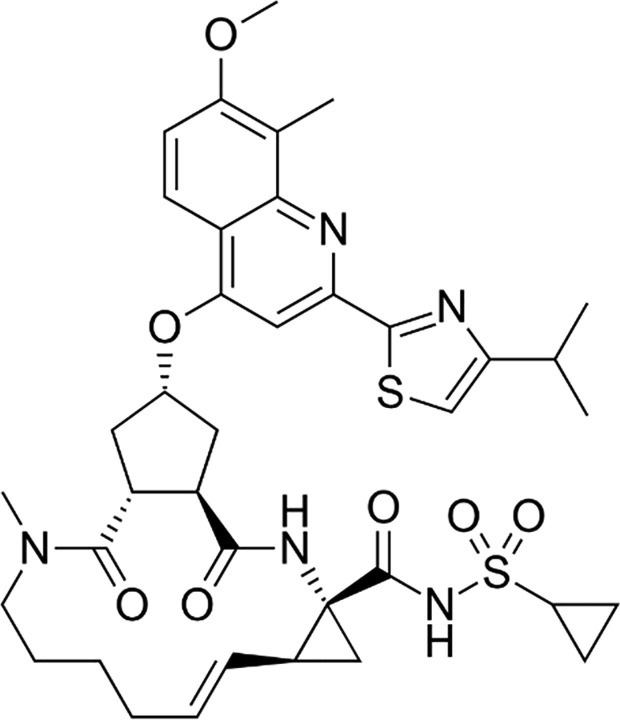
Graphical abstract
Keywords: Simeprevir, Sofosbuvir, Hepatitis C, COVID-19, Spectrophotometry
Abstract
Simeprevir and sofosbuvir are direct-acting antiviral drugs approved for the treatment of chronic HCV infection. Reports demonstrate the similarities between HCV and SARS-CoV-2 in terms of structure and replication mechanism. Therefore, it is suggested that a combination of simeprevir and sofosbuvir may be considered for COVID-19 patients. To date, no spectrophotometric methods have been published for quantitative analysis of simeprevir and sofosbuvir in combination. In this work, two simple spectrophotometric methods allowed quantitative analysis of the studied drugs in the mixed form. The zero-order direct method allowed quantitative analysis of simeprevir at 333 nm, with sofosbuvir showing zero absorbance values. The dual wavelength method allowed quantitative analysis of sofosbuvir by measuring the difference in absorbance values at 259.40 and 276 nm, where the difference in absorbance values of simeprevir was zero. With the applied methods, the investigated drugs in the mixtures and tablets prepared in the laboratory were successfully analyzed quantitatively with acceptable results.
1. Introduction
Simeprevir (SMV), Fig. 1 , is a direct acting antiviral which has been FDA approved in 2013 for the treatment of HCV genotype 1 in combination with ribavirin and PEG-interferon. Sofosbuvir (SFV), Fig. 2 , is a direct acting antiviral which has been FDA approved in 2013 for the treatment of HCV genotype 1,2,3 and 4 in combination with ribavirin or PEG-interferon [1]. In November 2014, FDA approved the combination of simeprevir and sofosbuvir for the treatment of chronic HCV genotype-1 patients. It was the first ribavirin- and interferon-free regimen therapy [2]. The WHO guidelines for the screening, care and treatment of patients with HCV recommend simeprevir and sofosbuvir as combined treatment for HCV genotype 1 and 4 [3].
Fig. 1.
Structural formula of SMV.
Fig. 2.
Structural formula of SFV.
Recently, with the global outbreak of the COVID-19 and the similarities between HCV and SARS-CoV-2 in structure and replication, several studies have considered the activity of anti-HCV drugs such as simeprevir and sofosbuvir, remdesivir, daclatasvir, against SARS-CoV-2 [4], [5], [6], [7], [8].
