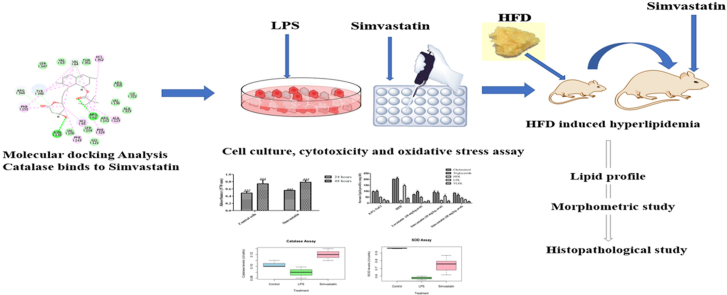
Abstract
Simvastatin is an established anti-hyperlipidemic drug and few studies have indicated its role in the mitigation of oxidative stress. However, a systematic study considering molecular binding/interaction of simvastatin with anti-oxidant enzymes followed by confirmational in vitro and in vivo studies have never been done. We investigated the molecular binding of simvastatin with multiple anti-oxidant enzymes and assessed their levels after the treatment of simvastatin in vitro and in vivo. This study is the first to show the molecular binding of simvastatin to catalase through molecular docking analysis. Moreover, the anti-oxidative properties of simvastatin have not been studied in Lipopolysaccharide (LPS) induced oxidative stress in HepG2 cells. We found that simvastatin effectively attenuated oxidative stress in LPS induced HepG2 cells and high-fat diet (HFD) fed hyperlipidemic rats by increasing the levels of antioxidant enzymes. The activity of catalase and superoxide dismutase (SOD) both increased significantly in oxidatively stressed HepG2 cells after the treatment with simvastatin (10 μM, 24 h). In addition to this, he original cell morphology of oxidatively stressed cells was restored by simvastatin, and an increase in antioxidant enzymes, catalase (0.08 U/cells to 0.12 U/cells), and SOD (0.57 U/cells to 0.74 U/cells) was also noted in HepG2 cells. Furthermore, a significant increase in the antioxidant enzymes such as Catalase, SOD, and reduced glutathione (GSH) was noted after simvastatin treatment in the HFD model. Moreover, we also observed degradation of by-products of lipid peroxidation thiobarbituric acid reactive substances (TBARs), nitric oxide (NO), and protein carbonyl levels. This indicates that simvastatin enhances anti-oxidant enzyme activities and can be repurposed for the treatment of oxidative stress in liver diseases in humans after extensive clinical trials.
Keywords: Oxidative stress, Simvastatin, Lipopolysaccharide, HepG2 cells, Anti-hyperlipidemia
Graphical abstract
Highlights
-
•
In silico, molecular docking analysis shows that simvastatin binds to the active site of the catalase enzyme.
-
•
Simvastatin attenuates LPS induced oxidative stress in HepG2 cells by increasing the amount of antioxidant enzymes catalase and SOD.
-
•
Simvastatin significantly reduces triglycerides, cholesterol, LDL, VLDL, and increases HDL level in HFD induced oxidative stress in Wistar rats.
-
•
Simvastatin can be repurposed for the treatment of oxidative stress in liver diseases.
1. Introduction
Simvastatin is a well-known statin used for lowering lipid and cholesterol levels and shows vasoprotective, immunomodulatory, and anti-inflammatory effects. Recently accumulated evidence has indicated the new role of simvastatin in the prevention and progression of liver cirrhosis, acute-onchronic liver failure (ACLF), and non-alcoholic fatty liver diseases (NAFLD) (Tripathi et al., 2018a; La Mura et al., 2013; Abraldes et al., 2007; Meireles et al., 2017). Interestingly, a new indication for simvastatin has gathered the attention of the scientific community and increased survival in patients with liver cirrhosis has been reported through double-blind randomized clinical trials showing beneficial effects of simvastatin (Sancho et al., 2013; Abraldes et al., 2009). Simvastatin facilitates reduction in liver fibrosis and prevents further liver damage by inhibiting the proliferation of hepatic stellate cells and ameliorating the phenotype of sinusoidal cells in the liver (Tripathi et al., 2018a; Tsochatzis and Bosch, 2017). In addition, it can reduce hepatic portal hypertension and hepatic inflammation, associated with high levels of oxidative stress (Rodrigues et al., 2019; Russo et al., 2012). Various compounds which are metabolized by the liver lead to the production of reactive oxygen species (ROS) that cause cellular damage and inflammation associated with liver diseases (Cichoż-Lach and Michalak, 2014). Hence, with antioxidants such as curcumin, and resveratrol, subsequent reduction in oxidative stress arebeing considered for the treatment of oxidative injury in the liver (Sakaguchi et al., 2010). The literature review provides few indications suggesting that simvastatin ameliorates oxidative stress in conditions such as hypercholesterolemia, diabetes, and heart diseases (Moutzouri et al., 2013; Zhu et al., 2005; Li et al., 2010; Abbas and Sakr, 2013; Mohamadin et al., 2011; PolanczykGoraca et al., 2012). Despite such evidences, molecular docking analysis of simvastatin with any anti-oxidant enzyme has never been reported and the underlying molecular mechanism is largely unexplored. This motivated us to investigate molecular interactions of simvastatin with anti-oxidant enzymes through computational analysis (ligand-molecular docking). Simvastatin is currently prescribed for hypercholesterolemia thus, the only known molecular docking for simvastatin is as competitive inhibition of HMG-CoA β-Hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase (Toppo et al., 2021). In this study, we have shown that simvastatin has a selective affinity towards catalase suggesting plausible interaction and participation of the drug in an anti-oxidative mechanism. There has been an upsurge in the literature suggesting that strong correlation exists between obesity, diabetes, cardiac diseases, hyperlipidemia, and chronic liver diseases. Considering the recent developments, it was necessary to investigate the role of simvastatin in hepatic cell lines. Thus, for the first time, we have explored the role of simvastatin in HepG2 cells under oxidative stress. We showed that the activity of the catalase enzyme is increased in oxidatively stressed HepG2 cells and hepatic tissues after the treatment with simvastatin. In addition, the anti-oxidative properties of simvastatin in hepatic tissue were also validated by using SOD, Catalase, and GSH activities, and reduction in lipid peroxidation was analyzed by TBARs, NO, and protein carbonyl levels in the HFD model. Interestingly, marked restoration of cellular morphology was noted in HepG2 cells after treatment with simvastatin. The histopathological findings showed that the normal structure of hepatic tissue was regained after treatment with simvastatin along with a reduction in fibrosis, sinusoidal spaces, and necrotic tissue. Further investigations are required to study the underlying molecular mechanisms and pathways involved in the activity of simvastatin as a potent anti-oxidant. Nonetheless, this study provides strong evidence that simvastatin can be used in the management of oxidative stress in liver diseases levels in metabolic diseases and liver diseases.
