Abstract
Background
Sarcopenia is an important factor in the postoperative outcome of gastrointestinal cancer patients. However, little research has been carried out on potential biomarkers of sarcopenia. Carnitine is an amino acid derivative that is stored in skeletal muscle and is essential for muscle energy metabolism. The primary purpose of this study was to investigate whether serum carnitine level is a biomarker of sarcopenia in preoperative patients with gastrointestinal cancer. The secondary purposes were (i) to examine the associations between carnitine, nutritional status, and albumin level, and (ii) to determine whether carnitine is a prognostic factor for postoperative complications.
Methods
One hundred fourteen patients scheduled to undergo gastroenterological surgery between August 2016 and January 2017 were enrolled. Their mean age was 68.4 ± 10.5, and 64.9% were male. Serum carnitine fractions [total carnitine (TC), free l‐carnitine (FC), and acylcarnitine (AC)] were measured prior to surgery. The correlation between carnitine level and a variety of clinical features was analysed, including skeletal muscle index (SMI), sarcopenia, prognostic nutritional index (PNI), and postoperative complications.
Results
Tumour locations included the oesophagus (n = 17), stomach (n = 16), pancreas (n = 20), bile duct (n = 9), liver [n = 33; primary liver cancer (n = 18), liver metastasis (n = 15)], and colorectal region (n = 19). TC and FC levels varied significantly by tumour location. TC and FC showed significant positive correlations with SMI [TC (r = 0.295, P = 0.0014), FC (r = 0.286, P = 0.0020)] and PNI [TC (P = 0.0178, r = 0.222), FC (P = 0.0067, r = 0.2526)]. These levels were significantly lower in the sarcopenia group (TC, P = 0.0124; FC, P = 0.0243). In addition, TC and FC showed significant positive correlations with ALB level [TC (P = 0.038 r = 0.19), FC (P = 0.016 r = 0.23)]. When patients were divided into high ALB (≥3.5 g/dL, 96 patients) and low ALB (<3.5 g/dL, 18 patients) groups, these correlations were no longer significant, but in the low ALB group there was a tendency towards a negative relationship between ALB level and both TC and FC. No significant relationship was found between postoperative complications and carnitine level.
Conclusions
This study suggests that carnitine level is a biomarker of sarcopenia and nutritional status. However, it did not find an association between carnitine level and postoperative complications.
Keywords: Carnitine, Sarcopenia, Metabolism, Prognostic nutritional index, Postoperative complication
Introduction
Skeletal muscle is not only the basis of movement in the human body, but it is also the body's largest site of metabolism and is itself an endocrine organ. Muscle dysfunction negatively affects physical activity, as well as other aspects of health and quality of life. Sarcopenia is a progressive and generalized skeletal muscle disorder characterized by a loss of skeletal muscle mass and strength. It is associated with an increased likelihood of adverse outcomes, including falls, fractures, physical disability, and mortality. 1 In the field of gastrointestinal oncological surgery, several studies have reported that sarcopenia is a negative prognostic factor for short and long‐term outcomes. 2 , 3 , 4 Muscle status is evaluated by assessing an individual's grip strength, gait speed, and lean muscle mass using dual‐energy X‐ray absorptiometry (DXA), bioelectrical impedance analysis (BIA), and skeletal muscle index (SMI). SMI is calculated based on the skeletal muscle area on a computed tomography (CT) scan at the level of the third lumbar vertebra (L3), which is frequently used in retrospective studies of gastrointestinal surgery. However, only a few biomarkers of muscle status have been reported. 5
In this study, we focused on carnitine level as a potential biomarker of sarcopenia. Carnitine (β‐hydroxy‐N‐trimethyl‐aminobutyric acid) is an amino acid derivative that plays an important role in energy production in the mitochondria of skeletal and cardiac muscles. Ninety‐five per cent of carnitine is stored in these muscle types, 6 with almost all of it located inside their cells. 7 Under normal circumstances, carnitine is mainly obtained from the diet; however, it is also synthesized from lysine and methionine in the liver, kidney, and brain. Red meat is the quintessential carnitine‐rich food. 7 In the muscle carnitine metabolic pathway, free l‐carnitine (FC) and acylcarnitine (AC) produce energy through fatty acid beta‐oxidation. FC transports long‐chain fatty acid derivatives into the mitochondria via carnitine palmitoyltransferase 1 (CPT 1), and AC modulates rises in intramitochondrial acyl‐CoA/CoA ratio via CPT 2. 7 This modulation protects the cell and reduces the inhibition of metabolic enzymes in the other primary energy production pathways, glucose and amino acid catabolism. 8 It has been reported that an increase in certain types of acylcarnitine is an indicator of abnormal fatty acid metabolism. 9
Metabolic pathway dysfunctions in mitochondria have been reported to be related to reduced muscle mass and loss of strength. 10 There are also reports of an association between low serum carnitine level and cancer cachexia 11 and of serum carnitine level as a possible muscle mass biomarker in chronic kidney disease patients. 12 Fatty acid oxidation is the main energy production pathway during low intensity (<50% of maximal oxygen consumption) exercise. 7 Based on the above findings, we hypothesized that carnitine is present at low levels under conditions of low muscle activity, low muscle volume, and malnutrition, as in the case of sarcopenia patients with gastrointestinal cancer. In the literature, no previous studies have examined the association of serum carnitine level with muscle condition or with nutritional status in gastrointestinal cancer patients. The primary purpose of this study was to investigate whether serum carnitine level is a biomarker of sarcopenia in preoperative patients with gastrointestinal cancer. The secondary purposes were to examine the association between carnitine and nutritional status and determine whether carnitine is a prognostic factor for postoperative complications.
