Abstract
Sarcopenia, or the age‐related loss of skeletal muscle mass and function, is an increasingly prevalent condition that contributes to reduced quality of life, morbidity, and mortality in older adults. Older adults display blunted anabolic responses to otherwise anabolic stimuli—a phenomenon that has been termed anabolic resistance (AR)—which is likely a casual factor in sarcopenia development. AR is multifaceted, but historically much of the mechanistic focus has been on signalling impairments, and less focus has been placed on the role of the vasculature in postprandial protein kinetics. The vascular endothelium plays an indispensable role in regulating vascular tone and blood flow, and age‐related impairments in vascular health may impede nutrient‐stimulated vasodilation and subsequently the ability to deliver nutrients (e.g. amino acids) to skeletal muscle. Although the majority of data has been obtained studying younger adults, the relatively limited data on the effect of blood flow on protein kinetics in older adults suggest that vasodilatory function, especially of the microvasculature, strongly influences the muscle protein synthetic response to amino acid feedings. In this narrative review, we examine evidence of AR in older adults following amino acid and mixed meal consumption, examine the evidence linking vascular dysfunction and insulin resistance to age‐related AR, review the influence of nitric oxide and endothelin‐1 on age‐related vascular dysfunction as it relates to AR, briefly review the potential causal role of arterial stiffness in promoting skeletal muscle microvascular dysfunction and AR, and provide a brief overview and future considerations for research examining age‐related AR.
Keywords: Anabolic resistance, Muscle protein synthesis, Ageing, Insulin, Vasodilation, Blood flow
Introduction
Sarcopenia is defined as the age‐related loss of skeletal muscle mass and function and is consistently associated with decreased quality of life, disability, co‐morbidity, hospitalization, and mortality. 1 , 2 , 3 , 4 , 5 , 6 , 7 Sarcopenia is increasingly prevalent with increased age, and data from masters athletes indicate that even in healthy, highly active individuals, muscle function may decline as early as age 30. 8 Consequently, sarcopenia is estimated to impact up to 24% of adults below the age of 70 and 43–58% of individuals over the age of 80. 9 Because of improvements in living conditions and health care in modern society, mortality is now driven primarily by morbidity and age‐associated injuries, which are more likely in those who display low skeletal muscle mass and quality. 10 , 11 , 12 Accordingly, the healthcare costs associated with sarcopenia are substantial, as individuals with sarcopenia are two times more likely to be hospitalized than those without, and the annual estimated cost of these hospitalizations is over $40bn in the USA alone. 13 Because the global age structure is expected to shift dramatically, such that individuals over the age of 80 years will outnumber those under the age of 5 by two to one by the year 2100, 14 there is a dire need to determine effective treatment strategies that are able to combat sarcopenia.
The protein content of skeletal muscle fibres is a primary determinant of skeletal muscle mass. Skeletal muscle protein is predominately regulated by the homeostatic balance between muscle protein synthesis (MPS) and muscle protein breakdown (MPB), which becomes dysregulated in older adults to result in a more negative protein balance and, ultimately, sarcopenia. 4 Notably, it does not appear that this derangement is driven by differences in basal muscle amino acid kinetics, 15 , 16 , 17 but via a phenomenon termed anabolic resistance (AR) whereby older adults demonstrate reduced MPS responses to anabolic stimuli, 18 including amino acid feedings. 19 Indeed, older adults have been shown to need nearly twice the relative isolated protein dose to maximally stimulate postprandial MPS. 16 Critically, evidence suggests that the potential for amino acid consumption to cause an anabolic response in skeletal muscle is dependent on nutrient delivery, which is a function of both nutritive (blood) flow and concentration. Indeed, multiple studies have demonstrated that a linear relationship exists between (skeletal muscle) blood flow and muscle fractional synthetic rates following amino acid administration. 20 , 21 Ageing is associated with progressive declines in macrovascular (large conduit arteries) and microvascular (arterioles and capillaries) function 22 that are likely to contribute to reduced nutrient delivery and thus impairments in postprandial skeletal muscle anabolism. 23 , 24 , 25 , 26 , 27 In summary, it is likely that AR is a primary driver of sarcopenia in ageing populations and that age‐related vascular dysfunction is an important, but overlooked contributory factor to this phenomenon (Figure 1). In this review, we will (i) examine evidence of AR following amino acid administration alone and (ii) following mixed meals, (iii) provide evidence linking insulin resistance and vascular dysfunction to age‐related AR, (iv) briefly review potential mechanisms of vascular dysfunction as they relate to age‐related AR, and lastly (v) provide a brief overview of important considerations and future research directions. As this review will focus on the role of the vasculature following meal consumption on age‐related AR, readers are encouraged to review the contributions of downstream signalling, 28 , 29 nutrient interactions, 30 and the impact of resistance exercise on AR, 31 which may be mentioned briefly but are predominantly outside of the scope of this review.
Figure 1.
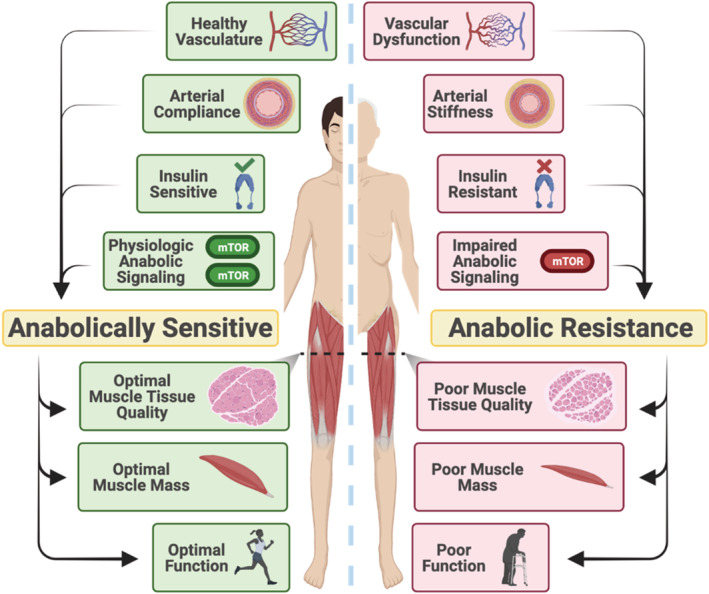
During the ageing process, there are gradual impairments in vascular function, anabolic signalling, arterial compliance, and insulin sensitivity. These impairments may ultimately lead and/or contribute to anabolic resistance, or a reduced ability to mount a muscle protein synthetic response to anabolic stimuli. Over time, anabolic resistance promotes sarcopenia, or the age‐related loss in muscle mass and function, resulting in a loss of functional ability and independence in older adults.
Anabolic resistance following amino acid administration
Age‐related alterations in protein kinetics are a complex, multifaceted phenomenon. Initial early work examining protein kinetics appeared to indicate that decrements in basal protein kinetics were driving age‐related decrements in muscle mass. 32 , 33 However, these early studies neglected to also quantify MPB and utilized indirect measurements of MPS. 17 More recent studies employing direct MPS measurements indicate that, despite differences in whole‐body protein kinetics, 34 there is little difference in basal, post‐absorptive skeletal MPS rates between younger and older adults. 15 , 16 , 17 , 34 , 35 Instead, divergent responses in older vs. younger adults appear to occur most dramatically during the dynamic changes following amino acid feedings or exercise, where blunted MPS responses are often observed in older adults. For example, Moore et al. investigated the amount of protein needed to maximize postprandial MPS in both younger and older adults and found that nearly twice (0.24 vs. 0.40 g/kg) and over twice (0.25 vs. 0.61 g/kg) the isolated protein dose were needed in older adults to maximize MPS when normalized to body weight and lean body mass, respectively. 16 An important caveat to these data, however, is the overlap in the confidence intervals for the protein doses associated with saturated MPS responses in the older vs. younger adults, which appears to be driven primarily by large variability in older adults. Katsanos et al. investigated the effect of age on muscle protein accretion and indicated that, when given a standard dose of 7 g essential amino acids (EAAs), older adults had diminished net phenylalanine uptake (9.9 vs. 25.1 mg/leg) in the 3.5 h period following consumption. 35 In a follow‐up study by the same group, Katsanos et al. demonstrated that the leucine content of the amino acid mixture is likely a key variable in the subsequent MPS response. In fact, while the older adults displayed an attenuated phenylalanine net balance following consumption of a 7 g EAA dose containing 26% leucine, net balance was not different from the younger adults following consumption of a 7 g EAA dose containing 41% leucine. 36 Together, these data suggest that (i) there are no real differences in basal protein kinetics between younger and older adults, (ii) older adults are able to mount similar maximal rates of MPS, but older adults exhibit AR whereby the protein synthetic response to the same submaximal amino acid dose is lower in older than younger adults, and therefore, (iii) larger absolute amino acids doses are needed to achieve similar anabolic responses to younger adults in older individuals. However, although this has been shown in multiple studies when examining amino acid intake in isolation, the results from studies examining age‐related differences in the postprandial MPS response to mixed meals have been less clear. Given that most individuals are unlikely to consume amino acids in isolation during the majority of their meals, examination of age‐related differences in postprandial protein kinetics following mixed meals is also extremely important.
