Highlights
-
•
Bcl-2 was suppressed more effectively by combined treatment in DLD-1 and HT-29 cells.
-
•
Casp-9,-3 were stimulated more effectively by combined treatment in both cell lines.
-
•
TACAs were supressed more effectively by combined treatment in both cell lines.
-
•
Anti-MUC1 combined with PtPz4, PtPz6 or cisPt is more effective than monotherapy.
Keywords: Anti-cancer treatment, Anti-MUC1, Apoptosis, Carbohydrate antigens, Cisplatin, Colon cancer, Platinum complexes
Abstract
The membrane-bound MUC1 mucin is overexpressed and aberrantly glycosylated in many epithelium origin cancers. One of the promising strategies in cancer therapy is combining monoclonal antibodies against cancer related antigens, like MUC1, with chemotherapeutics. In the study we evaluated the potency of cisplatin (cisPt), two pyrazole-platinum(II) complexes PtPz4, PtPz6, and anti-MUC1 mAb applied as monotherapy, as well as the chemotherapeutics administrated with antibody, towards apoptotic response and cancer-related carbohydrate antigens (TACAs) in DLD-1 and HT-29 colon cancer cells. To assess the impact of the tested compounds on the examined factors flow cytometry, RT-PCR, Western blotting and ELISA were utilized. The combined therapy was more potent than monotherapy towards Bcl-2, Bid, caspases and TACAs of both cell lines. Combined therapy applied in DLD-1 cells induced apoptosis, was more effective than monotherapy in relation to p53, Bcl-xL, Bax, and Bim. In HT-29 cells, anti-MUC1 administrated with the drugs was more potent than monotherapy towards Bad. The proposed anti-MUC1/cisPt and pyrazole-platinum(II) complexes PtPz4, PtPz6 combined therapy may be promising anti-colon cancer therapy.
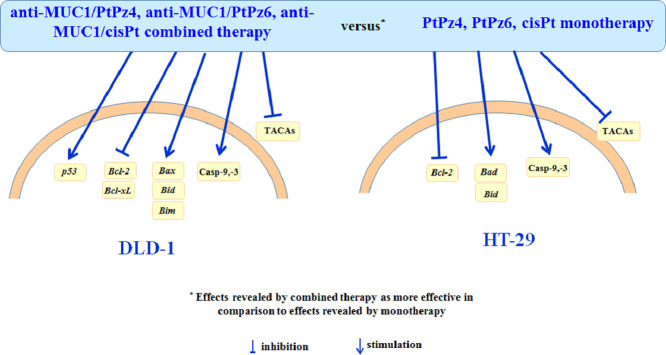
Graphical abstract
Introduction
Colon cancer is one of the leading types of malignancies that results in considerable number of deaths worldwide. Surgery, radiation therapy, and chemotherapy are the most common treatment strategies [3,17]. However, chemotherapy, considered as the most effective, can be seriously limited due to severe toxic side effects and chemoresistance [41]. Therefore, the development of novel, efficient, anti-colon cancer reagents seems to be an attractive goal in looking for potential anti-cancer drugs.
Recently, it has been established that utilizing of tumor antigen-specific monoclonal antibodies seems to be promising direction in therapies of many cancers [5]. The essential challenge is to target specific antigens that are not expressed or are expressed in much lower amounts in normal cells in comparison with malignant cells [7]. The membrane-bound MUC1 mucin is glycoprotein which exists on the apical surface of the most epithelial cells. In many types of cancer, including colon cancers, its abnormal overexpression and aberrant glycosylation can be observed. In malignancies, MUC1 becomes also redistributed over the entire cell surface due to a loss in apical-basal polarity [4,7]. MUC1 has been approved to reveal a role in tumorigenesis mainly by inhibition of cell death and promotion of metastasis [37,42]. Because of this, tumor associated MUC1 molecule is considered as a very attractive therapeutic target in cancer treatment. It is more likely that potential antibodies against tumor-associated MUC1 will bind to the antigen on malignant cells surface, not mucin found on normal cells. Currently, there are clinical trials, pre-clinical and experimental studies of many anti-MUC1 monoclonal antibodies [5,22,33]. However, thoroughly effective therapy with clinical advantage is yet to be achieved.
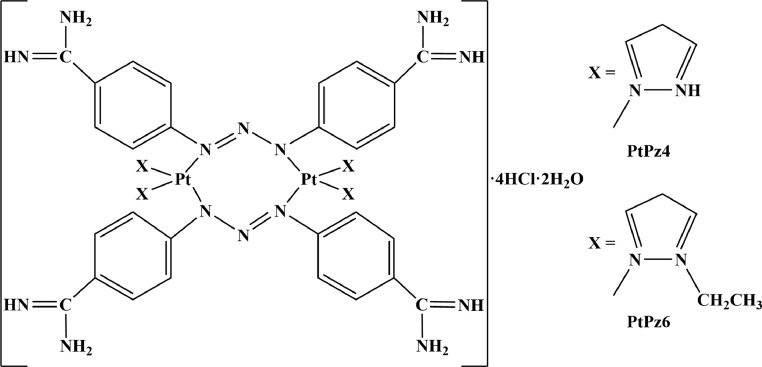
Herein, we decided to assess the effectiveness of anti-MUC1 mAb, two pyrazole-platinum(II) complexes (PtPz4, PtPz6) (Fig. 1), and cisplatin (cisPt) in monotherapy, as well as mAb combined with cisplatin and its derivatives towards DLD-1 and HT-29 colon cancer cells. Recently, pyrazole-platinum complexes has been revealed as promising, anti-cancer agents in breast cancer cells [10]. Potential, anti-cancer action of such platinum derivatives combined with anti-MUC1 mAb has been also demonstrated in AGS gastric cancer cells [36]. In the present study we compared the effectiveness of monotherapy with combined therapy against selected apoptosis-related factors and specific cancer-associated sugar antigens likely participating in cancer development.
Fig. 1.
Structures of pyrazole-platinum(II) complexes PtPz4 and PtPz6.
Materials and methods
Platinum complexes (PtPz4, PtPz6)
Two platinum complexes PtPz4 ([Pt2(pyrazole)4(berenil)2]•4HCl•2H2O) and PtPz6 ([Pt2(N-ethylpyrazole)4(berenil)2]•4HCl•2H2O) were synthesized as previously described [10]. The structures of the synthesized compounds were confirmed by 1H-NMR and 13C-NMR spectra recorded on the Brucker AC 200F (Germany) apparatus (1H–200 MHz and 13C–50 MHz) in deuterated dimethylsulfoxide (d6-DMSO). Chemical shifts are expressed as a δ value (ppm). The multiplicity of resonance peaks was indicated as singlet (s), doublet (d), triplet (t), quartet (q), and multiplet (m). Infrared spectra were recorded on the Perkin-Elmer Spectrum 100 FT-IR spectrometer (PerSeptive Biosystems, Houston, TX, USA) as KBr pellets (4000–450 cm−1). Mass spectra were recorded using a Mariner mass spectrometer (USA). Melting points were determined on the Buchi 535 (GER) melting-point apparatus, and were uncorrected. Elemental analysis of C, H, and N was performed on a Perkin-Elmer 240 analyzer (USA), and satisfactory results within ±0.4% of calculated values were obtained.
[Pt2(pyrazole)4(berenil)2]•4HCl•2H2O (PtPz4): Yield: 29.7%; lemon powder; mp 243–245 °C; 1H-NMR (DMSO-d6) δ (ppm): 11.84 (br, s, NH), 9.48 (br, s, amidine), 7.92 (d, J= 8.6 Hz, 8H, Ar), 7.67 (d, J= 8.3 Hz, 8H, Ar), 7.65 (d, J= 2.0 Hz, 4H, Pz), 6.40 (t, J =2.6 Hz, 4H, Pz); 13C-NMR (DMSO-d6) δ (ppm): 165.2 (amidine), 153.8 (Ar), 146.3 (Pz), 140.8 (Pz), 130.2 (Ar), 121.6 (Ar), 114.6 (Ar), 107.0 (Pz); IR (KBr, cm−1): 3220 (C=NH imine), 3127 (NH3+), 1686 (NCN/C=N imine), 1606 (triazene), 1567 (CN pyrazole ring), 1513 (NH3+), 1257 (triazene), 1174 (triazene), 854 (1,4-disubstituted aromatic), 525 (Pt-N); MS (ES, HR) m/z (M+) calcd. for C40H48Cl4N22Pt2 1366.2482, found 1366.2503; Anal. calcd. for C40H44N22Pt2•4HCl•2H2O: C, 34.20; H, 3.73; N, 21.93; found: C, 34.18; H, 3.76; N, 21.92.
[Pt2(N-ethylpyrazole)4(berenil)2]•4HCl•2H2O (PtPz6): Yield: 69.9%; lemon powder; mp 255–260 °C; 1H-NMR (DMSO-d6) δ (ppm): 9.48 (br, s, amidine), 7.92 (d, J =8.6 Hz, 8H, Ar), 7.67 (d, J =8.3 Hz, 8H, Ar), 7.83 (d, J= 2.0 Hz, 4H, Pz), 7.27 (d, J =7.5 Hz, 4H, Pz), 6.23 (t, J =2.6 Hz, 4H, Pz), 4.10 (q, J =7.2 Hz, 8H, CH2), 1.46 (t, J= 7.2 Hz, 12H, CH2); 13C-NMR (DMSO-d6) δ (ppm): 165.2 (amidine), 153.8 (Ar), 139.6 (Pz), 130.2 (Ar), 129.7 (Pz), 121.6 (Ar), 114.6 (Ar), 105.3 (Pz), 48.3 (CH2), 15.7 (CH3); IR (KBr, cm−1): 3360 (C=NH imine), 3109 (NH3+), 2924 (CH2/CH3), 1686 (NCN/C=N imine), 1606 (CN pyridine/triazene), 1572 (CN pyrazole ring), 1514 (NH3+), 1485 (CH2/CH3), 1257 (triazene), 1173 (triazene), 851 (1,4-disubstituted aromatic), 525 (Pt-N); MS (ES, HR) m/z (M+) calcd. for C48H64Cl4N22Pt2 1481.1620, found 1481.1820; Anal. calcd. for C48H60N22Pt2•4HCl•2H2O: C, 38.00; H, 4.52; N, 20.31, found: C, 37.99; H, 4.53; N, 20.36.
Cell culture
Human colorectal adenocarcinoma cells DLD-1 (CCL-221) and HT-29 (HTB-38) were purchased from American Type Culture Collection (Manassas, VA, USA). HT-29 cells were cultured in McCoy's 5a medium (Pan Biotech., Aidenbach, Lower Bavaria, Germany), and DLD-1 in RPMI 1640 medium (ATCC, Manassas, VA, USA) at 37°C, 5% CO2 in humidified air. Media were supplemented with Fetal Bovine Serum (10%) (FBS) (Gibco, Waltham, MA, USA), penicillin (100 U/mL) and streptomycin (100 μg/mL) (Sigma, St. Louis, MO, USA). The cells were seeded into six-well plates in 1 mL of growth medium, grown to 70% confluency, and then used for the further analyses. Dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO, USA) was used to dissolve cisplatin (Sigma, St. Louis, MO, USA), PtPz4 and PtPz6 (stock solutions). Next, the final concentration of 10 μM was obtained by dilution of tested compounds in supplemented medium. The cells were separated into following groups: cells incubated with FBS-free medium (control), medium with PtPz4, PtPz6, cisPt, or monoclonal anti-MUC1 antibody in concentration 5 μg/mL. In combined treatment, DLD-1 and HT-29 cancer cells were pretreated with anti-MUC1 mAb 5 h before addition of cisPt and two derivatives. After 24 h incubation, wells were washed with Phosphate Buffered Saline (PBS; pH 7.4) and cells were lysed at 4 °C with RIPA (Radio-Immunoprecipitation Assay) buffer (Sigma, St. Louis, MO, USA) with protease inhibitors (dilution 1:200) (Protease Inhibitor Coctail; Sigma, St. Louis, MO, USA) for 20 min. Then, after intense vortexing and centrifugation at 1000 x g for 5 min at 4 °C, the supernatants of cell lysates and culture media were collected, frozen at −70 °C and used for further analyses. For the measurements of protein concentration, BCA Protein Assay Kit (Pierce, USA) was applied. For real-time PCR, the wells were washed (3-times, sterile 10 mM PBS), collected and sonified (Sonics Vibra cell; Sonics & Materials, Leicestershire, UK) (10 W, 3-times for 15 s on ice). For RNA isolation, aliquots of the homogenate were used.
