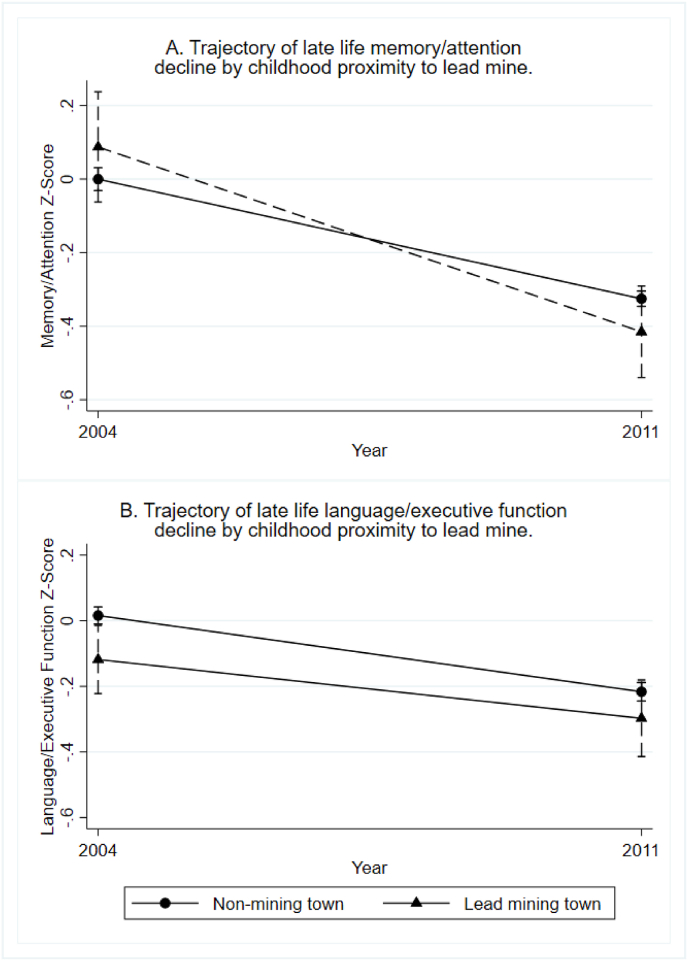
In Model 1, adjusting for no covariates, we observed no significant relationship between childhood lead exposure and baseline memory/attention (β = −0.03; CI = −0.18, 0.12) or rate of memory/attention decline (β = −0.14; CI = −0.28, 0.01). After adjusting for potentially confounding socio-economic and demographic factors in Model 2, the estimated effect of childhood lead exposure on the intercept of memory/attention was still not significant. However, the estimated slope effect increased slightly in magnitude and indicated a significantly steeper rate of decline in memory/attention for those who were exposed to lead as children (β = −0.16; CI = −0.32, 0.00). This significant effect on memory/attention decline was nearly identical in Model 3 after adjusting for adolescent IQ and Model 4 after adjusting for respondent education, net worth, self-rated health, hypertension, and heart disease. The steeper decline in memory/attention for lead-exposed participants is visualized in Panel A of Fig. 3.
Fig. 3.
Marginal effects of childhood exposure to lead-mining on trajectories of cognitive decline in late life: WLS Graduates and Siblings.
3.3. Association of childhood lead exposure with late life language/executive functioning
Estimated fixed effects from multilevel regression models of language/executive function are presented in Table 3. We observed a significant effect of childhood lead exposure on baseline language/executive function in late life, but not rate of decline. In Model 1, our bivariate regression estimates suggest that participants who grew up in a lead mining town had approximately a 1/4 standard deviation lower language/executive function score at baseline (β = −0.23; CI = −0.35, −0.11). This coefficient was attenuated, though was still negative and significant, in Model 2 after adjusting for potentially confounding childhood socio-economic and demographic variables (β = −0.15; CI = −0.27, −0.04). In Model 3, we found that the estimated coefficient for childhood lead exposure did not substantially change after adjusting for adolescent IQ, even though adolescent IQ itself is strongly associated with late life language/executive function. Further adjusting for adult socio-economic and health variables (Model 4) did not meaningfully affect the coefficient for childhood lead exposure. In the fully controlled model, living near a lead mine as a child is associated with approximately a 1/8th standard deviation deficit in baseline language/executive function (β = −0.13; CI = −0.24, −0.03).
