Abstract
Background
Influenza viruses pose significant disease burdens through seasonal outbreaks and unpredictable pandemics. Existing surveillance programs rely heavily on reporting of medically attended influenza (MAI). Continuously monitoring cause‐specific school absenteeism may identify local acceleration of seasonal influenza activity. The Oregon Child Absenteeism Due to Respiratory Disease Study (ORCHARDS; Oregon, WI) implements daily school‐based monitoring of influenza‐like illness‐specific student absenteeism (a‐ILI) in kindergarten through Grade 12 schools and assesses this approach for early detection of accelerated influenza and other respiratory pathogen transmission in schools and surrounding communities.
Methods
Starting in September 2014, ORCHARDS combines automated reporting of daily absenteeism within six schools and home visits to school children with acute respiratory infection (ARI). Demographic, epidemiological, and symptom data are collected along with respiratory specimens. Specimens are tested for influenza and other respiratory viruses. Household members can opt into a supplementary household transmission study. Community comparisons are possible using a pre‐existing and highly effective influenza surveillance program, based on MAI at five family medicine clinics in the same geographical area.
Results
Over the first 5 years, a‐ILI occurred on 6634 (0.20%) of 3,260,461 student school days. Viral pathogens were detected in 64.5% of 1728 children with ARI who received a home visit. Influenza was the most commonly detected virus, noted in 23.3% of ill students.
Conclusion
ORCHARDS uses a community‐based design to detect influenza trends over multiple seasons and to evaluate the utility of absenteeism for early detection of accelerated influenza and other respiratory pathogen transmission in schools and surrounding communities.
Keywords: absenteeism, children, influenza, population surveillance, school
Abbreviations
- 4k
4‐year‐old pre‐kindergarten
- a‐I
absenteeism due to illness
- a‐ILI
absenteeism due to influenza‐like illness
- ARI
acute respiratory infection
- a‐TOT
all‐cause absenteeism
- CDC
Centers for Disease Control and Prevention
- FDA
Food and Drug Administration
- FERPA
Family Educational Rights and Privacy Act
- Flu
influenza
- ILI
influenza‐like illness
- IRB
institutional review board
- IT
information technology
- MAI
medically attended influenza
- NP
nasopharyngeal
- OP
oropharyngeal
- ORCHARDS
Oregon Child Absenteeism Due to Respiratory Disease Study
- OSD
Oregon School District
- PCR
polymerase chain reaction
- RedCap
Research Electronic Data Capture
- RIDT
rapid influenza diagnostic test
- RP
human RNAse P
- RPP
respiratory pathogen panel
- SIS
student information system
- VTM
viral transport medium
- W‐IISP
Wisconsin Influenza Incidence Surveillance Project
- WIR
Wisconsin Immunization Registry
- WSLH
Wisconsin State Laboratory of Hygiene
1. INTRODUCTION
Influenza viruses contribute to significant disease burdens and economic costs through annual seasonal outbreaks and unpredictable pandemics. 1 , 2 , 3 Approaches for prevention and control of seasonal and pandemic influenza include vaccines, antiviral medications, and nonpharmaceutical interventions (NPIs), but their combined success is predicated on timely deployment. NPI, the first line of defense, 4 hinges on early detection and recognition of outbreaks. 4 , 5 , 6
Existing influenza surveillance programs rely on medical facilities to report cases of influenza‐like illness (ILI) and test‐confirmed influenza. 7 , 8 Even though influenza transmission among school‐aged children frequently precedes subsequent community transmission, 9 , 10 there have been no systematical evaluations of school‐based monitoring of influenza activity for complementing routine surveillance or serving as an early‐warning system for increased influenza activity in the wider community. Monitoring school absenteeism is feasible, as seasonal outbreaks occur between late fall and mid‐spring while schools are in session, 11 , 12 and most of the 13,588 school districts 13 across the United States collect daily absenteeism data using electronic school information systems. 14
During the 2009 influenza pandemic, there was high correlation (r = 0.92) reported between hospitalized influenza cases and school absenteeism due to ILI in one jurisdiction, 15 likely enhanced by the short, intense nature of the outbreak, which amplified absenteeism related to ILI. Conversely, monitoring all‐cause absenteeism was less effective due to the multifactorial nature of absenteeism. 16 , 17 , 18 The value of continuously monitoring cause‐specific absenteeism over the entire school year to identify local activity acceleration of seasonal influenza is not well understood.
The goal of Oregon Child Absenteeism Due to Respiratory Disease Study (ORCHARDS) is to develop and implement a system for daily school‐based monitoring of ILI‐specific student absenteeism in kindergarten through grade 12 (K‐12) schools and assess the system's usability for early detection of accelerated influenza and other respiratory pathogen transmission in schools and surrounding communities. The theoretical relationships between influenza infection in school‐aged children, K‐12 absenteeism, and influenza infection in the surrounding community, and study components and hypotheses are demonstrated in Figure 1.
FIGURE 1.
