Abstract
Background
The role of Numb, a protein that is important for cell fate and development and that, in human muscle, is expressed at reduced levels with advanced age, was investigated; adult mice skeletal muscle and its localization and function within myofibres were determined.
Methods
Numb expression was evaluated by western blot. Numb localization was determined by confocal microscopy. The effects of conditional knock out (cKO) of Numb and the closely related gene Numb‐like in skeletal muscle fibres were evaluated by in situ physiology, transmission and focused ion beam scanning electron microscopy, three‐dimensional reconstruction of mitochondria, lipidomics, and bulk RNA sequencing. Additional studies using primary mouse myotubes investigated the effects of Numb knockdown on cell fusion, mitochondrial function, and calcium transients.
Results
Numb protein expression was reduced by ~70% (P < 0.01) at 24 as compared with 3 months of age in gastrocnemius and tibialis anterior muscle. Numb was localized within muscle fibres as bands traversing fibres at regularly spaced intervals in close proximity to dihydropyridine receptors. The cKO of Numb and Numb‐like reduced specific tetanic force by 36% (P < 0.01), altered mitochondrial spatial relationships to sarcomeric structures, increased Z‐line spacing by 30% (P < 0.0001), perturbed sarcoplasmic reticulum organization and reduced mitochondrial volume by over 80% (P < 0.01). Only six genes were differentially expressed in cKO mice: Itga4, Sema7a, Irgm2, Vezf1, Mib1, and Tmem132a. Several lipid mediators derived from polyunsaturated fatty acids through lipoxygenases were up‐regulated in Numb cKO skeletal muscle: 12‐HEPE was increased by ~250% (P < 0.05) and 17,18‐EpETE by ~240% (P < 0.05). In mouse primary myotubes, Numb knockdown reduced cell fusion (~20%, P < 0.01) and delayed the caffeine‐induced rise in cytosolic calcium concentrations by more than 100% (P < 0.01).
Conclusions
These findings implicate Numb as a critical factor in skeletal muscle structure and function and suggest that Numb is critical for calcium release. We therefore speculate that Numb plays critical roles in excitation–contraction coupling, one of the putative targets of aged skeletal muscles. These findings provide new insights into the molecular underpinnings of the loss of muscle function observed with sarcopenia.
Keywords: Ageing, Sarcopenia, Excitation contraction coupling, Sarcomere, Mitochondria
Introduction
Ageing is associated with sarcopenia, a condition associated with deleterious changes in skeletal muscle that include progressive loss of muscle mass and reduced muscle quality reflected as impaired force generation and power. 1 Molecular mechanisms responsible for age‐related loss of muscle quality remain incompletely understood. Mounting evidence supports a role for decreased effectiveness of excitation–contraction coupling whereby the multiprotein complex called the triad couples membrane depolarization with calcium release. 2 Deficiency in calcium regulatory components contributes greatly to reduced muscle function during ageing. 3 , 4 , 5 Reduced store‐operated calcium entry function 6 , 7 has also been implicated. Altered balance in the generation of pro‐inflammatory lipid mediators (LMs) and anti‐inflammatory LMs during ageing leads to low‐grade systemic inflammation that may also contribute to the development of various age‐related disorders such as sarcopenia. 8
Determinants of calcium release during excitation–contraction coupling remain incompletely understood. Studies in cell culture suggest that one regulator of calcium release is the adaptor protein Numb. 9 Numb mRNA expression was reduced in muscle of older individuals, 10 raising questions regarding the role of reduced Numb expression in the pathogenesis of ageing‐related decline of muscle strength and power. Numb is a highly conserved protein expressed in all higher organisms. 11 A closely related gene, Numb‐like (NumbL), has also been identified. 11 Functions of Numb and NumbL overlap, but they are not fully redundant. 12 Numb has roles in development, cell fate commitment, termination of Notch, and Sonic Hedgehog signalling, 13 , 14 mitochondrial fission in kidney 15 and proliferation of skeletal muscle satellite cells. 16
The possibility that Numb could contribute to muscle function beyond its role in Pax7+ satellite cell biology has not been evaluated. We report that Numb is concentrated within skeletal muscle fibres of adult mice where its expression is required for optimal muscle force production, sarcomere structure and morphology of intermyofibrillar mitochondria. Studies with primary cultures of mouse myotubes link Numb to myoblast fusion, mitochondrial function, and calcium release from intracellular stores. Underlying mechanisms were explored using RNA sequencing and lipidomics profiling focused on signalling lipids formed from n‐3 and n‐6 polyunsaturated fatty acids (PUFA).
Methods
Animals
C57BL/6N mice used for determination of changes in Numb protein levels were obtained from the NIA Aged Rodent Colonies and euthanized at the ages indicated. C57BL/6 mice used for immunofluorescence studies were from Charles River Laboratories. Mice carrying a floxed allele for the Numb (fl‐Numb) and Numb‐like (fl‐NumbL) genes 17 were obtained from Jackson Laboratories (Bar Harbor, ME) (Numb tm1Zili NumbL tm1Zili /J, stock # 005384). The fl‐Numb and fl‐NumbL lines were heterozygous for one or both alleles at the time they were received and were crossed until homozygous for both floxed Numb and floxed NumbL; these animals are designated Numbf/f/NumbLf/f. HSA‐MCM mice, which express a mutated‐oestrogen receptor‐Cre recombinase (Mer‐Cre‐Mer) double fusion protein under the control of the human actin alpha‐1 (skeletal muscle) promoter, 18 were a generous gift from Dr. Karyn Esser, University of Florida. HSA‐MCM mice were crossed with Numbf/f/NumbLf/f mice through multiple rounds of breeding to generate HSA‐MCM/Numbf/f/NumbLf/f mice. Mice were genotyped using genomic DNA isolated from tail or ear snips. The primers for the floxed Numb allele were as described 17 while the following primers were used for the floxed NumbL allele: 5′GAGTTTCCGTACATGCTTTGGG and 5′GGAGACCTTCTCAATGGTCTGG. Cre genotyping was performed as previously described. 19 The study protocol was approved by the James J. Peters VA Medical Center Institutional Animal Care and Use Committee. All animal procedures were conducted in accordance with the requirements of Guide for the Care and Animal Use of Laboratory Animals.
Tamoxifen treatment
HSA‐MCM Numbf/f/NumbLfl/f mice were injected intraperitoneally with 0.2 mL of tamoxifen solution (10 mg/mL in peanut oil/5% ethanol) or vehicle for five consecutive days and weekly thereafter beginning on Day 10 after the first injection until they were sacrificed at 56 days after the first injection. These mice are referred to as Numb/NumbL cKO and vehicle‐treated controls, respectively. cKO, Numbf/f/NumbLfl/f mice treated with tamoxifen or vehicle were used as tamoxifen‐treated or vehicle‐treated genotype controls, respectively. Mice were 4 to 8 months of age at the time of the first injection of vehicle or tamoxifen.
Statistical analysis
Data are shown as mean value ± standard deviation (SD). Data were analysed by analysis of variance with post hoc Tukey honest significant difference test or by a two‐tailed unpaired t‐test. The statistical analyses were performed with OriginPro 2020 (Figures 5, 6 and S12) or Graph Pad Prism 9.0 (all other figures). A value for P < 0.05 was considered significant.
Figure 5.

Numb/NumbL KO leads to significant alterations in the levels of key lipid signalling mediators associated with the lipoxygenase pathway in gastrocnemius muscles. (A) 12‐HEPE; (B) 17,18‐EpETE; (C) 12‐HETE; (D) LTB4; (E) 13‐KODE; (F) AEA. Data are shown as mean ± SD, n = 5 for each group, two‐tailed Student's t‐test was applied for mean comparison with vehicle (Veh), *P < 0.05. Tam, tamoxifen; Veh, vehicle; (G) the most altered signalling LMs in Numb/NumbL cKO mice are derived from the 5‐, 12‐, or 15‐lipoxygenase (LOX) pathways. 13‐HODE, 13‐hydroxyoctadecadienoic acid; 13‐KODE, 13‐oxo‐9,11‐octadecadienoic acid; AA, arachidonic acid; AEA, N‐arachidonoylethanolamine; Ca‐NAT, Ca2+‐dependent N‐acyltransferase; EPA, eicosapentaenoic acid; FAAH, fatty acid amide hydrolase; HEPEs, hydroxyeicosapentaenoic acids; HETEs, hydroxyeicosatetraenoic acids; LA, linoleic acid; LTs, leukotrienes; NAPE‐PLD, N‐acyl‐phosphatidylethanolamine‐specific phospholipase D; NArPE, N‐arachidonoyl phosphatidylethanolamine. AEA is biosynthesized from membrane phospholipids through the action of a Ca2+‐dependent N‐acyltransferase (Ca‐NAT) and then of N‐acyl‐phosphatidylethanolamine‐specific phospholipase D (NAPE‐PLD). AEA is subsequently degraded by fatty acid amide hydrolase (FAAH) to generate AA.