2. Material and method
2.1. Molecular docking
The three-dimensional structure of the catalase, superoxide dismutase, nitric oxide and reduced glutathione enzymes were obtained from the RCSB PDB database. The PDB ID's are mentioned in Table 1. The molecular docking studies were performed with the help of the LibDock program (Discovery studio 2.0). In the first step, the protein preparation protocol was used. This protocol performs tasks such as removal of water molecules, adding the missing hydrogen and deleting alternate conformations. The active site was defined for the protein structure using a sphere object radius i.e., 9.0 Åo so as to include all the essential amino acid residues of active site for interaction with ligand. Simvastatin was docked into the active site of catalase, superoxide dismutase, nitric oxide and reduced glutathione. Noticeably simvastatin docked only in the active site of catalase, hence the docking score and potential interactions taking place between simvastatin and catalase enzyme's active site amino acids were analyzed. The results of molecular docking obtained from LibDock program were validated using AutoDock version 4.0. The 3 D structure of catalase enzyme as used in LibDock studies (PDB ID 2xq1) was employed to evaluate the docking potential of simvastatin. The ligand library was created for the identified drug in Autodock version 4.0 (Autodock vina) and the grid batch docking was also performed. In total ten poses were positioned for analysis of type of interaction (Leechalsit et al., 2019; Arya et al., 2021; Kesar et al., 2020).
Table 1.
PDB ID's of enzymes used in molecular docking.
| Enzyme | PDBID |
|---|---|
| Superoxide dismutase | 1IDS, 2APS, 1GV3, 1B06, 1WB8, 1MY6, 1IXB, 1BSM, 1Y67, 1ISC, 1P7G, 1MNG |
| Reduced glutathione | 5G5F |
| Catalase | 1DGB, 1DGF, 1DGG, 1DGH, 1E93, 1F4J, 1GWE, 2XQ1 |
| Nitric oxide | 1M7Z, 2FC2, 2FBZ, 1M7V, 2FC1, 4UQS, 5G6N, 5G6J, 5G6G |
2.2. Reagents
Dulbecco's Modified Eagle Media (DMEM) media, fetal bovine serum (FBS), penicillin-streptomycin, LPS, deoxycholic acid, propylthiouracil, and cholesterol were purchased from Sigma (St Louis, MO, USA). HepG2 cells were procured from National Centre for Cell Science (NCCS), Pune, India. EZcount™ MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) Cell Assay Kit (CCK003) was obtained from HiMedia. Cholesterol, HDL, and triglyceride estimation kit were purchased from ARKRAY (Autospan liquid glucose kit and Autospan liquid gold triglyceride kit).
2.3. Cell culture work
The received condition of cells was 90% confluent so cells were further passaged in split ratio of 1:3. HepG2 Cells were maintained DMEM media supplemented with 10% FBS with 1% antibiotic at 5% CO2 and 37 °C under aseptic conditions maintained in animal cell culture laboratory. Simvastatin was given at a concentration of 10 μM optimum concentration as per the reported literature (Haider and John, 2012; Raza et al., 2011). Cells were seeded in 6 well plate at the density of 5 × 104 cells/cm2 and LPS stock solution contained 1 mg/ml LPS concentration which was further diluted for working solution 100 μg/ml mixed in dimethyl sulfoxide (DMSO) as reported in the literature (Kanmani and Kim, 2019; Xu et al., 2015).
2.4. Cell culture assays
The assessment of cytotoxicity of simvastatin was performed according to the manufacturer's instructions given in the MTT assay kit (Saha et al., 2016). Cells were seeded in 96 well plate at the density of 1 × 104 cells per well to grow for 24 h and after that MTT reagent was added as per the protocol. The absorbance was noted at 570 nm using a spectrophotometer plate reader. Catalase and SOD assays were performed protocol used by Takahara et al. (1960) (Bak et al., 2012). Cells were seeded in 6 well plate at the density of 5 × 104 cells/cm2 and the levels of anti-oxidant enzymes were measured spectrophotometrically at 540 nm for catalase activity and 560 nm for the activity of enzyme SOD.
2.5. Animals and animal model
24 Wistar rats (male, n = 6) weighing 200–250 g were procured from Lala Lajpat Rai Veterinary and Animal Sciences, Hisar, India. All the experiments were conducted in accordance with the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and Institutional Animal Ethics Committee (IAEC), approval number: BV//2020–21/4011. The rats were placed in 12:12 h cycle in polypropylene cages under a standard condition of relative humidity 45–55% and 25 ± 2 °C. They were housed in the noiseless environment provided with ad libitum access to food and water. The hyperlipidemic model was established by the HFD administration as described. Rats of a different group (I-IV) were divided into normal pellet diet and HFD fed groups. Group I was served as control and fed with a normal chow diet (NCD) and groups (II-IV) were fed with an HFD [normal pellet diet (610 g/kg), deoxycholic acid (100 g), cholesterol (100 g/kg), D (−) fructose (90 g), propyl thiouracil (30g) in 1000 ml peanut oil] for 6 weeks. At the end (6 weeks) lipid profile was analyzed using commercial kits. Rat with elevated levels of triglyceride, cholesterol, low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), and reduced level of high-density lipoprotein (HDL) was considered hyperlipidemic (Lasker et al., 2019; Ferreira-Santos et al., 2020). Groups III-IV received simvastatin (10 & 20 mg/kg, oral) twice a day until a normal lipid profile was recorded.
2.6. Morphometric parameters
Rats were sacrificed, organs were isolated, weighed, and stored at −80 °C until analysis. The length of the tibia was recorded. The isolated liver was washed with chilly Krebs solution and absorbed the moisture with the help of filter paper. The liver hypertrophy index was determined using formula: (liver weight (g))/(tibial length (mm)) and body mass index by: ((body weight (g)/body length squared (cm2). For each rat, the abdominal adipose tissue was excised and weighed. The adiposity index was calculated as abdominal fat weight versus tibial length (g/mm) (Ferreira-Santos et al., 2020).
2.7. Histopathological study
Isolated hepatic tissue from rats was fixed in 10% neutral buffer formalin solution and embedded in paraffin wax. Tissue sections were sliced and stained with hematoxylin and eosin (H&E), as described previously, and observed under a microscope (Ferreira-Santos et al., 2020; Takaoka et al., 2001).
2.8. Statistical analysis
Statistical analysis and figures were made using Graph prism 7.0. and R. 2.13.0 software and one-way and two-way analysis of variance (ANOVA) was performed to calculate P-value and box plots were plotted.
