Abstract
Background
Reduced muscular strength, as measured by absolute grip strength, has been associated with increased risk of some site‐specific cancers. The ability of grip strength to predict other diseases may be affected by whether it is expressed in absolute or relative terms, but the evidence for cancer is scarce. This study compared the associations of absolute and relative grip strength with all‐cause and 15 site‐specific cancers.
Methods
A prospective cohort study was undertaken using data from the UK Biobank. The exposure variable was grip strength, in absolute form (kilogrammes) and relative to weight, body mass index (BMI), height. and body fat mass. The outcome was incident cancer, at 15 sites and overall. Cox proportional hazard models were performed to study the associations.
Results
This study included 445 552 participants, where 53.8% of the participants were women, with a mean (SD) age of 56.3 (8.11) years. During a median of 8.8 years follow‐up period, 48 886 (11.0%) patients were diagnosed with cancer. After adjusting for sociodemographic and lifestyle factors, as well as multiple testing, absolute grip strength was inversely and linearly associated with endometrial [hazard ratio (HR): 0.74, 95% confidence interval (CI): 0.69; 0.79, P value <0.001], gallbladder (HR: 0.81, 95% CI: 0.72; 0.92, P value = 0.001), liver (HR: 0.86, 95% CI: 0.79; 0.93, P value <0.001), kidney (HR: 0.93, 95% CI: 0.88; 0.99), and breast (HR: 0.93, 95% CI: 0.91; 0.96, P value = 0.031), as well as all‐cause cancer (HR: 0.97, 95% CI: 0.95; 0.98, P value <0.001). Eight cancer sites were inversely associated with HGS relative to weight and BMI: endometrium, liver, gallbladder, kidney, oesophagus, pancreas, colorectal, breast, and all‐cause cancer. Compared with absolute grip strength, grip strength relative to body fat mass had better discriminatory power for head and neck and breast cancer. Grip strength relative to BMI was marginally better than absolute grip strength in predicting stomach cancer.
Conclusions
Grip strength was associated with risk of several site‐specific cancers and all‐cause cancer. Head and neck and breast cancers might be better predicted by relative grip strength.
Keywords: Cancer, Handgrip, Muscle mass
Introduction
There were 19.3 million new cancer cases in 2020, 1 and by 2040, this number is expected to increase to 27.5 million. 2 To alleviate the burden of cancer, several public health guidelines have been developed. The current physical activity guidelines include recommendations that aim to increase and maintain muscular strength across the life span. 3
One of the most common muscle strength markers, in clinical and research settings, is handgrip strength (HGS) as it correlates well with overall strength. 4 , 5 HGS is a simple, non‐invasive and low‐cost method that has been associated with several chronic diseases and all‐cause mortality across different age groups. 6 , 7 , 8 HGS has been associated with a range of health outcomes such as all‐cause mortality, cardiovascular diseases, and some site‐specific cancers (colorectal, lung, and breast) as well as all‐cause cancer. 5 , 7 , 9 , 10 However, evidence regarding the association of grip strength with cancer has been mainly restricted to absolute HGS, with limited and conflicting evidence available for site‐specific cancers. 4 , 11 , 12
A meta‐analysis published in 2018, which included 309 413 participants and 9787 cases, found no association between HGS and overall cancer mortality. However, the categorization of strength and adjustment for covariates was heterogeneous between studies, and there was no differentiation between sites of cancer. 12 The Prospective Urban Rural Epidemiology (PURE) study, which included data from 139 691 participants across 17 countries, reported that absolute HGS (per 5 kg reduction in HGS) was associated with increased overall cancer risk, especially in participants from high‐income countries. 13 Some previous studies in UK Biobank reported associations of absolute HGS with all‐cause cancer, colorectal, lung, and breast cancer incidence and mortality. 11 Whilst similar results were reported by Yates et al., the authors concluded that the association between absolute HGS and all‐cause cancer mortality was less consistent than other diseases. 14 Individual study findings have also been inconsistent across cancer sites. 4 , 12 , 14 Hence, Wu et al., in a meta‐analysis that included 42 studies, did not find an association between HGS and overall cancer [hazard ratio (HR): 0.89, 95% confidence interval (CI): 0.66–1.20]. 4
Studies have shown that relative HGS might be a better indicator for muscle weakness, 15 as well as more predictive of cardiometabolic diseases. 16 Because of these, there is yet a consensus on how HGS should be used in clinical practice. 17 To our knowledge, all existing studies on HGS and cancer expressed HGS in absolute terms. The aims of this study, therefore, were to investigate the associations of HGS, expressed (i) in absolute terms (kilogrammes) and (ii) relative to anthropometric variables, with 15 cancer sites and all‐cause cancer and to compare risk prediction scores of HGS when differentially expressed.
Methods
Study design
Between April 2007 and December 2010, UK Biobank recruited approximately 502 000 participants, aged 37–73 years from the general population. 18 Participants attended 1 of 22 assessment centres across England, Wales, and Scotland, 19 where they completed a touch‐screen questionnaire, had physical measurements taken and provided biological samples, as described in detail elsewhere. 19 , 20 In this prospective population‐based study, 15 site‐specific cancers and all‐cause cancer incidence (fatal/non‐fatal) were the outcomes, HGS was the exposure variables; and socio‐demographic factors (age, ethnicity, area socioeconomic deprivation index), smoking status, sedentary behaviour, physical activity, height, diet (red and processes meat, oily fish, and alcohol), and multimorbidity were covariates. After excluding participants with cancer at baseline (n = 41 406), and with missing data for the exposure and covariates (n = 15 534), our sample was restricted to the 445 552 participants who had full data available.
