Abstract
Background
Bullous pemphigoid (BP) is a senile chronic autoimmune bullous skin disease with a high relapse rate, which significantly impairs patients’ quality of life and contributes to disease mortality. This observational case-control study explores the gene polymorphisms of cytokines and their clinical significance in Chinese patients with BP.
Methods
IL-1α (rs1800587), IL-1β (rs16944, rs1143627, rs1143634), IL-4 (rs2243250), IL-6 (rs1800795), IL-10 (rs1800896, rs1800871, rs1800872), IL-13 (rs1800925, rs20541), TNF-α (rs1799964, rs1800630, rs1799724, rs361525), IFN-γ (rs1799964, rs1800630, rs361525, rs1800629, rs4248160, rs1800750), and TGF-β1 (rs2317130, rs1800469, rs4803457) genes were genotyped in the healthy controls and BP patients, respectively. Expression of these cytokines in serum was measured. Medical profiles of patients, including baseline characteristics and prognosis, were statistically analyzed.
Results
We found that IL-1 β and IL-13 concentrations were higher in the BP patients’ sera compared to those in the controls. For IL-13, significant differences were found in the nucleotide ratio/genotype/haploid frequency/haplotype, respectively. IL-13 (rs20541, rs1800925) is related to gender, and the IL-13 genotype was significantly associated with recurrence.
Conclusions
BP is associated with IL-13 gene polymorphism and IL-13 concentration is elevated in blood circulation in patients with BP. Our results support that IL-13 is relevant in the pathogenesis of BP, suggesting that IL-13 could potentially represent a promising target for BP therapy and a prognostic marker.
Keywords: bullous pemphigod, cytokine, SNP, IL-13, recurrence, gender
Introduction
Bullous pemphigoid (BP) is a chronic autoimmune skin disease with severe pruritus most commonly identified in the elderly. It is characterized by urticarial, erythematous, or painful blisters in the skin and circulating autoantibodies against the hemidesmosomal molecules, BP180 and BP230, located in the basement membrane zone and basal keratinocytes of the skin (1). BP represents the most common autoimmune blistering disease, albeit rare, with a reported incidence ranging from approximately 4.5 to 14 per million per year (2, 3). Moreover, the prevalence rate has been increasing markedly in the past years (4, 5).
Histopathology of the BP lesion has revealed subepidermal blisters, typically with neutrophilic and eosinophilic infiltration. Although the exact mechanisms remain to be elucidated, it is well established that BP is triggered by the autoantibodies against BP180 (6) potentially via three pathways (7). First, direct binding of the antibodies to an extracellular noncollagenous domain (NC16A) of BP180 can cause a conformation change, thus preventing the natural assembly of hemidesmosomes. Second, binding of the antibodies may lead to signaling activation and hemidesmosomal disassembly in the basal keratinocytes, playing a significant role in pathophysiology of BP. Alternatively, binding of the autoreactive IgG1 or 4 recruits and activates neutrophils and eosinophils to the basement membrane and activation of complements, which can release and activate proteolytic enzymes including the neutrophil elastase (NE) and matrix metalloproterinase-9 to degrade the extracellular portion of BP180 (8, 9). A body of accumulating evidence has supported that the BP autoreactive antibodies mediated a cascade of inflammatory responses that could play a critical role in BP disease pathogenesis. These include activation of complements, aggregation of neutrophils and eosinophils, increase of cytokine expression, and secretion of proteolytic enzymes (1, 5, 10, 11).
Multiple cytokines appear indispensable in developing the local immune and inflammatory responses primarily by recruiting inflammatory cells and activating resident cells in BP pathogenesis (12) and regulating the humoral or cellular immune response (13). Significantly elevated levels of cytokines were observed in the blister fluid (14–16) and the sera from BP patients (17, 18). These cytokines function as proinflammatory cytokines that further stimulate cytokine expression through a series of signaling cascades (19). Immunohistochemical and in situ hybridization studies have shown a significant expression of Th2 cytokines such as IL-4, IL-5, and IL-13 in the mononuclear cells in the BP lesional or perilesional skin (20, 21). More recently, the concentration of some cytokines has been correlated with BP severity and disease activity (20, 22, 23). Given their importance in BP disease, these cytokines potentially serve as promising targets for developing novel, effective therapeutic strategies for BP.
Cytokine gene polymorphisms or variations may affect cytokine functions by regulating cytokine protein expression and cytokine release, modulating susceptibility to autoimmune diseases. Interestingly, gene polymorphisms of various cytokines have been reported in some other autoimmune diseases (24–26). However, the significance of cytokine polymorphisms in BP disease has not been adequately investigated. In a previous Chinese cohort study, a polymorphism in IL-1β was identified in female patients (27). Recently, some IL-13 variation has been identified as a protective marker from BP in Iranian patients (28). In order to explore the gene polymorphisms of cytokines and their clinical significance in Chinese patients with BP, we performed a retrospective case-control association study to elucidate genetic polymorphisms of various cytokines and their relationship with serum cytokine level, clinical manifestation, and prognosis of patients. Our goal was to identify the key cytokines most relevant to BP and explore the relationship of patients’ characteristics with gene polymorphisms of these cytokines.
Results
Characteristics of Bullous Pemphigoid Patients
The 61 BP patients tested for SNP consisted of 30 males and 31 females, ranging from 31 to 89 years, with a mean age of 69.36 ± 15.28. The 56 patients tested for cytokine serum level consisted of 27 males and 29 females, ranging from 31 to 96 years, with a mean age of 72.09 ± 15.24 years. There was no significant difference between patients and controls regarding age and gender in SNP and serum level analysis (P > 0.05). The frequencies of TGF-β1 (rs4803457) SNP in both patient and control groups deviated markedly from Hardy–Weinberg equilibrium and were excluded from further analysis, while the genotype frequencies of all other polymorphisms were within the equilibrium.
Allele and Genotype Frequencies of Cytokines
Gene variations of IL-1α, IL-1β, IL-4, IL-6, IL-10, IL-13, TNF-α, IFN-γ, and TGF-β1 were analyzed in BP patients compared to healthy controls to clarify whether these polymorphisms are associated with BP ( Supplementary Table 1 ). We found GG is the most common genotype of IL-13 in healthy control, while the AG and AA genotypes are considered as variants. In BP patients, the proportion of IL-13 rs20541 AG genotype was significantly higher (P=0.003). The proportion of IFN - γ rs2430561 TA or TT was much higher than that of the control group. For the allele of IL-13 rs1800925, the T ratio was higher than that of the control group, and the C ratio was lower than that of the control group. For the IL-13 rs20541 allele, the G ratio was lower, whereas the A ratio was higher than that of the control group ( Table 1 ).
Table 1.