The literature review of SMV [9], [10], [11], [12], [13] and SFV [14], [15], [16], [17], [18] revealed few published methods for determination of the drugs individually. There were only two published methods for the simultaneous determination goal [19], [20].
In this paper, we developed two simple spectrophotometric methods for determination of SMV and SFV in the laboratory prepared mixtures and tablets. Zero order method enabled quantification of SMV at 333 nm where SFV had zero absorbance values. The dual wavelength method was applied for quantification of SFV by measuring the difference in absorbance values at 259.40 and 276 nm at which the difference in absorbance values of SMV was equal zero.
2. Experimental
2.1. Materials and solvent
Pure SMV & SFV powders were supplied by Benchmark Health Company, Cairo, Egypt. Merospevir® tablets (150 mg SMV per tablet) and Mpiviropack® tablets (400 mg SFV per tablet) were purchased from local drugstore. Laboratory prepared tablets (150 mg SMV, 400 mg SFV, 20 mg talc powder, 15 mg maize starch and 7 mg magnesium stearate per tablet) were laboratory prepared. Ethanol, HPLC grade, was supplied by Sigma-Aldrich, Darmstadt, Germany. Di methyl sulfoxide (DMSO), analytical grade, was supplied by El-Nasr pharmaceutical chemicals company, Cairo, Egypt.
2.2. Apparatus
UV–Visible 1800 Spectrophotometer (Shimadzu Corp., Tokyo, Japan), equipped with 10 mm × 3.5 mL quartz cuvette.
2.3. Standard solutions
SMV and SFV standard stock solutions (100 μg/mL) were freshly prepared by transferring 10 mg of each drug powder into two separate 100-mL volumetric flasks with 10 mL DMSO and 40 mL ethanol, shaken vigorously, and complete to the volume with ethanol.
2.4. Procedures
2.4.1. Linearity and calibration graphs
Two serial dilutions of SMV and SFV were separately prepared by transferring aliquots equivalent to (30–450 µg) and (30–440 µg) of SMV and SFV, respectively, from their standard solutions (100 μg/mL) into a two sets of 10-mL volumetric flasks and diluted to volume with ethanol. The absorption spectra of the prepared solutions were measured against ethanol as a blank in the wavelength range from 200 to 400 nm.
2.4.1.1. Zero-order method
The absorption spectra of SMV were measured directly at 333 nm where there was no absorption interference from SFV. The absorbance values at 333 nm were plotted with SMV concentrations in µg/mL to develop the calibration plot. Regression equation was computed.
2.4.1.2. Dual wavelength method
The difference between absorbance values of SFV spectra at 259.40 and 276 nm was plotted against SFV concentrations to develop the calibration plot. Regression equation was derived.
2.4.2. Analysis of synthetic mixtures
Synthetic mixtures of SMV and SFV were laboratory prepared by transferring different concentrations of SMV and SFV to a set of 10 mL volumetric flasks and adjusting the volume to the mark with ethanol. The mixtures were analyzed following the described procedure and the concentration of the drugs was calculated.
2.4.3. Analysis of SMV and SFV in their single pharmaceutical tablets
Ten Merospevir® and MPIVIROPACK® tablets were separately weighed and grinded to fine powder. A weighed powder equivalent to one tablet of Merospevir® and Mpiviropack® were thoroughly mixed and accurately transferred to 100-mL volumetric flask with 10 mL DMSO and 40 mL ethanol. The samples were shaken vigorously for 20 min, filtered, and adjusted to 100 mL with ethanol. Further dilution with ethanol was made to prepare five samples with different concentrations. The samples were analyzed following the described procedures and the concentration of the drugs was measured.
2.4.4. Analysis of SMV and SFV in their laboratory prepared tablets
Ten laboratory prepared tablets were weighed and grinded to fine powder. A weighed powder equivalent to one tablet was accurately transferred to 100-mL volumetric flask with 10 mL DMSO and 40 mL ethanol. The sample were shaken vigorously for 20 min, filtered, and adjusted to 100 mL with ethanol. Further dilution with ethanol was made to prepare five samples with different concentrations. The samples were analyzed following the procedure mentioned for each method under the linearity and calibration graphs and the concentration of each drug was computed.