Anabolic resistance following mixed amino acid and carbohydrate administration
In 2000, Volpi et al. reported that, despite similar basal amino acid turnover in younger and older adults, administration of an amino acid–glucose (40 g crystalline amino acids and 40 g glucose) cocktail increased MPS in younger, but not older adults, despite similar decreases in MPB independent of age. 27 Thus, this work by Volpi et al. suggests that AR may actually be more pronounced when amino acids and carbohydrate are co‐administered. 27 However, in contrast to these findings, Kiskini et al. reported no differences between healthy older and young men in the MPS response to co‐ingestion of 20 g casein protein and 40 g carbohydrates, but did observe greater hyperglycaemia and hyperinsulinaemia in the older men. 37 In 2014, Gorissen et al. specifically examined whether carbohydrate co‐ingestion with protein influenced muscle protein accretion in healthy younger and older men. When MPS rates were examined over a 5 h postprandial period, there were no differences observed among the younger and older men, and carbohydrate co‐ingestion did not modulate the MPS response in either group. Importantly, if MPS rates were examined only at 2 h postprandially, MPS was only elevated in the younger and not older men, suggesting that the observation of AR may be, at least in part, a temporal phenomenon. 38 Indeed, Volpi et al. only examined the anabolic responses during a 3 h postprandial window. 27 Further, whereas the older adults in both the studies conducted by Gorissen et al. 38 and Kiskini et al. 37 had significantly greater insulin responses to protein and carbohydrate co‐ingestion compared with the young participants, the older adults in the study conducted by Volpi et al. 27 had similar postprandial insulin responses to the younger adults. Therefore, there were likely differences in the older adult populations used in these studies that may help explain the disparate results, such as in the degree of insulin sensitivity and/or AR. It is also worth noting that these studies rely on isolated nutrient sources and, although outside the scope of this review, additional work is needed to analyse amino acid and glucose kinetics following ingestion of whole food vs. isolated sources to more fully understand the impact of other bioactive compounds present in whole foods on MPS. The reader is referred to Burd et al. 30 for a more in depth discussion.
Vascular function, insulin, and muscle protein anabolism
While well known for its direct actions to promote glucose disposal in skeletal muscle and adipose tissue, insulin also plays a critical role in causing the redistribution of blood flow from non‐nutritive to nutritive capillary networks to improve nutrient delivery to skeletal muscle in response to nutrient consumption. 39 Indeed, insulin stimulates endothelial nitric oxide (NO) production in precapillary muscle arterioles through the phosphoinositide 3‐kinase–protein kinase B/AKT–endothelial NO synthase (eNOS) signalling pathway 40 , 41 and also regulates endothelin‐1 (ET‐1) synthesis and secretion from the vascular endothelium 42 via the mitogen‐activated protein kinase‐dependent signalling pathway (Figure 2). Thus, insulin plays a central role in increasing the capillary surface area available for nutrient exchange via nutritive capillary recruitment in an endothelium‐dependent manner. 43 Importantly, insulin‐mediated capillary recruitment occurs far prior to 44 and perhaps even independently 45 of changes in total limb blood flow. Insulin sensitivity decreases substantially across the age span, 46 and there is evidence that the vasculature also becomes less responsive to insulin resulting in lower insulin‐stimulated microvascular blood flow. 39 These reductions in vascular insulin sensitivity thus have critical implications for nutrient delivery and may contribute to subsequent morbidity such as type 2 diabetes and potentially sarcopenia. Accordingly, the decrements in microvascular function seen in type 2 diabetes far precede traditional signs of the disease. 47 Specifically, using Sprague Dawley rats, Premilovac et al. demonstrated that microvascular insulin resistance is antecedent and a contributor to impairments in muscle glucose uptake. 48 Further, Bradley et al. demonstrated that, when the NO synthase inhibitor l‐N G‐nitro arginine methyl ester was locally infused during a hyperinsulinaemic–euglycaemic clamp in hooded Wistar rats, the microvascular blood flow response to insulin was completely abolished, providing evidence that NO directly mediated microvascular dilation in response to insulin. 49
Figure 2.
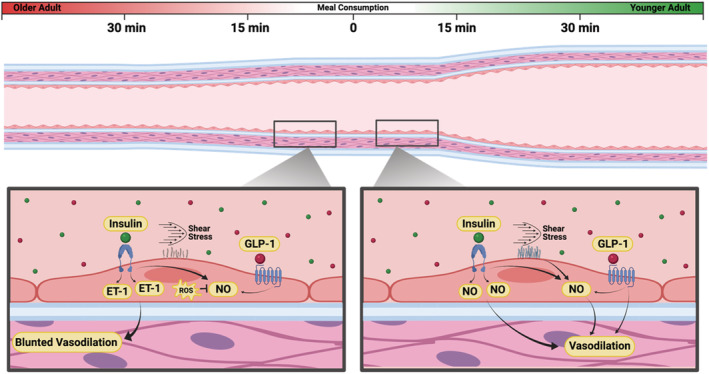
The impact of meal consumption in an older (left) and younger adult (right). In the older adult, there is an increased tendency for insulin to stimulate endothelin‐1 (ET‐1) release from the vascular endothelium, rather than nitric oxide (NO) as typically observed in healthy younger adults. Additionally, shear stress subsequent to an increase in blood flow stimulates the glycocalyx to release NO from the vascular endothelium in younger adults, whereas this effect is significantly reduced with ageing. Glucagon‐like peptide‐1 (GLP‐1) also stimulates vasodilation through NO‐dependent and NO‐independent mechanisms in a postprandial state. Finally, the NO that is produced in older adults is more likely to be scavenged by overproduced and/or unregulated reactive oxygen species (ROS) (i.e. oxidative stress). Consequently, meal consumption in younger adults is ultimately more likely to cause a robust vasodilatory response, thus enhancing the anabolic potential of meal consumption via greater nutrient delivery to skeletal muscle, when compared with older adults.