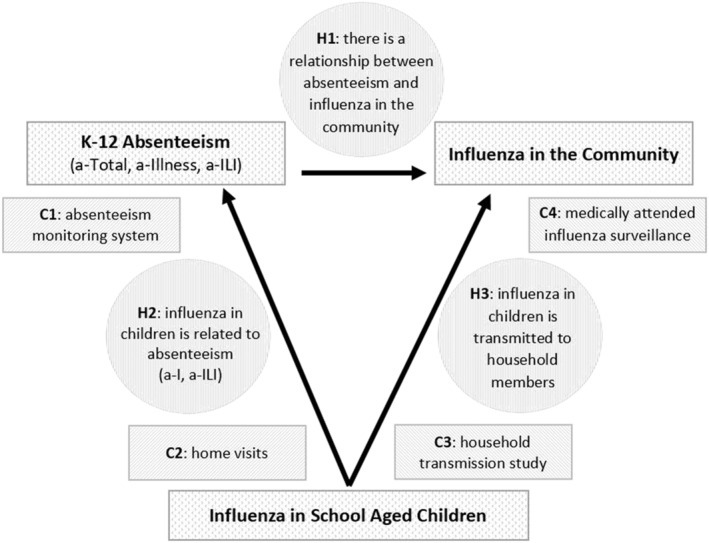
Theoretical framework of ORCHARDS demonstrating the relationships between influenza in school‐aged children, K‐12 school absenteeism, and medically attended influenza in the community. The relatedness of the four components (C1–C4) of ORCHARDS and the three primary hypotheses (H1–H3) are provided
2. METHODS
2.1. Location
The Oregon School District (OSD: www.oregonsd.org) encompasses the villages of Oregon and Brooklyn, decentralized subdivisions, and farms in Dane County, Wisconsin (latitude: 42.90 N, longitude: 89.43 W). 19 The region experiences distinct temperate seasonality.
2.2. Community
The OSD population is estimated at 20,094 and is less racially and ethnically diverse, wealthier, and more formally educated than the United States average, while ages of individual family members and mean household size are similar (Table 1). 20 The district is composed of 6 public schools enrolling 4091 students, including pre‐kindergarten students, in 2018–2019. 21 There are 3 elementary schools (K‐4: 1503 students), 1 intermediate school (5–6: 623), 1 middle school (7–8: 596), and 1 high school (9–12: 1145).
TABLE 1.
Demographic descriptions of the Oregon School District (OSD) as compared with Dane County, Wisconsin, and the United States
| Demographic feature | OSD | Dane County | Wisconsin | United States |
|---|---|---|---|---|
| Total population | 20,094 | 488,073 | 5,691,047 | 312,700,151 |
| White [nH a ] (%) | 90.7 | 84.7 | 87.0 | 74.8 |
| Black (%) | 2.5 | 5.2 | 6.2 | 13.6 |
| Am. Indian (%) | 0.2 | 0.4 | 0.8 | 1.7 |
| Asian (%) | 1.9 | 4.7 | 2.3 | 5.6 |
| Pacific Isl. (%) | 0 | 0 | 0 | 0.4 |
| Hispanic (%) | 3.3 | 5.9 | 5.9 | 16.3 |
| Ages (years) | ||||
| <5 | 5.9 | 6.2 | 6.2 | 6.5 |
| 5–19 | 21.3 | 18.7 | 17.2 | 20.4 |
| 20–44 | 33.6 | 39.2 | 36.0 | 33.6 |
| 45–64 | 30.3 | 25.7 | 27.7 | 26.4 |
| 65+ | 8.9 | 10.4 | 13.7 | 13.0 |
| Household | ||||
| Mean size | 2.69 | 2.33 | 2.43 | 2.58 |
| <$15,000 | 4.4 | 10.9 | 6.1 | 13.4 |
| $15,000–$35,000 | 10.5 | 18.6 | 33.1 | 22.3 |
| $35,000–$75,000 | 33.0 | 32.0 | 46.0 | 32.5 |
| >$75,000 | 52.0 | 38.4 | 14.8 | 31.7 |
| Education | ||||
| < High school | 4.1 | 2.1 | 9.9 | 14.4 |
| High school/GED | 29.7 | 20.6 | 33.3 | 28.5 |
| Some college | 30.3 | 27.0 | 30.5 | 28.9 |
| Bachelor's | 24.6 | 28.1 | 17.4 | 17.7 |
| Post BA/BS | 11.3 | 19.0 | 9.0 | 10.4 |
Note: Data from US Census.
nH, non‐Hispanic.
2.3. Timeframe
Initiation of ORCHARDS data collection occurred in phases. Absenteeism data collection commenced on September 2, 2014. Data collection from student home visits commenced on January 5, 2015. The household transmission substudy started on January 6, 2016. Medically attended influenza surveillance has been ongoing since October 2009. 8 All components of ORCHARDS, including laboratory testing, have continued year‐around without interruption until present.
2.4. Absenteeism monitoring system
We modified an existing absenteeism reporting system. Parents report unscheduled absences using an automated telephone system, providing the student's name and the reason for absence, including symptoms if the child has a cold or flu‐like illness. Uniform messaging is on each school's absentee line:
Please inform us if your child has any flu‐like symptoms such as fever with cough, sneezing, chills, sore throat, body aches, fatigue, runny nose, and/or stuffy nose.
In the event that a student is absent without a report, OSD attendance staff make repeated efforts to contact the home or parents/guardians before the end of the day.
2.5. Absenteeism definition
Because of variability among schools in terms of the number of class periods for which a child can be absent and for simplicity/generalizability of electronic data retrieval, we consider a student absent for the entire day if absent for any part of a school day.
2.6. Types of absenteeism
All‐cause or total absenteeism (a‐TOT) is an absence for any reason. Absence due to illness (a‐I) is an absence due to any reported illness. Absence due to ILI (a‐ILI) is an a‐I for which ILI symptoms are reported.
2.7. a‐ILI definition
We considered established definitions for ILI 22 , 23 and used a simplified version of the Centers for Disease Control and Prevention (CDC) standard definition. ILI for ORCHARDS is defined as presence of fever and at least one respiratory symptom (cough, sore throat, nasal congestion, or runny nose).