3. Results
3.1. Molecular docking
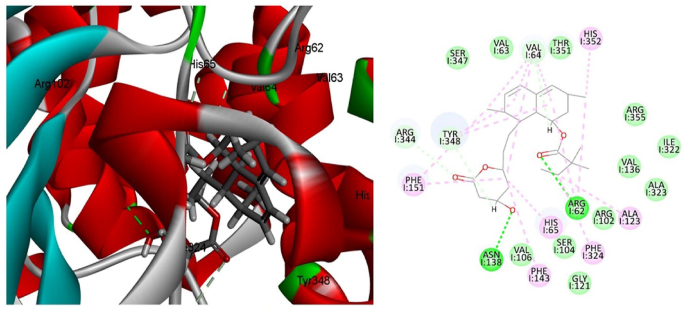
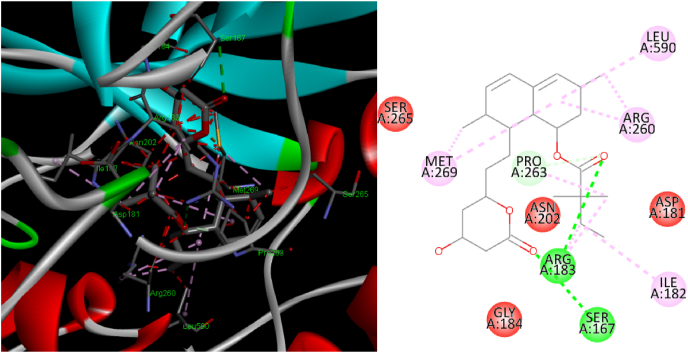
Molecular docking study of simvastatin was carried out on catalase, superoxide dismutase, nitric oxide and reduced glutathione enzymes and found that simvastatin was docked only in the active site of catalase enzyme with docking score of 133.63 kcal/mol. Among different type of interactions, the docked configuration of simvastatin displayed potential hydrogen bond interactions. Oxygen of the carbonyl and hydroxyl group showed hydrogen bond interaction with amino acid Arg62 and Asn138 respectively as shown in Fig. 1 & Table 2. Hydrogen bond interactions indicated that ligand simvastatin bind deep in the core of active site of enzyme. In addition to hydrogen bond interaction hydrophobic interactions were also observed between hydrophobic carbon functionalities present in the structure of simvastatin and hydrophobic amino acids. Moreover, carbon hydrogen bonds were also observed between active site amino acids and the carbon skeleton present in simvastatin. To confirm the results of docking analysis performed with LibDock, the docking study was performed using Autodock (binding energy of −5.88 kcal/mol). Similar binding mode and type of interactions were observed in case of Autodock The binding interactions are shown in Fig. 2. Here also simvastatin showed potential hydrogen bond interaction as observed in case of LibDock. Arg183 showed hydrogen bond interaction with carbonyl functionality present in the structure of simvastatin, however second hydrogen bond interaction was observed between aspargine and the carbonyl functionality present in the ring structure of simvastatin. Similar to LibDock study, hydrophobic interactions were also observed in case of Autodock. Carbon hydrogen bond formation was also observed in case of Autodock (Fig. 2).
Fig. 1.
Ligand interaction diagram of catalase with (a) 2D and (b) 3D poses of simvastatin (Discovery studio 2.0).
Table 2.
Molecular docking of simvastatin and catalase enzyme.
| S. No. | Compound | Binding energy (Kcal/mol) |
Amino acid resembles for hydrogen bond | H-bond distance |
|---|---|---|---|---|
| 1 | Simvastatin | 133.63 | O23-Arg62 O29-Asn138 |
2.344 2.265 |
Fig. 2.
Ligand interaction diagram of catalase with (a) 2D and (b) 3D poses of simvastatin (Autodock 4.0).
3.2. Simvastatin attenuates oxidative stress in LPS treated HepG2 cells
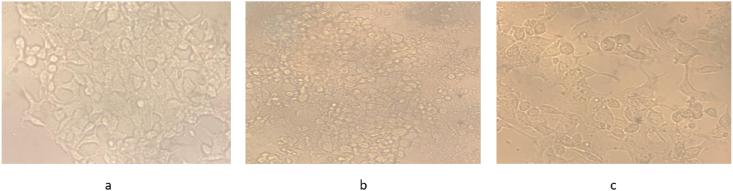
HepG2 cells treated with LPS for 24 h showed rounding of cells and presence of apoptotic bodies as compared to healthy cells (Fig. 3 a, b). Interestingly, addition of simvastatin improvement in morphology in comparison to LPS treated cells with only media change (Fig. 3c).
Fig. 3.
The morphology of oxidatively stressed HepG2 cells was restored after simvastatin (10 μM) treatment for 24 h. (a) Healthy Cells, (b) LPS treated cells (c) LPS + Simvastatin treated cells (under 20X).
3.3. Cytotoxicity assessment of simvastatin
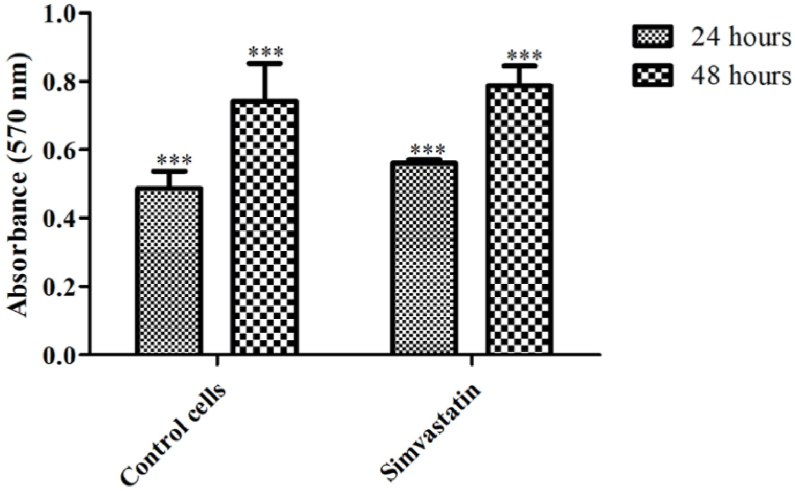
The cytotoxicity of the drug was tested using MTT assay. The assay was performed in 96 well ELISA plate and the readings were taken using an ELISA plate reader at different time intervals (as after 24 h & 48 h). As expected, the cell proliferation rates of HepG2 cells after 24 h were significantly different from cells at 48 h (Fig. 4). We note that control cells and simvastatin-treated cells show comparable growth suggesting that simvastatin is not cytotoxic at 10 μM concentration (Fig. 4).
Fig. 4.
Cell proliferation rates of HepG2 cells after 24 h and 48 h in control and simvastatin treated cells. ∗∗∗P < 0.001.