Comparison of the genotype and allele frequencies (gene polymorphisms) in the BP and control patients.
| Gene | Genotype/Allele | Patients | Control | χ2/OR (95% CI) | P-Value1 |
|---|---|---|---|---|---|
| IL-13 rs1800925 | CC | 41 | 65 | 3.1 | 0.212 |
| CT | 16 | 16 | |||
| TT | 3 | 1 | |||
| C Allele | 98 | 146 | 0.549 (0.280-1.077) | 0.078 | |
| T Allele | 22 | 18 | |||
| IL-13 rs20541 | GG | 27 | 58 | 11.85 | 0.003** |
| AG | 29 | 18 | |||
| AA | 4 | 9 | |||
| G Allele | 83 | 134 | 0.603 (0.353-1.028) | 0.062 | |
| A Allele | 37 | 36 | |||
| IFN – γ rs2430561 | TT | 48 | 63 | 5.119 | 0.077 |
| AT | 10 | 20 | |||
| AA | 3 | 0 | |||
| T Allele | 106 | 146 | 0.908 (0.449-1.834) | 0.787 | |
| A Allele | 16 | 20 |
1 **denotes P<0.01.
The frequencies of genotypes or alleles for IL-13 and INF-γ were listed in the table. The difference was compared using the Chi-square (χ2) test.
Linkage Disequilibrium and Haplotype Frequencies
Linkage disequilibrium (LD) was performed to investigate the relationship between polymorphisms in chromosome (chr) 2, chr5, chr1, chr6, chr12, chr19 among all cytokines ( Supplementary Figure 1 ). We found that rs1800871 and rs1800872 (D’=1, r2 = 1) in IL-10 are in complete linkage disequilibrium. Notably, rs16944 and rs1143627 in IL-1 (D’=1, r2 = 0.985), and rs1800630 and rs1799964 in TNF-α (D’=1, r2 = 0.759), rs2069718 and rs2430561 in IFN-γ (D’=1, r2 = 0.935), and rs2317130 and rs1800469 in TGF-β1 (D’=0.985, r2 = 0.957) have strong linkage disequilibrium. Linkage disequilibrium also exists in rs2069718 and rs2069705 in IFN-γ (D’=1, r2 = 0.501), and rs2317130 and rs4803457 in TGF-β1 (D’=1, r2 = 0.333). However, no LD is found in chr5 where IL-13 and IL-4 locate.
Haplotype frequencies were compared accordingly ( Supplementary Table 2 ). Interestingly, the haploid frequency of IL-13, IL-4 (rs1800925, rs20541, rs2243250) in chr5 was different between the patient and control group, and the proportion of C A C haplotype was more [P=0.05, OR & 95%CI: 47.626 (3.156-718.771)], though not significantly, in the BP group ( Table 2 ).
Table 2.
Haplotype patterns with their frequencies in the population.
| Haplotype | Case (Frequency) | Control (Frequency) | χ2 | P-value1 | Odds Ratio [95%CI] |
|---|---|---|---|---|---|
| C A C | 3.55 (0.030) | 0.10 (0.001) | 3.833 | 0.050* | 47.626 (3.156-718.771) |
| C A T | 16.18 (0.137) | 16.12 (0.101) | 0.870 | 0.351 | 1.417 (0.680-2.956) |
| C G C | 17.67 (0.150) | 31.26 (0.195) | 0.979 | 0.322 | 0.725 (0.383-1.373) |
| C G T | 58.61 (0.497) | 94.52 (0.591) | 2.450 | 0.118 | 0.682 (0.423-1.102) |
| T A C | 0.78 (0.007) | 2.54 (0.016) | 0.294 | 0.588 | 0.415 (0.052-3.311) |
| T A T | 16.49 (0.140) | 12.24 (0.077) | 2.925 | 0.087 | 1.960 (0.897-4.281) |
| T G T | 4.72 (0.040) | 3.12 (0.019) | 1.044 | 0.307 | 2.097 (0.492-8.947) |
| Global haplotype association p-value | 0.080 | ||||
1 *denotes P=0.05.
The frequencies of the haplotypes (IL-4 (rs2243250) and IL-13 (rs1800925, rs20541)) in the BP (Case) and control patients (Control) were compared using the Chi-square (χ2) test. The P values and odds ratios with a 95% confidence (95%CI) were calculated. A noticeable difference was found in the CAC(P=0.050) and TAT(P=0.087) haplotypes, respectively.
Serum Concentration of Cytokines in Patients and Its Relationship With Genotypes
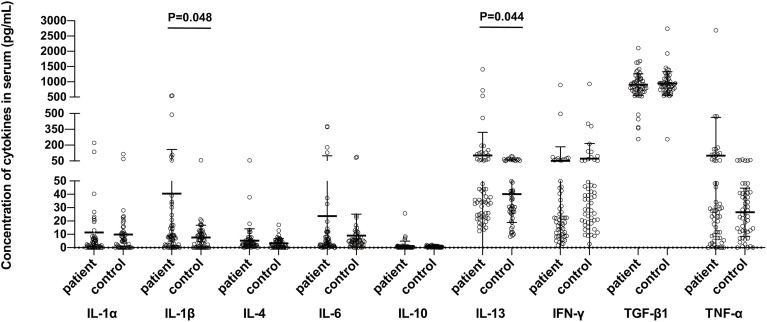
The serum concentrations of IL-1 β ((P=0.048) and IL-13 (P=0.044), but not of the IL-1α, IL-4, IL-6, IL-10, IFN-γ, TNF-α, or TGF-β, were found significantly higher in the sera of BP patients as compared to those of the controls ( Figure 1 ).
Figure 1.
Comparison of serum cytokine levels between bullous pemphigoid patients and control groups. The protein concentrations were measured in the BP patients and healthy control subjects using the commercially available ELISA kits. Statistical analysis revealed a significant difference in the concentrations of IL-1β (P=0.048) and IL-13 (P=0.044) between the patient and control groups, respectively.
We also evaluated the relationship between the different genotypes and serum levels of cytokines ( Supplementary Table 3 ). There is no significant correlation between the genotype and serum concetration of IL-13. A significant correlation was found between the IL-1α rs1800587 genotype and its cytokine concentrations. Patients with the AA genotype have a significantly higher IL-1α cytokine concentration than those with the GG genotype (P=0.027) or the GA genotype (P=0.024). The cytokine concentration of TNF-α rs1799964 genotypes is also significantly different (P<0.001).
Relationship Among Cytokine Gene Polymorphism, Cytokine Serum Level, and Clinical Characteristics
The possible association between cytokine serum level and polymorphic cytokine genotype of the patients was analyzed, respectively. Factors including age, gender, duration before treatment, percentage of eosinophils, number of eosinophils in whole blood, whether hospitalized, mucosa involvement, whether the lesion is extensive, whether complications occurred, DIF, and IIF results were compared. IL-13 is the only cytokine that showed a significant difference. We found that gender is significantly correlated with IL-13 rs20541 genotype (P = 0.027), and the ratio of AG in female is higher than that in male (AG & AA: P = 0.036, AG & GG: P = 0.073). Gender is significantly correlated with the rs1800925 genotype (P=0.030), and females had a higher frequency of CC genotypes than males (CC & TT: P=0.041, CC & CT: P=0.092). Moreover, the serum concentration level of IL-13 is higher in patients with high percentage or numbers of eosinophils, although without significant difference (P=0.082) ( Table 3 ).