Procedure
Hospital admissions were identified via record linkage to Health Episode Statistics records for England (1 June 2020) and Wales (31 March 2017) and to Scottish Morbidity Records for Scotland (31 March 2017). The International Classification of Diseases, 10th revision (ICD‐10) was used to define the following 15 cancers: all cancers (C00‐C97, D37, and D48), and oral (C00‐C14), oesophageal (C15), stomach (C16), colorectal (C18, C19, and C20), liver (C22), gallbladder (C23), pancreatic (C25), lung (C34), kidney (C64‐C65), bladder (C67), breast (C50), endometrial (C54), cervical (C53), ovarian (C56), and prostate (C61) cancer. Of these, 10 cancer sites were used for men and women; one site was specific to men (prostate) and four to women (breast, endometrium, cervix, and ovary). Potential confounders were identified a priori based on established relationships with cancer and muscular strength. Area‐based socioeconomic status was derived from postcode of residence, using the Townsend score. 21 Age at baseline was calculated from date of birth and date of baseline assessment. Medical history (physician diagnosis of depression, stroke, angina, heart attack, hypertension, cancer, diabetes, or long‐standing illness), ethnicity, smoking status (never, former, or current smoker), and female reproductive factors were collected from the self‐completed, baseline questionnaire. Dietary intake was collected via a food frequency questionnaire, with participants asked how many portions of red meat, processed meat, and fish they generally ate. Total time spent in discretionary sedentary behaviours was derived from the sum of self‐reported time spent driving, using a computer, and watching television. Anthropometric measurements, height, and weight were obtained during the baseline assessment by trained clinic staff using standard operating procedures and regularly calibrated equipment. Body fat was measured using the Tanita BC‐418 MA body composition analyser (fat mass divided by the total body mass). Further details of these measurements can be found in the UK Biobank online protocol (http://www.ukbiobank.ac.uk).
Exposures
The HGS was assessed using a Jamar J00105 hydraulic hand dynamometer (Patterson Medical, Sutton‐in‐Ashfield, UK), and the mean of the right and left hand values, expressed as kilogramme, was used in the analysis, as reported elsewhere. 9 , 22 Five representations of HGS were analysed: (1) absolute HGS in kg, (2) HGS divided by height, (3) HGS divided by weight, (4) HGS divided by BMI, (5) HGS divided by body fat mass (BFM) in kilogramme. All these variables were standardized using sex‐specific mean and standard deviation of the whole sample ([X − Mean] ÷ SD).
Statistical analyses
Continuous variables were summarized using mean and standard deviation, and categorical variables using frequencies and percentages. Non‐linear associations between HGS and cancer sites were visually explored using multivariable penalized cubic splines in Cox‐proportional hazard models. 23 Penalized spline is a technique that balances data fit and smoothness. 24 Spline curvature is penalized by the integrated second derivative. Knots were selected based on generalized cross‐validation and were equally spaced across the range of the exposure variable. The results were reported as hazard ratios (HRs) together with 95% confidence intervals (CIs). Analyses were adjusted for baseline age (at time of hand grip assessment), sex, ethnicity, Townsend deprivation index, height, smoking status, dietary intake (alcohol, red meat, oily fish, and processed meat), sedentary behaviour, physical activity, and comorbidities (longstanding illness, diabetes, hypertension, cardiovascular disease (CVD), cancer, and depression), as well as height when it was not included in the exposure. Additional covariates were added for breast, cervical, endometrial, and ovarian cancer: hormonal replacement (yes/no), contraceptive use (yes/no), and age at menarche. Finally, because of potentially inflated Type‐I errors due to multiple tests, we provided the adjusted P values (denoted as P adj) using Holm's method controlling family‐wise error rate. 25
We calculated Harrell's C‐index (which estimates the probability of concordance between observed and predicted responses) to compare the discriminatory power of HGS markers. 26 The proportional hazard assumption was checked by tests based on Schöenfeld residuals. All analyses were performed using R Statistical Software version 3.6.2 with the package survival. Statistical significance was set at α < 0.05.
Patient involvement
No patients were involved in setting the research question or the outcome measures.
Results
Characteristics of the study population
There were 445 552 participants included in the analysis. The median follow‐up period was 8.8 years [IQR 7.9–9.6]. During the follow‐up period, 48 886 (11.0%) people developed cancer. Table 1 presents the characteristics of the study population. In summary, 53.8% of the cohort were women, the mean (SD) age was 56.3 (8.11) years, and the majority were white. People with lower HGS had a higher mean weight and waist circumference than those with moderate and higher strength, as well as a higher prevalence of obesity. No substantial differences were observed in lifestyle variables. However, more people in the lower strength group had been diagnosed with diabetes and hypertension and they had a higher multimorbidity count compared with people in the moderate and higher strength groups.
Table 1.
