Abstract
The diffusion-limited reaction of nitric oxide (NO) and superoxide (O2−) produces peroxynitrite (ONOO−), a biological oxidant that has been implicated in a number of pathological conditions, including neurodegenerative disorders. We previously reported that incubation of PC12 cells with peroxynitrite triggers apoptosis by simultaneously inhibiting the PI3K/Akt survival pathway, and activating the p38 and JNK MAP kinase pathways. We also reported that peroxynitrite-treated Heat Shock Protein 90 (Hsp90) stimulates PC12 cell death. Here, we show that nitrated Hsp90 mediates peroxynitrite-induced apoptosis by regulating specific signaling pathways triggered by activation of the purine receptor P2X7 (P2X7R) and downstream activation of PTEN. Intracellular delivery of peroxynitrite-treated Hsp90 was sufficient to stimulate PC12 cell death. In contrast, intracellular delivery of peroxynitrite-treated Hsp90 in which the five tyrosine (Tyr) residues susceptible to nitration were replaced by nitration-resistant phenylalanine had no effect on PC12 cell survival. Further, only nitration of Hsp90 at Tyr 56 was necessary and sufficient to stimulate PC12 cell apoptosis, and incubation of PC12 cells with peroxynitrite resulted in Hsp90 nitration at Tyr 56. Inhibition of P2X7R or downstream inhibition of PTEN prevented PC12 cell death stimulated by both incubation with peroxynitrite and nitrated Hsp90 (Hsp90NY). Peroxynitrite, Hsp90NY, and P2X7R activation all increased p38 and JNK MAP kinases activity, while inhibiting the Akt survival pathway. These results suggest that, in undifferentiated PC12 cells, peroxynitrite triggers apoptosis via nitration of Hsp90 at Tyr 56, which in turn activates P2X7R and PTEN. These results contrast with observations in motor neurons where the nitration of either Tyr 33 or Tyr 56 in Hsp90 stimulates apoptosis, suggesting that the targets of peroxynitrite may be different in different cell types. However, uncovering the pathways through which peroxynitrite triggers cell death in neurodegenerative conditions will provide new potential targets for therapeutic treatment.
Keywords: Peroxynitrite, Nitrotyrosine, Hsp90, P2X7 receptor, Apoptosis
Graphical abstract
Highlights
-
•
Peroxynitrite and Hsp90 nitrated in Tyr residue 56 activated both p38 and JNK MAP kinases to trigger apoptosis.
-
•
Peroxynitrite and nitrated Hsp90 activate the ATP-gated P2X7 ion channel.
-
•
Activation of P2X7 receptor inhibits the PI3K/Akt pathway via activation of PTEN.
-
•
Nitration of a single residue in Hsp90 by peroxynitrite is sufficient to trigger PC12 cell apoptosis.
Abbreviations
- BBG
Brilliant Blue G
- BzATP
3′-O-(4-Benzoyl)benzoyladenosine 5′-triphosphate
- FGF
Fibroblast Growth Factor
- Hsp90
Heat Shock Protein 90
- Hsp90NY
Nitrated Hsp90
- JNK
c-Jun N-terminal Kinase
- MLK
Mixed Linage Kinase
- NGF
Nerve Growth Factor
- NitroTyr
Nitrotyrosine
- ONOO−
Peroxynitrite
- P2X7R
P2X7 Receptor
- PI3K
Phosphatidylinositol 3 kinase
- PTEN
Phosphate and Tensin Homolog
1. Introduction
Under inflammatory and pathological conditions, the production of nitric oxide and superoxide increases, resulting in the formation of peroxynitrite by a diffusion-limited reaction [[1], [2], [3]]. Peroxynitrite has been implicated in the induction of neuronal death in a number of conditions affecting the central nervous system, including Parkinson's disease, Alzheimer's disease, amyotrophic lateral sclerosis, stroke, multiple sclerosis, and spinal cord injury [1,[4], [5], [6], [7]]. Peroxynitrite can directly or indirectly oxidize biomolecules such as lipids and proteins, which in turn are responsible for the toxicity of the oxidant [4,[8], [9], [10], [11], [12]].
Peroxynitrite induces cell death through a variety of mechanisms, including apoptosis and necrosis in a concentration-dependent manner [11,[13], [14], [15], [16]]. Incubation of the pheochromocytoma-derived PC12 cell line with peroxynitrite, or stimulation of endogenous peroxynitrite generation triggers apoptosis [13,17,18]. Peroxynitrite-dependent induction of apoptosis requires the simultaneous inhibition and activation of intracellular signaling pathways [13,[19], [20], [21]]. Intriguingly, treatment with nerve growth factor (NGF) prior to peroxynitrite incubation provides protection against apoptosis in these cells by upregulating phosphatidylinositol 3 kinase (PI3K) activity [19]. In contrast, incubation with fibroblast growth factor (FGF) before peroxynitrite treatment, or NGF after peroxynitrite treatment enhances the toxicity of the oxidant [13,20], suggesting that peroxynitrite act on a number of signaling pathways that are differentially regulated by growth factors. We previously reported that peroxynitrite-induced apoptosis in PC12 cells is regulated by the simultaneous but independent inhibition of the PI3K/Akt survival pathway, and the activation of p38 and JNK MAP kinases [21]. Taken together, these studies suggest that peroxynitrite-mediated apoptosis is triggered by the activation of specific oxidative signaling pathways, rather than due to broad oxidative damage. However, the identity of the oxidative modification and the molecular targets that mediate peroxynitrite effects on signaling pathways are not fully understood.
Among peroxynitrite oxidative protein modifications, nitrotyrosine is one of the most widely used biomarkers of oxidative stress [8,[22], [23], [24]]. However, tyrosine (Tyr) nitration may be more than a simple byproduct of peroxynitrite production, it may have actual pathological consequences. Prevention of Tyr nitration is sufficient to protect PC12 cells and motor neurons from peroxynitrite-induced apoptosis, suggesting that nitration of one or more proteins plays a key role in the regulation of the signaling pathways triggering apoptosis [13,25]. One such target is the molecular chaperone heat shock protein 90 (Hsp90). Site-specific Tyr nitration of Hsp90 results in a toxic gain-of-function that stimulates motor neuron and PC12 cell death [26]. Hsp90 is a molecular chaperone responsible for the stabilization, activation, and inactivation of over 300 client proteins involved in all aspects of cellular metabolism, including the regulation of cell survival and death [[27], [28], [29], [30], [31]]. Peroxynitrite-mediated nitration of Hsp90 at Tyr 33 and/or Tyr 56 in motor neurons turns the chaperone into a mediator of cell death through the activation of the purine receptor P2X7 (P2X7R) and the Fas death pathway [26].
Collectively, these results suggest that nitration of specific tyrosine residues in Hsp90 plays a key role in peroxynitrite-induced apoptosis. Here, we investigated the hypothesis that nitration of Hsp90 mediates peroxynitrite-induced apoptosis, which requires the activation of the P2X7 receptor. To this end, we investigated whether peroxynitrite and intracellularly released site-specific nitrated Hsp90 activated the same signaling pathways to induce PC12 cell apoptosis.
2. Materials and Methods
2.1. Synthesis of peroxynitrite
Peroxynitrite was synthesized by the rapid addition of acidified hydrogen peroxide to sodium nitrate. The reaction was quenched with sodium hydroxide, and manganese dioxide was used to remove residual hydrogen peroxide, as previously described [32]. Peroxynitrite concentration was determined immediately prior to each experiment by spectrophotometry (ε302 = 1800 M−1cm−1).
2.2. PC12 cell culture and peroxynitrite treatment
PC12 cells were cultured on collagen-coated dishes in RPMI medium (Gibco/Invitrogen), supplemented with 10% horse serum, 5% FetalClone II (Hyclone), and 1% Pen-Strep by volume at 37 °C and 5% CO2 in a humidified atmosphere for 24 h prior to incubation with peroxynitrite. Cells were rinsed with warm PBS containing Ca2+- and Mg2+prior to incubation with 0.5 mM peroxynitrite for 3 min in 50 mM phosphate buffer saline, as previously described [13,21]. After treatment, the buffer was removed and replaced with complete RPMI medium. Decomposed peroxynitrite (ROA) was prepared by diluting peroxynitrite in 50 mM phosphate buffer saline for 24 h at room temperature before addition to the cells [13,21]. Viability was assessed 24 h after incubation with peroxynitrite by incubating the cells in phenol red-free RPMI medium (Gibco/Invitrogen) with a mixture of fluorescein diacetate (FDA; 15 μg/mL) and propidium iodide (PI; 5 μg/mL) for 15 min at 37 °C and 5% CO2 in a humidified atmosphere. Fluorescent images were captured with the RUNNER (Trophos, Marseilles, France). Cell counts were performed with the Metamorph software. Viability was calculated as the fraction of cells positive for FDA relative to the total number of cells (FDA + PI) in each well.
2.3. Production and purification of Hsp90 using gene code expansion
Human recombinant unmodified Hsp90, and nitroTyr-specific Hsp90 were produced using genetic code expansion and expressed in bacterial cultures by induction with l-arabinose for 2 h at 37 °C as previously described [26,33,34]. All recombinant proteins were purified using the Ni-NTA purification system (Invitrogen/Life Technologies) according to the manufacturer's instructions.
