Abstract
Background
Early lymph node tuberculosis (LNTB) diagnosis is still difficult. The majority of LN specimens require the undertaking of invasive and unpleasant procedures.
Purpose
To evaluate the diagnostic efficacy of pathology when combined with molecular tests for the diagnosis of LNTB in core needle biopsy (CNB) specimens and to compare that diagnostic efficacy with that deriving from tissue specimens’ examination alone.
Methods
We retrospectively analyzed the medical records of LNTB patients who met the inclusion criteria. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the curve (AUC) of pathology, molecular tests, and parallel test (positive result for either of these two assays) were calculated to evaluate their diagnostic efficacy compared with a composite reference standard.
Results
A total of 289 patients were included in the study. The overall sensitivity, specificity, PPV, NPV, and AUC of pathology, molecular tests, and parallel test were 94.5%, 97.2%, 99.6%, 71.4%, 0.96; 73.1%, 100.0%, 100.0%, 34.6%, 0.87; and 98.4%, 97.2%, 99.6%, 89.7%, 0.98, respectively. For CNB specimens, these values for pathology, molecular tests, and parallel test were 93.3%, 96.2%, 99.4%, 69.4%, 0.95; 76.4%, 100.0%, 100.0%, 40.0%, 0.88; and 99.4%, 96.2%, 99.4%,96.2%,0.98, while those same values for the tissue were 96.6%, 100.0%, 100.0%, 76.9%, 0.98; 67.1%, 100.0%, 100.0%, 25.6%, 0.84; and 96.6%, 100.0%, 100.0%, 76.9%,0.98, respectively.
Conclusion
The validity of pathology and molecular testing when using CNB specimens was similar to that of tissue specimens for relevant assessment approaches. For the LNTB diagnosis, CNB specimens were preferred for the simultaneous undertaking of pathological examination and molecular testing.
Keywords: pathology, molecular tests, CapitalBio assay, Xpert, lymph node tuberculosis, CNB, tissue, accuracy
Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB) infection, remains a major threat to human health.1 TB occurring in organs other than the lungs is called extrapulmonary tuberculosis (EPTB). Lymph node TB (LNTB) is the most common type of EPTB.2 In LNTB, lesions in the peripheral LN are the most easily observed.3 Although peripheral LNTB is not fatal in most cases,4 delayed treatment could result in significant lymphatic enlargement or abscess formation, seriously affecting the patient’s appearance and quality of life, and leading to social isolation.5 Early LNTB diagnosis is still difficult. The majority of LN specimens require the undertaking of invasive and unpleasant procedures.6 The fine needle aspiration (FNA) is still in limited use for the diagnosis of LNTB.7,8 Its alternative, the LN biopsy, can obtain the whole LN tissue with better diagnostic efficacy, but it is more invasive and has certain risks.9 On the other hand, core needle biopsy (CNB) allows for more of a specimen to be obtained, avoids the shortcomings of FNA without increasing the risk.10 Due to limitations such as the low sensitivity of acid-fast bacilli (AFB) smear and 2–6 weeks required in order to conduct an MTB culture, the AFB smear and the MTB culture using LN samples cannot provide early diagnosis.11 However, a pathological diagnosis is also very important for the diagnosis of LNTB, while the time to obtain pathological results is less than 1 week; a timeframe that does not exert a significant impact on the treatment of LNTB.
With the development of molecular detection technologies, the early and rapid diagnosis of infectious diseases, including TB, is now possible.12 The Xpert MTB/RIF (Xpert, Cepheid, Sunnyvale, CA, USA) is a star product.13,14 This test has been recommended as a diagnostic tool for LNTB by the World Health Organization.15 Moreover, the CapitalBio Mycobacterium real-time polymerase chain reaction (RT-PCR) assay (CapitalBio assay, CapitalBio Technology Inc., Beijing, China) is a rapid diagnostic test that can detect both TB and nontuberculous mycobacteria (NTM) infections with a single test.16 It should be noted that the diagnostic efficacy of this assay for TB is similar to that of Xpert.17,18
Studies including a pathological examination of CNB specimens combined with molecular tests as an approach to LNTB diagnosis, and comparing their efficacy with that provided by tissue specimens alone, are limited. The aim of this study was: (i) to evaluate the diagnostic efficacy of pathology when combined with molecular tests for the diagnosis of LNTB in CNB specimens, (ii) to compare that diagnostic efficacy with that deriving from tissue specimens’ examination alone, as well as (iii) to explore further diagnostic strategies for LNTB so as to provide clinicians and patients with optimal diagnostic protocols.
Materials and Methods
Study Design
At the Zhejiang Tuberculosis Diagnosis and Treatment Center, Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine, we conducted a retrospective study aiming to evaluate the role of pathology combined with molecular tests in the diagnosis of LNTB. We reviewed the clinical medical records of patients with suspected peripheral LNTB hospitalized at our center between January 2018 and December 2020. Patients with enlarged peripheral LNs (diagnosis by ultrasound), combined with TB-related symptoms, such as fever, night sweats, positive results for the tuberculin purified protein derivative (PPD) test and/or the gamma interferon (IFN-γ) release assay, or an evidence of a TB-infection in other body sites, were considered suspected LNTB. Patients who had LN CNB or biopsy performed to obtain specimens for pathology, MTB culture, and molecular tests (CapitalBio assay and/or Xpert) were included in this study. The clinician communicated with the patient about advantages and disadvantages associated with CNB and biopsy, and the patient decided whether to undergo CNB or biopsy. Patients who did not undergo invasive procedures or did not take the relevant tests, had uncertain pathological or molecular test results or were not followed up, were excluded. Human immunodeficiency virus (HIV) antibodies tests were performed on all patients. Written informed consent was obtained from all patients.