Figure 6.

Acute knockdown of Numb negatively impacted mitochondrial function. (A) Less energetic phenotype resulted from knockdown of Numb, (B) oxygen consumption rate, (C) extracellular acidification rate, (D) overall profiling of mitochondrial functions determined by a seahorse mitochondrial stress kit, (E) non‐mitochondrial oxygen consumption, (F) basal respiration, (G) maximal respiration, (H) spare capacity, and (I) ATP production. Data were normalized relative to total DNA levels. Shown are results from three independent experiments. Data are shown as mean ± SD. Two‐tailed unpaired t‐test was used for comparisons, *P < 0.05.
Results
Numb and NumbL expression and localization in muscle
Numb expression was investigated in skeletal muscle by western blot using gastrocnemius (GS), tibialis anterior (TA) and soleus muscles isolated from 3‐month‐old and 24‐month‐old C57BL/6N mice. Significantly lower levels of Numb protein were found in GS and TA from 24‐month‐old mice when compared with 3‐month‐old mice; in soleus, Numb expression was numerically reduced in 24‐month‐old mice, but the difference did not reach statistical significance (Supporting Information, Figure S1). These data suggest an age‐dependent decrease in the expression of Numb protein in mouse skeletal muscle. NumbL expression could be detected by qPCR and was approximately 5‐fold to 10‐fold lower than that of Numb (Figure S2).
To determine the localization of Numb in skeletal muscle, sections of mouse GS muscle from C57B6 mice were immunostained for Numb and dystrophin. Intense Numb staining was observed within myofibres where it was organized in distinct bands that traversed the myofibre at regular intervals (Figure 1A–C). A similar pattern of Numb distribution was observed by immunostaining of muscle fibres isolated from mouse hindlimb muscles (Figure 1D). Numb immunostaining did not colocalize with the Z‐line protein α‐actinin but showed a precisely ordered relationship to it indicating a spatial relationship of Numb to the sarcomere (Figure 1E). Numb appeared to be in close proximity to, although not overlapping with, the dihydropyridine receptor (DHPR) (Figure 1F).
Figure 1.

Numb protein localizes to the sarcomere of skeletal muscles. (A–C) Representative longitudinal sections of mouse gastrocnemius muscle immunostained for dystrophin (green, A) and Numb (red, B); the merged image is shown in panel (C). (D–F) Z‐stacks of confocal images of isolated muscle fibres stained for Numb (green) and with: dystrophin (D); actinin (E), and dihydropyridine receptor (DHPR) (F). The insert in (F) shows a higher magnification of the Numb/DHPR stained fibre. Nuclei (blue) are labelled with DAPI in every panel. Scale bar: 10 μm; 5 μm for the insert in (F).
Generation and characteristics of muscle‐specific inducible Numb conditional knock out
To understand the physiological role(s) of Numb in skeletal muscle fibres, mice in which Numb expression was conditionally and inducibly knocked out in muscle fibres were generated. Because NumbL may substitute for Numb in some biological contexts and might be up‐regulated after knocking out Numb, the conditional, inducible knockout was designed to inactivate both genes. Mice expressing a chimeric Cre recombinase sandwiched between two copies of a mutated‐oestrogen receptor ligand binding domain (MCM) under the control of a fragment of the human skeletal actin promoter (HSA‐MCM) 18 were crossed with mice homozygous for floxed Numb and NumbL alleles (Numbf/f/NumbLf/f). 17 The resulting HSA‐MCM Numbf/f/NumbLf/f mice were treated with tamoxifen (Numb/NumbL cKO) or vehicle for 5 days and then once weekly until the time of euthanasia at 56 days to prevent Numb re‐accumulation. This time point for examining effects of Numb knockdown was chosen to model effects related to the sustained reduction of Numb expression that occurs during ageing. After tamoxifen treatment, Numb protein levels were reduced in GS, TA, and soleus muscle by over 70% (Figure 2A–B) as compared with vehicle‐treated mice.
Figure 2.
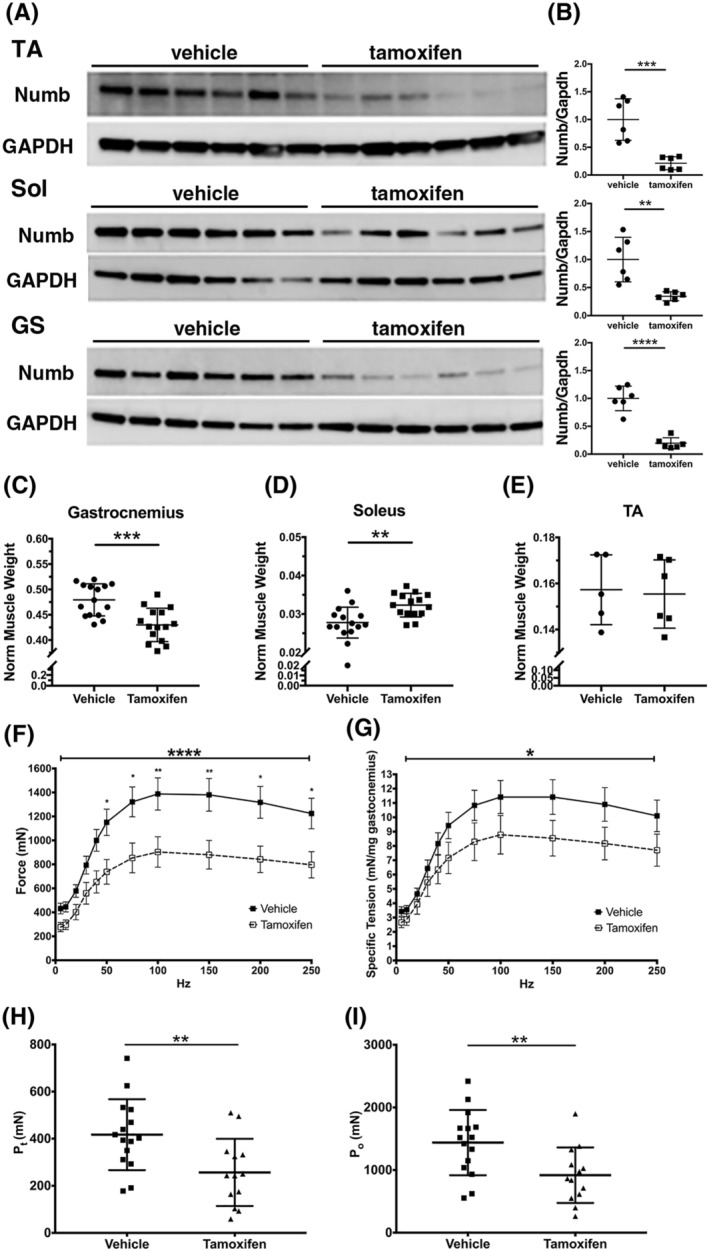
Numb/NumbL‐specific knockdown in skeletal muscle leads to drastic decrease in contractile muscle force. HSA‐MCM Numbfl/fl/NumbLfl/fl mice were treated with vehicle or tamoxifen and sacrificed 56 days post‐induction. (A) Western blot analysis showing reduced levels of Numb protein in tibialis anterior (TA), soleus (Sol), and gastrocnemius (GS) muscles (N = 6 per group). (B) Quantitation of blots shown in (A). Numb levels were normalized to GAPDH (loading control) and expressed relative to the mean level of Numb in vehicle‐treated samples (N = 6 per group). (C–E) Normalized muscle weights; wet muscle weight at time of euthanasia was normalized to pre‐induction body weight (N = 15 per group). (F–I) Force–frequency (F) and specific force–frequency (G) relationships during in situ physiological testing of the gastrocnemius muscle; (H) peak twitch force (Pt); (I) tetanic force (Po); N = 15 for vehicle and 13 for tamoxifen‐treated mice. Statistical analysis was performed with repeated measure one‐way analysis of variance with post hoc Tukey honestly significant difference test (F–G) (force–frequency, F = 4.753, DFn 10, DFd 260, drug × frequency interaction P < 0.0001; specific tension force–frequency, F = 2.150, DFn 10, DFd260, drug × frequency interaction P = 0.0213) or two‐tailed unpaired t‐tests (all other panels). ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05.