3.4. Simvastatin reduces oxidative stress levels in HepG2 cells
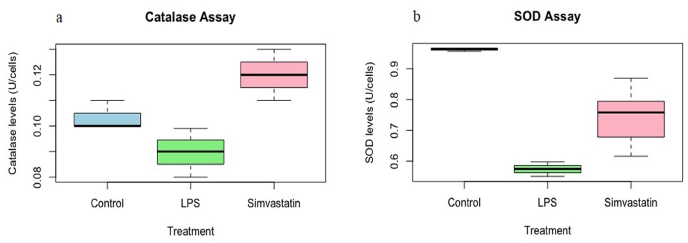
We observed that LPS treatment resulted in oxidative stress in HepG2 cells and significantly reduced the levels of catalase and SOD anti-oxidant enzymes (Fig. 5). Interestingly, the activity of catalase enzyme was increased at marginal significance range of 0.08 U per 50, 000 cells to 0.12 U per 50, 000 cells after treatment with simvastatin (Fig. 5 a). In addition, the activity of SOD was also found to increase from 0.57 U per 50, 000 cells to 0.74 U per 50, 000 cells in HepG2 cells (Fig. 5 b). One way ANOVA analysis in R script shows that catalase and SOD activities increased at statistically significant at levels after the treatment of simvastatin P < 0.05.
Fig. 5.
Anti-oxidant enzyme levels in HepG2 cells (a) Catalase activity (b) the SOD activity expressed as Units per 50,000 cells, P < 0.05.
3.5. Simvastatin attenuated increased lipid levels in HFD induced hyperlipidemia
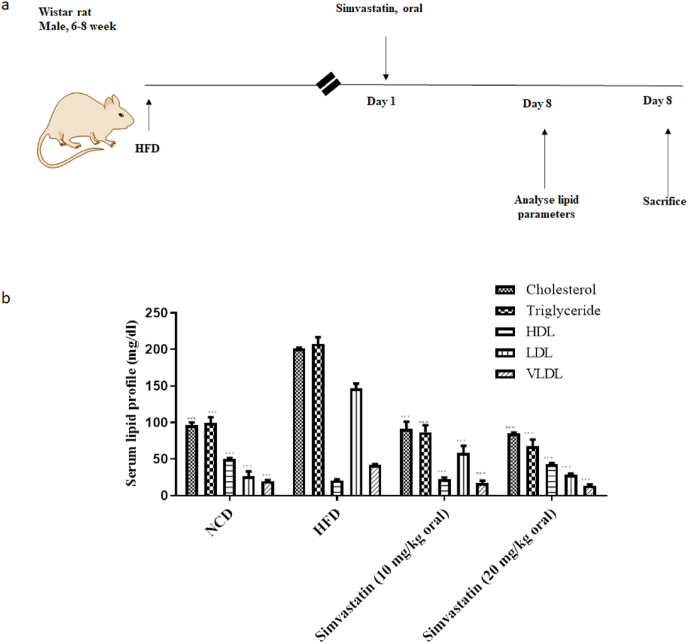
Considering molecular docking and in-vitro studies, it was thought worthy to perform in-vivo study which correlates both in silico and in-vitro results. HFD fed rats displayed an increase in plasma cholesterol (201 ± 1.41mg/dL to 91 ± 9.89 mg/dL), triglyceride (207.5 ± 9.19 to 86.5 ± 9.89 mg/dL), LDL (146.5 ± 6.64 mg/dL to 58.3 ± 9.75 mg/dL), VLDL (41.5 ± 1.83 mg/dL to 17.3 ± 3.25 mg/dL) and increase in HDL (21 ± 1.41 mg/dL to 22.5 ± 2.12 mg/dL) levels was noted when compared to control rats at 10 mg/kg dose of simvastatin. Higher dose of simvastatin lead to marked changes and better lipid profile; cholesterol (201 ± 1.41mg/dL to 85 ± 1.41 mg/dL), triglyceride (207.5 ± 9.19 to 67.5 ± 9.19 mg/dL), LDL (146.5 ± 6.64 mg/dL to 28.3 ± 1.83 mg/dL), VLDL (41.5 ± 1.83 mg/dL to 13.5 ± 1.83 mg/dL) and significant increase in HDL (21 ± 1.41 mg/dL to 43.5 ± 1.41 mg/dL) levels, Fig. 6.
Fig. 6.
Effects of simvastatin on lipid parameters in HFD fed rats (a) Shows the plan of the study and (b) Shows serum lipid profile. Values are mean ± s. d; ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.1.
3.6. Simvastatin attenuates oxidative stress in HFD induced hyperlipidemia
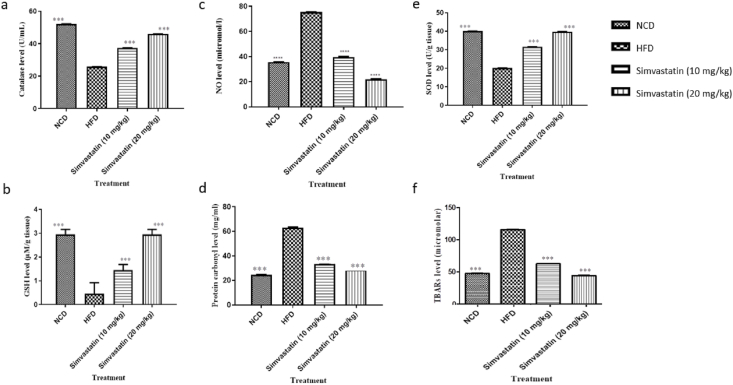
We evaluated the effect of simvastatin (10 mg/kg and 20 mg/kg) on the production of free radicals and damage to the lipid membrane by the formation of lipid peroxidation products in hepatic tissue. The increase in levels of antioxidant enzymes SOD, Catalase, GSH in hepatic tissues and reduction in lipid peroxidation levels analyzed by TBARs, NO, and protein carbonyl levels are shown in Fig. 7. In HFD rats, the activities of SOD, Catalase, GSH, levels in the positive group decreased significantly (P < 0.05) compared to the control. However, simvastatin treatment led to a significant increase (P < 0.05) in the activities of SOD, GSH, and catalase compared to the positive group. The increased amounts after simvastatin treatment at 10 mg/kg were noted for all anti-oxidant enzymes are Catalase (25.71 ± 0.034 U/gm tissue to 37.38 ± 0.032 U/gm tissue), SOD (20.17 ± 0.032 U/gm tissue to 31.56 ± 0.034 U/gm tissue), and GSH (0.45 ± 0.470 mmol/gm tissue to 1.45 ± 0.24 mmol/gm tissue) with respect to HFD rats. In addition, reduction in the values of degradation of by-products of lipid TBARs (115.95 ± 0.121 mmol/gm tissue to 62.72 ± 0.22 mmol/gm tissue), NO (75.40 mmol/gm tissue to 39.73 ± 0.27 mmol/gm tissue), protein carbonyl (63.15 ± 0.51 mg/ml to 33.45 ± 0.52 mg/ml). Further, better oxidative management was observed at a higher concentration of simvastatin (20 mg/kg). The activities of antioxidant enzymes, catalase (25.71 ± 0.034 U/gm tissue to 45.98 ± 0.053 U/gm tissue), SOD (20.17 ± 0.032 U/gm tissue to 39.78 ± 0.053 U/gm tissue), and GSH (0.45 ± 0.470 mmol/gm tissue to 2.95 ± 0.21 mmol/gm tissue) were increased with respect to HFD rats. In addition, reduction in the values of degradation of by-products of lipid TBARs (115.95 ± 0.121 mmol/gm tissue to 44.315 ± 0.23 mmol/gm tissue), NO (75.40 mmol/gm tissue to 22.01 ± 0.36 mmol/gm tissue), protein carbonyl (63.15 ± 0.51 mg/ml to 28.67 ± 0.43) with respect to HFD rats was noted, Fig. 7.
