Abstract
Background
Assessment of muscle quantity by sonographic muscle indices could help identify patients at risk for fatal outcome during coronavirus disease‐2019 (COVID‐19). The aim of this study was to explore sonographic muscle indices as predictors of COVID‐19 outcome and to test the feasibility of sonographic muscle measurement in an isolation context.
Methods
Muscle indices, derived from the psoas muscle or thigh muscles, were quantified by sonography in a cohort of patients without COVID‐19 to obtain reference values for low muscle quantity. Gender‐specific median of different muscle indices were defined as threshold value for low muscle quantity. The prognostic relevance of low muscle quantity, was prospectively explored in two cohorts of hospitalized COVID‐19 patients. Optimal muscle index cutoff values predictive for 30 day mortality during COVID‐19 were determined by receiver operating characteristic‐area under the curve and Youden index calculation. Muscle quantity and known prognostic factors of COVID‐19 were analysed by multivariable log‐regression.
Results
Compared with other muscle indices, the psoas muscle area index (PMAI) showed the most favourable characteristics to predict outcome of COVID‐19 disease. Sonographic morphometry of patients without COVID‐19 (n = 136) revealed a gender‐specific median for PMAI (male: 291.1 mm2/m2, female 260.6 mm2/m2) as threshold value of low muscle quantity. Subsequently, COVID‐19 patients (Cohort I: n = 58; Cohort II: n = 55) were prospectively assessed by bedside sonography. The studied COVID‐19 patients developed a critical course of disease in 22.4% (Cohort I: n = 13/58) and 34.5% (Cohort II: n = 20/55). Mortality rate reached 12.1% (Cohort I: n = 7/58) and 20.0% (Cohort I: n = 11/55) within 30 days of follow up. COVID‐19 patients with a PMAI below the gender‐specific median showed a higher 30 day mortality in both COVID‐19 cohorts (log rank, P < 0.05). The optimal PMAI cutoff value (206 mm2/m2) predicted 30 day mortality of hospitalized COVID‐19 patients with a sensitivity of 72% and specificity of 78.5% (receiver operating characteristic‐area under the curve: 0.793, 95% confidence interval 0.671–0.914, P = 0.008). Multivariable log‐regression analysis of PMAI, age, gender, BMI and comorbidities confirmed an independent association of low PMAI with 30 day mortality of COVID‐19 patients (P = 0.018).
Conclusions
Sonographic morphometry provides reliable muscle quantification under hygienic precautions and allows risk stratification of patients with COVID‐19.
Keywords: Muscle, Morphometry, COVID‐19, Ultrasound, Outcome
Introduction
The coronavirus disease 2019 (COVID‐19) pandemic caused by the Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) represents a major challenge with an initially reported lethality rate of 2.3% in China, which reached up to 14.8% in infected patients older than 80 years. 1 A high mortality rate affects older patients (>60 years of age), who represent about 90% of the overall death toll caused by the global COVID‐19 pandemic. 2 COVID‐19 infection specifically threatens vulnerable, mostly multimorbid older adults. 2 , 3 , 4 , 5 , 6 , 7
Assessment of muscle quantity could further help to identify patients at risk to develop fatal outcome during COVID‐19. Particularly reduced muscle mass and deteriorated muscle function has been identified as detrimental condition among older‐aged patients, 8 , 9 , 10 which has relevant impact on outcome of various diseases. 11 , 12 , 13 Pathological reduction of muscle mass is primarily caused by physical inactivity, catabolism, and age‐dependent maladaptation. 14 Reduced muscle quantity is an important finding associated with impaired mobility and muscle strength, 15 , 16 which is part of the frailty syndrome. 17 Patients affected by frailty face increased vulnerability resulting from a decline in reserve and function that leads to an inadequate capacity to deal with extrinsic and intrinsic stressors. 18 Frailty shows a prevalence of 15% in older patients (age >65 years) and a prevalence of even 25% in very old individuals (age >80 years) 17 and should be considered in COVID‐19 patients.
Radiological sectional imaging can be used reliably to measure muscle quantity and identify patients with low muscle mass. 19 The cross‐sectional assessment of several muscles at the lumbar vertebral level (L3) or assessment of the psoas muscles both normalized by the body height and the dual X‐ray absorptiometry are currently the most widely used techniques. 11 , 12 , 13 , 20 However, radiological imaging is relatively cost intensive and cumbersome in patients requiring isolation for COVID‐19.
Sonographic assessment of the psoas muscle area index (PMAI) as well as the thigh muscle thickness index (TMTI) are alternative approaches for bedside morphometry. 21 , 22 , 23 , 24 Particularly, sonographic morphometry of the psoas muscle shows a good correlation (r > 0.9, P < 0.05) with corresponding radiologic sectional imaging. 21 , 22
The aim of this study was to apply ultrasound‐based morphometry for a rapid, bedside assessment of muscle quantity in COVID‐19 patients. In the absence of an adequate evaluation of the patient's strength and physical performance, fast and easy muscle assessment helps to identify patients at risk to develop fatal outcomes.
We quantified the dimensions of the Musculus psoas major and of the thigh muscle group in patients attending our clinic to obtain morphometric reference values and subsequently assessed sonographic muscle indices in hospitalized COVID‐19 patients to predict outcome.
Patients and methods
Experimental design and study aims
This prospective observational study followed a three‐step design to evaluate different sonographic muscle indices representing muscle quantity as predictor for COVID‐19 outcome. (I) Sonographic muscle indices were explored in patients without COVID‐19 (reference cohort) in order to obtain benchmark muscle quantities regardless of the underlying acute or chronic pathology. Gender‐specific medians of different muscle indices were defined as threshold value for low muscle quantity. (II) Sonographic muscle indices were analysed in a first cohort of COVID‐19 patients. COVID‐19 patients were stratified by muscle index threshold values to explore association with outcome (e.g. mortality) during a prospective 30 day follow up. (III) Sonographic muscle indices were subsequently validated in a second cohort of COVID‐19 patients to confirm their association with mortality during a prospective 30 day follow up.
Additional analyses were designated to determine optimal threshold levels of sonographic muscle indices in COVID‐19 patients to predict 30 day mortality. Also association of muscle quantity with other mortality risk factors of COVID‐19 was explored. The flow chart and study design is shown in Supporting information, Figure S1.