Table 3.
Regression of language/executive function score on proximity to lead mine: WLS graduates and siblings 2004–2011 (N = 8238).
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Intercept | Slope | Intercept | Slope | Intercept | Slope | Intercept | Slope | |
| Constant | 0.01 | −0.22*** | −0.15** | −0.20*** | −0.08* | −0.20*** | −0.25*** | −0.21*** |
| (-0.05, 0.06) | (-0.24, −0.20) | (-0.24, −0.06) |
(-0.24, −0.15) | (-0.15, −0.02) |
(-0.25, −0.16) | (-0.34, −0.15) |
(-0.27, −0.14) |
|
| Lead mining town in childhood | −0.23*** | 0.03 | −0.15* | 0.06 | −0.13* | 0.05 | −0.13* | 0.05 |
| (-0.35, −0.11) |
(-0.08, 0.14) | (-0.27, −0.04) |
(-0.06, 0.19) | (-0.24, −0.03) |
(-0.07, 0.17) | (-0.24, −0.03) |
(-0.07, 0.17) | |
| Demographic variables | ||||||||
| Baseline age | −0.02*** | −0.01 | −0.01*** | −0.01 | −0.01*** | −0.01 | ||
| (-0.03, −0.01) |
(-0.01, 0.00) | (-0.02, −0.01) |
(-0.01, 0.00) | (-0.02, −0.01) |
(-0.01, 0.00) | |||
| Female | 0.13*** | −0.02 | 0.13*** | −0.03 | 0.18*** | −0.03 | ||
| (0.08, 0.18) | (-0.06, 0.01) | (0.08, 0.17) | (-0.07, 0.01) | (0.13, 0.23) | (-0.07, 0.01) | |||
| Childhood variables | ||||||||
| Parents' Education | ||||||||
| High school graduate | 0.19*** | 0.03 | 0.06** | 0.04 | 0.01 | 0.04 | ||
| (0.14, 0.24) | (-0.02, 0.07) | (0.02, 0.11) | (-0.01, 0.09) | (-0.04, 0.05) | (0.00, 0.09) | |||
| College | 0.38*** | −0.01 | 0.15*** | 0.01 | 0.02 | 0.03 | ||
| (0.32, 0.44) | (-0.06, 0.04) | (0.09, 0.21) | (-0.04, 0.07) | (-0.03, 0.08) | (-0.03, 0.08) | |||
| log(Parents' Wages) | 0.01 | −0.01 | 0.00 | −0.01 | 0.00 | −0.01 | ||
| (0.00, 0.02) | (-0.02, 0.00) | (0.00, 0.01) | (-0.02, 0.00) | (0.00, 0.01) | (-0.02, 0.00) | |||
| Parents >$50 non-wage income | 0.06* | −0.05 | 0.04 | −0.04 | 0.03 | −0.04 | ||
| (0.00, 0.12) | (-0.10, 0.00) | (-0.01, 0.09) | (-0.09, 0.01) | (-0.02, 0.07) | (-0.09, 0.01) | |||
| Rural | −0.11** | 0.02 | −0.09** | 0.02 | −0.07* | 0.02 | ||
| (-0.19, −0.03) |
(-0.03, 0.08) | (-0.15, −0.02) |
(-0.03, 0.07) | (-0.13, −0.01) |
(-0.04, 0.07) | |||
| Farm | −0.02 | −0.02 | 0.00 | −0.03 | 0.02 | −0.03 | ||
| (-0.09, 0.05) | (-0.09, 0.04) | (-0.06, 0.06) | (-0.10, 0.04) | (-0.04, 0.08) | (-0.10, 0.04) | |||
| Southwest WI | −0.04 | −0.05 | −0.03 | −0.05 | −0.03 | −0.05 | ||
| (-0.11, 0.02) | (-0.10, 0.01) | (-0.09, 0.02) | (-0.10, 0.00) | (-0.08, 0.03) | (-0.10, 0.00) | |||
| Highschool IQ Z-Score | 0.40*** | −0.04*** | 0.33*** | −0.04** | ||||
| (0.38, 0.42) | (-0.06, −0.02) | (0.31, 0.35) | (-0.06, −0.01) |
|||||
| Late life variables | ||||||||
| Education | ||||||||
| Some College | 0.22*** | −0.03 | ||||||
| (0.16, 0.27) | (-0.09, 0.02) | |||||||
| BA+ | 0.47*** | −0.06* | ||||||
| (0.41, 0.53) | (-0.11, −0.01) |
|||||||
| Net Worth | ||||||||
| Quartile 2 | 0.00 | 0.04 | ||||||
| (-0.06, 0.07) | (-0.03, 0.10) | |||||||
| Quartile 3 | 0.02 | 0.04 | ||||||
| (-0.04, 0.08) | (-0.02, 0.11) | |||||||
| Quartile 4 | 0.04 | 0.02 | ||||||
| (-0.02, 0.11) | (-0.04, 0.08) | |||||||