Based on the results of the pathological examination and those of the molecular tests, we were able to further analyze the first in combination with the second (parallel test). A positive parallel test result means a positive test for either the pathological examination or the molecular tests, and a negative parallel test result means that both the pathological examination and the molecular tests results are negative. This study complies with the Declaration of Helsinki. The present study was approved by the Human Research Ethics Committee of the Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine.
A composite reference standard (CRS) was used as the diagnostic gold standard for this study. The CRS includes a number of indicators: namely, the AFB smear, the MTB culture, clinical symptoms, ultrasound and imaging changes, the pathology results, other molecular testing results, the results of the IFN-γ release assay and those of the PPD test, as well as the effectiveness of the administered anti-TB treatment. Multiple positive indicators were needed to confirm LNTB as single indicators, except AFB smear and MTB culture, cannot confirm LNTB. Based on this reference standard, we organized the enrolled patients into three groups:
Confirmed LNTB
Patients with a positive AFB smear or MTB culture from their LN specimens;
Probable LNTB
Patients with TB-associated clinical symptoms, imaging changes indicative of LNTB, combined TB of other organs, positive immunological tests, positive results from the pathological examination or the molecular testing, response to anti-TB therapy;
Non-LNTB
Negative AFB smear and MTB culture, clearly other pathogenic infections or tumors, and improvement of disease without anti-TB therapy.
Confirmed LNTB and probable LNTB patients were considered to have clinical LNTB according to the CRS.
Diagnostic Specimen Collection and Handling
LN CNB specimens and tissue specimens were collected for pathological examination and molecular testing. CNB specimens were obtained by ultrasound-guided CNB using an 18G puncture needle, while tissue specimens were obtained by LN biopsy. The obtained LN specimens (≥3 punctures-full of CNB specimens and ≥1 cm3 of tissue specimens from biopsy) were aliquoted into three groups: (i) those to be used for an MTB culture, (ii) those to undergo a histological examination, and (ii) those to be submitted to molecular testing. As a result, one of the CNB specimens and one part of the tissue specimen were placed in 10% formalin solution and subsequently sent to the Pathology Department for histological processing and examination. The remaining CNB and tissue specimens were sent to the TB laboratory for molecular testing and MTB culturing.
MTB Culture
MTB culture of LN specimens used solid or liquid medium. Solid culture used Lowenstein–Jensen medium and liquid culture used the BACTEC MGIT 960 Mycobacteria Culture System (BD Diagnostic Systems, Sparks, MD), according to the system’s instructions.
Pathology
After receiving the specimens fixed in 10% formalin solution, the Pathology Department first rinsed them and then gradually dehydrated them with 80–100% alcohol. After dehydration, the specimens were soaked in paraffin wax and embedded so as to obtain wax blocks. The wax blocks were trimmed and sliced to a thickness of 4–6 μm. The sections were fully flattened, then placed on slides and subsequently baked at 60°C for 15–30 min in order to remove the paraffin wax. The obtained sections were subjected to pathological examination and antacid staining by an experienced pathology professional. TB was considered when granulomatous inflammation along with coagulative necrosis was observed. Histological results were available within 1 week.
Molecular Tests
Specimens were tested using CapitalBio assay and/or Xpert. For CapitalBio assay, the specimens were added to the buffer; a homogeneous suspension was obtained by grinding, and 1 mL was obtained. The 1-mL sample was added to 2 mL of the specimen preparation solution to obtain liquefied specimens. Then, 1 mL liquefied specimens were centrifuged to obtain sediment. Then, the sediment was used for nucleic acid extraction. Then, 2 µL extracted DNA was used according to the manufacturer’s instructions. A fluorescence quantification RT-PCR instrument (SLAN-96S Real-Time PCR System ZEESAN Xiamen CN) was used to amplify and detect IS6110 multicopy elements for MTB. The test results were available in ~3 hours.19 For Xpert, 1 mL of the specimen and 2 mL of the specimen reagent were mixed. The mixture was agitated for 20s and incubated at room temperature for 15 min. Next, 2 mL of the treated specimen solution was added to the Xpert reaction cartridge and placed in the assay module to initiate automatic assay. The system automatically read the MTB assay results within 2 hours.20 A positive test for either CapitalBio assay or Xpert was considered a positive molecular test.
Data Processing and Statistical Analysis
True positive (TP), false positive (FP), false negative (FN), and true negative (TN) values in a cross-tabulation were calculated using SPSS 24.0 (IBM Corp., Armonk, NY). Calculation of the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the curve (AUC) with 95% confidence interval (CI) of the pathology and molecular tests based on TP, FP, FN, TN values using MedCalc Statistical v15.2.2 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org). This study used CRS as the reference standard to evaluate the diagnostic efficacy of the relevant assays. Paired data were compared using McNemar’s test. Proportions were compared for different types of specimens using the Chi-square test or Fisher’s exact test. Differences between different AUCs were compared using the Z test. Venn diagram was generated using an interactive tool (https://bioinfogp.cnb.csic.es/tools/venny/index.html).21 P values < 0.05 were regarded as statistically significant differences.