Cell viability assay
The cell viability was assayed in accordance with Carmichael et al. [9], using MTT (3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide) (Sigma, St. Louis, MO, USA). Briefly, cells were cultured on six-well plates to achieve 70% of confluency and then exposed to (24 h) 2–100 μM concentrations of PtPz4, PtPz6, cisPt in 1 mL of MTT solution (0.5 mg MTT/mL of PBS) at 37 °C in 5% CO2. For anti-MUC1 monoclonal antibody 1.25–20 μg/mL concentrations of mAb were used. Absorbance was determined at a wavelength of 570 nm. The viability of the cells in the presence of studied compounds was calculated as a percentage of control cells (without tested compounds addition, 100% cell viability).
Annexin V binding assessment
Apoptosis Detection Kit II (BD Pharmingen, San Diego, Ca, USA) was used to evaluate apoptosis induced by the examined compounds. Briefly, cells were trypsinised, resuspended in proper medium, and then in binding buffer. Next, for cells staining FITC Annexin V and propidium iodide (PI) were applied (15 min at room temperature (RT)) in the dark. Cells cultured in a drug-free medium were a negative control. FACSCanto II cytometer (BD, San Jose, CA, USA) with FACSDiva software (BD Biosciences systems, San Jose, CA, USA) was used to analyze obtained results. Cells incubated with 3% formaldehyde in buffer for 30 min on ice (a positive control) were used to find the optimal parameter settings.
RNA isolation and quantitative real-time PCR
Total ribonucleic acid (RNA) from cells was isolated applying Total RNA Mini Plus Concentrator (A&A Biotechnology, Poland), according to the instructions provided. RNA purity and concentration was assessed spectrophotometrically (Nanodrop 2000, Thermo Scientific, Waltham, MA, USA). Equal amounts (1 μg) of total RNA were subjected to reverse transcription using the SensiFAST™ cDNA Synthesis Kit (Bioline, London, UK). 20 μL of the reaction mixture contained RNA template, 1 μl of Reverse Transcriptase, 4 μl of 5xTransAmp Buffer and DEPC-treated water. The conditions of incubation were: 10 min at 25 °C, 30 min at 45 °C, and 5 min at 70 °C. cDNA amplification was carried out using SensiFAST™ SYBR Kit (Bioline, London, UK) in the thermocycler CFX96 real-time system (BioRad, Hercules, CA, USA). Reaction mixture contained 2 μL of cDNA template (3-times diluted), 0.4 μL of each target-specific primer (10 μmol/L) purchased from Genomed (Warsaw, Poland), 2 × SensiFAST SYBR No-ROX Mix (5 μL) and DEPC-treated water (total volume 10 μL). The following primers (forward and reverse) were used: Bad, forward 5′- CCC AGA GTT TGA GCC GAG TG-3′, reverse 5′- CCC ATC CCT TCG TCG TCC T -3′; Bax, forward 5′- TTG CTT CAG GGT TTC ATC CA-3′, reverse 5′- CAG CCT TGA GCA CCA GTT TG -3′; Bcl-2, forward 5′- GCT GAA GAT TGA TGG GAT CG -3′, reverse 5′- TAC AGC ATG ATC CTC TGT CAA G -3′; Bcl-xL, forward 5′- TGA CGT GGA CAT CCG C -3′, reverse 5′- CTG GAA GGT GGA CAG CGA GC -3'; Bid, forward 5′- CCT ACC CTA GAG ACA TGG AGA AG -3′, reverse 5′- TTT CTG GCT AAG CTC CTC ACG -3′; Bim, forward 5′- TAG GTG AGC GGG AGG CTA GGG ATC A -3′, reverse 5′- GTG CAG GCT CGG ACA GGT AAA GGC -3′; caspase-3, forward 5′- CAG TGG AGG CCG ACT TCT TG -3′, reverse 5′- TGG CAC AAA GCG ACT GGA T -3′; caspase-9, forward 5′- CCC ATA TGA TCG AGG ACA TCC A -3′, reverse 5′- ACA ACT TTG CTG CTT GCC TGT TAG -3′; MUC1, forward 5′- TGC CTT GGC TGT CTG TCA GT -3′, reverse 5′- GTA GGT ATC CCG GGC TGG AA -3′; p53, forward 5′- TCT GAG TCA GGC CCT TCT G -3′; reverse 5′- GTT CCG AGA GCT GAA TGA GG -3′; GAPDH, forward 5′- GTG AAC CAT GAG AAG TAT GAC AA -3′, reverse 5′- CAT GAG TCC TTC CAC GAT AC -3′. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference gene. The PCR plate was subjected to the following conditions: 95 °C for 2 min (DNA polymerase activation), followed by 40 cycles of 10 s at 95 °C (denaturation), 15 s at 60 °C (annealing) and elongation (20 s at 72 °C). Each sample was examined in triplicate. To confirm single product formation, the melting curves were analyzed after amplification. The levels of target gene transcripts were normalized to GAPDH transcripts using the ∆∆Ct method.
Western blot analysis
Cell lysates were boiled for 5 min in sample buffer (dilution 4:1) containing 2.5% sodium dodecyl sulfate (SDS) and then equal amount of proteins (20 μg) were subjected to electrophoretic separation at 7.5%–13% polyacrylamide gels and transferred onto Immobilon-P transfer membrane (Millipore, Bedford, MA, USA) according to Towbin et al. [38]. Next, the membranes were blocked (1 h) in 5% non-fat milk in Tris-buffered saline (50 mM Tris-HCl, pH 7.5, 150 mM NaCl) with 0.05% Tween 20 (Sigma, St. Louis, MO, USA) (TBS-T) at RT and washed (TBS-T, 3-times). Thereafter, membranes were incubated overnight with respective primary monoclonal antibodies (Table 1) in TBS-T containing 3% bovine serum albumin (BSA) at a dilution ratio of 1:600 at 4 °C. The blots were washed three times (TBS-T) before incubated with a proper secondary horseradish peroxidase-conjugated antibodies diluted 1:2500 in TBS-T containing 5% non-fat milk. TBS-T buffer instead of monoclonal antibodies (negative control) was used to exclude non-specific bindings. In some experiments, the membranes were reused after stripping with a Restore Western Blot Stripping Buffer (Thermo Fisher Scientific, Waltham, MA, USA). The protein bands were developed by enhanced chemiluminescence with Westar Supernova, ECL substrate for Western blotting (Cyangen, Bologna, Italy). To quantify the intensity of the bands, densitometric analyses using an imaging densitometer (G:BOX, Syngene, Cambridge, UK) were performed and results were normalized for β-actin.
Table 1.
Antibodies used in the study.
| Antibody | Clone | Source |
|---|---|---|
| Anti-MUC1; extracellular domain (mouse IgG) Anti-Caspase-3 (mouse IgG) Anti-Caspase-9 (mouse IgG) Anti-Cleaved caspase-9 (rabbit IgG) Anti-Bax (rabbit IgG) Anti-Bcl-xL (mouse IgG) Anti-β-actin (rabbit IgG) Anti-mouse IgG peroxidase conjugated Anti-rabbit IgG peroxidase conjugated |
BC2 B-4 C9 E5Z7N D2E11 7B2.5 |
Abcam Santa Cruz Biotech Cell Sign Tech Cell Sign Tech Cell Sign Tech Santa Cruz Biotech Sigma Sigma Sigma |
ELISA tests
ELISA tests were applied to assess the relative level of Tn, T antigens, their sialylated forms, and MUC1 in cell lysates and culture media. The following biotinylated lectins (Vector, Burlingame, CA, USA) (5 μg/mL) were used to determine carbohydrate antigens: VVA (from Vicia villosa) recognizing Tn antigen (GalNAcα1-O-Ser/Thr), PNA (from Arachishy pogaea (peanut)) recognizing T antigen (Galβ1-3GalNAcα1-O-Ser/Thr), SNA (from Sambucus nigra) with specificity to sialyl Tn (SAα2-6Gal/GalNAc), and MAAII (from Maackia amurensis) with affinity to sialyl T (SAα2-3Gal). Anti-MUC1 monoclonal antibody (Table 1) was applied to detect MUC1 mucin. Microtiter plates (NUNC F96; Maxisorp, Roskilde, Denmark) were coated with 50 μL of cell lysates or culture medium (100 μg protein/mL) and incubated overnight (RT). Then, the wells were blocked with 100 μL of blocking ELISA reagent (Roche Diagnostics, Germany) for 1 h at RT, washed (PBS, 0.05% Tween (100 μL, 3-times)), and incubated with proper lectin or anti-MUC1 mAb for 2 h (RT). Thereafter, 100 μL of horseradish peroxidase avidin D (Vector, Burlingame, CA, USA) to detect carbohydrate antigens, or secondary horseradish peroxidase-conjugated anti-mouse IgG antibody to detect MUC1, were added for 1 h (RT). Colored reaction was developed with ABTS (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (Sigma, St. Louis, MO, USA) (100 μL). Absorbances were read after 20–40 min at a wavelength of 405 nm. Each sample was analyzed in triplicate. As negative controls, 1% BSA instead of the samples and washing buffer instead of lectins and primary antibody were applied.
Statistical analysis
The numerical data are presented as mean ± standard deviation (SD) from at least 3 independent experiments. The values were analyzed using Statistica package (StatSoft, Tulsa, OK, USA). One-way ANOVA followed by the Tuckey's multiple range post hock test was used to determine statistical difference. Data with P < 0.05 were considered significant.