Body weights of Numb/NumbL cKO mice were not significantly different from vehicle‐treated controls and tamoxifen did not alter body weights of genotype controls (Numbf/f/Numbf/f; Figure S3A and S3F). Individual muscle weights of Numb/NumbL cKO showed a small decrease for GS (Figure 2C), plantaris, biceps, and triceps (Figure S3B, D, and E), while no significant changes were observed for TA (Figure 2E) or EDL (Figure S3C). Soleus showed a small increase in weight (Figure 2D), possibly representing compensatory hypertrophy due to weakening of GS and plantaris. Administration of tamoxifen to genotype control Numbf/f/NumbLf/f mice did not alter muscle weights, with the exception of a small decrease in plantaris weight (Figure S3G–L).
Numb/NumbL cKO alters muscle contractile properties
The effect of Numb/NumbL cKO on GS muscle contractile properties was evaluated by in situ physiology. Compared with vehicle‐treated animals, Numb/NumbL cKO mice demonstrated significantly reduced absolute and specific force (mN/mg muscle) for both peak twitch and peak tetanic force (Figure 2F–I), while muscle fatigue, time to peak tension, and half‐relaxation time were not significantly altered (Figure S5A–C). No difference was observed between Numb/NumbL cKO male and female mice (Figure S4). Administration of tamoxifen to Numbf/f/Numbf/f genotype controls did not alter muscle contractile properties based on analysis of force–frequency curves (Figure S6).
Numb/NumbL cKO did not alter muscle cross‐sectional area or fibre types
Analysis of cross‐sectional area (CSA) of TA muscle showed no significant difference between Numb/NumbL cKO animals and vehicle‐treated mice (Figure S7A–C). Analysis of fibre‐type composition of TA muscles with antibodies against myosin heavy chain isoforms did not show any significant difference between Numb/NumbL cKO mice and vehicle injected mice (Figure S7D–H).
To exclude any tamoxifen effect that was independent of the Numb/NumbL knockout, muscle fibre CSA, distribution, and type were determined in TA muscles derived from Numbf/f/NumbLf/f mice treated as above. CSA was slightly (~3%) although significantly increased in tamoxifen‐treated mice as compared with vehicle‐treated controls (Figure S8A–C), while fibre‐type composition was not altered by tamoxifen administration (Figure S8D–E).
Numb/NumbL cKO perturbs muscle ultrastructure
To gain insights as to why muscle force production was reduced by Numb/NumbL cKO, muscle ultrastructure was examined in TA muscle by transmission electron microscopy. This analysis revealed multiple ultrastructural alterations in Numb/NumbL cKO mice (Figure 3B) as compared with controls (Figure 3A). Frequent discontinuities of Z‐discs were observed (Figure 3B, Arrow 1). Mitochondria were smaller and had lost their normal spatial organization relative to the sarcomere (Figure 3B, Arrow 2). Sarcoplasmic reticulum was disorganized with increased spacing between the linear clusters of sarcoplasmic reticulum interspersed between sarcomeres on longitudinal sections (Figure 3B, Arrow 3). Distances between Z‐lines were longer in TA muscle from Numb/NumbL cKO mice (Figure 3B, Arrow 4 and Figure 3E). The area represented by mitochondria as a fraction of total area was reduced in Numb/NumbL cKO mice (Figure 3F). Tamoxifen had no effect on the ultrastructure of TA muscle from Numbf/f/NumbLf/f mice (Figure S9).
Figure 3.

Numb knockout resulted in marked ultrastructural changes in skeletal muscle. Transmission electron microscopy was performed on longitudinal sections of mouse tibialis anterior muscle at ×5300. Representative images for HSA‐MCM/Numbf/f/NumbLf/f mice treated with vehicle (A) or tamoxifen (B) are shown. Arrows indicate (1) the positions of Z‐lines, (2) sarcoplasmic reticulum, (3) intermyofibrillar mitochondria, and (4) Z‐disc spacing. Shown between (A) and (B) are enlarged images of selected areas (box in panels A–B). (E–F) Quantitation of the distance between Z‐discs (E) and of mitochondria area/total area (F) (N = 3 per group). (C–D) Three‐dimensional reconstruction of mitochondria in TA muscle of control (C) and Numb/NumbL cKO mice (D). Shapes outlined in blue represent Z‐discs; solid shapes represent individual mitochondria. (G–H) Quantitation of maximum length (G) and total meshed volume (H) of mitochondria in (C–D) (N= 4 animals per group). Scale bars, 1 μm. *P < 0.05, **P < 0.01, two‐tailed unpaired t‐test.
To more closely examine changes in mitochondria, TA muscle from Numb/NumbL cKO and control were examined by focused ion beam scanning electron microscopy. Residual bodies were frequently observed in muscle from Numb/NumbL cKO mice (Figure S10) but not in controls. Three‐dimensional reconstruction of mitochondria showed that mitochondria in Numb/NumbL cKO mice were smaller than those in controls, typically did not cross the Z‐disc, and had less complex shapes (Figure 3D; Movies S1 and S2). Volume and length of mitochondria were reduced in Numb/NumbL cKO muscle (Figure 3G–H).
Numb/NumbL cKO alters the expression of a very select group of genes
To dissect potential molecular mechanisms by which Numb/NumbL cKO regulates muscle function, RNA sequencing was performed using RNA from GS muscle from tamoxifen or vehicle‐treated HSA‐MCM/Numbf/f/NumbLf/f male and female mice. RNA from GS muscle from male and female Numbf/f/NumbLf/f (control genotype) mice treated as above was also sequenced to serve as a drug treatment control. Four hundred and seven differentially expressed genes (DEGs) were identified in female HSA‐MCM/Numbf/f/NumbLf/f muscle after tamoxifen treatment (Table S1), while only 35 DEGs were found in tamoxifen‐treated females of the control genotype (Table S2). In male Numb/NumbL cKO mice, 187 genes were differentially regulated (Table S3), while 705 DEG were observed in tamoxifen‐treated male genotype control mice (Table S4 and Figure S11). After removing those genes altered by tamoxifen in genotype controls, 389 DEGs were identified in female Numb/NumbL cKO muscle (Figure 4A), (212 up‐regulated and 177 down‐regulated), while only 68 DEG were identified in male mice (Figure 4B) (39 down‐regulated and 29 up‐regulated).
Figure 4.
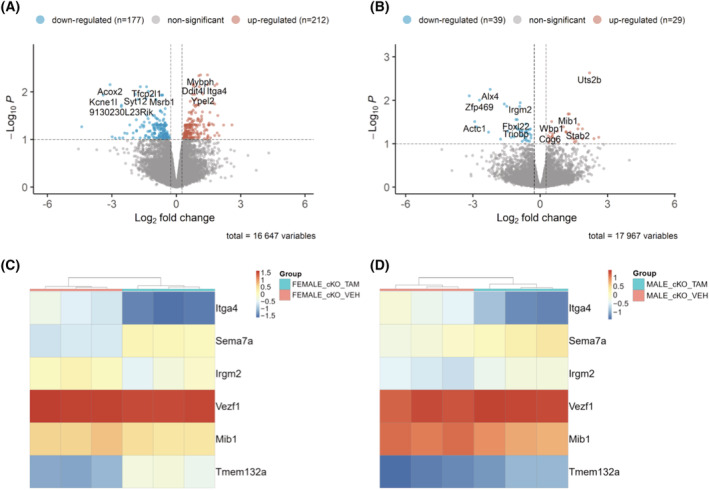
RNA sequencing identified genes that are differentially expressed after Numb/NumbL cKO. (A–B) Volcano plots showing mRNA expression levels for genes with fold‐changes greater than 1.2 and P values adjusted for FDR of less than 0.1 (A, females, B males). Differentially expressed genes are shown as light brown spots (up‐regulated) or light blue spots (down‐regulated). All other genes detected are shown as grey dots. Shown at the top of each panel are the values for numbers of up‐regulated and down‐regulated genes after removal of genes altered in tamoxifen‐treated genotype control female and male mice, respectively. The total number of transcripts analysed is shown under the X‐axis as total variables; (C–D) Heatmaps showing relative expression of genes altered in both female (C) and male (D) Numb/NumbL cKO mice.
Six genes were differentially regulated in both male and female Numb/NumbL cKO (Figure 4C and 4D and Table S5). Heatmaps showed that expression patterns for each of these genes were similar within each group (Figure 4C and 4D). Two genes were down‐regulated in both male and female mice: Sema7a, a cytoplasmic membrane protein involved in integrin signalling and Tmem132a, a gene with poorly understood function (Figure 4C and 4D). Three genes were up‐regulated in both male and female mice: Itga4, an α‐integrin subunit; Mib1, an E3 ubiquitin ligase that promotes internalization and activation of Notch receptors; and VezF1, a transcription factor that may be involved in angiogenesis and development (Figure 4C and 4D). Irgm2, a GTPase implicated in innate immune function, was up‐regulated in female mice but down‐regulated in male mice (Figure 4C and 4D).