Baseline characteristics by tertials of grip strength
| Lower HGS | Moderate HGS | Higher HGS | Overall | |
|---|---|---|---|---|
| Sociodemographic | ||||
| N (%) | 145 337 (32.6%) | 152 701 (34.3%) | 147 514 (33.1%) | 445 552 |
| Age, mean (SD) | 58.6 (7.58) | 56.7 (7.91) | 53.4 (7.97) | 56.3 (8.11) |
| Sex | ||||
| Females | 79 127 (54.4%) | 79 917 (52.3%) | 80 794 (54.8%) | 239 838 (53.8%) |
| Males | 66 210 (45.6%) | 72 784 (47.7%) | 66 720 (45.2%) | 205 714 (46.2%) |
| Townsend deprivation index | ||||
| Lower | 43 016 (29.6%) | 53 120 (34.8%) | 54 111 (36.7%) | 150 247 (33.7%) |
| Middle | 47 056 (32.4%) | 51 784 (33.9%) | 50 158 (34.0%) | 148 998 (33.4%) |
| Higher | 55 265 (38.0%) | 47 797 (31.3%) | 43 245 (29.3%) | 146 307 (32.8%) |
| Ethnicity | ||||
| White | 135 052 (92.9%) | 145 503 (95.3%) | 140 908 (95.5%) | 421 463 (94.6%) |
| Mixed | 2420 (1.7%) | 2081 (1.4%) | 2175 (1.5%) | 6676 (1.5%) |
| South Asian | 4881 (3.4%) | 2430 (1.6%) | 1521 (1.0%) | 8832 (2.0%) |
| Black | 2593 (1.8%) | 2240 (1.5%) | 2333 (1.6%) | 7166 (1.6%) |
| Chinese | 391 (0.3%) | 447 (0.3%) | 577 (0.4%) | 1415 (0.3%) |
| Anthropometric, mean (SD) | ||||
| Height (m) | 1.7 (0.09) | 1.7 (0.09) | 1.7 (0.10) | 1.7 (0.09) |
| Weight (kg) | 81.4 (15.58) | 77.8 (14.45) | 75.2 (17.04) | 78.1 (15.92) |
| Waist (cm) | 94.8 (12.86) | 90.1 (12.28) | 85.9 (13.69) | 90.2 (13.44) |
| Body fat percentage (%) | 4.2 (9.55) | 31.3 (8.15) | 28.5 (6.75) | 31.3 (8.54) |
| Body mass index (kg/m2) | 29.2 (5.43) | 27.2 (4.07) | 25.8 (4.07) | 27.4 (4.77) |
| BMI categories | ||||
| Underweight | 443 (0.3%) | 365 (0.2%) | 1424 (1.0%) | 2232 (0.5%) |
| Normal | 30 431 (20.9%) | 47 183 (30.9%) | 67 814 (46.0%) | 145 428 (32.6%) |
| Overweight | 59 897 (41.2%) | 72 249 (47.3%) | 57 704 (39.1%) | 189 850 (42.6%) |
| Obese | 54 566 (37.5%) | 32 904 (21.5%) | 20 572 (13.9%) | 108 042 (24.2%) |
| Lifestyle | ||||
| Smoking | ||||
| Never | 78 642 (54.1%) | 83 729 (54.8%) | 83 776 (56.8%) | 246 147 (55.2%) |
| Previous | 51 866 (35.7%) | 53 281 (34.9%) | 47 681 (32.3%) | 152 828 (34.3%) |
| Current | 14 829 (10.2%) | 15 691 (10.3%) | 16 057 (10.9%) | 46 577 (10.5%) |
| Alcohol intake | ||||
| Daily or almost daily | 26 001 (17.9%) | 32 400 (21.2%) | 32 484 (22.0%) | 90 885 (20.4%) |
| 3–4 times a week | 28 923 (19.9%) | 36 618 (24.0%) | 38 577 (26.2%) | 104 118 (23.4%) |
| Once or twice a week | 36 448 (25.1%) | 39 956 (26.2%) | 39 216 (26.6%) | 115 620 (25.9%) |
| 1–3 times a month | 17 093 (11.8%) | 16 848 (11.0%) | 15 851 (10.7%) | 49 792 (11.2%) |
| Special occasions only | 21 104 (14.5%) | 16 138 (10.6%) | 13 119 (8.9%) | 50 361 (11.3%) |
| Never | 15 768 (10.8%) | 10 741 (7.0%) | 8267 (5.6%) | 34 776 (7.8%) |
| Fruit and vegetable intake (portion/day), mean (SD) | 2.0 (0.83) | 2.0 (0.83) | 2.0 (0.83) | 2.0 (0.83) |
| Red meat (portion/week), mean (SD) | 2.1 (1.49) | 2.1 (1.43) | 2.1 (1.42) | 2.1 (1.45) |
| Processed meat (times/week), mean (SD) | 1.9 (1.06) | 1.9 (1.06) | 1.8 (1.07) | 1.9 (1.06) |
| Oily fish (times/week), mean (SD) | 1.6 (0.95) | 1.6 (0.92) | 1.6 (0.91) | 1.6 (0.93) |
| Sedentary time (h/day), mean (SD) | 5.2 (2.36) | 5.0 (2.24) | 4.9 (2.23) | 5.0 (2.28) |
| Physical activity (h/day), mean (SD) | 2.1 (1.94) | 1.8 (1.59) | 1.7 (1.43) | 1.8 (1.67) |
| Health | ||||
| Diabetes diagnosis | ||||
| No | 133 364 (91.8%) | 146 313 (95.8%) | 144 063 (97.7%) | 423 740 (95.1%) |
| Yes | 11 973 (8.2%) | 6388 (4.2%) | 3451 (2.3%) | 21 812 (4.9%) |
| Hypertension diagnosis | ||||
| No | 95 572 (65.8%) | 113 819 (74.5%) | 119 971 (81.3%) | 329 362 (73.9%) |
| Yes | 49 765 (34.2%) | 38 882 (25.5%) | 27 543 (18.7%) | 116 190 (26.1%) |
| Multimorbidty | ||||
| No illness | 40 045 (27.6%) | 57 711 (37.8%) | 68 780 (46.6%) | 166 536 (37.4%) |
| 1 + illness | 105 292 (72.4%) | 94 990 (62.2%) | 78 734 (53.4%) | 279 016 (62.6%) |
Data are shown in n (%) and mean (SD). SD, standard deviation. Data available 445 552.