Adequate nutrient delivery, which is the product of blood flow/tissue perfusion and amino acid concentration, is a key factor in determining the ability to derive a postprandial anabolic response. Because of insulin's effects on skeletal muscle perfusion, it has been well studied as a potential causal factor in AR. 21 , 50 Early work on the topic mainly examined the role of insulin in stimulating MPS without concomitant amino acid administration. These early studies demonstrated apparently conflicting findings regarding insulin's ability to stimulate MPS, but these are likely due to methodological differences primarily involving local vs. systemic insulin infusion that provide important mechanistic insight regarding insulin's limitations in stimulating MPS. For example, in a 2006 study examining whether local isolated insulin infusion could stimulate MPS in young healthy adults, Fujita et al. reported that both local low‐dose (0.05 mU/min per 100 mL) and high‐dose (0.30 mU/min per 100 mL) insulin infusion stimulated MPS to a lesser degree than an intermediate dose (0.15 mU/min per 100 mL). Specifically, during the low‐dose infusion, insulin did not increase blood flow, thus limiting nutrient delivery; whereas during the high‐dose infusion, insulin increased blood flow and also rapidly drove down arterial amino acid concentrations, thereby limiting skeletal muscle amino acid availability. 20 Similar to what was observed in the high‐dose infusion condition, several studies have shown that when insulin levels increase systemically in a physiological manner, whether by infusion or meal consumption, a marked decrease in amino acid availability occurs if additional amino acids are not simultaneously infused or consumed—an effect that is likely due to insulin signalling that is ubiquitous among the body's tissues. 51 , 52 Collectively, these studies demonstrate that physiological hyperinsulinaemia stimulates MPS by increasing muscle perfusion and amino acid delivery, provided amino acid availability remains adequate. 20 However, when amino acid availability is allowed to decrease, the effect of insulin on MPS becomes self‐limiting. 51 , 53
While the study by Fujita et al. was a vital step in furthering our understanding of insulin on protein kinetics, it did not include older adults. In the same year, Rasmussen et al. examined muscle protein metabolism during a hyperinsulinaemic (0.15 mU/min per 100 mL)–euglycaemic clamp via local insulin infusion and observed that, whereas younger adults experience significant increases in muscle blood flow and MPS in response to hyperinsulinaemia, older adults experience no changes in either. Importantly, the authors reported that the observed changes in MPS were highly related to changes in blood flow (r = 0.90) and leg amino acid delivery (r = 0.89). 21 While the participants in the study by Rasmussen et al. were otherwise healthy, the older adults displayed a resistance to the vasodilatory effects of insulin following infusion, thus ultimately limiting nutrient delivery. Finally, Fujita et al. examined whether age‐related decrements in insulin‐induced muscle protein anabolism could be overcome by supraphysiological hyperinsulinaemia. 54 The authors reported that in otherwise healthy older adults, a local physiological postprandial insulin dose (i.e. 0.15 mU/min per 100 mL) was unable to stimulate amino acid delivery into the muscle nor increase MPS, while a high dose (0.30 mU/min per 100 mL) was able to improve leg blood flow and induce an MPS response. Consequentially, the older adults needed roughly twice the insulin dose to stimulate vasodilation and MPS compared with a body mass index‐matched, younger cohort, providing further evidence of tissue‐specific, vascular insulin resistance in otherwise glucose‐tolerant older adults. Thus, the contributory role of insulin to AR seems to be mainly in its ability, or inability in the presence of age‐related AR, to stimulate vasodilation and thus improve nutrient (i.e. amino acid) delivery to the muscle. 54
Although the previous studies provide excellent insight into the influence of isolated insulin on protein kinetics, meals are often composed of both protein and carbohydrate, thus examining the interaction between amino acid and insulin concentrations in a postprandial state is critical for a full, ecologically valid understanding in any attempt to characterize AR. Cuthbertson et al. examined the muscle anabolic response to 0–20 and 0–40 g of EAA in younger and older men, respectively, while maintaining basal insulin and glucose concentrations. The authors reported that MPS was maximized in younger men in response to just 10 g of EAA, whereas MPS increased, but remained lower in the older men even at a 40 g dose of EAA. Further, anabolic signalling responses were similarly greater in the younger than older adults in response to EAA administration. 15 The postprandial plasma leucine concentration was markedly greater in the older men, perhaps signifying lower perfusion and delivery of amino acids into the muscle in agreement with the aforementioned 2006 study by Rasmussen et al. 21 Similarly, Condino et al. observed that older adults had significantly greater plasma EAA concentrations 60–180 min after consuming 8 g of EAA compared with the younger adults. 55 In another study examining the physiological interaction between amino acids and insulin on muscle protein metabolism in older adults, Volpi et al. observed blunted MPS responses in older adults following co‐administration of an amino acid and glucose cocktail, despite observing similar postprandial hyperinsulinaemia, similar rates of muscle glucose uptake, and similar decreases in MPB. 27 Consequently, the increase in net protein balance caused by amino acid and glucose administration was also blunted in the older compared with younger adults. Interestingly, leg blood flow actually decreased postprandially in the older adults, but increased in the younger adults. It is plausible that insulin may preferentially increase mitogen‐activated protein kinase and ET‐1 signalling and/or altered sympathetic nervous system activity in older adults, blunting the vasodilatory response to hyperinsulinaemia, although this possibility needs to be explored further. Regardless, while hyperinsulinaemia caused similar glucose uptake across age groups, there was clearly an alteration in the response of MPS and peripheral blood flow to the endogenous hyperinsulinaemia induced by glucose and amino acid co‐administration, 27 suggestive of impaired vasodilatory responses and an unresponsiveness of MPS to endogenous hyperinsulinaemia in otherwise healthy, glucose‐tolerant older adults. Collectively, these studies indicate that older adults exhibit diminished vasodilatory responses to insulin and have similar or greater postprandial plasma EAAs compared with younger adults, perhaps suggesting age‐related impairments in amino acid delivery that obstruct the anabolic effect of protein‐rich feedings.
With evidence mounting that AR may be partially attributable to an inability of insulin to promote adequate perfusion of skeletal muscle, Timmerman et al. further investigated the interaction between perfusion, or endothelial function, and protein kinetics by directly controlling for blood flow in two 2010 studies. 25 , 26 In the first study, Timmerman et al. investigated the role of eNOS in protein kinetics by infusing either isolated insulin, or insulin combined with the eNOS inhibitor N G‐monomethyl‐l‐arginine in 14 healthy, young adults. During the combined infusion of insulin and N G‐monomethyl‐l‐arginine, blood flow and capillary recruitment were significantly reduced. In addition, ET‐1 levels remained unchanged, whereas they significantly decreased in the isolated insulin group. Ultimately, fractional synthetic rate, MPS, and mTORC1 signalling significantly increased in response to isolated insulin but did not change during combined L‐NNMA and insulin infusion. However, the combined infusion did cause a decrease in proteolysis, which remained unchanged following isolated insulin, resulting in no differences in net protein balance between the two groups. 25
The second study by Timmerman et al. investigated the impact of blood flow on protein kinetics by again infusing either insulin alone or in combination with sodium nitroprusside (SNP) in older adults (71 years). SNP is an NO donor that promotes vasodilation independently of endothelial NO production. 56 Strikingly, the addition of SNP to insulin elicited a two‐fold increase in blood flow, a nine‐fold increase in microvascular perfusion, and a significantly greater increase in Akt phosphorylation, MPS (43–129 vs. 41–53 nmol/min per 100 mL), and net phenylalanine balance (−16 to 26 vs. −17 to −2 nmol/min per 100 mL) compared with isolated insulin. 26
In another study, Timmerman et al. examined whether an acute bout of aerobic exercise performed the night prior to consumption of 20 g of EAA and 35 g of sucrose could improve the postprandial anabolic response, skeletal muscle perfusion, or insulin signalling in older adults. 24 The authors illustrated that EAA and sucrose consumption alone failed to stimulate an increase in MPS, conduit artery blood flow, or skeletal muscle perfusion in older adults. However, the prior bout of aerobic exercise restored nutrient‐mediated increases in blood flow, muscle microvascular perfusion, and amino acid delivery and subsequently enhanced the MPS response to EAA and sucrose consumption. Interestingly, these differences were realized despite the observation of equivalent systemic insulin levels in the control and aerobic exercise conditions. Thus, the increase in skeletal muscle protein anabolism was due to an increase in microvascular perfusion and amino acid delivery subsequent to the prior bout of aerobic exercise and did not appear to be driven by an insulin‐related effect, although an exercise‐induced improvement in vascular insulin sensitivity cannot be ruled out. While largely outside the scope of this review, this study also describes a novel exercise and nutrient interaction and illustrates an underappreciated mechanism by which exercise may be expected to improve anabolism to nutrient consumption. When taking the aforementioned studies by the Timmerman group using either aerobic exercise 24 or SNP to increase perfusion 26 together, it is apparent that nutritive flow is likely a key determinant for deriving an anabolic response to amino acid consumption, providing further evidence that the vasodilatory role of insulin is potentially vital when examining age‐related AR. As noted by Timmerman et al., these data support a link between muscle protein anabolism and endothelial function that is a critical component of the complex response to nutrient consumption in older adults. 24 Collectively, these studies indicate that impairments in blood flow, specifically in the microvasculature, are contributing to AR and provide strong evidence for a link between muscle protein anabolism and endothelial microvascular function in older adults.
Glucagon‐like peptide‐1 (GLP‐1) is a gut‐derived, incretin hormone with insulin‐independent effects that is a key regulator of postprandial glucose metabolism following a feeding. 57 Additionally, in both humans and animal models, GLP‐1 has been shown to augment eNOS expression 58 and microvascular blood flow 59 and enhance insulin secretion 60 and muscle glucose uptake by enhancing skeletal muscle microvascular recruitment and blood flow independently of insulin. 61 Recent evidence has emerged to suggest that GLP‐1 may also augment postprandial MPS by enhancing microvascular blood flow through both NO‐dependent 62 and NO‐independent mechanisms 63 (Figure 2). For example, Abdulla et al. examined the acute effects of GLP‐1 infusion on muscle protein anabolism in response to amino acid infusion during a postprandial, euglycaemic–insulinaemic clamp. Importantly, both leg blood flow and skeletal muscle microvascular perfusion were also assessed. Consistent with previous studies, the authors reported that MPS was resistant to the effects of intravenous amino acid administration under postprandial insulin conditions in older adults. However, adjuvant GLP‐1 infusion rescued the MPS response to the amino acid infusion, which was observed alongside a significant improvement in skeletal muscle perfusion. 23 Thus, when considering experimental designs, GLP‐1 should be accounted for when executing and comparing studies examining postprandial anabolism and blood flow, as meal composition can greatly alter its response. 64 Further, these data provide additional evidence to reinforce the hypothesis that (micro)vascular dysfunction plays a key role in skeletal muscle AR.