Patient cohorts
Patients attending the University Medical Center between 20 March 2020 and 14 February 2021 were offered sonographic morphometry. Patients without COVID‐19 were analysed from March to August 2020 and served as reference cohort. Two independent COVID‐19 cohorts (COVID‐19 cohort I + II) were subsequently assessed. The two COVID‐19 cohorts consisted of hospitalized patients suffering from COVID‐19 during the first and second infection wave in Germany. Patients of the first COVID‐19 cohort were included between March and October 2020, and patients of the second COVID‐19 cohort participated between November 2020 and February 2021. Patients suffering from degenerative muscular diseases were not approached for this study.
Ethics
Written informed consent was obtained from the participants or from their authorized representative prior to the inclusion in the study. The study was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and its later amendments. The study protocol was approved by the ethics committee of the Landesärztekammer Rheinland‐Pfalz (accession number 2020‐15174).
Morphometric measurement
Patients of the reference cohort underwent muscle morphometry during routine diagnostic ultrasonography as indicated by the underlying medical condition. COVID‐19 patients received muscle morphometry during sonographic assessment of respiratory distress and infection within 48 h after admission.
Sonography was either performed at the ultrasound ward with an Aplico i800 ultrasound system (Canon, Tokyo Japan) or was performed as a bedside exam under isolation conditions using an ACUSON Freestyle ultrasound system (Siemens Healthcare, Erlangen, Germany) with a wireless ultrasound probe.
All patients lay in supine position for sonographic examination as body position could affect muscle dimensions. 25 The psoas muscle was depicted in the frontal plane above the iliac crest and the psoas muscle thickness was measured at the level of the caudal kidney pole to obtain reproducible results (Figure 1A).
Figure 1.
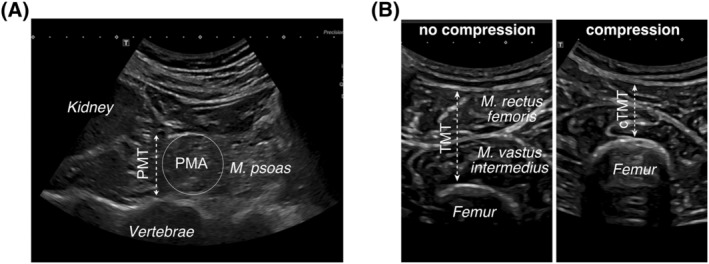
Sonographic muscle quantification. Transversal sonographic B‐mode section (A) acquired at the height of the iliac cristae. The lower kidney pole, psoas muscle thickness (PMT), psoas muscle area (PMA) and vertebrae are indicated. Transversal sonographic B‐mode section (B) of the upper thigh middle, showing the thigh muscle group (Musculus vastus intermedius, Musculus rectus femoris) and the femur. Thigh muscle thickness without compression (TMT) and with compression (cTMT) by the sonography probe are indicated. All morphometric variables are obtained from a person in supine position.
The extensor muscle thickness of the lower limb, including the M. rectus femoris and M. vastus intermedius, was measured in the frontal plane midway between the spina iliaca anterior and the cranial edge of the patella (Figure 1B). The combined extensor muscle thickness was assessed between the femur bone and the subcutaneous fat tissue, whereby the subcutaneous fat tissue was not included in the measurement.
Based on these measurements the psoas muscle thickness index (PMTI), PMAI, 21 , 22 and the TMTI were calculated as published. 21 , 26 The PMTI is obtained from the average bilateral psoas muscle thickness indexed to the body height of the patient (mm/m). PMAI incorporates the average bilateral psoas muscle area indexed to the squared body height of the patient (mm2/m2). The average bilateral psoas muscle area for the PMAI is approximated from the M. psoas diameter by the formula for a circular surface (Figure 1A). 21 The TMTI represents the average bilateral extensor muscle thickness of the lower limb ( M. rectus femoris and the M. vastus intermedius) indexed by the body height of the patient (mm/m). The same thigh muscle group dimensions measured under compression with the ultrasound detector resulted in the compressed TMTI (cTMTI) (Figure 1B). 26
Clinical parameters
Demographic data and comorbidities of patients were prospectively obtained from the hospital information system and chart reviews. All patients with COVID‐19 were followed for a maximum of 30 days after hospital admission and mortality was assessed during this follow up period. Later events were not considered. Severity of COVID‐19 was classified into an uncomplicated, complicated and critical clinical course. Patients with uncomplicated disease required no monitoring or oxygen supplementation, whereas patients affected by complicated COVID‐19 were in need for oxygen supplementation and critical COVID‐19 patients received invasive ventilation. 27 SARS‐CoV‐2 infection was confirmed by polymerase chain reaction (PCR) from respiratory samples (RealStar SARS‐CoV‐2 RT‐PCR Kit 1.0, Altona Diagnostics GmbH, Hamburg, Germany).
Statistical analysis and sample size calculation
Statistical analysis was carried out in order to identify an association between muscle quantity and COVID‐19 outcome. A flow chart of statistical analyses is incorporated in Figure S1. Quantitative data are expressed as median with interquartile range (IQR). Pairwise comparisons for quantitative variables were performed with an unpaired t test or Mann–Whitney U test, accordingly. Categorical variables are given as frequencies and percentages, respectively. Categorical variables of two or more patient groups were compared by χ 2 test. Univariate survival was assessed by Kaplan–Meier blot and log‐Rank comparison. Receiver operating characteristic‐area under the curve (ROC‐AUC) and Youden index were used to identify the optimal cutoff values for mortality prediction by muscle indices. Binary logistic regression was applied to explore multivariable associations with the 30 day morality rate. SPSS Version 26 (IBM, SPSS Statistics, Armonk NY, USA) was used for the statistical evaluation. The significance level was set at <0.05.
A case number planning was carried out based on the reported association of muscle indices with severe COVID‐19 courses. 28 A case number of 54 patients was calculated in order to show an influence of the muscle quantity on the course of a COVID disease. The case‐finding design is based on a test strength of 95% and an alpha error of 0.01 for the prediction of mortality by sonographic muscle quantification. Detailed information regarding the sample size calculation can be found in the supporting information.
Results
Reference patients
Aiming to define gender‐specific reference muscle indices, a total of n = 136 patients without COVID‐19 were assessed by ultrasound of whom 32.4% (44/136) were hospitalized. The main diagnoses requiring sonographic work up were liver diseases (50.7%, 69/136), malignant diseases (12.5%, 17/136), infectious diseases (9.6%, 13/136), and cardiovascular entities (6.9%, 9/136). Patients included into the reference cohort were male in 55.9% (n = 76/136), showed a median age of 60 years (49–69 years), and also presented with common comorbidities (Table 1).