Table 3.
Correlation of the IL-13 genotypes and IL-13 serum levels with clinical characteristics and demographic data in BP patients.
| Characteristics | IL-13 rs20541 | IL-13 rs1800925 | Serum IL-13 pg/mL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GG | AG | AA | P1 | CC | CT | TT | P | mean ± SD | P | ||
| Age | >60 | 8 | 11 | 2 | 0.953 | 13 | 6 | 3 | 0.313 | 132.67 ± 299.21 | 0.402 |
| ≤60 | 6 | 8 | 1 | 11 | 4 | 0 | 54.89 ± 56.55 | ||||
| Gender | female | 6 | 14 | 0 | 0.027* | 17 | 4 | 0 | 0.030* | 154.74 ± 324.86 | 0.138 |
| male | 8 | 5 | 3 | 7 | 6 | 3 | 44.73 ± 27.09 | ||||
| Duration before treatment | >5 months | 5 | 6 | 2 | 0.501 | 10 | 3 | 1 | 0.804 | 98.18 ± 168.97 | 0.823 |
| ≤5 months | 9 | 13 | 1 | 14 | 7 | 2 | 117.48 ± 307.39 | ||||
| EOS% | >5% | 6 | 9 | 1 | 0.943 | 12 | 5 | 0 | 0.264 | 31.37 ± 9.13 | 0.082 |
| ≤5% | 6 | 7 | 1 | 9 | 3 | 2 | 174.03 ± 337.68 | ||||
| EOS | >0.5×109/L | 7 | 10 | 1 | 0.848 | 14 | 5 | 0 | 0.147 | 31.01 ± 9.48 | 0.082 |
| ≤0.5×109/L | 5 | 5 | 1 | 6 | 3 | 2 | 174.03 ± 337.68 | ||||
| Hospitalization | no | 10 | 14 | 2 | 0.965 | 19 | 5 | 3 | 0.119 | 94.09 ± 143.72 | 0.646 |
| yes | 4 | 5 | 1 | 5 | 5 | 0 | 135.1 ± 382.8 | ||||
| Mucosa involvement | no | 8 | 12 | 2 | 0.921 | 16 | 4 | 3 | 0.127 | 132.21 ± 294.47 | 0.379 |
| yes | 6 | 7 | 1 | 8 | 6 | 0 | 48.29 ± 30.12 | ||||
| Extensive lesion | no | 6 | 9 | 1 | 0.891 | 11 | 4 | 2 | 0.719 | 60.09 ± 52.17 | 0.259 |
| >50% area | yes | 8 | 10 | 2 | 13 | 6 | 1 | 152.58 ± 342.1 | |||
| Complications | no | 13 | 17 | 3 | 0.811 | 22 | 9 | 3 | 0.855 | 115.29 ± 266.86 | 0.674 |
| yes | 1 | 2 | 0 | 2 | 1 | 0 | 57.82 ± 49.65 | ||||
| DIF IgG | – | 10 | 10 | 2 | 0.538 | 15 | 5 | 3 | 0.293 | 118.38 ± 298.19 | 0.794 |
| + | 4 | 9 | 1 | 9 | 5 | 0 | 95.64 ± 178.08 | ||||
| DIF C3 | – | 10 | 11 | 1 | 0.431 | 16 | 5 | 2 | 0.65 | 141.57 ± 311.76 | 0.308 |
| + | 4 | 8 | 2 | 8 | 5 | 1 | 51.1 ± 41.48 | ||||
| IIF | – | 5 | 10 | 2 | 0.491 | 10 | 6 | 2 | 0.503 | 202.63 ± 391.77 | 0.168 |
| + | 9 | 9 | 1 | 14 | 4 | 1 | 49.26 ± 37.98 | ||||
1*denotes P<0.05.
The IL-13 genotypes with alleles (GG, AG, AA for rs20541 and CC, CT, TT for rs1800925) were calculated and associated with BP patients’ data. A significant association with gender was found in the IL-13 rs20541 (P=0.027) and rs1800925 (P=0.030).
In the Kaplan-Meier analysis for the relationship between cytokines and BP’s recurrence ( Supplementary Figure 2 and Supplementary Table 4 ), IL-13 rs20541(P=0.048) and rs1800925 genotype (P<0.001) were significantly correlated with recurrence. For IL-13 rs20541, the ratio of AG in relapsed patients was significantly lower than that of AA genotype (P=0.025); for IL-13 rs1800925, the ratio of rs1800925 TT genotype in relapsed patients was higher than that of CC and CT genotype (CC &TT, P<0.001, CT & TT, P=0.025).
Discussion
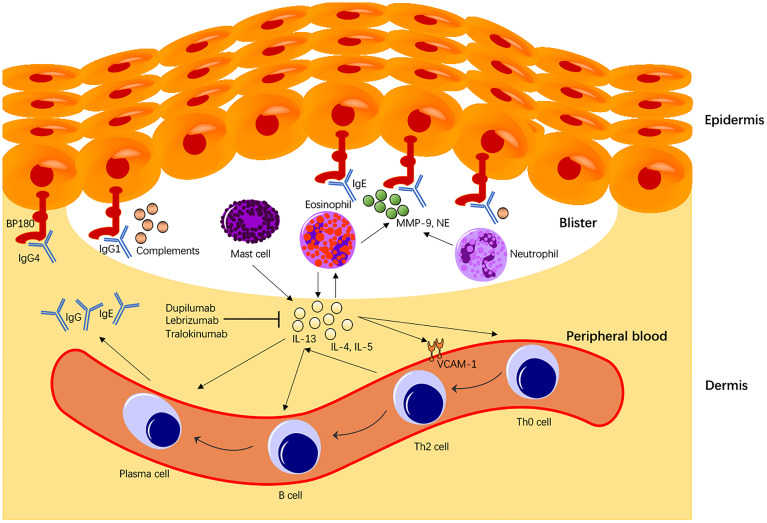
The current study revealed that IL-13 is correlated with BP in alleles, genotype, haplotype, and serum cytokine concentration. IL-13 is predominantly secreted by Th2 cells, although mast cells found in the inflammatory infiltrates of BP also produce bioactive IL-13 in vitro (29). IL-13 up-regulates gene expression VCAM-1, a cell adhesion molecule and marker of endothelial activation marker (30). VCAM-1 is a crucial mediator for transmigration of eosinophils and basophils expressing the ligand VLA-4 for VCAM-1. Additionally, IL-13 exerts profibrotic and proinflammatory effects on the human conjunctival fibroblasts by up-regulating the expression of the T cell co-stimulatory molecules CD80, CD40, and CD154, suggesting a significant role in the pathophysiology in mucous membrane pemphigoid (MMP) (31). IL-13 activates eosinophils to secrete various cytokines (including IL-13), promote B cell maturation into plasma cells, and mediate plasma cells to secrete IgE antibodies. Moreover, IL-13 can also transform Th0 cells into Th2 cells to maintain and enhance the entire autoimmune process ( Figure 2 ). In BP, IgE titer was reported to be correlated with disease activity (32). The pathogenic effect of BP180-specific IgE auto antibodies was mediated through the degranulation of eosinophils and mast cells (32, 33). IgE antibodies could also regulate Th2 cells through IL-4 and IL-13 stimulation (34), contributing to a further loss of tolerance against BP180 antigen. Th2 cells produce cytokines such as IL-4, IL-5, IL-13, and other chemokines (including eosinophil chemotactic factor and single-cell chemotactic protein), which are overexpressed in damaged BP skin in the early stage of the disease (35). Interestingly, eosinophils could participate in maintaining Th2-type responses by producing IL-4 and IL-13 (34). This is consistent with our finding that serum IL-13 concentration is positively correlated with the number of eosinophils, which produce even more IL-13 and further activate eosinophils in a “positive feedback” pattern.