| Fair/Poor Health | −0.11*** | −0.08 | ||||||
| (-0.17, −0.05) |
(-0.16, 0.00) | |||||||
| Hypertension | −0.03 | 0.02 | ||||||
| (-0.07, 0.00) | (-0.02, 0.05) | |||||||
| Heart Disease | 0.04 | −0.03 | ||||||
| (-0.01, 0.09) | (-0.09, 0.03) | |||||||
*p < 0.05; **p < 0.01; ***p < 0.001.
Standard errors are clustered at town level. Missing data for covariates were imputed.
We found no significant effect of lead exposure on rate of change in language/executive function score between waves in any of the models. This is apparent in the plotted marginal effects (based on Model 4) in Panel B of Fig. 3.
3.4. Sensitivity analyses
We examined how sensitive our results were to the geographic clustering of lead mining by re-estimating our regression models on a restricted sample of individuals who were living in Southwest Wisconsin in 1940. The results for memory/attention and language/executive function are presented in an online supplement (“Appendix B”). These models produced nearly identical results to those observed in our main analyses.
4. Discussion
Using prospective data collected over more than 70 years, we provide estimates of the association between childhood lead exposure and late life cognition. We found that, compared with their peers, participants who lived near a lead mine as children had significantly greater decline in memory/attention between ages 64 and 71 and significantly lower language/executive function at age 64. The magnitudes of these effects were considerable. Non-lead-exposed respondents experienced about a 1/5th standard deviation drop in memory/attention score between 2004 and 2011 (β = −0.19); lead-exposed respondents experienced memory/attention score decline that was nearly twice as large (β = −0.19 – 0.16 = −0.35), net of confounders. Similarly, in 2004, the difference in language/executive function between lead-exposed respondents and their peers net of confounders (β = −0.15) was approximately equal in size to the effect associated with having high school educated parents (β = 0.19). These negative effects persisted after controlling for potential socio-economic and demographic confounders. Assuming that unobserved factors did not confound this relationship, we interpret these results to indicate that childhood lead exposure may be an independent risk factor for poorer cognitive outcomes in late life.
The “chains of risk” model suggests that long-term consequences of childhood lead exposure could be explained via intervening cognitive, socio-economic, and health pathways (Reuben, 2018). Our data allowed us to test this hypothesis by adjusting for adolescent IQ and adult education, net worth, self-rated health, hypertension, and heart disease. Controlling for these variables in our regression models had almost no consequence for our estimates of the effects of childhood lead exposure. Instead, our analyses provided evidence for a possible latent influence of childhood exposures to lead on later life cognitive outcomes.