Results
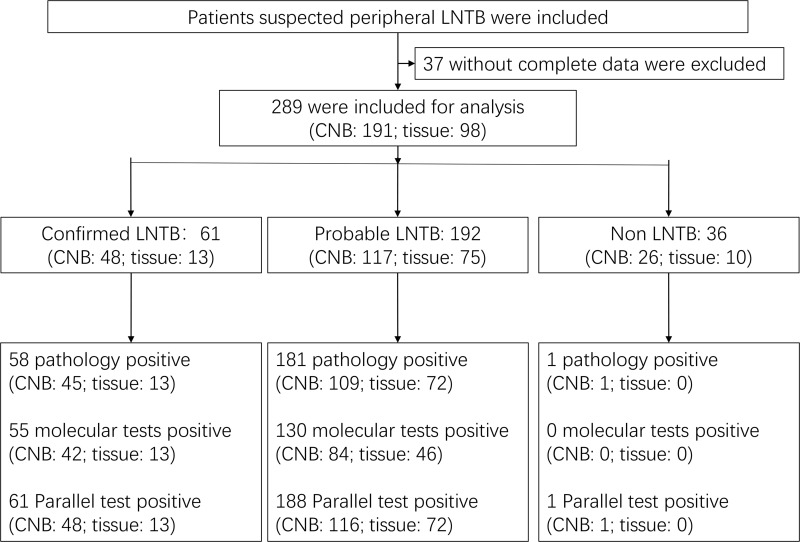
289 patients were finally included in this study after reviewing their clinical data. 37 patients with incomplete data were excluded, including 20 patients who were lost to follow-up (Figure 1). Each patient provided a CNB specimen or tissue specimen; as a result, a total of 289 specimens were collected, including 98 tissue specimens and 191 CNB specimens. The clinical characteristics of the patients are summarized in Table 1.
Figure 1.
Diagnostic classification of the study participants.
Abbreviations: LNTB, Lymph node tuberculosis; CNB, core needle biopsy.
Table 1.
Clinical Characteristics of the Included Patients
| Characteristics | All (n = 289) | Confirmed LNTB (n = 61) | Probable LNTB (n = 192) | Non-LNTB (n = 36) |
|---|---|---|---|---|
| Age (year, mean ± SD) | 39.7±19.0 | 47.7±20.7 | 36.4±16.4 | 44.8±23.1 |
| Male (n, %) | 127 (43.9) | 25 (41.0) | 81 (42.2) | 21 (58.3) |
| HIV positive (n, %) | 1 (0.3) | 1 (1.6) | 0 (0.0) | 0 (0.0) |
| Symptoms (n, %) | ||||
| Fever | 76 (26.3) | 19 (31.1) | 49 (25.5) | 8 (22.2) |
| Night sweat | 40 (13.8) | 17 (27.9) | 21 (10.9) | 2 (5.6) |
| Pain | 74 (25.6) | 21 (34.4) | 32 (16.7) | 21 (58.3) |
| Dysphagia | 9 (3.1) | 4 (6.6) | 3 (1.6) | 2 (5.6) |
| Weigh loss | 38 (13.1) | 19 (31.1) | 17 (8.9) | 2 (5.6) |
| LN sites (n, %) | ||||
| Cervical LN | 264 (91.3) | 57 (93.4) | 174 (90.6) | 33 (91.7) |
| Axillary LN | 23 (8.0) | 4 (6.6) | 16 (8.3) | 3 (8.3) |
| Inguinal LN | 2 (0.7) | 0 (0.0) | 2 (1.0) | 0 (0.0) |
| Other sites involved of TB (n, %) | ||||
| Pulmonary TB | 122 (42.2) | 26 (42.6) | 90 (46.9) | 6 (16.7) |
| Pleural TB | 13 (4.5) | 1 (1.6) | 11 (5.7) | 1 (2.8) |
| Specimen type (n, %) | ||||
| CNB | 191 (66.1) | 48 (78.7) | 117 (60.9) | 26 (72.2) |
| Tissue | 98 (33.9) | 13 (21.3) | 75 (39.1) | 10 (27.8) |
| Pathological antacid staining (+) (n, %) | 13 (4.5) | 13 (21.3) | 0 (0.0) | 0 (0.0) |
Abbreviations: LNTB, lymph node tuberculosis; HIV, human immunodeficiency virus; LN, lymph node; TB, tuberculosis; CNB, core needle biopsy.
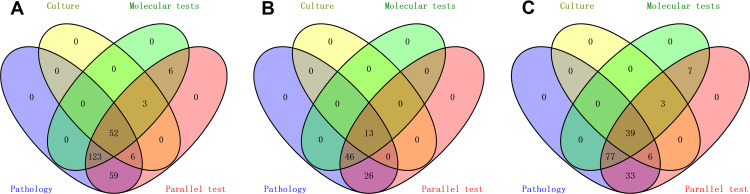
Based on the CRS, 61 specimens were defined as confirmed LNTB, 192 as probable LNTB, and 36 as non-LNTB (Figure 1). The distribution and the overlap of the positive findings deriving from the aforementioned methods were compared with the CRS in the different types of specimens, and are presented in Figure 2. The 36 non-LNTB patients were eventually diagnosed with lymphadenitis (n = 18), lymphomas (n = 9), metastatic tumors (n = 8) or NTM infection (n = 1). A patient with lymphoma had a pathological diagnosis of TB based on a CNB specimen, received an anti-TB treatment that proved ineffective, and eventually was diagnosed with lymphoma based on an LN biopsy at another hospital.