2.4. Peroxynitrite treatment of recombinant Hsp90
A freshly prepared dilution of peroxynitrite was quickly added to 30 μL of 1 mg/mL Hsp90 in PBS while gently vortexing to reach a final concentration of 0.5 mM peroxynitrite.
2.5. Intracellular delivery of proteins and viability determination
Intracellular delivery of recombinant unmodified Hsp90, Hsp90 with nitroTyr at position 33 (Hsp90NY33) or 56 (Hsp90NY56), or peroxynitrite-treated Hsp90, was accomplished by incubating 10 μg of protein with 4 μL of Chariot (Active Motif) permeating agent in 100 μL of H2O and 100 μL of PBS for 30 min at room temperature, as previously described [26,35]. Two hundred μL of the chariot-protein mixture was added to 1 × 106 PC12 cells, mixed gently in 400 μL RPMI, and incubated at 37 °C and 5% CO2 for 1 h. An additional 1.5 mL of complete RPMI medium supplemented with 10% horse serum and 5% FetalClone II was added to the mixture before incubating for an additional hour. Cells were plated in the appropriate dishes in complete RPMI medium. Viability was determined using either fluorescein diacetate (FDA)/propidium iodide (Molecular Probes) as described above and confirmed using either CyQuant Direct Proliferation Assay (Life Technologies), or the Live/Death™ Viability/Cytotoxicity Kit (LifeTechnologies) according to manufacturers’ instructions. No differences were found between the assays, and the viability reported is the combination of the results obtained with the different assays.
2.6. Infrared western blot analysis
Cells were rinsed with cold PBS and harvested on RIPA buffer supplemented with protease inhibitor cocktail (1:100 dilution, Sigma Aldrich), and 1 μg/mL PMSF (Sigma Aldrich). Samples were separated by PAGE, proteins transferred to a low-fluorescence background PVDF membrane, and blots visualized and quantified using Odyssey System (Li-Cor Biosciences) as previously described [25,26,36]. To determine the presence of nitroTyr and Hsp90NY in PC12 cells, cells were plated and cultured for 24 h at 37 °C in 5% CO2. Cells were incubated with 0.5 mM peroxynitrite or with recombinant Hsp90NY as described above. The cells were harvested and the protein process for western blot. Hsp90 was detected using antibodies to Hsp90 (Santa Cruz) and Hsp90 nitrated in residue 56 was visualized using a monoclonal antibody as previously described [26,37]. NitroY was visualized using a rabbit affinity-purified polyclonal antibody, as previously described [[37], [38], [39]]. Phospho-p38, p38, phospho-JNK, JNK, Phospho-Akt, and Akt were detected using antibodies from Cell Signaling Technology.
2.7. Transduction of PC12 cells with adenoviral and lentiviral vector particles
PC12 cell transduction with lentiviral particles expressing P2X7 shRNA, PTEN shRNA, control shRNA, or coGFP (Santa Cruz Biotechnology) was performed at a multiplicity of infection of 20. The lentiviral particles were added to the culture medium 2 h after plating. The efficiency of transduction was 85–90%. PC12 cells were transduced at a multiplicity of infection of 120 for 2 h with N-term myristolyation AKT1 (Upstate Biotechnology), cloned in an adenoviral vector co-expressing GFP (AdEasy™ System, ADDgene) as previously described [36]. The efficiency of transduction was 80–85% for adenovectors. The cells were incubated for additional 36 h before treatment with peroxynitrite, intracellular delivery of the proteins or incubation with BzATP. The total number of cells positive for green fluorescent protein (GFP) in each well was counted 4 h and 24 h after treatment. Viability was calculated as the number of GFP-positive cells at 24 h respect to those positive at 4 h. In each case the control group was used to standardize between experiments.
2.8. Statistical analyses
All statistical analyses were performed using one-way ANOVA followed by Holm-Sidak's multiple comparison test, using the Prism statistical analysis software (GraphPad Software Inc.). Homoscedasticity was tested using the Brown-Forsythe and the Bartlett's test. When necessary, the Kruskal-Wallis test followed by Dunn's multiple comparison test was used.
3. Results
3.1. Nitration of Hsp90 at Tyr 56 induces PC12 cell apoptosis
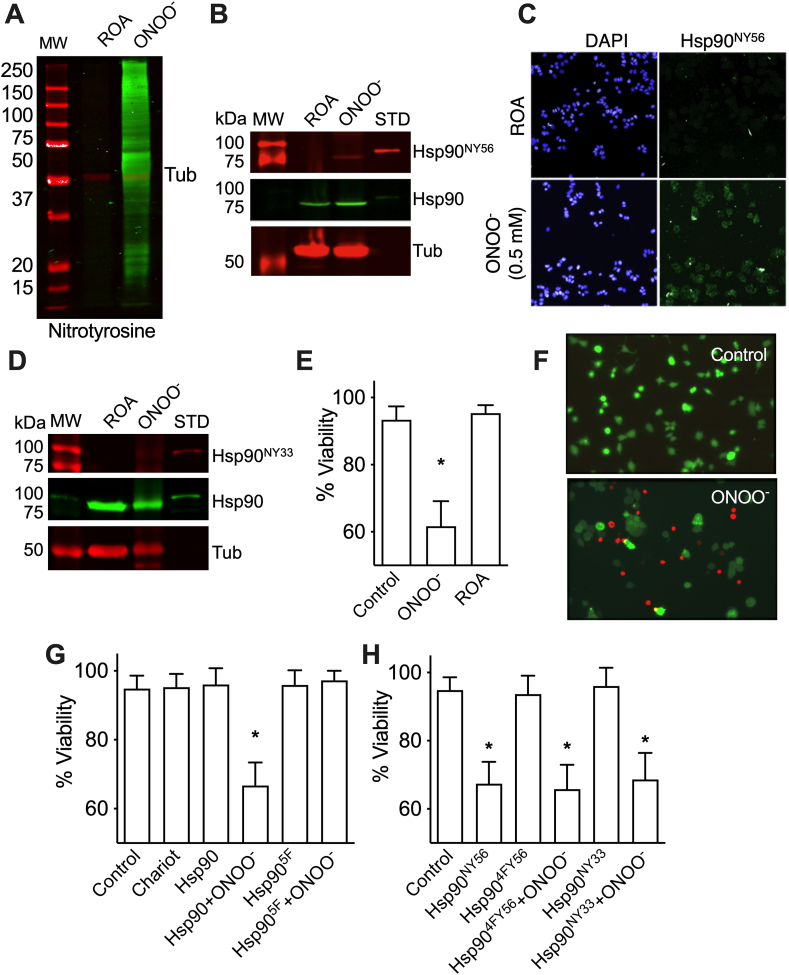
We previously showed that peroxynitrite-treated Hsp90 induces PC12 cell death, and that nitration of Hsp90 at Tyr 33 and Tyr 56 is sufficient to induce motor neuron death. Incubation of PC12 cells with 0.5 mM peroxynitrite caused protein Tyr nitration (Fig. 1A), including nitration of Hsp90 at Tyr 56 (Fig. 1B and C) and Tyr 33 (Fig. 1D, and Supplementary Figs. 1A and B), and stimulated approximately 40% cell death (Fig. 1E and F). In contrast, incubation with decomposed peroxynitrite did not affect cell viability (Fig. 1E and F) in agreement with previous reports [13,[19], [20], [21],25].
Fig. 1.
