Abstract
Sarcopenic obesity is a distinct condition of sarcopenia in the context of obesity, with the cumulative health risks of both phenotypes. Differential expression of microRNAs (miRNAs) has been reported separately in people with obesity and sarcopenia and may play a role in the pathogenesis of sarcopenic obesity. However, this has not been explored to date. This study aimed to identify differentially expressed miRNAs reported in serum, plasma, and skeletal muscle of people with obesity and sarcopenia and whether there are any commonalities between these conditions. We performed a systematic review on Embase and MEDLINE (PROSPERO, CRD42020224486) for differentially expressed miRNAs (fold change >1.5 or P‐value <0.05) in (i) sarcopenia or frailty and (ii) obesity or metabolic syndrome. The functions and targets of miRNAs commonly changed in both conditions, in the same direction, were searched using PubMed. Following deduplication, 247 obesity and 42 sarcopenia studies were identified for full‐text screening. Screening identified 36 obesity and 6 sarcopenia studies for final inclusion. A total of 351 miRNAs were identified in obesity and 157 in sarcopenia. Fifty‐five miRNAs were identified in both obesity and sarcopenia—by sample type, 48 were found in plasma and one each in serum and skeletal muscle. Twenty‐four miRNAs were identified from 10 of the included studies as commonly changed in the same direction (22 in plasma and one each in serum and skeletal muscle) in obesity and sarcopenia. The majority of miRNA‐validated targets identified in the literature search were members of the phosphoinositide 3‐kinase/protein kinase B and transforming growth factor‐β signalling pathways. The most common targets identified were insulin‐like growth factor 1 (miR‐424‐5p, miR‐483‐3p, and miR‐18b‐5p) and members of the SMAD family (miR‐483‐3p, miR‐92a‐3p, and miR‐424‐5p). The majority of commonly changed miRNAs were involved in protein homeostasis, mitochondrial dynamics, determination of muscle fibre type, insulin resistance, and adipogenesis. Twenty‐four miRNAs were identified as commonly dysregulated in obesity and sarcopenia with functions and targets implicated in the pathogenesis of sarcopenic obesity. Given the adverse health outcomes associated with sarcopenic obesity, understanding the pathogenesis underlying this phenotype has the potential to lead to effective screening, monitoring, or treatment strategies. Further research is now required to confirm whether these miRNAs are differentially expressed in older adults with sarcopenic obesity.
Keywords: MicroRNA, Sarcopenia, Obesity, Frailty, Metabolic syndrome
Introduction
Sarcopenic obesity is a condition of excess fat mass and sarcopenia. 1 , 2 Differing definitions of sarcopenia have been proposed with growing consensus on the importance of muscle function. 1 , 3 Sarcopenic obesity is more commonly found amongst older adults; however, it can also be found in younger adults during both acute and chronic disease, or intermittent weight cycling. 4 Dependent on the definition used, sarcopenic obesity is thought to range in prevalence from 2.75% to over 20%. 4 Of clinical importance, sarcopenic obesity may have the cumulative risk of both sarcopenia and obesity. 5 Growing evidence supports this with a greater risk of falls, hospitalization, worsening disability, and all‐cause mortality reported. 6 , 7 , 8
The aetiology of sarcopenic obesity is complex and not fully understood (see Batsis and Villareal and Zamboni et al. 2 , 9 for detailed reviews). Ageing is associated with changes in body composition including a loss of lean mass, increased body fat, and muscular fat infiltration, with a subsequent reduction in resting metabolic rate. 2 , 10 Reduced physical activity and malnutrition (including overnutrition or undernutrition and malabsorption) associated with ageing contribute to a gradual increase in body fat 2 and the development of sarcopenia. 1 Moreover, excess body fat or obesity can exacerbate sarcopenia. 1 , 11 Obesity is associated with low‐grade inflammation with the secretion of tumour necrosis factor, leptin, and C‐reactive protein. 2 , 12 Leptin elevates the levels of pro‐inflammatory cytokines, which cause a reduction in the anabolic effects of insulin‐like growth factor 1 (IGF‐1). 2 This inflammation leads to insulin resistance, further exacerbated by muscle catabolism, which promotes fat mass and loss of muscle mass. 2 , 12 As such, changes associated with ageing, obesity, and sarcopenia as well as interrelationships between these phenotypes can contribute to the pathogenesis of sarcopenic obesity.
MicroRNAs (miRNAs, miRs) are short, non‐coding RNAs that can regulate gene expression at a post‐transcriptional level. 13 To date, 2654 miRNAs have been discovered, which are predicted to regulate two‐thirds of the human genome. 14 , 15 Therefore, many miRNAs may modulate many physiological processes. There is evidence that ageing changes miRNA levels in the muscle and that these changes may have a detrimental impact on muscle quality and quantity. 16 , 17 However, the influence of obesity or adiposity on the miRNA profile of older adults and whether this translates into functional impairment has not yet been established. Evidence from rodent studies has demonstrated that adipose‐derived miRNAs can be transported via exosomes to a variety of host cells including myocytes, hepatocytes, and macrophages. 18 , 19 , 20 Likewise, skeletal muscle‐derived miRNAs can be taken up by adipose tissue. 21 Functionally, this inter‐organ crosstalk has been implicated in insulin resistance, adipogenesis, and lipid metabolism, 18 , 21 thus suggesting a role for miRNAs in the pathogenesis of sarcopenic obesity. MiRNAs are an exciting area of research due to the potential of antagomiRs, which are already been explored as pharmacological options in conditions such as cardiovascular disease and cancer. 22 , 23
The primary aim of this systematic review was to identify differentially expressed miRNAs reported in plasma, serum, or skeletal muscle of adults with obesity or sarcopenia to determine common miRNA changes between these phenotypes. As this is an emerging area with limited research, studies reporting (i) sarcopenia or frailty and (ii) obesity or metabolic syndrome were included because of similarities between definitions. A secondary aim of this review was to identify the targets and functions of these differentially expressed miRNAs.
Methods
Protocol registration
The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement was followed as a reference protocol standard. 24 A PRISMA flow chart is included. Our protocol was registered at the International Prospective Register of Systematic Reviews PROSPERO, with Registration Number CRD42020224486; available at https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020224486.
Bibliographical search and eligibility criteria
This systematic review consisted of two searches performed on MEDLINE and Embase (last searched 6 January 2021). Part 1 searched for studies of sarcopenia or frailty using the following terms: MicroRNA/miR/miRNA AND ‘sarcopenia’, ‘muscle strength’, ‘frail’, ‘ageing’/‘older adult’/‘aged’ AND ‘muscle’, ‘muscle weakness’, OR ‘dynapenia’. Part 2 searched for studies of obesity or metabolic syndrome using the following terms: MicroRNA/miR/miRNA combined with ‘obesity’ OR ‘metabolic syndrome’. The multipurpose function was used for keywords, and MeSH terms were used where available. Articles were limited to the English language and human studies. Eligible studies enrolled adult participants (>18 years) with sarcopenia, frailty, metabolic syndrome, or obesity and comparable non‐sarcopenic/frail or non‐obese/metabolic syndrome controls as outlined in Supporting Information, Table S1. Studies were excluded if the primary condition of interest was not sarcopenia, frailty, metabolic syndrome, or obesity but instead an unrelated disease or condition, for example, type 1 diabetes, cancer, or pregnancy, which may have confounded findings. Study groups containing some, but not all, type 2 diabetes participants were included. The considered biological fluids and tissues were serum, plasma, and skeletal muscle. Results on tissue samples or cell lines were excluded. Observational (cohort and case control) studies or intervention studies with relevant baseline results were included.
Study selection
Following deduplication, all selected titles and abstracts were screened to identify articles for full‐text screening. A second reviewer verified a random sample of articles included in the first sift. The second sift, which consisted of full‐text screening, was independently conducted by two reviewers to confirm that the criteria for the condition of interest, samples, age group, and outcome measure in the study met eligibility criteria. In cases where the same or similar results were reported in more than one study, the publication with the most information was included and the other rejected for duplication. Authors of papers with insufficient information relating to eligibility criteria or outcome measures for this review were contacted. If a reply was not received within one month, only the information reported in the paper was included (e.g. incomplete list of miRNAs) or else the paper was rejected if eligibility criteria remained unclear (e.g. age group). A more comprehensive list of miRNA results was obtained from one study following email correspondence. 25 Disagreements between reviewers that could not be solved with discussion were resolved with a third reviewer by consensus.
Data extraction
A standardized form was used to extract trial features (authors, published year, and country), patient characteristics (age and sex), RNA extraction and detection methods, and the subset of differentially expressed miRNAs between the two conditions. Extracted information was verified by a second reviewer.
Risk of bias in individual studies
Two reviewers assessed the quality and risk of bias of individual studies using the Newcastle–Ottawa Scale (NOS) with an additional star for validation of results within the study using either two measurement methods or two study groups (Table S2). Because of the type of studies included, all studies were given a star for the question on non‐response rate. Disagreements between reviewers that could not be solved through discussion were resolved with a third reviewer by consensus.
Summary measures
The outcome measure was differentially expressed miRNAs in human skeletal muscle, plasma, or serum with at least a 1.5‐fold change or P < 0.05 measured using RT‐qPCR, next‐generation sequencing, or microarray. Circulating (serum and plasma) miRNAs may be useful as non‐invasive biomarkers, whereas miRNAs found in muscle may provide a mechanistic insight into sarcopenic obesity.
Because of differences in nomenclature between studies, we used the information for previous miRNA IDs on miRBase (Release 22.1; October 2018) to clarify and update the nomenclature of included miRNAs, which did not specify whether they were ‐3p or ‐5p. 15 The BioVenn online interface was then used to identify potentially overlapping miRNAs. 26 Overlapping miRNAs that were differentially expressed in both obesity and sarcopenia were further classified by tissue/fluid type and as either differentially expressed in (i) the same direction (e.g. up‐regulated), (ii) different directions (e.g. up‐regulated in obesity and down‐regulated in sarcopenia), (iii) conflicting directions (e.g. both up‐regulated and down‐regulated in one condition but not the other), or (iv) unclear (e.g. differences in nomenclature limited interpretation).
Synthesis of results and additional analyses
MicroRNAs that were differentially expressed in the same direction in obesity and sarcopenia were identified for further investigation. A literature search was conducted on PubMed to identify validated target genes or functions of these miRNAs with regard to muscle, sarcopenia or frailty and obesity, metabolic syndrome, or insulin resistance. A narrative synthesis of the findings from the studies and in the context of sarcopenic obesity, their target genes, and metabolic pathways implicated is provided.
Results
Bibliographical search
The bibliographical search in MEDLINE and Embase retrieved 4097 obesity‐related papers and 2357 sarcopenia‐related papers published before 6 January 2021. Following deduplication, 2971 papers were screened for obesity and 2019 for sarcopenia. Following screening, 247 obesity studies and 42 sarcopenia studies were included for full‐text review. In total, 36 studies were identified for obesity and six for sarcopenia (Figure 1). MiRNAs dysregulated in both sarcopenia and obesity were identified from 10 studies. 25 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35
Figure 1.