Figure 2.
Immunological roles of IL-13 in BP pathogenesis. IL-13 secreted from Th2 cells, or mast cells activates eosinophils that produce more IL-13 molecules in a “positive feedback” pattern. IL-13 also mediates the transition of Th0 to Th2 cells, the primary cells expressing IL-13. Moreover, IL-13 promotes activated B cell maturation to plasma cells expressing BP IgE autoantibodies and enhances BP immunoglobulins (Igs) class switching. IL-13 also enhances VCAM-1 expression that promotes transmigration of eosinophils and basophils to the dermis. In BP, IL-13 also promotes B cell maturation and directly stimulates plasma cells to secrete BP autoantibodies that play a central role in the BP disease pathomechanism. Secretion of proteolytic enzymes including NE and Metalloproteinase-9 from eosinophils or neutrophils causes degradation of BP180 molecules, contributing to the BP disease pathology.
One of our major findings is that the serum IL-13 level in our BP patients was significantly higher than that in healthy subjects, supporting that IL-13 is implicated in the pathomechanism of BP via the Th2 cell-mediated immune response and eosinophil activation ( Figure 2 ). Consistent with this finding, CD3+CD4+ or CD3+CD8+ T cells expressing IL-13 and several other cytokines from the peripheral blood were found significantly higher in BP than those from healthy subjects (20). Interestingly, the percentage of cutaneous lymphocyte-associated antigen (CLA) positive cells expressing IL-13 (CLA+IL-13+) was significantly higher than that of peripheral CLA-IL-13+ cells. Conversely, treatment of BP patients with systemic steroids significantly reduced the frequency of CLA+IL-13+ T cells. CLA is considered as the marker for skin-homing memory T cells that will selectively accumulate into the skin. Similar results were shown in ocular MMP, where treatment with immunosuppressants led to a significant reduction of CD3+ IL-13+ cells in human conjunctival fibroblasts (36), suggesting that IL-13 plays a crucial role in the profibrotic and proinflammatory responses in BP and MMP. The IL-13 monoclonal antibodies (e.g., dupilumab, lebrikizumab, and tralokinumab) have been developed for not only allergic diseases (e.g., atopic dermatitis, asthma) (37) but various other autoimmune diseases (38). The monoclonal antibody therapy (dupilumab) with dual antagonistic effects on IL-4 and IL-13 has been trialed in pilot studies for BP treatment. A patient with severe and refractory BP disease was successfully treated with dupilumab, with no recurrence ten months after remission (38). A multicenter case meta-analysis of 13 patients reported that 92.3% of BP patients treated with dupilumab led to disease remission, and 53.8% of patients achieved complete remission, with no evident adverse reaction observed in those patients (39). In the treatment of BP, targeted therapy has achieved relieving pain or itching and preventing bullae formation. However, the optimal dose, treatment interval, and election of the treatment populations remain to be established in clinical trials.
Notably, we found that IL-1β is also significantly higher in the BP patients’ sera than in the control group, consistent with a previous study (17). This could be due to IL-1β gene polymorphisms linked to BP in a Chinese population (27). IL-1 is a key mediator of innate immunity and plays a central role in many human autoinflammatory diseases. Ameglio et al. have reported a positive correlation between the number of skin lesions in BP patients and the IL-1β level in the blister fluid (18). In our study, the IL-1 SNP polymorphism showed a positive correlation with the serum level of cytokines in BP patients ( Supplementary Table 3 ): The IL-1α cytokine concentration in patients with IL-1α rs1800587 AA genotype was higher than that of GG genotype (P=0.027) and GA genotype (P=0.024). IL-1 could play an essential role in BP development by inducing the synthesis and proteolytic enzyme activity of matrix metalloproteinase 9 in eosinophils and neutrophils (40, 41). The inflammatory process is initiated, further perpetuated, and enhanced by IL-1α and IL-1β (42).
Like IL-13, IL-4 is another crucial cytokine of Type 2 inflammation in which Th2 lymphocytes are the central cells of the adaptive immune response. However, we found that IL-4 is not significantly increased in sera of BP patients. Several published studies have demonstrated an elevated expression of IL-4 and IL-13 in the mononuclear cells in the skin tissue of patients with BP by immunohistochemistry or in situ hybridization (20, 21, 43), suggesting that IL-4 could have a pathogenic effect in the local, lesional skin. Both IL-4 and IL-13 regulate growth, survival, maturation, and activation of eosinophils (44) that contribute to the pathogenesis of BP (45). Additionally, several lines of evidence have shown that IL-4 and IL-13 could impair skin barrier function by suppressing the expression of tight junction proteins, filaggrin, loricrin, and involucrin (46–48). Other groups and we have previously shown a high incidence rate of local skin or systemic infections in BP (49–51). It will be interesting to investigate in the future whether these cytokines are involved in infections in BP patients.
Our further analysis on alleles and genotypes showed that BP patients were more likely to carry the IL-13 rs20541 allele A. IL-13 rs20541 genotypes in BP patients were significantly different from that of the control group, with a significantly higher proportion of AG genotype. However, a recent study has shown the IL-13 rs20541 variation could play a protective role in BP (28). The discrepancy could be derived from ethnicity differences, as differences in BP incidence and age of onset between ethnic groups have been documented (52). IL-13 rs20541 is located in the IL-13 gene on chr5q31 and is a common coding SNP in exon 4. Interestingly the polymorphism of this locus has been previously associated with several human diseases such as allergic rhinitis, asthma, eczema, and psoriatic arthritis in different ethnic groups (53–56). Our study also has shown a higher frequency of C-allele in IL-13 gene variation (rs1800925) in the healthy subjects than BP patients, although not statistically significant (P=0.078), suggesting this C allele, instead of T allele, could have a protective effect on the susceptibility to BP (28). Our finding that BP patients were likely to carry the IL-13 rs1800925 allele T supports that IL-13 gene polymorphisms might increase BP susceptibility. The IL-13 rs1800925 gene locates on chr5q31, which may cause a C to T transition at the 1112/1024/1055 sites. The C to T transition is also related to many other diseases, including asthma, eczema, psoriatic arthritis, and breast cancer (54–57). Furthermore, we found that the CAC haplotype in IL-13 may increase the susceptibility of BP on the haplotype analysis. Collectively, our results supports that IL-13 gene polymorphism is correlated with a higher risk of BP.