Although the results of the aforementioned studies are extremely insightful, they are all acute in nature and ultimately do not definitively demonstrate that (micro)vascular dysfunction contributes to a chronic inability to derive an anabolic response to anabolic stimuli. If skeletal muscle blood flow is a limiting factor in the ability to promote skeletal muscle anabolism in AR, individuals with a lower capacity for nutritive flow would be expected to be resistance to, and thus display impaired adaptation to, the anabolic effect of known anabolic stimuli (i.e. resistance exercise training). Moro et al. indirectly investigated this concept in 2019 by placing 19 male and female older adults, stratified by their pretraining capillary density, in a 12 week resistance training programme. 65 The authors found that the individuals with low pretraining capillary density were almost completely resistant to the anabolic effect of the resistance training programme, while the individuals with high pretraining capillary density saw significant improvements in appendicular skeletal muscle mass, skeletal muscle index, leg lean mass, and type II fibre cross‐sectional area. Additionally, the individuals with high pretraining capillary density showed a significant improvement in basal fractional synthetic rate, with no changes observed in the low pretraining capillary density group. 65 Thus, these initial findings suggest that older individuals with a lower capacity for skeletal muscle perfusion, and thus nutrient delivery, derive less benefit from chronic exposure to a known anabolic stimulus and lend support for the role of vascular function in AR. It is clear that additional longitudinal trials are needed in the context of AR to more fully understand this relationship.
While these studies provide strong evidence that AR and vascular dysfunction occur in tandem and that improvements in tissue perfusion occur alongside improvements in muscle anabolism in older adults, not all studies have supported the relationship between blood flow and MPS. Specifically, in 2014, Phillips et al. 66 indicated that muscle microvascular blood volume was not closely coupled to MPS nor did pharmacological enhancement of tissue perfusion enhance muscle anabolism. Using a between‐leg, bilateral comparison model, the authors infused an amino acid and glucose mixture and then infused the vasodilator methacholine into the femoral artery of one leg. The authors reported that microvascular blood flow increased 25% following administration of the amino acid and glucose alone and 79% in the leg that also received methacholine infusion. However, the increase in blood flow was not associated with additional increases in MPS or changes in net protein balance compared with amino acid and glucose administration alone. 66 On the surface, the results of this study appear to indicate that augmenting blood flow does not increase anabolism following feeding and thus that blood flow may not play a causal role in AR. However, several considerations are warranted before drawing such conclusions. For example, the study population consisted of young, healthy men, and therefore, feeding of amino acids and glucose alone enhanced blood flow and microvascular perfusion and caused a robust increase in muscle anabolism. Thus, it is highly likely that the MPS response to the feeding was saturated, even without concomitant methacholine administration. Such findings should not be readily extended to older adults, where these vascular and anabolic responses would be diminished, and dismissal of the role of vascular function (and skeletal muscle perfusion) in AR based on these data alone is unwarranted. Indeed, Mitchell et al. indicated that in response to EAA supplementation, and unlike their younger counterparts, older adults had completely absent macrovascular and microvascular responses following meal consumption, even with an equal insulin response between age groups. 67
However, in a study examining the effect of supplementation with the NO precursor sodium nitrate on the anabolic response to a protein feeding in older, type 2 diabetic participants, Kouw et al. reported no augmentation of MPS despite significant elevations in both plasma nitrate and nitrite. The authors concluded that microvascular function is likely not a key contributor to protein kinetics following protein consumption. 68 However, two major primary limitations to this study include the lack of a negative control group to confirm the presence of AR in their subject population and, perhaps most importantly, the lack of any blood flow or perfusion measurements. In fact, in direct opposition to a core underlying assumption made by Kouw et al., it has very recently been reported that despite significantly increasing plasma nitrate and nitrite, nitrate supplementation does not enhance skeletal muscle blood flow in the lower limbs of older adults. 69 Thus, it is possible that there was no or little change in microvascular perfusion in the subjects examined in the study by Kouw et al. Lastly, Mitchell et al. 70 showed that older adults consuming a combination of 3 g of arginine with 15 g EAAs elicited significant increases in microvascular blood flow without improvements in MPS when compared with EAAs alone. However, when examining the time course of both plasma EAA concentrations and changes in microvascular blood flow, it is apparent that EAA concentrations had peaked ~75 min prior to maximal improvements in microvascular blood flow. Thus, the improvement in microvascular blood flow occurred when EAA concentrations were returning near basal levels, thus limiting nutrient delivery‐related impacts on the MPS response. 70 Still, this study provides insight into the importance of early skeletal muscle capillary recruitment coincident with optimal amino acid availability following a meal to optimize the MPS response. This is especially apparent when juxtaposed by the previously described study of Abdulla et al., where GLP‐1 was infused alongside amino acids to immediately increase both microvascular perfusion and amino acid concentrations, which robustly enhanced the postprandial MPS response in older adults. 23 In sum, the studies that have examined the association of microvascular perfusion with postprandial skeletal MPS in populations who are characterized by impaired perfusion and diminished anabolism have seemingly demonstrated that augmenting microvascular perfusion does enhance postprandial MPS provided that the increase in microvascular perfusion occurs while postprandial amino acid availability is also elevated. Additional well‐controlled studies are needed to provide further confirmation of this association, but this evidence supports (micro)vascular function as an important factor in the ability to derive an anabolic response to meal consumption, a factor that is often compromised in the context of ageing.
Connecting age‐related anabolic resistance and vascular function
The endothelium was originally thought only to serve as a barrier between the wall of the vessel and the lumen, but is now known to be an active organ that lines the entire vascular system and plays a major role in maintaining vascular tone. 71 Ageing is associated with gradual impairments in vascular function, and dysfunction at the level of the endothelium is a primary contributing factor. Endothelial dysfunction is predominantly driven by altered bioavailability and signalling of endothelial‐derived signalling molecules such as NO and ET‐1 72 (Figure 2).
Endothelial dysfunction
Nitric oxide is a potent vasodilator and likely the most well‐known paracrine factor secreted from the endothelium. NO plays an important role in the maintenance of basal vascular tone. 73 When stimulated by shear stress, the glycocalyx lining the wall of the endothelium initiates a cell signalling cascade resulting in NO production. 74 Oxidative stress results when production of reactive oxygen species, which are otherwise vital for normal physiological function, overwhelms antioxidant capacity. 75 The main sources of reactive oxygen species are nicotinamide adenine dinucleotide phosphate oxidases and the mitochondria. 76 Superoxide is particularly damaging because it directly reduces NO bioavailability by scavenging NO to produce peroxynitrite. Peroxynitrite oxidizes tetrahydrobiopterin (BH4), the essential cofactor involved in NO formation, to dihydrobiopterin (BH2). Because eNOS has roughly equal affinity for BH4 and BH2, overproduction of BH2 promotes eNOS uncoupling, causing superoxide production (rather than NO) and perpetuating the process. 77 Because of the role of NO in maintenance of vascular tone and inhibition of platelet aggregation, secretion, and adhesion, decreased bioavailability ultimately leads to accumulation of lipids within the artery and formation of fibrous plaque, all of which eventually lead to wall thickening and promote adverse cardiovascular events. 78 Thus, endothelial function (and NO bioavailability) is an extremely important marker of overall health, as its impairment is a major contributor to the initiation and progression atherosclerosis and antecedent to traditional early signs of atherosclerosis and possibly also hypertension. 79 , 80 Endothelial function has also been shown to decline with age independent of traditional cardiovascular disease risk factors. 81 Ageing and endothelial dysfunction have been associated with impaired angiogenic responses to vascular endothelial growth factor, 82 likely due to NO's role in mediating vascular endothelial growth factor stimulated angiogenesis. 83 Therefore, endothelial dysfunction results in both impaired vasodilatory function via decreased NO bioavailability and impairments in angiogenesis, both of which are major contributors to microvascular dysfunction in ageing. 76 These aberrations may be particularly detrimental to skeletal muscle anabolism, due to the important, previously discussed link between anabolism and blood flow, 20 , 25 or, more specifically, microvascular perfusion and nutrient delivery. 23 , 26
Endothelin‐1 is a potent vasoconstrictor that acts as a paracrine molecule at the endothelium, playing a key role in vascular tone through binding at the ETA and ETB receptors on the endothelium and vascular smooth muscle cells. 84 , 85 , 86 , 87 Ageing is associated with the up‐regulation of ET‐1 21 , 88 and augmented ET‐1 mediated vasoconstriction, 87 , 89 and ET‐1 has been shown to impair glucose uptake into skeletal muscle 86 and interfere with insulin signalling in arterial smooth muscle cells. 90 At low concentrations, ET‐1 is also capable of potentiating the effects of other vasoconstrictive molecules, such as norepinephrine. 91 Accordingly, acute antagonism of ETA/B receptors results in greater leg blood flow in older vs. younger adults (29% vs. 10%) and improved endothelial function in obese and diabetic populations when compared with insulin‐sensitive individuals. 87 , 92 , 93 ET‐1 has been shown to inhibit both insulin receptor substrate 2 (IRS‐2) associated insulin‐stimulated phosphatidylinositol 3‐kinase and p85 phosphatidylinositol 3‐kinase subunit activity. 90 Shemyakin et al. indicated that blocking the ETA/B receptors in vivo resulted in a 63% increase in forearm blood glucose uptake, which was further doubled upon co‐infusion with insulin. As expected, ETA/B receptor blockade also resulted in 30% greater forearm blood flow. Insulin‐stimulated and basal glucose uptake was also decreased upon direct incubation of skeletal muscle cells with ET‐1. 86 In the aforementioned 2006 study by Rasmussen et al., greater blood flow and lower ET‐1 concentrations were reported at rest and during a hyperinsulinaemic–euglycaemic clamp in younger compared with older adults. Ultimately, after infusing insulin to levels commonly seen in the postprandial period, the authors reported that insulin was able to significantly elevate MPS in the younger, but not older subjects, with no changes in MPB. 21 It is possible in the previous study that either the elevated ET‐1 concentrations in the older adults prevented insulin‐induced vasodilation or insulin may have resulted in further ET‐1 synthesis, thus limiting vasodilation and increases in blood flow. Ultimately, these studies 21 , 86 , 90 collectively illustrate that age‐related increases in ET‐1 interfere with insulin‐stimulated vasodilation and glucose and amino acid delivery, contributing to blunted postprandial anabolic responses.
