Abstract
Coumarins are a family of benzopyrones largely distributed in the natural kingdom, being present in the seeds, fruits, flowers, or roots of various plant species. Natural coumarin compounds are found in significant concentrations in some herbs or spices used as nutraceuticals, but they are also present in cosmetics or household products, due to their pleasant odor. Therefore, an accidental exposure to high doses of coumarins, could lead to the development of harmful effects in some patients. This review summarizes the latest published data from preclinical and clinical studies with natural coumarins, focused on the investigation of general and specific toxicity, with the aim of a better understanding of the safety profile of these valuable compounds. Regulatory aspects concerning the use of natural coumarins in several world regions are also reviewed.
Keywords: coumarins, nutraceuticals, acute toxicity, hepatotoxicity, phototoxicity
Introduction
Nutraceuticals are bioactive substances which have become increasingly popular in the last 2 decades being used worldwide for health promotion and the prevention of various diseases. They are represented by numerous phytochemicals but also fatty acids, amino acids or probiotics/prebiotics and may be responsible for a variety of biological effects (Nahar et al., 2021).
In the larger category of nutraceuticals, natural coumarins play an important role, being present in high concentrations in several dietary plant species, like tonka beans (Dipteryx odorata (Aubl.) Forsyth f [Fabaceae]) where they have been originally discovered in 1820, or cinnamon (Cinnamomum verum J. Presl [Lauraceae]), but also in a variety of foodstuffs like olive and soy oils, coffee, nuts, wine, and green tea (Lončar et al., 2020).
Many natural coumarins have been successfully tested for an array of pharmacological properties like anti-inflammatory, antioxidant, antimicrobial, antidepressant, neuroprotective or antitumoral effects (Srikrishna et al., 2018). Additionally, several natural coumarins have served as scaffolds for the development of authorized drugs like warfarin, other drug candidates with different pharmacological properties being constantly developed (Bansal et al., 2013).
Although several articles and reviews focused on the presentation of important chemical and pharmacotherapeutic aspects regarding natural coumarins have been already published (Venugopala et al., 2013; Annunziata et al., 2020;Sharifi-Rad et al., 2021), the safety profile of coumarins was not thoroughly reviewed to present date. From a toxicological point of view, the presence of several natural coumarins in spices like cassia cinnamon which is widely used for preparation of pastries, cakes, or sweet biscuits, but also in cosmetics like perfumes or sunscreens, means that multiple routes of human exposure to natural coumarins have been described, with a possible oral, pulmonary, or skin absorption and subsequent development of toxic effects. Therefore, the aim of this review was to present the latest available data from preclinical and clinical studies with natural coumarins, regarding both general toxicity and specific organ toxicities, with additional mechanistic explanations, in order to increase the awareness of healthcare and food industry professionals for a safer use of these valuable compounds.
Natural Coumarins: Types and Sources
Structure and Classification
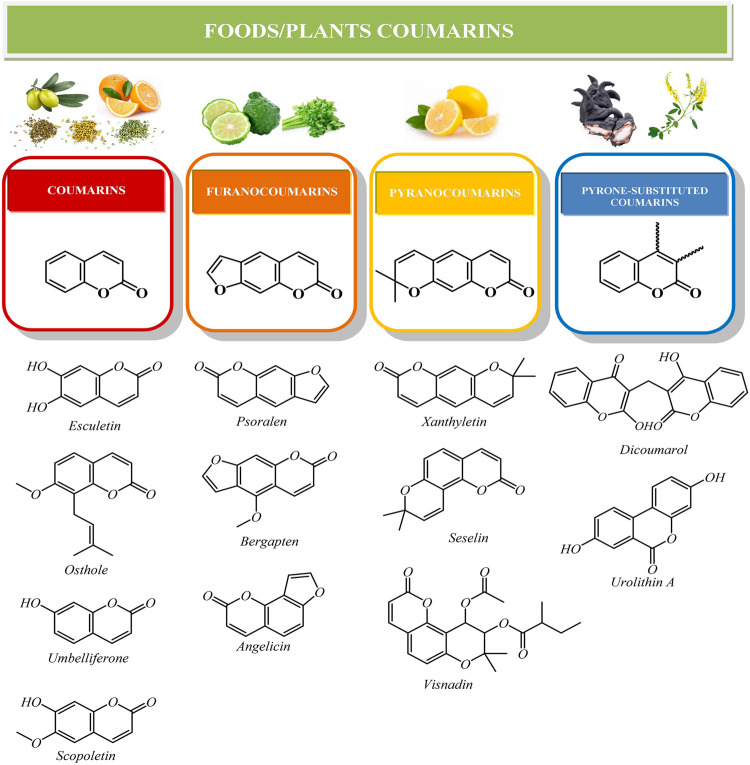
Coumarins are one of the most important classes of chemical compounds synthesized by plants, being part of the family of benzopyrones (Sarker and Nahar, 2017). Coumarin backbone (Figure 1) consists of an aromatic benzene ring in a conjugated system, which is fused with α-pyrone (lactone ring). The structure is rich in electrons and capable to react with different molecules as enzymes and receptors, which leads to potent medicinal effects (Önder, 2020).
FIGURE 1.
Chemical structures of the main classes of natural coumarins.
Currently, more than 1800 coumarin-derived compounds were described (Akkol et al., 2020; Lončar et al., 2020). Naturally occurring coumarins are subdivided in different classes based on their chemical diversity and complexity: simple coumarins (esculetin, scopoletin, umbelliferone), furanocoumarins (psoralen, bergapten, angelicin), pyranocoumarins (xanthyletin, seselin) and coumarins substituted in the pyrone ring, as biscoumarins (dicoumarol) or benzocoumarins (urolithins) (Annunziata et al., 2020) (Figure 1). Additionally, simple coumarins like umbelliferone or can be linked to a C15 terpene moiety, forming sesquiterpene coumarins like umbelliprenin (Gliszczynska and Brodelius, 2011).
Food and Herbal Sources of Coumarin Compounds
In nature, coumarins can be found in a free form or conjugated with other molecules like glycosides (Stringlis et al., 2019; Stassen et al., 2021). They are found in different parts of plants, such as roots, seeds, nuts, flowers and fruits of many species, being used as condiments (spices), herbal teas or medicines. In addition, coumarins can also be found in some widely used foods like oils (olive), coffee, nuts, wine, and tea (Lončar et al., 2020). Coumarins are even considered significant constituents of propolis that contribute to its pharmacological properties (esculin, daphnetin, fraxetin, umbelliferone, 4-methylumbelliferone, 4-hydroxycoumarin, scoparone, coumarin or herniarin) (Hroboňová et al., 2013).
The most significant natural coumarins in the field of phytochemistry, pharmacology, medicinal chemistry, and food science, together with their vegetal sources grouped in families are listed in Table 1.
TABLE 1.