3. Results and discussion
3.1. Spectral characteristics
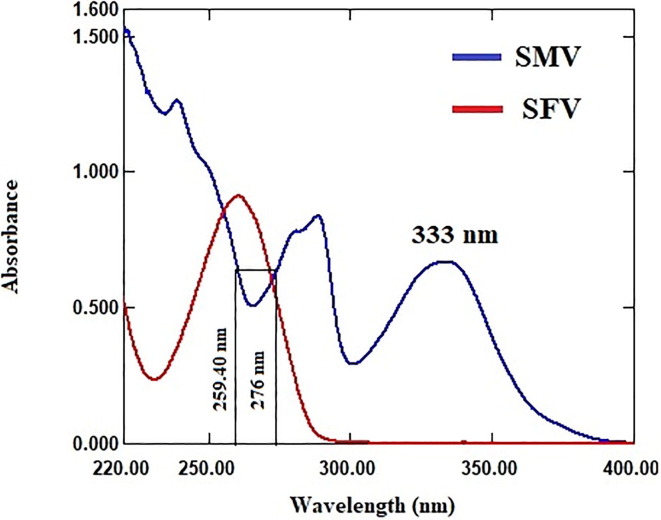
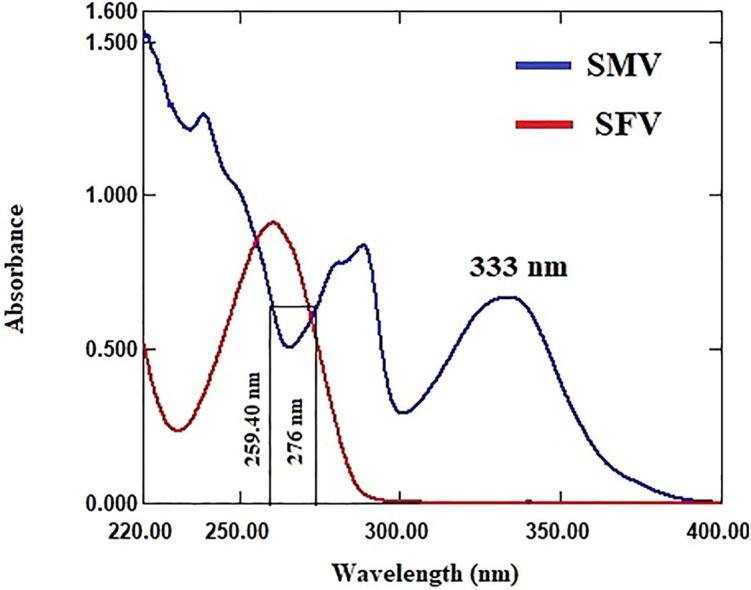
The UV absorption spectra of both SMV and SFV, Fig. 3 , SMV shows extended absorption peak at 333 nm without overlapping from SFV, which exhibits zero absorbance in the wavelength range from 400 to 300 nm. SMV could be directly determined in mixture with SFV using zero order absorption method. The absorbance values of zero order absorption spectra at 333 nm were directly proportional the concentration of SMV without any interference from SFV. On the other hand, SFV shows sever overlap spectra with SMV in the wavelength range from 300 to 200 nm, which hinders SFV direct determination. Therefore, it is recommended to overcome the contribution of SMV spectra by mathematical method to enable quantitative estimation of SFV.
Fig. 3.
Absorption spectra of SMV (25 μg/mL) and SFV (36 μg/mL).