2.8. Data system
OSD utilizes Infinite Campus®, 24 a commercially available, electronic student information system (SIS), to track student attendance. This system allows attendance staff to identify a student, select a period, and select a reason for absence from a modifiable, drop‐down pick list. The OSD Information Technology (IT) staff added an option for “a‐ILI.”
2.9. Data extraction and secure file transfer
An automated process within Infinite Campus® extracts daily counts of absent individuals by school, grade, and absence type (a‐TOT, a‐I, and a‐ILI). Data contain no personal identifiers, are compliant with the Family Educational Rights and Privacy Act (FERPA: 20 U.S.C. § 1232g; 34 CFR Part 99), 25 and are sent to a secure file transfer protocol (ftp) site at the University of Wisconsin Department of Family Medicine and Community Health. We track daily totals of absentee counts in each category. The data flow is illustrated in Figure 2.
FIGURE 2.
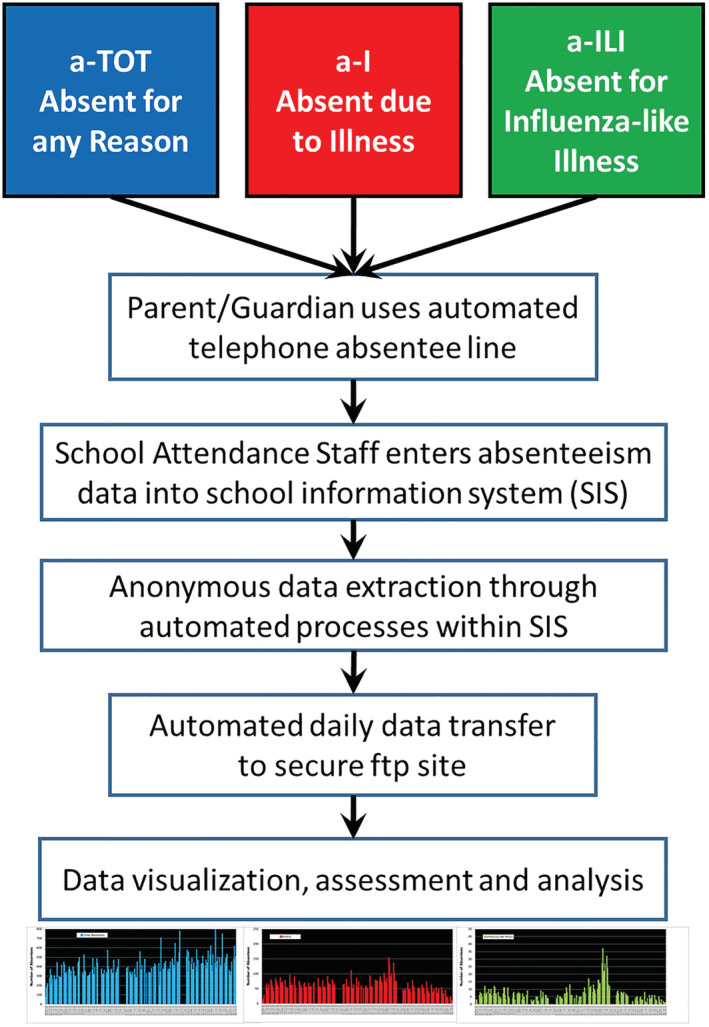
Flow diagram of absenteeism data from telephone reporting by parents/guardians, to entry into the student information system at the Oregon School District, to data transfer to the ORCHARDS research team
2.10. School incentive
Each school receives $4000 per year to defray costs associated with IT support and effort by the attendance staff.
2.11. Assessment of influenza and absenteeism in children through home visits
2.11.1. Contact and screening
Students are not required to be absent, and school does not need to be in session for home visits to occur. To comply with FERPA, interested parents/guardians call the study line to initiate a home visit. If a student meets the inclusion criteria, a 20‐min home visit occurs within 2 days of initial phone contact and within 7 days of symptom onset.
Inclusion criteria include:
student attends, or is eligible to attend (e.g., home schooled), a school within the OSD
has an illness characterized by ≥2 of 6 acute respiratory infection (ARI)/ILI symptoms (nasal discharge; nasal congestion; sneezing; sore throat; cough; and fever)
Exclusion criteria include:
illness onset ≥7 days before anticipated time of specimen collection
anatomical defect for which nasal specimen collection is contraindicated
student participated too recently (<7 days during peak influenza period and <30 days during other times, as determined by medically attended surveillance program)
If criteria are met, family members are invited to participate in an optional household transmission substudy. Participation is allowed even if individual members opt out or are unable to complete the entire study.
Inclusion criteria include:
individuals of any age/gender residing in the same household as ORCHARDS participant
able to provide appropriate consent/assent
Exclusion criteria include:
anatomical defect for which nasal sampling is contraindicated
household participated too recently (<7 days during peak influenza period and <30 days during other times, as determined by medically attended surveillance program)
2.11.2. Acquiring informed consent/assent
Research coordinators obtain written informed consent from parents/guardians and/or adult students, and assent from younger students using forms tailored to reading levels based on age.
2.11.3. Data collection
Demographic, epidemiologic, and symptom data are collected on an Acute Respiratory Infection and Influenza Surveillance Form (Data S1), based on a clinical instrument used since 2009 as part of the Wisconsin component of the Influenza Incidence Surveillance Project (W‐IISP). 8 Accordingly, collected data are comparable with data from other surveillance systems.