Main coumarins and their vegetal sources.
| Plant species | Coumarins | References |
|---|---|---|
| Apiaceae/Umbelliferae | ||
| Anethum graveolens (L.) | Esculetin, scopoletin, furanocoumarin, oxypeucedanin, oxypeucedanin hydrate, falcarindiol | Kovač-Bešović and Durić, (2003); Kaur and Arora, (2010) |
| (Dill) | ||
| Angelica archangelica (L.) | Angelicin, osthole, bergapten, imperatorin, isoimperatorin, oreoselone, oxypeucedanin, psoralen, umbelliferone, xanthotoxin, xanthotoxol, umbelliprenin | Kumar et al., 2013; Forycka and Buchwald, (2019) |
| (Angelica) | ||
| Apium graveolens (L.) | Esculetin, bergapten, celerin, celereoside, isoimperatorin, isopimpinellin, osthenol, seselin, scopoletin, psoralen, umbelliferone, xanthotoxin | Garg et al., 1979; Najda et al., 2015; Arsenov et al., 2021 |
| (Celery) | ||
| Coriandrum sativum (L.) (Coriander) | Coumarin 7-substituted derivatives | Sharifi-Rad et al. (2021) |
| Cuminum cyminum (L.) (Cumin) | Coumarin | Rebey et al. (2012) |
| Daucus carota (L.) | Bergapten, isopimpinellin, umbelliferone, xanthotoxin | Ozçelik and Kusmenoglu, (2004); Kenari et al., 2021 |
| (Carrot) | ||
| Foeniculum vulgare Mill. (Fennel) | Scopoletin, bergapten, imperatorin, 8-methoxypsoralen, psoralen | Kaur and Arora, (2010); Yang et al., 2015 |
| Pastinaca sativa (L.) | Angelicin, bergapten, isopimpinellin, oxypeucedanin hydrate, xanthotoxin, imperatorin, psoralen | Kenari et al. (2021) |
| (Parsnip) | ||
| Petroselinum crispum (Mill.) Fuss | Bergapten, oxypeucedanin, 8-metoxypsoralen, imperatorin, isoimperatorin, isopimpinellin, psoralen | Manderfeld et al., 1997; Arsenov et al., 2021 |
| (Parsley) | ||
| Pimpinella anisum (L.) (Aniseed) | Bergapten, scopoletin, umbelliferone, umbelliprenine | Sun et al. (2019) |
| Rutaceae | ||
| Aegle marmelos (L.) Corrêa | Angelicin, umbelliferone, scopoletin, marmesinin, 8-hydroxypsoralen, marmelosin | Avula et al. (2016) |
| (Bael fruit) | ||
| Citrus x aurantiifolia (Christm.) Swingle | Bergamottin, 5-geranyloxy-7-Methoxycoumarin, imperatorin, isoimperatorin, isopimpinellin, limettin, marmesin, oxypeucedanin hydrate, phellopterin, scoparone | Dugrand et al., 2013; Dugrand-Judek et al., 2015 |
| (Lime) | ||
| Citrus x limon (L.) Osbeck | Limettin, 5-geranyloxy-7-methoxycoumarin, oxypeucedanin hydrate, byakangelicol, oxypeucedanin, 8-geranyloxypsoralen, bergamottin, umbelliferone, heraclenin, phellopterin, osthole, auraptene, isopimpinellin, bergapten | Dugrand et al., 2013; Dugrand-Judek et al., 2015 |
| (Lemon) | ||
| Citrus x sinensis (L.) Osbeck | Herniarin, scopoletin, scoparone, umbelliferone, xanthyletin, bergaptol | Dugrand et al., 2013; Dugrand-Judek et al., 2015 |
| (Sweet orange) | ||
| Citrus x paradisi Macfad. (Grapefruit) | Bergamottin, auraptene, limettin, scopolin, bergapten, bergaptol, isopimpinellin, osthole | Dugrand et al., 2013; Dugrand-Judek et al., 2015 |
| Asteraceae/Compositae | ||
| Arnica montana (L.) (Arnica) | Scopoletin, umbelliferone | Kriplani et al. (2017) |
| Chamaemelum nobile (L.) All | Scopolin (7-β-d-glucopyranosyl-scopoletin), umbelliferone, herniarin, scopoletin | European Medicines Agency, (2011) |
| (Roman Chamomile) | ||
| Cichorium intybus (L.) (Chicory) | Umbelliferon, esculetin (6,7-dihydrocumarin) scopoletin, esculetin and cichorin | Das et al., 2016; Aisa et al., 2020 |
| Matricaria chamomilla (L.) | Umbelliferone, herniarin, skimmin, daphin, daphnetin | Petruľová-Poracká et al. (2013) |
| (Chamomille) | ||
| Fabaceae/Leguminosae | ||
| Dipteryx odorata (Aubl.) Forsyth f | Esculin, esculetin | Oliveros-Bastidas et al. (2013) |
| (Tonka Bean) | ||
| Glycyrriza glabra (L.) (Liquorice) | Glycycoumarin, isoglycycoumarin, licopyranocoumarin, isotrifoliol, glycyrol, glycyrurol, licoarylcoumarin, glycyrin | Zang, (2020) |
| Trigonella foenum-graecum (L.) (Fenugreek) | Hymecromone, trigocoumarin, trigoforin, scopoletin | Dini and Laneri, (2021) |
| Moraceae | ||
| Oleae europaea (L.) | Esculetin, scopoletin, esculin | Hashmi et al., 2015; European Medicines Agency, (2017) |
| (Olive) | ||
| Ficus carica (L.) | Umbelliferone, psoralen, bergapten | Ammar et al. (2015) |
| (Fig) | ||
| Araliaceae | ||
| Eleutherococcus senticosus (Rupr. & Maxim.) Maxim | Isofraxidin | Guo et al. (2019) |
| (Siberian Ginseng) | ||
| Lamiaceae/Labiadae | ||
| Ocimum basilicum (L.) | Esculetin, esculin, coumarin, ocimarin | Zahran et al. (2020) |
| (Basil) | ||
| Lauraceae | ||
| Cinnamomum sp. (Cinnamon) | Coumarin, scopoletin | Wang et al., 2013; Ananthakrishnan et al., 2018 |
| C. zeylanicum has a low content compared with other species | ||
| Persea americana Mill. (Avocado) | Scopoletin | Bhuyan et al. (2019) |
| Tiliaceae | ||
| Tilia cordata Mill. (Linden) | Scopoletin | Arcos et al. (2006) |
| Urticaceae | ||
| Urtica dioica (L.) | Umbelliferone, esculetin, scopoletin | Esposito et al., 2019; Repajić et al., 2021 |
| (Nettle) | ||
Safety Profile of Natural Coumarins
General Toxicity of Natural Coumarins
The general toxicity of natural coumarins was evaluated preclinically in several acute, subacute, subchronic or chronic tests. For coumarin, the acute oral LD50 in mice was found to be 196–780 mg/kg bw with signs of liver toxicity (Lake, 1999). In rats, acute LD50 values were 290–680 mg/kg bw after oral administration, while in guinea pigs the acute oral LD50 was 202 mg/bw (IARC, 2000). In a subchronic study, B6C3F1 mice were treated orally with 19–300 mg/kg bw coumarin for 13 weeks. Although no clinical signs of toxicity were observed, a reduction of the mean body weight gain and a centrolobular hepatocellular hypertrophy were noted for the highest dose. In Sprague-Dawley rats treated orally with 50–500 mg/kg bw coumarin for 13 weeks, several signs of liver toxicity were observed (Lake and Grasso, 1996). In a chronic study on CD-1 mice treated orally for 2 years with a diet containing 300–3,000 ppm coumarin, no signs of clinical toxicity were observed, with NOAEL of 3,000 ppm or 280 mg/kg bw/day for male mice. However, Sprague-Dawley rats administered 333–5,000 ppm coumarin for 2 years showed signs of anemia and increase of alkaline phosphatase and glutamic-pyruvic transaminase (Carlton et al., 1996).