PRISMA flow chart for obesity/metabolic syndrome and sarcopenia/frailty parts of the systematic review.
Studies were conducted in Korea, 25 China, 32 Singapore, 29 New Zealand, 28 , 30 the USA, 31 , 34 Spain, 27 and the UK. 33 The majority of obesity studies used World Health Organization criteria for obesity, 25 , 27 , 29 , 30 although the criteria for two studies were unclear. 28 , 35 The sarcopenia studies used Fried Frailty Score, 31 Asian Working Group for Sarcopenia, 32 and European Working Group on Sarcopenia in Older People 2010 33 , 34 criteria. Four studies were conducted in women only 27 , 28 , 30 , 34 and three studies in men only. 29 , 33 , 35 Two studies recruited both men and women, 25 , 31 and one study did not report the sex of participants. 32 Based on the reported average age, obese participants would not be defined as older adults, age ≥65 years. 25 , 27 , 28 , 29 , 30 Sarcopenia studies recruited older adults, 31 , 32 , 33 although one used a younger cut‐off of 60–85 years. 34 Only three studies validated their findings. 27 , 30 , 32
MicroRNAs reported as dysregulated in the context of sarcopenia and obesity
A total of 351 miRNAs were identified in obesity and 157 in sarcopenia (Figure 2). Fifty‐five potential miRNAs were identified in both obesity and sarcopenia. When examined by sample type, 48 overlapping miRNAs were identified in plasma and one each in serum and skeletal muscle (vastus lateralis). Sixteen plasma miRNAs were expressed in differing directions in obesity and sarcopenia. Eight plasma miRNAs in obesity, which were also present in sarcopenia, were expressed in conflicting directions. Two plasma miRNAs could not be determined with confidence because of the nomenclature in the studies (miR‐328 and miR‐215). Therefore, across six obesity 25 , 27 , 28 , 29 , 30 , 35 and four sarcopenia 31 , 32 , 33 , 34 studies, we manually identified 24 miRNAs differentially expressed in the same direction. Twenty‐two of these miRNAs were found in plasma and one each in serum and skeletal muscle (Table 1). Of these overlapping miRNAs, only miR‐23a‐3p was reported in more than one tissue (serum and plasma) of both sarcopenia and obesity; however, in plasma, conflicting directions were reported in obesity. The majority of overlapping miRNAs were identified in two studies, one of which used RT‐qPCR 29 and the other used RNA‐Seq. 31 Exosomal miRNAs were reported by three studies. 25 , 27 , 31 Twenty‐one of the 24 overlapping miRNAs were found in one study of frailty using plasma exosomes. 31
Figure 2.
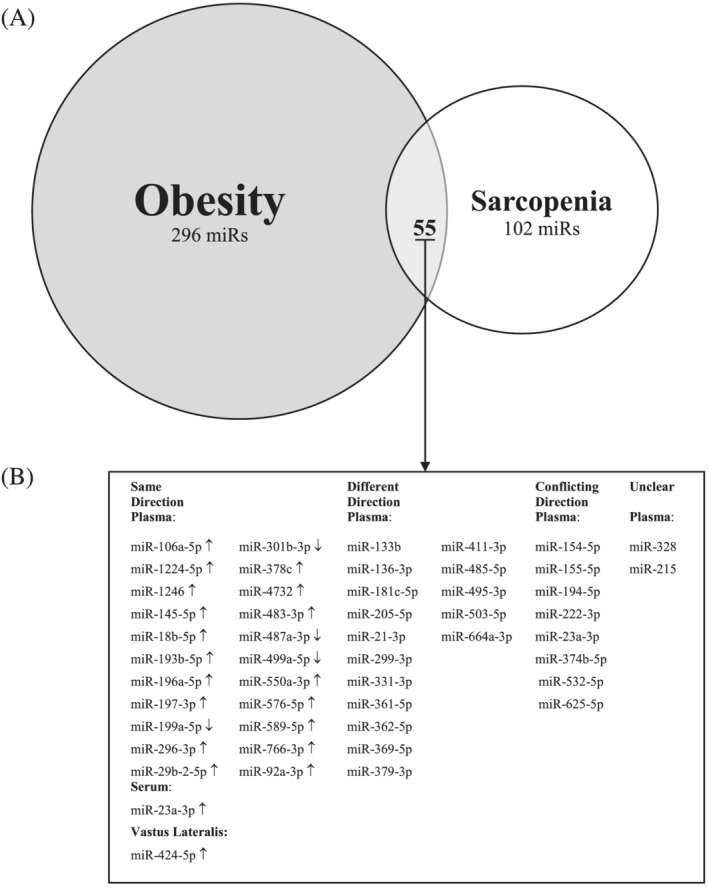
(A) Venn diagram of miRNAs commonly expressed in all tissues in both obesity and sarcopenia. (B) MiRNAs by sample type (plasma, serum, or vastus lateralis) found in both obesity and sarcopenia. ‘↑’ refers to overexpressed; ‘↓’ refers to underexpressed. Since the publication of several studies included in this review, some reported miRs have been removed from the latest version of miRBase (e.g. miR‐4461, miR‐4532, and miR‐6087); this does not affect overlapping miRs.
Table 1.
Summary characteristics of studies with overlapping miRNAs in the same direction
| Obese | Sarcopenia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MiRNA | Country | Obesity definition | N (%female) | Age (years) | Log2FC | Country | Sarcopenia definition | N (%female) | Age (years) | Log2FC | |
| Plasma | |||||||||||
| 1 | MiR‐106a‐5p | Spain 27 | BMI ≥ 30 kg/m2 |
Ob 12 (100%) Lean 19 (100%) |
Range 30–70 49.9 ± 11.3 45.1 ± 15.1 |
ND (Up) |
USA 31 |
Fried Frailty Phenotype |
Fr 7 (0%) N‐F 7 (71%) |
Range 71–89 85.6 ± 3.8 76 ± 6.5 |
1.81 |
| 2 | MiR‐18b‐5p | New Zealand 28 | ND |
Ob 11 (100%) Lean 12 (100%) |
41 ± 5 44 ± 9 |
ND (Up) |
USA 31 | Fried Frailty Phenotype |
Fr 7 (0%) N‐F 7 (71%) |
Range 71–89 85.6 ± 3.8 76 ± 6.5 |
4.00 |
| 3 | MiR‐193b‐5p | Singapore 29 | BMI ≥ 27.5 kg/m2 |
Ob 9 (0%) Lean 9 (0%) |
28.4 ± 1.6 a 23.2 ± 0.2 a |
1.89 | USA 31 | Fried Frailty Phenotype |
Fr 7 (0%) N‐F 7 (71%) |
Range 71–89 85.6 ± 3.8 76 ± 6.5 |
1.96 |
| 4 | MiR‐197‐3p | New Zealand 28 | ND |
Ob 11 (100%) Lean 12 (100%) |
41 ± 5 44 ± 9 |
ND (Up) |
USA 31 | Fried Frailty Phenotype |
Fr 7 (0%) N‐F 7 (71%) |
Range 71–89 85.6 ± 3.8 76 ± 6.5 |
1.85 |
| 5 | MiR‐199a‐5p | Singapore 29 | BMI ≥ 27.5 kg/m2 |
Ob 9 (0%) Lean 9 (0%) |
28.4 ± 1.6 a 23.2 ± 0.2 a |
−1.324 | USA 31 | Fried Frailty Phenotype |
Fr 7 (0%) N‐F 7 (71%) |
Range 71–89 85.6 ± 3.8 76 ± 6.5 |
−0.74 |
| 6 | MiR‐483‐3p | Singapore 29 | BMI ≥ 27.5 kg/m2 |
Ob 9 (0%) Lean 9 (0%) |
28.4 ± 1.6 a 23.2 ± 0.2 a |
1.413 | USA 31 | Fried Frailty Phenotype |
Fr 7 (0%) N‐F 7 (71%) |
Range 71–89 85.6 ± 3.8 76 ± 6.5 |
3.92 |
| 7 | MiR‐499 | New Zealand 28 | BMI > 30 kg/m2 |
Ob 80 (100%) Lean 80 (100%) |
52.5 ± 10.5 b 53.0 ± 13.5 b |
ND (Down) |
China 32 | AWGS |
S 93 (ND) N‐S 93 (ND) |
≥65 76.15 ± 0.58 a 76.19 ± 0.58 a |
Down |
| 8 | MiR‐550a‐3p | Singapore 29 | BMI ≥ 27.5 kg/m2 |
Ob 9 (0%) Lean 9 (0%) |
28.4 ± 1.6 a 23.2 ± 0.2 a |
0.782 | USA 31 | Fried Frailty Phenotype |
Fr 7 (0%) N‐F 7 (71%) |
Range 71–89 85.6 ± 3.8 76 ± 6.5 |
3.08 |
| 9 | MiR‐576‐5p | Singapore 29 | BMI ≥ 27.5 kg/m2 |
Ob 9 (0%) Lean 9 (0%) |
28.4 ± 1.6 a 23.2 ± 0.2 a |
0.501 | USA 31 | Fried Frailty Phenotype |
Fr 7 (0%) N‐F 7 (71%) |
Range 71–89 85.6 ± 3.8 76 ± 6.5 |
3.24 |
| 10 | MiR‐589‐5p | Singapore 29 | BMI ≥ 27.5 kg/m2 |
Ob 9 (0%) Lean 9 (0%) |
28.4 ± 1.6 a 23.2 ± 0.2 a |
0.274 | USA 31 | Fried Frailty Phenotype |
Fr 7 (0%) N‐F 7 (71%) |
Range 71–89 85.6 ± 3.8 76 ± 6.5 |
1.81 |
| 11 | MiR‐92a‐3p | Singapore 29 | BMI ≥ 27.5 kg/m2 |
Ob 9 (0%) Lean 9 (0%) |
28.4 ± 1.6 a 23.2 ± 0.2 a |
0.571 | USA 31 | Fried Frailty Phenotype |
Fr 7 (0%) N‐F 7 (71%) |
Range 71–89 85.6 ± 3.8 76 ± 6.5 |
0.78 |