There are several hypothesized biological pathways through which childhood lead exposure could have latent effects on late life cognition. One possibility is the remobilization of bone lead. Lead levels in the blood can increase quickly due to environmental exposure; however, after exposure ceases, blood lead levels return to normal in a matter of months (Hu et al., 1998). By contrast, after passing through the blood and soft tissue, lead can lie inert in bones for years or decades (Hu et al., 1998; Rabinowitz, 1991). During bone demineralization, which accelerates in late life, bone lead may re-enter the circulatory system and cause new organ damage decades after the initial exposure (Hu et al., 1998; Reuben, 2018). An alternative explanation is that childhood lead exposure causes epigenetic changes to genes responsible for maintaining brain function in later life. Laboratory studies in mice indicate that early lead exposure affects the expression of certain genes leading to a pathological accumulation of proteins and amyloid-β in the brain, which is considered to be an underlying cause of Alzheimer's Disease (Basha et al., 2005; Huang et al., 2011; Waseem Bihaqi S and Zawia, 2012). Further research is needed to clarify whether these hypothetical pathways are responsible for the latent effect of lead exposure on adult cognition.
Interestingly, there was no significant relationship between living near a lead mine in childhood and adolescent IQ, though a relationship with cognitive functioning emerged later in life. This may seem at odds with the extensive research documenting an association between lead exposure and intelligence among children (Bellinger, 2008; Bellinger et al., 1992; Lanphear et al., 2005). However, evidence from the Dunedin cohort showed a similar pattern to the one we observed in the WLS: There was no association between blood levels and early life cognitive functioning (Silva et al., 1988), but a significant relationship by age 38 (Reuben et al., 2017). There are several potential explanations for this finding. The association between blood levels and IQ in childhood may be moderated by economic resources. There is some evidence, for example, that children with adequate nutrition, including sufficient calcium intake, may be protected from cognitive deficits early in life, but still carry high concentrations of lead from bone stores (Mahaffey, 1990). Given that the WLS cohort is relatively high-SES (Herd et al., 2014), it is possible that they were largely protected from the short-term consequences of childhood lead exposure. Notably, the hypothesized biological pathways through which childhood lead exposure affects late life cognition—such as epigenetic changes or the remobilization of bone lead—may still operate even in absence of short-term effects on adolescent intelligence (Bolin et al., 2006; Dosunmu et al., 2012; Hu et al., 1998; Khalid & Abdollahi, 2019; Rabinowitz, 1991; Reuben, 2018).
Childhood lead exposure was not associated with deficits in memory/attention score at age 64, but children at high risk for lead exposure experienced significantly greater declines in memory/attention between ages 64 and 71, after adjusting for early life confounders and adolescent IQ. Rate of memory decline is a precursor for Alzheimer's Disease and AD-related dementia (Marden et al., 2017). Individuals who experience memory decline late in life have higher risk for dementia than those who had stable low memory scores (Zahodne et al., 2015). These results could indicate that lead-exposed children have higher risk of dementia in late life.
A different pattern of results was observed with language/executive function. Although childhood lead exposure was associated with baseline language/executive function, it did not predict decline in this cognitive skill over time. It is possible that the effect of childhood lead exposure on late life language/executive function occurs before age 64, which was the average baseline age in this study. Previous research on the effect of childhood environment on adult cognition has also found that some cognitive domains are more affected than others, perhaps because of the prolonged development of language/executive function during childhood, making it more susceptible to environmental influences (Greenfield & Moorman, 2019; Hackman et al., 2010).
Our study had numerous strengths. Using a novel combination of census and survey data, we were able to prospectively measure the effect of childhood lead exposure on late life cognition. Additionally, the rich cognitive, socio-economic, and health variables collected across the life course made the WLS valuable for testing potential confounders and mediators.