Figure 2.
Venn diagram of positive tests. (A) All specimens. (B) Tissue specimens. (C) Core needle biopsy specimens.
Diagnostic Accuracy of Pathology, Molecular Tests, and the Parallel Test
The overall sensitivity, specificity, PPV, NPV, and AUC of pathology in diagnosing LNTB were 94.5% (90.9–96.9%), 97.2% (85.5–99.9%), 99.6% (97.7–100.0%), 71.4% (56.7–83.4%), and 0.96 (0.93–0.98), respectively. The overall sensitivity, specificity, PPV, NPV, and AUC of the molecular tests were 73.1% (67.2–78.5%), 100.0% (90.3–100.0%), 100.0% (98.0–100.0%), 34.6% (25.6–44.6%), and 0.87 (0.82–0.90), respectively, while those same values for the parallel test were 98.4% (96.0–99.6%), 97.2% (85.5–99.9%), 99.6% (97.8–100.0%), 89.7% (75.8–97.1%), and 0.98 (0.95–0.99), respectively. Table 2 summarizes the results of these analyses.
Table 2.
Accuracy of Pathology, Molecular Tests, and Parallel Test (Positive Result for Either of the Two Assays) for the Diagnosis of Lymph Node Tuberculosis Against a Composite Reference Standard
| Test | Sample | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC |
|---|---|---|---|---|---|---|
| Pathology | All | 94.5 (90.9–96.9) | 97.2 (85.5–99.9) | 99.6 (97.7–100.0) | 71.4 (56.7–83.4) | 0.96 (0.93–0.98) |
| Tissue | 96.6 (90.4–99.3) | 100.0 (69.2–100.0) | 100.0 (95.8–100.0) | 76.9 (46.2–95.0) | 0.98 (0.93–1.00) | |
| CNB | 93.3 (88.4–96.6) | 96.2 (80.4–99.9) | 99.4 (96.5–100.0) | 69.4(51.9–83.7) | 0.95 (0.91–0.97) | |
| Molecular test | All | 73.1 (67.2–78.5) | 100.0 (90.3–100.0) | 100.0 (98.0–100.0) | 34.6 (25.6–44.6) | 0.87 (0.82–0.90) |
| Tissue | 67.1 (56.2–76.7) | 100.0 (69.2–100.0) | 100.0 (93.9–100.0) | 25.6 (13.0–42.1) | 0.84 (0.75–0.90) | |
| CNB | 76.4 (69.1–82.6) | 100.0 (86.8–100.0) | 100.0 (97.1–100.0) | 40.0 (28.0–52.9) | 0.88 (0.83–0.92) | |
| Parallel test | All | 98.4 (96.0–99.6) | 97.2 (85.5–99.9) | 99.6 (97.8–100.0) | 89.7 (75.8–97.1) | 0.98 (0.95–0.99) |
| Tissue | 96.6 (90.4–99.3) | 100.0 (69.2–100.0) | 100.0 (95.8–100.0) | 76.9 (46.2–95.0) | 0.98 (0.93–1.00) | |
| CNB | 99.4 (96.7–100.0) | 96.2 (80.4–99.9) | 99.4 (96.7–100.0) | 96.2 (80.4–99.9) | 0.98 (0.95–0.99) |
Abbreviations: PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve; CNB, core needle biopsy.
When using tissue specimens for testing, the sensitivity, specificity, PPV, NPV, and AUC of the histological examination were 96.6% (90.4–99.3%), 100.0% (69.2–100.0%), 100.0% (95.8–100.0%), 76.9% (46.2–95.0%), and 0.98 (0.93–1.00), respectively. On the other hand, the sensitivity, specificity, PPV, NPV, and AUC of the molecular tests were 67.1% (56.2–76.7%), 100.0% (69.2–100.0%), 100.0% (93.9–100.0%), 25.6% (13.0–42.1%), and 0.84 (0.75–0.90), respectively, while those same values for the parallel test were 96.6% (90.4–99.3%), 100.0% (69.2–100.0%), 100.0% (95.8–100.0%), 76.9% (46.2–95.0%), and 0.98 (0.93–1.00), respectively.
Finally, for the CNB specimens, the sensitivity, specificity, PPV, NPV, and AUC of the histological examination were 93.3% (88.4–96.6%), 96.2% (80.4–99.9%), 99.4% (96.5–100.0%), 69.4% (51.9–83.7%), and 0.95 (0.91–0.97), respectively, while those same values for the molecular testing were 76.4% (69.1–82.6%), 100.0% (86.8–100.0%), 100.0% (97.1–100.0%), 40.0% (28.0–52.9%), and 0.88 (0.83–0.92), respectively. When the parallel testing was examined on CNB specimens, its sensitivity, specificity, PPV, NPV, and AUC were 99.4% (96.7–100.0%), 96.2% (80.4–99.9%), 99.4% (96.7–100.0%), 96.2% (80.4–99.9%), and 0.98 (0.95–0.99), respectively. These results were summarized in Table 2.