Methods
Study population
This prospective clinical pilot study enrolled patients who were scheduled to undergo standard curative operations for gastrointestinal cancer at Shizuoka General Hospital from August 2016 to January 2017. The exclusion criteria were as follows: (i) patients with pathological diagnosis of a benign tumour; (ii) those who had undergone exploratory laparotomy, palliative surgery, or other non‐curative resection; and (iii) those taking carnitine supplements.
Sample size
The present investigation is a pilot study. To date, there have been no studies of carnitine levels in relation to gastrointestinal cancer, and it remains unknown whether these levels vary with the location of the malignancy. Thus, this study divided the whole cohort into the following groups according to tumour location: oesophageal cancer, gastric cancer, pancreatic cancer, bile duct cancer, primary liver cancer, liver metastasis, and colorectal cancer. The resulting sample sizes ranged from 5 to 20 cases per group.
Sample and data collection
Patients' blood samples were collected at 1 or 2 days before surgery, and three serum carnitine levels were measured using a carnitine enzymatic cycling assay: FC, AC, and total carnitine (TC) levels. The following data were prospectively collected and recorded: preoperative clinical data [sex, age, body mass index (BMI), albumin level (ALB level)], preoperative SMI, and prognostic nutritional index (PNI), clinical course date (postoperative complications), and pathological diagnosis.
Skeletal muscle index and sarcopenia diagnosis
Skeletal muscle index was used to evaluate muscle volume. SMI assessment was performed using a Synapse Vincent image analysis system (Fujifilm Medical, Tokyo, Japan). The cross‐sectional area of total skeletal muscle (TSM L3) (cm2) was measured using an axial image at the third lumbar vertebral level (L3). SMI (cm2/m2) was calculated as SMI = TSM L3/height2 (m2). Sarcopenia was defined according to the criteria of the Japan Society of Hepatology (JSH criteria), 13 and patients were classified into sarcopenia and non‐sarcopenia groups based on the SMI cut‐off values of 42 for men and 38 for women.
Prognostic nutritional index cut‐off value
PNI was used to evaluate an individual's nutritional status. It was calculated as 10 × serum ALB level (g/L) + 0.005 × total lymphocyte count (/mm3). In previous studies, PNI cut‐off values have varied depending on the type of surgery, cancer location, and study outcome. In this study, the cut‐off value was defined as the mean PNI value, and patients were divided into low and high‐PNI groups based on that value.
Postoperative complications
Postoperative complications were scored according to the Clavien–Dindo classification system. 14 Patients with a Clavien–Dindo grade of ≥III were considered to have complications.
Statistical analysis
Statistical analysis was carried out using JMP 11.0 software (SAS Institute Inc., Cary, NC, USA). A P value of 0.05 was considered statistically significant. Continuous variables are shown as mean ± standard deviation (SD), or as median, minimum, and maximum values. Analysis of variance (ANOVA) was used to determine the association between carnitine level and cancer location. Correlations between carnitine levels and continuous and categorical variables were assessed using Pearson's correlation coefficient and Student's t test, respectively.
Ethical approval
This study was performed at the Shizuoka General Hospital Department of Gastroenterological Surgery and was approved by the hospital's Ethical Committee on Clinical Studies (IRB approval code: SGHIRB#2015077). It was performed in accordance with the Declaration of Helsinki, and written informed consent was obtained preoperatively from all participants.