Absolute HGS and incident cancers
Absolute HGS was inversely associated with five cancer sites: endometrium (HR: 0.74, 95% CI: 0.69; 0.79, P value <0.001), gallbladder (HR: 0.81, 95% CI: 0.72; 0.92, P value = 0.001), liver (HR: 0.86, 95% CI: 0.79; 0.93, P value <0.001), kidney (HR: 0.93, 95% CI: 0.88; 0.99, P value = 0.031), and breast (HR: 0.94, 95% CI: 0.91; 0.96, P value = <0.001), as well as all‐cause cancer (HR: 0.97, 95% CI: 0.95; 0.98, P value <0.001) (Figure 1 and Table S1). There was no strong evidence to suggest nonlinear associations ( Figure S2).
Figure 1.
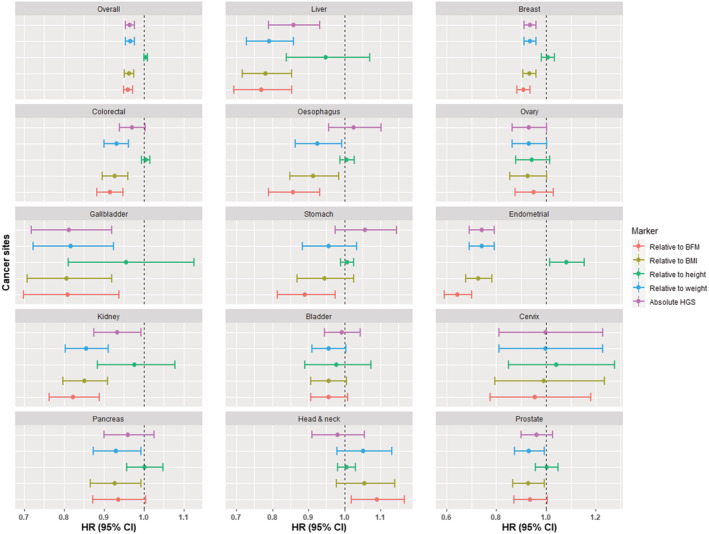
Association between relative grip strength and cancer incidence for 15 cancer. Data are presented in hazard ratio with 95% confidence intervals. Model was adjusted for age, sex, deprivation and ethnicity, height (except when height was part of the exposure), diet (red and process meat; oily fish and alcohol), smoking, physical activity sedentary behaviour, and comorbidity. Breast, cervix, endometrium and ovary also for age at menarche, hormonal replacement use, and contraceptive use. All P values were corrected for multiple testing by using the Holm's method. BMF, body fat mass; BMI, body mass index; HGS, hand grip strength.
Relative HGS and incident cancers
Eight cancer sites were inversely associated with HGS relative to weight and BMI: endometrium, liver, gallbladder, kidney, oesophagus, pancreas, colorectal, breast, and all‐cause cancer. The majority of these associations were linear (Table S1, Figures S2 and S3). The association patterns were similar for HGS relative to BFM, except that the association with stomach cancer was significant and with pancreatic cancer was not (Table S1 and Figure S5). HGS relative to height was inversely associated with only endometrial and lung cancer, as well as overall cancer (Table S1 and Figure S4). Prostate cancer was positively associated with almost all HGS markers (Figure 1 and Table S1) and head and neck cancer was positively associated with HGS relative to BFM.
C‐index
Table 2 shows the Harrell's C‐indices for prediction of overall and site‐specific cancers. There were no significant differences in C‐indices between HGS expressed in absolute and relative terms for most cancer sites. However, HGS relative to BFM was better than absolute HGS in predicting head and neck and breast cancer. Also, HGS relative to BMI was better than absolute HGS at predicting stomach cancer.
Table 2.