Despite these strengths, there were notable limitation to our analyses. The WLS did not have direct measurements of childhood blood lead levels. Instead, we relied on proximity to historical lead mines in childhood as a proxy measure. Living near a lead mine may be detrimental to cognition in ways that are not related to environmental lead exposure. Additionally, this measure does not account for other possible sources of lead exposure (e.g., exhaust from leaded gasoline), nor does it allow for variation in level of exposure among those living near a mine. These measurement issues may have biased our estimates.
Selective attrition may also have biased our results if childhood lead exposure was associated with survey retention. For instance, if childhood were associated with early mortality, then a disproportionate number of lead-exposed WLS respondents may not have survived to participate in the late-life follow up waves. To investigate this threat, we tested whether WLS participants who lived near lead mines as children were less likely to participate in the 2004 or 2011 survey rounds. We found no significant association between lead exposure and survey retention.
Our data are observational, and therefore susceptible to bias due to non-random exposure to lead mining as children. We attempted to mitigate this risk by adjusting for prospectively collected childhood socio-economic and demographic factors. Finally, although the WLS is a large population-based sample, it includes mostly those with at least a high school degree, reflecting approximately 80 percent of those graduating from high schools during this period. It also lacks racial diversity, reflecting the characteristics of this cohort in Wisconsin. These results may not be generalizable to other populations.
5. Conclusion
Our study shows that childhood lead exposure may be an independent risk factor for late life cognitive outcomes. As cohorts born in the 1970s—who experienced historically unprecedented levels of lead exposure as children—continue to age, this factor may quickly become an important determinant for the population health of older Americans. Further research is urgently needed to understand the biological mechanisms underlying the long-term consequences of childhood lead exposure, such as remobilization of bone lead and epigenetic modification. Enhanced knowledge of these processes may point toward interventions to improve cognitive outcomes among aging adults who experienced lead exposure.
Author statement
Mark Lee: Conceptualization, Methodology, Formal Analysis, Writing – Original Draft, Haena Lee: Conceptualization, Writing – Reviewing and Editing, John Robert Warren: Conceptualization, Funding Acquisition, Supervision, Writing – Reviewing and Editing, Pamela Herd: Conceptualization, Funding Acquisition, Supervision, Writing – Reviewing and Editing.
Funding
The Wisconsin Longitudinal Study is funded by the National Institute on Aging (NIA) (R01 AG041868; R01 AG060737; R01 AG050300). Support has also come from the Minnesota Population Center, which receives core funding (P2C HD041023) from the Eunice Kennedy Shriver National Institute for Child Health and Human Development (NICHD). Mark Lee was supported by a training grant from the NICHD (T32 HD095134). Haena Lee was supported by an NIA K99 Pathway to Independence award (K99 AG071834).
Ethical statement
Although much of the WLS data are publicly available, census-linked data are available to researchers with approved restricted data use agreements. Participants signed consent forms prior to any data collection and research procedures were approved by the University of Wisconsin's Institutional Review Board.
Declaration of competing interest
None.