Table 1.
Patient characteristics
| Characteristics | Reference cohort | COVID‐19 Cohort I | COVID‐19 Cohort II | Combined COVID‐19 Cohorts I + II |
|---|---|---|---|---|
| Patients (N) | 136 | 58 | 55 | 113 |
| Age (years) | 60 (49–69) | 67 (54–79) | 70 (57–79) | 69 (57–79) |
| <65 years | 87 (64.0%) | 26 (44.8%) | 20 (36.4%) | 46 (40.7%) |
| 65–80 years | 39 (28.7%) | 18 (31.0%) | 22 (40.0%) | 40 (38.4%) |
| >80 years | 10 (7.4%) | 14 (24.1) | 13 (23.6%) | 27 (23.9%) |
| Gender (male/female) | 76/60 (55.9%/44.1%) | 31/27 (53.4%/46.6%) | 38/17 (69.1%/30.9%) | 69/44 (69.1%/30.9%) |
| BMI (kg/m2) | 26.9 (23.6–30.3) | 27.2 (24.0–33.4) | 25.7 (22.9–27.8) | 26.1 (23.7–30.3) |
| BMI (>30 kg/m2) | 38 (27.9%) | 21 (36.2%) | 8 (14.5%) | 29 (25.7%) |
| Pre‐existing comorbidities | ||||
| Arterial hypertension | 50 (36.8%) | 34 (58.6%) | 39 (70.9%) | 73 (64.6%) |
| Cardiovascular disease | 37 (27.2%) | 20 (34.5%) | 34 (61.8%) | 54 (47.8%) |
| Diabetes mellitus | 29 (21.3%) | 13 (22.4%) | 25 (45.5%) | 38 (33.6%) |
| Chronic respiratory disease | 10 (7.4%) | 6 (10.3%) | 17 (30.9%) | 23 (20.4%) |
| Cerebrovascular disease | 12 (8.8%) | 14 (24.1%) | 14 (25.5%) | 28 (24.8%) |
| COVID‐19 severity | ||||
| Mild | NA | 28 (48.3%) | 16 (30.9%) | 44 (38.9%) |
| Complicated | NA | 17 (29.3%) | 19 (34.5%) | 36 (31.9%) |
| Critical | NA | 13 (22.4%) | 20 (34.5%) | 33 (29.2%) |
| Deceased | NR | 7 (12.1%) | 11 (20.0%) | 18 (15.9%) |
| Medical care | ||||
| Hospital care | 44 (32.4%) | 58 (100%) | 55 (100%) | 113 (100%) |
| Hospital care duration (days) | NR | 13 (8–21) | 19 (10–31) | 16 (8–25) |
| Intensive care | NR | 13 (22.8%) | 16 (29.1%) | 29 (25.9%) |
| Intensive care duration (days) | NR | 25 (8–37) | 17 (9–39) | 20 (9–38) |
| Invasive ventilation | NR | 12 (21.1%) | 11 (20%) | 23 (20.5%) |
| Invasive ventilation duration (days) | NR | 15 (5–35) | 22 (7–38) | 18 (6–35) |
| Vasopressor therapy | NR | 8 (14.3%) | 10 (18.2%) | 18 (16.2%) |
| Renal replacement therapy | NR | 3 (5.2%) | 6 (10.9%) | 9 (8.9%) |
| ECMO therapy | NR | 1 (1.8%) | 2 (3.6%) | 3 (2.7%) |
| Dexamethasone therapy | NR | 22 (37.9%) | 31 (56.4%) | 53 (46.9%) |
| Anticoagulation | ||||
| None | NR | 3 (5.2%) | 4 (7.3%) | 7 (6.3%) |
| Prophylactic LMWH | NR | 39 (68.4%) | 30 (54.5%) | 69 (61.6%) |
| Therapeutic LMWH | NR | 15 (26.3%) | 21 (38.1%) | 36 (32.1%) |
| Oral anticoagulation | NR | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
BMI, body mass index; LMWH, low molecular weight heparin, ECMO, extracorporeal membrane oxygenation, NA not applicable, NR, not rated. Patient characteristics presented as median (interquartile range) or number (%).
Sonographic assessment of the psoas muscle identified a median PMTI of 18.4 mm/m (16.2–20.2 mm/m) and a median PMAI of 268.4 mm2/m2 (207.3–321.8 mm2/m2). Thigh muscle dimensions resulted in a median cTMTI of 9.3 mm/m (6.8–12.0 mm/m) under compression and a median TMTI of 21.0 mm/m (16.9–26.1 mm/m) without compression, respectively.
Given that baseline muscle quantity is dependent on gender, 22 muscle indices were analysed for male and female patients accordingly (Table 2A). The gender related differences were significant for median PMAI (male: 291.1 vs. female: 260.6 mm/m2, P = 0.037) and median PMTI (male: 19.2 vs. female: 18.2 mm/m, P = 0.040). However, no significant differences were observed for median cTMTI (male: 9.4 vs. female: 8.9 mm/m, P = 0.676) and median TMTI (male: 21.2 vs. female: 20.5 mm/m, P = 0.998).
Table 2.