Results
Clinical characteristics
One hundred twenty Japanese patients scheduled to undergo gastrointestinal surgery between August 2016 and January 2017 were enrolled. Six patients were excluded from the study because (i) they had undergone palliative surgery or exploratory laparotomy, (ii) their course of treatment had been changed to radiofrequency ablation therapy for hepatocellular carcinoma, or (iii) they had received a pathological diagnosis of having a benign tumour (Figure 1 ). None of the patients were taking carnitine supplements. The patients' clinical characteristics (sex, age, ALB level, BMI, SMI, PNI, and tumour location) and carnitine levels (TC, FC, and AC) are shown in Table 1 . The study population had a slight male predominance of 64.9%. Mean ALB level was 4.0 g/dL, with a wide range from 2.4 to 5 g/dl. Mean BMI, SMI, and PNI were 22.3, 41.4, and 47.3, respectively. The variation in carnitine level by tumour location is shown in Figure 2 . TC and FC levels varied significantly by location (TC, P = 0.0005; FC, P = 0.0003). Patients with oesophageal cancer and liver metastasis had lower TC and FC levels, whereas those with colorectal cancer had higher levels.
Figure 1.
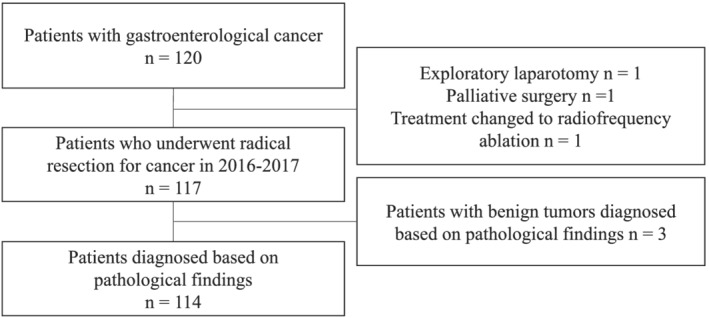
Patient selection flow chart.
Table 1.
Patient characteristics, tumour location, and carnitine level
| Variable | All patients (n = 114) |
|---|---|
| Sex | |
| Male | 74 (64.9%) |
| Female | 40 (33.1%) |
| Age, years | 68.4 ± 10.5 |
| Albumin g/dL | 4 (2.4–5.0) |
| BMI (kg/m2), mean ± SD | 22.3 ± 3.3 |
| SMI (cm2/m2), mean ± SD | 41.4 ± 7.6 |
| PNI, mean ± SD | 47.3 ± 6.2 |
| Tumour location | |
| Oesophagus | 17 (14.9%) |
| Stomach | 16 (14.0%) |
| Pancreas | 20 (17.5%) |
| Bile duct | 9 (7.9%) |
| Primary liver cancer | 18 (15.8%) |
| Liver metastasis | 15 (13.2%) |
| Colorectal cancer | 19 (16.7%) |
| Carnitine levels | |
| Total carnitine | 60.4 (25.5–102.5) |
| Free carnitine | 50.4 (20.6–93.5) |
| Acylcarnitine | 9.8 (3.9–24.5) |
BMI, body mass index; SMI, skeletal muscle index; PNI, prognostic nutritional index.
Figure 2.
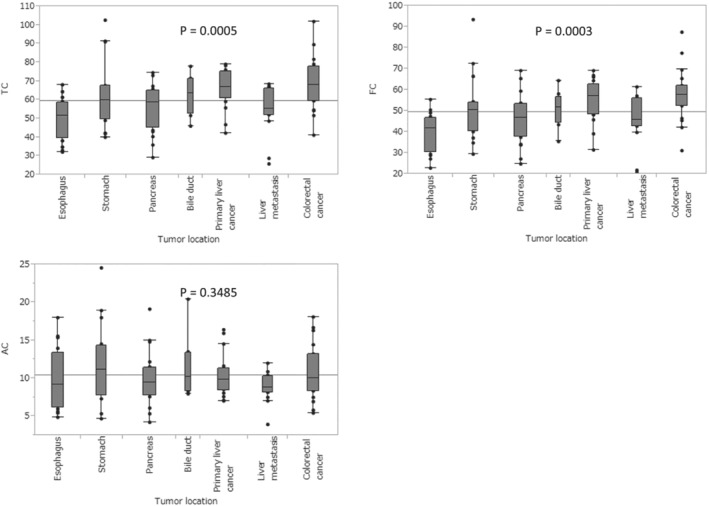
Analysis of variance for differences in carnitine level by tumour location.
Association of carnitine levels with age, body mass index, skeletal muscle index, and prognostic nutritional index
Correlation coefficients between carnitine level and age, BMI, SMI, and PNI, and their P values are shown in Table 2 . TC and FC levels were significantly correlated with SMI and PNI (Figure 3 ). No correlation was found between carnitine levels and age. AC was not significantly correlated with SMI or PNI. BMI was significantly correlated with all carnitine levels.
Table 2.