2.11.4. Respiratory specimen collection and handling
A nasal swab specimen is collected using a Puritan® Sterile Foam Tipped Applicator for rapid influenza diagnostic testing (RIDT). In addition, a nasopharyngeal (NP) or high oropharyngeal (OP) specimen is obtained using a Copan FLOQSwabs™ flocked swab. The NP/OP swab is placed into a 3.0‐ml Remel MicroTest™ M4RT® Transport viral transport medium (VTM) tube, placed into a small biohazard bag, and maintained at 2–8°C. Following processing for RIDT, the residual nasal swab is added to the VTM containing the NP/OP swab. A courier delivers the VTM tube to the Wisconsin State Laboratory of Hygiene (WSLH), usually within 24 h of collection.
2.11.5. Incentives and feedback
Student participants receive a $20 gift card at the end of the home visit. Research coordinators call parents/guardians with RIDT results within 24 h (usually less than 4 h) of the home visit. Laboratory confirmed results are mailed to families within 2 weeks of a home visit and are accepted as documentation for an excused absence by the OSD.
2.12. Within‐household influenza transmission substudy
2.12.1. Contact and screening
At the ORCHARDS home visit, recruited families receive a packet containing substudy information, instructions, consents/assents, and a collection kit for each household member. Each collection kit contains a data form, two nasal swabs, and two small biohazard bags, each of which contain a 3.0‐ml Remel MicroTest™ M4RT® VTM tube. VTM tubes and swabs for Day 0 and Day 7 are individually marked.
The research coordinator explains components of the family packet to all present household members and reviews consent/assent forms. A designated adult assures that all interested household participants sign the consent/assent forms prior to specimen collection. Household members collect their own specimens on Day 0 (within 24 h of home visit) and Day 7 (7 days after the initial collection). The timing (Day 7) for follow‐up data and specimen collection is based on prior influenza transmission studies. 29 , 30 , 31
2.12.2. Data collection
The ORCHARDS Household Study Form (Data S2) is used to collect data on:
Household composition: the number and characteristics of people sharing the household of an ORCHARDS participant. Information includes relationship to the ORCHARDS participant, age, gender, number of bedrooms in home, employment outside of home, school attendance, and daycare attendance.
Household member illness assessment on Day 0 (“Today” section): influenza vaccine status for the current season, and information about any ARI/ILI illnesses occurring over the past 7 days.
Household member illness assessment on Day 7, 1 week after the initial visit (“Follow‐up” section): information about any ARI/ILI illnesses occurring over the past 7 days.
2.12.3. Respiratory specimen collection and handling
Household members are instructed on specimen collection using a Copan FLOQSwabs™ flocked midturbinate swab (January 6, 2016, through October 15, 2017) or an anterior nasal Puritan® Sterile Foam Tipped Applicator (starting on October 16, 2017). Each household participant collects the specimen without staff on Day 0 and Day 7. Upon retrieval, research coordinators review forms for completion and ensure specimens were collected and correspond with the appropriate labels.
2.12.4. Household incentive for participation
Households completing the substudy receive a $50 gift card to local businesses.
2.13. Laboratory procedures
2.13.1. Rapid influenza diagnostic test
We use the Quidel® Sofia® Influenza A + B fluorescent immunoassay 32 for assessment of ORCHARDS participants, but not for household members. 32 RIDT is performed at a nearby clinical facility within 6 h of specimen collection.
2.13.2. Influenza rRT‐PCR
All specimens from students and household members (Day 0 and Day 7) are tested at WSLH for influenza A and B virus and Human RNase P (RP) using the in vitro diagnostic (IVD) Food and Drug Administration (FDA)‐approved CDC Human Influenza Virus Real‐time RT‐PCR Diagnostic Panel (Cat. # FluIVD03). 33 The detection of RP, with a cycle threshold (Ct) value < 38, determines specimen adequacy for influenza polymerase chain reaction (PCR) testing.
2.13.3. Multipathogen testing
All specimens from ORCHARDS students are tested for influenza A, influenza B, rhinovirus/enterovirus, adenovirus, parainfluenza virus, 1 , 2 , 3 , 4 seasonal coronavirus (HKU1, NL63, 229E, OC43), respiratory syncytial virus (A, B), human metapneumovirus and human bocavirus, and two atypical bacterial pathogens, Chlamydia pneumoniae and Mycoplasma pneumoniae, using the multiplexed PCR respiratory pathogen panel (RPP: Luminex NxTAG Respiratory Pathogen Panel). 34
2.13.4. Whole genome sequencing
For a subset of influenza‐positive subjects, we conduct next‐generation genome sequencing at the WSLH using the Illumina MiSeq™ platform. Sequence reads are trimmed using Trimmomatic with a 4‐bp sliding window quality score cutoff of Q30. 35 The trimmed sequence reads are mapped against vaccine strain HA reference sequences using Geneious Version 11.0.5. 36
2.13.5. Specimen archiving
Aliquots of all residual specimens are archived at −70°C at WSLH.
2.13.6. Validation of influenza vaccination
We validate influenza immunization status for all ORCHARDS students using the Wisconsin Immunization Registry (WIR). 37 Vaccination status at the time of specimen collection is determined using criteria provided by the US Advisory Committee on Immunization Practices. 38
2.13.7. Data security and integrity
All identifying information on subjects is secured using a separate password‐protected security REDCap (Research Electronic Data Capture) database. 39
2.13.8. Community engagement and study promotion
ORCHARDS recruits using a reminder within the absenteeism reporting system. Information is provided at OSD registration each August and through e‐mails to OSD families. We employ flyers at community sites, presentations at community events, postcards, and the study website (www.fammed.wisc.edu/orchards/) and Facebook page (www.facebook.com/orchardstudy/).