While many other clinical features appear not correlated with BP gene polymorphisms, we found that gender is significantly associated with the alleles of rs20541 and rs1800925, suggesting there is a difference in genetic predisposing factors between male and female patients. As mentioned previously, a published series with a Chinese population has shown a significant association of IL-1 gene alleles (-511T and -31C) with BP in female patients (27).
The expression level of several cytokines has been correlated with BP severity and disease activity (20, 22, 30). Conversely, BP treatment with immunosuppressants could improve BP severity and reduce the cytokine-producing T cells in the skin of BP or MMP (20, 31). More recently, the number of IL-13 expressing cells has been significantly associated with itch severity (58), further supporting that IL-13 is involved in BP pathophysiology. Our analysis of the correlation between IL-13 and recurrence found that both the rs20541 and rs1800925 genotypes significantly correlate with recurrence, suggesting that patients with some genotypes be genetically more susceptible. Relapse in BP remained a persistent issue for patients and clinicians and has contributed substantially to the morbidity and mortality from BP (36). Because high IL-13 expression and gene polymorphisms are associated with BP and clinical relapse of BP, we hypothesize that IL-13 gene polymorphisms have an effect on IL-13 gene expression. However, we found no significant correlation between serum IL-13 level and IL-13 gene polymorphism, highlighting that multiple other factors such as gender may participate and act synergistically in the regulation of IL-13 expression.
Limitations of our study could be the limited number of patients and samples obtained from a single medical center. The heterogeneity of patient treatment and disease status at the time of sample collection could further introduce biases to our conclusion. Moreover, we did not further investigate how gene polymorphisms may directly affect gene expression and disease pathogenesis in BP. Furthermore, it will be interesting to investigate whether there is a significantly elevated expression of the cytokines in the perilesional and lesional skin in future work. Nevertheless, our study presented a series of patients matched with a group of healthy subjects and whose clinical features align with those previously reported series, including characteristics such as age, sex, mucosal involvement, corticosteroid dose, and relapse rate (28, 36, 51, 59–61). Remarkably, our studies have identified that BP is associated with IL-13 gene polymorphism and an increased IL-13 in the peripheral blood. Our preliminary observation also supports that the IL-13 genotypes could be valuable in predicting disease relapse of BP. Future studies with a larger sample size in a multicenter research scenario will be required to validate our results.
Methods
Study Population and Design
The medical records of Chinese BP patients from the Peking Union Medical College Hospital between 2014 and 2019 were searched. All 68 BP patients were Chinese and genetically unrelated to each other. The control group comprised 86 healthy individuals, and the groups were selected to match age and sex for both SNP and serum level analyses. Informed consent was obtained from each participant, and among all these subjects, 61 patients and 86 healthy subjects were available for whole blood collection. 56 BP patients and 52 healthy subjects were available for serum collection. The SNP and serum level of cytokines were examined in 51 BP patients. Complete medical profiles were available only in 37 patients with SNP results and 36 patients with serum level results, and 21 patients were regularly followed up for more than one year.
BP was diagnosed based on clinical presentation, subepidermal blister on skin biopsy, compatible anti-BP180 antibody titer more than 9U/L, positive indirect immunofluorescence microscopy (IIF), or positive direct immunofluorescence microscopy (DIF) according to investigational assessment guidelines (62). Whole blood serum samples of the patients were collected immediately after diagnosis of BP, and the peri-procedural assessment was performed by using the initial data analysis of patients. Samples and medical records of the healthy group were collected at their regular health check. BP patients were followed up by telephone calls or in our outpatient office for prognostic information. The definition of relapse is the appearance of at least three new lesions in one month (including blisters, urticarial plaques, or eczematous lesions) or no less than one large (>10 cm in diameter) urticarial plaque or eczematous lesion that does not heal within one week, or the extension of original lesions or daily pruritus in the adequately managed patients within disease control (36).
The serum cytokine level, cytokine polymorphism, and clinical characteristics of the patient group and the healthy group were analyzed retrospectively. The Human Research Ethics Committee of Peking Union Medical College Hospital considered and approved this study (JS-2338). Informed consent was obtained from all individual participants included in the study.
Genotyping Genes and Measuring Serum Level Cytokines
We extracted genomic DNA from 5 mL of whole blood using a DNA isolation kit with magnetic beads (BioTeKe Corporation). The single nucleotide polymorphisms (SNPs) of cytokine genes including IL-1α (rs1800587), IL-1β (rs16944, rs1143627, rs1143634), IL-4 (rs2243250), IL-6 (rs1800795), IL-10 (rs1800896, rs1800871, rs1800872), IL-13 (rs1800925, rs20541), TNF-α (rs1799964, rs1800630, rs1799724, rs361525), IFN-γ (rs1799964, rs1800630, rs361525, rs1800629, rs4248160, rs1800750), and TGF-β1 (rs2317130, rs1800469, rs4803457) were determined by MassARRAY System analysis.
The DNA sequences containing each SNP of cytokine genes were amplified by polymerase chain reaction. The remaining deoxyribonucleoside triphosphate (dNTP) and primers were removed using Shrimp alkaline phosphatase enzyme. Subsequently, a single base extension primer, whose 3’ terminal base was close to the SNP site and which was completely complementary to the target fragment, was added so that the probe only extended one base at the SNP site. Besides, this primer had four ddNTPs instead of dNTPs so that the connected ddNTP corresponded to the allele of the SNP site. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) was used to detect the molecular weight difference between the extended product and the unextended primer and determine the specific base.
Serum samples were tested using the ELISA kits (human IL-1α IL-1β, IL-4, IL-6, IL-10, IL-13, TNF-α, IFN-γ, and TGF-β1 ELISA kits by Neobioscience Company) for cytokine concentrations.