Nitration of Hsp90 at Y56 precedes peroxynitrite triggered-PC12 cell death. PC12 cells were incubated with 0.5 mM peroxynitrite (ONOO−), or the products of peroxynitrite decomposition (ROA), as described in Materials and Methods. A) Representative infrared western blot showing nitroTyr staining (green) after treatment of PC12 cells with peroxynitrite. Tubulin (Tub) infrared signal was used as a loading control (red). B) Representative infrared western blot showing nitration of Hsp90 at Y56 (Hsp90NY56 in red, upper panel). In green, infrared signal for total Hsp90 (middle panel), Tubulin in red (lower panel). STD: Peroxynitrite-treated recombinant human Hsp90; MW: molecular weight marker. C) Representative image of PC12 cells 6 h post ROA or ONOO− treatment stained for Hsp90NY56 (green) and nuclei (DAPI, blue). D) Representative infrared western blot stained for Hsp90 specifically nitrated at Tyr 33 (Hsp90NY33 in red), tubulin (red), and for total Hsp90 (green). E) Viability of PC12 cells incubated in the absence (control) or presence of ONOO−, or ROA. F) Representative images of live (green) and death (red) PC12 cells 24 h post-treatment with ONOO− or ROA. G-H) PC12 cell viability was measured 24 h after the intracellular delivery of Hsp90 or Hsp90 in which all 5 residues prone to nitration were replaced by phenylalanine (Hsp905F) (G), or Hsp90 with nitroY at either position 33 (Hsp9033NY) or 56 (Hsp9056NY), or Hsp90 with Y56 and phenylalanine in the other 4 residues prone to nitration (Hsp904F56Y) (H), treated or not with ONOO−, and in the absence (Control) or presence of the permeant agent Chariot. In all cases, the values represent the mean ± SD (n = 3–5 with 3–8 replicates). *p < 0.01 vs control by Kruskal-Wallis test followed by Dunn's multiple comparison test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The relevance of Tyr nitration of Hsp90 in the induction of PC12 cell death downstream of peroxynitrite was evaluated by the intracellular release of peroxynitrite-treated human recombinant Hsp90, and a recombinant chaperone in which the 5 residues identified to be nitrated by peroxynitrite were replaced by nitration-resistant phenylalanine (Hsp905F), using the membrane permeating agent Chariot (Active Motif). The conditions used for the delivery of nitrated Hsp90 (Hsp90NY) render 2–5% nitrated chaperone respect to total cellular Hsp90, as previously described [26,35]. These levels of Hsp90NY are similar to those found endogenously nitrated in peroxynitrite-treated PC12 cells, and in motor neurons after endogenous production of peroxynitrite induced by trophic factor deprivation [26]. While the release of peroxynitrite-treated Hsp90 stimulated approximately 40% PC12 cell death (Fig. 1G), the release of peroxynitrite-treated Hsp905F had no effects on PC12 cell viability (Fig. 1G), suggesting that nitration of one or more of these Tyr residues is necessary for the induction of cell death. Intracellular release of recombinant Hsp90 produced by genetic code expansion to replace the Tyr residue in position 56 by nitroTyr (Hsp90NY56) [26,[33], [34], [35],40], was sufficient to trigger PC12 cell death (Fig. 1H). Surprisingly, intracellular release of Hsp90 with nitroTyr in position 33 (Hsp90NY33) did not affect PC12 cell viability, but Hsp90NY33 treated with peroxynitrite stimulated PC12 cell death (Fig. 1H). In contrast, intracellular release of peroxynitrite-treated recombinant Hsp90 carrying Tyr 56 and phenylalanine in the other four sites prone to nitration stimulated PC12 cell death (Fig. 1H). These results demonstrate that nitration of Hsp90 at Tyr 56 is necessary and sufficient to trigger PC12 cell death.
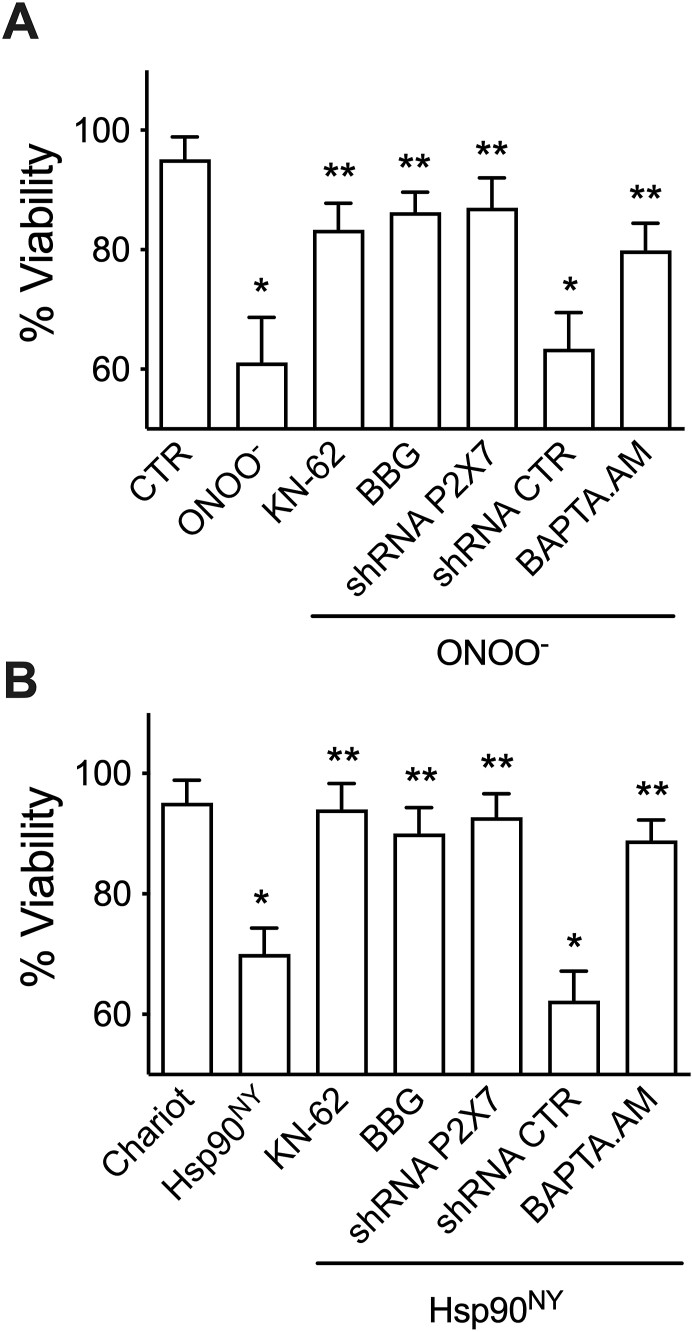
3.2. Peroxynitrite- and Hsp90NY-induced PC12 cell death requires P2X7 receptor activation
We previously reported that Hsp90NY activates the P2X7R to trigger motor neuron apoptosis. We also showed that Hsp90 nitrated at Tyr 56 interacts with the P2X7R in PC12 cells [26]. To determine whether peroxynitrite and Hsp90NY trigger PC12 cell death by activation of the P2X7R, we investigated the effects of blocking the activity of the receptor on the survival of PC12 cell treated with peroxynitrite, or after intracellular release of Hsp90NY. The P2X7R inhibitors KN-62 and BBG prevented both peroxynitrite- (Fig. 2A), and Hsp90NY-induced PC12 cell death (Fig. 2B). In addition, decreasing expression of P2X7R using shRNA had similar effects (Fig. 2A and B). The P2X7R is a selective calcium channel [26,41,42]. In agreement with this function, peroxynitrite- and Hsp90NY-induced PC12 cell death was prevented by the intracellular calcium chelator BAPTA-AM (Fig. 2A and B). These results suggest that peroxynitrite induces PC12 cell death through nitration of Hsp90, which triggers an influx of calcium mediated by the activation of P2X7R.
Fig. 2.
Activation of P2X7R mediates both peroxynitrite- and Hsp90NY-triggered PC12 cell death. PC12 cell were incubated for 1 h with the P2X7R inhibitors KN-62 (1 μM) and BBG (10 μM), and the intracellular calcium chelator BAPTA.AM (1 μM; BAPTA) followed by treatment with peroxynitrite (A), or Hsp90NY was intracellularly released using Chariot (B), followed by incubation with the inhibitors for additional 24 h before assessing cell viability. For the down-regulation experiments, cells were transduced with a shRNA to P2X7R (shRNA P2X7R) or a scrambled sequence (shRNA CTR) 24 h before treatment with peroxynitrite, and cell viability assessed after additional 24 h. The values represent the mean ± SD (n = 3–5 with 3–8 replicates). *p < 0.001 vs control and ** vs ONOO− or Hsp90NY by one-way ANOVA followed by Holm-Sidak's multiple comparison test.
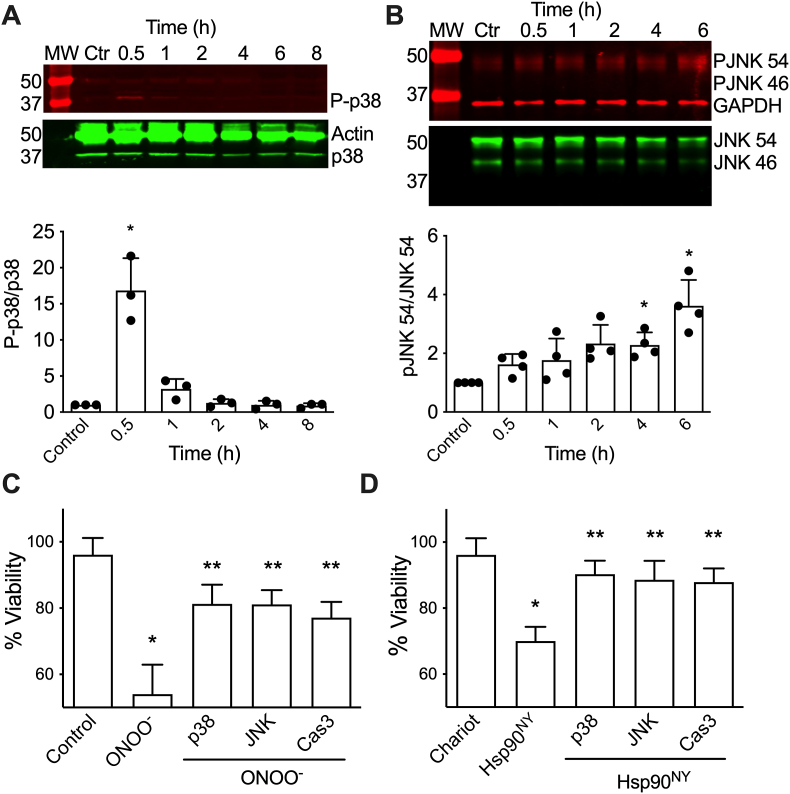
3.3. Hsp90NY stimulate PC12 cell death by activation of p38 and JNK MAPKs
Collectively, these results suggest that Hsp90 is a key target for peroxynitrite nitration and may be the mediator that activates downstream death signaling cascades leading to cell death. We reason that if Hsp90NY56 is the downstream target of peroxynitrite in the induction of apoptosis, the same signaling pathways should be activated by peroxynitrite and Hsp90NY56. Apoptosis triggered by peroxynitrite requires the simultaneous activation of p38 and JNK MAP kinases and the inactivation of the Akt pathway [21]. Activation of p38 and JNK was detected by infrared western blot after intracellular release of Hsp90NY. Increased activation of p38 was evident at 30 min post intracellular release, returning to basal activity after 1–2 h (Fig. 3A). Progressive activation of JNK 54 was observed 30 min to 6 h after Hsp90NY release (Fig. 3B). In contrast, no changes in the phosphorylation of JNK 46 were detected. There was no significant difference in total p38 or the JNK protein over the same time period.