Osthole (7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one) was another natural coumarin tested for acute or subchronic toxicity. The acute intraperitoneal LD50 in mice was 710 mg/kg bw, the clinical signs of toxicity being hyperventilation, tremor, and photophobia. In a subchronic study, osthole was administered to Wistar rats in doses of 5–50 mg/kg bw for 45 days, by oral route. The results showed pulmonary hemorrhage and mild inflammatory processes in kidneys and liver of the animals treated with higher doses of osthole (Shokoohinia et al., 2017).
The acute toxicity of esculetin (6,7-dihydroxycoumarin) was evaluated in mouse after oral and intraperitoneal administration. The results showed a low acute toxicity with an oral LD50 of over 2000 mg/kg bw, the intraperitoneal LD50 being 1,450 mg/kg bw (Tubaro et al., 1988). The subchronic or chronic toxicity of esculetin was not assessed.
Auraptene, a coumarin from Citrus species was tested for acute oral toxicity in rats in doses of 125–2000 mg/kg bw, not causing mortality or clinical signs of toxicity. In a subacute test, auraptene was administered to rats orally in doses of 125–250 mg/kg bw for 28 days, with no observed hematological, histopathological, or biochemical modifications (Vakili et al., 2017). The most significant aspects concerning toxicity of natural coumarins are listed in Table 2.
TABLE 2.
Toxicological aspects concerning natural coumarins.
| Type of toxicity | Type of study (preclinical model/clinical test/case report) | Findings | References |
|---|---|---|---|
| General toxicity | Acute toxicity of coumarin in mice | Oral LD50 of 196–780 mg/kg with signs of liver toxicity | Lake, (1999) |
| Subchronic toxicity of coumarin in Sprague-Dawley rats | Signs of liver toxicity after 13 weeks of oral administration of doses over 50 mg/kg | Lake and Grasso, (1996) | |
| Acute toxicity of osthole in mice | Intraperitoneal LD50 of 710 mg/kg | Shokoohinia et al. (2017) | |
| Acute toxicity of esculetin in mice | Low toxicity with oral LD50 over 2000 mg/kg | Tubaro et al. (1988) | |
| Acute toxicity of auraptene in rats | No mortality or signs of toxicity after oral administration of 125–2000 mg/kg | Vakili et al. (2017) | |
| Hepatotoxicity | Subchronic toxicity of coumarin in rats | Vacuolar degeneration and necrosis of hepatocytes after oral administration of 0.75% coumarin | Lake and Grasso, (1996) |
| Acute hepatotoxicity of psoralen in rats and mice | Cholestatic liver injuries in rats only after oral administration of 80 mg/kg | Wang et al. (2019) | |
| Randomized control trial of coumarin in lymphedema | Under 1% incidence of hepatotoxicity in patients after oral administration of 400 mg coumarin for 14 months | Casley-Smith and Casley-Smith, (1995) | |
| Case report of cinnamon supplements toxicity | Hepatotoxicity with abdominal pain and liver enzymes elevation in a 73-year-old patient | Brancheau et al. (2015) | |
| Dermatological toxicity | Case reports of fig toxicity | Photoallergic reaction to furanocoumarins confirmed by histopathological test | Bonamonte et al. (2010) |
| Case reports of cinnamon flavored products toxicity | Contact stomatitis cause by cinnamon flavored chewing-gum | Calapai et al. (2014) | |
| Reproductive and developmental toxicity | In vitro test of coumarin on zebrafish embryos | No modifications caused by coumarin in the developing larvae or their internal structures | Aspatwar et al. (2020) |
| In vivo test of coumarin on Wistar rats | No effect of coumarin on parental fertility or development of rat pups | Api et al. (2019) |
Hepatotoxicity of Coumarins
Previous studies have shown that coumarin and especially its 3,4-epoxide intermediate can cause vacuolar degeneration, necrosis, and apoptosis of hepatocytes in the liver of rats fed with a 0.75% coumarin diet for 4 weeks. The histopathological modifications were accompanied by significant increases in serum bilirubin and alanine aminotransferase activity (Lake and Grasso, 1996).
A recent study investigated in depth the hepatotoxic potential of psoralen, a furanocoumarin from Fructus Psoraleae (the seed of Cullen corylifolium (L.) Medik [Fabaceae]) in rats and mice. The oral administration of psoralen in doses of 80 mg/kg bw in rats and 320 mg/kg in mice produced cholestatic liver injuries in rats but not in mice. In rat liver, psoralen decreased the expression of BSEP and MRP2, suggesting an inhibition of bile acid excretion and also reduced the expression of SULT2A1, an enzyme involved in the clearance of bile acids from the organism (Wang et al., 2019).
In humans, unlike in rats, the major metabolic pathway of coumarins is 7-hydroxylation, catalyzed by CYP2A6 enzyme which leads to the formation of 7-hydroxycoumarin, excreted by urine as conjugates with glucuronic acid or sulphate anion. However, in humans with genetic polymorphism of CYP2A6, with the apparition of the inactivating CYP2A6*2 allele, the 7-hydroxylation is deficient, leading to the accumulation of toxic 3,4-coumarin epoxide. Although the genetic polymorphism of the mentioned isoform of cytochrome P450 system is more frequent in Asians, affecting 20% of the population (Mizutani, 2003), its precise correlations with known cases of coumarin-induced liver toxicity were not thoroughly investigated.
In the clinical trials investigating possible beneficial effects of coumarin in lymphedema, data concerning possible hepatotoxic effects were often contradictory. Initially, in one clinical trial, two cases of hepatotoxicity were observed in 1,106 patients taking 400 mg coumarin daily for 14 months), suggesting a low incidence (below 1%) of this adverse effect (Casley-Smith and Casley-Smith, 1995). However, another smaller scale clinical trial highlighted an incidence rate of 9% of hepatotoxicity induced by coumarin use also for lymphedema treatment (Loprinzi et al., 1997). The large variations of the incidence of coumarin-induced hepatotoxicity observed in clinical trials are not fully understood, but they can be partially explained by differences in study designs and protocols for quantifying and interpreting the side effects.