2.13.9. IRB and project oversight
This study was approved by the Human Subjects Committees of the Education and Social/Behavioral Sciences institutional review board (IRB) (initial approval on September 4, 2013) and the University of Wisconsin Health Sciences IRB (initial approval on December 5, 2013, with additional approvals as the protocol expanded and modified). The study is compliance with the Health Insurance Portability and Accountability Act of 1996 (HIPAA) and FERPA. The US Office of Management and Budget has approved all forms used in this study.
2.13.10. Reference data—Assessment of influenza in the surrounding community
An independent surveillance system assesses medically attended influenza (MAI) at five family practice clinics within or adjoining the OSD. W‐IISP, sponsored by CDC and organized by the ORCHARDS team, has conducted active surveillance in medically attended patients with ARI/ILI since October 2009. 8 , 40 Specific clinic locations include Belleville, Oregon, Madison (two sites), and Verona. Together, the clinics recorded 412,752 ambulatory visits between June 29, 2014, and June 29, 2019, and assessed 4069 individual ARI patients.
NP/OP swabs are collected from a subset of ambulatory patients with ARI/ILI. Specimens are tested at the WSLH for influenza A and B using the rRT‐PCR 33 and, for other viruses, using a multiplexed PCR RPP. 34 Virological characterization is available for all (n = 4069) individual patient visits.
3. RESULTS
The entire school district, including all schools, have participated since the initiation of ORCHARDS. Absenteeism data have been captured for all students, excluding pre‐kindergarten (for whom absenteeism is not recorded), since September 2014. The number of enrolled K‐12 students per year has increased from 3588 (2014/2015) to 3867 (2018/2019). Over the first 5 years of ORCHARDS, from September 2014 through June 2019, we evaluated 3,260,461 student days. Total absenteeism accounted for 301,427 (9.2%), a‐I for 58,126 (1.8%), and a‐ILI for 6634 (0.2%) of potential student days. The daily counts for each type of absenteeism, showing the variability, are depicted in Figure 3. Annual levels of absenteeism were similar across all 5 years (Table 2).
FIGURE 3.
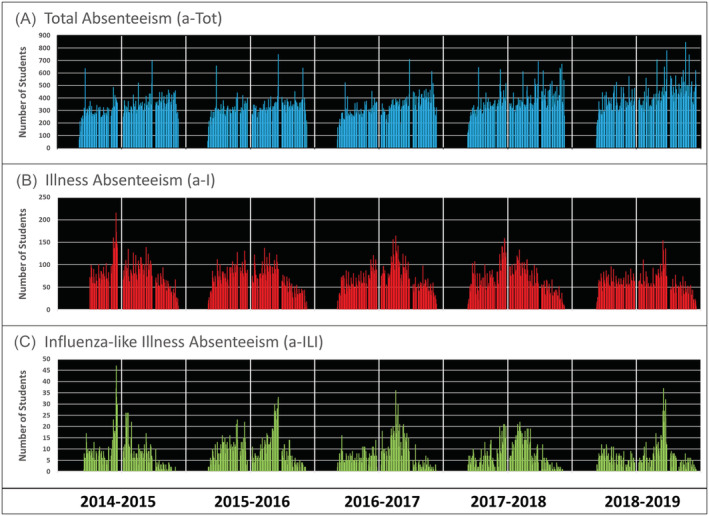
Daily counts of all absent students (a‐TOT: Panel A), students for whom an illness is reported (a‐I: Panel B), and students absent with influenza‐like illness (a‐ILI: Panel C) occurring over five consecutive school years at the Oregon School District, Wisconsin, USA, from September 2014 through June 2019. Vertical white bars demonstrate the timing of July 1 (thin bars) and January 1 (heavy bars)
TABLE 2.
Enrollment estimates and absenteeism by defined type for the Oregon School District, Oregon, Wisconsin: 2014–2019
| 2014–2015 | 2015–2016 | 2016–2017 | 2017–2018 | 2018–2019 | |
|---|---|---|---|---|---|
| Total estimated enrollment a | 3588 | 3713 | 3749 | 3828 | 3867 |
| Total curricular days | 176 | 177 | 175 | 176 | 174 |
| Total possible student days | 629,024 | 635,076 | 649,775 | 673,728 | 672,858 |
| a‐TOT days (% of total) | 56,477 (8.98%) | 55,871 (8.80%) | 56,511 (8.70%) | 63,476 (9.42%) | 69,092 (10.3%) |
| Average daily a‐TOT (std. dev.) | 320.9 (78.4) | 315.7 (79.2) | 322.9 (80.4) | 360.7 (96.3) | 397.1 (106.2) |
| a‐I days (% of total) | 12,024 (1.91%) | 12,158 (1.91%) | 11,922 (1.83%) | 11,710 (1.74%) | 10,312 (1.53%) |
| Average daily a‐I (std. dev.) | 68.3 (39.0) | 68.7 (27.0) | 68.1 (27.0) | 66.5 (30.1) | 59.3 (21.7) |
| a‐ILI days (% of total) | 1314 (0.21%) | 1609 (0.25%) | 1309 (0.20%) | 1261 (0.19%) | 1141 (0.17%) |
| Average daily a‐ILI (std. dev.) | 7.5 (7.3) | 9.1 (6.6) | 7.5 (5.9) | 7.2 (5.8) | 6.6 (5.5) |
| Influenza vaccination rate in ORCHARDS participants | 62.8% b | 46.7% | 48.8% | 41.6% | 54.7% |
Note: The percentage of participants that were fully vaccinated against influenza is provided for each academic year.