Fig. 3.
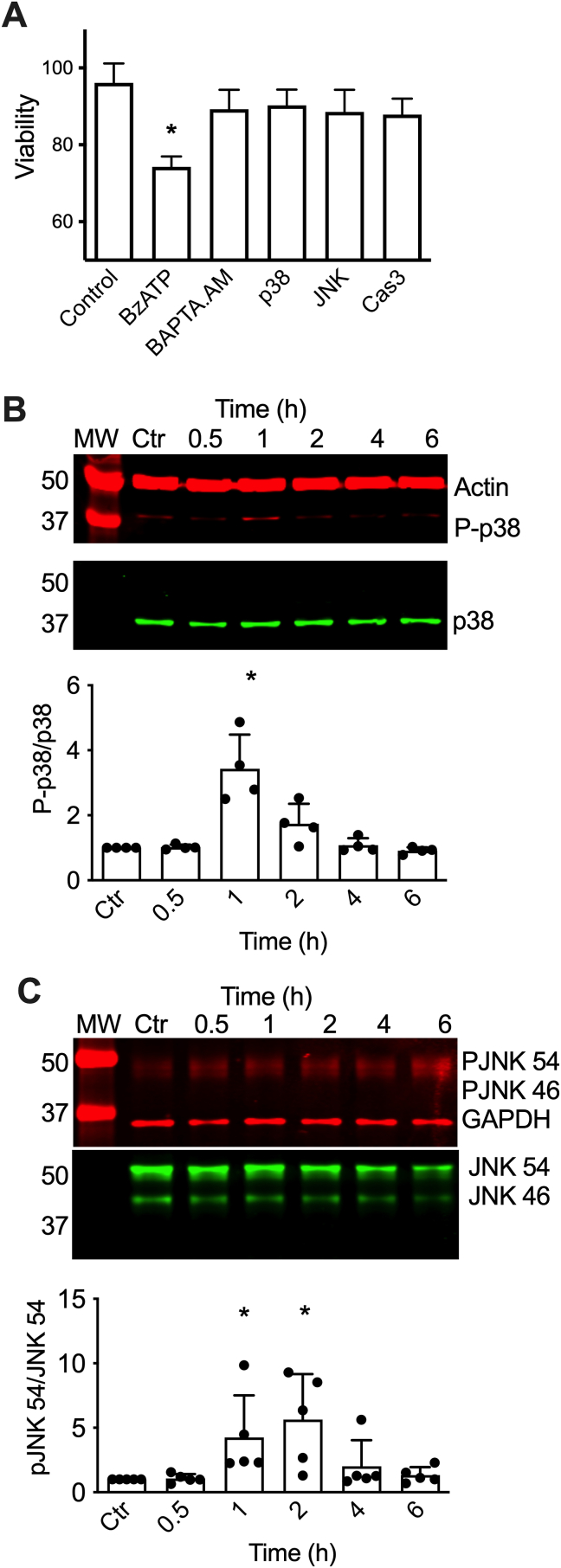
Peroxynitrite- and Hsp90NY-triggered PC12 cell death requires activation of p38 and JNK MAP kinases. A) Representative infrared western blot of an 8 h time course following p38 phosphorylation after intracellular release of Hsp90NY. Blots were stained for phospho-p38 (P-p38, red), and total p38 and actin (green). The bottom graph shows the quantitation of the infrared fluorescence intensity of the P-p38 versus total p38 bands, expressed as the signal ratio. MW: molecular weight marker. The columns represent the mean ± SD (n = 3, individual values show as dots). Control (Ctr): Cells incubated with Chariot alone. *p < 0.001 vs control by one-way ANOVA followed by Holm-Sidak's multiple comparison test. B) Representative infrared western blot of a 6 h time course for JNK phosphorylation after intracellular delivery of Hsp90NY. Blots were stained for phospho-JNK 54 (PJNK54), phospho-JNK 46 (PJNK 46), and GADPH (red), and total JNK 56 and JNK 46 (green). Bottom graph, quantitation of the infrared fluorescence signal ratio of PJNK 54 versus total JNK 54. No changes were detected in the phosphorylation of JNK 46 at any timepoint. The columns represent the mean ± SD (n = 3, individual values show as dots). Control (Ctr): Cells incubated with Chariot alone. *p < 0.05 vs control by Kruskal-Wallis test followed by Dunn's multiple comparison test. C) Viability of PC12 cell treated or not with peroxynitrite in the presence or absence of the p38 inhibitor SB203580 (1 μM; p38), the JNK inhibitor SP600125 (0.1 μM; JNK) and the selective caspase 3 inhibitor z-DVED-fmk (25 μM; Cas3) was evaluated 24 h after peroxynitrite-treatment. The values represent the mean ± SD (n = 3 with 8 replicates). *p < 0.001 vs control, ** vs ONOO− by one-way ANOVA test followed by Holm-Sidak's multiple comparison test. D) PC12 cell viability was assessed 24 h after the intracellular release of Hsp90NY in the presence or absence of the p38 inhibitor SB203580 (1 μM; p38), the JNK inhibitor SP600125 (0.1 μM; JNK), and the selective caspase 3 inhibitor z-DVED-fmk (25 μM; Cas3). In all cases, the values represent the mean ± SD (n = 3 with 3–8 replicates). *p < 0.001 vs control, ** vs Hsp90NY by one-way ANOVA followed by Holm-Sidak's multiple comparison test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In agreement, incubation of the cells with the p38 inhibitor SB203580, and the JNK inhibitor SP600125 prevented PC12 cell death stimulated by peroxynitrite and Hsp90NY (Fig. 3C and D). Similar results were obtained using a different set of inhibitors, p38 inhibitors SB202190 and SCIO 469, and JNK inhibitors L-JNKi1 and CEP1347 (Supplementary Figs. 2A and B). Furthermore, in both conditions, PC12 cell death was prevented by the selective caspase 3 inhibitor z-DEVD-fmk (Fig. 3C and D), providing strong evidence that both peroxynitrite treatment and intracellular Hsp90NY stimulate apoptosis. These results further suggest that Hsp90NY plays a role as peroxynitrite mediator in the induction of PC12 cell apoptosis through the activation of p38 and JNK MAPK.
3.4. Activation of P2X7R is sufficient to trigger PC12 cell death through a MAPK-Mediated mechanism
To determine if the activation of p38 and JNK required upstream activation of P2X7R, leading to PC12 cell apoptosis, P2X7R was activated using the ATP analog 3′-O-(4-benzoyl) benzoyl adenosine 5′-triphosphate (BzATP). As expected, incubation with BzATP stimulated PC12 cell death, which was prevented by co-incubation with the calcium chelator BAPTA-AM (Fig. 4A). PC12 cell death induced by activation of the P2X7R with BzATP was prevented by inhibition of p38, JNK, and caspase 3 activity (Fig. 4A), replicating peroxynitrite- and Hsp90NY-induced PC12 cell apoptosis. Similar results were obtained using the p38 inhibitors SB202190 and SCIO 469, and the JNK inhibitors L-JNKi1 and CEP1347 (Supplementary Fig. 2C). In addition, incubation of PC12 cells with BzATP stimulated p38 phosphorylation with a peak 1 h after treatment (Fig. 4B). Direct activation of the P2X7R also stimulated JNK 56 phosphorylation with a peak at 1–2 h and returned to basal levels at 4 h after treatment (Fig. 4C). In contrast, BzATP did not affected the phosphorylation of JNK 46 as described before for Hsp90NY. These results show that activation of P2X7R and downstream signaling recapitulates peroxynitrite- and Hsp90NY-induced PC12 cell apoptosis.
Fig. 4.