Correlation coefficients and P value between carnitine levels and age, BMI, SMI, and PNI
| TC | FC | AC | ||||
|---|---|---|---|---|---|---|
| Variable | r | P value | r | P value | r | P value |
| Age | −0.03 | 0.73 | −0.03 | 0.79 | −0.004 | 0.96 |
| BMI (kg/m2) | 0.35 | 0.0002 | 0.3 | 0.0013 | 0.3 | 0.0015 |
| SMI (cm2/m2) | 0.3 | 0.0014 | 0.29 | 0.002 | 0.13 | 0.18 |
| PNI | 0.22 | 0.018 | 0.25 | 0.007 | −0.026 | 0.79 |
BMI, body mass index; PNI, prognostic nutritional index; SMI, skeletal muscle index.
Figure 3.
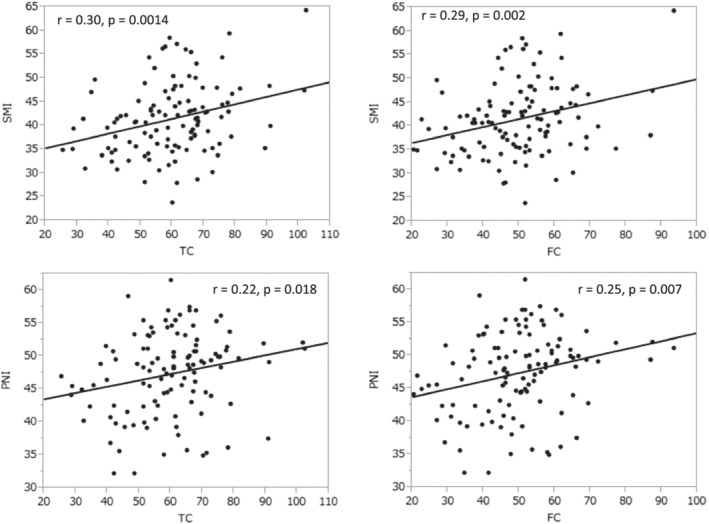
Correlations of total carnitine (TC) and free carnitine (FC) with skeletal muscle index (SMI) and prognostic nutritional index (PNI).
Association of carnitine level with albumin level
The correlation between each of the carnitine levels and ALB level is shown in Figure 4 . TC and FC were significantly correlated with ALB level (TC: P = 0.038 r = 0.19, FC: P = 0.016 r = 0.23). There was a large range in plasma ALB level (from 2.4 to 5 g/dL, mean 4.0), so we divided patients into a high ALB group (≥3.5 g/dL, 96 patients) and a low ALB group (<3.5 g/dL, 18 patients) for further analysis. After this division, neither group showed significant correlations between ALB level and TC or FC level. However, in the high ALB group, the correlation coefficients (r value) both between ALB and TC and between ALB and FC were positive, but in the low ALB group, both of these correlation coefficients were negative.
Figure 4.
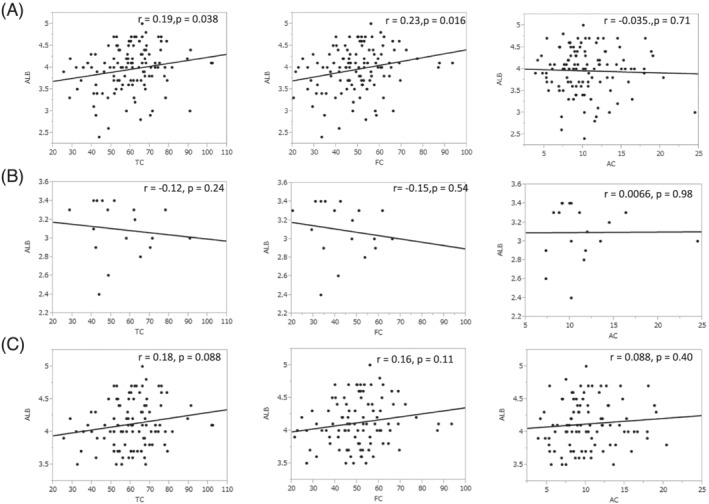
Associations between carnitine level and albumin (ALB) level. (A) Associations between carnitine level and ALB level for all patients, (B) associations between carnitine level and ALB level in low ALB group, defined as serum ALB level <3.5, 18 patients. (C) Associations between carnitine level and ALB level in high ALB group, defined as serum ALB level ≥3.5 g/dL, 96 patients.
Association of carnitine levels with sarcopenia, nutritional status, and surgical outcome
The TC and FC levels were significantly lower in the sarcopenia group than in the non‐sarcopenia group, as classified by JSH criteria (TC, P = 0.0124; FC, P = 0.0243). The low‐PNI group [<47.3 (mean value)] had lower TC and AC levels than the high‐PNI group (TC, P = 0.0003; FC, P = 0.0001). However, carnitine levels were not significantly different between patients with and without postoperative complications (TC, P = 0.997; FC, P = 0.872; AC, P = 0.411) (Table 3). There was also no significant difference between the sarcopenia and non‐sarcopenia groups in relation to postoperative complications in this study cohort (P = 0.07). Similarly, there was no significant difference between the low‐PNI and high‐PNI groups in relation to postoperative complications (P = 0.92).