Alterations in lipid mediator levels occur after Numb/NumbL cKO
Accumulating evidence links LM synthesized by cyclooxygenase and by 5, 12, or 15 lipoxygenases (LOX) in regulation of skeletal muscle mass and function. 8 , 20 , 21 To understand how Numb/NumbL cKO influenced levels of LM, levels of 158 LMs, primarily derived from omega‐6 and omega‐3 PUFAs including arachidonic acid (AA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), were determined using LC/MS. Forty‐eight LMs were detected in all samples in all groups. The change in tissue levels of each lipid mediator was calculated by dividing its relative area [(peak area of analyte/peak area of Internal Standard) in the LC chromatogram] by the average of that measured in vehicle‐treated Numbf/f/NumbLf/f mice. Normalized levels for these LMs are shown in Table S6. In Numb/NumbL cKO mice, there was a significant increase in the levels of 12‐hydroxyeicosapentaenoic acid (12‐HEPE, P = 0.035), 17,18‐epoxyeicosatetraenoic acid (17,18‐EpETE, P = 0.041), 12‐hydroxyeicosatetraenoic acid (12‐HETE, P = 0.024), leukotriene B4 (LTB4, P = 0.090), 13‐oxo‐9,11‐octadecadienoic acid (13‐KODE, P = 0.032), and N‐arachidonoylethanolamine (AEA, P = 0.032) (Figure 5A–F). Tamoxifen did not change levels of these LMs in Numb(f/f)/NumbL(f/f) controls (Figure 5A–F). Of note, 12‐HEPE, 12‐HETE, LTB4, and 13‐KODE are derived from AA, linoleic acid (LA), and EPA, respectively, via LOX‐mediated pathways (Figure 5G). These data suggest a role for changes in levels of these LMs in phenotypes of Numb/NumbL cKO mice.
Acute Numb KO reduces fusion of mouse primary myoblasts in vitro
To further investigate the mechanism of Numb on the functions of skeletal muscle, mouse primary myoblasts were treated with a vivo‐morpholino against Numb. Treatment with Numb morpholino for 48 h led to approximately 30% reduction in Numb protein levels (Figure S12A–B) and 15% reduction in fusion index (Figure S12C–D), suggesting that Numb plays an important role in myoblast fusion.
Acute Numb knockdown reduces mitochondrial functional capacity of mouse primary myoblasts
To specifically determine whether the physiological and ultrastructural changes in the cKO mice could be associated with mitochondrial dysfunction, mitochondrial function was compared between control and Numb morpholino‐treated primary mouse myotubes using the Seahorse energy phenotype test kit and mitochondrial stress test kit. Compared with control, the Numb‐knockdown group had lower mitochondrial and glycolytic energy production although the balance between the two was preserved (Figure 6A). In Numb‐knockdown myotubes, oxygen consumption rate (Figure 6B) and extracellular acidification rate (Figure 6C) were decreased at both baseline and when mitochondria were stressed. Following the phenotype test, the mitochondrial stress test was used to determine the effect of Numb knockdown on mitochondrial respiration. Numb knockdown reduced basal respiration (Figure 6F), maximal respiration (Figure 6G), spare capacity (Figure 6H), and ATP‐linked respiration (Figure 6I) but did not alter non‐mitochondrial oxygen consumption (Figure 6E).
Effect of Numb on cytosolic calcium transients
Because of the importance of intracellular calcium homeostasis to the control of muscle function, and the prominent role of excitation–contraction coupling in muscle contractile force generation, one explanation for some of our findings in the Numb/NumbL cKO mice would be a decreased release of calcium ions from sarcoplasmic reticulum stores during excitation–contraction coupling. To assess the role of Numb in cytosolic calcium homeostasis, caffeine‐induced calcium transients were measured in cultured mouse primary myotubes treated with a Numb‐targeted vivo‐morpholino to down‐regulate this gene. Control cells were treated with a biologically inactive, control vivo‐morpholino. In cells treated with the control morpholino, the baseline Fura‐2 signal was stable; addition of 20 mM caffeine to cultures stimulated a robust increase in fluorescence (Figure 7A). In contrast, in myotubes treated with a Numb‐targeting vivo‐morpholino, the development of spontaneous calcium release events was observed as small calcium oscillations as if calcium‐induced calcium release (CICR) was overactive in these fibres. Moreover, the caffeine‐induced calcium release transient observed in Numb‐knockdown cells appeared to be reduced by more than 50% (Figure 7A) and the time taken for responding to caffeine stimulation (time to peak) was significantly prolonged by Numb knockdown (Figure 7A–B).
Figure 7.

Numb KO elicits spontaneous CICR events and reduces caffeine‐induced SR Ca2+ release. Primary myoblasts were treated with either a control or Numb‐targeting vivo‐morpholino. The cells were loaded with Fura‐2AM, and intracellular calcium transients induced by 20 mM caffeine were monitored based on F350/F375 readings. (A) Representative tracings of F350/F375 over time for individual wells of cells treated with either Numb morpholino or control morpholino. (B) Time to peak F350/F375 was measured. Twenty to 30 myotubes were tested for each group. Two‐tailed unpaired t‐test was applied for comparison with the control, **P < 0.01. Data are from three to four independent experiments.
Discussion
The major conclusions supported by our study are that loss of Numb from skeletal muscle fibres results in significant loss of the capacity of muscle to generate force and that Numb protein levels decline with advancing age in mouse GS muscle. When coupled with prior observations that levels of Numb mRNA were reduced in muscle biopsy samples taken from men 60–75 years old, 10 these data strongly support a causal linkage between reductions in Numb protein levels in skeletal muscle and sarcopenia‐related loss of muscle force generating capability. While a role for NumbL in the physiological and ultrastructural phenotype observed in the Numb/NumbL cKO mice cannot be excluded, it seems unlikely that NumbL participates in function or homeostasis of healthy myofibres given the comparatively low expression of NumbL mRNA in adult skeletal muscle. This conclusion would be consistent with prior reports that mice carrying a loss‐of‐function mutation of NumbL had no appreciable muscle phenotype and that the mutation did not alter tissue repair. 16 On the basis of these observations, we propose that the physiological deficits observed in the Numb/NumbL knockout mice studied herein resulted essentially from decreased levels of Numb in muscle fibres.
Immunohistochemistry localized Numb close to DHPR, a component of the triad, suggesting spatial and functional relationships between Numb and the function of the triad in the release calcium from SR stores as part of excitation–contraction coupling. Some support for this interpretation stems from the finding that knockdown of Numb in primary mouse myotubes reduced release of calcium from intracellular stores as determined by the caffeine‐induced calcium release assay. The observation that in mouse primary myotubes, Numb‐knockdown results in spontaneous and transient rises in cytosolic calcium that is intriguingly similar to self‐pacing calcium transients in beating cardiac myocytes, suggest that Numb knockdown could alter the regulation of CICR via triads, which is normally tightly controlled by membrane potential, and is therefore under the control of voltage and membrane depolarization and is not normally spontaneously triggered as it is in the heart. 22 , 23
Our data do not provide information as to the mechanism by which Numb regulates appropriate calcium release from the SR, but the localization of Numb near the DHPR supports several possibilities. Numb may bind to one or more triad proteins to change their capacity to release calcium from SR stores; alternatively, the altered mitochondrial function observed in myotubes in which Numb expression was reduced may increase release of reactive oxygen species (ROS) which are deleterious to ryanodine receptor function. 3 Because caffeine acts via CICR to release calcium from the SR and enhanced spontaneous CICR was observed as if these muscles behaved like cardiac myocytes, it is also possible that the sensitivity of RyR‐1 to CICR is significantly shifted to the left (i.e. super or hypersensitivity to calcium). In other words, it is possible that in these muscle cells, even at very low intracellular calcium levels, the RyR‐1 is exquisitely sensitive to calcium, triggering CICR. It is possible that the spontaneous activity could lead to a partial depletion of the SR calcium stores, which in turn could result in the reduced overall response to caffeine. There are many other possibilities, such as altered ATPase, store‐operate calcium entry function, as well as direct modifications within the contractile machinery, and an increased contribution of IP3 receptors to the oscillatory calcium pattern observed. These other possibilities were not studied here.