| 12 | MiR‐1224‐5p | Singapore 29 | BMI ≥ 27.5 kg/m2 |
Ob 9 (0%) Lean 9 (0%) |
28.4 ± 1.6 a 23.2 ± 0.2 a |
0.987 | USA 31 | Fried Frailty Phenotype |
Fr 7 (0%) N‐F 7 (71%) |
Range 71–89 85.6 ± 3.8 76 ± 6.5 |
0.49 |
| 13 | MiR‐1246 | Singapore 29 | BMI ≥ 27.5 kg/m2 |
Ob 9 (0%) Lean 9 (0%) |
28.4 ± 1.6 a 23.2 ± 0.2 a |
1.254 | USA 31 | Fried Frailty Phenotype |
Fr 7 (0%) N‐F 7 (71%) |
Range 71–89 85.6 ± 3.8 76 ± 6.5 |
3.23 |
| 14 | MiR‐145‐5p | New Zealand 28 | ND |
Ob 11 (100%) Lean 12 (100%) |
41 ± 5 44 ± 9 |
Up | USA 31 | Fried Frailty Phenotype |
Fr 7 (0%) N‐F 7 (71%) |
Range 71–89 85.6 ± 3.8 76 ± 6.5 |
3.23 |
| 15 | MiR‐196a‐5p | Singapore 29 | BMI ≥ 27.5 kg/m2 |
Ob 9 (0%) Lean 9 (0%) |
28.4 ± 1.6 a 23.2 ± 0.2 a |
0.885 | USA 31 | Fried Frailty Phenotype |
Fr 7 (0%) N‐F 7 (71%) |
Range 71–89 85.6 ± 3.8 76 ± 6.5 |
1.48 |
| 16 | MiR‐296‐3p |
Singapore 29 |
BMI ≥ 27.5 kg/m2 |
Ob 9 (0%) Lean 9 (0%) |
28.4 ± 1.6 a 23.2 ± 0.2 a |
1.049 | USA 31 | Fried Frailty Phenotype |
Fr 7 (0%) N‐F 7 (71%) |
Range 71–89 85.6 ± 3.8 76 ± 6.5 |
0.20 |
| 17 | MiR‐29b‐2‐5p | New Zealand 28 | ND |
Ob 11 (100%) Lean 12 (100%) |
41 ± 5 44 ± 9 |
ND (Up) |
USA 31 | Fried Frailty Phenotype |
Fr 7 (0%) N‐F 7 (71%) |
Range 71–89 85.6 ± 3.8 76 ± 6.5 |
3.68 |
| 18 | MiR‐301b‐3p | Singapore 29 | BMI ≥ 27.5 kg/m2 |
Ob 9 (0%) Lean 9 (0%) |
28.4 ± 1.6 a 23.2 ± 0.2 a |
−0.973 | USA 31 | Fried Frailty Phenotype |
Fr 7 (0%) N‐F 7 (71%) |
Range 71–89 85.6 ± 3.8 76 ± 6.5 |
−1.00 |
| 19 | MiR‐378c | Singapore 29 | BMI ≥ 27.5 kg/m2 |
Ob 9 (0%) Lean 9 (0%) |
28.4 ± 1.6 a 23.2 ± 0.2 a |
0.676 | USA 31 | Fried Frailty Phenotype |
Fr 7 (0%) N‐F 7 (71%) |
Range 71–89 85.6 ± 3.8 76 ± 6.5 |
2.41 |
| 20 | MiR‐4732‐5p | Singapore 29 | BMI ≥ 27.5 kg/m2 |
Ob 9 (0%) Lean 9 (0%) |
28.4 ± 1.6 a 23.2 ± 0.2 a |
0.88 | USA 31 | Fried Frailty Phenotype |
Fr 7 (0%) N‐F 7 (71%) |
Range 71–89 85.6 ± 3.8 76 ± 6.5 |
2.97 |
| 21 | MiR‐487a‐3p | Singapore 29 | BMI ≥ 27.5 kg/m2 |
Ob 9 (0%) Lean 9 (0%) |
28.4 ± 1.6 a 23.2 ± 0.2 a |
−1.3 | USA 31 | Fried Frailty Phenotype |
Fr 7 (0%) N‐F 7 (71%) |
Range 71–89 85.6 ± 3.8 76 ± 6.5 |
−1.03 |
| 22 | MiR‐766‐3p | New Zealand 28 | ND |
Ob 11 (100%) Lean 12 (100%) |
41 ± 5 44 ± 9 |
ND (Up) |
USA 31 | Fried Frailty Phenotype |
Fr 7 (0%) N‐F 7 (71%) |
Range 71–89 85.6 ± 3.8 76 ± 6.5 |
1.14 |
| Serum | |||||||||||
| 23 | MiR‐23a‐3p | Korea 25 | BMI ≥ 35 kg/m2 |
Ob 16 (56%) Lean 18 (72%) |
Range 30–59 31.3 ± 8.76 38.6 ± 7.9 |
2.81 | USA 34 | EWGSOP 2010 |
S 12 (100%) N‐S 51 (100%) |
Range 60–85 ND ND |
1.66 (NS) |
| Vastus lateralis | |||||||||||
| 24 | MiR‐424‐5p | ND 35 | ND |
Ob 5 (0%) Lean 5 (0%) |
ND |
ND (Up) |
UK 33 | EWGSOP 2010 |
S 5 (0%) N‐S 59 (0%) |
Range 68–76 | Up |
AWGS, Asian Working Group for Sarcopenia; BMI, body mass index; EWGSOP, European Working Group on Sarcopenia in Older People; Fr, frail; IQR, inter‐quartile range; ND, not documented; N‐F, non‐frail; N‐S, non‐sarcopenic; NS, not statistically significant; Ob, obese; S, sarcopenic; SD, standard deviation; SEM, standard error of the mean.
Age is presented as mean ± SD unless specified.
±SEM.
Median ± IQR.
Two miRNAs may also be commonly expressed in obesity and sarcopenia, but differences in nomenclature limited our understanding. Plasma miR‐328‐3p 29 and miR‐328 28 were down‐regulated and up‐regulated in obesity, respectively, and miR‐328 was down‐regulated in sarcopenia. 32 Plasma miR‐215 was up‐regulated in both obesity 28 and sarcopenia, 31 and miR‐215‐5p was also up‐regulated in obesity. 29 However, as we could not determine whether miR‐215 was ‐3p or ‐5p using the previous ID section on miRBase, we classified this miRNA as an unclear match. A list of the top externally validated circulating (plasma or serum) miRNAs in obesity or sarcopenia is available in Table S3.
Assessment of risk of bias
The majority of studies scored ≤6 on the NOS (2, 25 3, 28 , 35 4, 31 5, 29 and 6, 33 , 34 ), two received a star for validation (4* 27 and 6* 30 ), and one study scored >6 (8* 32 ). All studies lost a mark for failing to comment on the representativeness of cases, 25 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 five of six obesity studies lost a mark for failing to adequately define how controls were selected, 25 , 27 , 28 , 29 , 35 and only four studies received marks for adequately describing how exposure was ascertained. 29 , 32 , 33 , 34 All studies, except three, 27 , 29 , 35 received a mark for adequately describing the case definition.
Validated target genes, metabolic pathways, and functions of microRNAs
Validated target genes of the miRNAs of interest, in relation to sarcopenia, obesity, and related conditions (e.g. insulin resistance, inflammation, and cachexia) where possible, were identified by conducting a literature search using PubMed (Table 2). The majority of validated targets identified in the literature search were members of the phosphoinositide 3‐kinase/protein kinase B (PI3K/AKT) and transforming growth factor‐β (TGF‐β) signalling pathways. The most common targets identified were IGF‐1 (miR‐424‐5p, miR‐483‐3p, and miR‐18b‐5p) and members of the SMAD family (miR‐483‐3p, miR‐92a‐3p, and miR‐424‐5p). MiRNAs also targeted phosphatase and tensin homologue (PTEN) (miR‐296‐3p and miR‐499) and peroxisome proliferator‐activated receptor‐γ coactivator 1‐α (PGC‐1α) both directly (miR‐23a‐3p) and indirectly (miR‐499 via Fnip). The forkhead box protein (FOXO) family was targeted by miR193b‐5p. AMP‐activated protein kinase (AMPK) was also targeted directly (miR‐1224‐5p) and indirectly (miR‐499 via Fnip). The majority of commonly expressed miRNAs were involved in protein homeostasis, mitochondrial dynamics, determination of muscle fibre type, insulin resistance, and adipogenesis.
Table 2.