C‐indices of absolute and relative HGS in predicting cancer incidence
| Absolute HGS (95% CI) | Relative HGS (95% CI) | Difference (95% CI) | P value | |
|---|---|---|---|---|
| Handgrip to weight | ||||
| Overall | 0.6506 (0.6478; 0.6533) | 0.6515 (0.6487; 0.6543) | −0.0009 (−0.0013; −0.0006) | <0.001 |
| Head and neck | 0.6774 (0.6580; 0.6959) | 0.6753 (0.6558; 0.6943) | 0.0020 (0.0001; 0.0039) | 0.035 |
| Oesophagus | 0.7686 (0.7539; 0.7828) | 0.7687 (0.7540; 0.7827) | −0.0001 (−0.0016; 0.0015) | 0.945 |
| Bladder | 0.7742 (0.7642; 0.7840) | 0.7741 (0.7641; 0.7839) | 0.0001 (−0.0004; 0.0006) | 0.742 |
| Colorectal | 0.6691 (0.6613; 0.6767) | 0.6686 (0.6609; 0.6763) | 0.0004 (−0.0005; 0.0013) | 0.384 |
| Gallbladder | 0.6743 (0.6450; 0.7023) | 0.6770 (0.6476; 0.7050) | −0.0026 (−0.0063; 0.0010) | 0.154 |
| Kidney | 0.7111 (0.6973; 0.7243) | 0.7091 (0.6953; 0.7226) | 0.0019 (−0.0006; 0.0045) | 0.135 |
| Pancreas | 0.6979 (0.6837; 0.7116) | 0.6979 (0.6837; 0.7117) | 0.0000 (−0.0016; 0.0016) | 0.984 |
| Stomach | 0.7369 (0.7195; 0.7533) | 0.7375 (0.7200; 0.7542) | −0.0006 (−0.0025; 0.0013) | 0.552 |
| Lung | 0.8209 (0.8135; 0.8281) | 0.8212 (0.8138; 0.8284) | −0.0003 (−0.0006; −0.0001) | 0.003 |
| Prostate | 0.6809 (0.6757; 0.6861) | 0.6807 (0.6755; 0.6859) | 0.0002 (−0.0002; 0.0005) | 0.332 |
| Breast | 0.5470 (0.5401; 0.5539) | 0.5552 (0.5483; 0.5620) | −0.0082 (−0.0121; −0.0043) | <0.001 |
| Endometrium | 0.6497 (0.6339; 0.6653) | 0.6497 (0.6338; 0.6652) | 0.0001 (−0.0003; 0.0004) | 0.761 |
| Handgrip to height | ||||
| Overall | 0.6502 (0.6475; 0.6530) | 0.6515 (0.6487; 0.6543) | −0.0013 (−0.0016; −0.0009) | <0.001 |
| Head and neck | 0.6765 (0.6571; 0.6953) | 0.6753 (0.6558; 0.6943) | 0.0012 (0.0001; 0.0023) | 0.039 |
| Oesophagus | 0.7686 (0.7539; 0.7826) | 0.7687 (0.7540; 0.7827) | −0.0001 (−0.0005; 0.0003) | 0.549 |
| Bladder | 0.7740 (0.7639; 0.7838) | 0.7741 (0.7641; 0.7839) | −0.0001 (−0.0006; 0.0003) | 0.517 |
| Colorectal | 0.6680 (0.6602; 0.6756) | 0.6686 (0.6609; 0.6763) | −0.0007 (−0.0015; 0.0001) | 0.104 |
| Gallbladder | 0.6678 (0.6384; 0.6961) | 0.6770 (0.6476; 0.7050) | −0.0091 (−0.0170; −0.0013) | 0.023 |
| Kidney | 0.7079 (0.6940; 0.7214) | 0.7091 (0.6953; 0.7226) | −0.0013 (−0.0034; 0.0009) | 0.250 |
| Pancreas | 0.5438 (0.5370; 0.5506) | 0.5552 (0.5483; 0.5620) | −0.0114 (−0.0160; −0.0067) | <0.001 |
| Stomach | 0.6805 (0.6753; 0.6857) | 0.6807 (0.6755; 0.6859) | −0.0002 (−0.0006; 0.0002) | 0.284 |
| Lung | 0.7332 (0.7149; 0.7509) | 0.7356 (0.7174; 0.7531) | −0.0024 (−0.0054; 0.0006) | 0.111 |
| Prostate | 0.6970 (0.6829; 0.7109) | 0.6979 (0.6837; 0.7117) | −0.0009 (−0.0024; 0.0007) | 0.284 |
| Breast | 0.6319 (0.6163; 0.6470) | 0.6497 (0.6338; 0.6652) | −0.0177 (−0.0293; −0.0062) | 0.003 |
| Endometrium | 0.8212 (0.8138; 0.8283) | 0.8212 (0.8138; 0.8284) | −0.0001 (−0.0003; 0.0002) | 0.655 |
| Handgrip to BMI | ||||
| Overall | 0.6503 (0.6475; 0.6531) | 0.6515 (0.6487; 0.6543) | −0.0012 (−0.0016; −0.0009) | <0.001 |
| Head and neck | 0.6774 (0.6580; 0.6959) | 0.6753 (0.6558; 0.6943) | 0.0020 (0.0002; 0.0039) | 0.033 |
| Oesophagus | 0.7687 (0.7540; 0.7829) | 0.7687 (0.7540; 0.7827) | 0.0000 (−0.0016; 0.0015) | 0.986 |
| Bladder | 0.7741 (0.7640; 0.7839) | 0.7741 (0.7641; 0.7839) | 0.0000 (−0.0006; 0.0005) | 0.860 |
| Colorectal | 0.6686 (0.6608; 0.6762) | 0.6686 (0.6609; 0.6763) | −0.0001 (−0.0010; 0.0009) | 0.867 |
| Gallbladder | 0.6730 (0.6436; 0.7011) | 0.6770 (0.6476; 0.7050) | −0.0040 (−0.0088; 0.0008) | 0.102 |
| Kidney | 0.7099 (0.6961; 0.7232) | 0.7091 (0.6953; 0.7226) | 0.0008 (−0.0019; 0.0035) | 0.572 |