Table 3.
Association of carnitine levels with sarcopenia, nutrition status, surgical outcome
| Variable | TC | FC | AC |
|---|---|---|---|
| Sarcopenia | P = 0.0124 | P = 0.0243 | P = 0.102 |
| With sarcopenia group (n = 57) | 63.3 (29–102.5) | 52.2 (24.8–93.5) | 11 (4.2–24.5) |
| Without sarcopenia group (n = 57) | 56.6 (25.5–91.2) | 46.8 (20.6–77.2) | 9.8 (3.9–18.9) |
| Nutrition status a | P = 0.0003 | P = 0.0001 | P = 0.484 |
| Low PNI group (n = 66) | 54.3 (25.5–90.8) | 44.2 (20.6–69.5) | 10.2 (3.9–20.4) |
| High PNI group (n = 48) | 64 (38–102.5) | 53.4 (29.4–93.5) | 10.7 (4.7–19.1) |
| Surgical outcome | P = 0.997 | P = 0.872 | P = 0.411 |
| With postoperative complication (n = 37) | 59.9 (32–90.8) | 49.2 (22.8–77.2) | 10.9 (5.4–24.5) |
| Without postoperative complication (n = 77) | 59.9 (25.5–102.5) | 49.6 (20.6–93.5) | 10.2 (3.9–20.4) |
AC, acylcarnitine FC, free carnitine; PNI, prognostic nutritional index; TC, total carnitine.
Cut‐off value is mean PNI value.
Discussion
The key finding of this study is that serum TC and FC levels were significantly correlated with SMI. TC and FC levels were also significantly lower in the sarcopenia group than in the non‐sarcopenia group; however, AC level was not. To our knowledge, this is the first study evaluating the correlation between carnitine level and sarcopenia.
Carnitine is an amino acid derivative largely acquired through the diet, but it is also synthesized from lysine and methionine provided by the diet. It has been reported that deficits in dietary carnitine intake may largely be compensated for by high lysine and methionine levels in the diet, by the reabsorption of carnitine by the kidneys, and by a slow turnover rate of endogenous carnitine. 15 Carnitine synthesis has been reported to be regulated by muscle function. 16 In muscle, carnitine plays an important role in transporting fatty acids into mitochondria in the process of fatty acid oxidation (FAO). FAO is inhibited by acetyl‐CoA carboxylase 2 (ACC2), which catalyses malonyl‐CoA, a direct inhibitor of the carnitine transport of fatty acids into mitochondria. That is, reducing ACC2 activity increases carnitine activity, which in turn increases in fatty acid metabolism. In an interesting recent development, Ritterhoff et al. investigated the therapeutic significance of reduced ACC2 activity using heart failure model mice. The study revealed that ACC2‐deficient mice showed the accumulation of certain kinds of unproductive acylcarnitine, an indicator of abnormal fatty acid metabolism. Interestingly, they also found that mitochondrial oxidative capacity was sustained in female ACC2‐deficient mice, but not in male mice, a difference that they found was related to sex‐dependent regulation of the peroxisome proliferator‐activated receptor α signalling pathway in heart failure. This sustained oxidative capacity led to improved energetics in the heart muscle of the female mice. 16
Our study has revealed an association between ALB and carnitine. ALB is an important marker of nutrition status and a useful predictive marker of surgical risk. 17 Our study showed a significant correlation both between ALB and TC and between ALB and FC, suggesting that TC and FC are possible predictive markers of surgical risk. Interestingly, when patients were divided into groups by low and high ALB level, while the correlations between ALB level and both TC and FC were no longer significant in either group, there was a tendency towards a negative relationship between ALB level and both TC and FC in the low ALB group. These results suggest the possibility that compensatory carnitine synthesis had occurred in the low ALB group. Vegetarians have been reported to have lower serum ALB levels 18 but also higher l‐carnitine levels than non‐vegetarians. 15 Taken together, these findings suggest the possibility that compensatory carnitine synthesis occurs under low ALB conditions in humans.
The discovery of a biomarker for sarcopenia may be useful for assessing the risk of postoperative complications and their potential oncological outcome after surgical resection in patients with gastrointestinal cancer. Such an assessment could help to prevent sarcopenia and low nutrition status through preoperative nutritional intervention. Several studies have previously reported the effectiveness of preoperative rehabilitation and nutritional intervention (referred to as ‘prehabilitation’) for short‐term and long‐term oncological outcomes. 19 , 20 , 21 Thus, carnitine level has the potential to be a simple, objective, and quick test for determining whether such an intervention should be carried out.