Results
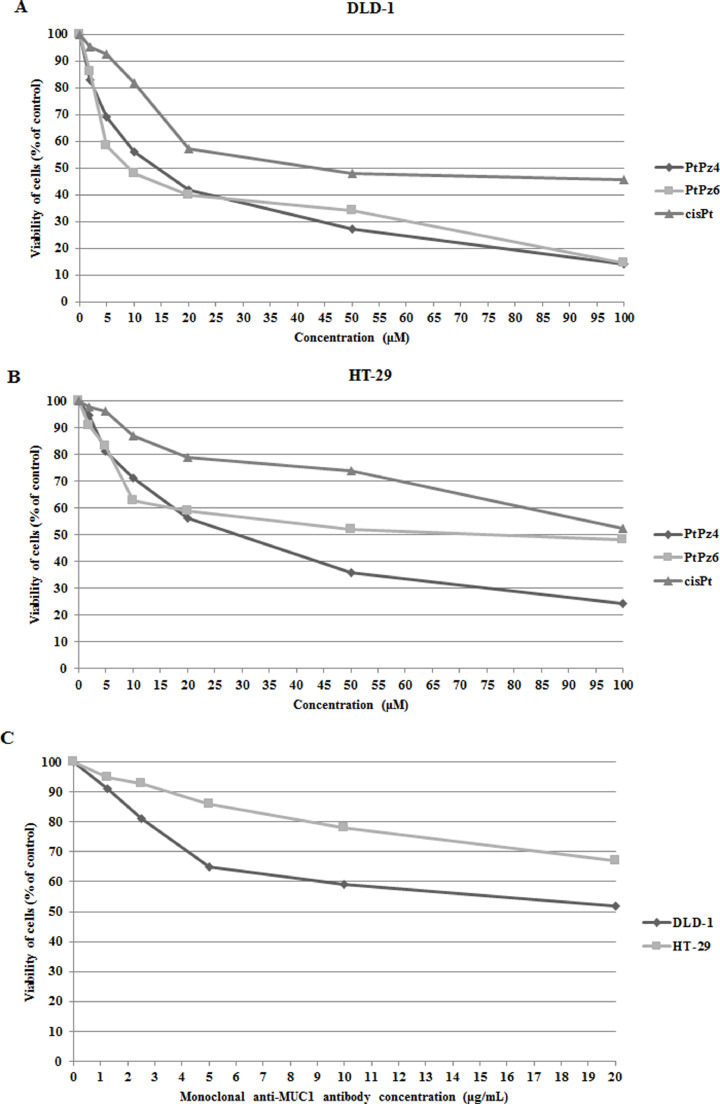
Effects of PtPz4, PtPz6, cisPt and anti-MUC1 on the viability of DLD-1 and HT-29 colon cancer cells
To analyze the effects of PtPz4, PtPz6, cisplatin, and anti-MUC1 on the viability of colon cancer cells, MTT assay was used. The IC50 values for cisplatin (reference drug) were 38 μM in DLD-1 cells and > 100 μM in HT-29 cells after 24 h of incubation (Fig. 2A,B). It was revealed that PtPz4 and PtPz6 drugs were more toxic than cisplatin in both cell lines. In DLD-1 cells IC50 for PtPz4 was 13 μM, for PtPz6 about 10 μM (Fig. 2A). In HT-29 cancer cells IC50 for PtPz4 and PtPz6 were, respectively 27 and 71 μM (Fig. 2B). IC50 values for anti-MUC1 were established as > 20 μg/mL for both colon cancer cell lines (Fig. 2C). In all experiments in our study we decided to use 10 μM concentration of PtPz4, PtPz6 and cisPt, whereas for anti-MUC1 5 μg/mL.
Fig. 2.
Viability of DLD-1 colon cancer cells (A), HT-29 colon cancer cells (B) treated for 24 h with 0–100 μM of PtPz4, PtPz6, cisPt, and viability of both cell lines (C) treated for 24 h with 0–20 μg/mL anti-MUC1 monoclonal antibody. Mean values ±SD are the mean of triplicate culture.
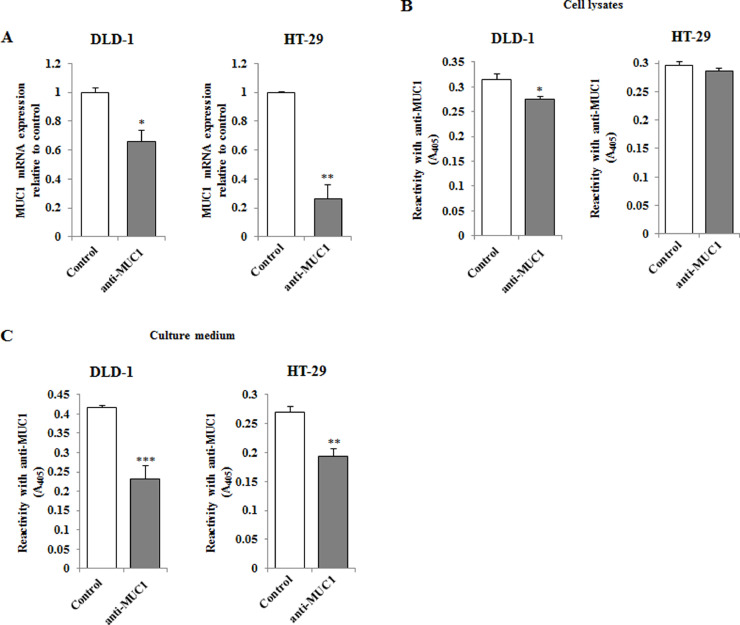
Inhibitory effect of anti-MUC1 monoclonal antibody on MUC1
RT-PCR and ELISA assays were used to evaluate the effect of anti-MUC1 mAb action on MUC1 mucin expression in DLD-1 and HT-29 colon cancer cells. In both studied cell lines a significant decrease of MUC1 mRNA expression compared to uncreated control was observed after incubation (24 h) of the cells with monoclonal antibody (5 μg/mL) (Fig. 3A). Higher suppression of MUC1 expression was revealed in HT-29 cells. The inhibition of protein expression was observed in DLD-1 cell lysates (Fig. 3B) and in culture medium of both cell lines (Fig. 3C).
Fig. 3.
The effect of anti-MUC1 mAb onMUC1 mRNA (A), MUC1 glycoprotein expression in cell lysates (B) and culture medium (C) of DLD-1 and HT-29 colon cancer cells. The cancer cells were incubated for 24 h with 5 μg/mL of anti-MUC1. mRNA was assessed by RT-PCR. The result is presented as a relative fold change in mRNA expression of gene in comparison of gene in control where expression was set as 1. ±SD are the mean of triplicate cultures. *P < 0.05; **P < 0.01. MUC1 glycoprotein expression was assessed by ELISA tests. The results are expressed as absorbance at 405 nm after reactivity with anti-MUC1 monoclonal antibody. Values ±SD are the mean from three independent assays. *P < 0.05, **P < 0.01, ***P < 0.001.
Impact of PtPz4, PtPz6, cisPt, and anti-MUC1 on apoptosis
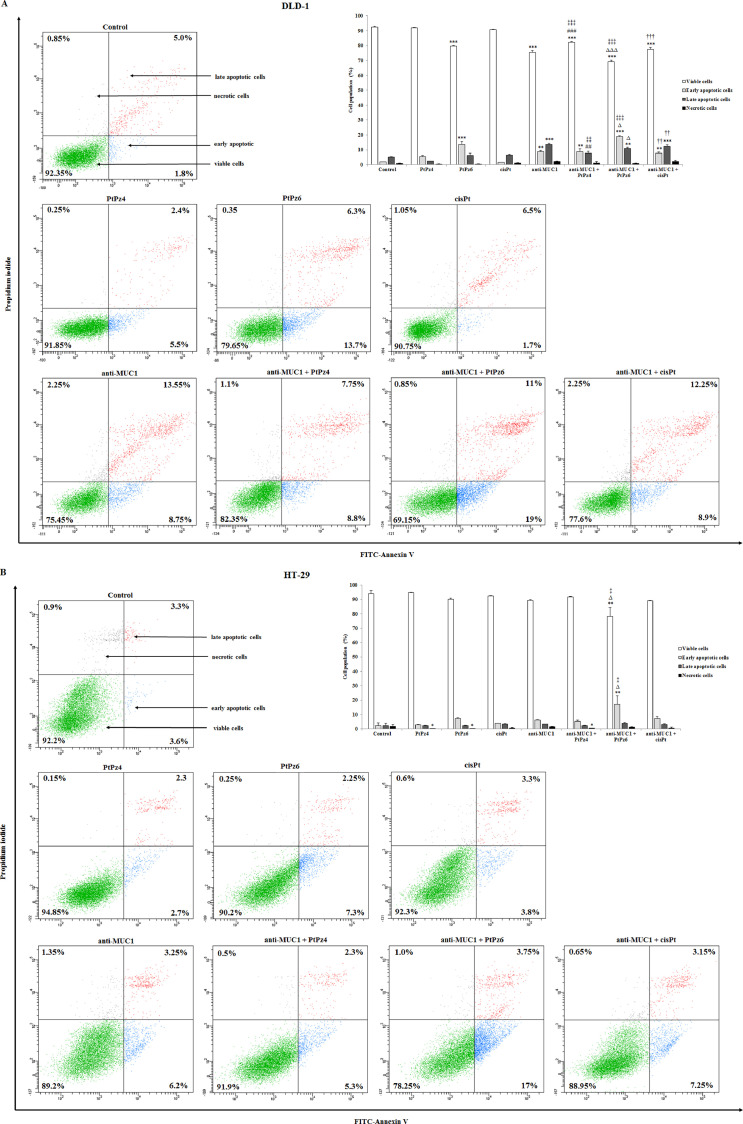
The double-labeling Annexin V and PI procedure was used to assess the influence of examined compounds on apoptosis in DLD-1 and HT-29 colon cancer cells. Annexin V was able to establish cells at all stages of the programmed cell death due to detection of phosphatidylserine exposed at the outer cell membrane (Annexin V+/PI−). Propidium iodide identified late apoptotic (Annexin V+/PI+) and necrotic cells (Annexin V−/PI+) because of staining cells with disrupted cell membranes.
After 24 h of incubation of DLD-1 cancer cells with the tested compounds used in monotherapy, we revealed significant induction of apoptosis upon PtPz6 (13.7% of early apoptotic cells versus 1.8% in untreated control) and anti-MUC1 (22.3% of apoptotic cells). Combined therapy of mAb with PtPz4 increased the number of apoptotic cells to 16.55%, mAb combined with PtPz6 increased to 30%, and mAb combined with cisPt to 21.15%. Administration of PtPz6 with anti-MUC1 occurred to be more effective than monotherapy with mAb (19% of early apoptotic cells versus 8.75%, respectively) (Fig. 4A). In case of HT-29 cancer cells, significant increase of the number of apoptotic cells was observed only after combined action of anti-MUC1 and PtPz6 (17% of early apoptotic cells treated with mAb and PtPz6 versus 3.6% in untreated control, 7.3% in PtPz4 monotherapy, and 6.2 in anti-MUC1 monotherapy) (Fig. 4B). Generally, PtPz6 platinum derivative seems to be the most effective towards apoptosis in both cell lines.
Fig. 4.
Flow cytometry analysis of DLD-1 colon cancer cells (A) and HT-29 colon cancer cells (B) after 24 h incubation with PtPz4 (10 μM), PtPz6 (10 μM), cisPt (10 μM), anti-MUC1 (5 μg/mL), PtPz4 + anti-MUC1 (10 μM + 5 μg/mL), PtPz6 + anti-MUC1 (10 μM + 5 μg/mL), and cisPt + anti-MUC1 (10 μM + 5 μg/mL) and successive staining with Annexin V and propidium iodide. The data are presented as mean percentage values from 3 independent experiments (n = 3) done in duplicate. *P < 0.05, **P < 0.01, ***P < 0.001 compared to the untreated control; ##P < 0.01, ###P < 0.001 compared to PtPz4 monotherapy; ΔP < 0.05, ΔΔΔP < 0.001 compared to PtPz6 monotherapy; ††P < 0.01, †††P < 0.001 compared to cisPt monotherapy; ‡P < 0.05, ‡‡P < 0.01, ‡‡‡P < 0.001 compared to anti-MUC1 monotherapy.