Only a few reports signaled the apparition of hepatotoxicity in patients taking coumarin rich foods or dietary supplements. A case report study showed that cinnamon supplements used for a week by a 73-year-old patient caused a hepatitis-like syndrome with abdominal pain and liver enzymes elevation, the causality relation between the adverse effect and the ingested drug/supplement being confirmed by Naranjo algorithm (Brancheau et al., 2015). However, other molecules present in the chemical composition of cinnamon could also contribute to the hepatotoxic effect.
Anticoagulant Effect and Risk of Hemorrhage
The anticoagulant effect of natural coumarins was firstly noticed in the first decades of the 20th century when cattle feeding on molded sweet clover (Melilotus spp.) died of severe hemorrhage. An investigation found that in sweet clover infected with specific fungi (Aspergillus spp., Penicilium spp.), the naturally present coumarin was converted by the fungi into 4-hydroxycoumarin which can spontaneously form dicoumarol, a potent anticoagulant which inhibits hepatic synthesis of several coagulation factors, acting as a vitamin K antagonist (Yarnell and Abascal, 2009).
However, the presence of dicoumarol in plants is relatively rare apart from molded sweet clover, being cited only in sweet vernal grass (Anthoxantum odoratum L [Poaceae]) (Polya, 2003), therefore the risk of an accidental anticoagulant effect with hemorrhage after ingesting dietary plants rich in coumarins is probably rather low. The structural characteristics (hydroxy groups) that enable dicoumarol to effectively block vitamin K epoxide reductase (VKOR) are not present in the molecule of coumarin. Furthermore, a small scale clinical study with coumarin administered orally to patients with chronic venous insufficiency in doses of 90 mg/day for 6 weeks, failed to demonstrate any effect of coumarin on coagulation parameters (Köstering et al., 1985). Even though other natural coumarins could present anticoagulant effects, there are no sufficient studies to reach a clear conclusion.
Dermatological Toxicity
Among natural coumarins, several compounds like psoralen, bergapten and xanthotoxin, all belonging to furanocoumarin class, present in large concentrations in celery or limes, caused a limited number of skin phototoxic reactions in humans (Wagstaff, 1991). Thus, several cases of photoallergic reactions were also demonstrated in patients exposed to furanocoumarins from fig, the adverse reaction being confirmed by histopathological examination of patch tests (Bonamonte et al., 2010). However, a typical furanocoumarin intake from food sources is several times below the lowest dose capable of producing phototoxic effects, but the risk of exposure increases in case of inappropriate storage or processing of foods (Guth et al., 2011).
Additionally, a case of severe exacerbation of rosacea induced by cinnamon dietary supplements was reported in a 68-year-old patient but the adverse effect could not be attributed to a specific coumarin present in the chemical composition of the supplement administered with the purpose of lowering glycemia (Campbell et al., 2008). Also, several case reports signaled the development of contact dermatitis caused by cinnamon-flavored toothpaste, chewing gum and mouthwash (Calapai et al., 2014).
Moreover, in case of furanocoumarins, the capacity to form interstrand crosslinks with DNA and to alter DNA transcription may favor the development of skin melanoma, but further research is necessary to ascertain the validity of this hypothesis (Melough and Chun, 2018).
Reproductive and Developmental Toxicity
Several in vitro and in vivo models evaluated the reproductive and developmental toxicity of coumarins. A study on zebrafish (Danio rerio) embryos showed that coumarin caused malformations of head and tail of zebrafish embryos but the calculated LC50 was 855 μM, suggesting that in humans, under normal therapeutic conditions, teratogenicity could be rather low for coumarin, unlike warfarin (Weigt et al., 2012). Moreover, a recent study investigated the effects of a series of coumarin derivatives on zebrafish embryos, finding no modifications in the developing larvae and no apparent damage to their internal structures (Aspatwar et al., 2020). Additionally, an in vivo study showed that oral administration of high doses of coumarin to male and female Wistar rats, prior and during mating phase, produced no adverse effects concerning parental fertility or the development of rat pups (Api et al., 2019). In humans, no data concerning reproductive and developmental toxicity of natural coumarins have been published so far.
Drug Interactions
Coumarin derivatives are a class of chemically diverse compounds, capable of interfering with the metabolism or the effects of other drugs. Thus, several constituents from grapefruit juice like bergapten, a furanocoumarin derivative, are inhibitors of CYP3A4 liver microsomal enzyme, capable of reducing the metabolism of several associated drugs like calcium channel blockers (nitrendipine) or statins (simvastatin) with the augmentation of their adverse effects. Nevertheless, other chemical compounds like naringenin, a flavanone also present in the chemical composition of grapefruit juice are additionally responsible for this pharmacokinetic interaction (EMA, 2012).
Moreover, a recent study evaluated in vivo in rats and rabbits with alloxan-induced diabetes, the pharmacokinetic and pharmacodynamic interactions of cinnamon bark powder and pioglitazone, an oral antidiabetic drug from the class of thiazolidinediones. The results showed that cinnamon was able to inhibit CYP3A4 enzyme activity, increasing AUC of pioglitazone which is metabolized by the same isoform. Additionally, the antidiabetic effect of cinnamon, demonstrated in several studies, could increase the hypoglycemic effect of pioglitazone, an adjustment of the dose being recommended in human patients (Mamindla et al., 2017).
Another individual coumarin molecule, osthole was tested in vitro on rat and human liver microsomes regarding the effects on CYP2C11/CYP2C9 enzymes. The results showed a potent CYP2C9 inhibition in human liver microsomes with Ki values between 13.12 and 21.93 µM, also proving an influence of genotype on the pharmacokinetics of osthole. Thus, the presence of CYP2C9*3 allele caused the strongest enzymatic inhibitory activity of osthole in this experimental model (He et al., 2020).
Regulatory Aspects
A variety of foods and herbs used as nutraceuticals may have a high concentration of natural coumarins, therefore regulatory authorities around the world took legislative actions in order to avoid possible toxicities in the general public.
Initially, in the European Union, a limit of 2 mg/kg coumarin for foods prepared with natural spices and herbs was imposed. Several years later, the European Food Safety Authority (EFSA) recommended a maximum level of 0.5 mg/kg in foods. Based on various animal data, extrapolated to humans, a tolerable daily intake (TDI) of 0.1 mg/kg bw coumarin was calculated (EFSA, 2004). Nowadays, in the European Union, the presence of coumarins in food is regulated by the Decision No 1334/2008 of the European Parliament and Council which states that coumarin cannot be added to food as an additive. However, the Annex III of the document, stipulates that coumarin may be allowed in specific foods prepared with cinnamon as a flavor but with maximum admitted levels (e.g., 50 mg/kg for traditional bakery products and 5 mg/kg for desserts) (European Commission, 2008).