Morphometric variables
| Panel a | Reference cohort | ||
|---|---|---|---|
| Morphometric variables | Total | Male | Female |
| Patients (N) | 136 | 76 | 60 |
| Body height (cm) | 170 (165–178) | 176 (170–182) | 167 (163–170) |
| PMA (mm2) | 780.3 (606.1–965.3) | 881.6 (708.4–1024.9) | 683.7 (534.1–831.3) |
| PMAI (mm2/m2) | 268.4 (207.2–321.8) | 291.1 (224.8–328.4) | 260.6 (191.5–297.8) |
| PMT (mm) | 31.5 (27.8–35.0) | 33.5 (30.0–36.0) | 29.5 (26.0–32.5) |
| PMTI (mm/m) | 18.4 (16.2–20.2) | 19.2 (16.9–20.4) | 18.2 (15.6–19.4) |
| TMT (mm) (no compression) | 35.8 (28.5–45.0) | 37.0 (28.8–46.5) | 33.8 (27.5–43.3) |
| TMTI (mm/m) (no compression) | 21.0 (16.9–26.1) | 21.2 (16.8–25.6) | 20.4 (16.9–26.2) |
| cTMT (mm) (compression) | 15.8 (11.5–20.8) | 16.3 (12.0–21.3) | 15.0 (11.0–20.0) |
| cTMTI (mm/m) (compression) | 9.2 (6.8–12.0) | 9.4 (6.8–12.2) | 8.9 (6.8–12.0) |
| Panel b | COVID‐19 Cohort I | ||
|---|---|---|---|
| Morphometric variables | Total | Male | Female |
| Patients (N) | 58 | 31 | 27 |
| Body height (cm) | 170 (165–176) | 176 (173–180) | 166 (160–170) |
| PMA (mm2) | 719.0 (497.9–1018.7) | 730.8 (573.3–1114.1) | 702.2 (436.0–974.7) |
| PMAI (mm2/m2) | 240.4 (176.4–331.7) | 246.9 (197.6–330.0) | 224.1 (155.8–367.1) |
| PMT (mm) | 30.2 (25.0–36.0) | 30.5 (27.0–37.5) | 29.9 (23.6–35.0) |
| PMTI (mm/m) | 17.5 (14.9–20.5) | 17.7 (15.6–20.5) | 16.9 (14.0–21.5) |
| TMT (mm) (no compression) | 25.3 (19.5–33.0) | 25.0 (18.5–32.5) | 25.5 (20.0–37.0) |
| TMTI (mm/m) (no compression) | 15.1 (11.5–20.6) | 14.2 (10.6–18.6) | 15.8 (12.2–21.7) |
| cTMT (mm) (compression) | 11.8 (9.0–15.7) | 10.5 (9.0–15.0) | 13.0 (9.0–17.0) |
| cTMTI (mm/m) (compression) | 6.8 (5.1–9.6) | 6.2 (5.0–8.8) | 7.8 (5.9–9.8) |
| Panel c | COVID‐19 cohort II | ||
|---|---|---|---|
| Morphometric variables | Total | Male | Female |
| Patients (N) | 55 | 38 | 17 |
| Body height (cm) | 175 (166–188) | 176 (173–180) | 165 (160–170) |
| PMA (mm2) | 788.9 (573.3–1,125‐5) | 913.0 (594.2–1164.4) | 683.7 (510.9–962.9) |
| PMAI (mm2/m2) | 258.3 (194.0–373‐9) | 289.2 (195.5–373.9) | 251.5 (176.8–353.7) |
| PMT (mm) | 31.5 (27.0–37.5) | 34.0 (27.5–38.5) | 29.5 (25.5–35.0) |
| PMTI (mm/m) | 18.13 (15.7–21.8) | 19.0 (15.8–21.8) | 17.8 (15.0–21.2) |
| TMT (mm) (no compression) | 24.0 (10.4–31.0) | 23.5 (20.5–31.5) | 24.0 (20.5–28.0) |
| TMTI (mm/m) (no compression) | 14.4 (11.2–18.0) | 13.6 (10.2–18.0) | 15.4 (11.5–16.5) |
| cTMT (mm) (compression) | 12.0 (8.5–16.0) | 12.5 (8.5–15.0) | 11.0 (9.0–16.0) |
| cTMTI (mm/m) (compression) | 6.8 (5.0–9.2) | 6.9 (4.8–8.6) | 6.5 (5.6–9.4) |
| Panel d | Combined COVID‐19 Cohorts I + II | ||
|---|---|---|---|
| Morphometric variables | Total | Male | Female |
| Patients (N) | 113 | 69 | 44 |
| Body height (cm) | 172 (166–180) | 176 (173–180) | 165 (160–170) |
| PMA (mm2) | 730.8 (5,435–1078.4) | 744.2 (575.7–1114.1) | 688.4 (472.9–968.8) |
| PMAI (mm2/m2) | 251.5 (190.1–353‐7) | 259.9 (196.3–341.6) | 238.9 (172.7–361.5) |
| PMT (mm) | 30.5 (26.2–37.0) | 32.0 (27.5–37.9) | 29.5 (24.4–35.0) |
| PMTI (mm/m) | 17.7 (15.6–21.1) | 18.2 (15.8–20.8) | 17.3 (14.8–21.4) |
| TMT (mm) (no compression) | 24.5 (20.0–32.0) | 24.0 (19.5–31.5) | 24.8 (20.3–32.3) |
| TMTI (mm/m) (no compression) | 14.6 (11.5–19.2) | 13.8 (10.9–18.0) | 15.5 (11.9–20.3) |
| cTMT (mm) (compression) | 12.0 (9.0–16.0) | 11.5 (9.0–15.0) | 12.3 (9.0–16.5) |
| cTMTI (mm/m) (compression) | 6.8 (5.1–9.4) | 6.7 (5.0–7.1) | 7.5 (5.7–9.8) |
BMI; body mass index; cTMTI, compressed tight muscles thickness index; PMA, psoas muscle area; PMAI, psoas muscle area index; PMTI, psoas muscle thickness index; TMT, tight muscles thickness. Patient characteristics are presented as median (interquartile range) or number (%).
COVID‐19 patients
Sonographic muscle indices were subsequently explored in hospitalized patients suffering from COVID‐19 (COVID‐19 Cohort I). The first COVID‐19 cohort showed a balanced gender distribution (male/female, n = 31/27) and a median age of 67 years (54–79 years). They presented with comorbidities, such as arterial hypertension (n = 34/58, 58.6%), cardiovascular disease (n = 20/58, 34.5%) and diabetes mellitus (n = 13/58, 22.4%). Obesity (BMI > 30 kg/m2) was present in 36.2% (n = 21/58) (Table 1). Throughout hospitalization, the patients developed a mild (n = 28/58, 48.3%), complicated (n = 17/58, 29.3%), or critical (n = 13/58, 22.4%) course of COVID‐19. Seven COVID‐19 patients (n = 7/58, 12.1%) died during a follow up of 30 days (Table 1).
Sonographic assessment of the psoas muscle revealed a median PMAI of 240.4 mm2/m2 (176.4–331.7 mm2/m2) and a median PMTI of 17.5 mm/m (14.9–20.5 mm/m). Thigh muscle assessment showed a median TMTI of 15.1 mm/m (11.5–20.6 mm/m) and a median cTMTI of 6.8 mm/m (5.1–9.6 mm/m). All median morphometric indices of the COVID‐19 patients were lower compared with the reference cohort (Table 2, Panel b).