Functions and predicted targets of miRNAs that are differentially expressed in the same direction in obesity and sarcopenia
| MiRNA (family) | Cluster | ↑↓ | Function in relation to obesity/adiposity/insulin resistance or sarcopenia/muscle/exercise | Sample | Target |
|---|---|---|---|---|---|
| Plasma | |||||
| MiR‐106a‐5p (miR‐17) | MiR‐106a, miR‐18b, miR‐20b, miR‐19b‐2, miR‐92a‐2, miR‐363 | ↑ |
Down‐regulated in polycystic ovary syndrome (PCOS) 36 Elevated in aged muscles (mice) and dexamethasone‐treated myotubes; agomir results in down‐regulation of both myogenic regulatory factors (MyoD, MyoG, and MyHC) and phosphorylation of AKT and decreased myotube size 37 |
Plasma exosomes 36 C2C12 cells 37 Mice 37 |
PIK3R1 37 |
| MiR‐1224‐5p (miR‐1224) | N/A | ↑ | Up‐regulated in the liver of obese and high‐fat diet‐fed mice, contributes to hepatic lipid accumulation by targeting AMPKα1 38 | Mice 38 | AMPKα1 38 |
| MiR‐1246 (miR‐1246) | N/A | ↑ |
Down‐regulated in patients with chronic obstructive pulmonary disease (COPD) and emphysema (n = 20) 39 and amyotrophic lateral sclerosis (ALS) patients (n = 14) 40 Up‐regulated in diabetic nephropathy patients (n = 23); positively correlated with BMI 41 |
Plasma 40 |
|
| MiR‐145‐5p (miR‐145) | MiR‐145, miR‐143 | ↑ |
Limited studies on obesity/sarcopenia Up‐regulated in normal‐weight women (n = 11) following a high‐energy/fat breakfast 42 |
Plasma 42 | |
| MiR‐18b‐5p (miR‐17) | MiR‐106a, miR‐18b, miR‐20b, miR‐19b‐2, miR‐92a‐2, miR‐363 | ↑ |
Limited studies on obesity/sarcopenia Up‐regulated in PCOS 43 and relapsing multiple sclerosis (MS), may be involved in inflammatory pathways 44 SORBS2 identified as a target in diabetic nephropathy model cells 45 Targets and inhibits IGF‐1, suppressing the activation of p‐AKT, p‐MEK, and p‐ERK1/2 in vitro 46 |
HGMCs/HRGECs 45 HRECs 46 |
SORBS2 45 IGF‐1 46 |
| MiR‐193b‐5p (miR‐193) | MiR‐193b, miR‐365a | ↑ |
Limited studies on obesity/sarcopenia Weak negative correlations with BMI, plasma glucose levels, and insulin response to OGTT in younger adults 47 Targets and decreases expression of FoxO3 in cells, regulating cell cycle and cell proliferation 48 |
Subcutaneous adipose tissue 47 BRL‐3A 48 |
FoxO3 48 |
| MiR‐196a‐5p (miR‐196) | N/A | ↑ | High level of expression in myoblasts, suppresses mitochondrial biogenesis and its master regulator, PGC1β, and ND4. Suppresses osteoclast formation induced by RANKL in Raw264.7 cells 49 |
C2C12 cells 49 Raw264.7 cells 49 |
|
| MiR‐197‐3p (miR‐197) | N/A | ↑ |
Increased after high‐intensity resistance exercise in young adults 50 Up‐regulation inhibits GIP and GLP‐1 production through suppression of PCSK1/3 51 |
Serum 50 STC‐1 cells 51 |
|
| MiR‐199a‐5p (miR‐199) | MiR‐214 | ↓ |
Overexpression of AKT down‐regulates miR‐199a‐5p with a subsequent increase in targets Sirt1 and HiF‐1α in cardiomyocytes 52 Down‐regulated in mild and terminal‐stage ALS 53 and patients with Parkinson's disease 54 Up‐regulated in middle‐aged adults with T2DM; in vitro studies showed that miR‐199a regulates cellular glucose uptake by targeting and suppressing GLUT4 55 Up‐regulated in rat pancreatic β‐cells exposed to high glucose, promotes apoptosis and ROS formation, suppresses SIRT1 56 Inhibition results in decreased myogenic differentiation and increased MyoD1 and Pax7 in human myoblasts. High levels inhibit WNT signalling in HEK293T cells. Overexpression in zebrafish results in disorganization and detachment of myofibres 57 |
Cardiomyocytes 52 Serum 53 Plasma 55 Induced pluripotent stem cells 54 Rat pancreatic β‐cells 56 Myoblasts, HEK293T cells, zebrafish 57 |
HiF‐1α 52 GLUT4 55 |
| MiR‐296‐3p (miR‐296) | MiR‐296, miR‐298 | ↑ | Up‐regulated in PCOS; reduction in miR‐296‐3p promotes cell proliferation 59 |
Human granulosa cells 59 Human granulosa‐like tumour cells 59 |
PTEN 59 |
| MiR‐29b‐2‐5p (miR‐29) | MiR‐29b‐2, miR‐29c | ↑ |
Limited studies in the context of muscle/obesity Targets STAT3 in a fibroblast cell line 60 |
L929 cells 60 | STAT3 60 |
| MiR‐301b‐3p (miR‐130) | MiR‐301b, miR‐130 | ↓ | Decreased during myogenic differentiation; may be involved in muscle differentiation by regulating Rb1cc1 61 | Chicken myoblasts 61 | Rb1cc1 61 |
| MiR‐378c | N/A | ↑ | Studies not identified in the context of muscle/obesity | ||
| MiR‐4732‐5p (miR‐4732) | MiR‐4732, miR‐144, miR‐451a, miR‐451b | ↑ | Studies not identified in the context of muscle/obesity | ||
| MiR‐483‐3p (miR‐483) | N/A | ↑ |
Up‐regulated in hyperglycaemic mice and cardiomyocytes. Overexpression down‐regulates IGF‐1, thus promoting apoptosis in hyperglycaemic cardiomyocytes 62 Overexpression inhibits bovine myoblast cell proliferation through the IGF1/PI3K/AKT pathway; knockdown of miR‐483 enhances the expression of myogenic maker genes MyoD1, MyoG, and MyHC 63 Elevated in Duchenne's muscular dystrophy 64 |
Mice, H9c2 cell line 62 Bovine myoblasts 63 Serum 64 |
IGF‐1 62 , 63 |
| MiR‐487a‐3p (miR‐154) | MiR‐1185‐1, miR‐1185‐2, miR‐381, miR‐487a, miR‐487b, miR‐539, miR‐889, miR‐544a, miR‐655, miR‐382, miR‐154, miR‐496, miR‐377, miR‐134, miR‐668, miR‐485, miR‐323b | ↓ | Studies not identified in the context of muscle/obesity | ||
| MiR‐499a (miR‐499) MyomiR |
MiR‐499a, miR‐499b Encoded in slow myosin heavy chain genes (Myh7b)—restricted to T1 fibres (expressed in T1 fibres only) |
↓ |
Elevated in patients and carriers (mothers) with Duchenne's muscular dystrophy 65 and COPD (n = 103) and significantly correlated with NF‐κB p50 66 Affected by aerobic exercise—no changes after acute bout in young men 67 ; decreased following acute bout with weight vest with/without nutritional supplementation 68 ; increased in male marathon runners (n = 21) after competitive marathon competition 69 Increased after essential amino acid (EAA) ingestion in young adults (n = 7) 70 Associated with a slow muscle fibre phenotype in human muscle 71 Double knockout miR‐499/miR‐208b mice lost slow Type I myofibres with a concomitant increase in fast Type IIx/d and IIb myosin isoforms; forced expression of miR‐499 converted fast myofibres to slow. Sox6 helps mediate the actions of miR‐499 on slow myofibre gene programming 72 Targets Thrap1 to promote slow muscle fibre type 73 Targets TGF‐βR1, a known regulator of skeletal myoblast development. Knockdown of TGF‐βR1 inhibits myogenic differentiation in C2C12 cells 74 Targets PRDM16, which subsequently promotes myogenic, rather than brown adipogenic, differentiation in mouse skeletal muscle stem cells (SMSCs) 75 Promotes mitochondrial function. Targets Fnip1, a negative regulator of mitochondrial function in myocytes, which leads to activation of PGC‐1α. Fnip1 inhibition stimulates oxygen consumption rates, a sign of mitochondrial function, in myocytes. Mice with muscular dystrophy bred with miR‐499 mice exhibit improved mitochondrial capacity, restored slow‐oxidative muscle fibre programming and greater muscle functionality assessed with treadmill distance 76 Knockdown of p21, a target of miR‐499, decreases mitochondrial fission and cell death in cardiomyocytes exposed to doxorubicin, anti‐tumour drug 77 PTENP1, a target gene of miR‐499, expression is enhanced in diabetic and obese mouse models resulting in impaired AKT/GSK activation and glycogen synthesis contributing to insulin resistance 78 Down‐regulation was observed in diabetic mouse models. Down‐regulation in vitro was shown to impair the insulin signalling, AKT/GSK pathway and glycogen synthesis. PTEN was identified as a target 79 |
Serum 67 Mice 71 , 72 , 76 , 77 , 78 , 79 SMSCs 75 H9c2 cells 77 |
Sox6 72 p21 77 TGF‐βR1 74 PRDM16 75 Fnip1 76 PTEN 79 PTENP1 78 |
| MiR‐550a‐3p (miR‐550) | MiR‐550a‐1, miR‐550b‐1 | ↑ |
Limited studies in muscle/obesity Down‐regulated in patients with sporadic ALS 80 Associated with parameters of bone formation and microstructure parameters (mineral apposition ratio, bone surface, trabecular bone volume) 81 Down‐regulated in postmenopausal women with fractures older than 6 months; excellent discrimination of patients with low traumatic fractures 82 |
Peripheral blood 80 |
|
| MiR‐576‐5p (miR‐576) | N/A | ↑ | Studies not identified in the context of muscle/obesity | ||
| MiR‐589‐5p (miR‐589) | N/A | ↑ |
Limited studies in muscle/obesity Decreased upon TGF‐β stimulation in control fibroblasts, with no effect seen in COPD fibroblasts 83 |
Fibroblasts 83 | |
| MiR‐766‐3p (miR‐766) | N/A | ↑ |
Decreased in older (60–73 years; n = 51) compared with younger (19–42 years; n = 55) or long‐lived (90–102 years; n = 51) adults. 84 Overexpressed in older adult human dermal fibroblasts (HDFs) 85 Decreased after 12 weeks of endurance training in young men (n = 32) 86 Increased in sedentary T2DM adults (40–70 years; n = 24) who undertook either 4 month resistance or aerobic training 87 |
PBMCs 84 HDFs 85 HeLa cells 85 |
SIRT6 85 |
| MiR‐92a‐3p (miR‐92a) |
MiR‐17, miR‐18a, miR‐19a, miR‐20a, miR‐19b‐1, miR‐92a‐1 |
↑ |
Anti‐miR, MRG‐110, was tested in adult men and found to counteract the repression of known miR‐92a‐3p targets, ITGA5 and CD93. Elevated levels of DDIT4, an inhibitor of mTOR, were found in cells treated with MRG‐110 88 In a systematic review, down‐regulated following bariatric surgery 89 Decreased following 20 week aerobic exercise training (n = 20), 90 12 week endurance training in young men (n = 32), 86 and a 6 week cycling training in young men (n = 24) 91 No change following 5 month aerobic training in obese older adults (n = 33); however changes in miR‐92a positively correlated with changes in gait speed following intervention 92 MiR‐92a targets SMAD7, inhibition of miR‐92a led to increased mitochondrial content and oxygen consumption of brown adipocytes; inhibition of miR‐92a led to promotion of SMAD7 and subsequent suppression of p‐SMAD3/SMAD3. Inhibition of miR‐92a promoted differentiation of brown adipocytes. 93 Negatively correlated with BAT activity in young adults (n = 41); down‐regulated in the serum exosomes of mice with active BAT 94 Gradually up‐regulated with age (22, 40, 59, and 70 years) in men and women 95 |
Whole blood 88 CD4+ T cells 88 C2C12 cells 93 Vastus lateralis 91 Mice 94 |
ITGA5 88 CD93 88 SMAD7 93 |
| Serum | |||||
| MiR‐23a‐3p (miR‐23) | Mir‐23a, miR‐27a, miR‐24‐2 | ↑ |
Significantly down‐regulated in SAT and VAT of obese participants and significantly correlated with measures of adiposity (BMI, waist circumference, insulin measures). Involved in the regulation of PTEN, although the molecular mechanism is unclear 96 In young men (n = 7), increased following resistance or endurance exercise and protein ingestion 97 Increased following EAA ingestion alone 70 Decreased after an acute bout of endurance exercise in young adults (n = 9) 98 Up‐regulated in ALS. Targets PGC‐1α with subsequent effects on mitochondrial biogenesis and activity 99 Protects muscles from atrophy by targeting atrogin‐1/MAFbx1 and MURF‐1. Overexpression counteracts muscle atrophy induced by dexamethasone in myotubes and glucocorticoids in mice 100 |
VAT, SAT 96 Adipocytes 96 C2C12 cells 100 |
Atrogin‐1/MAFbx1 100 MURF‐1 100 |
| Vastus lateralis | |||||
| MiR‐424‐5p (miR‐322) | MiR‐424, miR‐503, miR‐542, miR‐450a‐2, miR‐450a‐1, miR‐450b | ↑ |
Down‐regulated in young women with PCOS (n = 24). 43 No difference between obese (n = 21) and NW (n = 19) women but correlated with waist circumference 102 Increased in cachectic cancer patients 103 Up‐regulated in muscle wasting conditions—ICU‐acquired weakness and COPD. Overexpression causes a reduction in muscle diameter of mice 33 Saturated fat/high‐fat diet impairs insulin signalling (INSR and IRS‐1) and up‐regulated miR‐424‐5p in hepatocytes and mice. Overexpression causes a significant decrease in insulin‐induced glycogen synthesis in hepatocytes. INSR is a direct target 104 Targets IGF‐1 in mice and human myocytes 105 |
Serum 43 SAT 102 Plasma 102 Hepatocytes 104 C2C12 cells 105 Human myoblasts 105 |
SMAD7 33 INSR 104 IGF‐1 105 |
↑, up‐regulated in sarcopenia/obesity; ↓, down‐regulated in sarcopenia/obesity; HGMCs, human glomerular mesangial cells; HRECs, human retinal endothelial cells; HRGECs, human renal glomerular endothelial cells; PBMCs, peripheral blood mononuclear cells.