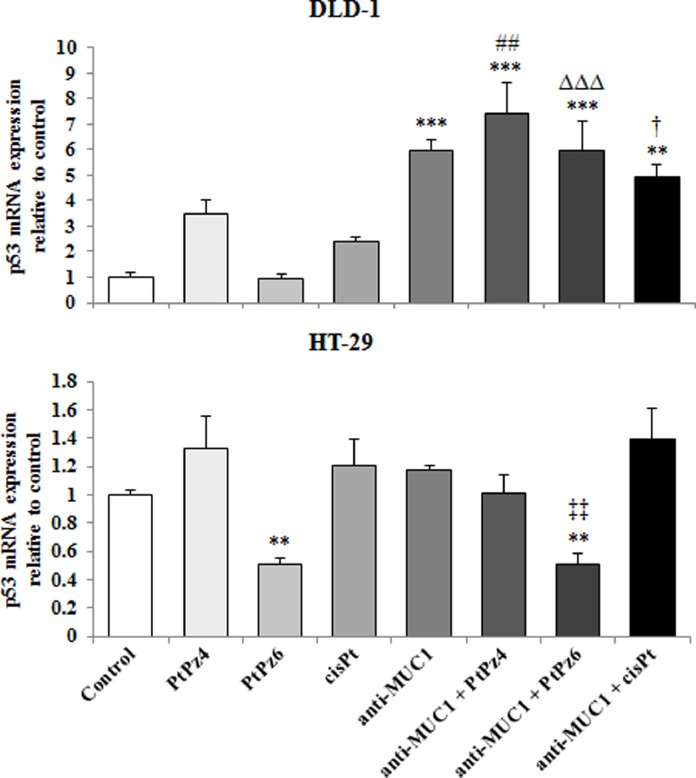
The effect of PtPz4, PtPz6, cisPt, and anti-MUC1 on p53 mRNA
p53 is accepted as one of the most important tumor suppressors. RT-PCR analysis revealed that in DLD-1 cells, p53 mRNA increased as a result of anti-MUC1 action as well as after combined treatment of mAb and the drugs, when compared with untreated control as well as when compared with PtPz4, PtPz6, cisPt monotherapy. The highest induction effect was observed for anti-MUC administrated together with PtPz4. However, in HT-29 cell line, PtPz6 and anti-MUC1/PtPz6 suppressed p53 mRNA expression (Fig. 5).
Fig. 5.
The effect of PtPz4, PtPz6, cisPt, and anti-MUC1 on p53 mRNA expression in DLD-1 and HT-29 colon cancer cells. The cells were incubated for 24 h with PtPz4 (10 μM), PtPz6 (10 μM), cisPt (10 μM), anti-MUC1 (5 μg/mL), PtPz4 + anti-MUC1 (10 μM + 5 μg/mL), PtPz6 + anti-MUC1 (10 μM + 5 μg/mL), and cisPt + anti-MUC1 (10 μM + 5 μg/mL). p53 gene expression was assessed by RT-PCR. The result is presented as a relative fold change in mRNA expression of gene in comparison of gene in control where expression was set as 1. ±SD are the mean of triplicate cultures. ** P < 0.01; *** P < 0.001 compared to untreated control; ##P < 0.01 compared to PtPz4 monotherapy; ΔΔΔP < 0.001 compared to PtPz6 monotherapy; †P < 0.05 compared to cisPt monotherapy; ‡‡P < 0.01 compared to anti-MUC1 monotherapy.
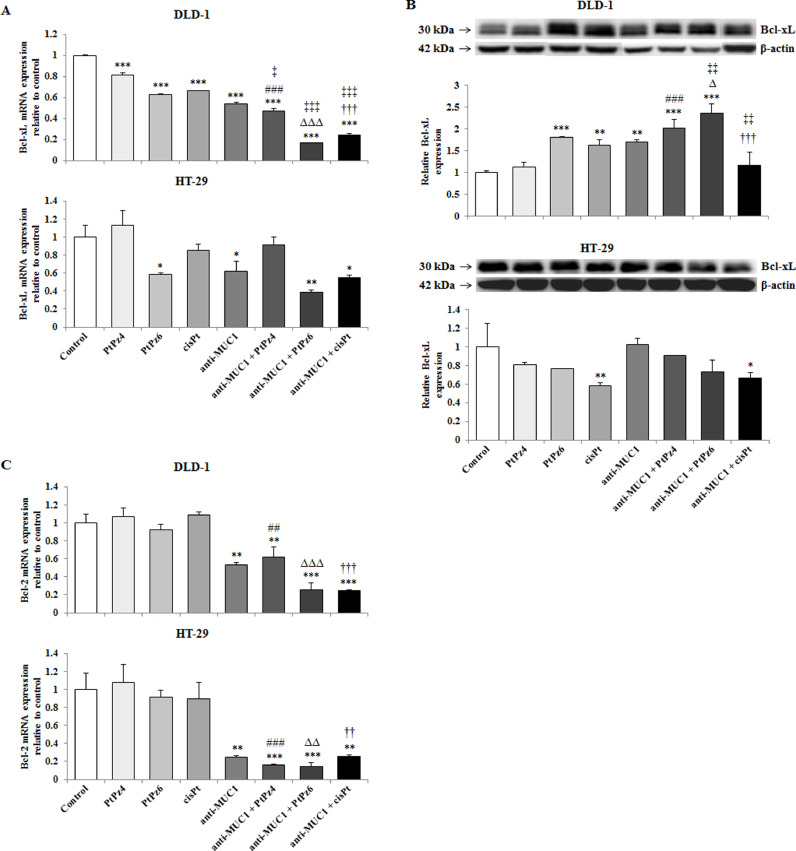
The effect of PtPz4, PtPz6, cisPt, and anti-MUC1 on anti-apoptotic factors
Two crucial pro-survival proteins, Bcl-xL and Bcl-2 were assessed. In DLD-1 cancer cells, all the drugs, in monotherapy and administrated with ant-MUC1, revealed suppressing effect on Bcl-xL mRNA, with the strongest action of anti-MUC1/PtPz6, in comparison to untreated control. Combined therapy was more effective than PtPz4, PtPz6, cisPt, mAb monotherapy. For HT-29 cells, suppression of Bcl-xL mRNA was observed only in case of PtPz6, anti-MUC1, and anti-MUC1 combined with PtPz6 and cisPt, when compared with untreated cells (Fig. 6A). Surprisingly, Bcl-xL protein expression in DLD-1 cancer cells, analyzed by Western blotting, was induced by PtPz6, cisPt, anti-MUC1, and mAb administrated with both cisPt derivatives in comparison with untreated control and monotherapy. In HT-29 cancer cells, decrease of Bcl-xL protein expression was seen only for cisPt and mAb combined with this drug (Fig. 6B, Supplementary Fig. 1). Bcl-2 was analyzed only on mRNA level. In both colon cancer cell lines, significant suppression of Bcl-2 was observed in case of anti-MUC1 and mAb administrated together with all examined drugs in comparison with untreated cells and PtPz4, PtPz6, cisPt monotherapy. The stronger effects were observed for HT-29 cell line (Fig. 6C).
Fig. 6.
The effect of PtPz4, PtPz6, cisPt, and anti-MUC1 on anti-apoptotic Bcl-xL mRNA (A), Bcl-xL protein (B), and Bcl-2 mRNA (C) expressions in DLD-1 and HT-29 colon cancer cells. The cells were incubated for 24 h with PtPz4 (10 μM), PtPz6 (10 μM), cisPt (10 μM), anti-MUC1 (5 μg/mL), PtPz4 + anti-MUC1 (10 μM + 5 μg/mL), PtPz6 + anti-MUC1 (10 μM + 5 μg/mL), and cisPt + anti-MUC1 (10 μM + 5 μg/mL). mRNAs were determined by RT-PCR. The results are presented as a relative fold change in mRNA expression of gene in comparison of gene in control where expression was set as 1. ±SD are the mean of triplicate cultures. *P < 0.05, **P < 0.01, ***P < 0.001 compared to untreated control; ##P < 0.01, ###P < 0.001 compared to PtPz4 monotherapy; ΔΔP < 0.01, ΔΔΔP < 0.001 compared to PtPz6 monotherapy; ††P < 0.01, †††P < 0.001 compared to cisPt monotherapy; ‡P < 0.05, ‡‡‡P < 0.001 compared to anti-MUC1 monotherapy. Bcl-xL protein expression in cell lysates was assessed by Western blotting. The intensities of the bands were quantified by densitometric analysis. Data show the mean ±SD of the relative expression levels (from 3 assays) standardized to β-actin. *P < 0.05, **P < 0.01, ***P < 0.001 compared to the untreated control; ###P < 0.001 compared to PtPz4 monotherapy; ΔP < 0.05 compared to PtPz6 monotherapy; †††P < 0.001 compared to cisPt monotherapy; ‡‡P < 0.01 compared to anti-MUC1 monotherapy.
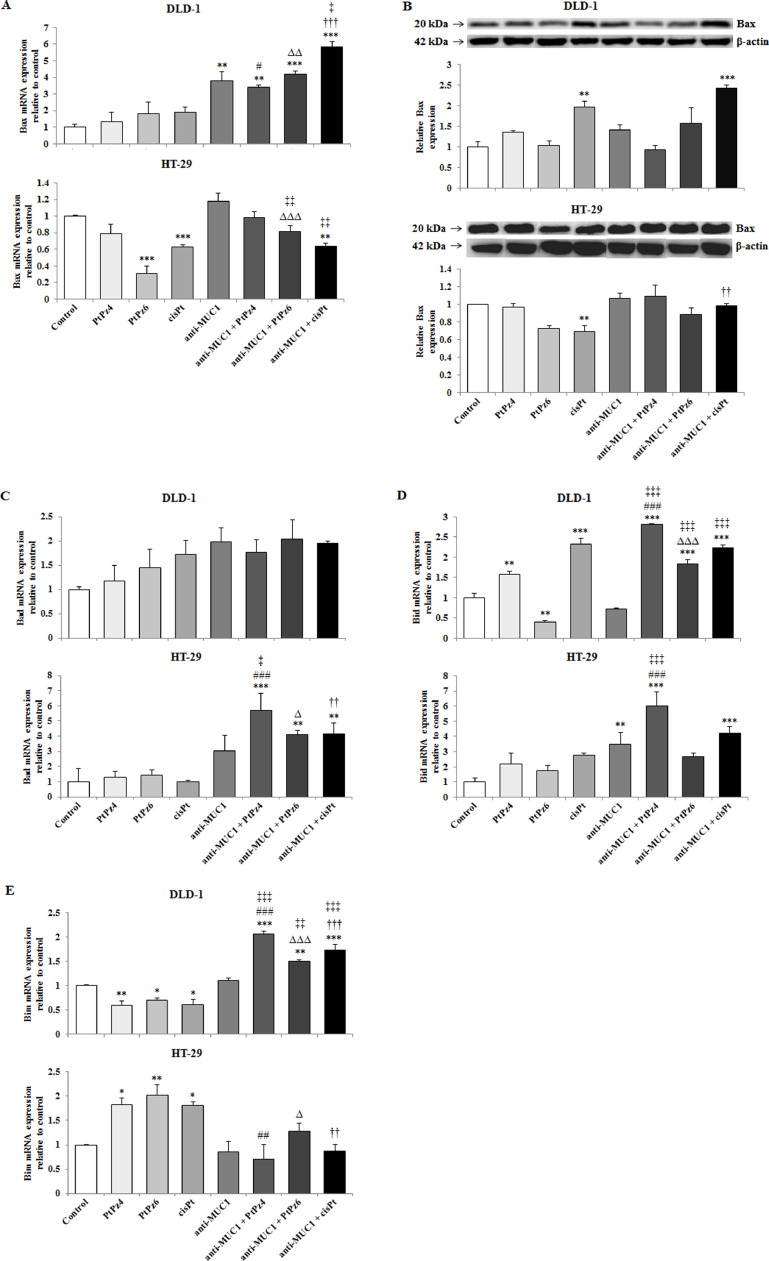
The effect of PtPz4, PtPz6, cisPt, and anti-MUC1 on pro-apoptotic factors
In the next step we checked if examined compounds initiate apoptosis by activation of pro-apoptotic factors, Bax, Bad, Bid, and Bim. Bax expression was examined on both, mRNA and protein level as Bad, Bid, and Bim only on mRNA level. In DLD-1 cells Bax mRNA expression was significantly induced by anti-MUC1, and cisPt, PtPz4, PtPz6 provided with mAb, in comparison with untreated cells as well as in comparison with PtPz4, PtPz6, cisPt monotherapy. mAb/cisPt action was also more effective than mAb monotherapy. In HT-29 cells, Bax mRNA significant stimulation was not observed. Suppression of Bax gene was seen in case of PtPz6, cisPt, and anti-MUC1 combined with cisPt (Fig. 7A). The results of Western blotting analysis revealed the stimulation of Bax protein in DLD-1 cells by cisPt, and mAb/cisPt in comparison with untreated control and cisPt monotherapy (Fig. 7B, Fig. 2). Bax protein expression in HT-29 cells was inhibited by cisPt in comparison with untreated cells (Fig. 7B, Supplementary Fig. 2).