Lipid mediators are also regulators of calcium release from intracellular stores of C2C12 cell‐derived myotubes 24 and smooth muscle. 25 Mo et al. demonstrated that nanomolar levels of PGE2 and agonists of its signalling pathway accelerate myogenic differentiation and proliferation of C2C12 muscle cells, primary mouse, and primary human muscle cells, while concomitantly causing intracellular calcium oscillations. 8 , 21 , 23 It is thus noteworthy that Numb knockdown up‐regulates concentrations of several LMs generated by LOXs and its downstream pathways. How these changes in LMs occur is not clear, but it is notable that increased intracellular calcium concentration such as might occur due to calcium leakage from intracellular calcium storages enhances the activities of LOXs and their upstream phospholipases resulting in increased production of LMs, which could further exaggerate dysregulation of cytosolic calcium, and eventually contribute to the development of muscle weakness.
The elevations in LMs in muscle of mice with Numb/NumbL knockouts may have broader implications. Potent signalling lipids synthesized by oxidation of the PUFA precursors through cyclooxygenase and LOX are implicated in regulating skeletal muscle mass and function. 8 , 20 , 21 Most ω‐3 PUFA (EPA, DHA, etc.) metabolites are anti‐inflammatory while those derived from ω‐6 PUFA (e.g. AA and LA) are considered to be pro‐inflammatory. 26 The enzymes 5/12/15 LOX play a significant role in age‐related muscle atrophy by different mechanisms such as regulating inflammation, protein degradation, 27 and ROS generation. 28 In our lipidomics studies, the GS muscles of Numb/NumbL cKO mice demonstrated significantly elevated levels for LTB4 and 12‐HETE, which are pro‐inflammatory eicosanoids derived from AA through the 5/12‐LOX pathways. Increased levels were also observed for 13‐KODE, a potent ligand for PPAR‐γ that is generated through the LA/13‐HODE pathway that is associated with ROS production. 29 One interpretation of the data is that prolonged exposure to these LMs leads to a chronic inflammatory condition and ultimately contributes to ageing‐related muscle phenotypes in the Numb/NumbL cKO mice model. It should be noted, however, that mRNA for pro‐inflammatory cytokines or chemokines were not up‐regulated in GS muscle by Numb/NumbL cKO suggesting that any pro‐inflammatory signalling elicited by depletion of Numb may be limited to increased levels of LMs such as LTB4. Anti‐inflammatory changes in LMs were also observed in muscle after Numb/NumbL cKO including increased 12‐HEPE which belongs to the ω‐3 PUFA precursor EPA pathways and is thought to attenuate inflammatory response in macrophages. 30 The increased 12‐HEPE levels in the GS of the Numb/NumbL cKO mice may be a compensatory or adaptive mechanism. Further studies are needed to determine the functional role(s) of LMs in the development of weakness due to Numb and NumbL knockouts and age‐related loss of muscle function.
Many studies have linked ageing, mitochondrial dysfunction, and sarcopenia. 31 , 32 Reasons for the reduced size of mitochondria in Numb/NumbL cKO mice are unclear, but perhaps, the accumulation of ROS and lipid damage could be a contributing factor. In addition, the accumulation of residual bodies in muscles of these mice suggests impaired quality control through mitochondrial fission and mitophagy. Analysis of cellular respiration of primary mouse myotubes depleted of Numb suggested reduced mitochondrial oxygen consumption rate, indicative of an overall decrease in mitochondrial respiration and ATP generating capacity. Of interest, it has been reported that depletion of Numb through a conditional knockout increased mitochondrial fragmentation in the kidney through Drp1, 15 a critical protein in mitochondrial fission. In contrast to results reported herein, in other studies of kidney mitochondria from cells lacking Numb, mitochondrial membrane potential and ATP generating capabilities were not altered. 15 Further studies are required to understand both the mechanisms and functional implications of the altered mitochondrial properties.
Surprisingly, the number and list DEGs were quite different between male and female genotype controls. One possible explanation is the much higher levels of oestrogens in female mice such that addition of a tamoxifen elicited minimal effects on this already activated programme of oestrogen‐regulated genes within skeletal muscle. Moreover, knockouts of Numb/NumbL in muscle fibres resulted in only six shared gene expression changes. How these gene alterations contribute to the muscle weakness observed in Numb/NumbL cKO mice is unclear. It remains possible that some of these DEGs reflect a compensatory response of skeletal muscle to the consequences of Numb/NumbL cKO. Down‐regulated genes were Sema7a and Tmem132a, neither of which has been previously implicated in ageing or muscle weakness. Sema7a is involved in immunity, axon guidance, and integrin signalling 33 and may be linked to osteoporosis. 34 Tmem132a is one of five variants of the transmembrane 132 family of proteins. Little is known regarding its biology; of interest, it was recently shown to bind WLS, a Wnt ligand stabilizing protein. Tmem132a augments WLS‐Wnt ligand interactions thereby increasing Wnt signalling. 35 Up‐regulated genes were Itga4, Mib1, and VezF1. Itga4 (integrin A4 subunit) is a cell surface adhesion molecule; it is possible that up‐regulation of Itga4 may be a compensatory mechanism to stabilize structural integrity of skeletal muscle. VezF1 is a transcriptional regulator implicated in regulating angiogenesis; VezF1 knockdown has been linked to impaired cardiac muscle contractility without alteration of Ca2+ release kinetics. 36 We are not aware of prior studies of the function of VezF1 in skeletal muscle; elevation of VezF1 in Numb/NumbL cKO mice may be an adaptive response to overcome the deleterious effects on homeostasis or contractility of the knockouts. Mib1 (Mindbomb, E3 ubiquitin protein ligase) is known to positively regulate Notch signalling by ubiquitinating Notch receptors leading to their endocytosis. Recent data have shown that Mib1 is up‐regulated in skeletal muscle satellite cells by both androgen and oestrogen dependent signalling and that absence of Mib1 in myofibres leads to depletion of satellite cells due to impaired cell cycle exit of proliferating satellite cells. 37 Mib1 has been suggested to ubiquitinate and target for proteasomal degradation other proteins such as the anti‐apoptotic protein DAPK1, although implications for muscle fibre biology remain unclear. Our findings also demonstrate the importance of including genotype control mice treated with tamoxifen or vehicle when studying effects of inducible knockouts on skeletal muscle gene expression to exclude the confounding effect of changes due to tamoxifen.
An unexpected finding was the reduced fusion index observed in myotubes formed from Numb depleted primary mouse myoblasts. The mechanisms underlying this observation are unclear. Some understanding of the roles of Numb in progenitor cells of the myogenic lineage have been gleaned using a conditional knockout under the Pax7 promoter. 16 These studies showed reduced ability of skeletal muscle to undergo repair after cardiotoxin injury associated with reduce proliferation of skeletal muscle satellite cells associated with up‐regulation of myostatin.
In summary, Numb protein levels decrease in skeletal muscle with age. Loss of Numb in skeletal muscle myofibres reduces contractile function, sarcomere length, and mitochondrial size. Impaired release of calcium from intracellular stores may contribute to poor contractile function in the absence of Numb. Altered levels of LMs result from loss of Numb and may be key drivers of some of the physiological characteristics of Numb‐deficient muscle. Changes in levels of genes encoding an integrin (Itga4), a molecule involved in integrin signalling (Sema7a), and architectural changes in Z‐lines suggest one common and potentially related structural theme in Numb‐deficient skeletal muscle fibres. Accumulation of residual bodies, small mitochondria, and prior evidence linking Numb to mitochondrial fission 15 further implicate Numb as having central roles in homeostasis of mitochondria in skeletal muscle and other tissues. A role for such perturbations in the well‐described age‐related decline in mitochondrial function is an attractive direction for future investigations.
Conflict of interest
The authors of this manuscript declare no conflict of interest or competing financial interests.
Funding
This work is supported by the Department of Veterans Affairs Rehabilitation Research and Development Service (B9212C and B2020C to W.A.B., CDA‐2 IK2RX002781‐01 to Z.A.G., and B7756R to C.C.), by NIH‐National Institutes of Aging (R01AG060341 to C.C. and M.B. and PO1 AG039355 to M.B.), and the George W. and Hazel M. Jay professorship (M.B.). Some of this work was performed at the Simons Electron Microscopy Center and National Resource for Automated Molecular Microscopy located at the New York Structural Biology Center, supported by grants from the Simons Foundation (SF349247), NYSTAR, and the NIH National Institute of General Medical Sciences (GM103310) with additional support from NIH (RR029300). The NYU Genome Technology Center is partially supported by the Cancer Center Support Grant (P30CA016087) at the Laura and Isaac Perlmutter Cancer Center. The work reported herein does not represent the views of the US Department of Veterans Affairs or the US Government.