Direct activation of P2X7R triggers PC12 cell apoptosis and phosphorylation of p38 and JNK MAPKs. A) Viability of PC12 cells was assessed after 24 h incubation with BzATP (1 mM) in the presence or absence of the calcium chelator BAPTA.AM (1 μM), p38 inhibitor SB203580 (1 μM), the JNK inhibitor SP600125 (0.1 μM) and the selective caspase 3 inhibitor z-DVED-fmk (25 μM) The values represent the mean ± SD (n = 3–5 with 8 replicates). *p < 0.001 vs control by one-way ANOVA followed by Holm-Sidak's multiple comparison test. B–C) PC12 cells were cultured with BzATP before protein was harvested at the indicated times and processed for quantitative infrared western blot. B) Blots were stained for phospho-p38 (P-p38) and actin (red), and total p38 (green). MW: molecular weight marker. Bottom graph, quantitation of the infrared fluorescence signal ratio of P-p38 versus total p38. The columns represent the mean ± SD (n = 4, individual values show as dots). Control (Ctr): Untreated cells. *p < 0.02 vs. control by Kruskal-Wallis test followed by Dunn's multiple comparison test. C) Blots were stained for phospho-JNK 54 (PJNK54), phospho-JNK 46 (PJNK 46), and GADPH (red), and total JNK 56 and JNK 46 (green). Bottom graph, quantitation of the infrared fluorescence signal ratio of PJNK 54 versus total JNK 54. No changes were detected in the phosphorylation of JNK 46 at any timepoint. The columns represent the mean ± SD (n = 5, individual values show as dots). Control (Ctr): Untreated cells. *p < 0.02 vs. control by Kruskal-Wallis test followed by Dunn's multiple comparison test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.5. Activation of P2X7R leads to the inhibition of the PI3K/Akt pathway in PC12 cells through stimulation of PTEN
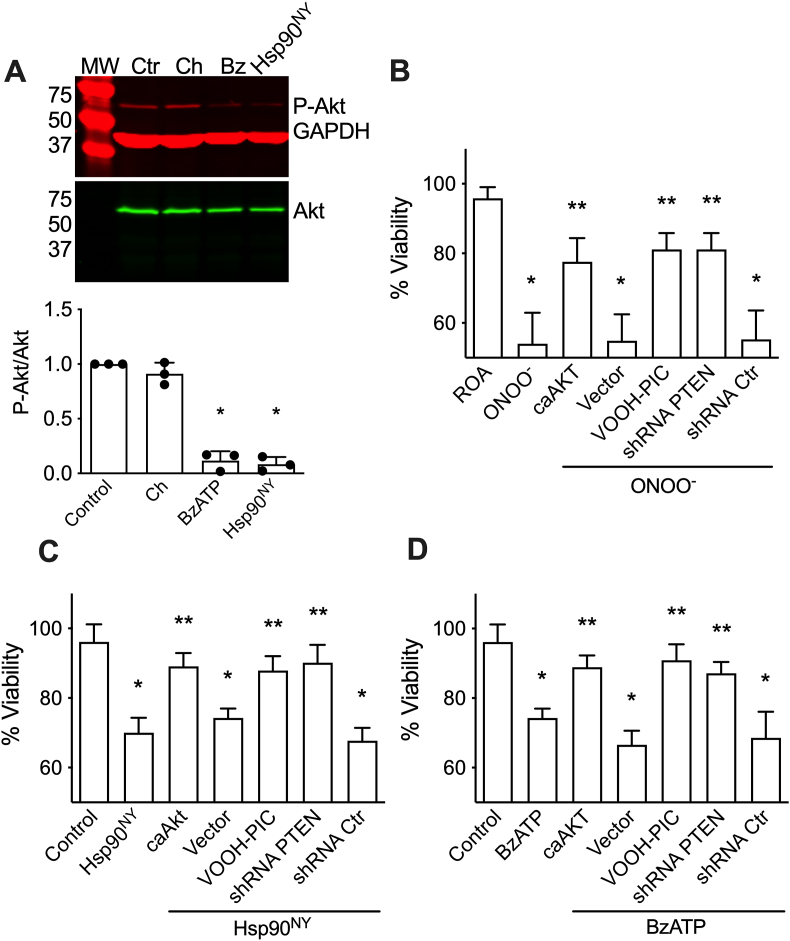
Peroxynitrite-induced PC12 cell apoptosis requires not only activation of p38 and JNK but also the inhibition of the PI3K/Akt pathway [21]. Next, we investigated the effects of Hsp90NY and P2X7R activation on the PI3K/Akt cell survival pathway. Hsp90NY triggered the dephosphorylation of Akt without affecting total Akt levels 16 h after treatment (Fig. 5A). Similarly, incubation of PC12 cells with BzATP resulted in a decrease in Akt phosphorylation 16 h after treatment (Fig. 5A).
Fig. 5.
Activation of PTEN mediates decreased Akt phosphorylation and PC12 cell death triggered by peroxynitrite, nitrated Hsp90, and activation of P2X7R. A) Representative infrared western blots of Phospho-Akt (P-Akt) and GAPDH (red) and total Akt (green) from homogenates of PC12 cells cultured in the presence of BzATP for 16 h, or 16 h after Hsp90NY intracellular delivery. Untreated PC12 cells (Ctr) or cell incubated with Chariot alone (Ch) were used as controls. MW: molecular weight marker. Bottom graph, quantitation of the infrared fluorescence signal ratio of P-Akt versus total Akt. The columns represent the mean ± SD (n = 3, individual values show as dots). *p < 0.05 vs control by Kruskal-Wallis test followed by Dunn's multiple comparison test. B-D) PC12 cells were transduced with an adenovector expressing constitutively active AKT and GFP (caAkt) or GFP alone (vector), shRNA to PTEN (shRNA PTEN) or a scramble shRNA (shRNA Ctr), or incubated with the PTEN inhibitor VOOH-PIC (2 μM) before peroxynitrite treatment (ONOO−) (B), release of nitrated Hsp90 (Hsp90NY) (C), or incubation with BzATP (1 μM) (D), and cell viability assessed 24 h post-treatment. The columns represent the mean ± SD (n = 3–6 with 3–8 replicates). *p < 0.001 vs ROA or control, ** vs ONOO−, Hsp90NY, or BzATP by one-way ANOVA followed by Holm-Sidak's multiple comparison test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The role of Akt dephosphorylation in PC12 cell apoptosis triggered by Hsp90NY and activation of the P2X7R was investigated using a constitutive active Akt. Expression of constitutive active Akt in PC12 cells prevented cell death after incubation with peroxynitrite (Fig. 5B), intracellular release of Hsp90NY (Fig. 5C), and activation of P2X7R (Fig. 5D), while transfection with the empty vector had no effect on survival in any condition. Because the mechanism inducing PI3K/Akt downregulation by peroxynitrite and Hsp90NY remains unknown, we investigated the role of PTEN, a well-known inhibitor of the PI3K/Akt [43,44]. To investigate whether PTEN was involved in the activation of peroxynitrite- and Hsp90NY-induced apoptosis, PC12 cells were incubated with the PTEN inhibitor VOOH-PIC for 1 h prior to treatment with peroxynitrite, Hsp90NY release, or incubation with BzATP. Inhibition of PTEN significantly protected PC12 cells against apoptosis triggered by peroxynitrite (Fig. 5B), Hsp90NY (Fig. 5C), and activation of P2X7R (Fig. 5D). Similar results were observed after incubation of the cells with a lentiviral vector expressing a shRNA to induce PTEN down-regulation, but not with a vector expressing scrambled shRNA, used as control (Fig. 5B, C and D). These findings suggest that peroxynitrite inhibits the Akt survival pathway through Hsp90 nitration, followed by P2X7R-dependent activation of PTEN. In addition, these results also reveal that activation of PTEN is necessary for the stimulation of PC12 cell apoptosis by peroxynitrite and Hsp90NY.
4. Discussion
Peroxynitrite acts on a multitude of biomolecules that are key players in a number of signaling pathways, and it can thereby influence critical cellular processes [[8], [9], [10],45]. However, the molecular targets of peroxynitrite are not well described and could differ among different cell types. We first identified nitroTyr as a relevant oxidative post-translational modification in peroxynitrite-induced apoptosis in undifferentiated PC12 cells and motor neurons [25]. More recently, the nitration of one of two residues in the ATPase domain of Hsp90 was determined to be necessary and sufficient to make the chaperone toxic to motor neurons, raising the possibility that Hsp90 mediates peroxynitrite-induced apoptosis [26]. The hypothesis addressed here is that nitrated Hsp90 mediates the activation of the apoptotic signaling pathways in peroxynitrite-induced PC12 cells death. Our results reveal that nitration of Hsp90 at Tyr 56 by peroxynitrite is necessary and sufficient to trigger apoptosis in undifferentiated PC12 cells. These results contrast with previous observations in motor neurons, where nitration at Tyr 33 or/and Tyr 56 was equally potent in triggering apoptosis [26], raising the possibility that the mechanisms involved in the stimulation of apoptosis by each modification are different, and cell-type dependent. Supporting this possibility, we showed that Hsp90 nitrated at Tyr 33 downregulates mitochondrial metabolism in PC12 cells without inducing release of cytochrome c from the mitochondria [35]. In contrast, while nitration of Hsp90 at Tyr 56 induces PC12 cell apoptosis, we showed that it has no effect on mitochondrial activity and membrane potential when tested in PC12 cell homogenates [35], highlighting that different forms of nitrated Hsp90 play distinct roles depending on the conditions and cell type.