The second independent COVID‐19 cohort, which primarily originated from the second COVID‐19 wave in Germany, was assessed to validate our findings (COVID‐19 Cohort II). The second COVID‐19 cohort showed no significantly different baseline characteristics compared to the first COVID‐19 cohort (Table 1). Eleven patients (n = 11/55, 20.0%) of the second COVID‐19 cohort died during a follow up of 30 days (Table 1). In line with our previous findings, the second COVID‐19 cohort also presented with lower morphometric indices compared to the reference cohort (Table 2, Panel c).
Muscle quantity associated with outcome of COVID‐19 patients
We explored the impact of muscle quantity on prognosis in COVID‐19 patients. Hence, median muscle indices derived from the reference cohort were used as cutoff value to stratify COVID‐19 patients (Table 2). COVID‐19 patients with a PMAI and PMTI below the gender‐specific median showed a significantly higher mortality during a 30 day follow up (both log rank p = 0.025) (Figure 2A and 2C), whereas COVID‐19 patients with a TMTI or cTMTI above the gender‐specific median had a higher 30 day mortality (both log rank P > 0.05) (Figure 2E and 2G).
Figure 2.
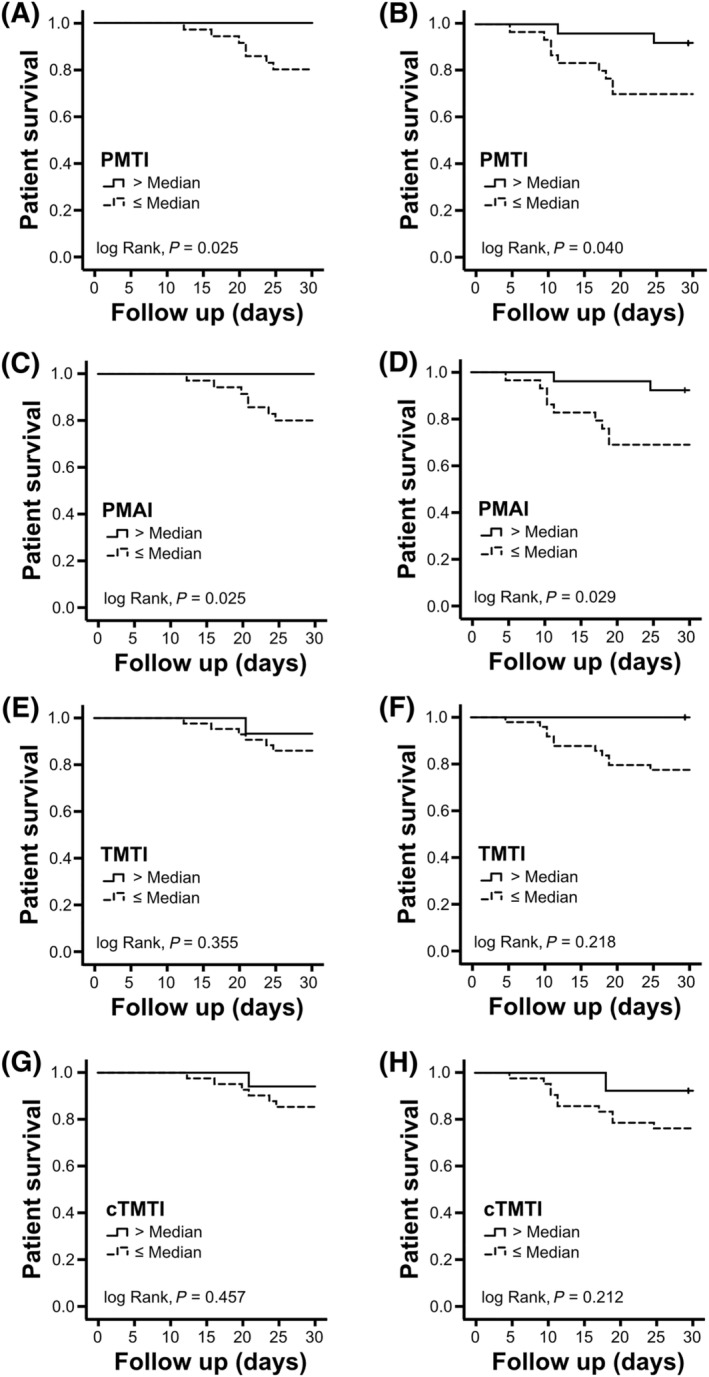
Patient survival according to muscle indices. Kaplan–Meier survival curves are displayed for COVID‐19 Cohort I (n = 58) (A, C, E, G) and COVID‐19 Cohort II (n = 55) (B, D, F, H) after stratification by morphometric muscle, psoas muscle thickness index (PMTI), psoas muscle area index (PMAI), thigh muscle thickness index (TMTI), and thigh muscle thickness index under compression (cTMTI) as indicated. Stratification of both COVID‐19 cohorts was based on gender‐specific median cutoff values derived from the reference cohort without COVID‐19: PMTI (male: 19.2 mm/m, female: 18.2 mm/m), PMAI (male: 291.1 mm2/m2, female: 260.6 mm2/m2), TMTI (male: 21.2 mm/m, female: 20.4 mm/m), and cTMTI (male: 9.4 mm/m, female: 8.9 mm/m).
The morphometric cutoff values from the reference cohort were also validated by a second COVID‐19 cohort. In line with our previous results COVID‐19 patients with a PMAI and PMTI below the gender‐specific median showed a significantly higher mortality during a 30 day follow up (Figure 2B and 2D), whereas the gender‐specific median of TMTI and cTMTI was not associated with outcome in the first COVID‐19 cohort (Figure 2F and 2H).
Given that the reference cohort does not resemble characteristics of COVID‐19 patients, we went on to validate morphometric cutoffs values entirely derived from COVID‐19 patients. Gender specific median muscle indices obtained from the first COVID‐19 cohort were analysed for their correlation with mortality (Table 2). The resulting stratification by PMAI and PMTI below the gender‐specific median was associated with a significantly higher mortality in both COVID‐19 cohorts ( Figure S2A–S2D). Thigh muscle indices on the other hand were not consistently related to 30 day mortality in both COVID‐19 cohorts ( Figure S2E–S2H).
Additional ROC‐AUC analyses of muscle indices from both COVID‐19 cohorts were applied to estimate sensitivity and specificity for mortality prediction. However, no gender specific estimation could be applied, due to the limited mortality events in female COVID‐19 patients. The different muscle indices achieved a ROC‐AUC between 0.778 and 0.793 within the entire first COVID‐19 cohort and a ROC‐AUC between 0.723 and 0.814 in the entire second COVID‐19 cohort (Table 3, Figure 3A and 3B).