Comparison of the Diagnostic Accuracy of Pathology, Molecular Tests, and the Parallel Test
We compared the differences in the diagnostic efficacy when using different assays for the same type of specimen. Overall, the diagnostic efficacy of pathology was significantly better than that of the molecular tests (P < 0.05; Table 3). Parallel tests improved the diagnostic efficacy over single tests, and significantly improved the diagnostic efficacy over the molecular tests (P < 0.05), but did not achieve statistically significant improvement for this same efficacy over pathology (P > 0.05). For tissue specimens, the comparison results were consistent with those concluded for the overall comparison: the diagnostic efficacies of pathology and of the parallel tests were significantly better than that of the molecular tests (P < 0.05), while the diagnostic efficacies of pathology and of the parallel tests were consistent. For CNB specimens, the accuracy of pathology was superior to that of the molecular tests, but the difference was not statistically significant (P > 0.05). Moreover, no statistically significant difference was observed in the parallel testing being compared to pathology (P > 0.05), while when compared to the molecular testing, a statistical significance was indeed observed (P < 0.05). All undertaken comparisons are summarized in Table 3.
Table 3.
Comparison of the Diagnostic Efficiency Between Pathology, Molecular Tests, and Parallel Test for the Diagnosis of Lymph Node Tuberculosis
| Sample Type | Test | Sensitivity (P-value) | Specificity (P-value) | PPV (P-value) | NPV (P-value) | AUC (P-value) |
|---|---|---|---|---|---|---|
| Overall | Pathology vs Molecular test | < 0.001 | 0.314 | 0.379 | < 0.001 | < 0.001 |
| Pathology vs Parallel test | 0.016 | 1.000 | 0.977 | 0.034 | 0.188 | |
| Molecular test vs Parallel test | < 0.001 | 0.314 | 0.389 | < 0.001 | < 0.001 | |
| Tissue | Pathology vs Molecular test | < 0.001 | 1.000 | 1.000 | 0.001 | < 0.001 |
| Pathology vs Parallel test | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | |
| Molecular test vs Parallel test | < 0.001 | 1.000 | 1.000 | 0.001 | < 0.001 | |
| CNB | Pathology vs Molecular test | < 0.001 | 0.313 | 0.366 | 0.005 | 0.053 |
| Pathology vs Parallel test | 0.003 | 1.000 | 0.965 | 0.009 | 0.199 | |
| Molecular test vs Parallel test | < 0.001 | 0.313 | 0.381 | < 0.001 | 0.003 |
Abbreviations: PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve; CNB, core needle biopsy.
We then further compared the diagnostic efficacy of the same test across the different types of specimens. The diagnostic efficacy of pathology in tissue specimens was – as expected – superior to that in CNB specimens, whereas the diagnostic efficacy of the molecular testing was – on the contrary – lower in tissue than in CNB specimens. However, the difference in the diagnostic efficacy in tissue and CNB specimens was not significant for either the histological examination or the molecular testing (P > 0.05; Table 4). When the pathological and the molecular testing were applied in parallel, the diagnostic efficacy was consistent in both the tissue and the CNB specimens.
Table 4.
Comparison of the Diagnostic Efficiency of Pathology, Molecular Tests, and Parallel Test Using Different Specimen Types for the Diagnosis of Lymph Node Tuberculosis
| Test | Sensitivity (P-value) | Specificity (P-value) | PPV (P-value) | NPV (P-value) | AUC (P-value) |
|---|---|---|---|---|---|
| Pathology (Tissue vs CNB) | 0.280 | 0.529 | 0.458 | 0.609 | 0.188 |
| Molecular test (Tissue vs CNB) | 0.111 | 1.000 | 1.000 | 0.136 | 0.346 |
| Parallel test (Tissue vs CNB) | 0.089 | 0.529 | 0.472 | 0.062 | 0.843 |
Abbreviations: PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve; CNB, core needle biopsy.
Discussion
Early and effective LNTB treatment is conducive to the prognosis of patients.22 In general, LN specimens are most likely to be obtained by invasive procedures, while the way to achieve optimal diagnostic performance with minimal trauma remains an issue to be explored. Early stage LNTB is mainly characterized by LN enlargement, and the options for obtaining specimens at this time are either the performance of an LN puncture or of an LN biopsy.23 LN puncture can be categorized into FNA and CNB based on the thickness of the puncture needle. FNA, CNB and tissue specimens (through biopsy) can all be examined histologically, but FNA specimens contain way less tissue and can only be examined cytopathologically in most cases,7,24 while CNB specimens contain more tissue and can be examined histopathologically in the same way as tissue specimens.25 In terms of pathology, CNB and tissue specimens are more effective than FNA specimens in establishing diagnoses, while their differences in terms of diagnostic usefulness (as assessed through pathology) remain to be further clarified. The results of this study showed that the sensitivity, specificity, and AUC when using tissue specimens for pathological testing were 96.6%, 100.0%, and 0.98, respectively; on the other hand, those same values for the CNB specimens were 93.3%, 96.2%, and 0.95, respectively. Pathology had excellent efficacy in both tissue and CNB specimens, with tissue specimens being superior but not significantly different than the CNB ones. Moreover, recent studies have shown that pathology has the highest positive rate in LNTB, which is consistent with our results.26 The amount of biopsy-deriving tissue specimens was sufficient enough to allow for the examination of the entire LN structure, while the CNB specimens were – of course – smaller compared to biopsies and the chances the puncture site not sampling the LN lesion was significant.