Acknowledgements
We thank David Van Riper for GIS support and Jonas Helgertz, Carol Roan, and Joe Savard for their work in producing and disseminating the data used in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ssmph.2022.101037.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Agnew A., Heyl A. Recent developments in the Wisconsin-Illinois-Iowa lead-zinc district. Proceedings of the Iowa Academy of Science. 1946;53(1):225–231. [Google Scholar]
- Basha M.R., Wei W., Bakheet S.A., et al. The fetal basis of amyloidogenesis: Exposure to lead and latent overexpression of amyloid precursor protein and β-amyloid in the aging brain. Journal of Neuroscience. 2005;25(4):823–829. doi: 10.1523/JNEUROSCI.4335-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger D.C. Very low lead exposures and children's neurodevelopment. Current Opinion in Pediatrics. 2008;20(2):172–177. doi: 10.1097/MOP.0b013e3282f4f97b. [DOI] [PubMed] [Google Scholar]
- Bellinger D.C., Stiles K.M., Needleman H.L. Low-level lead exposure, intelligence and academic achievement: A long-term follow-up study. Pediatrics. 1992;90(6):855–861. [PubMed] [Google Scholar]
- Bello O., Naidu R., Rahman M.M., Liu Y., Dong Z. Lead concentration in the blood of the general population living near a lead–zinc mine site, Nigeria: Exposure pathways. The Science of the Total Environment. 2016;542:908–914. doi: 10.1016/j.scitotenv.2015.10.143. [DOI] [PubMed] [Google Scholar]
- Berglund Å.M.M., Ingvarsson P.K., Danielsson H., Nyholm N.E.I. Lead exposure and biological effects in pied flycatchers (Ficedula hypoleuca) before and after the closure of a lead mine in northern Sweden. Environmental Pollution. 2010;158(5):1368–1375. doi: 10.1016/j.envpol.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Beyer W.N., Franson J.C., French J.B., et al. Toxic exposure of songbirds to lead in the southeast Missouri lead mining district. Archives of Environmental Contamination and Toxicology. 2013;65(3):598–610. doi: 10.1007/s00244-013-9923-3. [DOI] [PubMed] [Google Scholar]
- Bolin C.M., Basha R., Cox D., et al. Exposure to lead (Pb) and the developmental origin of oxidative DNA damage in the aging brain. The FASEB Journal. 2006;20(6):788–790. doi: 10.1096/fj.05-5091fje. [DOI] [PubMed] [Google Scholar]
- Brandt J., Spencer M., Folstein M. The telephone interview for cognitive status. Cognitive and Behavioral Neurology. 1988;1(2):111–117. [Google Scholar]
- Caito S., Aschner M. In: Neurotoxicity of metals. Advances in neurobiology. Aschner M., Costa L.G., editors. Springer International Publishing; 2017. Developmental neurotoxicity of lead; pp. 3–12. [DOI] [PubMed] [Google Scholar]
- Choudhari R., Sathwara N.G., Shivgotra V.K., et al. Study of lead exposure to children residing near a lead–zinc mine. Indian Journal of Industrial Medicine. 2010;14(2):58–62. doi: 10.4103/0019-5278.72243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Cohen J., Aiken L.S., West S.G. The problem of units and the circumstance for POMP. Multivariate Behavioral Research. 1999;34(3):315–346. doi: 10.1207/S15327906MBR3403_2. [DOI] [Google Scholar]
- Dong C., Taylor M.P., Zahran S. The effect of contemporary mine emissions on children's blood lead levels. Environment International. 2019;122:91–103. doi: 10.1016/j.envint.2018.09.023. [DOI] [PubMed] [Google Scholar]
- Dosunmu R., Alashwal H., Zawia N.H. Genome-wide expression and methylation profiling in the aged rodent brain due to early-life Pb exposure and its relevance to aging. Mechanism of Ageing and Development. 2012;133(6):435–443. doi: 10.1016/j.mad.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan K.B., Cornwell C.R., Courtney J.G., Ettinger A.S. Blood lead levels in U.S. Children ages 1-11 Years, 1976-2016. Environmental Health Perspectives. 2021;129(3):37003. doi: 10.1289/EHP7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield E.A., Moorman S.M. Childhood socioeconomic status and later life cognition: Evidence from the Wisconsin longitudinal study. Journal of Aging and Health. 2019;31(9):1589–1615. doi: 10.1177/0898264318783489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman D.A., Farah M.J., Meaney M.J. Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nature Reviews Neuroscience. 2010;11(9):651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henmon V., Nelson M. Houghton-Mifflin Company; 1954. The henmon-nelson tests of mental ability, manual for administration. [Google Scholar]