Our results also reveal that peroxynitrite and Hsp90NY activated the same signaling pathways to trigger PC12 cell apoptosis. Downstream of peroxynitrite, Hsp90NY-induced PC12 cell apoptosis proceeded through the activation of p38 and JNK MAPKs, and inactivation of the PI3K/Akt survival pathway, before the activation of caspases, as described for peroxynitrite-induced apoptosis [21]. Considering that these are well-described pathways inducing PC12 cell apoptosis, their involvement in both conditions is not surprising [21,[46], [47], [48], [49]]. However, both peroxynitrite and Hsp90NY activated the purinergic receptor P2X7, which in turn led to PTEN-dependent apoptosis. This mechanism was recapitulated by direct activation of the P2X7R by the agonist BzATP. Hsp90 is a normal component of the P2X7R complex that decreases the sensitivity of the receptor to agonist-dependent activation [50,51]. However, Hsp90NY triggers agonist-independent P2X7R activation [26]. We also showed that Hsp90 nitrated at Tyr 56 interacts with the P2X7R complex in PC12 cells, suggesting that this nitrated form of Hsp90 may compete with and displace unmodified Hsp90 from the complex to activate the P2X7R, ultimately leading to PC12 cell apoptosis [26]. P2X7R is a calcium channel, but continuous stimulation of the receptor leads to the activation of a cationic pore that allows the passage of molecules with molecular weight of less than approximately 1000 Da [52]. However, the pore does not seem to be involved in the toxicity of peroxynitrite or Hsp90NY since apoptosis triggered by peroxynitrite, Hsp90NY, and BzATP was prevented by calcium chelation, suggesting that the opening of the channel is sufficient to stimulate PC12 cell apoptosis.
As discussed above, activation of JNK and p38 MAP kinase is required for the stimulation of PC12 cell apoptosis by peroxynitrite [21]. Hsp90NY stimulated p38 phosphorylation in PC12 cells with a pattern similar to that previously described for peroxynitrite [21]. While the increase in p38 phosphorylation peaked 30 min after peroxynitrite incubation, the peak was evident 4 h after Hsp90NY release. A possible explanation for this delay is that the oxidized chaperone needs time to build up in the cytoplasm after release in order to produce a substantial effect on p38 phosphorylation. This conclusion is further supported by the observation that JNK phosphorylation stimulated by Hsp90NY was also delayed compared with peroxynitrite treatment, while the induction of JNK phosphorylation by activation of P2X7R followed the same pattern as previously described in the presence of peroxynitrite [21]. Calcium influx in PC12 cells has been demonstrated to activate RAS, leading to the stimulation of MLK, which is a known activator of the p38 and JNK pathways [21,53]. This evidence provides a possible link between calcium influx through P2X7R and the activation of p38 and JNK MAPKs seen in peroxynitrite- and Hsp90NY-induced apoptosis.
Hsp90NY and P2X7R activation also resulted in the down-regulation of the PI3K/Akt pathway, in agreement with peroxynitrite effect on the pathway [21]. Interestingly, we also observed the activation of PTEN, an inhibitor of the PI3K/Akt pathway that acts through the dephosphorylation of phosphatidylinositol (3,4,5)-triphosphate (PIP3) to phosphatidylinositol (4,5)-bi-phosphate (PIP2) [54]. A link between P2X7R and PTEN was established in cancer cell proliferation [[55], [56], [57]]. Recent reports show that inhibition of Hsp90 in motor neurons activates P2X7R, and in turn calcium-dependent activation of PTEN [36]. Our results provide further evidence for the activation of PTEN by a P2X7R-mediated calcium influx, as the intracellular calcium chelator BAPTA-AM and inhibition of PTEN prevented PC12 cells apoptosis triggered by P2X7R activation. Collectively, these results suggest that PTEN plays a central role in the regulation of peroxynitrite-induced PC12 cell apoptosis. Inactivation of the PI3K by nitration of unknown targets was proposed as a mechanism of peroxynitrite-induced apoptosis [19]. The activation of the PI3K/Akt pathway by NGF before incubation with peroxynitrite prevents apoptosis [13,19]. NGF may afford protection by preventing Akt inactivation mediated by PTEN, which agrees with our results showing that expression of constitutively activated Akt prevented PC12 cell apoptosis triggered by Hsp90NY and P2X7R activation, rather than prevention of the inactivation of PI3K by peroxynitrite.
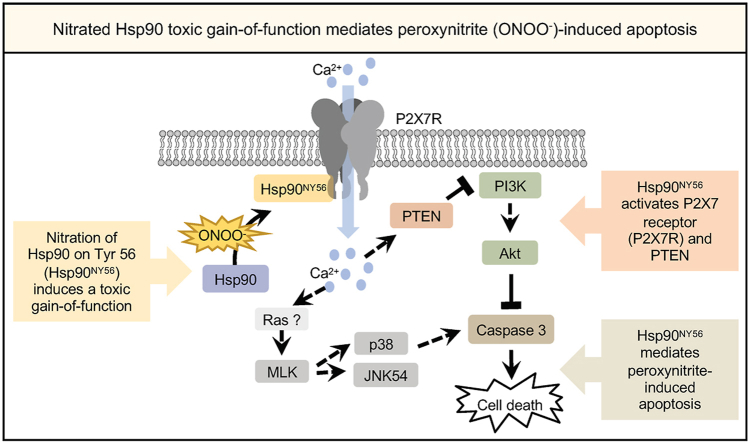
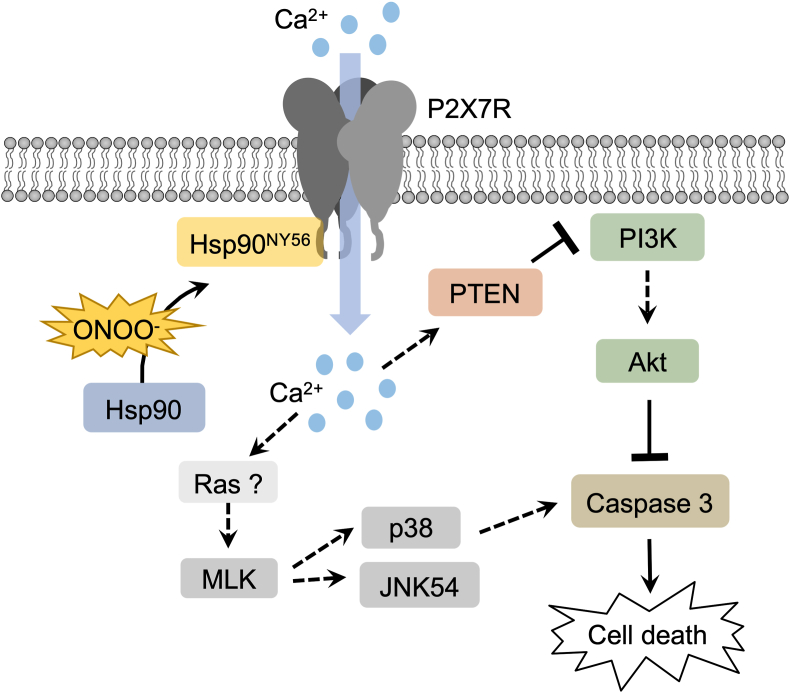
Therefore, our current understanding of the peroxynitrite-induced apoptotic pathway in PC12 cells includes the nitration of Hsp90 at Tyr 56, P2X7R activation leading to a calcium influx, MAPK activation, with Ras and MLK being possible mediators in the calcium-dependent activation of p38 and JNK MAPKs, and the inhibition of the PI3K/Akt pathway due to PTEN activation (Fig. 6).
Fig. 6.
Nitration of Tyr 56 in Hsp90 by peroxynitrite induces PC12 cells apoptosis by activation of P2X7R and downstreamactivationof PTEN. Nitration of Hsp90 at Tyr 56 by peroxynitrite leads to the activation of the P2X7R complex, inducing a calcium influx that triggers the simultaneous activation of p38 and JNK 54, and the inhibition of the PI3K/Akt pathway through activation of PTEN, ultimately inducing PC12 cell apoptosis.
In summary, our results strongly support the interpretation that nitration of Hsp90 at Tyr 56 by peroxynitrite is sufficient for the induction of PC12 cell apoptosis, and that downstream P2X7R activation is required for peroxynitrite-induced apoptosis in PC12 cells. In addition, our results provide further understanding of the pathways involved in peroxynitrite-induced apoptosis in PC12 cells, and possible other cell types. We also show for the first time that nitration of Hsp90 mediates peroxynitrite activation of the P2X7R, followed by PTEN dephosphorylation of PIP3, which is responsible for the inhibition of PI3K and downstream Akt. This pathway may be highly relevant in pathological conditions, since P2X7R antagonists improve recovery after spinal cord injury, a condition in which Hsp90 is endogenously nitrated [26,58,59]. Peroxynitrite and nitroTyr are found in a number of neurodegenerative diseases and are now accepted mediators in pathologies such as Parkinson's disease [12,24,60,61]. A better understanding of the proteins and signaling pathways regulated by peroxynitrite and Tyr nitration may provide new targets for the development of therapeutic strategies for a wide range of pathological conditions.