Fig. 7.
The effect of PtPz4, PtPz6, cisPt, and anti-MUC1 on pro-apoptotic Bax mRNA (A), Bax protein (B), Bad mRNA (C), Bid mRNA (D), and Bim mRNA (E) expressions in DLD-1 and HT-29 colon cancer cells. The cells were incubated for 24 h with PtPz4 (10 μM), PtPz6 (10 μM), cisPt (10 μM), anti-MUC1 (5 μg/mL), PtPz4 + anti-MUC1 (10 μM + 5 μg/mL), PtPz6 + anti-MUC1 (10 μM + 5 μg/mL), and cisPt + anti-MUC1 (10 μM + 5 μg/mL). mRNAs were assessed by RT-PCR. The result is presented as a relative fold change in mRNA expression of gene in comparison of gene in control where expression was set as 1. ±SD are the mean of triplicate cultures. *P < 0.05, **P < 0.01, ***P < 0.001 compared to untreated control; #P<0.05, ##P<0.01, ###P<0.001 compared to PtPz4 monotherapy; ΔP<0.05, ΔΔP<0.01, ΔΔΔP<0.001 compared to PtPz6 monotherapy; ††P<0.01, †††P<0.001 compared to cisPt monotherapy; ‡P<0.05, ‡‡P<0.01, ‡‡‡P < 0.001 compared to anti-MUC1 monotherapy. Bax protein expression in cell lysates was determined by Western blotting. The intensities of the bands were quantified by densitometric analysis. Data show the mean ±SD of the relative expression levels (from 3 assays) standardized to β-actin. **P < 0.01, ***P < 0.001 compared to the untreated control; ††P < 0.01 compared to cisPt monotherapy.
In HT-29 cells, Bad mRNA expression was stimulated by combined therapy, in comparison with untreated control as well as when compared with PtPz4, PtPz6, cisPt monotherapy, with the strongest effect for mAb/PtPz4 action. Combining anti-MUC1 with PtPz4 occurred to be also more effective than mAb monotherapy (Fig. 7C).
In Fig. 7D we can observe that PtPz4, cisPt, and the drugs combined with the mAb significantly stimulated Bid mRNA expression in DLD-1 line, when compared with untreated control. Anti-MUC1/PtPz4 and anti-MUC1/PtPz6 action was more potent than PtPz4, and PtPz6 monotherapy. Combined action was more efficient than anti-MUC1 monotherapy. PtPz6 inhibited Bid gene expression. In HT-29 cells, Bid mRNA increased after mAb, mAb/PtPz4, mAb/cisPt in comparison with the control. Additionally, after mAb/PtPz4 treatment, the increase was significant in comparison with PtPz4 and mAb monotherapy (Fig. 7D).
Bim mRNA expression was differently affected by examined compounds in colon cancer cell lines. In DLD-1, stimulatory effect after the action of mAb combined with PtPz4, PtPz6, and cisPt, in comparison with the untreated control as well as with PtPz4, PtPz6, cisPt, and anti-MUC1 monotherapy, with the strongest effect for PtPz4, was demonstrated. Meanwhile, in HT-29 cancer cells, increased Bim gene expression was revealed after PtPz4, PtPz6 and cisPt monotherapy (Fig. 7E).
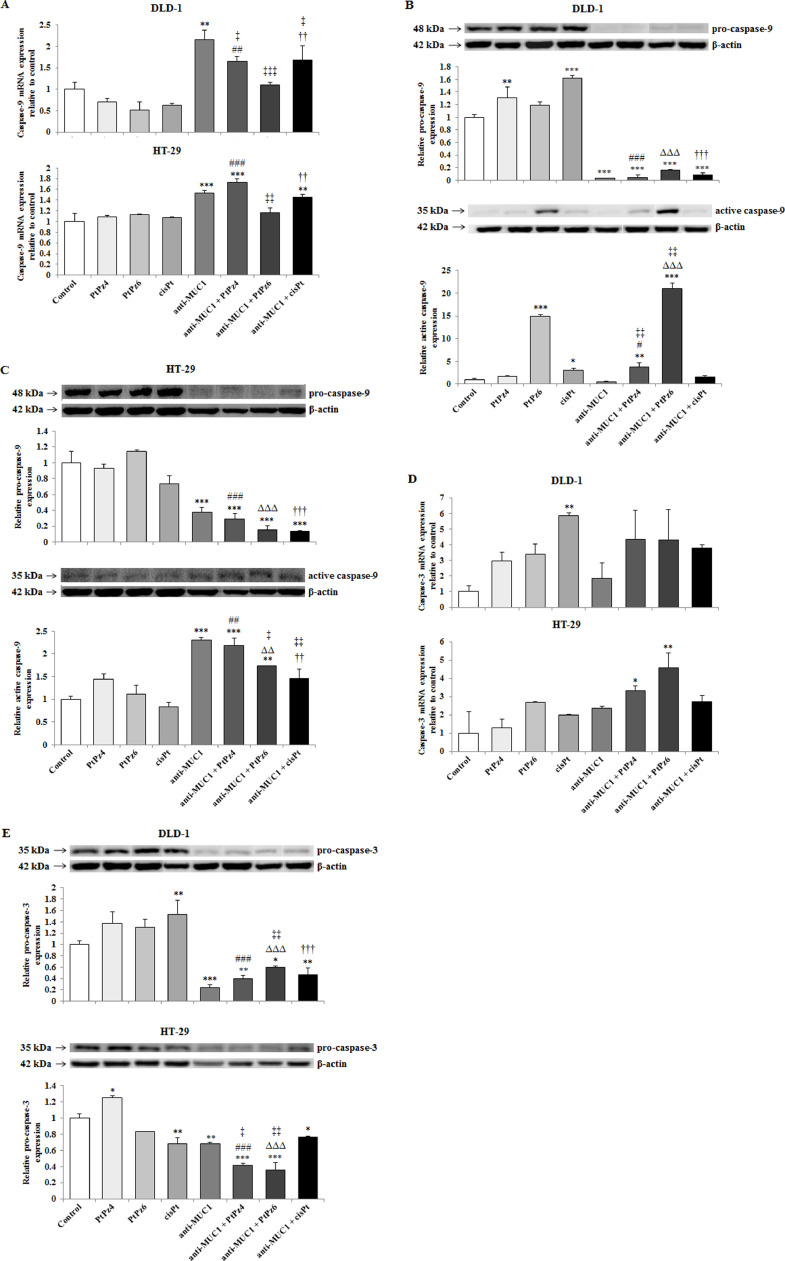
The effect of PtPz4, PtPz6, cisPt, and anti-MUC1 on caspases
Two caspases - initiatory caspase-9 and executive caspase-3 were determined in our study. In DLD-1 cell line, caspase-9 mRNA was significantly stimulated by anti-MUC1 action in comparison with untreated control and by anti-MUC1/PtPz4, anti-MUC1/cisPt when compared with PtPz4, and cisPt monotherapy. In HT-29 cells, caspase-9 expression was stimulated by anti-MUC1, anti-MUC1/PtPz4, anti-MUC1/cisPt action in comparison with untreated cells. In case of PtPz4 and cisPt combined therapy was more effective than PtPz4 and cisPt monotherapy (Fig. 8A). In Western blotting, pro-caspase-9 and activated caspase-9 expression were assessed. In DLD-1 cancer cells, pro-caspase-9 expression increased after PtPz4, and cisPt treatment and strongly decreased as a result of anti-MUC1 and combined therapy. Meantime, activated form of caspase-9 increased after PtPz6 and cisPt monotherapy as well as a result of combined action of mAb with PtPz4 and PtPz6. Stronger effects of combined therapy than PtPz4, PtPz6, anti-MUC1 monotherapy were observed (Fig. 8B, Supplementary Fig. 3). In HT-29 cells, pro-caspase-9 was inhibited by mAb, and anti-MUC1 administrated with all the examined drugs in comparison with untreated cells as well when compared with PtPz4, PtPz6, cisPt monotherapy. Simultaneously, strong stimulation of cleaved caspase-9 expression was revealed as a result of anti-MUC1 and combined therapy, when compared with control and monotherapy (Fig. 8C, Supplementary Fig. 3).
Fig. 8.
The effect of PtPz4, PtPz6, cisPt, and anti-MUC1 on caspase-9 mRNA in DLD-1 and HT-29 cells (A), pro- and active caspase-9 in DLD-1 (B), pro- and active caspase-9 in HT-29 cells (C) caspase-3 mRNA in DLD-1 and HT-29 cells (D), and pro-caspase-3 protein in both colon cancer cells (E). The cells were incubated for 24 h with PtPz4 (10 μM), PtPz6 (10 μM), cisPt (10 μM), anti-MUC1 (5 μg/mL), PtPz4 + anti-MUC1 (10 μM + 5 μg/mL), PtPz6 + anti-MUC1 (10 μM + 5 μg/mL), and cisPt + anti-MUC1 (10 μM + 5 μg/mL). mRNAs were determined by RT-PCR. The result is presented as a relative fold change in mRNA expression of gene in comparison of gene in control where expression was set as 1. ±SD are the mean of triplicate cultures. *P < 0.05, **P < 0.01, ***P < 0.001 compared to untreated control; ##P < 0.01, ###P < 0.001 compared to PtPz4 monotherapy; ††P < 0.01 compared to cisPt monotherapy; ‡P < 0.05, ‡‡P < 0.01, ‡‡‡P < 0.001 compared to anti-MUC1 monotherapy. Pro- and active caspase-9 and pro-caspase-3 expressions in cell lysates of both cell lines were evaluated by Western blotting. The intensities of the bands were quantified by densitometry. Data show the mean ±SD of the relative expression levels (from 3 assays) standardized to β-actin. *P < 0.05, **P < 0.01, ***P < 0.001 compared to the untreated control; #P < 0.05, ##P < 0.01, ###P < 0.001 compared to PtPz4 monotherapy; ΔΔP < 0.01, ΔΔΔP < 0.001 compared to PtPz6 monotherapy; ††P < 0.01, †††P < 0.001 compared to cisPt monotherapy; ‡P < 0.05, ‡‡P < 0.01 compared to anti-MUC1 monotherapy.