Dual wavelength method is based on measuring the difference in absorbance values of the drug of interest at two wavelengths where the difference in absorbance values for any other overlapped drug in the mixture is equal zero. So, for determination of SFV in mixture with SMV by dual wavelength method, several pairs of wavelength at which the difference of absorbance values of SMV is equal zero were tested. Accepted linearity of SFV was obtained by measuring the difference in absorbance values at 259.40 and 276 nm. The difference in absorbance values at 259.40 and 276 nm is directly proportional to the SFV concentrations without contribution from SMV, Fig. 3.
3.2. Methods validation
The described methods were validated according to the guidelines of ICH [21]. Data listed in Table 1 represents the limits of detection (LOD) & limits of quantitation (LOQ), accuracy, precision, linearity, and regression parameters. The described methods succeeded to selectivity analyze SMV and SFV in the laboratory prepared mixtures as shown in Table 2 .
Table 1.
Regression and validation data for quantitative analysis of SMV and SFV by the proposed methods.
| Parameters | SMV | SFV |
|---|---|---|
| Wavelength (nm) | 333 | 259.40 & 276 |
| Linearity range (µg/mL) | 3–45 | 3–44 |
| Slope | 0.0263 | 0.0130 |
| Intercept | 0.0085 | −0.0001 |
| Coefficient of determination (r2) | 0.9997 | 0.9997 |
| LOD (µg/mL) | 0.888 | 0.803 |
| LOQ (µg/mL) | 2.692 | 2.434 |
| Accuracy (%R)a | 100.25 | 100.06 |
| Repeatability precision (RSD)b | 0.768 | 0.595 |
| Intermediate precision (RSD)b | 0.927 | 0.801 |
Average of 9 determinations (3 concentrations repeated 3 times).
RSD of 9 determinations (3 concentrations repeated 3 times).
Table 2.
Application of the proposed methods for the determination of SMV and SFV in their laboratory prepared mixtures.
| Taken (µg/mL) |
%Recovery |
||
|---|---|---|---|
| SMV | SFV | SMV | SFV |
| 3 | 8 | 99.49 | 99.13 |
| 6 | 16 | 98.54 | 100.05 |
| 9 | 24 | 100.34 | 101.31 |
| 12 | 32 | 100.92 | 100.26 |
| 15 | 40 | 100.00 | 98.67 |
| Mean ± RSD | 99.86 ± 0.901 | 99.89 ± 1.031 | |
3.3. Application of the methods
The described methods exhibited successful quantification of SMV and SFV in the synthetic mixtures (Table 2) and in single pharmaceutical tablets (Table 3 ) as well as in the laboratory prepared tablets (Table 4 ) without any interference from each other or tablet matrix.
Table 3.
Application of the proposed methods for the determination of SMV and SFV in their single-ingredient tablets.
| Taken (µg/mL) |
%Recovery |
||
|---|---|---|---|
| Merospevir® tablets | MPIVIROPACK® tablets | SMV | SFV |
| 3 | 8 | 100.13 | 100.10 |
| 6 | 16 | 99.11 | 99.66 |
| 9 | 24 | 99.07 | 100.99 |
| 12 | 32 | 100.98 | 100.50 |
| 15 | 40 | 99.24 | 100.02 |
| Mean ± RSD | 99.71 ± 0.836 | 100.26 ± 0.508 | |
Table 4.
Application of the proposed methods for the determination of SMV and SFV in their laboratory prepared tablets.
| Taken (µg/mL) |
%Recovery |
||
|---|---|---|---|
| SMV | SFV | SMV | SFV |
| 3 | 8 | 100.51 | 101.06 |
| 6 | 16 | 100.76 | 99.90 |
| 9 | 24 | 100.34 | 99.71 |
| 12 | 32 | 99.97 | 100.14 |
| 15 | 40 | 99.49 | 100.98 |
| Mean ± RSD | 100.21 ± 0.494 | 100.36 ± 0.620 | |
4. Conclusion
In this work, we developed the first spectrophotometric methods for quantitative analysis of SMV and SFV in their mixture. The applied methods were successfully determined the drugs under the study in the laboratory prepared mixtures and tablets without any interferences.