Discussion
In this systematic review, we identified 24 miRNAs that are differentially expressed in both sarcopenia and obesity. These findings are particularly novel as miRNAs have not yet been explored in the context of sarcopenic obesity. The common dysregulation of the miRNAs identified in this review may therefore provide clues to understand the pathogenesis of sarcopenic obesity. To address this aim, a search was subsequently undertaken to understand the functions of these 24 miRNAs in relation to muscle/sarcopenia and adiposity/obesity. For some miRNAs, there were limited or no studies in the context of obesity or sarcopenia, and therefore, their relevance in relation to sarcopenic obesity is still unclear at present (miR‐29b‐2‐5p, miR‐378c, miR‐4732‐5p, miR‐487a‐3p, miR‐550a‐3p, miR‐576‐5p, and miR‐589‐5p). Other miRNAs have been shown to be differentially regulated in related diseases or metabolic responses, for example, in chronic obstructive pulmonary disease or amyotrophic lateral sclerosis (miR‐1246), 39 , 40 in response to a high‐fat meal (miR‐145‐5p) 42 or exercise (miR‐766‐3p). 86 , 87 However, we found that the majority of these commonly expressed miRNAs were involved in protein homeostasis, mitochondrial dynamics, determination of muscle fibre type, insulin resistance, and adipogenesis—processes implicated in the development of sarcopenic obesity. 2 , 9 The targets identified were predominantly found in the PI3K/AKT and TGF‐β pathways.
Protein homeostasis
IGF‐1 is one of the most important mediators of muscle growth and repair 106 ; however, IGF‐1 declines with age. 107 MiRNAs identified to be up‐regulated in both obesity and sarcopenia target IGF‐1, leading to its inhibition. The miRNAs miR‐18b‐5p, miR‐483‐3p, and miR‐424‐5p target IGF‐1 in vitro. 46 , 62 , 63 , 105 Functionally, in vitro studies have shown that miR‐483‐3p inhibits bovine myoblast cell proliferation through the IGF1/PI3K/AKT pathway 63 and promotes apoptosis in hyperglycaemic cardiomyocytes. 62 Up‐regulation of miR‐483‐3p causes a reduction in muscle diameter in mice and is also up‐regulated in muscle wasting conditions in humans. 33 These studies therefore suggest that up‐regulation of these miRNAs could be detrimental to muscle metabolism.
We found that miRNAs implicated in both obesity and sarcopenia regulate several targets of the PI3K/AKT pathway, which is involved in protein homeostasis. 108 Downstream of AKT, FoxO3 is targeted by miR‐193b‐5p. 48 The FOXO family is implicated in many processes included cell cycle, apoptosis, autophagy, and muscle atrophy. 48 , 108 In muscle, FOXO proteins are important mediators of two major proteolytic cellular pathways—the autophagy–lysosome and ubiquitin–proteasome systems. 108 These pathways are critical for quality control of sarcomeric proteins. 108 Interestingly, miR‐23a‐3p targets the muscle atrophy genes atrogin‐1/MAFbx1 and MURF‐1, which are downstream targets of FOXO. 100 Ectopic overexpression of miR‐23a‐3p counteracts muscle atrophy in dexamethasone‐treated myotubes and glucocorticoid‐treated mice. 100 In addition to direct targeting, miRNAs have been reported to target regulators of the FOXO family. MiR‐199a‐5p, which is down‐regulated in sarcopenia and obesity, targets and suppresses Sirt1, 56 , 58 which is responsible for the deacetylation of FOXO; suppression of Sirt1 results in cellular senescence in vitro. 58 Up‐regulation of miR‐199a‐5p promotes apoptosis and ROS formation in vitro. 56 Dysregulation in the PI3K/AKT/FOXO pathway may have implications on muscle atrophy and muscle quality control with implications for sarcopenia.
In mature adult muscle, TGF‐β is a potent regulator of muscle atrophy, which impairs skeletal muscle regeneration through inhibition of satellite cell proliferation, myofibre fusion, and expression of some muscle‐specific genes. 109 Multiple miRNAs commonly expressed in sarcopenia and obesity target this pathway. MiR‐499 targets TGF‐βR1, a receptor for TGF‐β. 74 Knockdown of this receptor inhibits myogenic differentiation in C2C12 cells. 74 Downstream of TGF‐βR1, Smad4 is down‐regulated by miR‐483‐3p to induce apoptosis in vitro. 110 Two miRNAs up‐regulated in obesity and sarcopenia, miR‐424‐5p 33 and miR‐92a‐3p, 93 target Smad7, a strong inhibitor of the TGF‐β pathway, which in turn inhibits SMAD2/3. Therefore, in sarcopenia and obesity, the TGF‐β pathway may be inhibited by miR‐483‐3p but promoted by miR‐424‐5p, miR‐92a‐3p, and the down‐regulation of miR‐499. It is unclear what effect this may have in relation to the pathogenesis of sarcopenic obesity, but known targets of TGF‐β pathway include the muscle atrophy genes, atrogin‐1 and MuRF‐1.
Mitochondrial dynamics
MicroRNAs dysregulated in both obesity and sarcopenia regulate mitochondrial biogenesis. MiR‐196a‐5p is highly expressed in myoblasts and suppresses PGC1β, a regulator of mitochondrial biogenesis. 49 MiR‐499a‐5p, which is down‐regulated in sarcopenia/obesity, targets Fnip1, which in turn inhibits AMPK with subsequent reduced activation of PGC‐1α. 76 Functionally, inhibition of Fnip1 by miR‐499a‐5p results in improved mitochondrial function in myocytes and improved mitochondrial capacity in mice with muscular dystrophy. 76 In adults with amyotrophic lateral sclerosis, of which mitochondrial dysfunction is considered an important factor in its pathogenesis, miR‐23a‐3p is elevated 99 similar to adults with obesity and sarcopenia. 25 , 34 MiR‐23a‐3p targets PGC‐1α with inhibition of its downstream signalling of mitochondrial biogenesis. 99 Likewise, miR‐92a‐3p 93 and miR‐424‐5p 33 target SMAD7, an antagonist of SMAD2/3. Functionally, miR‐92a‐3p inhibits mitochondrial content and oxygen consumption of brown adipocytes. 93 MiR‐499a‐5p also inhibits mitochondrial fission and apoptosis in cardiomyocytes exposed to an anti‐tumour drug by targeting p21, thus preventing cardiotoxicity. 77 Taken together, the dysregulation observed in these miRNAs in obesity and sarcopenia may lead to impaired mitochondrial function.
Fibre‐type switching
Ageing is associated with a switch from a fast muscle fibre phenotype to one of a slow muscle fibre type, 111 whereas obesity is associated with a greater proportion of fast, Type II, muscle fibres. 112 Overexpression of miR‐499 in vitro is associated with conversion of fast myofibres to slow through targeting of Sox6 and Thrap1. 72 , 73 In mice, knockout of miR‐499 and miR‐208b results in a loss of slow Type I myofibres and an increase in fast Type IIx/d and IIb myofibres. 72 In humans, miR‐499 is associated with a slow muscle fibre phenotype 71 and is elevated in patients with Duchenne's muscular dystrophy 65 and chronic obstructive pulmonary disease. 66 It is interesting that miR‐499 is underexpressed in both sarcopenia and obesity in light of the different muscle fibre properties of obesity and sarcopenia. However, it must be noted that this miRNA was reported in plasma rather than skeletal muscle where levels may be different.
Insulin resistance
Obesity is associated with insulin resistance, which can also impair muscle regeneration. 2 , 12 Several miRNAs identified as commonly up‐regulated in sarcopenia and obesity affect glucose metabolism and are associated with insulin resistance. 47 , 51 , 104 MiR‐197‐3p can regulate glucose metabolism by suppressing PCSK1/3 to inhibit GIP and GLP‐1 production, incretin hormones implicated in the pathogenesis of diabetes. 51 Overexpression of miR‐424‐5p is associated with decreased insulin‐induced glycogen synthesis in hepatocytes. 104 In young adults, miR‐193b‐5p is also negatively correlated with BMI, plasma glucose levels, and insulin response. 47 In contrast, down‐regulation of two miRNAs in obesity and sarcopenia may be beneficial for glucose metabolism—miR‐199a‐5p and miR‐499. 55 , 78 MiR‐199a‐5p is up‐regulated in diabetes, and in vitro studies have shown that miR‐199a‐5p targets and represses GLUT4, a glucose transporter isoform that increases glucose transport in response to insulin. 55 MiR‐499 targets both PTEN and PTENP1; PTENP1 can act as a ‘sink’ for miR‐499 to allow glucose metabolism. 78 It is unclear what effect these miRNAs may have in relation to the pathogenesis sarcopenic obesity.
Adiposity and adipogenesis
Gains in body fat and intramyocellular lipid deposition are characteristic of ageing, obesity, and sarcopenia. 2 , 10 , 111 MiRNAs differentially expressed in both obesity and sarcopenia were associated with parameters of adiposity. Body mass index is correlated with miR‐1246 41 and miR‐193b‐5p 47 in adults. MiR‐92a is negatively correlated with brown adipose tissue activity in young adults, 94 and inhibition of miR‐92a up‐regulates brown adipocyte differentiation in vitro. 93 MiR‐499a‐5p promotes myogenic rather than adipogenic differentiation in skeletal muscle stem cells. 75 MiR‐1224‐5p contributes to hepatic lipid accumulation in mice by targeting AMPKα1. 38
Limitations
The limitations of this study must be considered. Firstly, the heterogeneity and low quality of the studies identified in this review must be acknowledged. In some cases, matches were found between younger or female obese studies and older or predominantly male sarcopenia studies. As such, it is unclear how the interaction of age or sex has impacted our findings. It is known that age affects miRNA profiles, so perhaps older obese adults have differing miRNA profiles than younger obese adults, likewise men and women may exhibit differing profiles within the same condition. Secondly, we only included studies that had significantly different miRNAs, and therefore, we may have missed studies with non‐significant miRNAs, which may dispute our findings. However, this approach is commonly accepted. 113 , 114 Thirdly, because of the large number of overlapping miRNAs identified, we chose to discuss miRNAs, which were commonly dysregulated in the same direction in both conditions. The overlapping miRNAs identified were not externally validated, and therefore, our results should be viewed with caution and in need of further validation. MiRNAs that were reported as being expressed in conflicting directions in obesity may be due to differences between study methodology. Therefore, these miRNAs also warrant consideration in future research. Because of the large number of overlapping miRNAs, we chose to search for functions and targets in the context of obesity and sarcopenia and therefore may have omitted findings from other conditions, which may be relevant to sarcopenic obesity. However, a strength of our approach is that we focused on sarcopenia, obesity, and related conditions or diseases to focus our narrative review. It is possible that frailty and sarcopenia have differing miRNA profiles; however, because of limited studies and a similar clinical manifestation, it was deemed that information available on frailty may be useful in this context. In addition, the majority of miRNAs identified in sarcopenia/frailty were found in exosomes. There is evidence to suggest that some miRNAs appear to be preferentially recruited to exosomes whereas others are retained within the original cell. 115 However, because of a limited number of studies conducted in sarcopenia, we therefore opted to use a more open definition and a less specific outcome measure to avoid missing potentially relevant findings.