Total estimated enrollment excluding 4k students (for whom absenteeism data are not submitted). Data extracted from Wisconsin Department of Public Instruction, Wisconsin Information System for Education data Dashboard: https://wisedash.dpi.wi.gov/Dashboard/portalHome.jsp.
Home visits did not start until January 5, 2015. The 2014–2015 vaccination rate is likely biased due to the late season sampling frame.
We completed 1728 home visits for children with ARI. Children ranged in age from 4 to 18 years (mean ± std. dev. = 9.9 ± 2.5 years). There were more male (57%) than female (43%) participants. Home visits occurred, on average, 56.3 ± 46.5 h after onset of symptoms. The number of home visits per day was positively correlated with a‐TOT (r s = 0.20; p < 0.001), a‐I (r s = 0.41; p < 0.001), and a‐ILI (r s = 0.40; p < 0.001). Most children (79%) reported school absenteeism due to the current illness episode, and about half (853/1728: 49%) were fully vaccinated against influenza at the time of the home visit.
Pathogens were detected in 1115 (65%) specimens; the majority (99%) of these were viral. Codetections of viruses were noted in 66 students (6% of individuals with virus detections). Influenza was the most commonly detected virus, noted in 402/1728 (23%) students, followed by rhinovirus/enterovirus. The numbers of viruses detected are provided in Table 3.
TABLE 3.
Detections of viruses from ORCHARDS participants through home visits (January 5, 2015, to June 30, 2019)
| Respiratory virus detected | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ad | CoV | FluA (H1) | FluA (H3) | FluB | hMPV | PIV | R/E | RSV | |
| Number of detections | 32 | 169 | 77 | 224 | 98 | 74 | 77 | 368 | 47 |
| Percent | 2.7 | 14.3 | 6.5 | 18.9 | 8.3 | 6.3 | 6.5 | 31.1 | 3.9 |
Note: The counts include dual detections (n = 64) and triple detections (n = 2). Not shown are data for influenza A (unable to subtype: n = 3), Mycoplasma pneumoniae (n = 12), and Chlamydia pneumoniae (n = 1). Percentages are expressed as detections of specified virus groups divided by all detections.
Abbreviations: Ad, adenovirus; CoV, coronavirus; Flu, influenza virus; hMPV, human metapneumovirus; PIV, parainfluenza virus; R/E, rhinovirus/enterovirus; RSV, respiratory syncytial virus.
4. DISCUSSION
In contrast to routine surveillance relying on MAI, ORCHARDS uses a community‐based design. Most cases of influenza do not present for medical attendance 40 or result in hospitalization or death. 41 , 42 Influenza attack rates are much higher in school‐aged children than for any other demographic group, 40 and influenza significantly contributes to school absenteeism. 43 This is reflected by the prominence of influenza detections in ORCHARDS participants, of whom 79% reported school absenteeism. Accordingly, ORCHARDS is designed to detect and evaluate—over multiple seasons—temporal trends of influenza detection among school‐aged children who are central to community‐wide influenza transmission and who are less represented among MAI cases.
A number of studies evaluating absenteeism and influenza predate ORCHARDS 15 , 16 , 17 , 18 , 44 but have been limited by evaluating single outbreaks. Influenza does not follow a regular pattern but rather encompasses outbreaks of variable magnitudes and temporal patterns due to differing influenza types, subtypes, and clades. 45 , 46 Moreover, influenza outbreaks can occur at any time over a fairly wide seasonal range, 47 thus making assessments over several seasons necessary to evaluate for effects of timing. Finally, an observational approach allows accumulation of multiple periods of planned and unplanned (weather‐related) school breaks that may allow evaluation of school closure for outbreak response.
ORCHARDS takes advantage of a long‐standing and highly effective influenza surveillance system as a “gold standard” for daily comparability. This parallel system is based on MAI surveillance at five family medicine clinics overlapping with the study catchment area and using very similar data instruments and identical laboratory methods. The community involvement, longitudinal nature, and external comparability make ORCHARDS a unique study platform to evaluate the role of school‐aged children on influenza transmission and the utility of cause‐specific absenteeism monitoring for identifying influenza outbreaks.
AUTHOR CONTRIBUTIONS
Jonathan Temte: Conceptualization; data curation; funding acquisition; investigation; methodology; supervision. Shari Barlow: Conceptualization; funding acquisition; methodology; project administration; resources; supervision. Maureen Goss: Conceptualization; data curation; investigation; methodology; project administration; validation. Emily Temte: Conceptualization; data curation; investigation; methodology; project administration; validation. Cristalyne Bell: Data curation; project administration; validation. Cecilia He: Data curation; project administration; validation. Caroline Hamer: Data curation; project administration. Amber Schemmel: Data curation; project administration. Brad Maerz: Data curation; project administration. Lily Comp: Data curation; project administration; validation. Mitchell Arnold: Data curation; project administration; validation. Kimberly Breunig: Data curation; project administration; validation. Sarah Clifford: Data curation; project administration. Erik Reisdorf: Conceptualization; resources. Peter Shult: Conceptualization; resources. Mary Wedig: Conceptualization; resources. Thomas Haupt: Conceptualization; resources. James Conway: Conceptualization; methodology. Ronald Gangnon: Formal analysis; visualization. Ashley Fowlkes: Conceptualization; methodology. Amra Uzicanin: Conceptualization; methodology.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/irv.12920.
