Introduction
Keratoacanthoma (KA), a cutaneous, often spontaneously regressing tumor characteristically presenting as an umbilicated nodule with a central keratin plug, occurs most frequently in lightly pigmented individuals with sun damage. Here we report an unusual case of KA with a large pedunculated morphology, diagnosed in an African American man, that arose in hidradenitis suppurativa (HS), a relapsing inflammatory skin disease affecting hair follicles in intertriginous areas.
Case report
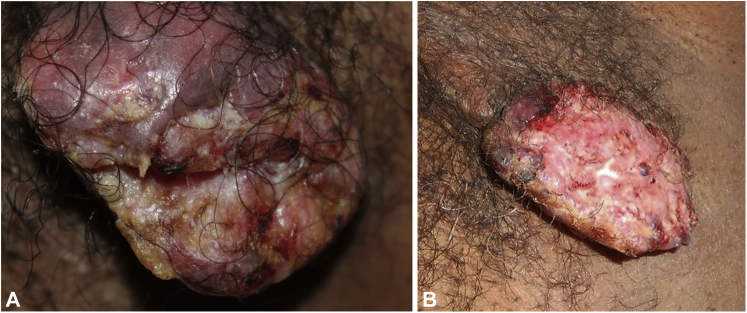
A 60-year-old African American man with no prior cancer history was referred to teledermatology with a left axillary pedunculated eroded mass, measuring 2.6 × 3.9 cm, of 3 to 4 months’ duration, after no improvement was seen during treatment with oral antibiotics. The mass was multicolored, with a rough, lumpy texture, lacking sinus tracts (Fig 1, A). The patient reported that the mass had nearly doubled in size over the past week, with mild bleeding, itching, and tenderness. His past medical history was notable for 40 pack years of smoking and daily use of oral prednisone (10 mg) for 5 years for nasal polyps before presentation.
Fig 1.
Pedunculated, eroded keratoacanthoma in the axilla (A) at the time of teledermatology consultation and (B) in the clinic 2 weeks later.
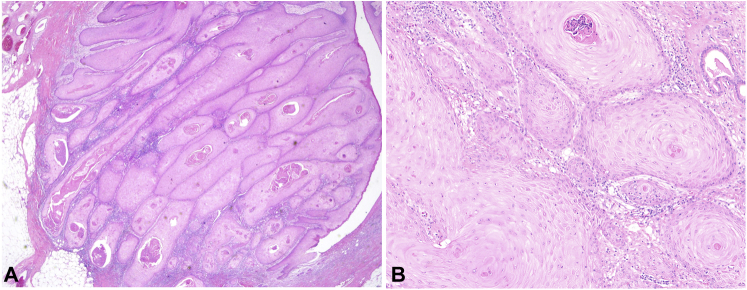
Teledermatology recommended a clinic visit for a biopsy. At the time of biopsy 2 weeks later, the mass had enlarged to 5 × 3 cm (Fig 1, B). The biopsy showed a pedunculated neoplasm composed of lobules of squamous keratinocytes with glassy-appearing cytoplasm, large but monomorphous vesicular nuclei, and foci of compact hyperkeratosis without flattening of the cells at the interface between the tumor and the cornified areas, more in keeping with KA than with conventional squamous cell carcinoma (SCC) (Fig 2). Targeted sequencing of the tumor revealed a NOTCH1 c.2740+1G>T truncating mutation and a loss of heterozygosity of the 9q chromosome on which NOTCH1 resides. No mutations were identified in TP53, PIK3CA, or any RAS isoform. The tumor was excised 2 weeks after the biopsy with 1-cm margins; no recurrence or further skin cancer was reported.
Fig 2.
Hematoxylin and eosin stain of patient’s keratoacanthoma. A, Low-power (×40) view demonstrates a lobular proliferation of keratinocytes with abundant eosinophilic cytoplasm. B, Higher-power view (×200) demonstrates the presence of only mild cytologic atypia of neoplastic keratinocytes and neutrophilic microabscesses within the tumor.
The patient reported a history of recurrent, painful, spontaneously resolving “bumps” in his armpits and groin since his 20s. Four months later, during flareups, HS was diagnosed. The patient died 5 years later of unrelated causes.
Discussion
There have been 3 reports of KA co-occurring with HS, all in White patients.1, 2, 3 The patients reported by Fenske et al1 and Tarnowski2 had Dowling-Degos disease and acne conglobate, respectively, in addition to HS, and multiple KAs of the lower extremities. Because their KAs were characteristically nodular with keratotic centers and their locations did not overlap with the locations of HS, these cases do not appear to be KAs arising directly in HS. Lesage et al3 described a patient in whom KA developed after a clinical trial of infliximab, but the morphology and site of the tumor were not reported. To our knowledge, this is the first report of an axillary KA and the first report of a KA with a pedunculated morphology.