Table 3.
Morphometric prediction of 30 day mortality (ROC‐AUC)
| Morphometric parameter | COVID‐19 Cohort I | COVID‐19 Cohort II | ||||||
|---|---|---|---|---|---|---|---|---|
| AUC | SD | 95% CI | P | AUC | SD | 95% CI | P | |
| PMAI | 0.793 | 0.062 | 0.671–0.914 | 0.008 | 0.723 | 0.064 | 0.599–0.848 | <0.001 |
| PMTI | 0.783 | 0.063 | 0.659–0.906 | 0.011 | 0.722 | 0.064 | 0.598–0.847 | <0.001 |
| cTMTI (compression) | 0.793 | 0.099 | 0.598–0.987 | 0.008 | 0.727 | 0.070 | 0.590–0.864 | 0.001 |
| TMTI (no compression) | 0.778 | 0.113 | 0.556–0.999 | 0.012 | 0.814 | 0.065 | 0.687–0.940 | <0.001 |
Receiver operator curve (ROC) analysis of muscle indices. AUC, area under the curve; cTMTI, compressed tight muscle thickness index; PMAI, psoas muscle area index; PMTI, psoas muscle thickness index; TMTI, tight muscle thickness index; 95% CI, 95% confidence interval.
Figure 3.
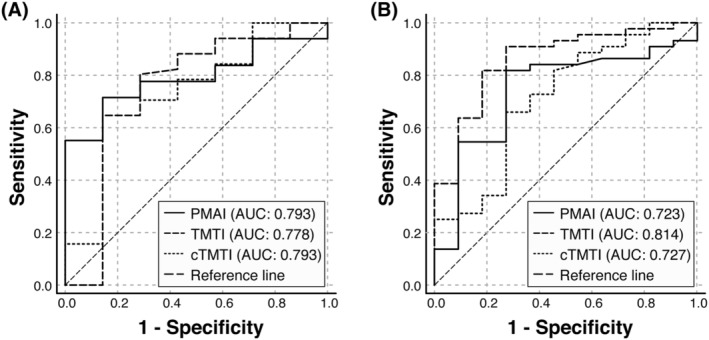
Morphometric survival prediction (ROC‐AUC). ROC‐AUC analysis of the psoas muscle area index (PMAI), thigh muscle thickness index (TMTI) and thigh muscle thickness index under compression (cTMTI) to predict 30 day mortality of hospitalized COVID‐19 Cohort I (n = 58) (A) and COVID‐19 Cohort II (n = 55) (B). AUC, area under the curve; ROC, receiver operator curve.
The optimal cutoff value of PMAI (206 mm2/m2) and PMTI (16.1 mm/m) derived from the first COVID‐19 cohort could both predict 30 day mortality of the second COVID‐19 cohort with a sensitivity of 75% and a specificity of 73%. Corresponding optimal cutoff values of TMTI (11.6 mm/m) and cTMTI (6.0 mm/m) from the first COVID‐19 cohort were applied to the second COVID‐19 cohort to predict 30 day mortality. Mortality prediction achieved a sensitivity of 68% and specificity of 64% for cTMTI and a sensitivity of 82% and a specificity of 73% for TMTI, respectively.
Muscle quantity related factors in COVID‐19 patients
We finally explored outcome‐relevant anthropometric and demographic parameters as well as comorbidities associated with muscle quantity in COVID‐19 patients to identify prognostic cofounders. The entire COVID‐19 cohort was divided by the gender‐specific median PMAI and these parameters were compared between both groups (Table 4). Factors associated with muscle quantity included patient age, body mass index (BMI), and comorbidities such as arterial hypertension, diabetes mellitus and cerebrovascular disease. As expected, the 30 day mortality was higher (P = 0.001, χ 2 test) and hospital stay was prolonged (P = 0.02, Mann–Whitney U test) in patients with a lower muscle quantity (Table 4).
Table 4.
Muscle quantity related factors in COVID‐19 patients
| Characteristics | PMAI ≤median a | PMAI >median a | P value |
|---|---|---|---|
| Patients (N) | 53 | 60 | |
| Age (years) | 73 (63–84) | 66 (54–78) | 0.001 c |
| Gender (male/female) | 33/20 (62.3/37.7%) | 36/24 (60.0/40.0%) | 0.975 b |
| Body height (cm) | 170 (168–180) | 173 (165–180) | 0.810 d |
| Body weight (kg) | 80 (67–90) | 80 (70–93) | 0.041 c |
| BMI (kg/m2) | 26.1 (23.0–27.8) | 26.8 (23.9–30.6) | 0.024 c |
| BMI (>30 kg/m2) | 12 (22.6%) | 17 (28.3%) | 0.137 b |
| Pre‐existing comorbidities | |||
| Arterial hypertension | 39 (73.6%) | 34 (56.7%) | 0.002 b |
| Cardiovascular disease | 35 (52.8%) | 26 (43.3%) | 0.093 b |
| Diabetes mellitus | 20 (37.7%) | 18 (30.0%) | 0.162 b |
| Chronic respiratory disease | 12 (22.8%) | 11 (18.3%) | 0.161 b |
| Cerebrovascular disease | 16 (30.2%) | 12 (20.0%) | 0.346 b |
| COVID‐19 severity/outcome | 0.088 b | ||
| Uncomplicated | 19 (35.8%) | 25 (41.7%) | |
| Complicated | 13 (24.5%) | 23 (38.3%) | 0.803 b |
| Critical | 21 (39.6%) | 12 (20.0%) | 0.092 b |
| Deceased | 15 (28.3%) | 3 (5.1%) | 0.001 b |
| Medical care | |||
| Hospital care | 53 (100%) | 60 (100%) | 1.000 d |
| Hospital care duration (days) | 19 (11–26) | 12 (7–21) | 0.020C |
| Intensive care | 17 (32.1%) | 12 (20.3%) | 0.290 b |
| Intensive care duration (days) | 25 (14–47) | 13 (8–25) | 0.100 c |
| Invasive ventilation | 14 (26.4%) | 9 (15.3%) | 0.239 b |
| Ventilation duration (days) | 22 (7–39) | 11 (5–22) | 0.217 d |
| Vasopressor therapy | 11 (21.6%) | 7 (11.7%) | 0.313 b |
| Renal replacement therapy | 5 (10.0%) | 4 (7.9%) | |
| ECMO | 3 (5.8%) | 0 (0.0%) | 0.255 d |
| No anticoagulation | 4 (7.5%) | 3 (5.1%) | |
| Prophylaktische LMWH/heparin | 29 (54.7%) | 40 (66.7%) | 0.859 b |
| Therapeutische LMWH/heparin | 20 (37.7%) | 17 (28.3%) | 0.794 b |
| Dexamethasone | 26 (49.1%) | 27 (45.0%) | 0.257 b |
Patient characteristics are presented as median (interquartile range) or number (%).