Acknowledgements
The authors are very thankful to Dr. Lloyd Green for his always valuable advice and his generosity in providing the PC12 cells. This work was supported by the NINDS, National Institutes of Health Grant R01NS102479 (to MCF) and R01NS36716 (to AGE). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2022.102247.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Beckman J.S., Beckman T.W., Chen J., Marshall P.A., Freeman B.A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U.S.A. 1990;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nauser T., Koppenol W.H. The rate constant of the reaction of superoxide with nitrogen monoxide: approaching the diffusion limit. J. Phys. Chem. 2002;106(16):4084–4086. [Google Scholar]
- 3.Padmaja S., Huie R.E. The reaction of nitric oxide with organic peroxyl radicals. Biochem. Biophys. Res. Commun. 1993;195:539–544. doi: 10.1006/bbrc.1993.2079. [DOI] [PubMed] [Google Scholar]
- 4.Beckman J.S., Koppenol W.H. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 1996;271(5 Pt 1):C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 5.Macfadyen A.J., Reiter C., Zhuang Y., Beckman J.S. A novel superoxide dismutase-based trap for peroxynitrite used to detect entry of peroxynitrite into erythrocyte ghosts. Chem. Res. Toxicol. 1999;12(3):223–229. doi: 10.1021/tx980253u. [DOI] [PubMed] [Google Scholar]
- 6.Sakurai M., Fukuyama N., Takizawa S., Abe K., Hayashi T., Shinohara Y., Nakazawa H., Tabayashi K. Inductions of 3-L-nitrotyrosine in motor neurons after transient spinal cord ischemia in rabbits. J. Cerebr. Blood Flow Metabol. 1998;18(11):1233–1238. doi: 10.1097/00004647-199811000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Bruijn L.I., Beal M.F., Becher M.W., Schulz J.B., Wong P.C., Price D.L., Cleveland D.W. Elevated free nitrotyrosine levels, but not protein-bound nitrotyrosine or hydroxyl radicals, throughout amyotrophic lateral sclerosis (ALS)-like disease implicate tyrosine nitration as an aberrant in vivo property of one familial ALS-linked superoxide dismutase 1 mutant. Proc. Natl. Acad. Sci. USA. 1997;94(14):7606–7611. doi: 10.1073/pnas.94.14.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franco M.C., Estevez A.G. Tyrosine nitration as mediator of cell death. Cell. Mol. Life Sci. 2014;71(20):3939–3950. doi: 10.1007/s00018-014-1662-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams L., Franco M.C., Estevez A.G. Reactive nitrogen species in cellular signaling. Exp. Biol. Med. 2015;240(6):711–717. doi: 10.1177/1535370215581314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramdial K., Franco M.C., Estevez A.G. Cellular mechanisms of peroxynitrite-induced neuronal death. Brain Res. Bull. 2017 doi: 10.1016/j.brainresbull.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Radi R. Oxygen radicals, nitric oxide, and peroxynitrite: redox pathways in molecular medicine. Proc. Natl. Acad. Sci. U.S.A. 2018;115(23):5839–5848. doi: 10.1073/pnas.1804932115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estevez A.G., Radi R., Barbeito L., Shin J.T., Thompson J.A., Beckman J.S. Peroxynitrite-induced cytotoxicity in PC12 cells: evidence for an apoptotic mechanism differentially modulated by neurotrophic factors. J. Neurochem. 1995;65(4):1543–1550. doi: 10.1046/j.1471-4159.1995.65041543.x. [DOI] [PubMed] [Google Scholar]
- 14.Lin K.T., Xue J.Y., Nomen M., Spur B., Wong P.Y. Peroxynitrite-induced apoptosis in HL-60 cells. J. Biol. Chem. 1995;270(28):16487–16490. doi: 10.1074/jbc.270.28.16487. [DOI] [PubMed] [Google Scholar]
- 15.Bonfoco E., Krainc D., Ankarcrona M., Nicotera P., Lipton S.A. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc. Natl. Acad. Sci. U.S.A. 1995;92(16):7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salgo M.G., Squadrito G.L., Pryor W.A. Peroxynitrite causes apoptosis in rat thymocytes. Biochem. Biophys. Res. Commun. 1995;215(3):1111–1118. doi: 10.1006/bbrc.1995.2578. [DOI] [PubMed] [Google Scholar]
- 17.Troy C.M., Derossi D., Prochiantz A., Greene L.A., Shelanski M.L. Downregulation of Cu/Zn superoxide dismutase leads to cell death via the nitric oxide-peroxynitrite pathway. J. Neurosci. 1996;16(1):253–261. doi: 10.1523/JNEUROSCI.16-01-00253.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Troy C.M., Shelanski M.L. Down-regulation of copper/zinc superoxide dismutase causes apoptotic death in PC12 neuronal cells. Proc. Natl. Acad. Sci. U.S.A. 1994;91(14):6384–6387. doi: 10.1073/pnas.91.14.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spear N., Estevez A.G., Barbeito L., Beckman J.S., Johnson G.V. Nerve growth factor protects PC12 cells against peroxynitrite-induced apoptosis via a mechanism dependent on phosphatidylinositol 3-kinase. J. Neurochem. 1997;69(1):53–59. doi: 10.1046/j.1471-4159.1997.69010053.x. [DOI] [PubMed] [Google Scholar]
- 20.Spear N., Estevez A.G., Johnson G.V., Bredesen D.E., Thompson J.A., Beckman J.S. Enhancement of peroxynitrite-induced apoptosis in PC12 cells by fibroblast growth factor-1 and nerve growth factor requires p21Ras activation and is suppressed by Bcl-2. Arch. Biochem. Biophys. 1998;356(1):41–45. doi: 10.1006/abbi.1998.0741. [DOI] [PubMed] [Google Scholar]
- 21.Shacka J.J., Sahawneh M.A., Gonzalez J.D., Ye Y.Z., D'Alessandro T.L., Estevez A.G. Two distinct signaling pathways regulate peroxynitrite-induced apoptosis in PC12 cells. Cell Death Differ. 2006;13(9):1506–1514. doi: 10.1038/sj.cdd.4401831. [DOI] [PubMed] [Google Scholar]
- 22.Ischiropoulos H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem. Biophys. Res. Commun. 2003;305(3):776–783. doi: 10.1016/s0006-291x(03)00814-3. [DOI] [PubMed] [Google Scholar]
- 23.Bartesaghi S., Radi R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 2018;14:618–625. doi: 10.1016/j.redox.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bandookwala M., Sengupta P. 3-Nitrotyrosine: a versatile oxidative stress biomarker for major neurodegenerative diseases. Int. J. Neurosci. 2020;130(10):1047–1062. doi: 10.1080/00207454.2020.1713776. [DOI] [PubMed] [Google Scholar]
- 25.Ye Y., Quijano C., Robinson K.M., Ricart K.C., Strayer A.L., Sahawneh M.A., Shacka J.J., Kirk M., Barnes S., Accavitti-Loper M.A., Radi R., Beckman J.S., Estevez A.G. Prevention of peroxynitrite-induced apoptosis of motor neurons and PC12 cells by tyrosine-containing peptides. J. Biol. Chem. 2007;282(9):6324–6337. doi: 10.1074/jbc.M610800200. [DOI] [PubMed] [Google Scholar]
- 26.Franco M.C., Ye Y., Refakis C.A., Feldman J.L., Stokes A.L., Basso M., Melero Fernandez de Mera R.M., Sparrow N.A., Calingasan N.Y., Kiaei M., Rhoads T.W., Ma T.C., Grumet M., Barnes S., Beal M.F., Beckman J.S., Mehl R., Estevez A.G. Nitration of Hsp90 induces cell death. Proc. Natl. Acad. Sci. U.S.A. 2013;110(12):E1102–E1111. doi: 10.1073/pnas.1215177110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pratt W.B., Morishima Y., Osawa Y. The Hsp90 chaperone machinery regulates signaling by modulating ligand binding clefts. J. Biol. Chem. 2008;283(34):22885–22889. doi: 10.1074/jbc.R800023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wandinger S.K., Richter K., Buchner J. The Hsp90 chaperone machinery. J. Biol. Chem. 2008;283(27):18473–18477. doi: 10.1074/jbc.R800007200. [DOI] [PubMed] [Google Scholar]
- 29.Zhao R., Davey M., Hsu Y.-C., Kaplanek P., Tong A., Parsons A.B., Krogan N., Cagney G., Mai D., Greenblatt J., Boone C., Emili A., Houry W.A. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the Hsp90 chaperone. Cell. 2005;120(5):715–727. doi: 10.1016/j.cell.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 30.Dezwaan D.C., Freeman B.C. HSP90: the Rosetta stone for cellular protein dynamics? Cell Cycle. 2008;7(8):1006–1012. doi: 10.4161/cc.7.8.5723. [DOI] [PubMed] [Google Scholar]