The presentation of a KA in an area that is not exposed to the sun provides an opportunity to elucidate the driver genes of KA without the confounding factor of ultraviolet radiation-induced mutations. NOTCH1 loss-of-function mutations were found to be drivers of KA in humans,4 and impaired NOTCH1 signaling was found to have a role in the pathogenesis of HS in mice.5 Because only tumor sequencing was performed, it is unknown whether the patient’s NOTCH1 mutation was somatic or germline, but the shared molecular mechanism of KA and HS leads us to speculate that a germline NOTCH1 mutation was the predisposing factor leading to both his KA and his HS. A search for “hidradenitis suppurativa NOTCH 1” in PubMed did not reveal any reports of patients with germline NOTCH1 mutations. The absence of other mutations is consistent with previous reports of KAs having a low mutational burden,4 and the loss of heterozygosity at chromosome 9q is in accordance with previous studies showing that KAs have a higher frequency of loss of heterozygosity at 9q compared with the closely related SCCs.6
It is well known that the risk factors for KA, in addition to ultraviolet exposure, include chronic inflammation or trauma. KAs have been reported to arise in lichen planus and tattoos.7,8 The morphology and histopathology of the reported non-sun-exposed KAs, which presented with indurated lesions that showed central keratinization histologically, were consistent with those of typical sun-exposed KAs. Because there have been no other case reports of KA arising directly in HS, it is not known whether our patient’s atypical KA morphology was a result of previous epithelial damage due to HS or to other mechanisms.
There is debate about whether KA is a variant of SCC. They are often difficult to distinguish histologically, and certain forms of follicular SCC are thought to be high-grade forms of KA; in fact, NOTCH1 loss-of-function mutations were also found to be drivers in conventional cutaneous and head and neck SCCs.6 More than 100 cases of SCC arising in HS have been reported, all in the gluteal, perianal, and perineal regions.9,10 Because SCC seems to occur exclusively in extra-axillary HS, Maclean and Coleman10 argued that extra-axillary HS is more prone to malignancy than axillary HS and should be treated as a premalignant condition. Thus, the incidence of KA but not of SCC arising in HS in the axilla might support the hypothesis that KA is a less malignant version of SCC.
Our case of a KA with atypical morphology occurring in a non-sun-exposed site highlights the similarity of genetic mutations observed in KA arising from an inflammatory condition due to a likely underlying germline mutation and those in classic KAs arising on sun-exposed skin. Although axillary HS is thought to be less prone to developing malignancy, our case report shows that neoplasms in axillary HS are possible, but the presentation may be unusual. Given that the NOTCH1 pathway has been reported to have a role in both HS and KA, further studies are needed to explore whether patients with HS may be at increased risk for KA.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Fenske N.A., Groover C.E., Lober C.W., Espinoza C.G. Dowling-Degos disease, hidradenitis suppurativa, and multiple keratoacanthomas. A disorder that may be caused by a single underlying defect in pilosebaceous epithelial proliferation. J Am Acad Dermatol. 1991;24(5):888–892. doi: 10.1016/0190-9622(91)70141-N. [DOI] [PubMed] [Google Scholar]
- 2.Tarnowski W.M. Multiple keratoacanthomata. Response of a case to systemic chemotherapy. Arch Dermatol. 1966;94(1):74–80. doi: 10.1001/archderm.1966.01600250080016. [DOI] [PubMed] [Google Scholar]
- 3.Lesage C., Adnot-Desanlis L., Perceau G., et al. Efficacy and tolerance of prolonged infliximab treatment of moderate-to-severe forms of hidradenitis suppurativa. Eur J Dermatol. 2012;22(5):640–644. doi: 10.1684/ejd.2012.1795. [DOI] [PubMed] [Google Scholar]
- 4.Lim Y.H., Fisher J.M., Bosenberg M.W., Choate K.A., Ko C.J. Keratoacanthoma shares driver mutations with cutaneous squamous cell carcinoma. J Invest Dermatol. 2016;136(8):1737–1741. doi: 10.1016/j.jid.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Melnik B.C., Plewig G. Impaired Notch-MKP-1 signalling in hidradenitis suppurativa: an approach to pathogenesis by evidence from translational biology. Exp Dermatol. 2013;22(3):172–177. doi: 10.1111/exd.12098. [DOI] [PubMed] [Google Scholar]
- 6.Clausen O.P.F., Aass H.C.D., Beigi M., et al. Are keratoacanthomas variants of squamous cell carcinomas? A comparison of chromosomal aberrations by comparative genomic hybridization. J Invest Dermatol. 2006;126(10):2308–2315. doi: 10.1038/sj.jid.5700375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giesecke L.M., Reid C.M., James C.L., Huilgol S.C. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44(4):267–269. doi: 10.1046/j.1440-0960.2003.00006.x. [DOI] [PubMed] [Google Scholar]
- 8.Kluger N., Minier-Thoumin C., Plantier F. Keratoacanthoma occurring within the red dye of a tattoo. J Cutan Pathol. 2008;35(5):504–507. doi: 10.1111/j.1600-0560.2007.00833.x. [DOI] [PubMed] [Google Scholar]
- 9.Kohorst J.J., Shah K.K., Hallemeier C.L., Baum C.L., Davis M.D.P. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45(4):519–526. doi: 10.1097/DSS.0000000000001713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maclean G.M., Coleman D.J. Three fatal cases of squamous cell carcinoma arising in chronic perineal hidradenitis suppurativa. Ann R Coll Surg Engl. 2007;89(7):709–712. doi: 10.1308/003588407X209392. [DOI] [PMC free article] [PubMed] [Google Scholar]