Conclusions
The pathogenesis underlying sarcopenic obesity is not fully understood. This is the first study to examine the potential role of miRNAs in the context of sarcopenic obesity and thus offers a novel perspective on this topic. We have provided an overview of the field and identified a panel of miRNAs, which may be implicated in sarcopenic obesity. Given the synergistic effect of sarcopenia and obesity on the risk of adverse health outcomes (falls, hospitalization, worsening disability, and all‐cause mortality), understanding the pathogenesis of sarcopenic obesity has the potential to lead to effective screening, monitoring, or treatment strategies. However, this systematic review was exploratory, and further work is now required to validate the findings presented here in older adults with sarcopenic obesity.
Conflict of interest
None declared.
Funding
K.G.‐W. is funded by Science Foundation Ireland (SFI) FFFP (19/FFP/6709), Irish Research Council (IRC) (IRCLA/2017/101), Health Research Board (HRB) (COV19‐2020‐060), and Dunhill Medical Trust (R545/0217). A.D. is funded by FIDELIO, an MSCA Innovative Training Network, receiving funding from the European Union's Horizon 2020 Framework Programme under Grant Agreement No. 860898. This work was supported by a studentship from the Medical Research Council (MRC) and Versus Arthritis as part of the Medical Research Council Versus Arthritis Centre for Integrated Research into Musculoskeletal Ageing (CIMA) (MR/R502182/1). The MRC Versus Arthritis Centre for Integrated Research into Musculoskeletal Ageing is a collaboration between the University of Liverpool, the University of Sheffield, and Newcastle University.
Supporting information
Table S1. Eligible definitions/criteria for conditions studied.
Table S2. Interpretation of Newcastle‐Ottawa Quality Assessment Scale for Case Control Studies in the context of this study.
Table S3. Top externally validated circulating (plasma or serum) miRNAs in obesity and sarcopenia.
Acknowledgements
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 116
Dowling L., Duseja A., Vilaca T., Walsh J. S., and Goljanek‐Whysall K. (2022) MicroRNAs in obesity, sarcopenia, and commonalities for sarcopenic obesity: a systematic review, Journal of Cachexia, Sarcopenia and Muscle, 13, 68–85, 10.1002/jcsm.12878
References
- 1. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol 2018;14:513–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhasin S, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA, et al. Sarcopenia definition: the position statements of the Sarcopenia Definition and Outcomes Consortium. J Am Geriatr Soc 2020;68:1410–1418. [DOI] [PubMed] [Google Scholar]
- 4. Donini LM, Busetto L, Bauer JM, Bischoff S, Boirie Y, Cederholm T, et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin Nutr 2020;39:2368–2388. [DOI] [PubMed] [Google Scholar]
- 5. Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res 2004;12:887–888. [DOI] [PubMed] [Google Scholar]
- 6. Zhang X, Xie X, Dou Q, Liu C, Zhang W, Yang Y, et al. Association of sarcopenic obesity with the risk of all‐cause mortality among adults over a broad range of different settings: a updated meta‐analysis. BMC Geriatr 2019;19:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gandham A, Mesinovic J, Jansons P, Zengin A, Bonham MP, Ebeling PR, et al. Falls, fractures, and areal bone mineral density in older adults with sarcopenic obesity: a systematic review and meta‐analysis. Obes Rev 2021;22:e13187, 10.1111/obr.13187 [DOI] [PubMed] [Google Scholar]
- 8. Rossi AP, Bianchi L, Volpato S, Bandinelli S, Guralnik J, Zamboni M, et al. Dynapenic abdominal obesity as a predictor of worsening disability, hospitalization, and mortality in older adults: results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2017;72:1098–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zamboni M, Rubele S, Rossi AP. Sarcopenia and obesity. Curr Opin Clin Nutr Metab Care 2019;22:13–19. [DOI] [PubMed] [Google Scholar]
- 10. Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez‐Mieyer P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr 2009;90:1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Carvalho DHT, Scholes S, Santos JLF, de Oliveira C, Alexandre TDS. Does abdominal obesity accelerate muscle strength decline in older adults? Evidence from the English Longitudinal Study of Ageing. J Gerontol A Biol Sci Med Sci 2019;74:1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol (1985) 2007;102:919–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ambros V. microRNAs: tiny regulators with great potential. Cell 2001;107:823–826. [DOI] [PubMed] [Google Scholar]
- 14. Friedman RC, Farh KK‐H, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 2009;19:92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kozomara A, Birgaoanu M, Griffiths‐Jones S. miRBase: from microRNA sequences to function. Nucleic Acids Res 2019;47(D1:D155–D162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sannicandro AJ, Soriano‐Arroquia A, Goljanek‐Whysall K. Micro(RNA)‐managing muscle wasting. J Appl Physiol 2019;127:619–632. [DOI] [PubMed] [Google Scholar]
- 17. Drummond MJ, McCarthy JJ, Sinha M, Spratt HM, Volpi E, Esser KA, et al. Aging and microRNA expression in human skeletal muscle: a microarray and bioinformatics analysis. Physiol Genomics 2011;43:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang Z, Xu A. Adipose extracellular vesicles in intercellular and inter‐organ crosstalk in metabolic health and diseases. Front Immunol 2021;12:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Y, Wang C, Wei M, Yang G, Yuan L. Multifaceted roles of adipose tissue‐derived exosomes in physiological and pathological conditions. Front Physiol 2021;12:669429, 10.3389/fphys.2021.669429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kita S, Maeda N, Shimomura I. Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. J Clin Invest 2019;129:4041–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vechetti IJ Jr, Peck BD, Wen Y, Walton RG, Valentino TR, Alimov AP, et al. Mechanical overload‐induced muscle‐derived extracellular vesicles promote adipose tissue lipolysis. FASEB J 2021;35:e21644, 10.1172/JCI129193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Rooij E, Kauppinen S. Development of microRNA therapeutics is coming of age. EMBO Mol Med 2014;6:851–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez‐Torres M, Patel K, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med 2013;368:1685–1694. [DOI] [PubMed] [Google Scholar]
- 24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71, 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bae YU, Kim Y, Lee H, Kim H, Jeon JS, Noh H, et al. Bariatric surgery alters microRNA content of circulating exosomes in patients with obesity. Obesity (Silver Spring) 2019;27:264–271. [DOI] [PubMed] [Google Scholar]
- 26. Hulsen T, de Vlieg J, Alkema W. BioVenn – a web application for the comparison and visualization of biological lists using area‐proportional Venn diagrams. BMC Genomics. 2008;9(1):488. 10.1186/1471-2164-9-488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Santamaria‐Martos F, Benitez ID, Latorre J, Lluch A, Moreno‐Navarrete JM, Sabater M, et al. Comparative and functional analysis of plasma membrane‐derived extracellular vesicles from obese vs. nonobese women. Clin Nutr 2020;39:1067–1076. [DOI] [PubMed] [Google Scholar]
- 28. Jones A, Danielson KM, Benton MC, Ziegler O, Shah R, Stubbs RS, et al. miRNA signatures of insulin resistance in obesity. Obesity (Silver Spring) 2017;25:1734–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Choi H, Koh HWL, Zhou L, Cheng H, Loh TP, Parvaresh Rizi E, et al. Plasma protein and microRNA biomarkers of insulin resistance: a network‐based integrative ‐omics analysis. Front Physiol 2019;10:379, 10.3389/fphys.2019.00379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Manning P, Munasinghe PE, Bellae Papannarao J, Gray AR, Sutherland W, Katare R. Acute weight loss restores dysregulated circulating microRNAs in individuals who are obese. J Clin Endocrinol Metab 2019;104:1239–1248. [DOI] [PubMed] [Google Scholar]
- 31. Ipson BR, Fletcher MB, Espinoza SE, Fisher AL. Identifying exosome‐derived MicroRNAs as candidate biomarkers of frailty. J Frailty Aging 2018;7:100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. He N, Zhang YL, Zhang Y, Feng B, Zheng Z, Wang D, et al. Circulating microRNAs in plasma decrease in response to sarcopenia in the elderly. Front Genet 2020;11:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Connolly M, Paul R, Farre‐Garros R, Natanek SA, Bloch S, Lee J, et al. miR‐424‐5p reduces ribosomal RNA and protein synthesis in muscle wasting. J Cachexia Sarcopenia Muscle 2018;9:400–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen Z, Bemben MG, Bemben DA. Bone and muscle specific circulating microRNAs in postmenopausal women based on osteoporosis and sarcopenia status. Bone 2019;120:271–278. [DOI] [PubMed] [Google Scholar]
- 35. Samjoo I, Safdar A, Hamadeh M, Akhtar M, Raha S, Timmons J, et al. Endurance training‐mediated differential regulation of miRNAs in skeletal muscle of lean and obese men. FASEB J 2010;24(S1):806.14–806.14. 10.1096/fasebj.24.1_supplement.806.14 [DOI] [Google Scholar]
- 36. Jiang X, Li J, Zhang B, Hu J, Ma J, Cui L, et al. Differential expression profile of plasma exosomal microRNAs in women with polycystic ovary syndrome. Fertil Steril 2021;115:782–792. [DOI] [PubMed] [Google Scholar]
- 37. Li X, Zhu Y, Zhang H, Ma G, Wu G, Xiang A, et al. MicroRNA‐106a‐5p inhibited C2C12 myogenesis via targeting PIK3R1 and modulating the PI3K/AKT signaling. Genes (Basel) 2018;9:333, 10.3390/genes9070333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen T, Yan D, Cheng X, Ji X, Bian J, Yin W. miR‐1224‐5p enhances hepatic lipogenesis by targeting adenosine monophosphate–activated protein kinase α1 in male mice. Endocrinology 2018;159:2008–2021. [DOI] [PubMed] [Google Scholar]