The gender‐specific median PMAI (male: 246.9 mm2/m2, female: 224.1 mm2/m2) derived from the COVID‐19 Cohort I was applied.
χ 2 test.
Mann–Whitney U test.
Student's t test.
Age, gender, BMI, and comorbidities, which qualified as baseline parameters at hospital admission, were eventually included into a multivariable log‐regression analysis together with PMAI. In this model, an independent association of PMAI with 30 day mortality was observed in our combined cohort (n = 113) of hospitalized COVID‐19 patients (P = 0.018) (Table 5).
Table 5.
Variables associated with 30 day mortality
| Variables | Odds ratio (SD) | Wald | P |
|---|---|---|---|
| PMAI (mm2/m2) | 0.139 (0.27–0.717) | 5.557 | 0.018 |
| Gender | 0.398 (0.105–1.506) | 1.841 | 0.175 |
| Age (years) | 0.984 (0.935–1.035) | 0.397 | 0.529 |
| BMI (kg/m2) | 0.921 (0.817–1.039) | 1.780 | 0.182 |
| Arterial hypertension | 6.746 (0.635–71.685) | 2.506 | 0.113 |
| Cardiovascular disease | 1.361 (0.357–5.183) | 0.202 | 0.652 |
| Diabetes mellitus | 2.385 (0.716–7.946) | 2.002 | 0.157 |
| Cerebrovascular disease | 1.407 (0.407–4.860) | 0.292 | 0.939 |
BMI, body mass index; OR, odds ratio; PMAI, psoas muscle area index; SD, standard deviation. Multivariable log‐regression analysis of factors associated with 30 day mortality in COVID‐19 patients (n = 113).
Discussion
Sonographic morphometry uncovered an association of 30 day mortality with muscle quantity in COVID‐19 patients. This association was subsequently validated by an independent COVID‐19 cohort during the ongoing SARS‐CoV‐2 pandemic. A lower PMAI correlated consistently with 30 day mortality of COVID‐19 patients, which was confirmed by a multivariable analysis including established prognostic factors, such as age, gender, BMI, and common comorbidities. However, the limited number of COVID‐19 patients in this study does not allow a detailed assessment of muscle quantity in context of all established prognostic factors. 5 , 7 The small number of patients only provides explorative data to support sonographic muscle assessment for risk stratification of patients with COVID‐19, which should undergo external validation.
Particularly, the well‐documented relation between gender, age and low muscle mass requires further analyses to unravel the individual impact of these factors on COVID‐19 outcome. 2 , 9 , 10 In addition, diabetes mellitus and cerebrovascular comorbidities contribute to reduced muscle quantity, 29 which are associated with more severe COVID‐19 as well. 5 , 6 , 7 We therefore argue that reduced muscle quantity primarily represents a multifactorial maladaptation, which reflects the impaired capacity of vulnerable patients to cope with COVID‐19. Low muscle quantity is a prognostic factor of high relevance as immobility and malnutrition have been identified as driving mechanisms of muscle loss. 10 , 14 These contributors are aggravated in hospitalized COVID‐19 patients under isolation conditions in general and in particular during respiratory support. 30 Not only baseline muscle quantity but also acute muscle loss during COVID‐19 are potential contributors to overall COVID‐19 morbidity. 30 , 31 This highlights the need for adequate care, including adequate alimentary support, facing limited personnel resources during the COVID‐19 pandemic. Sonographic screening for muscle loss could eventually provide the basis of targeted alimentary interventions in COVID‐19 patients. For this purpose, bedside sonography is a convenient muscle screening tool as it is feasible under hygienic precautions, which avoids transportation of COVID‐19 to a diagnostic site.
Despite the advantages of ultrasound, we are aware that sonographic muscle quantification faces some limitations. This includes the observer‐dependent method in contrast to a standardized radiologic scanning or dual X‐ray absorptiometry. 21 , 22 , 32 Hence inter‐observer variations could be relevant, but muscle indices from our reference cohort were in line with previously published PMT (range 31.7–61.2 mm), PMA (range 251–2,177 mm2) and PMAI (range 104–761 mm2/m2). 22 , 23
In addition, sonographic morphometry has not been investigated in the general European population and morphometric reference values from our study are based on a small selection of cohorts. For this reason, sonographic cutoff values of muscle quantity may not account for other COVID‐19 patients and should undergo external validation to obtain reliable gender‐specific cutoff values in particular.
Muscle quantification does not directly assess body function, which is primarily quantified by clinical questionnaires, walk speed, and muscle strength tests to determine frailty. 8 Given that functional testing was not applicable in critical COVID‐19 patients receiving respiratory support, sonography remained the most practical approach to assess functional capacity in these patients. Particularly as muscle quantity is strongly correlated with some functional parameters, such as grip strength and walk speed. 15 , 16 Additional sonographic analysis of muscle density or echogenicity could further improve assessment of muscular function by estimating muscle fat content. 33 However, we did not measure muscle quality in our prospective study and are therefore unable to judge its impact on outcome of COVID‐19. This has to be assessed in future studies and may implement another dimension into sonographic risk stratification of these highly vulnerable patients.
In conclusion, this study underlines the feasibility of bedside ultrasonography to assess muscle dimensions as a surrogate of reduced muscle quantity. Sonographic morphometry hereby allowed stratification for mortality risk in patients with COVID‐19. Our results can serve as a planning basis for larger‐scale prospective studies, which are mandatory to define sonographic parameters of low muscle quantity and their impact on the course of COVID‐19 in context with other confounding factors.
Conflict of interest
The authors declare no conflicts of interest regarding the content of this manuscript.
Supporting information
Figure S1. Flow chart and study design.
Figure S2. Patient survival according to muscle indices (internal reference).
Acknowledgements
No funding was granted for this project.