Many studies have reported the importance of muscle status (strength, performance, and volume) for various surgical and oncological outcomes. 3 , 4 , 5 Muscle status is evaluated using a variety of parameters. Grip strength, chair stand test, DXA, BIA, SMI, and gait speed are listed in the criteria for the European Working Group on Sarcopenia in Older People, which were revised in 2019. 22 Furthermore, detailed evaluations of skeletal muscle composition, including intramuscular adipose tissue content 23 , 24 and visceral‐to‐subcutaneous adipose tissue area ratio 25 , 26 have also been reported to be prognostic factors for the surgical outcome and survival of patients with gastrointestinal cancer. However, the assessment of these parameters requires investments in labour, measuring instruments, specialized software, time, and cost. Moreover, a patient's condition may lead to errors in muscle strength measurement and in the gait speed test, and SMI and other parameters utilizing CT data are prone to measurement bias in retrospective studies. Furthermore, the cut‐off values of these parameters vary by race. The JSH criteria were developed based on multiple studies of sarcopenia in Japanese patients. 13 A simple and objective parameter that can be easily carried out by a variety of institutions is required to facilitate a more universal concept of sarcopenia.
This study focused on serum carnitine levels. There have been several suggestive studies on the clinical benefits of carnitine supplementation. Hiatt et al. showed that carnitine supplementation (propionyl‐l‐carnitine) in patients with disabling claudication improved walking distance and speed as well as symptoms of physical fatigue in a double‐blind, randomized, placebo‐controlled trial. 27 Gramignano et al. reported that patients with carcinoma had symptoms of fatigue and nutritional status improved by carnitine supplementation. 28 However, Primassin et al. have pointed out that the efficacy and even the safety of carnitine supplementation are unclear and that controlled studies have given no substantial insight into this issue. 29 In a Phase III trial conducted by the Eastern Cooperative Oncology Group (ECOG), the effectiveness of carnitine supplementation in improving symptoms of fatigue could not be established. 30 The ECOG study is the only one that has measured serum carnitine levels. In that study, mean AC, FC, and TC levels were 11.35, 37.2, and 46.28 in the carnitine supplementation group and 10.68, 35.10, and 43.56 in the placebo group. The mean FC and TC levels in that study were lower than those in ours, which may be due to differences in the characteristics of the patients studied. Our study included only patients with resectable cancer, whereas the ECOG study included mainly patients with unresectable cancer [chemotherapy (78.1%) and radiotherapy (18.7%)]. The degree of cancer progression may be associated with a decrease in carnitine level. In our study, the mean FC and TC levels were lower in the liver metastasis group than in the other groups, except for the oesophageal cancer group.
Previous studies have reported that both sarcopenia and PNI are predictors of postoperative complications. 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 While this study found that carnitine was significantly correlated with sarcopenia and PNI, it did not identify carnitine as a predictor of postoperative complications. This appears to be due to the selection of patients included in the study. While most of the previous studies reporting sarcopenia and PNI as predictors of postoperative complications were limited to a single type of cancer and surgery, 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 this study included a variety of cancer locations and types of surgery, and these subgroups had small sample sizes. This limitation may be overcome by further studies of carnitine based on a larger sample size of these various cancer locations and surgery types.
There are several additional limitations to this study. The overall sample size was small. In addition, the study did not evaluate factors that may affect carnitine metabolism, such as degree of cancer progression, chronic systemic disease (heart, liver, or renal dysfunction), or diet.
Conclusion
This pilot study has revealed that total carnitine and free carnitine levels are associated with sarcopenia, indicating that serum carnitine is a potential biomarker for sarcopenia. It has also revealed that total carnitine and free carnitine levels are associated with the nutritional status of preoperative patients undergoing gastrointestinal cancer surgery. However, this study did not find an association between carnitine levels and postoperative complications.
Conflict of interest
None declared.
Funding
This work was supported in part by a Grant‐in‐Aid for Medical Research from the Shizuoka Prefectural Hospital Organization.