Supporting information
Figure S1. Expression of Numb protein in mouse muscle. (a) Representative western blots showing Numb protein levels in gastrocnemius (GS), tibialis anterior (TA) and soleus (Sol) muscles from 3 and 24‐month‐old C57BL/6 N mice from the NIA Aged Rodent Colonies. GS blot: shown are non‐contiguous lanes from a single representative western blot. TA and soleus: shown are contiguous lanes from a single representative western blot. (b) Graphs represent quantification of Numb levels for the blots shown in (a). Numb protein levels were normalized using ß‐tubulin (ß‐Tub). Data are presented as mean values ± STD. **, p < 0.01, t‐test. GS, n = 3/group; TA, n = 6/group; Sol, n = 4 (3 months) and n = 3 (24 months).
Figure S2. Expression of Numb and NumbL genes in mouse muscle. Total RNA was isolated from gastrocnemius muscle using RNeasy kits (Qiagen) and reverse transcribed using the High‐Capacity cDNA reverse transcription kit. Numb and NumbL expression were determined by qPCR using specific TaqMan primers and probes and TaqMan universal Master mix (N = 6). mRNA expression was calculated with the ‐2DDCt method using GAPDH as a housekeeping gene. NumbL expression was normalized relative to Numb. Statistical differences were analyzed by a two‐tailed t‐test; ****, p<0.0001. Data are expressed as mean values ± STD.
Figure S3. Body and tissue weights. a‐e: data show body or muscle weights for HSA‐MCM Numbf/f/NumbLf/f mice at 56 days after the beginning of treatment with either tamoxifen or vehicle. a, Change in body weight (%) of vehicle or tamoxifen treated b‐e, Normalized muscle weight of Plantaris (b), EDL (c), Biceps (d) and Triceps (e) (N = 15/group).
f‐l: data show body or muscle weights for Numbf/f/NumbLf/f mice (genotype controls) at 56 days after the beginning of treatment with either tamoxifen or vehicle. f, Percent body weight change of vehicle or tamoxifen treated Numbf/f/NumbLf/f mice 56 days after the beginning of the treatment. g‐j, Normalized muscle weight of Gastrocnemius (g), Soleus (h), Plantaris (i), EDL (l), Biceps (k) and Triceps (j). (N = 9 for vehicle‐treated and N = 11 for tamoxifen‐treated animals). All muscle weights were normalized to pre‐induction body weight. Statistical differences were analyzed by two‐tailed t‐tests; ***, p < 0.0005, * p < 0.05.
Figure S4. Force‐frequency plots generated by in‐situ physiological testing of gastrocnemius muscle from male or female mice at 56 days after beginning treatment with tamoxifen or vehicle. No difference was observed in force‐frequency relationships between genders. Group sizes were: N = 8 for vehicle and tamoxifen treated females, N = 7 for vehicle treated males, N = 5 for tamoxifen treated males.
Figure S5. In‐situ physiology analysis of gastrocnemius muscle isolated from vehicle and tamoxifen treated HSA‐MCM Numbf/f/NumbLf/f mice 56 days after the beginning of the treatment. N = 15 for the vehicle group and 13 for the tamoxifen group. a, Time to peak tension. b, Half relaxation time. c, Fatigue index. No statistically significant differences were found for these parameters (two tailed t‐test p > 0.05).
Figure S6. Specific tension‐frequency curves determined by in‐situ testing of gastrocnemius muscle in Numbf/f/NumbLf/f mice (genotype controls) treated with tamoxifen or vehicle for 56 days after the beginning of the treatment. (N = 8 animals for the vehicle group and N = 5 for the tamoxifen group). Statistical analysis was performed with a repeated measure two way ANOVA. Standard error bars are displayed in the upward direction only to improve the ease with which data points can be seen in the figure. There was no significant interaction effect (F value = 0.5832, DFn 10, DFd 110, p = 0.8247 or main effect of tamoxifen (F value = 0.1479 with DFn 11, DFd 110, p = 0.7079).
Figure S7. Effects of Numb/NumbL cKO on fiber cross sectional area (CSA) and fiber type distribution. Tibialis anterior (TA) muscle was isolated from HSA‐MCM Numbfl/fl/NumbLfl/fl mice treated with vehicle or tamoxifen. a, Representative hematoxylin‐eosin stained cryosections. b, Mean fiber cross sectional area. c, Distribution of fiber cross sectional area in vehicle and tamoxifen treated TA. CSA was determined for 150 fibers on one image per animal for 3 animals per group. d‐e, Representative immunostaining of muscle fiber types in TA from vehicle (d) or tamoxifen treated animals (e) f‐g, Percent muscle fiber types in vehicle and tamoxifen treated animals. All fibers on a cross‐section were scored; one section was analyzed for each of 3 animals per group. Scale bar: a, 400 μm; d‐e, 25 μm. No significant differences were observed between vehicle and tamoxifen groups (two‐tailed unpaired t‐test).
Figure S8. Tibialis anterior (TA) muscle was isolated from Numbfl/fl/NumbLfl/fl mice (genotype controls) treated with vehicle or tamoxifen for 56 days. a, Hematoxylin‐eosin stained TA muscle from vehicle (left panel) and tamoxifen treated (right panel) animal. b, Mean fiber cross sectional area. c, Distribution of fiber cross sectional area (CSA). 150 fibers were scored/animal. Data are mean values ± SEM for 3 animals per group. d, Representative images of cryosections immunostained for detection of muscle fiber types in TA from vehicle (left panel) and tamoxifen treated animals (right panel) e, Percent muscle fiber types in vehicle and tamoxifen treated animals. A total of 2400 fibers were scored/animals (N=3/group). * < 0.05, two‐tailed unpaired t‐test. Scale bar: a: 400 μm, d: 25 μm.
Figure S9. Representative transmission Electron Microscopy images acquired at 5800x of tibialis anterior muscle from genotype control (Numbf/f/NumbLf/f) mice are shown for vehicle treated (a) and tamoxifen‐treated (b) mice. Mitochondrial area as a fraction of total area was determined for 3 sections for each of 3 different mice for each gender (N = 6 vehicle and 6 tamoxifen‐treated mice) (c). Mitochondrial and total area was determined using the Measure tool of NIH ImageJ. (d) Distance between Z‐discs (μm) (N = 3/per group). Tamoxifen treatment, tissue harvest and TEM were performed as described in Materials and Methods.
Figure S10. Representative FIB‐SEM Image of tibialis anterior muscle from HSA‐MCM/(Numbf/f/NumbLf/f mice treated with tamoxifen showing an example of residual bodies noted in the female Numb/NumbL cKO animals. The pop‐out image shows an enlarged image of the area of interest delineated by a box in the original image. Tamoxifen treatment, tissue harvest and FIB‐SEM were performed as described in Materials and Methods. The scale bar represents 1 micron.
Figure S11. RNA sequencing genes that are differentially expressed genes after tamoxifen treatment in genotype controls (Numbf/f/NumbLf/f mice). (a, b). Volcano plots showing mRNA expression levels for genes that had fold‐changes > 1.2 with p values adjusted for FDR < 0. Light brown spots indicate upregulated genes, light blue spots downregulated genes. All other genes detected are shown as grey dots. Values for numbers of up and downregulated genes are show at the top right and top left of each panel, respectively. The total number of transcripts analyzed is shown under the X‐Axis as Total Variables Heatmaps showing relative expression of genes altered in tamoxifen‐treated females (c) or males (d) Numb(f/f)/NumbL(f/f) mice. (e) Table shows relative expression, log2 fold‐change in expression and adjusted p value for the indicated genes for female (c) and male (d) mice.
Figure S12. (a) Numb Western blot results after morpholino treatment for 48 h; (b) quantification of Numb Western blot results using ImageJ; (c) Downregulation of Numb significantly inhibit primary myoblast myogenic differentiation. (d) Treatment with a Numb morpholino significantly reduces fusion index. n = 3–4, **p < 0.01 and ***p < 0.001 compared with control, unpaired t‐test.
Data S1. Supporting Information.
Table S1. Supporting Information.
Table S2. Supporting Information.
Table S3. Supporting Information.
Table S4. Supporting Information.
Table S5. Supporting Information.
Table S6. Normalized levels of lipid mediators in gastrocnemius muscle in tamoxifen‐treated mice by the average of that in vehicle‐treated mice.