| Pancreas | 0.5446 (0.5377; 0.5514) | 0.5552 (0.5483; 0.5620) | −0.0106 (−0.0150; −0.0062) | <0.001 |
| Stomach | 0.6810 (0.6758; 0.6862) | 0.6807 (0.6755; 0.6859) | 0.0003 (0.0000; 0.0006) | 0.046 |
| Lung | 0.7367 (0.7184; 0.7545) | 0.7356 (0.7174; 0.7531) | 0.0011 (−0.0021; 0.0043) | 0.501 |
| Prostate | 0.6975 (0.6833; 0.7112) | 0.6979 (0.6837; 0.7117) | −0.0004 (−0.0021; 0.0013) | 0.619 |
| Breast | 0.6482 (0.6323; 0.6638) | 0.6497 (0.6338; 0.6652) | −0.0015 (−0.0040; 0.0010) | 0.232 |
| Endometrium | 0.8209 (0.8135; 0.8281) | 0.8212 (0.8138; 0.8284) | −0.0003 (−0.0005; −0.0001) | 0.003 |
| Handgrip to BFM | ||||
| Overall | 0.6506 (0.6478; 0.6533) | 0.6515 (0.6487; 0.6543) | −0.0009 (−0.0013; −0.0006) | <0.001 |
| Head and neck | 0.6783 (0.6589; 0.6968) | 0.6753 (0.6558; 0.6943) | 0.0030 (0.0003; 0.0057) | 0.031 |
| Oesophagus | 0.7692 (0.7545; 0.7837) | 0.7687 (0.7540; 0.7827) | 0.0006 (−0.0018; 0.0029) | 0.638 |
| Bladder | 0.7742 (0.7641; 0.7839) | 0.7741 (0.7641; 0.7839) | 0.0000 (−0.0005; 0.0005) | 0.947 |
| Colorectal | 0.6692 (0.6614; 0.6769) | 0.6686 (0.6609; 0.6763) | 0.0005 (−0.0006; 0.0017) | 0.338 |
| Gallbladder | 0.6724 (0.6429; 0.7007) | 0.6770 (0.6476; 0.7050) | −0.0046 (−0.0108; 0.0017) | 0.152 |
| Kidney | 0.7113 (0.6976; 0.7244) | 0.7091 (0.6953; 0.7226) | 0.0022 (−0.0012; 0.0056) | 0.201 |
| Pancreas | 0.5503 (0.5434; 0.5572) | 0.5552 (0.5483; 0.5620) | −0.0049 (−0.0089; −0.0008) | 0.019 |
| Stomach | 0.6809 (0.6757; 0.6861) | 0.6807 (0.6755; 0.6859) | 0.0002 (−0.0002; 0.0006) | 0.408 |
| Lung | 0.7362 (0.7177; 0.7546) | 0.7356 (0.7174; 0.7531) | 0.0006 (−0.0032; 0.0044) | 0.771 |
| Prostate | 0.6975 (0.6834; 0.7112) | 0.6979 (0.6837; 0.7117) | −0.0004 (−0.0021; 0.0013) | 0.667 |
| Breast | 0.6617 (0.6455; 0.6783) | 0.6497 (0.6338; 0.6652) | 0.0120 (0.0077; 0.0164) | <0.001 |
| Endometrium | 0.8208 (0.8134; 0.8281) | 0.8212 (0.8138; 0.8284) | −0.0004 (−0.0007; −0.0001) | 0.004 |
BMI, body mass index; CI, confidence interval; HGS, handgrip strength.
Discussions
This paper reports the associations between HGS, in absolute and relative terms, and incident site‐specific and all‐cause cancer and explores the relative performance of these emerging risk markers in cancer risk prediction. Eight cancer sites were inversely associated with strength relative to weight, BMI, and BFM. Meanwhile, five cancer sites were inversely associated with absolute HGS. HGS expressed in relative terms modestly improved the prediction of head and neck, stomach, and breast cancer.
Comparisons with other studies
The association patterns shown in this study are generally consistent with previous studies. HGS (per 5 kg decreases) was previously associated with lung, breast and colorectal cancer. 11 In our study, both absolute and relative HGS, apart from HGS relative to height, were associated with breast cancer. Only relative HGS was associated with colorectal cancer and, whilst absolute HGS was associated with incident lung cancer in the partially adjusted models, it was not in the fully adjusted model including comorbidities.
To date, all studies have focused on absolute HGS, with equivocal results with most evidence relating to all‐cause cancer. 11 , 13 Gale et al. found a 19% decrease in overall cancer risk per 1‐SD increase of HGS, 27 but García‐Hermoso et al. did not find the same association for cancer mortality (HR: 0.97, 95% CI: 0.92; 1.02). 12 A previous large‐scale study showed a positive association between HGS and cancer mortality, but only in high‐income countries, 13 consistent with our finding that, in the UK population, absolute and relative HGS were associated with lower risk of all‐cause cancer.