Supporting information
Data S1. Supporting Information
Data S2. Supporting Information
ACKNOWLEDGMENTS
We would like to acknowledge Dr. Yenlik Zheteyeva, MD, MPH, formerly of CDC's Division of Global Migration and Quarantine, for her contributions to the study design and implementation. We are also grateful to Rich Griesser, Tim Davis, Tonya Danz, and Erika Hanson (Virology Laboratory Staff at the Wisconsin State Laboratory of Hygiene) for their assistance with specimen testing throughout ORCHARDS. Special thanks for Dr. Brian Bussler, Superintendent, Jon Tanner, Information Technology Director, and the attendance staff at OSD. Finally, a multitude of student participants of ORCHARDS and their families made this research possible.
This study has been supported by Centers for Disease Control and Prevention (www.cdc.gov) through cooperative agreement # 5U01CK000542‐02‐00. The findings and conclusions in this study are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Temte JL, Barlow S, Goss M, et al. The Oregon Child Absenteeism Due to Respiratory Disease Study (ORCHARDS): Rationale, objectives, and design. Influenza Other Respi Viruses. 2022;16(2):340-350. doi: 10.1111/irv.12920
Funding information Centers for Disease Control and Prevention, Grant/Award Number: 5U01CK000542‐02‐00
DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are not publicly available because the study is ongoing but may be available from the corresponding author upon reasonable request.
REFERENCES
- 1. CDC . Disease burden of influenza. https://www.cdc.gov/flu/about/burden/index.html. Accessed 6/22/2020.
- 2. Putri WCWS, Muscatello DJ, Stockwell MS, Newall AT. Economic burden of seasonal influenza in the United States. Vaccine. 2018. Jun 22;36(27):3960‐3966. 10.1016/j.vaccine.2018.05.057 Epub 2018 May 22 [DOI] [PubMed] [Google Scholar]
- 3. Monto AS, Fukuda K. Lessons from influenza pandemics of the last 100 years. Clin Infect Dis. 2019. Aug 17. pii:;70(5):951, ciz803‐957. 10.1093/cid/ciz803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qualls N, Levitt A, Kanade N, et al. Community mitigation guidelines to prevent pandemic influenza—United States, 2017. MMWR Recomm Rep. 2017. Apr 21;66(1):1‐34. 10.15585/mmwr.rr6601a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lowe L, Dopson SA, Budd AP. Pandemic influenza readiness report on laboratory and epidemiology capacity—United States and territories, 2015. Health Secur. 2018. Jul/Aug;16(4):239‐243. 10.1089/hs.2018.0021 [DOI] [PubMed] [Google Scholar]
- 6. Meltzer MI, Gambhir M, Atkins CY, Swerdlow DL. Standardizing scenarios to assess the need to respond to an influenza pandemic. Clin Infect Dis. 2015. May 1;60(Suppl 1):S1‐S8. 10.1093/cid/civ088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. CDC . Weekly U.S. Influenza Surveillance Report. https://www.cdc.gov/flu/weekly/index.htm. Accessed 8/30/2019.
- 8. Fowlkes A, Dasgupta S, Chao E, et al. Estimating influenza incidence and rates of influenza‐like illness in the outpatient setting. Influenza Other Respi Viruses. 2013;7(5):694‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sasaki A, Hoen AG, Ozonoff A, et al. Evidence‐based tool for triggering school closures during influenza outbreaks, Japan. EID. 2009;15(11):1841‐1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Egger JR, Hoen AG, Brownstein JS, et al. Usefulness of school absenteeism data for predicting influenza outbreaks, United States. Letter to Editor. EID. August 2012;18(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Uscher‐Pines L, Schwartz HL, Ahmed F, et al. School practices to promote social distancing in K‐12 schools: review of influenza pandemic policies and practices. BMC Public Health. 2018. Mar 27;18(1):406. 10.1186/s12889-018-5302-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Temte JL, Meiman JG, Gangnon RE. School sessions are correlated with seasonal outbreaks of medically attended respiratory infections: electronic health record time series analysis, Wisconsin 2004–2011. Epidemiol Infect Jan. 147:e127. 10.1017/S0950268818003424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. National Center for Education Statistics . Number of public school districts and public and private elementary and secondary schools: selected years, 1869–70 through 2010–11. https://nces.ed.gov/programs/digest/d12/tables/dt12_098.asp. Accessed 10/20/2019.
- 14. Best K‐12 Student Information Systems. https://www.g2.com/categories/k-12-student-information-systems. Accessed 10/20/2019.
- 15. Williams NJ, Ghosh TS, Bisgard KM, Vogt RL. Comparison of 3 school‐based influenza surveillance indicators: lessons learned from 2009 pandemic influenza A (H1N1)‐Denver Metropolitan Region, Colorado. J Public Health Manag Pract. 2013;19(2):119‐125. [DOI] [PubMed] [Google Scholar]
- 16. Besculides M, Heffernan R, Mostashari F, Weiss D. Evaluation of school absenteeism data for early outbreak detection. New York City BMC Public Health. 2005;5(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schmidt WP, Pebody R, Mangtani P. School absence data for influenza surveillance: a pilot study in the United Kingdom. Euro Surveill. 2010;15(3) pii::19467. [PubMed] [Google Scholar]
- 18. Aldridge RW, Hayward AC, Field N, et al. Decipher My Data project and schools. Are school absences correlated with influenza surveillance data in England? Results from Decipher My Data—a research project conducted through scientific engagement with schools. PLoS ONE. 2016;11(3):e0146964. 10.1371/journal.pone.0146964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oregon School District . https://www.oregonsd.org/. Accessed 10/20/2019.
- 20. United States Census . Quick Facts. https://www.census.gov/quickfacts/oregonvillagewisconsin. Accessed 10/20/2019.