Caspase-3 mRNA increased significantly in DLD-1 cells as a result of cisPt action and in HT-29 cells for mAb administrated with PtPz4 and PtPz6 in comparison to untreated cells (Fig. 8D). In Western blotting analysis only pro-caspase-3 was revealed (Fig. 8E, Supplementary Fig. 4). In DLD-1 line, the stimulation of pro-caspase-3 expression was detected after monotherapy with cisPt. Meanwhile, anti-MUC1 and mAb administrated with the drugs statistically decreased expression of this protein. The stronger effect for combined therapy than for monotherapy was observed. In HT-29 cells, the stimulation of pro-caspase-3 protein was observed after PtPz4 monotherapy. After cisPt, mAb, and mAb administrated with the drugs, the inhibition of pro-caspase-3 was revealed, in comparison with untreated control. Combined action of mAb and PtPz4, PtPz6 was more effective than PtPz4, PtPz6, mAb monotherapy.
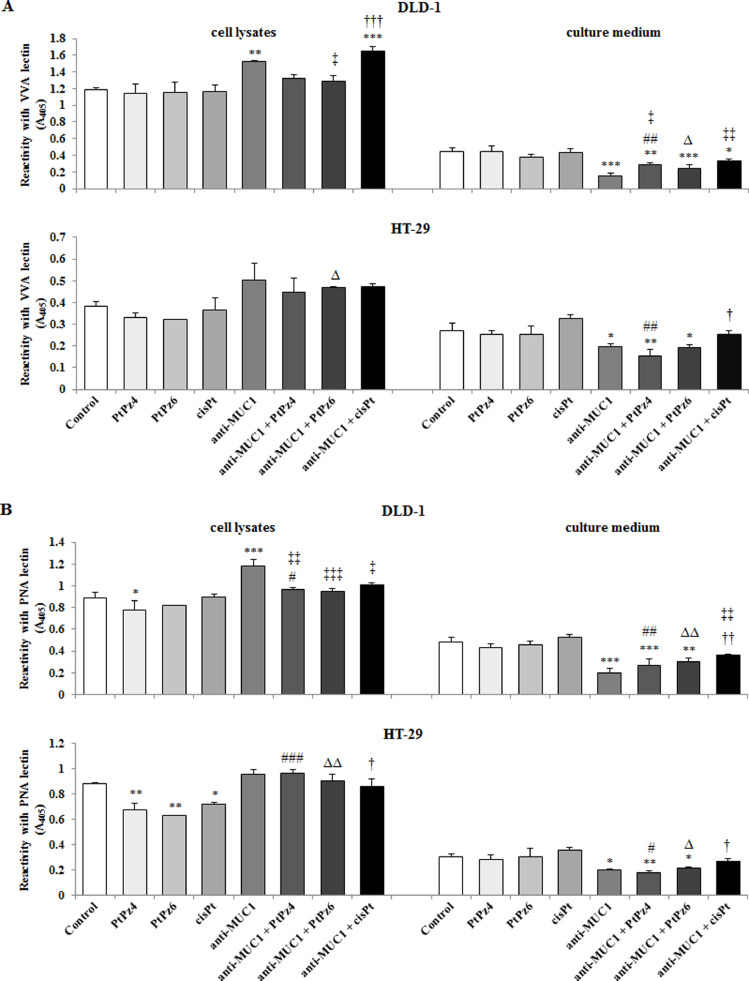
The effect of PtPz4, PtPz6, cisPt, and anti-MUC1 on cancer related carbohydrate antigens expression
ELISA-like test with biotinylated lectins was used to assess the expression of tumor-associated carbohydrate antigens (TACAs). The following sugar antigens were determined in cell lysates and culture medium: Tn (GalNAcα1-O-Ser/Thr), T (Galβ1-3GalNAcα1-O-Ser/Thr), sialyl Tn (SAα2-6Gal/GalNAc), and sialyl T (SAα2-3Gal). To detect Tn antigen, VVA lectin (from Vicia villosa) was applied. In cell lysates of DLD-1 cell line, the significant increase of Tn antigen level was revealed after action of anti-MUC1 and mAb administrated with cisPt, in comparison with untreated control and for mAb/cisPt in comparison with cisPt monotherapy. In HT-29 cells, the stimulation of Tn antigen expression was observed as a result of anti-MUC1 and PtPz6 treatment in comparison with monotherapy (Fig. 9A). Meantime, in culture medium of DLD-1 cancer cells, the inhibitory effect on this antigen expression after action of anti-MUC1 as well as the drugs combined with mAb, in comparison with untreated cells, was revealed. For PtPz4 and PtPz6 combined therapy was more potent than monotherapy. In HT-29 cells, mAb, mAb/PtPz4, and mAb/PtPz6 action suppressed Tn structure expression when compared with control. For PtPz4 and cisPt combined with anti-MUC1, the inhibition was more effective than monotherapy (Fig. 9A).
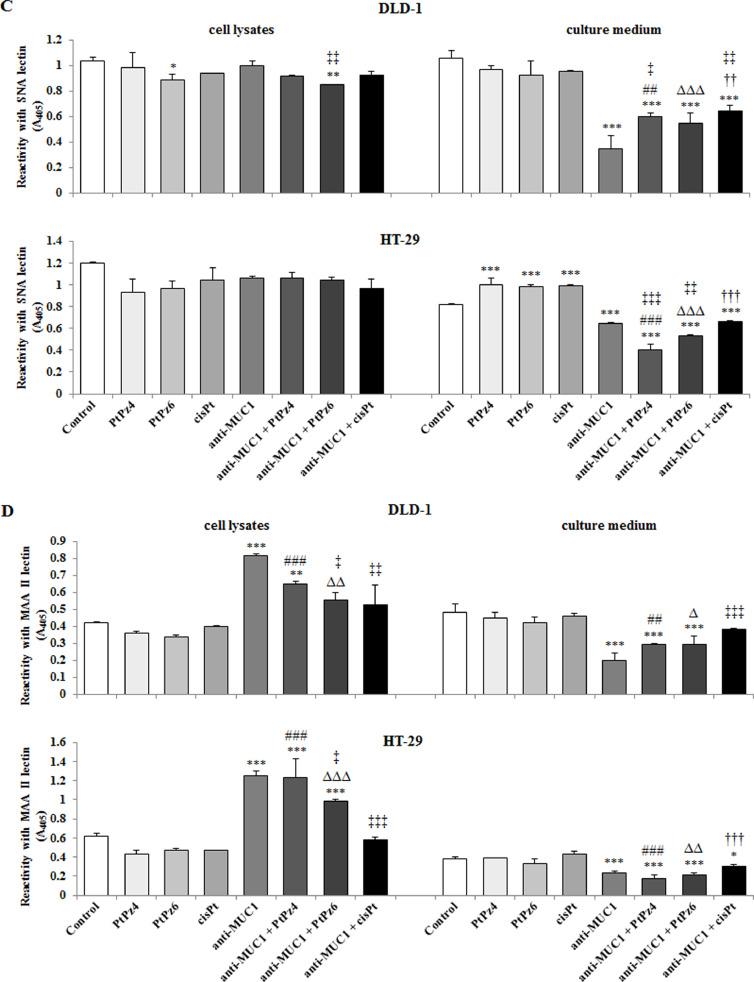
Fig. 9.
The effect of PtPz4, PtPz6, cisPt, and anti-MUC1 on cancer related Tn antigen (detected by VVA lectin) (A), T antigen (detected by PNA lectin) (B), sialyl Tn (detected by SNA lectin) (C), and sialyl T (detected by MAAII lectin) (D) in DLD-1 and HT-29 colon cancer cells. The cells were incubated for 24 h with PtPz4 (10 μM), PtPz6 (10 μM), cisPt (10 μM), anti-MUC1 (5 μg/mL), PtPz4 + anti-MUC1 (10 μM + 5 μg/mL), PtPz6 + anti-MUC1 (10 μM + 5 μg/mL), and cisPt + anti-MUC1 (10 μM + 5 μg/mL). The relative expressions of antigens in cell lysates and culture medium were assessed by ELISA tests. The results are expressed as absorbance at 405 nm after reactivity with biotinylated lectins. Values ±SD are the mean from three independent assays. *P < 0.05, **P < < 0.01, ***P < 0.001 compared to the untreated control; #P < 0.05, ##P < 0.01, ###P < 0.001 compared to PtPz4 monotherapy; ΔP < 0.05, ΔΔP < 0.01, ΔΔΔP < 0.001 compared to PtPz6 monotherapy; †P < 0.05, ††P < 0.01, †††P < 0.001 compared to cisPt monotherapy; ‡P < 0.05, ‡‡P < 0.01, ‡‡‡P < 0.001 compared to anti-MUC1 monotherapy.
PNA lectin (from Arachis hypogaea (peanut)) was applied to detect T antigen. In DLD-1 cell lysates, PtPz4 decreased T structure expression, while anti-MUC1 statistically increased T antigen level in comparison with untreated control. In HT-29 cells, monotherapy with PtPz4, PtPz6, and cisPt significantly inhibited T antigen expression. Combined therapy increased this antigen expression in comparison with monotherapy (Fig. 9B). In culture medium of both cell lines, anti-MUC1 as well as antibody combined with PtPz4, PtPz6 decreased the level of T sugar antigen in comparison with untreated control. When compared with monotherapy, mAb administrated with the drugs, more effectively suppressed T antigen expression (Fig. 9B).
In DLD-1 cell lysates, sialylated form of Tn antigen (sTn), detected by SNA lectin (from Sambucus nigra), was inhibited by PtPz6 and the drug combined with anti-MUC1. There was no effect of such action in HT-29 line (Fig. 9C). In the culture medium of both cell lines, significant inhibition of sTn antigen, after anti-MUC1, as well as after mAb combined with all the examined drugs, in comparison with untreated cells, was demonstrated. Combined therapy was more effective than PtPz4, PtPz6, cisPt monotherapy. Apart from that, in HT-29 culture medium, monotherapy with cisPt and its both derivatives stimulated sTn antigen expression. In case of anti-MUC1/PtPz4 and anti-MUC1/PtPz6 action, the inhibitory effects were more potent than anti-MUC1 monotherapy (Fig. 9C).
Sialyl T antigen (sT) was determined by MAAII lectin (from Maackia amurensis). In both cell line lysates, anti-MUC1 and mAb combined with PtPz4, significantly increased sT antigen expression in comparison with untreated control. Combined anti-MUC1/PtPz4, anti-MUC1/PtPz6 action was more effective than monotherapy. Additionally, in HT-29 cells, significant increase was observed also in case of mAb/PtPz6 therapy when compared with control and monotherapy (Fig. 9D). Anti-MUC1 and antibody administrated with PtPz4/PtPz6 decreased sT antigen expression in culture medium of both cell lines, in comparison with untreated control and monotherapy. Additionally, in HT-29 line, such effect was also observed for mAb/cisPt action (Fig. 9D).
Discussion
MUC1 is reported to be up to 10 times higher in many cancers in comparison with normal cells. Moreover, it is aberrantly glycosylated and specifically distributed over the whole cell surface and within the cytoplasm, nuclei, and mitochondria of malignant cells [4,7,31,32]. Thus, MUC1 fulfills all the requirements to be targeted molecule in mAb-based anti-cancer therapy. Inhibition of its activity could potentially be a supportive strategy in malignant treatment [5,31]. mAbs are able to induce signal arrest and point to apoptosis in targeted cancer cells by binding with their receptors what can lead to the conversion of the receptor or disturbance with ligand binding [8,26]. It was demonstrated that anti-MUC1 applied in pancreatic cancer model induced the translocation of MUC1 protein from the membrane into the cytoplasm and thus inactivation of MUC1 oncogenic signaling was achieved [40]. Hisatsune et al. [19] stated, that MUC1 on the cancer cells can be internalized by mAb through the macropinocytic pathway, in the process mediated by MUC1-cytoplasmic tail. It was also reported that anti-MUC1 applied in pancreatic cancer cells caused internalization of EGFR, what resulted in suppression of proliferation and migration of cancer cells [20].