In the USA, coumarin was used as a food flavor until 1954, when its addition to food was banned by the FDA on suspicion of hepatotoxicity (Abraham et al., 2010). Therefore, any food with added coumarin is considered to be “adulterated under the act” being strictly prohibited in the US (Food and Drug Administration, 1999).
In Australia, coumarin itself was authorized for the treatment of lymphedema in 1993, but in 1996 the Australian regulatory authorities suspended the drug due to the apparition of ten cases of hepatotoxicity with two fatalities. Currently, due to the extensive use of coumarin as an ingredient in cosmetic products (sunscreens), the Australian regulatory authorities imposed a limit of maximum 0.001% coumarin in topical cosmetics, which is considered safe, with a maximum estimated exposure of below 0.02 mg/kg (Therapeutic Goods Administration, 2019).
The regulatory aspects concerning the safe use of coumarins in foods or cosmetics are quite variable worldwide. Moreover, some important aspects have not been regulated at all, like the maximum admitted level of coumarin in cinnamon itself. As a consequence, some foods, and spices with a high content of coumarin which can lead to potential toxicity are still used nowadays. The best example is the cheaper cassia cinnamon (Cinnamomum aromaticum Nees [Lauraceae]) which often replaces true cinnamon (Cinnamomum verum J. Presl [Lauraceae]) as flavor used in bakery products, generating coumarin levels over 50 mg/kg, well above the upper limits set by the regulatory authorities (Yarnell and Abascal, 2009). Further studies aimed at a better understanding of the bioavailability of natural coumarins from foods and cosmetics, but also harmonization measures at international level regarding regulatory aspects are needed, for a safer use of these compounds.
Conclusion
Natural coumarins present in a variety of foods and herbs are a class of chemically diverse compounds with important biological effects, useful for health promotion and the prevention of various diseases. The most important adverse effects of coumarins are represented by hepatotoxicity favored by the ingestion of large doses and possible genetic polymorphism of CYP2A6 and dermatological phototoxic reactions. A better understanding of the safety profile of coumarins present in nutraceuticals is necessary for a safer use of these valuable natural compounds.
Author Contributions
Conceptualization and methodology: SH, OV and LF Validation and formal analysis: CM, DM and CI.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abraham K., Wöhrlin F., Lindtner O., Heinemeyer G., Lampen A. (2010). Toxicology and Risk Assessment of Coumarin: Focus on Human Data. Mol. Nutr. Food Res. 54 (2), 228–239. 10.1002/MNFR.200900281 [DOI] [PubMed] [Google Scholar]
- Aisa H. A., Xin X.-l., Tang D. (2020). Chemical Constituents and Their Pharmacological Activities of Plants from Cichorium Genus. Chin. Herbal Medicines 12 (3), 224–236. 10.1016/J.CHMED.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkol E. K., Genç Y., Karpuz B., Sobarzo-Sánchez E., Capasso R. (2020). Coumarins and Coumarin-Related Compounds in Pharmacotherapy of Cancer. Cancers 12 (7), 1959–2025. 10.3390/cancers12071959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammar S., Contreras M. d. M., Belguith-Hadrich O., Bouaziz M., Segura-Carretero A. (2015). New Insights into the Qualitative Phenolic Profile of Ficus Carica L. Fruits and Leaves from Tunisia Using Ultra-high-performance Liquid Chromatography Coupled to Quadrupole-Time-Of-Flight Mass Spectrometry and Their Antioxidant Activity. RSC Adv. 5 (26), 20035–20050. 10.1039/C4RA16746E [DOI] [Google Scholar]
- Ananthakrishnan R., Chandra P., Kumar B., Rameshkumar K. B. (2018). Quantification of Coumarin and Related Phenolics in Cinnamon Samples from South India Using UHPLC-ESI-QqQLIT-MS/MS Method. Int. J. Food Properties 21 (1), 50–57. 10.1080/10942912.2018.1437629 [DOI] [Google Scholar]
- Annunziata F., Pinna C., Dallavalle S., Tamborini L., Pinto A. (20202020). An Overview of Coumarin as a Versatile and Readily Accessible Scaffold with Broad-Ranging Biological Activities. Int. J. Mol. Sci. 21 (13), 4618. 10.3390/ijms21134618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Api A. M., Belmonte F., Belsito D., Biserta S., Botelho D., Bruze M., et al. (2019). RIFM Fragrance Ingredient Safety Assessment, Coumarin, CAS Registry Number 91-64-5. Food Chem. Toxicol. 130 (Suppl. 1), 110522. 10.1016/j.fct.2019.05.030 [DOI] [PubMed] [Google Scholar]
- Arcos M. L. B., Cremaschi G., Werner S., Coussio J., Ferraro G., Anesini C. (2006). Tilia Cordata Mill. Extracts and Scopoletin (Isolated Compound): Differential Cell Growth Effects on Lymphocytes. Phytother Res. 20 (1), 34–40. 10.1002/PTR.1798 [DOI] [PubMed] [Google Scholar]
- Arsenov D., Župunski M., Pajević S., Nemeš I., Simin N., Alnuqaydan A. M., et al. (2021). Roots of Apium graveolens and Petroselinum Crispum-Insight into Phenolic Status against Toxicity Level of Trace Elements. Plants 10 (9), 1785. 10.3390/PLANTS10091785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspatwar A., Berrino E., Bua S., Carta F., Capasso C., Parkkila S., et al. (2020). Toxicity Evaluation of Sulfamides and Coumarins that Efficiently Inhibit Human Carbonic Anhydrases. J. Enzyme Inhib. Med. Chem. 35 (1), 1765–1772. 10.1080/14756366.2020.1822829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avula B., Chittiboyina A. G., Wang Y. H., Sagi S., Raman V., Wang M., et al. (2016). Simultaneous Determination of Aegeline and Six Coumarins from Different Parts of the Plant Aegle Marmelos Using UHPLC-PDA-MS and Chiral Separation of Aegeline Enantiomers Using HPLC-ToF-MS. Planta Med. 82 (06), 580–588. 10.1055/S-0042-103160 [DOI] [PubMed] [Google Scholar]