- 21. Wisconsin Department of Public Instruction . Wisconsin Information System for Education data Dashboard. https://wisedash.dpi.wi.gov/Dashboard/portalHome.jsp. Accessed 10/20/2019.
- 22. Thomas RE. Is influenza‐like illness a useful concept and an appropriate test of influenza vaccine effectiveness? Vaccine. 2014. Apr 17;32(19):2143‐2149. 10.1016/j.vaccine.2014.02.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Casalegno JS, Eibach D, Valette M, et al. Performance of influenza case definitions for influenza community surveillance: based on the French influenza surveillance network GROG, 2009–2014. Euro Surveill. 2017. Apr 6;22(14) pii::30504. 10.2807/1560-7917.ES.2017.22.14.30504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Infinite Campus . https://www.infinitecampus.com. Accessed 10/27/2017.
- 25. U.S. Department of Education . Family Educational Rights and Privacy Act (FERPA). https://www2.ed.gov/policy/gen/guid/fpco/ferpa/index.html. Accessed 8/30/2019.
- 26. Jackson GG, Dowling HF, Spiesman IG, Boand AV. Transmission of the common cold to volunteers under controlled conditions. Arch Intern Med. 1958;101(2):267‐278. [DOI] [PubMed] [Google Scholar]
- 27. Jackson GG, Dowling HF, Anderson TO, Riff L, Saporta J, Turck M. Susceptibility and immunity to common upper respiratory viral infections—the common cold. Ann Intern Med. 1960;55:719‐738. [DOI] [PubMed] [Google Scholar]
- 28. Jackson GG, Dowling HF, Muldoon RL. Present concepts of the common cold. Am J Public Health. 1962;52(6):940‐945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ng S, Lopez R, Kuan G, et al. The timeline of influenza virus shedding in children and adults in a household transmission study of influenza in Managua, Nicaragua. Pediatr Infect Dis J. 2016;35(5):583‐586. 10.1097/INF.0000000000001083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lau MS, Cowling BJ, Cook AR, Riley S. Inferring influenza dynamics and control in households. Proc Natl Acad Sci U S A. 2015;112(29):9094‐9099. 10.1073/pnas.1423339112 Epub 2015 Jul 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsang TK, Lau LLH, Cauchemez S, Cowling BJ. Household transmission of influenza virus. Trends Microbiol. 2016. Feb;24(2):123‐133. 10.1016/j.tim.2015.10.012 Epub 2015 Nov 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Quidel . Sofia Influenza A + B FIA. https://www.quidel.com/immunoassays/rapid-influenza-tests/sofia-influenza-fia. Accessed 10/20/2019.
- 33. CDC . CDC Human Influenza Virus Real‐time RT‐PCR Diagnostic Panel. Package Insert LB‐029, R‐0. 2011.
- 34. Luminex . NxTAG® Respiratory Pathogen Panel. https://www.luminexcorp.com/nxtag-respiratory-pathogen-panel/#overview. Accessed 10/20/2019.
- 35. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina Sequence Data. Bioinformatics. 2014;30(15):2114‐2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kearse M, Moir R, Wilson A, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647‐1649.WGS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wisconsin Department of Health Services . Wisconsin Immunization Registry. https://www.dhs.wisconsin.gov/immunization/wir.htm. Accessed 10/20/2019.
- 38. Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep. 2019;68(RR‐3):1‐21. 10.15585/mmwr.rr6803a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. UW Institute for Clinical and Translational Research . REDCap. https://ictr.wisc.edu/redcap/. Accessed 10/20/2019.
- 40. Fowlkes A, Steffens A, Temte J, et al. Influenza Incidence Surveillance Project Working Group. Incidence of medically attended influenza during pandemic and post‐pandemic seasons through the Influenza Incidence Surveillance Project, 2009–13. Lancet Respir Med. 2015. Sep;3(9):709‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rolfes MA, Foppa IM, Garg S, et al. Annual estimates of the burden of seasonal influenza in the United States: a tool for strengthening influenza surveillance and preparedness. Influenza Other Respi Viruses. 2018;12(1):132‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reed C, Chaves SS, Daily Kirley P, et al. Estimating influenza disease burden from population‐based surveillance data in the United States. PLoS ONE. 2015;10(3):e0118369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McLean HQ, Peterson SH, King JP, Meece JK, Belongia EA. School absenteeism among school‐aged children with medically attended acute viral respiratory illness during three influenza seasons, 2012–2013 through 2014–2015. Influenza Other Respi Viruses. 2017;11(3):220‐229. 10.1111/irv.12440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cheng CK, Cowling BJ, Lau EH, Ho LM, Leung GM, Ip DK. Electronic school absenteeism monitoring and influenza surveillance, Hong Kong. Emerg Infect Dis. 2012;18(5):885‐887. 10.3201/eid1805.111796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Allena JD, Rossa TM. H3N2 influenza viruses in humans: viral mechanisms, evolution, and evaluation. Hum Vaccin Immunother. 2018;14(8):1840‐1847. 10.1080/21645515.2018.1462639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. CDC . Types of influenza viruses. https://www.cdc.gov/flu/about/viruses/types.htm. Accessed 10/20/2019.
- 47. Bjørnstada ON, Viboudc C. Timing and periodicity of influenza epidemics. Proc Natl Acad Sci U S A. 2016. Nov 15;113(46):12899‐12901. 10.1073/pnas.1616052113 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information
Data S2. Supporting Information
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available because the study is ongoing but may be available from the corresponding author upon reasonable request.