Statistical Analysis
SPSS statistical software program (version 26.0) and GraphPad Prism 8 software were used for statistical analysis in this study. The difference of cytokine serum levels between groups was assessed using the Student’s t-test or Mann-Whitney U test (2 groups) or one-way analysis of variance test (ANOVA, or Kruskal-Wallis test, more than 2 groups). During the analysis of SNPs, the Hardy-Weinberg equilibrium test was conducted with the Chi-square (χ2) test. Genotype distribution, allele frequency, linkage disequilibrium (LD), and haplotype analysis were calculated by SHEsis online software (http://analysis.biox.cn/myAnalysis.php). The χ2 test (or Fisher’s exact) is used to determine the relationship between SNP genotypes, BP occurrence, genotypes of SNP, and clinical characteristics of patients. Kaplan-Meier analysis was performed to analyze the related factors of recurrence. P<0.05 was considered to have a significant difference.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Human Research Ethics Committee of Peking Union Medical College Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors contributed to either conceptualization. Methodology: YW, XM, and LL. Software: YW and XM. Validation: YL and YY. Formal analysis: YW. Investigation: YW, YL, and YY. Resources: LL. Data curation: YW and XM. Writing—original draft preparation: YW. Writing—review and editing: XM and LL. Visualization: YW. Supervision: LL and HJ. Project administration: HJ and LL. Funding acquisition: LL. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81972945), the National Key Research and Development Program of China Grant No. 2016YFC0901500, Milstein Medical Asian American Partnership Foundation, and Education Reform Projects of Peking Union Medical College (2016zlgc0106).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.824110/full#supplementary-material
References
- 1. Ludwig RJ, Vanhoorelbeke K, Leypoldt F, Kaya Z, Bieber K, McLachlan SM, et al. Mechanisms of Autoantibody-Induced Pathology. Front Immunol (2017) 8:603. doi: 10.3389/fimmu.2017.00603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Colbert RL, Allen DM, Eastwood D, Fairley JA. Mortality Rate of Bullous Pemphigoid in a US Medical Center. J Invest Dermatol (2004) 122(5):1091–5. doi: 10.1111/j.0022-202X.2004.22504.x [DOI] [PubMed] [Google Scholar]
- 3. Kasperkiewicz M, Zillikens D, Schmidt E. Pemphigoid Diseases: Pathogenesis, Diagnosis, and Treatment. Autoimmunity (2012) 45(1):55–70. doi: 10.3109/08916934.2011.606447 [DOI] [PubMed] [Google Scholar]
- 4. Kridin K, Ludwig RJ. The Growing Incidence of Bullous Pemphigoid: Overview and Potential Explanations. Front Med (Lausanne) (2018) 5:220. doi: 10.3389/fmed.2018.00220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmidt E, Zillikens D. Pemphigoid Diseases. Lancet (2013) 381(9863):320–32. doi: 10.1016/S0140-6736(12)61140-4 [DOI] [PubMed] [Google Scholar]
- 6. Giudice GJ, Emery DJ, Zelickson BD, Anhalt GJ, Liu Z, Diaz LA. Bullous Pemphigoid and Herpes Gestationis Autoantibodies Recognize a Common non-Collagenous Site on the BP180 Ectodomain. J Immunol (1993) 151(10):5742–50. doi: 10.1016/0923-1811(93)90940-Q [DOI] [PubMed] [Google Scholar]
- 7. Kasperkiewicz M, Zillikens D. The Pathophysiology of Bullous Pemphigoid. Clin Rev Allergy Immunol (2007) 33(1-2):67–77. doi: 10.1007/s12016-007-0030-y [DOI] [PubMed] [Google Scholar]
- 8. Stahle-Backdahl M, Inoue M, Guidice GJ, Parks WC. 92-kD Gelatinase is Produced by Eosinophils at the Site of Blister Formation in Bullous Pemphigoid and Cleaves the Extracellular Domain of Recombinant 180-kD Bullous Pemphigoid Autoantigen. J Clin Invest (1994) 93(5):2022–30. doi: 10.1172/JCI117196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verraes S, Hornebeck W, Polette M, Borradori L, Bernard P. Respective Contribution of Neutrophil Elastase and Matrix Metalloproteinase 9 in the Degradation of BP180 (Type XVII Collagen) in Human Bullous Pemphigoid. J Invest Dermatol (2001) 117(5):1091–6. doi: 10.1046/j.0022-202x.2001.01521.x [DOI] [PubMed] [Google Scholar]
- 10. Haeberle S, Wei X, Bieber K, Goletz S, Ludwig RJ, Schmidt E, et al. Regulatory T-Cell Deficiency Leads to Pathogenic Bullous Pemphigoid Antigen 230 Autoantibody and Autoimmune Bullous Disease. J Allergy Clin Immunol (2018) 142(6):1831–42 e7. doi: 10.1016/j.jaci.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 11. Liu Z. Immunopathology of Bullous Pemphigoid, an Autoimmune and Inflammatory Skin Blistering Disease. Keio J Med (2003) 52(2):128–33. doi: 10.2302/kjm.52.128 [DOI] [PubMed] [Google Scholar]
- 12. Nakashima H, Fujimoto M. Expression of Cytokines and Chemokines in Bullous Pemphigoid. Expert Rev Dermatol (2014) 4(4):321–7. doi: 10.1586/edm.09.23 [DOI] [Google Scholar]
- 13. Didona D, Di Zenzo G. Humoral Epitope Spreading in Autoimmune Bullous Diseases. Front Immunol (2018) 9:779. doi: 10.3389/fimmu.2018.00779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. D'Auria L, Pietravalle M, Mastroianni A, Ferraro C, Mussi A, Bonifati C, et al. IL-5 Levels in the Serum and Blister Fluid of Patients With Bullous Pemphigoid: Correlations With Eosinophil Cationic Protein, RANTES, IgE and Disease Severity. Arch Dermatol Res (1998) 290(1-2):25–7. doi: 10.1007/s004030050272 [DOI] [PubMed] [Google Scholar]
- 15. Schmidt E, Bastian B, Dummer R, Tony HP, Brocker EB, Zillikens D. Detection of Elevated Levels of IL-4, IL-6, and IL-10 in Blister Fluid of Bullous Pemphigoid. Arch Dermatol Res (1996) 288(7):353–7. doi: 10.1007/BF02507102 [DOI] [PubMed] [Google Scholar]
- 16. Margaroli C, Bradley B, Thompson C, Brown MR, Giacalone VD, Bhatt L, et al. Distinct Compartmentalization of Immune Cells and Mediators Characterizes Bullous Pemphigoid Disease. Exp Dermatol (2020) 29(12):1191–8. doi: 10.1111/exd.14209 [DOI] [PubMed] [Google Scholar]