Acknowledgements
We are grateful to Tom Rando and Rob Krauss for helpful discussions. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 38
De Gasperi R., Mo C., Azulai D., Wang Z., Harlow L. M., Du Y., Graham Z., Pan J., Liu X.‐h., Guo L., Zhang B., Ko F., Raczkowski A. M., Bauman W. A., Goulbourne C. N., Zhao W., Brotto M., and Cardozo C. P. (2022) Numb is required for optimal contraction of skeletal muscle, Journal of Cachexia, Sarcopenia and Muscle, 13, 454–466, 10.1002/jcsm.12907
Rita De Gasperi, Chenglin Mo, and Marco Brotto contributed equally to the design of the research and data interpretation.
References
- 1. Brotto M, Abreu EL. Sarcopenia: pharmacology of today and tomorrow. J Pharmacol Exp Ther 2012;343:540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calderon JC, Bolanos P, Caputo C. The excitation‐contraction coupling mechanism in skeletal muscle. Biophys Rev 2014;6:133–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andersson DC, Betzenhauser MJ, Reiken S, Meli AC, Umanskaya A, Xie W, et al. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab 2011;14:196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lamboley CR, Wyckelsma VL, McKenna MJ, Murphy RM, Lamb GD. Ca(2+) leakage out of the sarcoplasmic reticulum is increased in type I skeletal muscle fibres in aged humans. J Physiol 2016;594:469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dowling JJ, Lawlor MW, Dirksen RT. Triadopathies: an emerging class of skeletal muscle diseases. Neurotherapeutics 2014;11:773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thornton AM, Zhao X, Weisleder N, Brotto LS, Bougoin S, Nosek TM, et al. Store‐operated Ca(2+) entry (SOCE) contributes to normal skeletal muscle contractility in young but not in aged skeletal muscle. Aging (Albany NY) 2011;3:621–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pan Z, Brotto M, Ma J. Store‐operated Ca2+ entry in muscle physiology and diseases. BMB Rep 2014;47:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mo C, Wang Z, Bonewald L, Brotto M. Multi‐staged regulation of lipid signaling mediators during myogenesis by COX‐1/2 pathways. Int J Mol Sci 2019;20:4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim SY, Yang D, Myeong J, Ha K, Kim SH, Park EJ, et al. Regulation of calcium influx and signaling pathway in cancer cells via TRPV6‐Numb1 interaction. Cell Calcium 2013;53:102–111. [DOI] [PubMed] [Google Scholar]
- 10. Carey KA, Farnfield MM, Tarquinio SD, Cameron‐Smith D. Impaired expression of Notch signaling genes in aged human skeletal muscle. J Gerontol A Biol Sci Med Sci 2007;62:9–17. [DOI] [PubMed] [Google Scholar]
- 11. Yan B. Numb—from flies to humans. Brain Dev 2010;32:293–298. [DOI] [PubMed] [Google Scholar]
- 12. Garcia‐Heredia JM, Carnero A. NUMB and NUMBL differences in gene regulation. Oncotarget 2018;9:9219–9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gulino A, Di Marcotullio L, Screpanti I. The multiple functions of Numb. Exp Cell Res 2010;316:900–906. [DOI] [PubMed] [Google Scholar]
- 14. Spana EP, Kopczynski C, Goodman CS, Doe CQ. Asymmetric localization of numb autonomously determines sibling neuron identity in the Drosophila CNS. Development 1995;121:3489–3494. [DOI] [PubMed] [Google Scholar]
- 15. Liu Z, Li H, Su J, Xu S, Zhu F, Ai J, et al. Numb depletion promotes Drp1‐mediated mitochondrial fission and exacerbates mitochondrial fragmentation and dysfunction in acute kidney injury. Antioxid Redox Signal 2019;30:1797–1816. [DOI] [PubMed] [Google Scholar]
- 16. George RM, Biressi S, Beres BJ, Rogers E, Mulia AK, Allen RE, et al. Numb‐deficient satellite cells have regeneration and proliferation defects. Proc Natl Acad Sci U S A 2013;110:18549–18554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilson A, Ardiet DL, Saner C, Vilain N, Beermann F, Aguet M, et al. Normal hemopoiesis and lymphopoiesis in the combined absence of numb and numblike. J Immunol 2007;178:6746–6751. [DOI] [PubMed] [Google Scholar]
- 18. McCarthy JJ, Srikuea R, Kirby TJ, Peterson CA, Esser KA. Inducible Cre transgenic mouse strain for skeletal muscle‐specific gene targeting. Skeletal Muscle 2012;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wen PH, De Gasperi R, Sosa MA, Rocher AB, Friedrich VL Jr, Hof PR, et al. Selective expression of presenilin 1 in neural progenitor cells rescues the cerebral hemorrhages and cortical lamination defects in presenilin 1‐null mutant mice. Development 2005;132:3873–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mo C, Zhao R, Vallejo J, Igwe O, Bonewald L, Wetmore L, et al. Prostaglandin E2 promotes proliferation of skeletal muscle myoblasts via EP4 receptor activation. Cell Cycle 2015;14:1507–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lipina C, Hundal HS. Lipid modulation of skeletal muscle mass and function. J Cachexia Sarcopenia Muscle 2017;8:190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pouvreau S, Royer L, Yi J, Brum G, Meissner G, Rios E, et al. Ca(2+) sparks operated by membrane depolarization require isoform 3 ryanodine receptor channels in skeletal muscle. Proc Natl Acad Sci U S A 2007;104:5235–5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheng H, Lederer WJ. Calcium sparks. Physiol Rev 2008;88:1491–1545. [DOI] [PubMed] [Google Scholar]
- 24. Mo C, Romero‐Suarez S, Bonewald L, Johnson M, Brotto M. Prostaglandin E2: from clinical applications to its potential role in bone‐ muscle crosstalk and myogenic differentiation. Recent Pat Biotechnol 2012;6:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saito F, Hori MT, Ideguchi Y, Berger M, Golub M, Stern N, et al. 12‐Lipoxygenase products modulate calcium signals in vascular smooth muscle cells. Hypertension 1992;20:138–143. [DOI] [PubMed] [Google Scholar]
- 26. DiNicolantonio JJ, O'Keefe JH. Importance of maintaining a low omega‐6/omega‐3 ratio for reducing inflammation. Open Heart 2018;5:e000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bhattacharya A, Hamilton R, Jernigan A, Zhang Y, Sabia M, Rahman MM, et al. Genetic ablation of 12/15‐lipoxygenase but not 5‐lipoxygenase protects against denervation‐induced muscle atrophy. Free Radic Biol Med 2014;67:30–40. [DOI] [PubMed] [Google Scholar]
- 28. Zuo L, Christofi FL, Wright VP, Bao S, Clanton TL. Lipoxygenase‐dependent superoxide release in skeletal muscle. J Appl Physiol 1985;2004:661–668. [DOI] [PubMed] [Google Scholar]
- 29. Vangaveti V, Baune BT, Kennedy RL. Hydroxyoctadecadienoic acids: novel regulators of macrophage differentiation and atherogenesis. Ther Adv Endocrinol Metab 2010;1:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang C, Liu W, Yao L, Zhang X, Zhang X, Ye C, et al. Hydroxyeicosapentaenoic acids and epoxyeicosatetraenoic acids attenuate early occurrence of nonalcoholic fatty liver disease. Br J Pharmacol 2017;174:2358–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Del Campo A, Contreras‐Hernandez I, Castro‐Sepulveda M, Campos CA, Figueroa R, Tevy MF, et al. Muscle function decline and mitochondria changes in middle age precede sarcopenia in mice. Aging (Albany NY) 2018;10:34–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Migliavacca E, Tay SKH, Patel HP, Sonntag T, Civiletto G, McFarlane C, et al. Mitochondrial oxidative capacity and NAD(+) biosynthesis are reduced in human sarcopenia across ethnicities. Nat Commun 2019;10:5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jongbloets BC, Ramakers GM, Pasterkamp RJ. Semaphorin7A and its receptors: pleiotropic regulators of immune cell function, bone homeostasis, and neural development. Semin Cell Dev Biol 2013;24:129–138. [DOI] [PubMed] [Google Scholar]
- 34. Koh JM, Oh B, Lee JY, Lee JK, Kimm K, Kim GS, et al. Association study of semaphorin 7a (sema7a) polymorphisms with bone mineral density and fracture risk in postmenopausal Korean women. J Hum Genet 2006;51:112–117. [DOI] [PubMed] [Google Scholar]
- 35. Li B, Niswander LA. TMEM132A, a novel Wnt signaling pathway regulator through Wntless (WLS) interaction. Front Cell Dev Biol 2020;8:599890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paavola J, Alakoski T, Ulvila J, Kilpio T, Siren J, Perttunen S, et al. Vezf1 regulates cardiac structure and contractile function. EBioMedicine 2020;51:102608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim JH, Han GC, Seo JY, Park I, Park W, Jeong HW, et al. Sex hormones establish a reserve pool of adult muscle stem cells. Nat Cell Biol 2016;18:930–940. [DOI] [PubMed] [Google Scholar]
- 38. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Expression of Numb protein in mouse muscle. (a) Representative western blots showing Numb protein levels in gastrocnemius (GS), tibialis anterior (TA) and soleus (Sol) muscles from 3 and 24‐month‐old C57BL/6 N mice from the NIA Aged Rodent Colonies. GS blot: shown are non‐contiguous lanes from a single representative western blot. TA and soleus: shown are contiguous lanes from a single representative western blot. (b) Graphs represent quantification of Numb levels for the blots shown in (a). Numb protein levels were normalized using ß‐tubulin (ß‐Tub). Data are presented as mean values ± STD. **, p < 0.01, t‐test. GS, n = 3/group; TA, n = 6/group; Sol, n = 4 (3 months) and n = 3 (24 months).