- 31.Wiech H., Buchner J., Zimmermann R., Jakob U. Hsp90 chaperones protein folding in vitro. Nature. 1992;358(6382):169–170. doi: 10.1038/358169a0. [DOI] [PubMed] [Google Scholar]
- 32.Robinson K.M., Beckman J.S. Synthesis of peroxynitrite from nitrite and hydrogen peroxide. Methods Enzymol. 2005;396:207–214. doi: 10.1016/S0076-6879(05)96019-9. [DOI] [PubMed] [Google Scholar]
- 33.Neumann H., Hazen J.L., Weinstein J., Mehl R.A., Chin J.W. Genetically encoding protein oxidative damage. J. Am. Chem. Soc. 2008;130(12):4028–4033. doi: 10.1021/ja710100d. [DOI] [PubMed] [Google Scholar]
- 34.Cooley R.B., Feldman J.L., Driggers C.M., Bundy T.A., Stokes A.L., Karplus P.A., Mehl R.A. Structural basis of improved second-generation 3-nitro-tyrosine tRNA synthetases. Biochemistry. 2014;53(12):1916–1924. doi: 10.1021/bi5001239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franco M.C., Ricart K.C., Gonzalez A.S., Dennys C.N., Nelson P.A., Janes M.S., Mehl R.A., Landar A., Estevez A.G. Nitration of Hsp90 on tyrosine 33 regulates mitochondrial metabolism. J. Biol. Chem. 2015;290(31):19055–19066. doi: 10.1074/jbc.M115.663278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strayer A.L., Dennys-Rivers C.N., Ricart K.C., Bae N., Beckman J.S., Franco M.C., Estevez A.G. Highlight article: ligand-independent activation of the P2X7 receptor by Hsp90 inhibition stimulates motor neuron apoptosis. Exp. Biol. Med. 2019;244(11):901–914. doi: 10.1177/1535370219853798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye Y., Quijano C., Robinson K.M., Ricart K.C., Strayer A.L., Sahawneh M.A., Shacka J.J., Kirk M., Barnes S., Accavitti-Loper M.A., Radi R., Beckman J.S., Estevez A.G. Prevention of peroxynitrite-induced apoptosis of motor neurons and PC12 cells by tyrosine-containing peptides. J. Biol. Chem. 2007;282:6324–6337. doi: 10.1074/jbc.M610800200. [DOI] [PubMed] [Google Scholar]
- 38.Ye Y.Z., Strong M., Huang Z.Q., Beckman J.S. Antibodies that recognize nitrotyrosine, nitric oxide. P T B. 1996;269:201–209. doi: 10.1016/s0076-6879(96)69022-3. [DOI] [PubMed] [Google Scholar]
- 39.Viera L., Ye Y.Z., Estevez A.G., Beckman J.S. Immunohistochemical methods to detect nitrotyrosine. Methods Enzymol. 1998;301:373–381. doi: 10.1016/s0076-6879(99)01101-5. [DOI] [PubMed] [Google Scholar]
- 40.Hammill J.T., Miyake-Stoner S., Hazen J.L., Jackson J.C., Mehl R.A. Preparation of site-specifically labeled fluorinated proteins for 19F-NMR structural characterization. Nat. Protoc. 2007;2(10):2601–2607. doi: 10.1038/nprot.2007.379. [DOI] [PubMed] [Google Scholar]
- 41.Gandelman M., Levy M., Cassina P., Barbeito L., Beckman J.S. P2X7 receptor-induced death of motor neurons by a peroxynitrite/FAS-dependent pathway. J. Neurochem. 2013;126(3):382–388. doi: 10.1111/jnc.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burnstock G. Physiopathological roles of P2X receptors in the central nervous system. Curr. Med. Chem. 2015;22(7):819–844. doi: 10.2174/0929867321666140706130415. [DOI] [PubMed] [Google Scholar]
- 43.Jiang B.H., Liu L.Z. PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochim. Biophys. Acta. 2008;1784(1):150–158. doi: 10.1016/j.bbapap.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Weng L., Brown J., Eng C. PTEN induces apoptosis and cell cycle arrest through phosphoinositol-3-kinase/Akt-dependent and -independent pathways. Hum. Mol. Genet. 2001;10(3):237–242. doi: 10.1093/hmg/10.3.237. [DOI] [PubMed] [Google Scholar]
- 45.Radi R. Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 2013;288(37):26464–26472. doi: 10.1074/jbc.R113.472936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Z., Maroney A.C., Dobrzanski P., Kukekov N.V., Greene L.A. The MLK family mediates c-Jun N-terminal kinase activation in neuronal apoptosis. Mol. Cell Biol. 2001;21(14):4713–4724. doi: 10.1128/MCB.21.14.4713-4724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan C.Y., Greene L.A. Prevention of PC12 cell death by N-acetylcysteine requires activation of the Ras pathway. J. Neurosci. 1998;18(11):4042–4049. doi: 10.1523/JNEUROSCI.18-11-04042.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stefanis L., Troy C.M., Qi H., Shelanski M.L., Greene L.A. Caspase-2 (Nedd-2) processing and death of trophic factor-deprived PC12 cells and sympathetic neurons occur independently of caspase-3 (CPP32)-like activity. J. Neurosci. 1998;18(22):9204–9215. doi: 10.1523/JNEUROSCI.18-22-09204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park D.S., Morris E.J., Stefanis L., Troy C.M., Shelanski M.L., Geller H.M., Greene L.A. Multiple pathways of neuronal death induced by DNA-damaging agents, NGF deprivation, and oxidative stress. J. Neurosci. 1998;18(3):830–840. doi: 10.1523/JNEUROSCI.18-03-00830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adinolfi E., Kim M., Young M.T., Di Virgilio F., Surprenant A. Tyrosine phosphorylation of HSP90 within the P2X7 receptor complex negatively regulates P2X7 receptors. J. Biol. Chem. 2003;278(39):37344–37351. doi: 10.1074/jbc.M301508200. [DOI] [PubMed] [Google Scholar]
- 51.Kim M., Jiang L.H., Wilson H.L., North R.A., Surprenant A. Proteomic and functional evidence for a P2X7 receptor signalling complex. EMBO J. 2001;20(22):6347–6358. doi: 10.1093/emboj/20.22.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coddou C., Yan Z., Obsil T., Huidobro-Toro J.P., Stojilkovic S.S. Activation and regulation of purinergic P2X receptor channels. Pharmacol. Rev. 2011;63(3):641–683. doi: 10.1124/pr.110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosen L.B., Ginty D.D., Weber M.J., Greenberg M.E. Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of Ras. Neuron. 1994;12(6):1207–1221. doi: 10.1016/0896-6273(94)90438-3. [DOI] [PubMed] [Google Scholar]
- 54.Hopkins B.D., Hodakoski C., Barrows D., Mense S.M., Parsons R.E. PTEN function: the long and the short of it. Trends Biochem. Sci. 2014;39(4):183–190. doi: 10.1016/j.tibs.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghalali A., Wiklund F., Zheng H., Stenius U., Hogberg J. Atorvastatin prevents ATP-driven invasiveness via P2X7 and EHBP1 signaling in PTEN-expressing prostate cancer cells. Carcinogenesis. 2014;35(7):1547–1555. doi: 10.1093/carcin/bgu019. [DOI] [PubMed] [Google Scholar]
- 56.Miraglia E., Hogberg J., Stenius U. Statins exhibit anticancer effects through modifications of the pAkt signaling pathway. Int. J. Oncol. 2012;40(3):867–875. doi: 10.3892/ijo.2011.1223. [DOI] [PubMed] [Google Scholar]
- 57.Mistafa O., Ghalali A., Kadekar S., Hogberg J., Stenius U. Purinergic receptor-mediated rapid depletion of nuclear phosphorylated Akt depends on pleckstrin homology domain leucine-rich repeat phosphatase, calcineurin, protein phosphatase 2A, and PTEN phosphatases. J. Biol. Chem. 2010;285(36):27900–27910. doi: 10.1074/jbc.M110.117093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peng W., Cotrina M.L., Han X., Yu H., Bekar L., Blum L., Takano T., Tian G.F., Goldman S.A., Nedergaard M. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc. Natl. Acad. Sci. USA. 2009;106(30):12489–12493. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X., Arcuino G., Takano T., Lin J., Peng W.G., Wan P., Li P., Xu Q., Liu Q.S., Goldman S.A., Nedergaard M. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat. Med. 2004;10(8):821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- 60.Benner E.J., Banerjee R., Reynolds A.D., Sherman S., Pisarev V.M., Tsiperson V., Nemachek C., Ciborowski P., Przedborski S., Mosley R.L., Gendelman H.E. Nitrated alpha-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS One. 2008;3(1):e1376. doi: 10.1371/journal.pone.0001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiong Y., Rabchevsky A.G., Hall E.D. Role of peroxynitrite in secondary oxidative damage after spinal cord injury. J. Neurochem. 2007;100(3):639–649. doi: 10.1111/j.1471-4159.2006.04312.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.