To date, molecular tests are widely used for the diagnosis of TB.14,27,28 The exploration of molecular tests in the diagnosis of LNTB has not stopped either.27 In fact, many studies have shown good efficacy of the molecular tests in the diagnosis of LNTB,29 and obtaining LN specimens for molecular tests is a very important step in obtaining microbiological evidence for the diagnosis of LNTB. However, molecular tests could present with different efficacy in different types of specimens.18,30 Studies assessing differences in the validity of molecular tests by using CNB specimens and tissue specimens are limited. This aspect was also evaluated in the present study, and the results have shown that the sensitivity, specificity, and AUC of the molecular assays using tissue specimens are 67.1%, 100.0%, and 0.84, respectively; while those same values for CNB specimens were 76.4%, 100.0%, and 0.88, respectively. The validity of the molecular tests when using CNB specimens was higher than that of tests using tissue specimens, but still no statistically significant differences were observed between them. These results may occur due to the fact that: (i) the bacterial content of the tissue is very low, (ii) the distribution of the pathogens in tissue specimens is not relatively uniform, and (iii) the processing of tissue specimens is more difficult. On the other hand, CNB specimens are much easier to homogenize, and molecular tests are characterized by improved validity for CNB specimens. Previous studies had also compared the efficacy of FNA specimens with those of tissue specimens for molecular testing, again suggesting that the use of FNA specimens for molecular testing is more appropriate.6
We, herein, further examined the role of pathology when combined with molecular tests for the diagnosis of LNTB; a role that has been explored in only a few other studies. The results of our study have shown that the sensitivity, specificity, and AUC of the parallel testing using tissue specimens for the diagnosis of LNTB was 96.6%, 100.0%, and 0.98, respectively; and that those same values for the parallel testing using CNB specimens were 99.4%, 96.2%, and 0.98, respectively. Although the pathological validity of CNB specimens was lower than that of tissue specimens, the CNB specimens’ molecular testing validity was higher than that of tissue specimens, while the validity of the two tests combined can be equal to that of the tissue specimens. Although there was no significant benefit from the parallel testing in tissue specimens, the molecular testing can provide microbiological evidence and information on rifampicin-resistance,2,31 while in tissue specimens we have even managed to achieve an early identification of eight patients with rifampicin-resistant TB; a diagnosis that would have not been possible with pathology.
The present study showed that the overall sensitivity, specificity, and AUC of the histological examination and the molecular testing for LNTB was 94.5%, 97.2%, 0.96, and 73.1%, 100.0%, and 0.87, respectively. Overall, the histopathological diagnosis of LNTB was efficient, while the molecular tests were moderately effective in diagnosing LNTB. In tissue specimens, the validity of pathology was significantly better than that of the molecular testing, while in CNB specimens, the diagnostic advantage of pathology did not reach any statistical significance. These results suggested that pathology was still the most effective option in the diagnosis of LNTB, and that it was still necessary to obtain histopathological findings from wherever possible, in the diagnosis of LNTB. Histopathological results are usually available within 1 week; a longer process than that required for the molecular testing, but a more informative one. The time lost while waiting for the histopathological results does not have a serious impact on the course of LNTB, as the diagnosis of the latter will still be classified as “early” even a week after the biopsy.
The results of this study had very good reference value for the development of diagnostic strategies for LNTB. During the early stage LNTB, when no LN specimens can be obtained by non-invasive methods, we would recommend the performing of CNB first (as it would be less invasive) and the obtaining of specimens for simultaneous histopathological and molecular testing. Our recommendation is based on our finding that the diagnostic efficacy of pathology combined with molecular testing in CNB specimens seems to be consistent with that in tissue specimens, but CNB is less invasive, less risky, and more acceptable to patients, which is beneficial to them. If there was no evidence of TB after the assessment of the CNB specimen, LNTB should be considered highly unlikely as a diagnosis and an LN biopsy should be recommended as the next step, with the aim of excluding other LN diseases.
Some shortcomings existed in this study. Firstly, the patient selection may have been biased due to limitations of retrospective study design. Secondly, not all patients in this study had their specimens assessed by both molecular tests and, as a result, the actual diagnostic efficacy might have been underestimated when combining the two molecular tests in our analysis. Thirdly, this study used the CRS as the reference standard, which contains multiple indicators, and the number of indicators evaluated might be different for each patient, which might also be a source of bias. Fourthly, the positive rate of AFB smear and MTB culture in this study was low, and most of the patients were probable LNTB after a comprehensive evaluation, and the number of non-LNTB patients was also low, which may be related to the fact that we are a TB center in Zhejiang Province, where TB is clustered. Patients considered non-LNTB after initial evaluation in other hospitals did not come to our center. This may also be a source of bias in patient selection. Finally, this study was conducted in a high TB burden area, and therefore it may not be applicable to other areas.