Kremer W. M., Labenz C., Kuchen R., Sagoschen I., Bodenstein M., Schreiner O., Wörns M. A., Sivanathan V., Weinmann A., Galle P. R., and Sprinzl M. F. (2022) Sonographic assessment of low muscle quantity identifies mortality risk during COVID‐19: a prospective single‐centre study, Journal of Cachexia, Sarcopenia and Muscle, 13, 169–179, 10.1002/jcsm.12862
References
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases drom the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 2. Bonanad C, García‐Blas S, Tarazona‐Santabalbina F, Sanchis J, Bertomeu‐González V, Fácila L, et al. The effect of age on mortality in patients with COVID‐19: a meta‐analysis with 611,583 subjects. J Am Med Dir Assoc 2020;21:915–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan WJ, Liang WH, He JX, Zhong NS. Cardiovascular comorbidity and its impact on patients with COVID‐19. Eur Respir J 2020;55:2001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fang H, Liu Q, Xi M, Xiong D, He J, Luo P, et al. Impact of comorbidities on clinical prognosis in 1280 patients with different types of COVID‐19. J Invest Med 2020;69:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li J, He X, Yuan Y, Zhang W, Li X, Zhang Y, et al. Meta‐analysis investigating the relationship between clinical features, outcomes, and severity of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pneumonia. Am J Infect Control 2021;49:82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID‐19 in China. Clin Res Cardiol 2020;109:531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Izcovich A, Ragusa MA, Tortosa F, Lavena Marzio MA, Agnoletti C, Bengolea A, et al. Prognostic factors for severity and mortality in patients infected with COVID‐19: a systematic review. PLoS ONE 2020;15:e0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyère O. Health outcomes of sarcopenia: a systematic review and meta‐analysis. PLoS ONE 2017;12:e0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta‐analysis and systematic review. Eur J Cancer 2016;57:58–67. [DOI] [PubMed] [Google Scholar]
- 12. Benz E, Trajanoska K, Lahousse L, Schoufour JD, Terzikhan N, De Roos E, et al. Sarcopenia in COPD: a systematic review and meta‐analysis. Eur Respir Rev 2019;28:190049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Durand F, Buyse S, Francoz C, Laouénan C, Bruno O, Belghiti J, et al. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol 2014;60:1151–1157. [DOI] [PubMed] [Google Scholar]
- 14. Cruz‐Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019;393:2636–2646. [DOI] [PubMed] [Google Scholar]
- 15. Cawthon PM, Peters KW, Shardell MD, McLean RR, Dam TT, Kenny AM, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci 2014;69:567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McLean RR, Shardell MD, Alley DE, Cawthon PM, Fragala MS, Harris TB, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci 2014;69:576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dent E, Morley JE, Cruz‐Jentoft AJ, Woodhouse L, Rodríguez‐Mañas L, Fried LP, et al. Physical frailty: ICFSR International Clinical Practice Guidelines for Identification and Management. J Nutr Health Aging 2019;23:771–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med 2011;27:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shen W, Punyanitya M, Wang Z, Gallagher D, Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross‐sectional image. J Appl Physiol (1985) 2004;97:2333–2338. [DOI] [PubMed] [Google Scholar]
- 20. Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta‐analysis. PLoS ONE 2017;12:e0186990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takai Y, Katsumata Y, Kawakami Y, Kanehisa H, Fukunaga T. Ultrasound method for estimating the cross‐sectional area of the psoas major muscle. Med Sci Sports Exerc 2011;43:2000–2004. [DOI] [PubMed] [Google Scholar]
- 22. Kobayashi K, Maruyama H, Kiyono S, Ogasawara S, Suzuki E, Ooka Y, et al. Application of transcutaneous ultrasonography for the diagnosis of muscle mass loss in patients with liver cirrhosis. J Gastroenterol 2018;53:652–659. [DOI] [PubMed] [Google Scholar]
- 23. Hari A, Berzigotti A, Štabuc B, Caglevič N. Muscle psoas indices measured by ultrasound in cirrhosis—preliminary evaluation of sarcopenia assessment and prediction of liver decompensation and mortality. Dig Liver Dis 2019;51:1502–1507. [DOI] [PubMed] [Google Scholar]
- 24. Hida T, Ando K, Kobayashi K, Ito K, Tsushima M, Kobayakawa T, et al. Editors' choice ultrasound measurement of thigh muscle thickness for assessment of sarcopenia. Nagoya J Med Sci 2018;80:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yasuda T, Toyoda S, Inoue T, Nakajima T. Muscle thickness of anterior mid‐thigh in hospitalized patients: comparison of supine and standing postures. Arch Rehabil Res Clin Transl 2020;2:100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tandon P, Low G, Mourtzakis M, Zenith L, Myers RP, Abraldes JG, et al. A model to identify sarcopenia in patients with cirrhosis. Clin Gastroenterol Hepatol 2016;14:1473–1480, e3. [DOI] [PubMed] [Google Scholar]
- 27. Kluge S, Kluge S, Janssens U, Welte T, Weber‐Carstens S, Schälte G, et al. S2k guideline—recommendations for inpatient therapy of patients with COVID‐19. Pneumologie 2021;75:88–112. [DOI] [PubMed] [Google Scholar]
- 28. Ufuk F, Demirci M, Sagtas E, Akbudak IH, Ugurlu E, Sari T. The prognostic value of pneumonia severity score and pectoralis muscle area on chest CT in adult COVID‐19 patients. Eur J Radiol 2020;131:109271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Distefano G, Goodpaster BH. Effects of exercise and aging on skeletal muscle. Cold Spring Harb Perspect Med 2018;8:a029785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Welch C, Greig C, Masud T, Wilson D, Jackson TA. COVID‐19 and acute sarcopenia. Aging Dis 2020;11:1345–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morley JE, Kalantar‐Zadeh K, Anker SD. COVID‐19: a major cause of cachexia and sarcopenia? J Cachexia Sarcopenia Muscle 2020;11:863–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ishida Y, Kanehisa HI, Carroll JF, Pollock ML, Graves JE, Leggett SH. Body fat and muscle thickness distributions in untrained young females. Med Sci Sports Exerc 1995;27:270–274. [PubMed] [Google Scholar]
- 33. Akazawa N, Kishi M, Hino T, Tsuji R, Tamura K, Hioka A, et al. Intramuscular adipose tissue in the quadriceps is more strongly related to recovery of activities of daily living than muscle mass in older inpatients. J Cachexia Sarcopenia Muscle 2021;12:891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow chart and study design.
Figure S2. Patient survival according to muscle indices (internal reference).