Fig. 7.
Antioxidant enzyme activities observed after simvastatin treatments (a) Catalase (b) SOD (c) GSH (d) TBARs (e) NO (f) Protein carbonyl level in hyperlipidemic rats. Doses of simvastatin used in the study are 10 mg/kg and 20 mg/kg. Values are mean ± s. d; ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.1.
3.7. Simvastatin attenuated morphometric parameters
It was noted that body weight was markedly increased in HFD fed rats at the end of the study and significant changes were observed when compared to the treated group. Treatment with simvastatin (10 mg/kg and 20 mg/kg) was able to reduce the bodyweight significantly and aided in the maintenance of optimal body weight as shown in Table 3. Also, the abdominal fat, BMI, and liver index were significantly reduced after simvastatin treatment in HFD fed rats. As expected, a marked increase in adiposity index was also noted in HDF rats (it increased from 0.17 g/mm to 0.438 g/mm tibial length). After simvastatin treatment it improved to 0.182 g/mm after simvastatin treatment at high dose (20 mg/kg) and 0.283 gm/mm after simvastatin treatment at low dose (10 mg/kg). In addition, the liver index increased from 0.272 gm/mm to 0.472 gm/mm in HFD hyperlipidemic rats. We noted a reduction in this increase to 0.278 gm/mm after simvastatin treatment at high dose (20 mg/kg) and 0.338 gm/mm after simvastatin treatment at low dose (10 mg/kg).
Table 3.
Effects of simvastatin on morphometric parameters.
|
Groups |
Parameters |
|||
|---|---|---|---|---|
| Body weight (g) | Body mass index (g/cm2) | Adiposity index (g/mm tibial length) |
Liver index (g/mm tibial length) | |
| NCD fed | 240 ± 0.02∗∗∗ | 0.66 ± 0.12∗∗∗ | 0.178 ± 0.42∗∗∗ | 0.272 ± 0.24∗∗∗ |
| HFD fed | 450 ± 0.78∗∗∗ | 1.24 ± 0.19∗∗∗ | 0.438 ± 0.65∗∗∗ | 0.472 ± 0.21∗∗∗ |
| Simvastatin (10 mg/kg) | 290 ± 0.56∗∗∗ | 0.80 ± 0.45∗∗∗ | 0.283 ± 0.76∗∗∗ | 0.338 ± 0.89∗∗∗ |
| Simvastatin (20 mg/kg) | 240 ± 0.38∗∗∗ | 0.66 ± 0.76∗∗∗ | 0.182 ± 0.19∗∗∗ | 0.267 ± 0.01∗∗∗ |
Values are mean ± s. d; ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.1.
3.8. Histopathology
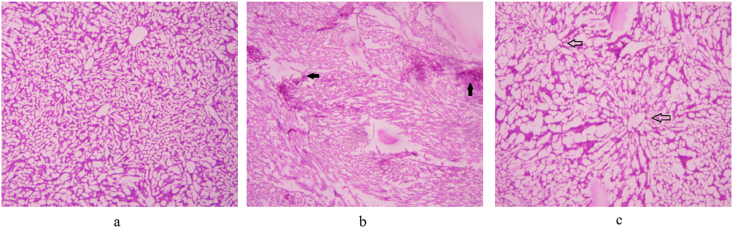
The hepatic tissue in control rats showed normal architecture with negligible fat accumulation and infiltration (arrows). The cords of hepatic cells were finely separated by blood sinusoids lined by flattened endothelial cells and von Kupfer cells (Fig. 8a). The hepatic tissue of HFD fed rats (Fig. 8b) showed sinusoidal dilation with an accumulation of fat droplets and increased inflammatory cell infiltration as compared to control rats (filled arrows).
Fig. 8.
Histopathology of hepatic tissue (a) NCD-fed rats (b) HFD fed rats (c) Simvastatin-fed rats (20 mg/kg). The arrows indicate change in morphological parameters.
Supplementation of simvastatin (20 mg/kg) showed normal architecture with reduced infiltration, proper central vein, and compact arrangement of hepatocytes without fatty lobulation (Fig. 8c).
4. Discussion
Anti-hyperlipidemic drug simvastatin has recently shown promising results as an effective anti-oxidant and anti-inflammatory candidate in both acute and chronic liver disease animal models (Tripathi et al., 2018a; La Mura et al., 2013; Abraldes et al., 2007; Meireles et al., 2017; Sancho et al., 2013). This motivated us to further investigate the molecular activity of simvastatin and thus, we performed in-silico molecular docking of simvastatin with anti-oxidant enzymes followed by validation of amelioration of oxidative stress levels in in vivo and in vitro anti-oxidant studies. We performed the molecular docking of simvastatin with multiple enzymes related to anti-oxidative activities through LibDock analysis using Discover Studio 2.0 and validated the results using Autodock software. Interestingly, we noted the selective affinity of simvastatin for catalase enzyme, and not to other enzymes such as SOD, GSH, and NO. It was noted that simvastatin interacts with to active site of catalase at Arg-62 and Asn-132 site with 133.63 kcal/mol through LibDock analysis, Fig. 1. The review of the literature shows that catalase binds with other compounds and simvastatin binds only to inhibitors of HMG-CoA reductase in particular (Yekta et al., 2017; Huo et al., 2020; Shahraki et al., 2020; Nazir et al., 2018). However, the binding of simvastatin with catalase has never been reported earlier. Thus, this study is the first to show that simvastatin interacts with catalase enzyme in-silico suggesting its plausible role in exhibiting anti-oxidant effect. Further investigations to better understand the underlying molecular mechanisms of simvastatin and catalase enzyme and other anti-oxidant activities are warranted.