The HGS has been suggested as a good risk marker for other diseases, such as cardiovascular disease, irrespective of which HGS marker is used. 6 HGS is a cheap and easy measure to incorporate into clinical practice. 28 In our study, absolute HGS was a predictor of five site‐specific cancers as well as all‐cause cancer. Better prediction for some site‐specific cancers was achieved by using relative HGS. Further studies should explore the clinical utility of using absolute and relative HGS in the prevention and early detection of cancers.
The main finding of the current study was that when comparing numerous different ways to express HGS—absolute and relative to height, weight, BMI, and BFM—relative HGS only showed a modestly improvement in prediction of two groups of cancers. These findings could have important public health implications in terms of the operationalization of HGS in predicting cancer risk, which warrants further studies. 6 This study demonstrates that the most basic form of reporting grip strength, namely in absolute units (kg), is largely sufficient for predicting cancer outcomes in clinical practice and further adjustment might not be needed.
Limitations of this study
UK Biobank is not representative of the general population in terms of deprivation and lifestyle. 18 , 19 However, effect size estimates were generally consistent with population representative cohorts. 29 As in all observational studies, residual confounding is possible, and association may not imply causation. Nonetheless, we minimized the risk of reverse causation using a two‐year landmark analysis. Even though UK Biobank has large sample size, there were small numbers of events for some site‐specific cancers which, therefore, might be underpowered.
Conclusion
HGS was associated with a higher risk of several cancer sites and all‐cause cancer. HGS expressed in relative terms modestly improved the prediction of head and neck and breast cancers. Therefore, expressing grips strength in it most simple unit (kg) appears adequate for predicting most cancer outcomes.
Conflict of interest
None to declare.
Funding
UK Biobank was established and funded by the Wellcome Trust, Medical Research Council, Department of Health, Scottish Government, and the Northwest Regional Development Agency. It has also had funding from the Welsh Assembly Government and the British Heart Foundation. All authors had final responsibility for submission for publication. SPS was funded by the Chilean Government PhD scholarship programme.
Supporting information
Table S1. Association between HGS z‐scores and cancer incidence
Figure S1. Association between absolute HGS and cancer incidence
Figure S2. Association between HGS relative to body weight and cancer incidence
Figure S3. Association between HGS relative to height and cancer incidence
Figure S4. Association between HGS relative to body mass index and cancer incidence
Figure S5. Association between HGS relative to body fat mass and cancer incidence
Acknowledgements
We are grateful to UK Biobank participants. This research has been conducted using the UK Biobank resource under application number 7155 and we express our gratitude to the participants and those involved in building the resource.
Parra‐Soto S., Pell J. P., Celis‐Morales C., and Ho F. K. (2022) Absolute and relative grip strength as predictors of cancer: prospective cohort study of 445 552 participants in UK Biobank, Journal of Cachexia, Sarcopenia and Muscle, 13, 325–332, 10.1002/jcsm.12863
Carlos Celis‐Morales, Frederick K. Ho, and Jill P. Pell contributed equally to this work and are joint senior authors.
Contributor Information
Solange Parra‐Soto, Email: s.parra-soto.1@research.gla.ac.uk.
Jill P. Pell, Email: jill.pell@glasgow.ac.uk.
Carlos Celis‐Morales, Email: Carlos.Celis@glasgow.ac.uk.
Frederick K. Ho, Email: frederick.ho@glasgow.ac.uk.
References
- 1. World Health Organization 2020. Latest global cancer data: cancer burden rises to 19.3 million new cases and 10.0 million cancer deaths in 2020 [https://www.iarc.who.int/faq/latest‐global‐cancer‐data‐2020‐qa/]
- 2. Global Cancer Control New global cancer data: GLOBOCAN 2018 2018. [https://www.uicc.org/news/new‐global‐cancer‐data‐globocan‐2018]
- 3. Rock CL, Thomson C, Gansler T, Gapstur SM, McCullough ML, Patel AV, et al. American Cancer Society Guideline for Diet and Physical Activity for cancer prevention. CA‐Cancer J Clin 2020;70:245–271. [DOI] [PubMed] [Google Scholar]
- 4. Wu Y, Wang W, Liu T, Zhang D. Association of grip strength with risk of all‐cause mortality, cardiovascular diseases, and cancer in community‐dwelling populations: a meta‐analysis of prospective cohort studies. J Am Med Dir Assoc 2017;18:517–551. [DOI] [PubMed] [Google Scholar]
- 5. Buckner SL, Dankel SJ, Bell ZW, Abe T, Loenneke JP. The association of handgrip strength and mortality: what does it tell us and what can we do with it? Rejuv Res 2019;22:230–234. [DOI] [PubMed] [Google Scholar]