- 17. Kowalski EH, Kneibner D, Kridin K, Amber KT. Serum and Blister Fluid Levels of Cytokines and Chemokines in Pemphigus and Bullous Pemphigoid. Autoimmun Rev (2019) 18(5):526–34. doi: 10.1016/j.autrev.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 18. Ameglio F, D'Auria L, Bonifati C, Ferraro C, Mastroianni A, Giacalone B. Cytokine Pattern in Blister Fluid and Serum of Patients With Bullous Pemphigoid: Relationships With Disease Intensity. Br J Dermatol (1998) 138(4):611–4. doi: 10.1046/j.1365-2133.1998.02169.x [DOI] [PubMed] [Google Scholar]
- 19. Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and Chemokines: At the Crossroads of Cell Signalling and Inflammatory Disease. Biochim Biophys Acta (2014) 1843(11):2563–82. doi: 10.1016/j.bbamcr.2014.05.014 [DOI] [PubMed] [Google Scholar]
- 20. Teraki Y, Hotta T, Shiohara T. Skin-Homing Interleukin-4 and -13-Producing Cells Contribute to Bullous Pemphigoid: Remission of Disease is Associated With Increased Frequency of Interleukin-10-Producing Cells. J Invest Dermatol (2001) 117(5):1097–102. doi: 10.1046/j.0022-202x.2001.01505.x [DOI] [PubMed] [Google Scholar]
- 21. Giomi B, Caproni M, Calzolari A, Bianchi B, Fabbri P. Th1, Th2 and Th3 Cytokines in the Pathogenesis of Bullous Pemphigoid. J Dermatol Sci (2002) 30(2):116–28. doi: 10.1016/s0923-1811(02)00067-1 [DOI] [PubMed] [Google Scholar]
- 22. Delli FS, Sotiriou E, Lazaridou E, Apalla Z, Lallas A, Vakirlis E, et al. Total IgE, Eosinophils, and Interleukins 16, 17A, and 23 Correlations in Severe Bullous Pemphigoid and Treatment Implications. Dermatol Ther (2020) 33(6):e13958. doi: 10.1111/dth.13958 [DOI] [PubMed] [Google Scholar]
- 23. Nin-Asai R, Muro Y, Sekiya A, Sugiura K, Akiyama M. Serum Thymus and Activation-Regulated Chemokine (TARC/CCL17) Levels Reflect the Disease Activity in a Patient With Bullous Pemphigoid. J Eur Acad Dermatol Venereol (2016) 30(2):327–8. doi: 10.1111/jdv.12719 [DOI] [PubMed] [Google Scholar]
- 24. Moravvej H, Tabatabaei-Panah PS, Ebrahimi E, Esmaeili N, Ghaderian SMH, Ludwig RJ, et al. TNF-Alpha -308G/A Gene Polymorphism in Bullous Pemphigoid and Alopecia Areata. Hum Antibodies (2018) 26(4):201–7. doi: 10.3233/HAB-180339 [DOI] [PubMed] [Google Scholar]
- 25. Trajkov D, Mishevska-Perchinkova S, Karadzova-Stojanoska A, Petlichkovski A, Strezova A, Spiroski M. Association of 22 Cytokine Gene Polymorphisms With Rheumatoid Arthritis in Population of Ethnic Macedonians. Clin Rheumatol (2009) 28(11):1291–300. doi: 10.1007/s10067-009-1238-4 [DOI] [PubMed] [Google Scholar]
- 26. Tu Y, Fan G, Zeng T, Cai X, Kong W. Association Between Polymorphism Within Interleukin Related Genes and Graves' Disease: A Meta-Analysis of 22 Case-Control Studies. Oncotarget (2017) 8(58):98993–9002. doi: 10.18632/oncotarget.20114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang YT, Liu HN, Yu CW, Lin MW, Huang CH, Chen CC, et al. Cytokine Gene Polymorphisms in Bullous Pemphigoid in a Chinese Population. Br J Dermatol (2006) 154(1):79–84. doi: 10.1111/j.1365-2133.2005.06938.x [DOI] [PubMed] [Google Scholar]
- 28. Tabatabaei-Panah PS, Moravvej H, Alirajab M, Etaaty A, Geranmayeh M, Hosseine F, et al. Association Between TH2 Cytokine Gene Polymorphisms and Risk of Bullous Pemphigoid. Immunol Invest (2020) 1(1):1–13. doi: 10.1080/08820139.2020.1832113 [DOI] [PubMed] [Google Scholar]
- 29. Burd PR, Thompson WC, Max EE, Mills FC. Activated Mast Cells Produce Interleukin 13. J Exp Med (1995) 181(4):1373–80. doi: 10.1084/jem.181.4.1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ying S, Meng Q, Barata LT, Robinson DS, Durham SR, Kay AB. Associations Between IL-13 and IL-4 (mRNA and Protein), Vascular Cell Adhesion Molecule-1 Expression, and the Infiltration of Eosinophils, Macrophages, and T Cells in Allergen-Induced Late-Phase Cutaneous Reactions in Atopic Subjects. J Immunol (1997) 158(10):5050–7. [PubMed] [Google Scholar]
- 31. Saw VP, Offiah I, Dart RJ, Galatowicz G, Dart JK, Daniels JT, et al. Conjunctival Interleukin-13 Expression in Mucous Membrane Pemphigoid and Functional Effects of Interleukin-13 on Conjunctival Fibroblasts In Vitro. Am J Pathol (2009) 175(6):2406–15. doi: 10.2353/ajpath.2009.090579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cozzani E, Gasparini G, Di Zenzo G, Parodi A. Immunoglobulin E and Bullous Pemphigoid. Eur J Dermatol (2018) 28(4):440–8. doi: 10.1684/ejd.2018.3366 [DOI] [PubMed] [Google Scholar]
- 33. Maglie R, Hertl M. Pharmacological Advances in Pemphigoid. Curr Opin Pharmacol (2019) 46:34–43. doi: 10.1016/j.coph.2018.12.007 [DOI] [PubMed] [Google Scholar]
- 34. Moyle M, Cevikbas F, Harden JL, Guttman-Yassky E. Understanding the Immune Landscape in Atopic Dermatitis: The Era of Biologics and Emerging Therapeutic Approaches. Exp Dermatol (2019) 28(7):756–68. doi: 10.1111/exd.13911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gounni Abdelilah S, Wellemans V, Agouli M, Guenounou M, Hamid Q, Beck LA, et al. Increased Expression of Th2-Associated Chemokines in Bullous Pemphigoid Disease. Role of Eosinophils in the Production and Release of These Chemokines. Clin Immunol (2006) 120(2):220–31. doi: 10.1016/j.clim.2006.03.014 [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Mao X, Wang Y, Zeng Y, Liu Y, Jin H, et al. Relapse of Bullous Pemphigoid: An Update on This Stubborn Clinical Problem. Ann Med (2018) 50(3):234–9. doi: 10.1080/07853890.2018.1443346 [DOI] [PubMed] [Google Scholar]
- 37. Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 Pathway in Atopic Diseases. Expert Rev Clin Immunol (2017) 13(5):425–37. doi: 10.1080/1744666X.2017.1298443 [DOI] [PubMed] [Google Scholar]
- 38. Kaye A, Gordon SC, Deverapalli SC, Her MJ, Rosmarin D. Dupilumab for the Treatment of Recalcitrant Bullous Pemphigoid. JAMA Dermatol (2018) 154(10):1225–6. doi: 10.1001/jamadermatol.2018.2526 [DOI] [PubMed] [Google Scholar]
- 39. Abdat R, Waldman RA, de Bedout V, Czernik A, McLeod M, King B, et al. Dupilumab as a Novel Therapy for Bullous Pemphigoid: A Multicenter Case Series. J Am Acad Dermatol (2020) 83(1):46–52. doi: 10.1016/j.jaad.2020.01.089 [DOI] [PubMed] [Google Scholar]