Conclusions
Pathology had excellent diagnostic efficacy, while the molecular tests had moderate efficacy in providing a diagnosis for LNTB. The validity of pathology combined with the molecular testing when using CNB specimens was similar to that of tissue specimens for relevant assessment approaches. Parallel tests improved the validity of LNTB diagnosis, particularly for CNB specimens. For the LNTB diagnosis, CNB is less invasive, less risky, and more acceptable to patients. Therefore, CNB specimens were preferred for the simultaneous undertaking of pathological examination and molecular testing.
Acknowledgments
We would like to express our feelings to the patients and colleagues in our department.
Funding Statement
Guocan Yu, 20201203B183, Hangzhou Science and Technology Bureau, http://kj.hangzhou.gov.cn. The funders do not have a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Sharing Statement
Data will be made available on reasonable request.
Ethics Approval and Consent to Participate
All patients gave written informed consent and the study was approved by the Human Research Ethics Committee of Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine. This study complies with the Declaration of Helsinki.
Disclosure
The authors declare that they have no conflict of interest.
References
- 1.World Health Organization. Global Tuberculosis Report 2021; 2021. [Google Scholar]
- 2.Kohli M, Schiller I, Dendukuri N, et al. Xpert MTB/RIF Ultra and Xpert MTB/RIF assays for extrapulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2021;1(1):Cd012768. doi: 10.1002/14651858.CD012768.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho OH, Park KH, Kim T, et al. Paradoxical responses in non-HIV-infected patients with peripheral lymph node tuberculosis. J Infect. 2009;59(1):56–61. doi: 10.1016/j.jinf.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 4.Mouba JF, Miloundja J, Mimbila-Mayi M, Ndjenkam FT, N’Zouba L. [Cervical lymph node tuberculosis in Libreville: epidemiology, diagnosis, and therapy]. Sante. 2011;21(3):165–168. French. doi: 10.1684/san.2011.0269 [DOI] [PubMed] [Google Scholar]
- 5.Chahed H, Hachicha H, Berriche A, et al. Paradoxical reaction associated with cervical lymph node tuberculosis: predictive factors and therapeutic management. Int J Infect Dis. 2017;54:4–7. doi: 10.1016/j.ijid.2016.10.025 [DOI] [PubMed] [Google Scholar]
- 6.Yu G, Zhong F, Ye B, Xu X, Chen D, Shen Y. Diagnostic accuracy of the Xpert MTB/RIF assay for lymph node tuberculosis: a systematic review and meta-analysis. Biomed Res Int. 2019;2019:4878240. doi: 10.1155/2019/4878240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sellami M, Charfi S, Chaabouni MA, et al. Fine needle non-aspiration cytology for the diagnosis of cervical lymph node tuberculosis: a single center experience. Braz J Otorhinolaryngol. 2019;85(5):617–622. doi: 10.1016/j.bjorl.2018.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou J, Li F, Meng L, et al. Fine needle aspiration cytology for lymph nodes: a three-year study. Br J Biomed Sci. 2016;73(1):28–31. doi: 10.1080/09674845.2016.1144947 [DOI] [PubMed] [Google Scholar]
- 9.Houlden C, Woodfield J. The validity of the macroscopic appearance of lymph node biopsy in the diagnosis of tuberculosis. Trop Doct. 2015;45(4):221–224. doi: 10.1177/0049475515586501 [DOI] [PubMed] [Google Scholar]
- 10.Ryu YJ, Cha W, Jeong WJ, Choi SI, Ahn SH. Diagnostic role of core needle biopsy in cervical lymphadenopathy. Head Neck. 2015;37(2):229–233. doi: 10.1002/hed.23580 [DOI] [PubMed] [Google Scholar]
- 11.Fontanilla JM, Barnes A, von Reyn CF. Current diagnosis and management of peripheral tuberculous lymphadenitis. Clin Infect Dis. 2011;53(6):555–562. doi: 10.1093/cid/cir454 [DOI] [PubMed] [Google Scholar]
- 12.Altez-Fernandez C, Ortiz V, Mirzazadeh M, Zegarra L, Seas C, Ugarte-Gil C. Diagnostic accuracy of nucleic acid amplification tests (NAATs) in urine for genitourinary tuberculosis: a systematic review and meta-analysis. BMC Infect Dis. 2017;17(1):390. doi: 10.1186/s12879-017-2476-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu G, Ye B, Chen D, et al. Comparison between the diagnostic validities of Xpert MTB/RIF and interferon-γ release assays for tuberculous pericarditis using pericardial tissue. PLoS One. 2017;12(12):e0188704. doi: 10.1371/journal.pone.0188704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Y, Yu G, Zhong F, Kong X, Cheungpasitporn W. Diagnostic accuracy of the Xpert MTB/RIF assay for bone and joint tuberculosis: a meta-analysis. PLoS One. 2019;14(8):e0221427. doi: 10.1371/journal.pone.0221427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denkinger CM, Schumacher SG, Boehme CC, Dendukuri N, Pai M, Steingart KR. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2014;44(2):435–446. doi: 10.1183/09031936.00007814 [DOI] [PubMed] [Google Scholar]