CRediT authorship contribution statement
Sherif Ramzy: Conceptualization, Methodology, Writing – original draft. Ahmed H. Abdelazim: Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Eletreby R., Elakel W., Said M., El Kassas M., Seif S., Elbaz T., El Raziky M., Abdel Rehim S., Zaky S., Fouad R., Gamal Eldeen H., Abdo M., Korany M., Yosry A., El Serafy M., El-Sayed M.H., ElShazly Y., Waked I., Doss W., Esmat G. Real life Egyptian experience of efficacy and safety of Simeprevir/Sofosbuvir therapy in 6211 chronic HCV genotype IV infected patients. Liver Int. 2017;37(4):534–541. doi: 10.1111/liv.13266. [DOI] [PubMed] [Google Scholar]
- 2.Sclair S.N., Hernandez M.D.P., Vance E., Gilinski D., Youtseff H., Toro M., Antoine M., Jeffers L.J., Peyton A. Sofosbuvir and simeprevir combination therapy for HCV genotype 1 infection: Results of a single-center VA experience. Gastroenterol. Hepatol. 2016;12:490–497. [PMC free article] [PubMed] [Google Scholar]
- 3.World health organization (WHO) guidelines for the screening, care and treatment of persons with chronic hepatitis C infection, Updated version, April,2016. Guidelines. [PubMed]
- 4.G.R. de Medeiros, I.C.C. Farias, J.V.C. Farias, P.R. de Macedo, Anti-HCV antiviral therapies as an alternative for the treatment of Covid-19, Res. Soc. Dev. 9(2020) e9489109406-e9489109406. 10.33448/rsd-v9i10.9406. [DOI]
- 5.Alothaid H., Aldughaim M.S.K., El Bakkouri K., AlMashhadi S., Al-Qahtani A.A. Similarities between the effect of SARS-CoV-2 and HCV on the cellular level, and the possible role of ion channels in COVID19 progression: a review of potential targets for diagnosis and treatment. Channels. 2020;14(1):403–412. doi: 10.1080/19336950.2020.1837439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo H.S., Hui K.P.Y., Lai H.-M., He X.u., Khan K.S., Kaur S., Huang J., Li Z., Chan A.K.N., Cheung H.-Y., Ng K.-C., Ho J.C.W., Chen Y.W., Ma B., Cheung P.-H., Shin D., Wang K., Lee M.-H., Selisko B., Eydoux C., Guillemot J.-C., Canard B., Wu K.-P., Liang P.-H., Dikic I., Zuo Z., Chan F.K.L., Hui D.S.C., Mok V.C.T., Wong K.-B., Mok C.K.P., Ko H.o., Aik W.S., Chan M.C.W., Ng W.-L. Simeprevir potently suppresses SARS-CoV-2 replication and synergizes with remdesivir. ACS Cent. Sci. 2021;7(5):792–802. doi: 10.1021/acscentsci.0c01186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shyr Z.A., Cheng Y.-S., Lo D.C., Zheng W. Drug combination therapy for emerging viral diseases. Drug Discovery Today. 2021;26(10):2367–2376. doi: 10.1016/j.drudis.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gammeltoft K.A., Zhou Y., Duarte Hernandez C.R., Galli A., Offersgaard A., Costa R., Pham L.V., Fahnøe U., Feng S., Scheel T.K.H., Ramirez S., Bukh J., Gottwein J.M. Hepatitis C Virus Protease Inhibitors Show Differential Efficacy and Interactions with Remdesivir for Treatment of SARS-CoV-2 In Vitro. Antimicrob. Agents Chemother. 2021;65(9) doi: 10.1128/AAC.02680-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanwelkenhuysen I., De Vries R., Timmerman P., Verhaeghe T. Determination of simeprevir: A novel, hepatitis C protease inhibitor in human plasma by high-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. B. 2014;958:43–47. doi: 10.1016/j.jchromb.