- Bansal Y., Sethi P., Bansal G. (2013). Coumarin: a Potential Nucleus for Anti-inflammatory Molecules. Med. Chem. Res. 22 (7), 3049–3060. 10.1007/S00044-012-0321-6 [DOI] [Google Scholar]
- Bhuyan D. J., Alsherbiny M. A., Perera S., Low M., Basu A., Devi O. A., et al. (2019). The Odyssey of Bioactive Compounds in Avocado (Persea Americana) and Their Health Benefits. Antioxidants (Basel) 8 (10), 426. 10.3390/ANTIOX8100426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonamonte D., Foti C., Lionetti N., Rigano L., Angelini G. (2010). Photoallergic Contact Dermatitis to 8-methoxypsoralen in Ficus Carica. Contact Dermatitis 62 (6), 343–348. 10.1111/J.1600-0536.2010.01713.X [DOI] [PubMed] [Google Scholar]
- Brancheau D., Patel B., Zughaib M. (2015). Do Cinnamon Supplements Cause Acute Hepatitis? Am. J. Case Rep. 16, 250–254. 10.12659/AJCR.892804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calapai G., Miroddi M., Mannucci C., Minciullo P., Gangemi S. (2014). Oral Adverse Reactions Due to Cinnamon-Flavoured Chewing Gums Consumption. Oral Dis. 20 (7), 637–643. 10.1111/odi.12170 [DOI] [PubMed] [Google Scholar]
- Campbell T. M., Neems R., Moore J. (2008). Severe Exacerbation of Rosacea Induced by Cinnamon Supplements. J. Drugs Dermatol. 7 (6), 586–587. 10.1016/b978-84-8086-334-6.50203-0 [DOI] [PubMed] [Google Scholar]
- Carlton B. D., Aubrun J. C., Simon G. S. (1996). Effects of Coumarin Following Perinatal and Chronic Exposure in Sprague-Dawley Rats and CD-1 Mice. Fundam. Appl. Toxicol. 30 (1), 145–151. 10.1006/FAAT.1996.0051 [DOI] [PubMed] [Google Scholar]
- Casley-Smith J. R., Casley-Smith J. R. (1995). Frequency of Coumarin Hepatotoxicity. Med. J. Aust. 162 (7), 391. 10.5694/J.1326-5377.1995.TB139958.X [DOI] [PubMed] [Google Scholar]
- Dini I., Laneri S. (2021). Spices, Condiments, Extra Virgin Olive Oil and Aromas as Not Only Flavorings, but Precious Allies for Our Wellbeing. Antioxidants (Basel) 10 (6), 868. 10.3390/ANTIOX10060868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugrand A., Olry A., Duval T., Hehn A., Froelicher Y., Bourgaud F. (2013). Coumarin and Furanocoumarin Quantitation in Citrus Peel via Ultraperformance Liquid Chromatography Coupled with Mass Spectrometry (UPLC-MS). J. Agric. Food Chem. 61 (45), 10677–10684. 10.1021/JF402763T [DOI] [PubMed] [Google Scholar]
- Dugrand-Judek A., Olry A., Hehn A., Costantino G., Ollitrault P., Froelicher Y., et al. (2015). The Distribution of Coumarins and Furanocoumarins in Citrus Species Closely Matches Citrus Phylogeny and Reflects the Organization of Biosynthetic Pathways. PLoS ONE 10 (11), e0142757. 10.1371/JOURNAL.PONE.0142757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (2004). Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) Related to Coumarin. EFSA J. 2 (12), 104. 10.2903/J.EFSA.2004.104 [DOI] [Google Scholar]
- EMA (2012). Assessment report on Citrus bergamia Risso et Poiteau, aetheroleum. Available at: www.ema.europa.eu .
- Esposito S., Bianco A., Russo R., Di Maro A., Isernia C., Isernia P. V. (20192019). Therapeutic Perspectives of Molecules from Urtica Dioica Extracts for Cancer Treatment. Molecules 24 (15), 2753. 10.3390/MOLECULES24152753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission (2008). European Parliament and Council Directive No. 1334/2008 on the Flavourings and Certain Food Ingredients with Flavouring Properties for Use in and on Food and Amending Council Regulation (EEC) No. 1601/91, Regulations (EC) No. 2232/96 and (EC) No. 110/2008 and Directive 2000/13/EC. Official J. Eur. Community L354, 34–50. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:354:0034:0050:en:PDF. [Google Scholar]
- European Medicines Agency (2011). Chamomillae Romanae Flos. Available at: https://www.ema.europa.eu/en/medicines/herbal/chamomillae-romanae-flos .
- European Medicines Agency (2017). Olea Europaea. Available at: https://www.ema.europa.eu/en/medicines/herbal/oleae-folium .
- Food and Drug Administration (1999). “Food and Drugs.” in US Code Federal Regulations. Title 21, Part 189, Subpart 189.130, 549. [Google Scholar]
- Forycka A., Buchwald W. (2019). Variability of Composition of Essential Oil and Coumarin Compounds of Angelica Archangelica L. Herba Pol. 65 (4), 62–75. 10.2478/HEPO-2019-0027 [DOI] [Google Scholar]
- Garg S. K., Gupta S. R., Sharma N. D. (1979). Coumarins from Apium graveolens Seeds. Phytochemistry 18 (9), 1580–1581. 10.1016/S0031-9422(00)98508-X [DOI] [Google Scholar]
- Gliszczyńska A., Brodelius P. E. (2011). Sesquiterpene Coumarins. Phytochem. Rev. 11, 77–96. 10.1007/S11101-011-9220-6 [DOI] [Google Scholar]
- Guo S., Wei H., Li J., Fan R., Xu M., Chen X., et al. (2019). Geographical Distribution and Environmental Correlates of Eleutherosides and Isofraxidin in Eleutherococcus Senticosus from Natural Populations in Forests at Northeast China. Forests 10 (10), 872. 10.3390/F10100872 [DOI] [Google Scholar]
- Guth S., Habermeyer M., Schrenk D., Eisenbrand G. (2011). Update of the Toxicological Assessment of Furanocoumarins in Foodstuffs (Update of the SKLM Statement of 23/24 September 2004)--Opinion of the Senate Commission on Food Safety (SKLM) of the German Research Foundation (DFG). Mol. Nutr. Food Res. 55 (5), 807–810. 10.1002/MNFR.201100011 [DOI] [PubMed] [Google Scholar]