Arterial stiffness and muscle microvascular dysfunction
During each cardiac cycle, a pulse wave is sent from the heart that travels through the aorta and into the descending arterial tree. The rich elastin content of the arteries early in the vascular tree allows for a buffering of this pulse wave prior to it reaching the progressively more resistant, distal vessels. 94 Therefore, in young, healthy individuals, there are points of mismatched stiffness along the arterial tree that result in portions of the pulsatile energy being reflected back proximally towards the heart at each region of mismatched impedance, protecting the microcirculation from excessive pulsatility. 95 During ageing, the large elastic arteries become more fibrous and less elastic, resulting in stiffening 96 —which is one of the earliest signs of unfavourable structural changes within the vessel wall of the artery. 94 The gold standard non‐invasive assessment of arterial stiffness is carotid–femoral pulse wave velocity (cfPWV), which is measured as the quotient of distance and the measured pulse transit time ( ) from a point on the carotid and femoral artery using tonometry (Figure 3). As the large elastic arteries stiffen to a point similar to or exceeding that of the large muscular arteries, there is a more distal shift in pulse wave reflection 97 and a reduction in protective partial wave reflections, promoting damage to the microvasculature via excessive pressure transfer. 95 The microvascular damage caused by elevated arterial stiffness has been well documented in both the brain 98 and kidneys. 99 Although far less attention has been paid to the microvasculature of skeletal muscle, there are data suggesting that arterial stiffness may promote microvascular dysfunction in this tissue, too. Cooper et al. 100 examined the association of cfPWV and forearm vascular reactive hyperaemia, a measure of microvascular function in the skeletal muscle of the forearm, in 1458 adults from the Jacksonville Heart Study. The authors reported that greater aortic stiffness was associated with lower skeletal muscle microvascular function and that this relationship persisted even after adjustment for traditional cardiovascular risk factors. 100 These findings are supported by data from the Framingham Heart Study, which also suggests that, when controlling for cardiovascular disease risk factors, cfPWV is a significant predictor of forearm microvascular function. 101 Collectively, these studies indicate that age‐related increases in arterial stiffness likely promote skeletal muscle microvasculature dysfunction and may therefore be a significant contributor to age‐related AR. However, studies are still needed to test this hypothesis.
Figure 3.
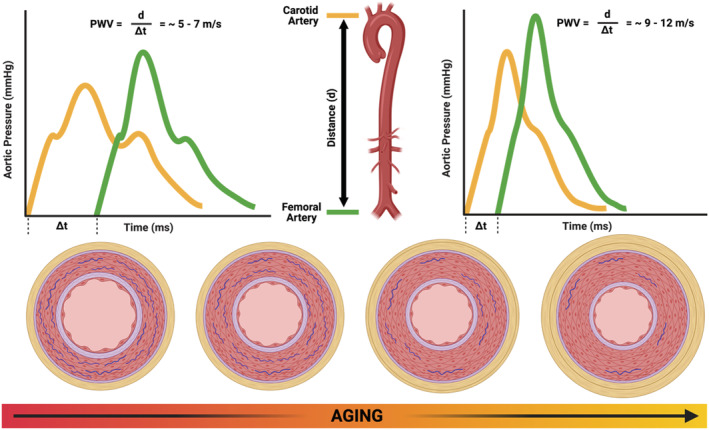
Ageing is associated with alterations in the vascular smooth muscle cells, loss of elastin, and increased collagen deposition in the large elastic arteries. Carotid–femoral pulse wave velocity is measured by dividing the distance (d) between a point on the carotid and femoral artery by the time it takes (Δt) for a pulse wave to travel from the carotid to femoral tonometry points in m/s. In younger adults, there are multiple points of impedance mismatch that reflect portions of the pulsatile energy back towards the heart, buffering the pulsatile energy travelling along the vessel, slowing the overall speed of the pulse wave, and protecting the microcirculation. In older adults, there is a more distal point of wave reflection due to stiffening of the elastic arteries, resulting in faster pulse wave transit times and excessive transmission of pulsatile energy into the microcirculation.
Overview and future considerations
Overall, while the majority of evidence supports the presence of age‐related AR, there is a general lack of consensus regarding the degree to which AR is present and the mechanisms that cause it. Regardless, a better understanding of AR is critical in the effort to combat sarcopenia. There are numerous factors that may explain the observation of impaired postprandial MPS responses to feedings in older adults. These factors include, but are not limited to, methodological differences in both study design and techniques employed, as well as differences in participant characteristics. Methodological considerations for studies moving forward include consideration of a temporal phenomenon regarding the postprandial increase in MPS, as MPS appears to increase more slowly in older than younger adults. Accordingly, when studies have examined MPS responses across longer postprandial windows (i.e. 5 h), the presence of AR is not as readily observed. 38 Furthermore, differences in the precursor and muscle protein pools that are studied, as well as the utilization of direct and indirect precursor–product methodologies, may explain between‐study differences regarding the presence of AR. Finally, it appears that older, otherwise healthy, adults are capable of mounting similar maximal MPS responses as younger adults. The current literature suggests that where AR is most evident, however, is in the response to suboptimal protein or amino acid feedings. In other words, age‐related AR can likely be overcome by simply providing additional protein or EAAs. Because older adults are very likely to experience appetite loss (i.e. anorexia of ageing), however, this may not represent a consistently viable nutritional strategy in older adults. Thus, exploration of methods to overcome AR in order to restore the anabolic response to suboptimal protein doses is still needed.
In general, the majority of the research regarding protein metabolism has been primarily focused on anabolic signalling and has neglected blood flow as a potential causal explanation for the deficits in the anabolic response seen in older adults. However, emerging evidence suggests that vascular dysfunction, specifically microvascular function, may indeed play a role in age‐related AR, as the inability to effectively elicit vasodilation can result in poor tissue perfusion and nutritive flow (Figure 4). Further, as discussed herein, insulin acts to enhance microvascular perfusion and nutritive flow in the postprandial period. While insulin appears to be permissive and not necessary to mount a significant postprandial anabolic response to meals containing amino acids in younger adults, it is plausible that postprandial hyperinsulinaemia may become more important and serve as a compensatory mechanism to enhance nutrient delivery and/or overcome blunted postprandial MPS responses caused by age‐related decreases in insulin sensitivity. Both age‐associated vascular dysfunction and insulin resistance exist on a continuum, and as such, we would suggest that it is critical that future studies report these characteristics in their study populations to more readily allow between‐study comparisons, or design studies to examine how differences in these characteristics specifically influence postprandial anabolic responses in older adults. Furthermore, as shown in studies examining the influence of increasing 24 or decreasing physical activity 102 , 103 , 104 on skeletal muscle anabolism, physical activity status is a critical modifier of postprandial MPS. Thus, the existence of age‐related AR may be explained in full or in part, by age‐related differences in activity patterns (e.g. sedentary behaviour and physical activity). However, again, additional studies are needed to directly explore this possibility, and studies comparing age‐related differences in skeletal muscle metabolism should certainly characterize physical activity status with validated methods. Additionally, although there has been a recent increase in papers assessing the effect of blood flow on postprandial skeletal muscle anabolism primarily in younger adults, future studies should assess the influence of known pharmacological vasodilators on postprandial haemodynamics and skeletal muscle anabolism in older adults. It would be especially important to understand if, by increasing skeletal muscle blood flow in ageing adults, nutrient delivery can be augmented such that an optimal postprandial anabolic response can be elicited using a submaximal amino acid or protein dose (as often consumed by older adults). It would also be beneficial to explore the role of more accessible vasodilators that can enhance NO bioavailability, such as dietary nitrates, to this same end. Finally, chronic oxidative stress and inflammation have been shown to lead to sarcopenia through impairments in anabolic signalling, 105 , 106 mitochondrial dysfunction, 105 , 106 and endothelial dysfunction 107 ; thus, the inclusion of exercise‐based or physical activity‐based lifestyle interventions 108 known to reduce oxidative stress, 108 , 109 , 110 initiated prior to or early in middle age, may be effective at ultimately combating AR.