- 39. Cazorla‐Rivero S, Mura‐Escorche G, Gonzalvo‐Hernandez F, Mayato D, Cordoba‐Lanus E, Casanova C. Circulating miR‐1246 in the progression of chronic obstructive pulmonary disease (COPD) in patients from the BODE cohort. Int J Chron Obstruct Pulmon Dis 2020;15:2727–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saucier D, Wajnberg G, Roy J, Beauregard AP, Chacko S, Crapoulet N, et al. Identification of a circulating miRNA signature in extracellular vesicles collected from amyotrophic lateral sclerosis patients. Brain Res 2019;1708:100–108. [DOI] [PubMed] [Google Scholar]
- 41. Kim H, Bae YU, Jeon JS, Noh H, Park HK, Byun DW, et al. The circulating exosomal microRNAs related to albuminuria in patients with diabetic nephropathy. J Transl Med 2019;17:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Quintanilha BJ, Pinto Ferreira LR, Ferreira FM, Neto EC, Sampaio GR, Rogero MM. Circulating plasma microRNAs dysregulation and metabolic endotoxemia induced by a high‐fat high‐saturated diet. Clin Nutr 2020;39:554–562. [DOI] [PubMed] [Google Scholar]
- 43. Butler AE, Ramachandran V, Cunningham TK, David R, Gooderham NJ, Benurwar M, et al. Increased microRNA levels in women with polycystic ovarian syndrome but without insulin resistance: a pilot prospective study. Front Endocrinol 2020;11:754, 10.3389/fendo.2020.571357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mohamed MS, Nahrery E, Shalaby N, Hussein M, Aal RAE, Mohamed MM. Micro‐RNA 18b and interleukin 17A profiles in relapsing remitting multiple sclerosis. Mult Scler Relat Disord 2019;28:226–229. [DOI] [PubMed] [Google Scholar]
- 45. Jie R, Zhu P, Zhong J, Zhang Y, Wu H. LncRNA KCNQ1OT1 affects cell proliferation, apoptosis and fibrosis through regulating miR‐18b‐5p/SORBS2 axis and NF‐κB pathway in diabetic nephropathy. Diabetol Metab Syndr 2020;12:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu JH, Wang YH, Wang W, Shen W, Sang YZ, Liu L, et al. MiR‐18b suppresses high‐glucose‐induced proliferation in HRECs by targeting IGF‐1/IGF1R signaling pathways. Int J Biochem Cell Biol 2016;73:41–52. [DOI] [PubMed] [Google Scholar]
- 47. Meerson A, Traurig M, Ossowski V, Fleming J, Mullins M, Baier L. Human adipose microRNA‐221 is upregulated in obesity and affects fat metabolism downstream of leptin and TNF‐α. Diabetologia 2013;56:1971–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang C, Nan B, Ye H, Yan H, Wang M, Yuan Y. MiR‐193b‐5p protects BRL‐3A cells from acrylamide‐induced cell cycle arrest by targeting FoxO3. Food Chem Toxicol 2021;150:112059, 10.1016/j.fct.2021.112059 [DOI] [PubMed] [Google Scholar]
- 49. Takafuji Y, Tatsumi K, Kawao N, Okada K, Muratani M, Kaji H. MicroRNA‐196a‐5p in extracellular vesicles secreted from myoblasts suppresses osteoclast‐like cell formation in mouse cells. Calcif Tissue Int 2021;108:364–376. [DOI] [PubMed] [Google Scholar]
- 50. Vogel J, Niederer D, Engeroff T, Vogt L, Troidl C, Schmitz‐Rixen T, et al. Effects on the profile of circulating miRNAs after single bouts of resistance training with and without blood flow restriction—a three‐arm, randomized crossover trial. Int J Mol Sci 2019;20:3249, 10.3390/ijms20133249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang Y, Huang S, Li P, Chen Q, Li Y, Zhou Y, et al. Pancreatic cancer‐derived exosomes suppress the production of GIP and GLP‐1 from STC‐1 cells in vitro by down‐regulating the PCSK1/3. Cancer Lett 2018;431:190–200. [DOI] [PubMed] [Google Scholar]
- 52. Rane S, He M, Sayed D, Yan L, Vatner D, Abdellatif M. An antagonism between the AKT and beta‐adrenergic signaling pathways mediated through their reciprocal effects on miR‐199a‐5p. Cell Signal 2010;22:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dobrowolny G, Martone J, Lepore E, Casola I, Petrucci A, Inghilleri M, et al. A longitudinal study defined circulating microRNAs as reliable biomarkers for disease prognosis and progression in ALS human patients. Cell Death Dis 2021;7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tolosa E, Botta‐Orfila T, Morato X, Calatayud C, Ferrer‐Lorente R, Marti MJ, et al. MicroRNA alterations in iPSC‐derived dopaminergic neurons from Parkinson disease patients. Neurobiol Aging 2018;69:283–291. 10.1016/j.neurobiolaging.2018.05.032 [DOI] [PubMed] [Google Scholar]
- 55. Yan S‐T, Li C‐L, Tian H, Li J, Pei Y, Liu Y, et al. MiR‐199a is overexpressed in plasma of type 2 diabetes patients which contributes to type 2 diabetes by targeting GLUT4. Mol Cell Biochem 2014;397:45–51. [DOI] [PubMed] [Google Scholar]
- 56. Lin N, Li X, Zhang H, Yang Z, Su Q. microRNA‐199a‐5p mediates high glucose‐induced reactive oxygen species production and apoptosis in INS‐1 pancreatic β‐cells by targeting SIRT1. Eur Rev Med Pharmacol Sci 2017;21:1091–1098. [PubMed] [Google Scholar]
- 57. Alexander M, Kawahara G, Motohashi N, Casar J, Eisenberg I, Myers J, et al. MicroRNA‐199a is induced in dystrophic muscle and affects WNT signaling, cell proliferation, and myogenic differentiation. Cell Death Differ 2013;20:1194–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shi L, Han Q, Hong Y, Li W, Gong G, Cui J, et al. Inhibition of miR‐199a‐5p rejuvenates aged mesenchymal stem cells derived from patients with idiopathic pulmonary fibrosis and improves their therapeutic efficacy in experimental pulmonary fibrosis. Stem Cell Res Ther 2021;12:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu J, Ding J, Qu B, Liu J, Song X, Suo Q, et al. CircPSMC3 alleviates the symptoms of PCOS by sponging miR‐296‐3p and regulating PTEN expression. J Cell Mol Med 2020;24:11001–11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li C, Wang Z, Zhang J, Zhao X, Xu P, Liu X, et al. Crosstalk of mRNA, miRNA, lncRNA, and circRNA and their regulatory pattern in pulmonary fibrosis. Mol Ther Nucleic Acids 2019;18:204–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xue J, Xue J, Zhang J, Li D, Jiang L. miR‐130b‐3p/301b‐3p negatively regulated Rb1cc1 expression on myogenic differentiation of chicken primary myoblasts. Biotechnol Lett 2017;39:1611–1619. [DOI] [PubMed] [Google Scholar]
- 62. Qiao Y, Zhao Y, Liu Y, Ma N, Wang C, Zou J, et al. miR‐483‐3p regulates hyperglycaemia‐induced cardiomyocyte apoptosis in transgenic mice. Biochem Biophys Res Commun 2016;477:541–547. [DOI] [PubMed] [Google Scholar]
- 63. Song C, Yang Z, Dong D, Xu J, Wang J, Li H, et al. miR‐483 inhibits bovine myoblast cell proliferation and differentiation via IGF1/PI3K/AKT signal pathway. J Cell Physiol 2019;234:9839–9848. [DOI] [PubMed] [Google Scholar]
- 64. Coenen‐Stass AML, Sork H, Gatto S, Godfrey C, Bhomra A, Krjutskov K, et al. Comprehensive RNA‐sequencing analysis in serum and muscle reveals novel small RNA signatures with biomarker potential for DMD. Mol Ther Nucleic Acids 2018;13:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mousa NO, Abdellatif A, Fahmy N, Zada S, El‐Fawal H, Osman A. Circulating microRNAs in Duchenne muscular dystrophy. Clin Neurol Neurosurg 2020;189:105634, 10.1016/j.clineuro.2019.105634 [DOI] [PubMed] [Google Scholar]
- 66. Donaldson A, Natanek SA, Lewis A, Man WD, Hopkinson NS, Polkey MI, et al. Increased skeletal muscle‐specific microRNA in the blood of patients with COPD. Thorax 2013;68:1140–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhou Q, Shi C, Lv Y, Zhao C, Jiao Z, Wang T. Circulating microRNAs in response to exercise training in healthy adults. Front Genet 2020;11:256, 10.3389/fgene.2020.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Margolis LM, McClung HL, Murphy NE, Carrigan CT, Pasiakos SM. Skeletal muscle myomiR are differentially expressed by endurance exercise mode and combined essential amino acid and carbohydrate supplementation. Front Physiol 2017;8:182, 10.3389/fphys.2017.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Baggish AL, Park J, Min PK, Isaacs S, Parker BA, Thompson PD, et al. Rapid upregulation and clearance of distinct circulating microRNAs after prolonged aerobic exercise. J Appl Physiol (1985) 2014;116:522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Drummond MJ, Glynn EL, Fry CS, Dhanani S, Volpi E, Rasmussen BB. Essential amino acids increase microRNA‐499, ‐208b, and ‐23a and downregulate myostatin and myocyte enhancer factor 2C mRNA expression in human skeletal muscle. J Nutr 2009;139:2279–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gan Z, Rumsey J, Hazen BC, Lai L, Leone TC, Vega RB, et al. Nuclear receptor/microRNA circuitry links muscle fiber type to energy metabolism. J Clin Invest 2013;123:2564–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, et al. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell 2009;17:662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Xu M, Chen X, Chen D, Yu B, Li M, He J, et al. MicroRNA‐499‐5p regulates skeletal myofiber specification via NFATc1/MEF2C pathway and Thrap1/MEF2C axis. Life Sci 2018;215:236–245. [DOI] [PubMed] [Google Scholar]
- 74. Wu J, Yue B, Lan X, Wang Y, Fang X, Ma Y, et al. MiR‐499 regulates myoblast proliferation and differentiation by targeting transforming growth factor β receptor 1. J Cell Physiol 2019;234:2523–2536. [DOI] [PubMed] [Google Scholar]
- 75. Jiang J, Li P, Ling H, Xu Z, Yi B, Zhu S. MiR‐499/PRDM16 axis modulates the adipogenic differentiation of mouse skeletal muscle satellite cells. Hum Cell 2018;31:282–291. [DOI] [PubMed] [Google Scholar]