Takagi A., Hawke P., Tokuda S., Toda T., Higashizono K., Nagai E., Watanabe M., Nakatani E., Kanemoto H., and Oba N. (2022) Serum carnitine as a biomarker of sarcopenia and nutritional status in preoperative gastrointestinal cancer patients, Journal of Cachexia, Sarcopenia and Muscle, 13, 287–295, 10.1002/jcsm.12906
References
- 1. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simonsen C, de Heer P, Bjerre ED, Suetta C, Hojman P, Pedersen BK, et al. Sarcopenia and postoperative complication risk in gastrointestinal surgical oncology: a meta‐analysis. Ann Surg 2018;268:58–69. [DOI] [PubMed] [Google Scholar]
- 3. Kamarajah SK, Bundred J, Tan BHL. Body composition assessment and sarcopenia in patients with gastric cancer: a systematic review and meta‐analysis. Gastric Cancer 2019;22:10–22. [DOI] [PubMed] [Google Scholar]
- 4. Levolger S, van Vugt JLA, de Bruin RWF, IJzermans JNM. Systematic review of sarcopenia in patients operated on for gastrointestinal and hepatopancreatobiliary malignancies. BJS Br J Surg 2015;102:1448–1458. [DOI] [PubMed] [Google Scholar]
- 5. Miyoshi K, Shimoda M, Udo R, Oshiro Y, Suzuki S. Urinary titin N‐terminal fragment concentration is an indicator of preoperative sarcopenia and nutritional status in patients with gastrointestinal tract and hepatobiliary pancreatic malignancies. Nutrition 2020;79‐80:110957, 10.1016/j.nut.2020.110957 [DOI] [PubMed] [Google Scholar]
- 6. Gnoni A, Longo S, Gnoni GV, Giudetti AM. Carnitine in human muscle bioenergetics: can carnitine supplementation improve physical exercise? Molecules 2020;25:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Role of carnitine in disease. Nutr Metab 2010;7:30, 10.1186/1743-7075-7-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tanphaichitr V, Leelahagul P. Carnitine metabolism and human carnitine deficiency. Nutr Burbank Los Angel Cty Calif 1993;9:246–254. [PubMed] [Google Scholar]
- 9. Ruiz M, Labarthe F, Fortier A, Bouchard B, Thompson Legault J, Bolduc V, et al. Circulating acylcarnitine profile in human heart failure: a surrogate of fatty acid metabolic dysregulation in mitochondria and beyond. Am J Physiol Heart Circ Physiol 2017;313:768–781. [DOI] [PubMed] [Google Scholar]
- 10. Kemp PR, Paul R, Hinken AC, Neil D, Russell A, Griffiths MJ. Metabolic profiling shows pre‐existing mitochondrial dysfunction contributes to muscle loss in a model of ICU‐acquired weakness. J Cachexia Sarcopenia Muscle 2020;11:1321–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Silvério R, Laviano A, Rossi Fanelli F, Seelaender M. l‐carnitine and cancer cachexia: clinical and experimental aspects. J Cachexia Sarcopenia Muscle 2011;2:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel SS, Molnar MZ, Tayek JA, Ix JH, Noori N, Benner D, et al. Serum creatinine as a marker of muscle mass in chronic kidney disease: results of a cross‐sectional study and review of literature. J Cachexia Sarcopenia Muscle 2013;4:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res 2016;46:951–963. [DOI] [PubMed] [Google Scholar]
- 14. Dindo D, Demartines N, Clavien P‐A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin TJ, Tang SC, Liao PY, Dongoran RA, Yang JH, Liu CH. A comparison of l‐carnitine and several cardiovascular‐related biomarkers between healthy vegetarians and omnivores. Nutrition 2019;66:29–37. [DOI] [PubMed] [Google Scholar]
- 16. Ritterhoff J, McMillen TS, Villet O, Young S, Kolwicz SC Jr, Senn T, et al. Increasing fatty acid oxidation elicits a sex‐dependent response in failing mouse hearts. J Mol Cell Cardiol 2021;158:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Evans DC, Guenter P, Jensen G, Malone A. Response to “low albumin levels should be interpreted, but not ignored”. Nutr Clin Pract 2021;36:504, Epub 2021 Feb 12. [DOI] [PubMed] [Google Scholar]
- 18. Caso G, Scalfi L, Marra M, Covino A, Muscaritoli M, McNurlan MA, et al. Albumin synthesis is diminished in men consuming a predominantly vegetarian diet. J Nutr 2000;130:528–533. [DOI] [PubMed] [Google Scholar]
- 19. Trépanier M, Minnella EM, Paradis T, Awasthi R, Kaneva P, Schwartzman K, et al. Improved disease‐free survival after prehabilitation for colorectal cancer surgery. Ann Surg 2019;270:493–501. [DOI] [PubMed] [Google Scholar]
- 20. Nakajima H, Yokoyama Y, Inoue T, Nagaya M, Mizuno Y, Kadono I, et al. Clinical benefit of preoperative exercise and nutritional therapy for patients undergoing hepato‐pancreato‐biliary surgeries for malignancy. Ann Surg Oncol 2019;26:264–272. [DOI] [PubMed] [Google Scholar]