Figure 4.
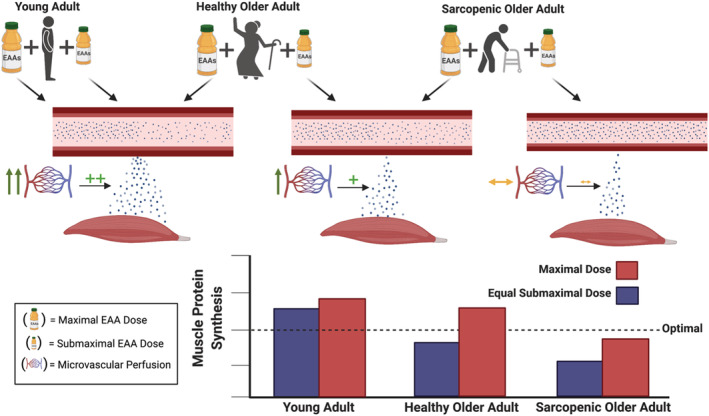
The postprandial skeletal muscle protein synthetic (MPS) response is dependent on amino acid delivery, which is the product of amino acid availability (e.g. concentrations) and blood flow (e.g. perfusion). In healthy younger adults, submaximal doses of essential amino acids (EAAs) are able to optimally stimulate MPS, whereby increasing to a maximal dose of EAAs does not result in further increases in MPS. In older healthy adults, submaximal doses of EAAs are often not able to optimally stimulate MPS, but when maximal doses are given, these individuals are often able to saturate the MPS response. In older sarcopenic adults, neither submaximal nor maximal doses of EAAs are able to optimally stimulate MPS. We propose that a rate‐limiting factor for older adults consuming submaximal and older sarcopenic adults consuming maximal EAA doses to be an inability of the meal consumption to promote adequate skeletal muscle perfusion, resulting in high circulating amino acid concentrations in these populations, but poor delivery and consequently impaired increases in MPS.
Conflict of interest
E.M.R. declares that she has no conflicts of interest. A.A.F. is listed as an inventor on US Patent 9364463 B2 entitled ‘Use of amino acid supplementation for improved muscle recovery’ and US Patent Application 20200253908 entitled ‘Use of amino acid supplementation for improved muscle protein synthesis’. N.D.M.J. currently serves on the American Heart Association (AHA) Research Committee, as well as two AHA research‐related subcommittees. Within the last year, N.D.M.J. has received compensation as a book reviewer, as well as a chapter author, for Human Kinetics.
Funding
N.F.B. and N.D.M.J. are supported by a research grant from the National Strength and Conditioning Association Foundation. D.D.C. is currently supported by a National Institutes of Health (NIH) Clinical Research Loan Repayment Award, and his salary is supported by funding through the U.S. Army Research Institute of Environmental Medicine and the Arkansas Geriatric Education Collaborative Geriatric Junior Faculty Development Award. A.A.F. is also supported by research grants from the U.S. Army Research Institute of Environmental Medicine and the Meat & Poultry Research & Education. N.D.M.J. is also currently supported by an American Heart Association (AHA) research grant (18AIREA33960528) and an NIH Clinical Research Loan Repayment Award.
Acknowledgements
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle.
Banks N. F., Rogers E. M., Church D. D., Ferrando A. A., and Jenkins N. D. M. (2022) The contributory role of vascular health in age‐related anabolic resistance, Journal of Cachexia, Sarcopenia and Muscle, 13, 114–127, 10.1002/jcsm.12898
References
- 1. Beaudart C, Rizzoli R, Bruyere O, Reginster JY, Biver E. Sarcopenia: burden and challenges for public health. Arch Public Health 2014;72:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jenkins ND, Buckner SL, Cochrane KC, Bergstrom HC, Palmer TB, Johnson GO, et al. Age‐related differences in rates of torque development and rise in EMG are eliminated by normalization. Exp Gerontol 2014;57:18–28. [DOI] [PubMed] [Google Scholar]
- 3. Jenkins ND, Housh TJ, Palmer TB, Cochrane KC, Bergstrom HC, Johnson GO, et al. Relative differences in strength and power from slow to fast isokinetic velocities may reflect dynapenia. Muscle Nerve 2015;52:120–130. [DOI] [PubMed] [Google Scholar]
- 4. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011;12:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moon SJ, Kim TH, Yoon SY, Chung JH, Hwang HJ. Relationship between stage of chronic kidney disease and sarcopenia in Korean aged 40 years and older using the Korea National Health and Nutrition Examination Surveys (KNHANES IV‐2, 3, and V‐1, 2), 2008–2011. PLoS One 2015;10:e0130740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta‐analysis of general population studies. J Diabetes Metab Disord 2017;16:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Volpato S, Bianchi L, Cherubini A, Landi F, Maggio M, Savino E, et al. Prevalence and clinical correlates of sarcopenia in community‐dwelling older people: application of the EWGSOP definition and diagnostic algorithm. J Gerontol A Biol Sci Med Sci 2014;69:438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gava P, Kern H, Carraro U. Age‐associated power decline from running, jumping, and throwing male masters world records. Exp Aging Res 2015;41:115–135. [DOI] [PubMed] [Google Scholar]
- 9. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 10. Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int 2010;21:543–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, et al. Sarcopenia: aging‐related loss of muscle mass and function. Physiol Rev 2019;99:427–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tieland M, Trouwborst I, Clark BC. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle 2018;9:3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goates S, Du K, Arensberg MB, Gaillard T, Guralnik J, Pereira SL. Economic impact of hospitalizations in US adults with sarcopenia. J Frailty Aging 2019;8:93–99. [DOI] [PubMed] [Google Scholar]
- 14. Vollset SE, Goren E, Yuan CW, Cao J, Smith AE, Hsiao T, et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet 2020;396:1285–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 2005;19:422–424. [DOI] [PubMed] [Google Scholar]
- 16. Moore DR, Churchward‐Venne TA, Witard O, Breen L, Burd NA, Tipton KD, et al. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci 2015;70:57–62. [DOI] [PubMed] [Google Scholar]
- 17. Volpi E, Sheffield‐Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 2001;286:1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burd NA, Gorissen SH, van Loon LJ. Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev 2013;41:169–173. [DOI] [PubMed] [Google Scholar]
- 19. Prod'homme M, Balage M, Debras E, Farges MC, Kimball S, Jefferson L, et al. Differential effects of insulin and dietary amino acids on muscle protein synthesis in adult and old rats. J Physiol 2005;563:235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fujita S, Rasmussen BB, Cadenas JG, Grady JJ, Volpi E. Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin‐induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab 2006;291:E745–E754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, et al. Insulin resistance of muscle protein metabolism in aging. FASEB J 2006;20:768–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res 2018;123:849–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abdulla H, Phillips BE, Wilkinson DJ, Limb M, Jandova T, Bass JJ, et al. Glucagon‐like peptide 1 infusions overcome anabolic resistance to feeding in older human muscle. Aging Cell 2020;19:e13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Timmerman KL, Dhanani S, Glynn EL, Fry CS, Drummond MJ, Jennings K, et al. A moderate acute increase in physical activity enhances nutritive flow and the muscle protein anabolic response to mixed nutrient intake in older adults. Am J Clin Nutr 2012;95:1403–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Timmerman KL, Lee JL, Dreyer HC, Dhanani S, Glynn EL, Fry CS, et al. Insulin stimulates human skeletal muscle protein synthesis via an indirect mechanism involving endothelial‐dependent vasodilation and mammalian target of rapamycin complex 1 signaling. J Clin Endocrinol Metab 2010;95:3848–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Timmerman KL, Lee JL, Fujita S, Dhanani S, Dreyer HC, Fry CS, et al. Pharmacological vasodilation improves insulin‐stimulated muscle protein anabolism but not glucose utilization in older adults. Diabetes 2010;59:2764–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose‐induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab 2000;85:4481–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baar K. Training for endurance and strength: lessons from cell signaling. Med Sci Sports Exerc 2006;38:1939–1944. [DOI] [PubMed] [Google Scholar]