A comprehensive study of simvastatin and anti-oxidant enzymes including in-silico, in-vitro, and in vivo studies under one platform has never been done earlier. Moreover, currently, there are no cell cultures available that can simulate high-fat diet rat model studies. Emerging research studies have shown that there is a strong link between metabolic diseases, obesity, hyperlipidemia, and fatty liver diseases. In addition, recent clinical trials are now focusing on exploring the effect of simvastatin in the amelioration of liver cirrhosis (https://clinicaltrials.go, 2968; https://clinicaltrials.go, 1504; https://clinicaltrials.go; Francis and Forman, 2021; Marrache and Rockey, 2021; Kaplan et al., 2021). Considering the recent developments, it was necessary to investigate the role of simvastatin in cell lines. Thus, the findings were validated through in-vitro analysis in LPS induced oxidative stress in HepG2 cells and HFD induced oxidative stress in hyperlipidemic rats. Various studies show that the bacterial endotoxins, LPS as one of the compounds capable of inducing oxidative stress in different cells of different organs and lead to the production of toxins (Belegri et al., 2018). LPS causes the accumulation of ROS resulting in cell cytotoxicity. Besides all these, there are counter processes or different reactions in order to balance the effect of oxidative stress. Thus, catalase, peroxides, and SOD are considered as indexes to assess the level of oxidative stress in HepG2 cells (Kaplan et al., 2021; Belegri et al., 2018). Thus, we investigated the role of simvastatin at a concentration of 10 μM as per the reported literature (Bak et al., 2012; Lasker et al., 2019) for the amelioration of LPS induced oxidative stress. Interestingly, we found that simvastatin restored the cellular morphology of HepG2 cells under LPS induced oxidative stress, Fig. 2. The cytotoxicity of simvastatin (10 μM) was tested using MTT assay and it was observed that administration of simvastatin had no significant effect on cell growth and proliferation and thus, it can be considered as non-toxic to the cells, Fig. 3. We noted that catalase and SOD activities significantly increased in simvastatin treated HepG2 cells under LPS induced oxidative stress, Fig. 4. This is a significant observation as catalase is a critical enzyme for anti-oxidative mechanisms and its molecular interaction followed by its concomitant increase in in vitro assays is a noteworthy observation and findings of this study. One-way ANOVA analysis in the R script shows that these enzymes increased at statistically significant levels (P < 0.05) and further molecular levels interactions are needed to understand the underlying molecular mechanism.
We further validated the anti-oxidative properties of simvastatin in HFD induced oxidative stress in hyperlipidemic rats. As expected, simvastatin reduced levels of triglycerides and LDL and increased HDL levels in hyperlipidemic rats, Fig. 5. An appreciable improvement in lipid profile at 20 mg/kg dose of simvastatin was noted as compared to a low dose of 10 mg/kg. We also observed an increase in the levels of antioxidant enzymes in oxidatively stressed tissues after treatment with simvastatin, Fig. 6. Similar indications of anti-oxidant properties of simvastatin in a diabetic rat model using simvastatin and clinical trials in heart and hypercholesterolemia were also observed, though the exhaustive study (Sancho et al., 2013; Tsochatzis and Bosch, 2017; Kesar et al., 2020; Tripathi et al., 2018b). The results obtained in this study are in accordance with the recent work of Tripathi et al., in 2018 and Rodrigues et al., in 2019 in liver diseases (Tripathi et al., 2018a; Sakaguchi et al., 2010). The role of simvastatin in the amelioration of liver diseases has only been studied in high-fat models. We have shown the effect of simvastatin in in-vitro liver cell cultures under oxidative stress and correlated that to similar results in in-vivo high-fat rat models. In addition, we are first to show that simvastatin binds to catalase, suggesting its promising role in reducing oxidative stress and amelioration of chronic liver diseases. The new findings of this work suggest that simvastatin can be re-purposed as a therapeutic candidate in combating oxidative stress-induced liver diseases. However, further in-depth molecular pathway analysis is required to better understand the underlying molecular mechanism of simvastatin in antioxidant mechanism under oxidative stress conditions.
5. Conclusion
In conclusion, we found that simvastatin interacts with catalase using in-silico/molecular docking approach and concomitantly reduces oxidative stress levels in LPS induced hepatic cells and HFD induced oxidative stress in tissues. Simvastatin does not interfere with cellular proliferation and metabolism, which may be explained by [difference observed in cell proliferation rates of simvastatin treated HepG2 cells at two-time intervals, suggesting its non-cytotoxicity] or [its non-cytotoxic effect in MTT assay]. As expected, treatment with simvastatin improved the lipid profile in HFD fed hyperlipidemic rats. It also restored the original morphology of the HepG2 cells. Histopathological studies revealed that hepatic tissues showed improved structure as well. This was further validated by increased levels of antioxidant enzymes catalase, GSH and SOD in HepG2 cells along with a reduction in levels of TBARs, NO, and protein carbonyls in HFD rats. The anti-oxidant properties of simvastatin have never been reported in human liver cell lines. This study including molecular docking and in-vivo and in-vitro anti-oxidant approaches suggests that simvastatin can be repurposed to reduce oxidative stress in liver diseases.
Formatting of funding sources
The authors also gratefully acknowledge support for this research from SERB (SRG/2019/002105).
CRediT authorship contribution statement
Kanika Verma: and. Shikha Makwana: performed in vitro and in vivo studies. Sarvesh Paliwal: conceptualized and supervised entire study, Dr. Vartika Paliwal: conducted molecular docking studies. Dr. Smita Jain: and. Swati Paliwal: and Prof. Swapnil Sharma: drafted and reviewed the manuscript.
Declaration of competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are highly thankful to Prof. Aditya Shastri, Vice-Chancellor, Banasthali Vidyapith, Banasthali, Rajasthan India for providing pivotal support and facilities for the successful compilation of work. We thank Prof. Dipjyoti Chakraborty for his support throughout the project. The authors also gratefully to Science and Engineering Research Board (SERB), India for providing the grant support for Startup Research Grant (SRG/2019/002105).
Contributor Information
Swati Paliwal, Email: swatzpaliwal@gmail.com.
Swapnil Sharma, Email: skspharmacology@gmail.com.