- 6. Ho FKW, Celis‐Morales CA, Petermann‐Rocha F, Sillars A, Welsh P, Welsh C, et al. The association of grip strength with health outcomes does not differ if grip strength is used in absolute or relative terms: a prospective cohort study. Age Ageing 2019;48:684–691. [DOI] [PubMed] [Google Scholar]
- 7. Welsh CE, Celis‐Morales CA, Ho FK, Brown R, Mackay DF, Lyall DM, et al. Grip strength and walking pace and cardiovascular disease risk prediction in 406,834 UK Biobank participants. Mayo Clin Proc 2020;95:879–888. [DOI] [PubMed] [Google Scholar]
- 8. Yeung CHC, Au Yeung SL, Fong SSM, Schooling CM. Lean mass, grip strength and risk of type 2 diabetes: a bi‐directional Mendelian randomisation study. Diabetologia 2019;62:789–799. [DOI] [PubMed] [Google Scholar]
- 9. Celis‐Morales CA, Lyall DM, Anderson J, Iliodromiti S, Fan Y, Ntuk UE, et al. The association between physical activity and risk of mortality is modulated by grip strength and cardiorespiratory fitness: evidence from 498 135 UK‐Biobank participants. Eur Heart J 2017;38:116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ntuk UE, Celis‐Morales CA, Mackay DF, Sattar N, Pell JP, Gill JMR. Association between grip strength and diabetes prevalence in black, South‐Asian, and white European ethnic groups: a cross‐sectional analysis of 418 656 participants in the UK Biobank study. Diabet Med 2017;34:1120–1128. [DOI] [PubMed] [Google Scholar]
- 11. Celis‐Morales CA, Welsh P, Lyall DM, Steell L, Petermann F, Anderson J, et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ 2018;361:k1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garcia‐Hermoso A, Ramirez‐Velez R, Peterson MD, Lobelo F, Cavero‐Redondo I, Correa‐Bautista JE, et al. Handgrip and knee extension strength as predictors of cancer mortality: a systematic review and meta‐analysis. Scand J Med Sci Spor 2018;28:1852–1858. [DOI] [PubMed] [Google Scholar]
- 13. Leong DP, Teo KK, Rangarajan S, Lopez‐Jaramillo P, Avezum A Jr, Orlandini A, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015;386:266–273. [DOI] [PubMed] [Google Scholar]
- 14. Yates T, Zaccardi F, Dhalwani NN, Davies MJ, Bakrania K, Celis‐Morales CA, et al. Association of walking pace and handgrip strength with all‐cause, cardiovascular, and cancer mortality: a UK Biobank observational study. Eur Heart J 2017;38:3232–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alley DE, Shardell MD, Peters KW, McLean RR, Dam TT, Kenny AM, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci 2014;69:559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Churilla JR, Summerlin M, Richardson MR, Boltz AJ. Mean combined relative grip strength and metabolic syndrome: 2011–2014 National Health and Nutrition Examination Survey. J Strength Cond Res 2020;34:995–1000. [DOI] [PubMed] [Google Scholar]
- 17. Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 2011;40:423–429. [DOI] [PubMed] [Google Scholar]
- 18. Collins R. What makes UK Biobank special? Lancet 2012;379:1173–1174. [DOI] [PubMed] [Google Scholar]
- 19. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Palmer LJ. UK Biobank: bank on it. Lancet 2007;369:1980–1982. [DOI] [PubMed] [Google Scholar]
- 21. Townsend P, Phillimore M, Beattie A. Health and Deprivation: Inequality and the North. London: Routledge; 1988. [Google Scholar]
- 22. Arnold CM, Warkentin KD, Chilibeck PD, Magnus CRA. The reliability and validity of handheld dynamometry for the measurement of lower‐extremity muscle strength in older adults. J Strength Cond Res 2010;24:815–824. [DOI] [PubMed] [Google Scholar]
- 23. Eisen EA, Agalliu I, Thurston SW, Coull BA, Checkoway H. Smoothing in occupational cohort studies: an illustration based on penalised splines. Occup Environ Med 2004;61:854–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eilers PHC, Marx BD. Flexible smoothing with B‐splines and penalties. Stat Sci 1996;11:89–102. [Google Scholar]
- 25. p.adjust {stats} Adjust P‐values for multiple comparisons [https://stat.ethz.ch/R‐manual/R‐devel/library/stats/html/p.adjust.html]
- 26. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 27. Gale CR, Martyn CN, Cooper C, Sayer AA. Grip strength, body composition, and mortality. Int J Epidemiol 2007;36:228–235. [DOI] [PubMed] [Google Scholar]
- 28. Yeung SSY, Reijnierse EM, Trappenburg MC, Hogrel JY, McPhee JS, Piasecki M, et al. Handgrip strength cannot be assumed a proxy for overall muscle strength. J Am Med Dir Assoc 2018;19:703–709. [DOI] [PubMed] [Google Scholar]
- 29. Batty GD, Gale CR, Kivimäki M, Deary IJ, Bell S. Comparison of risk factor associations in UK Biobank against representative, general population based studies with conventional response rates: prospective cohort study and individual participant meta‐analysis. BMJ 2020;368:m131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Association between HGS z‐scores and cancer incidence
Figure S1. Association between absolute HGS and cancer incidence
Figure S2. Association between HGS relative to body weight and cancer incidence
Figure S3. Association between HGS relative to height and cancer incidence
Figure S4. Association between HGS relative to body mass index and cancer incidence
Figure S5. Association between HGS relative to body fat mass and cancer incidence