- 29. Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin‐resistant states. Cold Spring Harb Perspect Biol 2014;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burd NA, Beals JW, Martinez IG, Salvador AF, Skinner SK. Food‐first approach to enhance the regulation of post‐exercise skeletal muscle protein synthesis and remodeling. Sports Med 2019;49:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walker DK, Dickinson JM, Timmerman KL, Drummond MJ, Reidy PT, Fry CS, et al. Exercise, amino acids and aging in the control of human muscle protein synthesis. Med Sci Sports Exerc 2011;43:2249–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Welle S, Thornton C, Jozefowicz R, Statt M. Myofibrillar protein synthesis in young and old men. Am J Physiol 1993;264:E693–E698. [DOI] [PubMed] [Google Scholar]
- 33. Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy‐chain and sarcoplasmic protein in humans. Am J Physiol 1997;273:E790–E800. [DOI] [PubMed] [Google Scholar]
- 34. Hirsch KR, Church DD, Kim IY, Park S, Wolfe RR, Ferrando AA. Comparison of basal whole‐body protein kinetics and muscle protein synthesis between young and older adults. Physiol Rep 2020;8:e14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Katsanos CS, Kobayashi H, Sheffield‐Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr 2005;82:1065–1073. [DOI] [PubMed] [Google Scholar]
- 36. Katsanos CS, Kobayashi H, Sheffield‐Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 2006;291:E381–E387. [DOI] [PubMed] [Google Scholar]
- 37. Kiskini A, Hamer HM, Wall BT, Groen BB, de Lange A, Bakker JA, et al. The muscle protein synthetic response to the combined ingestion of protein and carbohydrate is not impaired in healthy older men. Age (Dordr) 2013;35:2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gorissen SH, Burd NA, Hamer HM, Gijsen AP, Groen BB, van Loon LJ. Carbohydrate coingestion delays dietary protein digestion and absorption but does not modulate postprandial muscle protein accretion. J Clin Endocrinol Metab 2014;99:2250–2258. [DOI] [PubMed] [Google Scholar]
- 39. Clark MG, Wallis MG, Barrett EJ, Vincent MA, Richards SM, Clerk LH, et al. Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Endocrinol Metab 2003;284:E241–E258. [DOI] [PubMed] [Google Scholar]
- 40. Cardillo C, Nambi SS, Kilcoyne CM, Choucair WK, Katz A, Quon MJ, et al. Insulin stimulates both endothelin and nitric oxide activity in the human forearm. Circulation 1999;100:820–825. [DOI] [PubMed] [Google Scholar]
- 41. Jonk AM, Houben AJ, de Jongh RT, Serne EH, Schaper NC, Stehouwer CD. Microvascular dysfunction in obesity: a potential mechanism in the pathogenesis of obesity‐associated insulin resistance and hypertension. Physiology (Bethesda) 2007;22:252–260. [DOI] [PubMed] [Google Scholar]
- 42. Muniyappa R, Yavuz S. Metabolic actions of angiotensin II and insulin: a microvascular endothelial balancing act. Mol Cell Endocrinol 2013;378:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Manrique C, Lastra G, Sowers JR. New insights into insulin action and resistance in the vasculature. Ann N Y Acad Sci 2014;1311:138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, et al. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 2004;53:1418–1423. [DOI] [PubMed] [Google Scholar]
- 45. Coggins M, Lindner J, Rattigan S, Jahn L, Fasy E, Kaul S, et al. Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes 2001;50:2682–2690. [DOI] [PubMed] [Google Scholar]
- 46. Shou J, Chen PJ, Xiao WH. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol Metab Syndr 2020;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Horton WB, Barrett EJ. Microvascular dysfunction in diabetes mellitus and cardiometabolic disease. Endocr Rev 2021;42:29–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Premilovac D, Bradley EA, Ng HL, Richards SM, Rattigan S, Keske MA. Muscle insulin resistance resulting from impaired microvascular insulin sensitivity in Sprague Dawley rats. Cardiovasc Res 2013;98:28–36. [DOI] [PubMed] [Google Scholar]
- 49. Bradley EA, Richards SM, Keske MA, Rattigan S. Local NOS inhibition impairs vascular and metabolic actions of insulin in rat hindleg muscle in vivo. Am J Physiol Endocrinol Metab 2013;305:E745–E750. [DOI] [PubMed] [Google Scholar]
- 50. Fujita S, Rasmussen BB, Cadenas JG, Drummond MJ, Glynn EL, Sattler FR, et al. Aerobic exercise overcomes the age‐related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes 2007;56:1615–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Biolo G, Wolfe RR. Insulin action on protein metabolism. Baillieres Clin Endocrinol Metab 1993;7:989–1005. [DOI] [PubMed] [Google Scholar]
- 52. Heslin MJ, Newman E, Wolf RF, Pisters PW, Brennan MF. Effect of hyperinsulinemia on whole body and skeletal muscle leucine carbon kinetics in humans. Am J Physiol 1992;262:E911–E918. [DOI] [PubMed] [Google Scholar]
- 53. Biolo G, Williams BD, Fleming RY, Wolfe RR. Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes 1999;48:949–957. [DOI] [PubMed] [Google Scholar]
- 54. Fujita S, Glynn EL, Timmerman KL, Rasmussen BB, Volpi E. Supraphysiological hyperinsulinaemia is necessary to stimulate skeletal muscle protein anabolism in older adults: evidence of a true age‐related insulin resistance of muscle protein metabolism. Diabetologia 2009;52:1889–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Condino AM, Aquilani R, Pasini E, Iadarola P, Viglio S, Verri M, et al. Plasma kinetic of ingested essential amino acids in healthy elderly people. Aging Clin Exp Res 2013;25:711–714. [DOI] [PubMed] [Google Scholar]
- 56. Barton M, Cosentino F, Brandes RP, Moreau P, Shaw S, Luscher TF. Anatomic heterogeneity of vascular aging: role of nitric oxide and endothelin. Hypertension 1997;30:817–824. [DOI] [PubMed] [Google Scholar]
- 57. Drucker DJ. The cardiovascular biology of glucagon‐like peptide‐1. Cell Metab 2016;24:15–30. [DOI] [PubMed] [Google Scholar]
- 58. Ding L, Zhang J. Glucagon‐like peptide‐1 activates endothelial nitric oxide synthase in human umbilical vein endothelial cells. Acta Pharmacol Sin 2012;33:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sjoberg KA, Rattigan S, Jeppesen JF, Lundsgaard AM, Holst JJ, Kiens B. Differential effects of glucagon‐like peptide‐1 on microvascular recruitment and glucose metabolism in short‐ and long‐term insulin resistance. J Physiol 2015;593:2185–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Meloni AR, DeYoung MB, Lowe C, Parkes DG. GLP‐1 receptor activated insulin secretion from pancreatic β‐cells: mechanism and glucose dependence. Diabetes Obes Metab 2013;15:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Subaran SC, Sauder MA, Chai W, Jahn LA, Fowler DE, Aylor KW, et al. GLP‐1 at physiological concentrations recruits skeletal and cardiac muscle microvasculature in healthy humans. Clin Sci (Lond) 2014;127:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W, et al. Glucagon‐like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide‐dependent mechanism. Diabetes 2012;61:888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Smits MM, Muskiet MH, Tonneijck L, Kramer MH, Diamant M, van Raalte DH, et al. GLP‐1 receptor agonist exenatide increases capillary perfusion independent of nitric oxide in healthy overweight men. Arterioscler Thromb Vasc Biol 2015;35:1538–1543. [DOI] [PubMed] [Google Scholar]
- 64. Parvaresh Rizi E, Loh TP, Baig S, Chhay V, Huang S, Caleb Quek J, et al. A high carbohydrate, but not fat or protein meal attenuates postprandial ghrelin, PYY and GLP‐1 responses in Chinese men. PLoS One. 2018;13:e0191609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Moro T, Brightwell CR, Phalen DE, McKenna CF, Lane SJ, Porter C, et al. Low skeletal muscle capillarization limits muscle adaptation to resistance exercise training in older adults. Exp Gerontol 2019;127:110723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Phillips BE, Atherton PJ, Varadhan K, Wilkinson DJ, Limb M, Selby AL, et al. Pharmacological enhancement of leg and muscle microvascular blood flow does not augment anabolic responses in skeletal muscle of young men under fed conditions. Am J Physiol Endocrinol Metab 2014;306:E168–E176. [DOI] [PubMed] [Google Scholar]