- Herd P., Carr D., Roan C. Cohort profile: Wisconsin longitudinal study (WLS) International Journal of Epidemiology. 2014;43(1):34–41. doi: 10.1093/ije/dys194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hox J.J., Moerbeek M., van de Schoot R. 2nd ed. Routledge; 2010. Multilevel analysis: Techniques and applications. [Google Scholar]
- Huang H., Bihaqi S.W., Cui L., Zawia N.H. In vitro Pb exposure disturbs the balance between Aβ production and elimination: The role of AβPP and neprilysin. NeuroToxicology. 2011;32(3):300–306. doi: 10.1016/j.neuro.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Rabinowitz M., Smith D. Bone lead as a biological marker in epidemiologic studies of chronic toxicity: Conceptual paradigms. Environmental Health Perspectives. 1998;106(1):1–8. doi: 10.1289/ehp.981061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid M., Abdollahi M. Epigenetic modifications associated with pathophysiological effects of lead exposure. Journal of Environmental Science and Health Part C. 2019;37(4):235–287. doi: 10.1080/10590501.2019.1640581. [DOI] [PubMed] [Google Scholar]
- Lanphear B.P., Hornung R., Ho M., Howard C.R., Eberly S., Knauf K. Environmental lead exposure during early childhood. The Journal of Pediatrics. 2002;140(1):40–47. doi: 10.1067/mpd.2002.120513. [DOI] [PubMed] [Google Scholar]
- Lanphear B.P., Hornung R., Khoury J., et al. Low-level environmental lead exposure and children's intellectual function: An international pooled analysis. Environmental Health Perspectives. 2005;113(7):894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Ma Z., van der Kuijp T.J., Yuan Z., Huang L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. The Science of the Total Environment. 2014;468–469:843–853. doi: 10.1016/j.scitotenv.2013.08.090. [DOI] [PubMed] [Google Scholar]
- Little R.J.A., Rubin D.B. 2nd ed. John Wiley & Sons, Inc.; 2002. Statistical analysis with missing data. [Google Scholar]
- Mahaffey K.R. Environmental lead toxicity: Nutrition as a component of intervention. Environmental Health Perspectives. 1990;89:75–78. doi: 10.1289/ehp.908975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marden J.R., Tchetgen Tchetgen E.J., Kawachi I., Glymour M.M. Contribution of socioeconomic status at 3 life-course periods to late-life memory function and decline: Early and late predictors of dementia risk. American Journal of Epidemiology. 2017;186(7):805–814. doi: 10.1093/aje/kwx155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar M., Bellinger D.C., Gregas M., Abanilla K., Bacic J., Needleman H.L. Low-level environmental lead exposure in childhood and adult intellectual function: A follow-up study. Environmental Health. 2011;10(1):24. doi: 10.1186/1476-069X-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke H.W., Reagan P.L. Soil is an important pathway of human lead exposure. Environmental Health Perspectives. 1998;106:217–229. doi: 10.2307/3433922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M.L., Kim D., Galeano M.A.O., Paul C.J., Hull A.P., Morgan S.P. The relationship between early childhood blood lead levels and performance on end-of-grade tests. Environmental Health Perspectives. 2007;115(8):1242–1247. doi: 10.1289/ehp.9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman S.M., Carr K., Greenfield E.A. Childhood socioeconomic status and genetic risk for poorer cognition in later life. Social Science & Medicine. 2018;212:219–226. doi: 10.1016/j.socscimed.2018.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman S.M., Greenfield E.A., Garcia S. School context in adolescence and cognitive functioning 50 Years later. Journal of Health and Social Behavior. 2019;60(4):493–508. doi: 10.1177/0022146519887354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C., Sampson R.J., Winter A.S. Environmental inequality: The social causes and consequences of lead exposure. Annual Review of Sociology. 2018;44(1):263–282. doi: 10.1146/annurev-soc-073117-041222. [DOI] [Google Scholar]