List of abbreviations
- ACLF
Acute-on chronic liver failure
- DMEM
Dulbecco's Modified Eagle Media
- FBS
Fetal bovine serum
- NAFLD
Non-alcoholic fatty liver diseases
- HFD
High fat diet
- SOD
Superoxide dismutase
- GSH
Reduced glutathione
- NO
Nitric oxide
- TBARs
Thiobarbituric acid reactive substances
- PDB
Protein data bank
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- CPCSEA
Committee for the purpose of control and supervision of experiments on animals
- IAEC
Institutional animal ethics committee
- LPS
Lipopolysaccharide
- LDL
Low density lipoprotein
- VLDL
Very low-density lipoprotein
- HDL
High-density lipoprotein
- NaCl
Sodium chloride
- BMI
Body mass index
- ANOVA
One way analysis of variance
- TG
Triglyceride
- ELISA
Enzyme link immunosorbent assay
- NASH
Non-alcoholic steatohepatitis
- HMG
CoA β-Hydroxy β-methylglutaryl-CoA
- O2
Molecular oxygen
- ROS
Reactive oxygen species
- O2
Superoxide
- H2O2
Hydrogen peroxide
- OH•
Hydroxyl free radicals
- NCD
Normal chaw diet
References
- Abbas A.M., Sakr H.F. Simvastatin and vitamin E effects on cardiac and hepatic oxidative stress in rats fed on high fat diet. J. Physiol. Biochem. 2013;69:737–750. doi: 10.1007/s13105-013-0250-y. [DOI] [PubMed] [Google Scholar]
- Abraldes J.G., Rodríguez-Vilarrupla A., Graupera M., Zafra C., García-Calderó H., García-Pagán J.C., Bosch J. Simvastatin treatment improves liver sinusoidal endothelial dysfunction in CCl4 cirrhotic rats. J. Hepatol. 2007;46:1040–1046. doi: 10.1016/j.jhep.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Abraldes J.G., Albillos A., Banares R., Turnes J., Gonzalez R., Pagan J.C.G., Bosch J. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterol. 2009;136:1651–1658. doi: 10.1053/j.gastro.2009.01.043. [DOI] [PubMed] [Google Scholar]
- Arya R., Paliwal S., Gupta S.P., Sharma S., Madan K., Mishra A., Verma K., Chauhan N. In-silico studies and biological activity of potential BACE-1 inhibitors. Comb. Chem. High Throughput Screen. 2021;24:729–736. doi: 10.2174/1386207323999200918151331. [DOI] [PubMed] [Google Scholar]
- Bak M.J., Jun M., Jeong W.S. Antioxidant and hepatoprotective effects of the red ginseng essential oil in H2O2-treated HepG2 cells and CCl4-treated mice. Int. J. Mol. Sci. 2012;13:2314–2330. doi: 10.3390/ijms13022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belegri E., Eggeles L., Fleur S.E., Boelen A. One-week exposure to a free choice high fat-high-sugar diet does not interfere with the lipopolysaccharide-induced acute phase response in the hypothalamus of male rats. Front. Endrocrinol. 2018 doi: 10.3389/fendo.2018.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichoż-Lach H., Michalak A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014;20:8082. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-Santos P., Aparicio R., Carron R., Montero M.J., Sevilla M.A. Lycopene-supplemented diet ameliorates metabolic syndrome induced by fructose in rats. J. Funct.Foods. 2020;73:104098. [Google Scholar]
- Francis P., Forman L.M. Statins show promise against progression of liver disease. Clin. Liver Dis. 2021;18:280–287. doi: 10.1002/cld.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider R., John A. Implications of altered glutathione metabolism in aspirin-induced oxidative stress and mitochondrial dysfunction in HepG2 cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0036325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://clinicaltrials.gov/ct2/show/NCT03654053?term=NCT03654053&draw=2&rank=1
- https://clinicaltrials.gov/ct2/show/NCT03150459
- https://clinicaltrials.gov/ct2/show/NCT02968810
- Huo M., Zhao L., Wang T., Zong W., Liu R. Binding mechanism of maltol with catalase investigated by spectroscopy, molecular docking, and enzyme activity assay. J. Mol. Recogn. 2020;33 doi: 10.1002/jmr.2822. [DOI] [PubMed] [Google Scholar]
- Kanmani P., Kim H. Protective effects of lactic acid bacteria against TLR4 induced inflammatory response in hepatoma HepG2 cells through modulation of toll-like receptor negative regulators of mitogen-activated protein kinase and NF-κB signaling. Front. Immunol. 2019;9:1537. doi: 10.3389/fimmu.2018.01537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D.E., Mehta R., Garcia-Tsao G., Albrecht J., Aytaman A., Baffy G., Bajaj J., Hernaez R., Hunt K., Ioannou G., Johnson K. SACRED: effect of simvastatin on hepatic decompensation and death in subjects with high-risk compensated cirrhosis: statins and Cirrhosis: reducing Events of Decompensation. Contemp. Clin. Trials. 2021;104:106367. doi: 10.1016/j.cct.2021.106367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesar S., Paliwal S., Mishra P., Madan K., Chauhan M., Chauhan N., Verma K., Sharma S. Identification of novel rho-kinase-II inhibitors with vasodilatory activity. ACS Med. Chem. 2020;11:1694–1703. doi: 10.1021/acsmedchemlett.0c00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Mura V., Pasarin M., Meireles C.Z., Miquel R., Vilarrupla A.R., Hide D., Sancho J.G., Pagan J.C.G., Bosch J., Abraldes J.G. Effects of simvastatin administration on rodents with lipopolysaccharide induced liver microvascular dysfunction. Hepatology. 2013;57:1172–1181. doi: 10.1002/hep.26127. [DOI] [PubMed] [Google Scholar]
- Lasker S., Rahman M., Parvez F., Zamila M., Miah P., Nahar K., Kabir F., Sharmin S.B., Shuban S., Ahsan S., Alam A. High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Sci. Rep. 2019;9:1–15. doi: 10.1038/s41598-019-56538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leechalsit R., Pingaew R., Prachayasittikul V., Worachartcheewan A., Prachayasittikul S., Ruchirawat S., Synthesis V. Prachayasittikul. Molecular docking, and QSAR study of bis-sulfonamide derivatives as potential aromatase inhibitors. Bioorg. Med. Chem. 2019;27:115040. doi: 10.1016/j.bmc.2019.08.001. [DOI] [PubMed] [Google Scholar]