- Hashmi M. A., Khan A., Hanif M., Farooq U., Perveen S. (2015). Traditional Uses, Phytochemistry, and Pharmacology ofOlea europaea(Olive). Evidence-Based Complement. Altern. Med. 2015, 1–29. 10.1155/2015/541591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Zhang Y., Zhao D., Jiang J., Xie B., Ma L., et al. (2020). Osthole Inhibited the Activity of CYP2C9 in Human Liver Microsomes and Influenced Indomethacin Pharmacokinetics in Rats. Xenobiotica 50 (8), 939–946. 10.1080/00498254.2020.1734882 [DOI] [PubMed] [Google Scholar]
- Hroboňová K., Lehotay J., Čižmárik J., Sádecká J. (2013). Comparison HPLC and Fluorescence Spectrometry Methods for Determination of Coumarin Derivatives in Propolis. J. Liquid Chromatogr. Relat. Tech. 36 (4), 486–503. 10.1080/10826076.2012.660724 [DOI] [Google Scholar]
- IARC (2000). “Coumarin,” in International Agency for Research on Cancer. Monographs on the Evaluation of Carcinogenic Risks to Humans. Editor World Health Organisation (Lyon, France: IARC; ), 77, 193–227. [Google Scholar]
- Kaur G. J., Arora D. S. (2010). Bioactive Potential of Anethum Graveolens, Foeniculum Vulgare and Trachyspermum Ammi Belonging to the Family Umbelliferae - Current Status. J. Med. Plants Res. 4 (2), 087–094. 10.5897/JMPR09.018 [DOI] [Google Scholar]
- Kenari H. M., Kordafshari G., Moghimi M., Eghbalian F., TaherKhani D. (2021). Review of Pharmacological Properties and Chemical Constituents of Pastinaca Sativa. J. Pharmacopuncture 24 (1), 14–23. 10.3831/KPI.2021.24.1.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köstering H., Bandura B., Merten H. A., Wieding J. U. (1985). The Behavior of Blood Clotting and its Inhibitors under Long Term Treatment with 5,6-Benzo-Alpha-Pyrone (Coumarin). Double Blind Study. Arzneimittelforschung 35 (8), 1303–1306. [PubMed] [Google Scholar]
- Kovač-Bešović E. E., Durić K. (2003). Thin Layer Chromatography-Application in Qualitative Analysis on Presence of Coumarins and Flavonoids in Plant Material. Bosn J. Basic Med. Sci. 3 (3), 19–26. 10.17305/bjbms.2003.3523 [DOI] [PubMed] [Google Scholar]
- Kriplani P., Guarve K., Baghael U. S. (2017). Arnica montana L. - a Plant of Healing: Review. J. Pharm. Pharmacol. 69 (8), 925–945. 10.1111/JPHP.12724 [DOI] [PubMed] [Google Scholar]
- Kumar D., Bhat Z. A., Kumar V., Shah M. Y. (2013). Coumarins from Angelica Archangelica Linn. And Their Effects on Anxiety-like Behavior. Prog. Neuropsychopharmacol. Biol. Psychiatry 40 (1), 180–186. 10.1016/J.PNPBP.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Lake B. G. (1999). Coumarin Metabolism, Toxicity and Carcinogenicity: Relevance for Human Risk Assessment. Food Chem. Toxicol. 37 (4), 423–453. 10.1016/S0278-6915(99)00010-1 [DOI] [PubMed] [Google Scholar]
- Lake B. G., Grasso P. (1996). Comparison of the Hepatotoxicity of Coumarin in the Rat, Mouse, and Syrian Hamster: a Dose and Time Response Study. Fundam. Appl. Toxicol. 34 (1), 105–117. 10.1006/FAAT.1996.0181 [DOI] [PubMed] [Google Scholar]
- Lončar M., Jakovljević M., Šubarić D., Pavlić M., Buzjak Služek V., Cindrić I., et al. (2020). Coumarins in Food and Methods of Their Determination. Foods 9 (5), 645. 10.3390/foods9050645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loprinzi C. L., Sloan J., Kugler J. (1997). Coumarin-induced Hepatotoxicity. J. Clin. Oncol. 15 (9), 3167–3168. 10.1200/JCO.1997.15.9.3167 [DOI] [PubMed] [Google Scholar]
- Mamindla S., Koganti V. S. R. G. P., Ravouru N., Koganti B. (2017). Effect of Cinnamomum cassia on the Pharmacokinetics and Pharmacodynamics of Pioglitazone. Curr. Clin. Pharmacol. 12 (1), 41–49. 10.2174/1574884712666170207152020 [DOI] [PubMed] [Google Scholar]
- Manderfeld M. M., Schafer H. W., Davidson P. M., Zottola E. A. (1997). Isolation and Identification of Antimicrobial Furocoumarins from Parsley. J. Food Prot. 60 (1), 72–77. 10.4315/0362-028X-60.1.72 [DOI] [PubMed] [Google Scholar]
- Melough M. M., Chun O. K. (2018). Dietary Furocoumarins and Skin Cancer: A Review of Current Biological Evidence. Food Chem. Toxicol. 122, 163–171. 10.1016/J.FCT.2018.10.027 [DOI] [PubMed] [Google Scholar]
- Mizutani T. (2003). PM Frequencies of Major CYPs in Asians and Caucasians. Drug Metab. Rev. 35 (2–3), 99–106. 10.1081/DMR-120023681 [DOI] [PubMed] [Google Scholar]
- Nahar L., Xiao J., Sarker S. D. (2021). “Introduction of Phytonutrients,” in Handbook of Dietary Phytochemicals. Editors Xiao J., Sarker S. D., Asakawa Y. (Singapore: Springer; ), 1–17. 10.1007/978-981-15-4148-3_2 [DOI] [Google Scholar]
- Najda A., Dyduch J., Świca K., Kapłan M., Papliński R., Sachadyn-Król M., et al. (2015). Identification and Profile of Furanocoumarins from the Ribbed Celery (Apium Graveolens L Var. Dulce Mill./Pers.). Fstr 21 (1), 67–75. 10.3136/FSTR.21.67 [DOI] [Google Scholar]
- Oliveros-Bastidas A. d. J., Demuner A. J., Barbosa L. C. d. A. (2013). Chemical Characterization by GC-MS and Phytotoxic Potential of Non-polar and Polar Fractions of Seeds of Dioteryx Odorata (Aubl.) Willd. From Venezuelan Regions. Quím. Nova 36 (4), 502–506. 10.1590/S0100-40422013000400003 [DOI] [Google Scholar]
- Önder A. (2020). Anticancer Activity of Natural Coumarins for Biological Targets. Stud. Nat. Prod. Chem. 64, 85–109. 10.1016/B978-0-12-817903-1.00003-6 [DOI] [Google Scholar]
- Ozçelik B., Kusmenoglu Ş., Turkoz S., Abbasoglu U. (2004). Antimicrobial Activities of Plants from the Apicaceae. Pharm. Biol. 42 (7), 526–528. 10.3109/13880200490893311 [DOI] [Google Scholar]
- Petruľová-Poracká V., Repčák M., Vilková M., Imrich J. (2013). Coumarins of Matricaria Chamomilla L.: Aglycones and Glycosides. Food Chem. 141 (1), 54–59. 10.1016/J.FOODCHEM.2013.03.004 [DOI] [PubMed] [Google Scholar]
- Polya G. M. (2003). Protein and Non-protein Protease Inhibitors from Plants. Stud. Nat. Prod. Chem. 29, 567–641. 10.1016/S1572-5995(03)80015-7 [DOI] [Google Scholar]