- 16.Shen Y, Fang L, Xu X, Ye B, Yu G. CapitalBio Mycobacterium real-time polymerase chain reaction detection test: rapid diagnosis of Mycobacterium tuberculosis and nontuberculous mycobacterial infection. Int J Infect Dis. 2020;98:1–5. doi: 10.1016/j.ijid.2020.06.042 [DOI] [PubMed] [Google Scholar]
- 17.Zheng H, Zhong F, Yu G, Shen Y. Comparison of the diagnostic efficacy of the CapitalBio Mycobacterium real-time polymerase chain reaction detection test and Xpert MTB/RIF in smear-negative pulmonary tuberculosis. Eur J Clin Microbiol Infect Dis. 2021;40(5):969–977. doi: 10.1007/s10096-020-04113-1 [DOI] [PubMed] [Google Scholar]
- 18.Yu G, Shen Y, Ye B, Chen D, Xu K. Comparison of CapitalBio™ Mycobacterium nucleic acid detection test and Xpert MTB/RIF assay for rapid diagnosis of extrapulmonary tuberculosis. J Microbiol Methods. 2020;168:105780. doi: 10.1016/j.mimet.2019.105780 [DOI] [PubMed] [Google Scholar]
- 19.Sun L, Yao L, Fu G, Lin L, Zhu E, Huang J. A comparison of the accuracy of the CapitalBio Mycobacterium real-time polymerase chain reaction and the Xpert MTB/RIF assay for the diagnosis of tuberculous meningitis. Int J Infect Dis. 2021;104:92–96. doi: 10.1016/j.ijid.2020.12.044 [DOI] [PubMed] [Google Scholar]
- 20.Kay AW, González Fernández L, Takwoingi Y, et al. Xpert MTB/RIF and Xpert MTB/RIF ultra assays for active tuberculosis and rifampicin resistance in children. Cochrane Database Syst Rev. 2020;8(8):Cd013359. doi: 10.1002/14651858.CD013359.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu G, Wang X, Zhu P, Shen Y, Zhao W, Zhou L. Comparison of the efficacy of metagenomic next-generation sequencing and Xpert MTB/RIF in the diagnosis of tuberculous meningitis. J Microbiol Methods. 2021;180:106124. doi: 10.1016/j.mimet.2020.106124 [DOI] [PubMed] [Google Scholar]
- 22.Blaikley JF, Khalid S, Ormerod LP. Management of peripheral lymph node tuberculosis in routine practice: an unselected 10-year cohort. Int J Tuberc Lung Dis. 2011;15(3):375–378. [PubMed] [Google Scholar]
- 23.Ramos CG, Goldani LZ. Biopsy of peripheral lymph nodes: a useful tool to diagnose opportunistic diseases in HIV-infected patients. Trop Doct. 2011;41(1):26–27. doi: 10.1258/td.2010.100145 [DOI] [PubMed] [Google Scholar]
- 24.Suresh PK, Poojary S, Basavaiah SH, Kini JR, Lobo FD, Sahu KK. Utility of fine-needle aspiration cytology in the diagnosis of HIV lymphadenopathy. Diagn Cytopathol. 2019;47(10):1011–1017. doi: 10.1002/dc.24255 [DOI] [PubMed] [Google Scholar]
- 25.Oh KH, Woo JS, Cho JG, Baek SK, Jung KY, Kwon SY. Efficacy of ultrasound-guided core needle gun biopsy in diagnosing cervical lymphadenopathy. Eur Ann Otorhinolaryngol Head Neck Dis. 2016;133(6):401–404. doi: 10.1016/j.anorl.2016.01.013 [DOI] [PubMed] [Google Scholar]
- 26.Sharif N, Ahmed D, Mahmood RT, et al. Comparison of different diagnostic modalities for isolation of Mycobacterium Tuberculosis among suspected tuberculous lymphadenitis patients. Braz J Biol. 2021;83:e244311. doi: 10.1590/1519-6984.244311 [DOI] [PubMed] [Google Scholar]
- 27.Han Y, Xiao N, Huang S, Qin M, Che N, Liu Z. The application of Xpert Mycobacterium tuberculosis/Rifampicin, quantitative polymerase chain reaction and high resolution melting curve in the diagnosis of superficial lymph node TB. Curr Pharm Biotechnol. 2019;20(12):1044–1054. doi: 10.2174/1389201020666190716104131 [DOI] [PubMed] [Google Scholar]
- 28.Yu G, Shen Y, Zhong F, Ye B, Yang J, Chen G. Diagnostic accuracy of the loop-mediated isothermal amplification assay for extrapulmonary tuberculosis: a meta-analysis. PLoS One. 2018;13(6):e0199290. doi: 10.1371/journal.pone.0199290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaur M, Singh A, Sharma V, et al. Diagnostic performance of non-invasive, stool-based molecular assays in patients with paucibacillary tuberculosis. Sci Rep. 2020;10(1):7102. doi: 10.1038/s41598-020-63901-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohli M, Schiller I, Dendukuri N, et al. Xpert(®) MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst Rev. 2018;8(8):Cd012768. doi: 10.1002/14651858.CD012768.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Habous M, Elimam MA, Kumar R, Deesi ZA. Evaluation of GeneXpert Mycobacterium tuberculosis/Rifampin for the detection of Mycobacterium tuberculosis complex and rifampicin resistance in nonrespiratory clinical specimens. Int J Mycobacteriol. 2019;8(2):132–137. doi: 10.4103/ijmy.ijmy_83_19 [DOI] [PubMed] [Google Scholar]