- Li J., Sun Y.M., Wang L.F., Li Z.Q., Pan W., Cao H.Y. Comparison of effects of simvastatin versus atorvastatin on oxidative stress in patients with coronary heart disease. Clin. Cardiol. 2010;33:222–227. doi: 10.1002/clc.20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrache M.K., Rockey D.C. Statins for treatment of chronic liver disease. Curr. Opin. Gastroenterol. 2021;37:200–207. doi: 10.1097/MOG.0000000000000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meireles C.Z., Pasarin M., Lozano J.J., García-Calderó H., Gracia-Sancho J., García-Pagán J.C., Bosch J., Abraldes J.G. Simvastatin attenuates liver injury in rodents with biliary cirrhosis submitted to hemorrhage/resuscitation. Shock. 2017;47:370–377. doi: 10.1097/SHK.0000000000000734. [DOI] [PubMed] [Google Scholar]
- Mohamadin A.M., Elberry A.A., Gawad H.S.A., Morsy G.M., Abbasi F.A.A. Protective effects of simvastatin, a lipid lowering agent, against oxidative damage in experimental diabetic rats. J. Lipid Res. 2011:167958. doi: 10.1155/2011/167958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutzouri E., Liberopoulos E.N., Tellis C.C., Milionis H.J., Tselepis A.D., Elisaf M.S. Comparison of the effect of simvastatin versus simvastatin/ezetimibe versus rosuvastatin on markers of inflammation and oxidative stress in subjects with hypercholesterolemia. Atherosclerosis. 2013;23:P8–P14. doi: 10.1016/j.atherosclerosis.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Nazir N., Karim N., Abdel-Halim H., Khan I., Wadood S.F., Nisar M. Phytochemical analysis, molecular docking and antiamnesic effects of methanolic extract of Silybum marianum (L.) Gaertn seeds in scopolamine induced memory impairment in mice. J. Ethnopharmacol. 2018;210:198–208. doi: 10.1016/j.jep.2017.08.026. [DOI] [PubMed] [Google Scholar]
- Polanczyk A.P., Goraca, Demyanets A.S., Mittleboeck M., Domenig C., Neumayer C., Wojta J., Nanobachvili J., Huk I., Klinger M. Simvastatin decreases free radicals formation in the human abdominal aortic aneurysm wall via NF-κB. Eur. J. Vasc. Endovasc. Surg. 2012:133–137. doi: 10.1016/j.ejvs.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Raza H., John A., Benedict S. Acetylsalicylic acid-induced oxidative stress, cell cycle arrest, apoptosis and mitochondrial dysfunction in human hepatoma HepG2 cells. Eur. J. Pharmacol. 2011;668:15–24. doi: 10.1016/j.ejphar.2011.06.016. [DOI] [PubMed] [Google Scholar]
- Rodrigues G., Moreira A.J., Bona S., Schemitt E., Marroni C.A., Di Naso F.C., Dias A.S., Pires T.R., Picada J.N., Marroni N.P. Simvastatin reduces hepatic oxidative stress and endoplasmic reticulum stress in nonalcoholic steatohepatitis experimental model. Oxid. Med. Cell. Longev. 2019 doi: 10.1155/2019/3201873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo L., Sancho G.J., Caldero G.H., Marrone G., Pagan J.C.G., Cardena G.G., Bosch J. Addition of simvastatin to cold storage solution prevents endothelial dysfunction in explanted rat livers. Hepatology. 2012;55:921–930. doi: 10.1002/hep.24755. [DOI] [PubMed] [Google Scholar]
- Saha M.R., Dey P., Begum S., De B., Chaudhuri T.K., Sarker D.D., Das A.P., Sen A. Effect of Acacia catechu (L.f.) willd. on oxidative stress with possible implications in alleviating selected cognitive disorders. PLoS One. 2016;11:1–19. doi: 10.1371/journal.pone.0150574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S., Takahashi S., Sasaki T., Kumagai T., Nagata K. Progression of alcoholic or non-alcoholic steatohepatitis; common metabolic aspects of innate immune system and oxidative stress. Drug Metabol. Pharmacokinet. 2010:1011300126. doi: 10.2133/dmpk.dmpk-10-rv-087. 1011300126. [DOI] [PubMed] [Google Scholar]
- Sancho J.G., Caldero H.G., Hide D., Marrone G., Muntet S.G., Peralta C., Pagan J.C.G., Abraldes J.G., Bosch J. Simvastatin maintains function and viability of steatotic rat livers procured for transplantation. J. Hepatol. 2013;58:1140–1146. doi: 10.1016/j.jhep.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Shahraki S., Saeidifar M., Delarami H.S., Kazemzadeh H. Molecular docking and inhibitory effects of a novel cytotoxic agent with bovine liver catalase. J. Mol. Struct. 2020;1205:127590. [Google Scholar]
- Takaoka M., Kobayashi Y., Yuba M., Ohkita M., Matsumura Y. Effects of α-lipoic acid on deoxycorticosterone acetate–salt-induced hypertension in rats. Eur. J. Pharmacol. 2001;424:121–129. doi: 10.1016/s0014-2999(01)01120-7. [DOI] [PubMed] [Google Scholar]
- Toppo A.L., Yadav M., Dhagat S., Ayothiraman S., Eswari J.S. Molecular docking and ADMET analysis of synthetic statins form HMG-CoA reductase inhibition activity. Inter. J. Biochem. Biophysic. 2021;58:127–134. [Google Scholar]
- Tripathi D.M., Vilaseca M., Lafoz E., Garcia-Caldero H., Haute G.V., Fernández-Iglesias A., de Oliveira J.R., García-Pagán J.C., Bosch J., Gracia-Sancho J. Simvastatin prevents progression of acute on chronic liver failure in rats with cirrhosis and portal hypertension. Gastroenterol. 2018;155:1564–1577. doi: 10.1053/j.gastro.2018.07.022. [DOI] [PubMed] [Google Scholar]
- Tripathi N., Paliwal S., Sharma S., Verma K., Gururani R., Tiwari A., Verma A., Chauhan M., Singh A., Kumar D., Pant A. Discovery of novel soluble epoxide hydrolase inhibitors as potent vasodilators. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-32449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsochatzis E.A., Bosch J. Statins in cirrhosis—ready for prime time. Hepatology. 2017;66:697–699. doi: 10.1002/hep.29277. [DOI] [PubMed] [Google Scholar]
- Xu J., Li Y., Yang X., Liu Y., Chen Y., Chen M. Bilobol inhibits the lipopolysaccharide-induced expression and distribution of RhoA in HepG2 human hepatocellular carcinoma cells. Oncol. Lett. 2015;10:962–966. doi: 10.3892/ol.2015.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekta R., Dehghan G., Rashtbari S., Sheibani N., Moosavi-Movahedi A.A. Activation of catalase by pioglitazone: multiple spectroscopic methods combined with molecular docking studies. J. Mol. Recogn. 2017;30:e2648. doi: 10.1002/jmr.2648. [DOI] [PubMed] [Google Scholar]
- Zhu B., Shen H., Zhou J., Lin F., Hu Y. Effects of simvastatin on oxidative stress in streptozotocin-induced diabetic rats: a role for glomeruli protection. Nephron Exp. Nephrol. 2005;101:e1–e8. doi: 10.1159/000085712. [DOI] [PubMed] [Google Scholar]