- 67. Mitchell WK, Phillips BE, Williams JP, Rankin D, Smith K, Lund JN, et al. Development of a new Sonovue™ contrast‐enhanced ultrasound approach reveals temporal and age‐related features of muscle microvascular responses to feeding. Physiol Rep 2013;1:e00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kouw IW, Cermak NM, Burd NA, Churchward‐Venne TA, Senden JM, Gijsen AP, et al. Sodium nitrate co‐ingestion with protein does not augment postprandial muscle protein synthesis rates in older, type 2 diabetes patients. Am J Physiol Endocrinol Metab 2016;311:E325–E334. [DOI] [PubMed] [Google Scholar]
- 69. Hughes WE, Kruse NT, Ueda K, Feider AJ, Hanada S, Bock JM, et al. Dietary nitrate does not acutely enhance skeletal muscle blood flow and vasodilation in the lower limbs of older adults during single‐limb exercise. Eur J Appl Physiol 2020;120:1357–1369. [DOI] [PubMed] [Google Scholar]
- 70. Mitchell WK, Phillips BE, Wilkinson DJ, Williams JP, Rankin D, Lund JN, et al. Supplementing essential amino acids with the nitric oxide precursor, l‐arginine, enhances skeletal muscle perfusion without impacting anabolism in older men. Clin Nutr 2017;36:1573–1579. [DOI] [PubMed] [Google Scholar]
- 71. Sandoo A, van Zanten JJ, Metsios GS, Carroll D, Kitas GD. The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J 2010;4:302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 2011;120:357–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vallance P, Collier J, Moncada S. Effects of endothelium‐derived nitric oxide on peripheral arteriolar tone in man. Lancet 1989;2:997–1000. [DOI] [PubMed] [Google Scholar]
- 74. Davies PF. Flow‐mediated endothelial mechanotransduction. Physiol Rev 1995;75:519–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Incalza MA, D'Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol 2018;100:1–19. [DOI] [PubMed] [Google Scholar]
- 76. Ungvari Z, Tarantini S, Kiss T, Wren JD, Giles CB, Griffin CT, et al. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol 2018;15:555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tejero J, Shiva S, Gladwin MT. Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiol Rev 2019;99:311–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Widlansky ME, Gokce N, Keaney JF Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol 2003;42:1149–1160. [DOI] [PubMed] [Google Scholar]
- 79. Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004;109:III27–III32. [DOI] [PubMed] [Google Scholar]
- 80. Brandes RP. Endothelial dysfunction and hypertension. Hypertension 2014;64:924–928. [DOI] [PubMed] [Google Scholar]
- 81. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation 2003;107:139–146. [DOI] [PubMed] [Google Scholar]
- 82. Ungvari Z, Tucsek Z, Sosnowska D, Toth P, Gautam T, Podlutsky A, et al. Aging‐induced dysregulation of dicer1‐dependent microRNA expression impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci 2013;68:877–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ziche M, Morbidelli L. Nitric oxide and angiogenesis. J Neurooncol 2000;50:139–148. [DOI] [PubMed] [Google Scholar]
- 84. de Nucci G, Thomas R, D'Orleans‐Juste P, Antunes E, Walder C, Warner TD, et al. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium‐derived relaxing factor. Proc Natl Acad Sci U S A 1988;85:9797–9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Haynes WG. Endothelins as regulators of vascular tone in man. Clin Sci (Lond) 1995;88:509–517. [DOI] [PubMed] [Google Scholar]
- 86. Shemyakin A, Salehzadeh F, Bohm F, Al‐Khalili L, Gonon A, Wagner H, et al. Regulation of glucose uptake by endothelin‐1 in human skeletal muscle in vivo and in vitro. J Clin Endocrinol Metab 2010;95:2359–2366. [DOI] [PubMed] [Google Scholar]
- 87. Thijssen DH, Rongen GA, van Dijk A, Smits P, Hopman MT. Enhanced endothelin‐1‐mediated leg vascular tone in healthy older subjects. J Appl Physiol 1985;2007:852–857. [DOI] [PubMed] [Google Scholar]
- 88. Tokunaga O, Fan J, Watanabe T, Kobayashi M, Kumazaki T, Mitsui Y. Endothelin. Immunohistologic localization in aorta and biosynthesis by cultured human aortic endothelial cells. Lab Invest 1992;67:210–217. [PubMed] [Google Scholar]
- 89. Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin‐1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension 2007;50:403–409. [DOI] [PubMed] [Google Scholar]
- 90. Jiang ZY, Zhou QL, Chatterjee A, Feener EP, Myers MG Jr, White MF, et al. Endothelin‐1 modulates insulin signaling through phosphatidylinositol 3‐kinase pathway in vascular smooth muscle cells. Diabetes 1999;48:1120–1130. [DOI] [PubMed] [Google Scholar]
- 91. Thorin E, Webb DJ. Endothelium‐derived endothelin‐1. Pflugers Arch 2010;459:951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mather KJ, Lteif A, Steinberg HO, Baron AD. Interactions between endothelin and nitric oxide in the regulation of vascular tone in obesity and diabetes. Diabetes 2004;53:2060–2066. [DOI] [PubMed] [Google Scholar]
- 93. Mather KJ, Mirzamohammadi B, Lteif A, Steinberg HO, Baron AD. Endothelin contributes to basal vascular tone and endothelial dysfunction in human obesity and type 2 diabetes. Diabetes 2002;51:3517–3523. [DOI] [PubMed] [Google Scholar]
- 94. Cavalcante JL, Lima JA, Redheuil A, Al‐Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol 2011;57:1511–1522. [DOI] [PubMed] [Google Scholar]
- 95. Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end‐organ damage. J Appl Physiol 1985;2008:1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kim HL, Kim SH. Pulse wave velocity in atherosclerosis. Front Cardiovasc Med 2019;6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sugawara J, Hayashi K, Tanaka H. Distal shift of arterial pressure wave reflection sites with aging. Hypertension 2010;56:920–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility–Reykjavik study. Brain 2011;134:3398–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Fesler P, Safar ME, du Cailar G, Ribstein J, Mimran A. Pulse pressure is an independent determinant of renal function decline during treatment of essential hypertension. J Hypertens 2007;25:1915–1920. [DOI] [PubMed] [Google Scholar]
- 100. Cooper LL, Musani SK, Washington F, Moore J, Tripathi A, Tsao CW, et al. Relations of microvascular function, cardiovascular disease risk factors, and aortic stiffness in blacks: the Jackson Heart Study. J Am Heart Assoc 2018;7:e009515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, et al. Cross‐sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation 2005;112:3722–3728. [DOI] [PubMed] [Google Scholar]
- 102. Breen L, Stokes KA, Churchward‐Venne TA, Moore DR, Baker SK, Smith K, et al. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab 2013;98:2604–2612. [DOI] [PubMed] [Google Scholar]
- 103. Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 2007;297:1772–1774. [DOI] [PubMed] [Google Scholar]
- 104. McGlory C, von Allmen MT, Stokes T, Morton RW, Hector AJ, Lago BA, et al. Failed recovery of glycemic control and myofibrillar protein synthesis with 2 wk of physical inactivity in overweight, prediabetic older adults. J Gerontol A Biol Sci Med Sci 2018;73:1070–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Abrigo J, Elorza AA, Riedel CA, Vilos C, Simon F, Cabrera D, et al. Role of oxidative stress as key regulator of muscle wasting during cachexia. Oxid Med Cell Longev 2018;2018:2063179–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Meng SJ, Yu LJ. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci 2010;11:1509–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Jeon YK, Shin MJ, Saini SK, Custodero C, Aggarwal M, Anton SD, et al. Vascular dysfunction as a potential culprit of sarcopenia. Exp Gerontol 2021;145:111220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Covas MI, Elosua R, Fito M, Alcantara M, Coca L, Marrugat J. Relationship between physical activity and oxidative stress biomarkers in women. Med Sci Sports Exerc 2002;34:814–819. [DOI] [PubMed] [Google Scholar]
- 109. Hurley DM, Williams ER, Cross JM, Riedinger BR, Meyer RA, Abela GS, et al. Aerobic exercise improves microvascular function in older adults. Med Sci Sports Exerc 2019;51:773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Streese L, Guerini C, Buhlmayer L, Lona G, Hauser C, Bade S, et al. Physical activity and exercise improve retinal microvascular health as a biomarker of cardiovascular risk: a systematic review. Atherosclerosis 2020;315:33–42. [DOI] [PubMed] [Google Scholar]