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 10.Nannetti G., Pagni S., Parisi S.G., Alberti A., Loregian A., Palù G. Development of a simple HPLC–UV method for the determination of the hepatitis C virus inhibitor simeprevir in human plasma. J. Pharm. Biomed. Anal. 2016;121:197–203. doi: 10.1016/j.jpba.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Mohammed B.S., Hamad A.E., El‐Malla S.F., Derayea S.M. Sensitive spectrofluorimetric assay based on micelle enhanced protocol for the determination of hepatitis C antiviral agent (simeprevir): Application to dosage form and human plasma. Microchem. J. 2020;152:104372. doi: 10.1016/j.microc.2019.104372. [DOI] [Google Scholar]
- 12.Youssef A.F.A., Issa Y.M., Nabil K.M. Development and Validation of a New Method for the Determination of Anti-hepatitis C Agent Simeprevir in Human Plasma using HPLC with Fluorescence Detection. Curr. Anal. Chem. 2020;16(4):428–435. doi: 10.2174/1573411015666181217121619. [DOI] [Google Scholar]
- 13.Mohammed B.S., Hamad A.E., Derayea S.M. Stress stability study of simeprevir, a hepatitis C virus inhibitor, using feasible TLC-spectro-densitometry: application to pharmaceutical dosage form and human plasma. RSC Adv. 2020;10(36):21100–21107. doi: 10.1039/D0RA01172J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.S.AN. Abdel-Gawad, Simple chromatographic and spectrophotometric determination of sofosbuvir in pure and tablet forms, Eur. J. Chem. 7(2016) 375-379. 10.5155/eurjchem.7.3.375-379.1439. [DOI]
- 15.El-Yazbi A.F. Comparative validation of the determination of sofosbuvir in pharmaceuticals by several inexpensive ecofriendly chromatographic, electrophoretic, and spectrophotometric methods. J. AOAC Int. 2017;100:1000–1007. doi: 10.5740/jaoacint.16-0295. [DOI] [PubMed] [Google Scholar]
- 16.El Hamd M.A., Ali R., Marzouk A.A., Abdelmageed O.H. Validated ultraviolet-spectrometric method for determination of sofosbuvir in tablets formulation. J. Appl. Pharm. Sci. 2017;7:114–119. doi: 10.7324/JAPS.2017.70214. [DOI] [Google Scholar]
- 17.Rezk M.R., Basalious E.B., Amin M.E. Novel and sensitive UPLC–MS/MS method for quantification of sofosbuvir in human plasma: application to a bioequivalence study. Biomed. Chromatogr. 2016;30(9):1354–1362. doi: 10.1002/bmc.3690. [DOI] [PubMed] [Google Scholar]
- 18.Nebsen M., Elzanfaly E.S. Stability-indicating method and LC–MS-MS characterization of forced degradation products of sofosbuvir. J. Chromatogr. Sci. 2016;54(9):1631–1640. doi: 10.1093/chromsci/bmw119. [DOI] [PubMed] [Google Scholar]
- 19.Mohammed B.S., Derayea S.M., Hamad A.E. Feasible TLC-Spectro-Densitometry Technique for Simultaneous Determination of Two Hepatitis C Antiviral Drugs, Sofosbuvir and Simeprevir: Application to Combined Pharmaceutical Dosage Forms and Human Plasma. J. Chromatogr. Sci. 2021;59:576–583. doi: 10.1093/chromsci/bmab034. [DOI] [PubMed] [Google Scholar]
- 20.Mohamed S.H., Issa Y.M., Salim A.I. Simultaneous quantification of simeprevir sodium: A hepatitis C protease inhibitor in binary and ternary mixtures with sofosbuvir and/or ledipasvir utilizing direct and H-point standard addition strategies. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019;210:290–297. doi: 10.1016/j.saa.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Q.B. International Conference on Harmonization (ICH), Federal Register, 62(1997).