- 21. Bundred JR, Kamarajah SK, Hammond JS, Wilson CH, Prentis J, Pandanaboyana S. Prehabilitation prior to surgery for pancreatic cancer: a systematic review. Pancreatology 2020;20:1243–1250. [DOI] [PubMed] [Google Scholar]
- 22. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamaguchi Y, Kaido T, Okumura S, Ito T, Fujimoto Y, Ogawa K, et al. Preoperative intramuscular adipose tissue content is a novel prognostic predictor after hepatectomy for hepatocellular carcinoma. J Hepato‐Biliary‐Pancreat Sci 2015;22:475–485. [DOI] [PubMed] [Google Scholar]
- 24. Waki Y, Irino T, Makuuchi R, Notsu A, Kamiya S, Tanizawa Y, et al. Impact of preoperative skeletal muscle quality measurement on long‐term survival after curative gastrectomy for locally advanced gastric cancer. World J Surg 2019;43:3083–3093. [DOI] [PubMed] [Google Scholar]
- 25. Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 2015;63:131–140. [DOI] [PubMed] [Google Scholar]
- 26. Bian X, Dai H, Feng J, Ji H, Fang Y, Jiang N, et al. Prognostic values of abdominal body compositions on survival in advanced pancreatic cancer. Medicine (Baltimore) 2018;97:e10988, 10.1097/MD.0000000000010988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hiatt WR, Regensteiner JG, Creager MA, Hirsch AT, Cooke JP, Olin JW, et al. Propionyl‐l‐carnitine improves exercise performance and functional status in patients with claudication *. Am J Med 2001;110:616–622. [DOI] [PubMed] [Google Scholar]
- 28. Gramignano G, Lusso MR, Madeddu C, Massa E, Serpe R, Deiana L, et al. Efficacy of l‐carnitine administration on fatigue, nutritional status, oxidative stress, and related quality of life in 12 advanced cancer patients undergoing anticancer therapy. Nutrition 2006;22:136–145. [DOI] [PubMed] [Google Scholar]
- 29. Primassin S, Ter Veld F, Mayatepek E, Spiekerkoetter U. Carnitine supplementation induces acylcarnitine production in tissues of very long‐chain acyl‐CoA dehydrogenase‐deficient mice, without replenishing low free carnitine. Pediatr Res 2008;63:632–637. [DOI] [PubMed] [Google Scholar]
- 30. Cruciani RA, Zhang JJ, Manola J, Cella D, Ansari B, Fisch MJ. l‐carnitine supplementation for the management of fatigue in patients with cancer: an Eastern Cooperative Oncology Group Phase III, randomized, double‐blind, placebo‐controlled trial. J Clin Oncol 2012;30:3864–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boshier PR, Heneghan R, Markar SR, Baracos VE, Low DE. Assessment of body composition and sarcopenia in patients with esophageal cancer: a systematic review and meta‐analysis. Dis Esophagus 2018;31: 10.1093/dote/doy047 [DOI] [PubMed] [Google Scholar]
- 32. Rinninella E, Cintoni M, Raoul P, Pozzo C, Strippoli A, Bria E, et al. Muscle mass, assessed at diagnosis by L3‐CT scan as a prognostic marker of clinical outcomes in patients with gastric cancer: a systematic review and meta‐analysis. Clin Nutr 2020;39:2045–2054. [DOI] [PubMed] [Google Scholar]
- 33. Sun G, Li Y, Peng Y, Lu D, Zhang F, Cui X, et al. Can sarcopenia be a predictor of prognosis for patients with non‐metastatic colorectal cancer? A systematic review and meta‐analysis. Int J Colorectal Dis 2018;33:1419–1427. [DOI] [PubMed] [Google Scholar]
- 34. Filip B, Scarpa M, Cavallin F, Cagol M, Alfieri R, Saadeh L, et al. Postoperative outcome after oesophagectomy for cancer: nutritional status is the missing ring in the current prognostic scores. Eur J Surg Oncol 2015;41:787–794. [DOI] [PubMed] [Google Scholar]
- 35. Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre‐operative nutritional condition with post‐operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol 2002;28:396–400. [DOI] [PubMed] [Google Scholar]
- 36. Jiang N, Deng J‐Y, Ding X‐W, Ke B, Liu N, Zhang R‐P, et al. Prognostic nutritional index predicts postoperative complications and long‐term outcomes of gastric cancer. World J Gastroenterol 2014;20:10537–10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. BJS Br J Surg 2011;98:268–274. [DOI] [PubMed] [Google Scholar]
- 38. Ke M, Xu T, Li N, Ren Y, Shi A, Lv Y, et al. Prognostic nutritional index predicts short‐term outcomes after liver resection for hepatocellular carcinoma within the Milan criteria. Oncotarget 2016;7:81611–81620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg 2013;37:2688–2692. [DOI] [PubMed] [Google Scholar]
- 40. Bailón‐Cuadrado M, Pérez‐Saborido B, Sánchez‐González J, Rodríguez‐López M, Velasco‐López R, C. Sarmentero‐Prieto J, et al. Prognostic nutritional index predicts morbidity after curative surgery for colorectal cancer. Cir Esp Engl Ed 2019;97:71–80.4. [DOI] [PubMed] [Google Scholar]
- 41. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]