In the present study, performed on DLD-1 and HT-29 colon cancer cells, we applied BC2 anti-MUC1 monoclonal antibody, reacting with the MUC1 background protein with low effectiveness for glycosylation. We proved the action of mAb as we observed the decrease of MUC1 gene expression and inhibition of MUC1 released to the culture medium for both cell lines. Thus we assumed that these results confirmed the general, above mentioned conceptions of mAbs action and stated that the MUC1 activity could be diminished.
Cisplatin is a chemotherapeutic commonly used for colon cancer treatment. However, its therapeutic efficacy can be limited due to induced chemoresistance and serious side effects [28]. Exposure of cancer cells to chemotherapy can lead to adaptation of the cells through regulation of different signaling pathways engaged in cell proliferation and death [13,30]. It was reported that substitution of a pyrazole ring into divergent heteroaryl ring structures resulted in notable anti-cancer activities [12]. Applying cisplatin derivatives and combining the chemotherapeutic with other drugs have been greatly regarded to conquer drug-resistance and minimize toxicity [34]. Recently, two novel pyrazole-platinum(II) complexes PtPz4 ([Pt2(pyrazole)4(berenil)2]•4HCl•2H2O) and PtPz6 ([Pt2(N-ethylpyrazole)4(berenil)2]•4HCl•2H2O), combined with anti-MUC1 monoclonal antibody, have been examined against breast [10] and gastric [36] cancer cells. It was revealed that cisPt derivatives, administrated with mAb, occurred to be more effective than monotherapy. Due to aforesaid promising results, we decided to evaluate such therapy in DLD-1 and HT-29 colon cancer cells. DLD-1 cells were reported to be more sensitive to chemotherapeutic agents than HT-29 cells [39].
One of the features of cancer is the evasion of apoptosis [6]. The general action of cisPt and its derivatives is based on the ability to crosslink with DNA purine bases, leading to DNA damage, and afterwards inducing apoptotic response, which seems to play a crucial role in cancer treatment [11]. The combined therapy applied in our study was revealed to be more potent towards apoptosis in DLD-1 cells than in HT-29 cells. Additionally, we observed that combined therapy applied in DLD-1 cells induced programmed cell death much more effectively than the drugs used alone. Substitution of pyrazole ring with ethyl group (PtPz6) occurred to be the most sufficient in apoptosis induction, especially when applied with anti-MUC1 mAb. Similar results concerning apoptotic response have been revealed in gastric cancer cells when the same therapy was applied [36].
Chemotherapy can modulate different signaling pathways involved in apoptotic response [13,30]. p53 is considered as tumor suppressor which activation has been suggested as a mechanism for improving the cancer cells response to chemotherapy [2,24,30]. In the current study, in DLD-1 cells, combination of cisPt and its both derivatives with anti-MUC1 occurred to be much more effective towards p53 expression in comparison to monotherapy and the effects revealed for HT-29 colon cancer cells. Thus, such result seems to strongly support the rationality of combined treatment for DLD-1 cells.
Bcl-2 and Bcl-xL are anti-apoptotic Bcl-2 family members participating in the regulation of apoptosis [25]. It has been reported that Bcl-2, apart from increasing of cell survival, inhibits DNA repair systems [43]. Combined treatment applied in both colon cancer cell lines, inhibited Bcl-2 expression much more effectively than monotherapy, what strongly supports the anti-cancer action of used compounds. Interestingly, cisPt as well as PtPz4, PtPz6 used alone did not affect Bcl-2 mRNA. Anti-MUC1 used alone inhibited also Bcl-2 and Bcl-xL expression. Such results suggest participation of MUC1 mucin in regulation of Bcl-2 family activity, what was also demonstrated in breast cancer cells by Hiraki et al. [18]. Surprisingly, pro-survival Bcl-xL protein expression in DLD-1 cells was stimulated as a result of applied therapy. We suggest that such discrepancy, low Bcl-xL mRNA expression and simultaneous high level of Bcl-xL protein can be explained by kind of negative feedback, Bcl-xL protein can directly downregulate Bcl-xL expression.
Upregulation of pro-apoptotic proteins, including Bax, Bad, Bid and Bim is stated to be one more crucial goal in anti-cancer therapy. The mentioned factors are entangled in intrinsic and extrinsic apoptotic pathways. E.g. both Bax and Bak, when activated, undergo homo-oligomerization in the mitochondrial outer membrane, form pores and cytochrome c is released [29]. It was also demonstrated that Bid and Bim have the ability to directly activate Bax/Bad and cause cytochrome c release [23]. MUC1 was reported to interfere in such activation by blocking Bax dimerization [1]. Upregulation of Bax by anti-MUC1 administrated with cisPt and its Pt12 derivative was reported in MDA-MB-231 breast cancer cells [16]. Our outcomes concerning applied therapy towards noted above Bax, Bid, Bim, (but not Bad) pro-apoptotic factors approve rationality of combined treatment in DLD-1 cells. Monotherapy and combined therapy applied in HT-29 cancer cell line occurred to be not effective towards Bax. However, combined therapy, especially anti-MUC1/PtPz4 action, strongly stimulated Bad and Bid expression, what seems to be promising result, supporting the idea of utilizing mAbs administrated with chemotherapeutics in cancer therapy.
Commonly, cancer therapies are directed toward apoptosis induction to get rid of the cancer cells by engaging initiator caspases (caspase-9), and executioner caspases (caspase-3) which trigger damage of the cells. Anti-cancer therapies can target different caspase pathways or can act directly on caspases [6]. Direct engaging of caspase-9 or -3 triggers apoptosis downstream of mitochondrial outer membrane permeabilization, and caspase-3 activation can lead to a kind of positive feedback to the mitochondria by Bid cleavage [35]. We state that the therapy applied in our study aimed the goal towards caspases as combined action of mAb and platinum derivatives, applied in both cell lines, occurred to be more effective than monotherapy in caspase-9 activation as well as decreasing pro-caspase-3 expression. Surprisingly, we were not able to detect active form of caspase-3. At this point we can suggest that the concentration of active form of caspase-3 could be too low, below the sensitivity of Western blotting.
Recently we have revealed that application of anti-MUC1 as well as mAb combined with PtPz4, PtPz6 and cisPt in AGS gastric cancer cells inhibited the expression of TACAs (mucin-type, tumor-associated carbohydrate antigens) commonly overexpressed on MUC1 mucin and on the surface of cancer cells [36]. They are markedly distributed in large number of tumors, but, what is especially important, not on normal cells [21]. The most frequent TACAs established as a result of incomplete synthesis are Tn, T antigens and their sialylated forms [4]. They have been noted by the National Institutes of Health as important biomarkers of cancer prognosis [14]. Such antigens do not participate directly in apoptosis but by interacting with various factors taking part in different signaling pathways can be involved in promoting cancer development [21]. It has been reported that assembling metastatic breast and prostate cancer cells at the sites of the initial attachment is mediated by interplays between T antigen and galectin-3, and such adhesions promote metastatic cells survival and growth [15]. It was also demonstrated that the TNF superfamily of death receptors (e.g. Fas) carry glycoforms with possible roles in ligand binding and receptor oligomerization which can result in downstream of apoptotic signaling events [27]. In our work we checked the efficacy of applied therapy towards mentioned carbohydrate antigens in cell lysates and culture medium. Upon the received results we state that anti-MUC1 and mAb combined with the drugs was especially effective regarding TACAs present in culture medium of both cell lines. We suggest that observed lowered expression of specific carbohydrate antigens could e.g. reduce interactions with adhesive molecules participating in different signaling pathways inducing cancer progression. Thus, such results strongly support rationality of applied combined therapy. Interestingly, inhibition of the antigens in cell lysates by combined therapy was revealed only in DLD-1 cells in the regards to sialyl Tn structure, after mAb/PtPz6 treatment. To explain this, more detailed experiments should be performed. We can only suggest that specific pathways, regulating glycosylation enzymes could be triggered by applied therapy.
Conclusions
In conclusion, the proposed therapy utilizing anti-MUC1, two pyrazole-platinum(II) complexes PtPz4, PtPz6, and cisPt seems to be promising anti-colon cancer therapy. We demonstrated that the action of monoclonal antibody administrated with platinum complexes was especially potent toward Bcl-2, Bid, caspases in both cell lines. Additionally, such combined treatment was more efficient toward apoptosis induction and expression of p53, Bax, and Bim in DLD-1 cells than in HT-29 cells. Bad was more effectively influenced by combined therapy in HT-29 cells. Moreover, we revealed the inhibition of TACAs expression, especially on glycoproteins released to the culture medium of both cell lines, was more effective after combined therapy than after monotherapy.
Apart from that we can state that substitution of pyrazole ring with ethyl group (PtPz6) seemed to be more effective in comparison with PtPz4 toward apoptosis induction, Akt suppression, and TACAs reducing in both cell lines. In case of DLD-1 cells, PtPz6 was more potent in relation to Bcl-xL, Bcl-2 or caspase-9 activation in comparison with PtPz4. However, the exact explanation of the mechanism for both platinum derivatives action needs extra experiments to be performed, what is planned for the future.
We understand that not all presented results seem to be consistent. Some of the outcomes are going to be elucidated and in vivo analysis are planned to be performed in the future studies.
Funding
This work was funded by the Medical University of Bialystok (Grant No SUB/2/DN/20/001/2203).
CRediT authorship contribution statement
Katarzyna Supruniuk: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Funding acquisition. Robert Czarnomysy: Methodology, Investigation. Anna Muszyńska: Investigation. Iwona Radziejewska: Methodology, Formal analysis, Investigation, Writing – review & editing, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests.
Acknowledgments
The authors would like to thank Ms. Joanna Wosek (Department of Medical Chemistry, Medical University of Białystok, Poland) for technical support.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2022.101348.
Appendix. Supplementary materials
Supplementary Fig. 1. The effect of PtPz4, PtPz6, cisPt, and anti-MUC1 on anti-apoptotic Bcl-xL protein in DLD-1 and HT-29 colon cancer cells.
Supplementary Fig. 2. The effect of PtPz4, PtPz6, cisPt, and anti-MUC1 on pro-apoptotic Bax protein in DLD-1 and HT-29 colon cancer cells.
Supplementary Fig. 3. The effect of PtPz4, PtPz6, cisPt, and anti-MUC1 on pro- and active caspase-9 in DLD-1, pro- and active caspase-9 in HT-29 cells.
Supplementary Fig. 4. The effect of PtPz4, PtPz6, cisPt, and anti-MUC1 on pro-caspase-3 protein in both colon cancer cells.