Figure S2. Expression of Numb and NumbL genes in mouse muscle. Total RNA was isolated from gastrocnemius muscle using RNeasy kits (Qiagen) and reverse transcribed using the High‐Capacity cDNA reverse transcription kit. Numb and NumbL expression were determined by qPCR using specific TaqMan primers and probes and TaqMan universal Master mix (N = 6). mRNA expression was calculated with the ‐2DDCt method using GAPDH as a housekeeping gene. NumbL expression was normalized relative to Numb. Statistical differences were analyzed by a two‐tailed t‐test; ****, p<0.0001. Data are expressed as mean values ± STD.
Figure S3. Body and tissue weights. a‐e: data show body or muscle weights for HSA‐MCM Numbf/f/NumbLf/f mice at 56 days after the beginning of treatment with either tamoxifen or vehicle. a, Change in body weight (%) of vehicle or tamoxifen treated b‐e, Normalized muscle weight of Plantaris (b), EDL (c), Biceps (d) and Triceps (e) (N = 15/group).
f‐l: data show body or muscle weights for Numbf/f/NumbLf/f mice (genotype controls) at 56 days after the beginning of treatment with either tamoxifen or vehicle. f, Percent body weight change of vehicle or tamoxifen treated Numbf/f/NumbLf/f mice 56 days after the beginning of the treatment. g‐j, Normalized muscle weight of Gastrocnemius (g), Soleus (h), Plantaris (i), EDL (l), Biceps (k) and Triceps (j). (N = 9 for vehicle‐treated and N = 11 for tamoxifen‐treated animals). All muscle weights were normalized to pre‐induction body weight. Statistical differences were analyzed by two‐tailed t‐tests; ***, p < 0.0005, * p < 0.05.
Figure S4. Force‐frequency plots generated by in‐situ physiological testing of gastrocnemius muscle from male or female mice at 56 days after beginning treatment with tamoxifen or vehicle. No difference was observed in force‐frequency relationships between genders. Group sizes were: N = 8 for vehicle and tamoxifen treated females, N = 7 for vehicle treated males, N = 5 for tamoxifen treated males.
Figure S5. In‐situ physiology analysis of gastrocnemius muscle isolated from vehicle and tamoxifen treated HSA‐MCM Numbf/f/NumbLf/f mice 56 days after the beginning of the treatment. N = 15 for the vehicle group and 13 for the tamoxifen group. a, Time to peak tension. b, Half relaxation time. c, Fatigue index. No statistically significant differences were found for these parameters (two tailed t‐test p > 0.05).
Figure S6. Specific tension‐frequency curves determined by in‐situ testing of gastrocnemius muscle in Numbf/f/NumbLf/f mice (genotype controls) treated with tamoxifen or vehicle for 56 days after the beginning of the treatment. (N = 8 animals for the vehicle group and N = 5 for the tamoxifen group). Statistical analysis was performed with a repeated measure two way ANOVA. Standard error bars are displayed in the upward direction only to improve the ease with which data points can be seen in the figure. There was no significant interaction effect (F value = 0.5832, DFn 10, DFd 110, p = 0.8247 or main effect of tamoxifen (F value = 0.1479 with DFn 11, DFd 110, p = 0.7079).
Figure S7. Effects of Numb/NumbL cKO on fiber cross sectional area (CSA) and fiber type distribution. Tibialis anterior (TA) muscle was isolated from HSA‐MCM Numbfl/fl/NumbLfl/fl mice treated with vehicle or tamoxifen. a, Representative hematoxylin‐eosin stained cryosections. b, Mean fiber cross sectional area. c, Distribution of fiber cross sectional area in vehicle and tamoxifen treated TA. CSA was determined for 150 fibers on one image per animal for 3 animals per group. d‐e, Representative immunostaining of muscle fiber types in TA from vehicle (d) or tamoxifen treated animals (e) f‐g, Percent muscle fiber types in vehicle and tamoxifen treated animals. All fibers on a cross‐section were scored; one section was analyzed for each of 3 animals per group. Scale bar: a, 400 μm; d‐e, 25 μm. No significant differences were observed between vehicle and tamoxifen groups (two‐tailed unpaired t‐test).
Figure S8. Tibialis anterior (TA) muscle was isolated from Numbfl/fl/NumbLfl/fl mice (genotype controls) treated with vehicle or tamoxifen for 56 days. a, Hematoxylin‐eosin stained TA muscle from vehicle (left panel) and tamoxifen treated (right panel) animal. b, Mean fiber cross sectional area. c, Distribution of fiber cross sectional area (CSA). 150 fibers were scored/animal. Data are mean values ± SEM for 3 animals per group. d, Representative images of cryosections immunostained for detection of muscle fiber types in TA from vehicle (left panel) and tamoxifen treated animals (right panel) e, Percent muscle fiber types in vehicle and tamoxifen treated animals. A total of 2400 fibers were scored/animals (N=3/group). * < 0.05, two‐tailed unpaired t‐test. Scale bar: a: 400 μm, d: 25 μm.
Figure S9. Representative transmission Electron Microscopy images acquired at 5800x of tibialis anterior muscle from genotype control (Numbf/f/NumbLf/f) mice are shown for vehicle treated (a) and tamoxifen‐treated (b) mice. Mitochondrial area as a fraction of total area was determined for 3 sections for each of 3 different mice for each gender (N = 6 vehicle and 6 tamoxifen‐treated mice) (c). Mitochondrial and total area was determined using the Measure tool of NIH ImageJ. (d) Distance between Z‐discs (μm) (N = 3/per group). Tamoxifen treatment, tissue harvest and TEM were performed as described in Materials and Methods.
Figure S10. Representative FIB‐SEM Image of tibialis anterior muscle from HSA‐MCM/(Numbf/f/NumbLf/f mice treated with tamoxifen showing an example of residual bodies noted in the female Numb/NumbL cKO animals. The pop‐out image shows an enlarged image of the area of interest delineated by a box in the original image. Tamoxifen treatment, tissue harvest and FIB‐SEM were performed as described in Materials and Methods. The scale bar represents 1 micron.
Figure S11. RNA sequencing genes that are differentially expressed genes after tamoxifen treatment in genotype controls (Numbf/f/NumbLf/f mice). (a, b). Volcano plots showing mRNA expression levels for genes that had fold‐changes > 1.2 with p values adjusted for FDR < 0. Light brown spots indicate upregulated genes, light blue spots downregulated genes. All other genes detected are shown as grey dots. Values for numbers of up and downregulated genes are show at the top right and top left of each panel, respectively. The total number of transcripts analyzed is shown under the X‐Axis as Total Variables Heatmaps showing relative expression of genes altered in tamoxifen‐treated females (c) or males (d) Numb(f/f)/NumbL(f/f) mice. (e) Table shows relative expression, log2 fold‐change in expression and adjusted p value for the indicated genes for female (c) and male (d) mice.
Figure S12. (a) Numb Western blot results after morpholino treatment for 48 h; (b) quantification of Numb Western blot results using ImageJ; (c) Downregulation of Numb significantly inhibit primary myoblast myogenic differentiation. (d) Treatment with a Numb morpholino significantly reduces fusion index. n = 3–4, **p < 0.01 and ***p < 0.001 compared with control, unpaired t‐test.
Data S1. Supporting Information.
Table S1. Supporting Information.
Table S2. Supporting Information.
Table S3. Supporting Information.
Table S4. Supporting Information.
Table S5. Supporting Information.
Table S6. Normalized levels of lipid mediators in gastrocnemius muscle in tamoxifen‐treated mice by the average of that in vehicle‐treated mice.