- Navas-Acien A., Guallar E., Silbergeld E.K., Rothenberg S.J. Lead exposure and cardiovascular disease—a systematic review. Environmental Health Perspectives. 2007;115(3):472–482. doi: 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman H.L., Gatsonis C.A. Low-level lead exposure and the IQ of children: A meta-analysis of modern studies. JAMA. 1990;263(5):673–678. doi: 10.1001/jama.1990.03440050067035. [DOI] [PubMed] [Google Scholar]
- Pepp K., Siemering G., Ventura S. 2019. Digital Atlas of historic mining activity in southwestern Wisconsin. [DOI] [Google Scholar]
- Rabinowitz M.B. Toxicokinetics of bone lead. Environmental Health Perspectives. 1991;91:33–37. doi: 10.1289/ehp.919133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben A. Childhood lead exposure and adult neurodegenerative disease. Journal of Alzheimers Diseases JAD. 2018;64(1):17–42. doi: 10.3233/JAD-180267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben A., Caspi A., Belsky D.W., et al. Association of childhood blood lead levels with cognitive function and socioeconomic status at age 38 Years and with IQ change and socioeconomic mobility between childhood and adulthood. JAMA. 2017;317(12):1244–1251. doi: 10.1001/jama.2017.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris M.D., Dietrich K.N., Succop P.A., Berger O.G., Bornschein R.L. Early exposure to lead and neuropsychological outcome in adolescence. Journal of the International Neuropsychological Society. 2004;10(2):261–270. doi: 10.1017/S1355617704102154. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Lead, blood pressure, and cardiovascular disease in men and women. Environmental Health Perspectives. 1991;91:71–75. doi: 10.1289/ehp.919171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva P.A., Hughes P., Williams S., Faed J.M. Blood lead, intelligence, reading attainment, and behaviour in eleven year old children in Dunedin, New Zealand. Journal of Child Psychology and Psychiatry. 1988;29(1):43–52. doi: 10.1111/j.1469-7610.1988.tb00687.x. [DOI] [PubMed] [Google Scholar]
- Tombaugh T.N., Kozak J., Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Archives of Clinical Neuropsychology. 1999;14(2):167–177. doi: 10.1016/S0887-6177(97)00095-4. [DOI] [PubMed] [Google Scholar]
- Tong S., Baghurst P., McMichael A., Sawyer M., Mudge J. Lifetime exposure to environmental lead and children's intelligence at 11-13 years: The port pirie cohort study. BMJ. 1996;312(7046):1569–1575. doi: 10.1136/bmj.312.7046.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri N.D. Mechanisms of lead-induced hypertension and cardiovascular disease. American Journal of Physiology - Heart and Circulatory Physiology. 2008;295(2):H454–H465. doi: 10.1152/ajpheart.00158.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waseem Bihaqi S H., Zawia N. Alzheimer's disease biomarkers and epigenetic intermediates following exposure to Pb in vitro. Current Alzheimer Research. 2012;9(5):555–562. doi: 10.2174/156720512800617964. [DOI] [PubMed] [Google Scholar]
- Watson C.G., Klett W.G., Kucala T., Nixon C., Schaefer A., Gasser B. Prediction of the wais scores from the 1973 henmon-nelson revision. Journal of Clinical Psychology. 1981;37(4):840–842. doi: 10.1002/1097-4679(198110)37:4<840::AID-JCLP2270370427>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corporation; 1981. Wechsler adult intelligence scale-revised (WAIS-R) [Google Scholar]
- Wechsler D. Psychological Corporation; 1997. WAIS-III, wechsler adult intelligence scale: Administration and scoring manual. [Google Scholar]
- Zahodne L.B., Wall M.M., Schupf N., et al. Late-life memory trajectories in relation to incident dementia and regional brain atrophy. Journal of Neurology. 2015;262(11):2484–2490. doi: 10.1007/s00415-015-7871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.