References
- 1.Ahmad R., Alam M., Rajabi H., Kufe D. The MUC1‑C oncoprotein binds to the BH3 domain of the pro‑apoptotic BAX protein and blocks BAX function. J. Biol. Chem. 2012;287 doi: 10.1074/jbc.M112.357293. 20866‑20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amaral J.D., Xavier J.M., Steer C.J., Rodrigues C.M. Targeting the p53 pathway of apoptosis. Curr. Pharm. Des. 2010;16:2493–2503. doi: 10.2174/138161210791959818. [DOI] [PubMed] [Google Scholar]
- 3.Arnold M., Sierra M.S., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 4.Beckwith D.M., Cudic M. Tumor-associated O-glycans of MUC1: carriers of the glycol-code and targets for cancer vaccine design. Semin. Immunol. 2020;47 doi: 10.1016/j.smim.2020.101389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birrer M.J., Moore K.N., Betella I., Bates R.C. Antibody-drug conjugate-based therapeutics: state of the science. J. Natl. Cancer Inst. 2019;111:538–549. doi: 10.1093/jnci/djz035. [DOI] [PubMed] [Google Scholar]
- 6.Boice A., Bouchier-Hayes L. Targeting apoptotic caspases in cancer. Biochim. Biophys. Acta Mol. Cell Res. 2020;1867 doi: 10.1016/j.bbamcr.2020.118688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bose M., Mukherjee P. Potential of anti-MUC1 antibodies as a targeted therapy for gastrointestinal cancers. Vaccines. 2020;8:659. doi: 10.3390/vaccines8040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campoli M., Ferris R., Ferrone S., Wang X. Immunotherapy of malignant disease with tumor antigen-specific monoclonal antibodies. Clin. Cancer Res. 2010;16:11–20. doi: 10.1158/1078-0432.CCR-09-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmichael J., Degraff W., Gazdar A., Minna J., Mitchell J. Evaluation of a tetrazolium-based semi-automated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–942. [PubMed] [Google Scholar]
- 10.Czarnomysy R., Surażyński A., Muszyńska A., Gornowicz A., Bielawska A., Bielawski K. A novel serious of pyrazole-platinum(II) complexes as potential anti-cancer agents that induce cell cycle arrest and apoptosis in breast cancer cells. J. Enzym. Inhib. Med. Chem. 2018;33:1006–1023. doi: 10.1080/14756366.2018.1471687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dasari S., Tchounwou P.B. Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fahmy H.D., Khalifa N.M., Ismail M.M.F., El-Sahrawy H.M., Nossier E.S. Biological validation of novel polysubstituted pyrazole candidates with in vitro anticancer activities. Molecules. 2016;21:271. doi: 10.3390/molecules21030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farhood B., Najafi M., Salehi E., Goradel N.H., Nashtaei M.S., Khanlarkhani N., Mortezaee K. Disruption of the redox balance with either oxidative or anti-oxidative overloading as a promising target for cancer therapy. J. Cell. Biochem. 2019;120:71–76. doi: 10.1002/jcb.27594. [DOI] [PubMed] [Google Scholar]
- 14.Feng D., Shaikh A.S., Wang F. Recent advance in tumor-associated carbohydrate antigens (TACAs)-based antitumor vaccines. ACS Chem. Biol. 2016;11:850–863. doi: 10.1021/acschembio.6b00084. 10.1021/acschembio.6b00084. [DOI] [PubMed] [Google Scholar]
- 15.Glinsky V.V., Glinsky G.V., Glinskii O.V., Huxley V.H., Turk J.R., Mossine V.V., Deutscher S.L., Pienta K.J., Quinn T.P. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003;63:3805–3811. [PubMed] [Google Scholar]
- 16.Gornowicz A., Bielawska A., Czarnomysy R., Gabryel-Porowska H., Muszyńska A., Bielawski K. The combined treatment with novel platinum(II) complex and anti-MUC1 increases apoptotic response in MDA-MB-231 breast cancer cells. Mol. Cell. Biochem. 2015;408:103–113. doi: 10.1007/s11010-015-2486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta R., Bhatt L.K., Johnston T.P., Prabhavalkar K.S. Colon cancer stem cells: potential target for the treatment of colorectal cancer. Cancer Biol. Ther. 2019;20:1068–1082. doi: 10.1080/15384047.2019.1599660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiraki M., Maeda T., Mehrotra N., Jin C., Alam M., Bouillez A., Hata T., Tagde A., Keating A., Kharbanda S., Singh H., Kufe D. Targeting MUC1-C suppresses BCL2A1 in triple-negative breast cancer. Signal Transduct. Target. Ther. 2018;3:13. doi: 10.1038/s41392-018-0013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hisatsune A., Kawasaki M., Nakayama H., Mikami Y., Miyata T., Isohama Y., Katsuki H., Kim K.C. Internalization of MUC1 by anti-MUC1 antibody from cell membrane through the macropinocytic pathway. Biochem. Biophys. Res. Commun. 2009;388:677–682. doi: 10.1016/j.bbrc.2009.08.059. [DOI] [PubMed] [Google Scholar]
- 20.Hisatsune A., Nakayama H., Kawasaki M., Horie I., Miyata T., Isohama Y., Kim K.C., Katsuki H. Anti-MUC1 antibody inhibits EGF receptor signaling in cancer cells. Biochem. Biophys. Res. Commun. 2011;405:377–381. doi: 10.1016/j.bbrc.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 21.Hossain F., Andreana P.R. Developments in carbohydrate-based cancer therapies. Pharmaceuticals. 2019;12:84. doi: 10.3390/ph12020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiedler W., De Dosso S., Cresta S., Weidmann J., Tessari A., Salzberg M., Dietrich B., Baumeister H., Goletz S., Gianni L., Sessa C. A phase I study of PankoMab-GEX, a humanised glyco-optimised monoclonal antibody to a novel tumour-specific MUC1 glycopeptide epitope in patients with advanced carcinomas. Eur. J. Cancer. 2016;63:55–63. doi: 10.1016/j.ejca.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Kim H., Tu H.C., Ren D., Takeuchi O., Jeffers J.R., Zambetti G.P., Hsieh J.J., Cheng E.H. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol. Cell. 2009;36:487–499. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacroix M., Riscal R., Arena G., Linares L.K., Cam L.L. Metabolic functions of the tumor suppressor p53: implications in normal physiology, metabolic disorders, and cancer. Mol. Metab. 2020;33:2–22. doi: 10.1016/j.molmet.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laulier C., Lopez B.S. The secret life of Bcl-2: apoptosis-independent inhibition of DNA repair by Bcl-2 family members. Mutat. Res. 2012;751:247–257. doi: 10.1016/j.mrrev.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Li G., Wang S., Xue X., Qu X., Liu H.P. Monoclonal antibody-related drugs for cancer therapy. Drug Discov. Ther. 2013;7:178–184. doi: 10.5582/ddt.2013.v7.5.178. [DOI] [PubMed] [Google Scholar]
- 27.Li Y., Luo S., Dong W., Song X., Zhou H., Zhao L., Jia L. Alpha-2, 3-sialyltransferases regulate the multidrug resistance of chronic myeloid leukemia through miR-4701-5p targeting ST3GAL1. Lab. Investig. 2016;96:731–740. doi: 10.1038/labinvest.2016.50. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y., Yang C., Zhao Y., Chi Q., Wang Z., Sun B. Overexpressed methyltransferase-like 1 (METTL1) increased chemosensitivity of colon cancer cells to cisplatin by regulating miR-149-3p/S100A4/p53 axis. Aging. 2019;11:12328–12341. doi: 10.18632/aging.102575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo X., O’Neill K.L., Huang K. The third model of Bax/Bak activation: a Bcl-2 family feud finally resolved? F1000Res. 2020;9:935. doi: 10.12688/f1000research.25607.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mortezaee K., Salehi E., Mirtavoos-Mahyari H., Motevaseli E., Najafi M., Farhood B., Rosengren R.J., Sahebkar A. Mechanisms of apoptotic modulation by curcumin: implications for cancer therapy. J. Cell. Physiol. 2019;234:12537–12550. doi: 10.1002/jcp.28122. [DOI] [PubMed] [Google Scholar]
- 31.Nabavinia M.S., Gholoobi A., Charbgoo F., Nabavinia M., Ramezani M., Abnous K. Anti-MUC1 aptamer: a potential opportunity for cancer treatment. Med. Res. Rev. 2017;37:1518–1539. doi: 10.1002/med.21462. [DOI] [PubMed] [Google Scholar]
- 32.Pichinuk E., Chalik M., Benhar I., Ginat-Koton R., Ziv R., Smorodinsky N.I., Haran G., Garbar C., Bensussan A., Meeker A., Guillaume T., Rubinstein D.B., Wreschner D.H. In vivo anti-MUC1+ tumor activity and sequences of high-affinity anti-MUC1-SEA antibodies. Cancer Immunol. Immunother. 2020;69:1337–1352. doi: 10.1007/s00262-020-02547-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Runcie K., Budman D.R., John V., Seetharamu N. Bi-specific and tri-specific antibodies – the next big thing in solid tumor therapeutics. Mol. Med. 2018;24:50. doi: 10.1186/s10020-018-0051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen F., Feng L., Zhou J., Zhang H., Xu Y., Jiang R., Zhang H., Chen Y. Overexpression of CASC11 in ovarian squamous cell carcinoma mediates the development of cancer cell resistance to chemotherapy. Gene. 2019;710:363–366. doi: 10.1016/j.gene.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 35.Slee E.A., Keogh S.A., Martin S.J. Cleavage of BID during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release. Cell Death Differ. 2000;7:556–565. doi: 10.1038/sj.cdd.4400689. [DOI] [PubMed] [Google Scholar]
- 36.Supruniuk K., Czarnomysy R., Muszyńska A., Radziejewska I. Combined action of anti-MUC1 monoclonal antibody and pyrazole-platinum(II) complexes reveals higher effectiveness towards apoptotic response in comparison to monotherapy in AGS gastric cancer cells. Pharmaceutics. 2021;13:968. doi: 10.3390/pharmaceutics13070968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Supruniuk K., Radziejewska I. MUC1 is an oncoprotein with a significant role in apoptosis (review) Int. J. Oncol. 2021;59:68. doi: 10.3892/ijo.2021.5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Towbin T., Stachelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U. S. A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varghese V., Magnani L., Harada-Shoji N., Mauri F., Szydlo R.M., Yao S., Lam E.W.F., Kenny L.M. FOXM1 modulates 5-FU resistance in colorectal cancer through regulating TYMS expression. Sci. Rep. 2019;9:1–16. doi: 10.1038/s41598-018-38017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu G., Maharjan S., Kim D., Kim J.N., Park B.K., Koh H., Moon K., Lee Y., Kwon H.J. A novel monoclonal antibody targets mucin1 and attenuates growth in pancreatic cancer model. Int. J. Mol. Sci. 2018;19:2004. doi: 10.3390/ijms19072004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yadav S.S., Kumar M., Varshney A., Yadava P.K. KLF4 sensitizes the colon cancer cell HCT-15 to cisplatin by altering the expression of HMGB1 and hTERT. Life Sci. 2019;220:169–176. doi: 10.1016/j.lfs.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Zeng Y., Zhang Q., Zhang Y., Lu M., Liu Y., Zheng T., Feng S., Hao M., Shi H. MUC1 predicts colorectal cancer metastasis: a systematic review and meta-analysis of case controlled studies. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng H.C. The molecular mechanisms of chemoresistance in cancers. Oncotarget. 2017;8:59950–59964. doi: 10.18632/oncotarget.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. The effect of PtPz4, PtPz6, cisPt, and anti-MUC1 on anti-apoptotic Bcl-xL protein in DLD-1 and HT-29 colon cancer cells.
Supplementary Fig. 2. The effect of PtPz4, PtPz6, cisPt, and anti-MUC1 on pro-apoptotic Bax protein in DLD-1 and HT-29 colon cancer cells.
Supplementary Fig. 3. The effect of PtPz4, PtPz6, cisPt, and anti-MUC1 on pro- and active caspase-9 in DLD-1, pro- and active caspase-9 in HT-29 cells.
Supplementary Fig. 4. The effect of PtPz4, PtPz6, cisPt, and anti-MUC1 on pro-caspase-3 protein in both colon cancer cells.