- 40. Han YP, Downey S, Garner WL. Interleukin-1alpha-Induced Proteolytic Activation of Metalloproteinase-9 by Human Skin. Surgery (2005) 138(5):932–9. doi: 10.1016/j.surg.2005.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu Z. Bullous Pemphigoid: Using Animal Models to Study the Immunopathology. J Investig Dermatol Symp Proc (2004) 9(1):41–6. doi: 10.1111/j.1087-0024.2004.00841.x [DOI] [PubMed] [Google Scholar]
- 42. Dinarello CA. Interleukin-1 in the Pathogenesis and Treatment of Inflammatory Diseases. Blood (2011) 117(14):3720–32. doi: 10.1182/blood-2010-07-273417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rico MJ, Benning C, Weingart ES, Streilein RD, Hall RP, 3rd. Characterization of Skin Cytokines in Bullous Pemphigoid and Pemphigus Vulgaris. Br J Dermatol (1999) 140(6):1079–86. doi: 10.1046/j.1365-2133.1999.02907.x [DOI] [PubMed] [Google Scholar]
- 44. Sastre B, Rodrigo-Muñoz JM, Garcia-Sanchez DA, Cañas JA, Del Pozo V. Eosinophils: Old Players in a New Game. J Investig Allergol Clin Immunol (2018) 28(5):289–304. doi: 10.18176/jiaci.0295 [DOI] [PubMed] [Google Scholar]
- 45. Amber KT, Valdebran M, Kridin K, Grando SA. The Role of Eosinophils in Bullous Pemphigoid: A Developing Model of Eosinophil Pathogenicity in Mucocutaneous Disease. Front Med (Lausanne) (2018) 5:201. doi: 10.3389/fmed.2018.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wawrzyniak P, Wawrzyniak M, Wanke K, Sokolowska M, Bendelja K, Rückert B, et al. Regulation of Bronchial Epithelial Barrier Integrity by Type 2 Cytokines and Histone Deacetylases in Asthmatic Patients. J Allergy Clin Immunol (2017) 139(1):93–103. doi: 10.1016/j.jaci.2016.03.050 [DOI] [PubMed] [Google Scholar]
- 47. Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, Debenedetto A, et al. Cytokine Modulation of Atopic Dermatitis Filaggrin Skin Expression. J Allergy Clin Immunol (2007) 120(1):150–5. doi: 10.1016/j.jaci.2007.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and Involucrin Expression is Down-Regulated by Th2 Cytokines Through STAT-6. Clin Immunol (2008) 126(3):332–7. doi: 10.1016/j.clim.2007.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lehman JS, Khunger M, Lohse CM. Infection in Autoimmune Bullous Diseases: A Retrospective Comparative Study. J Dermatol (2013) 40(8):613–9. doi: 10.1111/1346-8138.12175 [DOI] [PubMed] [Google Scholar]
- 50. Phoon YW, Fook-Chong SM, Koh HY, Thirumoorthy T, Pang SM, Lee HY. Infectious Complications in Bullous Pemphigoid: An Analysis of Risk Factors. J Am Acad Dermatol (2015) 72(5):834–9. doi: 10.1016/j.jaad.2015.01.029 [DOI] [PubMed] [Google Scholar]
- 51. Chen J, Mao X, Zhao W, Zhang B, Chen X, Yu C, et al. Assessment of the Characteristics and Associated Factors of Infectious Complications in Bullous Pemphigoid. Front Immunol (2020) 11:1607. doi: 10.3389/fimmu.2020.01607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kridin K, Bergman R. Ethnic Variations in the Epidemiology of Bullous Pemphigoid in Israel. Int J Dermatol (2018) 57(1):34–9. doi: 10.1111/ijd.13813 [DOI] [PubMed] [Google Scholar]
- 53. Ying XJ, Zhao SW, Wang GL, Xie J, Xu HM, Dong P. Association of Interleukin-13 SNP Rs20541 With Allergic Rhinitis Risk: A Meta-Analysis. Gene (2013) 521(2):222–6. doi: 10.1016/j.gene.2013.03.088 [DOI] [PubMed] [Google Scholar]
- 54. Bottema RW, Nolte IM, Howard TD, Koppelman GH, Dubois AE, de Meer G, et al. Interleukin 13 and Interleukin 4 Receptor-Alpha Polymorphisms in Rhinitis and Asthma. Int Arch Allergy Immunol (2010) 153(3):259–67. doi: 10.1159/000314366 [DOI] [PubMed] [Google Scholar]
- 55. Miyake Y, Kiyohara C, Koyanagi M, Fujimoto T, Shirasawa S, Tanaka K, et al. Case-Control Study of Eczema Associated With IL13 Genetic Polymorphisms in Japanese Children. Int Arch Allergy Immunol (2011) 154(4):328–35. doi: 10.1159/000321825 [DOI] [PubMed] [Google Scholar]
- 56. Eder L, Chandran V, Pellett F, Pollock R, Shanmugarajah S, Rosen CF, et al. IL13 Gene Polymorphism is a Marker for Psoriatic Arthritis Among Psoriasis Patients. Ann Rheum Dis (2011) 70(9):1594–8. doi: 10.1136/ard.2010.147421 [DOI] [PubMed] [Google Scholar]
- 57. Erdei E, Kang H, Meisner A, White K, Pickett G, Baca C, et al. Polymorphisms in Cytokine Genes and Serum Cytokine Levels Among New Mexican Women With and Without Breast Cancer. Cytokine (2010) 51(1):18–24. doi: 10.1016/j.cyto.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hashimoto T, Kursewicz CD, Fayne RA, Nanda S, Shah SM, Nattkemper L, et al. Pathophysiologic Mechanisms of Itch in Bullous Pemphigoid. J Am Acad Dermatol (2020) 83(1):53–62. doi: 10.1016/j.jaad.2019.07.060 [DOI] [PubMed] [Google Scholar]
- 59. Bernard P, Reguiai Z, Tancrède-Bohin E, Cordel N, Plantin P, Pauwels C, et al. Risk Factors for Relapse in Patients With Bullous Pemphigoid in Clinical Remission: A Multicenter, Prospective, Cohort Study. Arch Dermatol (2009) 145(5):537–42. doi: 10.1001/archdermatol.2009.53 [DOI] [PubMed] [Google Scholar]
- 60. Fichel F, Barbe C, Joly P, Bedane C, Vabres P, Truchetet F, et al. Clinical and Immunologic Factors Associated With Bullous Pemphigoid Relapse During the First Year of Treatment: A Multicenter, Prospective Study. JAMA Dermatol (2014) 150(1):25–33. doi: 10.1001/jamadermatol.2013.5757 [DOI] [PubMed] [Google Scholar]
- 61. Brick KE, Weaver CH, Savica R, Lohse CM, Pittelkow MR, Boeve BF, et al. A Population-Based Study of the Association Between Bullous Pemphigoid and Neurologic Disorders. J Am Acad Dermatol (2014) 71(6):1191–7. doi: 10.1016/j.jaad.2014.07.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Feliciani C, Joly P, Jonkman MF, Zambruno G, Zillikens D, Ioannides D, et al. Management of Bullous Pemphigoid: The European Dermatology Forum Consensus in Collaboration With the European Academy of Dermatology and Venereology. Br J Dermatol (2015) 172(4):867–77. doi: 10.1111/bjd.13717 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.