- 76. Liu J, Liang X, Zhou D, Lai L, Xiao L, Liu L, et al. Coupling of mitochondrial function and skeletal muscle fiber type by a miR‐499/Fnip1/AMPK circuit. EMBO Mol Med 2016;8:1212–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wan Q, Xu T, Ding W, Zhang X, Ji X, Yu T, et al. miR‐499‐5p attenuates mitochondrial fission and cell apoptosis via p21 in doxorubicin cardiotoxicity. Front Genet 2018;9:734, 10.3389/fgene.2018.00734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang L, Zhang N, Wang Z, D‐M A, Z‐Y C, H‐P P. Pseudogene PTENP1 functions as a competing endogenous RNA (ceRNA) to regulate PTEN expression by sponging miR‐499‐5p. Biochemistry (Mosc) 2016;81:739–747. [DOI] [PubMed] [Google Scholar]
- 79. Wang L, Zhang N, Pan HP, Wang Z, Cao ZY. MiR‐499‐5p contributes to hepatic insulin resistance by suppressing PTEN. Cell Physiol Biochem 2015;36:2357–2365. [DOI] [PubMed] [Google Scholar]
- 80. Liguori M, Nuzziello N, Introna A, Consiglio A, Licciulli F, D'Errico E, et al. Dysregulation of MicroRNAs and target genes networks in peripheral blood of patients with sporadic amyotrophic lateral sclerosis. Front Mol Neurosci 2018;11:288, 10.3389/fnmol.2018.00288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Feichtinger X, Muschitz C, Heimel P, Baierl A, Fahrleitner‐Pammer A, Redl H, et al. Bone‐related circulating microRNAs miR‐29b‐3p, miR‐550a‐3p, and miR‐324‐3p and their association to bone microstructure and histomorphometry. Sci Rep 2018;8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kocijan R, Muschitz C, Geiger E, Skalicky S, Baierl A, Dormann R, et al. Circulating microRNA signatures in patients with idiopathic and postmenopausal osteoporosis and fragility fractures. J Clin Endocrinol Metab 2016;101:4125–4134. [DOI] [PubMed] [Google Scholar]
- 83. Ong J, Faiz A, Timens W, van den Berge M, Terpstra M, Kok K, et al. Marked TGF‐β‐regulated miRNA expression changes in both COPD and control lung fibroblasts. Sci Rep 2019;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Owczarz M, Połosak J, Domaszewska‐Szostek A, Kołodziej P, Kuryłowicz A, Puzianowska‐Kuźnicka M. Age‐related epigenetic drift deregulates SIRT6 expression and affects its downstream genes in human peripheral blood mononuclear cells. Epigenetics. 2020;15(12):1336–1347. 10.1080/15592294.2020.1780081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sharma A, Diecke S, Zhang WY, Lan F, He C, Mordwinkin NM, et al. The role of SIRT6 protein in aging and reprogramming of human induced pluripotent stem cells. J Biol Chem 2013;288:18439–18447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nielsen S, Akerstrom T, Rinnov A, Yfanti C, Scheele C, Pedersen BK, et al. The miRNA plasma signature in response to acute aerobic exercise and endurance training. PLoS ONE 2014;9:e87308, 10.1371/journal.pone.0087308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Olioso D, Dauriz M, Bacchi E, Negri C, Santi L, Bonora E, et al. Effects of aerobic and resistance training on circulating micro‐RNA expression profile in subjects with type 2 diabetes. J Clin Endocrinol Metab 2019;104:1119–1130. [DOI] [PubMed] [Google Scholar]
- 88. Abplanalp WT, Fischer A, John D, Zeiher AM, Gosgnach W, Darville H, et al. Efficiency and target derepression of anti‐miR‐92a: results of a first in human study. Nucleic Acid Ther 2020;30:335–345. [DOI] [PubMed] [Google Scholar]
- 89. Langi G, Szczerbinski L, Kretowski A. Meta‐analysis of differential miRNA expression after bariatric surgery. J Clin Med 2019;8:1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Barber JL, Zellars KN, Barringhaus KG, Bouchard C, Spinale FG, Sarzynski MA. The effects of regular exercise on circulating cardiovascular‐related microRNAs. Sci Rep 2019;9:7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Keller P, Vollaard NB, Gustafsson T, Gallagher IJ, Sundberg CJ, Rankinen T, et al. A transcriptional map of the impact of endurance exercise training on skeletal muscle phenotype. J Appl Physiol (1985) 2011;110:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhang T, Brinkley TE, Liu K, Feng X, Marsh AP, Kritchevsky S, et al. Circulating MiRNAs as biomarkers of gait speed responses to aerobic exercise training in obese older adults. Aging (Albany NY) 2017;9:900–913, 10.18632/aging.101199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang Z, Jiang H, Li X, Chen X, Huang Y. MiR‐92a regulates brown adipocytes differentiation, mitochondrial oxidative respiration, and heat generation by targeting SMAD7. J Cell Biochem. 2020;121(8‐9):3825–3836. 10.1002/jcb.29539 [DOI] [PubMed] [Google Scholar]
- 94. Chen Y, Buyel JJ, Hanssen MJ, Siegel F, Pan R, Naumann J, et al. Exosomal microRNA miR‐92a concentration in serum reflects human brown fat activity. Nat Commun 2016;7:11420, 10.1038/ncomms11420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhang H, Yang H, Zhang C, Jing Y, Wang C, Liu C, et al. Investigation of microRNA expression in human serum during the aging process. J Gerontol A Biol Sci Med Sci 2015;70:102–109. [DOI] [PubMed] [Google Scholar]
- 96. Lozano‐Bartolomé J, Llauradó G, Portero‐Otin M, Altuna‐Coy A, Rojo‐Martínez G, Vendrell J, et al. Altered expression of miR‐181a‐5p and miR‐23a‐3p is associated with obesity and TNFα‐induced insulin resistance. J Clin Endocrinol Metab 2018;103:1447–1458. [DOI] [PubMed] [Google Scholar]
- 97. Camera DM, Ong JN, Coffey VG, Hawley JA. Selective modulation of microRNA expression with protein ingestion following concurrent resistance and endurance exercise in human skeletal muscle. Front Physiol 2016;7:87, 10.3389/fphys.2016.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Russell AP, Lamon S, Boon H, Wada S, Guller I, Brown EL, et al. Regulation of miRNAs in human skeletal muscle following acute endurance exercise and short‐term endurance training. J Physiol 2013;591:4637–4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Russell AP, Wada S, Vergani L, Hock MB, Lamon S, Leger B, et al. Disruption of skeletal muscle mitochondrial network genes and miRNAs in amyotrophic lateral sclerosis. Neurobiol Dis 2013;49:107–117. [DOI] [PubMed] [Google Scholar]
- 100. Wada S, Kato Y, Okutsu M, Miyaki S, Suzuki K, Yan Z, et al. Translational suppression of atrophic regulators by microRNA‐23a integrates resistance to skeletal muscle atrophy. J Biol Chem 2011;286:38456–38465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Dai Y, Zheng C, Li H. Inhibition of miR‐23a‐3p promotes osteoblast proliferation and differentiation. J Cell Biochem 2019;122:1251, 10.1002/jcb.29497 [DOI] [PubMed] [Google Scholar]
- 102. Gasparotto AS, Borges DO, Sassi MGM, Milani A, Rech DL, Terres M, et al. Differential expression of miRNAs related to angiogenesis and adipogenesis in subcutaneous fat of obese and nonobese women. Mol Biol Rep 2019;46:965–973. [DOI] [PubMed] [Google Scholar]
- 103. van de Worp W, Schols A, Dingemans AC, Op den Kamp CMH, Degens J, Kelders M, et al. Identification of microRNAs in skeletal muscle associated with lung cancer cachexia. J Cachexia Sarcopenia Muscle 2020;11:452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Min KH, Yang WM, Lee W. Saturated fatty acids‐induced miR‐424‐5p aggravates insulin resistance via targeting insulin receptor in hepatocytes. Biochem Biophys Res Commun 2018;503:1587–1593. [DOI] [PubMed] [Google Scholar]
- 105. Connolly M, Garfield BE, Crosby A, Morrell NW, Wort SJ, Kemp PR. miR‐322‐5p targets IGF‐1 and is suppressed in the heart of rats with pulmonary hypertension. FEBS Open Bio 2018;8:339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Perrini S, Laviola L, Carreira MC, Cignarelli A, Natalicchio A, Giorgino F. The GH/IGF1 axis and signaling pathways in the muscle and bone: mechanisms underlying age‐related skeletal muscle wasting and osteoporosis. J Endocrinol 2010;205:201–210. [DOI] [PubMed] [Google Scholar]
- 107. Bidlingmaier M, Friedrich N, Emeny RT, Spranger J, Wolthers OD, Roswall J, et al. Reference intervals for insulin‐like growth factor‐1 (IGF‐I) from birth to senescence: results from a multicenter study using a new automated chemiluminescence IGF‐I immunoassay conforming to recent international recommendations. J Clin Endocrinol Metab 2014;99:1712–1721. [DOI] [PubMed] [Google Scholar]
- 108. Sandri M, Barberi L, Bijlsma AY, Blaauw B, Dyar KA, Milan G, et al. Signalling pathways regulating muscle mass in ageing skeletal muscle: the role of the IGF1‐Akt‐mTOR‐FoxO pathway. Biogerontology 2013;14:303–323. [DOI] [PubMed] [Google Scholar]
- 109. Burks TN, Cohn RD. Role of TGF‐β signaling in inherited and acquired myopathies. Skelet Muscle 2011;1:19, 10.1186/2044-5040-1-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Anderson BA, McAlinden A. miR‐483 targets SMAD4 to suppress chondrogenic differentiation of human mesenchymal stem cells. J Orthop Res 2017;35:2369–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cruz‐Jentoft AJ, Sayer AA. Sarcopenia. The Lancet 2019;393:2636–2646. [DOI] [PubMed] [Google Scholar]
- 112. Wade AJ, Marbut MM, Round JM. Muscle fibre type and aetiology of obesity. Lancet 1990;335:805–808. [DOI] [PubMed] [Google Scholar]
- 113. Ortiz‐Dosal A, Rodil‐Garcia P, Salazar‐Olivo LA. Circulating microRNAs in human obesity: a systematic review. Biomarkers 2019;24:499–509. [DOI] [PubMed] [Google Scholar]
- 114. Yanai K, Kaneko S, Ishii H, Aomatsu A, Ito K, Hirai K, et al. MicroRNAs in sarcopenia: a systematic review. Front Med (Lausanne) 2020;7:180, 10.3389/fmed.2020.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Guduric‐Fuchs J, O'Connor A, Camp B, O'Neill CL, Medina RJ, Simpson DA. Selective extracellular vesicle‐mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genomics 2012;13:357, 10.1186/1471-2164-13-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Eligible definitions/criteria for conditions studied.
Table S2. Interpretation of Newcastle‐Ottawa Quality Assessment Scale for Case Control Studies in the context of this study.
Table S3. Top externally validated circulating (plasma or serum) miRNAs in obesity and sarcopenia.


