AUTHOR CONTRIBUTIONS
Wan Yang: Conceptualization‐equal; formal analysis‐equal; investigation‐equal; methodology‐equal; visualization‐equal; writing — original draft‐equal. Jeffrey Shaman: investigation‐equal; writing — review & editing‐equal.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/irv.12961.
To the Editor—Recent studies have reported reductions in COVID‐19 mRNA vaccine effectiveness (VE) against infection and, to a lesser extent, against severe disease for the Delta SARS‐CoV‐2 variant of concern (VOC). These reductions in VE—particularly against infection—have been attributed to waning immunity, which appears to be supported by two lines of evidence: (1) laboratory studies comparing neutralizing ability of vaccinee sera against different variants and (2) estimated VE against Delta that decreases as the time since vaccination increases. 1 , 2 Notably, the reduction of vaccinee sera neutralizing ability against Delta is smaller than Beta (2–8‐fold reduction against Delta vs. 10–40‐fold against Beta), 3 , 4 yet more breakthrough infections have been observed for Delta, 5 coincidental with its later emergence and circulation. These findings have prompted administration of a third (i.e., booster) vaccine dose, and preliminary results have shown restored VE shortly after this boosting. 6 However, it remains unclear why the substantial reduction in VE occurred mostly when Delta became the predominant circulating variant and why VE against severe disease is more preserved than infection.
We hypothesize that in‐host viral replication dynamics and delays in immune response play a key role in VE. Compared to ancestral variants, Delta not only carries mutations enabling some degree of immune escape but also faster replication that leads to a much higher viral load in infected individuals (e.g., 10 to 1000 times higher than ancestral variants 7 , 8 ). As such, by the time adaptive immunity ramps up in response to infection, generated antibody titers need to be 2 to 3 orders of magnitude higher in order to neutralize the abundance of Delta virions. For example, if we combine neutralization reduction and faster replication, a 4‐fold reduction in antibody affinity and a 100‐fold increase in viral load require antibody titers 400 times higher, as opposed to 4 times higher as suggested by antibody neutralizing experiments alone. If Delta replication initially outpaces elevation of antibody titers, symptomatic infection may be more likely; however, as adaptive immunity continues to ramp up, it will ultimately overpower Delta and prevent more severe disease outcomes. This hypothesis would explain the loss of protection against symptomatic infection by Delta over time, but the resilience of protection against severe, critical, and fatal disease. 2
To illustrate this hypothesis, we simulated in‐host viral dynamics for a hypothetical wildtype infection and a Delta‐like VOC infection among unvaccinated, not recently vaccinated, and recently vaccinated or boosted individuals. As shown in Figure 1, the Delta‐like VOC generates reduced but still substantially high viral loads in non‐recent vaccinees (vs. naïve/unvaccinated) due to its faster replication rate and the slight delay of immune response. Further, viral load is substantially reduced in recently vaccinated or boosted individuals due to higher circulating titers and a faster and stronger immune response (Figure 1). These simulated results are consistent with the observed higher probability of Delta breakthrough but lower probability of severe infection, as well as the restored VE following the boosting.
FIGURE 1.
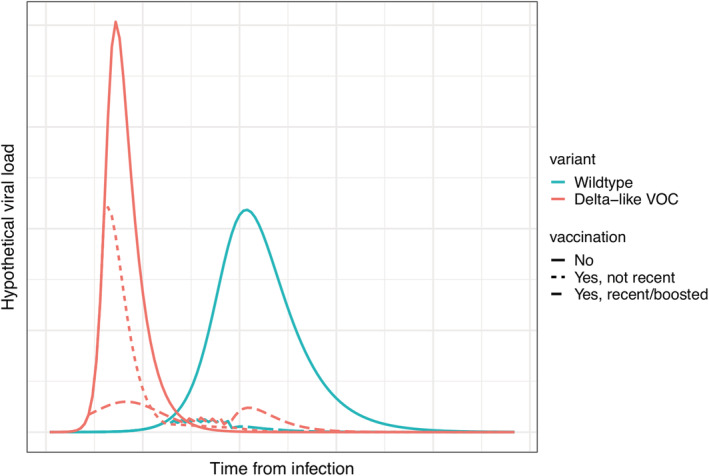
Simulated in‐host viral dynamics. The simulation assumes (1) a basic reproduction number R 0 of 2.4 and an infectious period D of 6 days for the wildtype infection and an R 0 of 4 and D of 4 days for a Delta‐like VOC infection; (2) lower antibody affinity for the Delta‐like VOC leading to a 15% reduction in immune response; and (3) for the recently vaccinated or boosted, immune response is 25% faster and stronger than those not recently vaccinated. VOC, variant of concern
Given the above, we call for more robust assessment of SARS‐CoV‐2 variant impact on immunity and VE. Specifically, viral replication dynamics need to be incorporated when assessing sensitivity to antibody neutralization. For instance, in addition to assays using the same starting viral titer, neutralizing experiments can be performed using harvested virus from cell cultures inoculated with the same amount of virus and grown for a certain period of time (e.g., roughly the time from infection to immune response). While such a study design also has limitations (e.g., in vitro cell culture may not fully represent in vivo replication dynamics), it may better represent the combined outcome of in‐host viral dynamics and delayed immune response. Such findings can better inform public health response to new variants like the newly detected Omicron variant.
DATA AVAILABILITY STATEMENT
All data are presented in the letter.
REFERENCES
- 1. Goldberg Y, Mandel M, Bar‐On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385(24):e85. doi:10.1056/NEJMoa2114228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID‐19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407‐1416. doi:10.1016/S0140‐6736(21)02183‐8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garcia‐Beltran WF, Lam EC, St Denis K, et al. Multiple SARS‐CoV‐2 variants escape neutralization by vaccine‐induced humoral immunity. Cell. 2021;184(9):2372‐2383.e2379. doi:10.1016/j.cell.2021.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wall EC, Wu M, Harvey R, et al. Neutralising antibody activity against SARS‐CoV‐2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397(10292):2331‐2333. doi:10.1016/S0140‐6736(21)01290‐3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS‐CoV‐2 infection in Qatar. N Engl J Med. 2021;385(24):e83. doi:10.1056/NEJMoa2114114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID‐19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398(10316):2093‐2100. doi:10.1016/S0140‐6736(21)02249‐2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Teyssou E, Delagrèverie H, Visseaux B, et al. The Delta SARS‐CoV‐2 variant has a higher viral load than the Beta and the historical variants in nasopharyngeal samples from newly diagnosed COVID‐19 patients. J Infect. 2021;83(4):E1‐E3. doi:10.1016/j.jinf.2021.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li B, Deng A, Li K, et al. Viral infection and transmission in a large, well‐traced outbreak caused by the SARS‐CoV‐2 Delta variant. medRxiv. 2021. doi:10.1101/2021.07.07.21260122 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are presented in the letter.