- Rebey I. B., Zakhama N., Karoui I. J., Marzouk B. (2012). Polyphenol Composition and Antioxidant Activity of Cumin (Cuminum Cyminum L.) Seed Extract Under Drought. J. Food Sci. 77 (6), C734–C739. 10.1111/J.1750-3841.2012.02731.X [DOI] [PubMed] [Google Scholar]
- Repajić M., Cegledi E., Zorić Z., Pedisić S., Elez Garofulić I., Radman S., et al. (2021). Bioactive Compounds in Wild Nettle (Urtica Dioica L.) Leaves and Stalks: Polyphenols and Pigments upon Seasonal and Habitat Variations. Foods 10 (1), 190. 10.3390/FOODS10010190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker S. D., Nahar L. (2017). “Progress in the Chemistry of Naturally Occurring Coumarins,” in Progress in the Chemistry of Organic Natural Products. Editors Kinghorn A., Falk H., Gibbons S., Kobayashi J. (Cham: Springer; ), 106, 241–304. 10.1007/978-3-319-59542-9_3 [DOI] [PubMed] [Google Scholar]
- Sharifi-Rad J., Cruz-Martins N., López-Jornet P., Lopez E. P.-F., Harun N., Yeskaliyeva B., et al. (2021). Natural Coumarins: Exploring the Pharmacological Complexity and Underlying Molecular Mechanisms. Oxidative Med. Cell Longevity 2021, 1–19. 10.1155/2021/6492346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokoohinia Y., Bazargan S., Miraghaee S., Javadirad E., Farahani F., Hosseinzadeh L. (2017). Safety Assessment of Osthole Isolated from Prangos Ferulacea: Acute and Subchronic Toxicities and Modulation of Cytochrome P450. Jundishapur J. Nat. Pharm. Prod. 12 (3), 12. 10.5812/JJNPP.63764 [DOI] [Google Scholar]
- Srikrishna D., Godugu C., Dubey P. K. (2018). A Review on Pharmacological Properties of Coumarins. Mini Rev. Med. Chem. 18 (2), 113–141. 10.2174/1389557516666160801094919 [DOI] [PubMed] [Google Scholar]
- Stassen M. J. J., Hsu S. H., Pieterse C. M. J., Stringlis I. A. (2021). Coumarin Communication along the Microbiome-Root-Shoot Axis. Trends Plant Sci. 26 (2), 169–183. 10.1016/J.TPLANTS.2020.09.008 [DOI] [PubMed] [Google Scholar]
- Stringlis I. A., de Jonge R., Pieterse C. M. J. (2019). The Age of Coumarins in Plant-Microbe Interactions. Plant Cel Physiol 60 (7), 1405–1419. 10.1093/PCP/PCZ076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., Shahrajabian M. H., Cheng Q. (2019). Anise (Pimpinella Anisum L.), a Dominant Spice and Traditional Medicinal Herb for Both Food and Medicinal Purposes. Cogent Biol. 5 (1), 1673688. 10.1080/23312025.2019.1673688 [DOI] [Google Scholar]
- Therapeutic Goods Administration (2019). Australian Government, Department of Health, Therapeutic Goods Administration. Coumarin for Use in Topical Listed Medicines. Available at: https://www.tga.gov.au/sites/default/files/safety-review-coumarin-listed-medicines.pdf .
- Tubaro A., Del Negro P., Ragazzi E., Zampiron S., Della Loggia R. (1988). Anti-inflammatory and Peripheral Analgesic Activity of Esculetin In Vivo . Pharmacol. Res. Commun. 20 (Suppl. 5), 83–85. 10.1016/S0031-6989(88)80847-6 [DOI] [PubMed] [Google Scholar]
- Vakili T., Iranshahi M., Arab H., Riahi B., Roshan N. M., Karimi G. (2017). Safety Evaluation of Auraptene in Rats in Acute and Subacute Toxicity Studies. Regul. Toxicol. Pharmacol. 91, 159–164. 10.1016/J.YRTPH.2017.10.025 [DOI] [PubMed] [Google Scholar]
- Vasudeva N., Das S., Sharma S. (2016). Cichorium Intybus: A Concise Report on its Ethnomedicinal, Botanical, and Phytopharmacological Aspects. Drug Dev. Ther. 7 (1), 1. 10.4103/2394-6555.180157 [DOI] [Google Scholar]
- Venugopala K. N., Rashmi V., Odhav B. (2013). Review on Natural Coumarin lead Compounds for Their Pharmacological Activity. Biomed. Res. Int. 2013, 963248. 10.1155/2013/963248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff D. J. (1991). Dietary Exposure to Furocoumarins. Regul. Toxicol. Pharmacol. 14 (3), 261–272. 10.1016/0273-2300(91)90029-U [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang H., Jiang J. M., Zheng D., Chen Y. Y., Wan S. J., et al. (2019). Hepatotoxicity Induced by Psoralen and Isopsoralen from Fructus Psoraleae: Wistar Rats Are More Vulnerable Than ICR Mice. Food Chem. Toxicol. 125, 133–140. 10.1016/J.FCT.2018.12.047 [DOI] [PubMed] [Google Scholar]
- Wang Y. H., Avula B., Nanayakkara N. P., Zhao J., Khan I. A. (2013). Cassia Cinnamon as a Source of Coumarin in Cinnamon-Flavored Food and Food Supplements in the United States. J. Agric. Food Chem. 61 (18), 4470–4476. 10.1021/JF4005862 [DOI] [PubMed] [Google Scholar]
- Weigt S., Huebler N., Strecker R., Braunbeck T., Broschard T. H. (2012). Developmental Effects of Coumarin and the Anticoagulant Coumarin Derivative Warfarin on Zebrafish (Danio rerio) Embryos. Reprod. Toxicol. 33 (2), 133–141. 10.1016/j.reprotox.2011.07.001 [DOI] [PubMed] [Google Scholar]
- Yang I. J., Lee D. U., Shin H. M. (2015). Anti-inflammatory and Antioxidant Effects of Coumarins Isolated from Foeniculum Vulgare in Lipopolysaccharide-Stimulated Macrophages and 12-O-Tetradecanoylphorbol-13-Acetate-Stimulated Mice. Immunopharmacol Immunotoxicol 37 (3), 308–317. 10.3109/08923973.2015.1038751 [DOI] [PubMed] [Google Scholar]
- Yarnell E., Abascal K. (2009). Plant Coumarins: Myths and Realities. Altern. Complement. Therapies 15 (1), 24–30. 10.1089/ACT.2009.15104 [DOI] [Google Scholar]
- Zahran E. M., Abdelmohsen U. R., Khalil H. E., Desoukey S. Y., Fouad M. A., Kamel M. S. (2020). Diversity, Phytochemical and Medicinal Potential of the Genus Ocimum L. (Lamiaceae). Phytochem. Rev. 19 (4), 907–953. 10.1007/S11101-020-09690-9 [DOI] [Google Scholar]
- Zang Y. (2020). Pharmacological Activities of Coumarin Compounds in Licorice: A Review. Nat. Product. Commun. 15 (9), 1934578X2095395. 10.1177/1934578X20953954 [DOI] [Google Scholar]