Abstract
Background
Sequential treatment with different generations of anaplastic lymphoma kinase (ALK) inhibitors have been widely applied to ALK-positive lung cancer; however, resistance mutations inevitably developed. Further characterization of ALK resistance mutations may provide key guidance to subsequent therapies. Here we explored the emergence of secondary ALK mutations during sequential ALK tyrosine kinase inhibitor (TKI) treatment in a real-world study of Chinese lung adenocarcinoma (ADC) patients.
Methods
A clinical-genomic database was queried for lung ADC patients with at least one ALK inhibitor treatment and at least one plasma sample collected following ALK inhibitor treatment. Targeted genome profiling was performed with a 139-gene panel in baseline tumor tissue and serial plasma samples of patients.
Results
A total of 116 patients met inclusion criteria. ALK G1202R was more common in patients with echinoderm microtubule-associated protein-like 4 (EML4)-ALK v3 fusion, whereas ALK L1196M was more common in v1. TP53 mutant patients were significantly associated with harboring multiple ALK resistance mutations (P = 0.03) and v3+/TP53 mutant patients had the highest rate of multiple ALK resistance mutations. The sequential use of ALK TKI led to an increased incidence of concurrent ALK mutations along the lines of therapies. Alectinib had a lower rate (9%) harboring ALK resistance mutation as first-line ALK TKI compared with crizotinib (36%). ALK compound mutations identified included ALK D1203N/L1196M, ALK G1202R/L1196M, and ALK G1202R/F1174C, which may be lorlatinib resistant. Using paired pretreatment and post-treatment samples, we identified several ALK-independent resistance-related genetic alterations, including PTPRD and CNKN2A/B loss, MYC, MYCN and KRAS amplification, and EGFR19del.
Conclusions
Sequential postprogression plasma profiling revealed that increased lines of ALK inhibitors can accelerate the accumulation of ALK resistance mutations and may lead to treatment-refractory compound ALK mutations. The selection for optimal first-line TKI is very important to achieve a more efficacious long-term strategy and prevent the emergence of on-target resistance, which may provide guidance for clinical decision making.
Key words: ALK inhibitors, lung adenocarcinoma, circulating tumor DNA, real-world data
Highlights
-
•
ALK resistance mutations were differentially enriched in the setting of EML4-ALK v1/v3 and TP53 status.
-
•
Serial liquid biopsies NGS depicted accumulation of multiple ALK secondary mutations during sequential ALK treatments.
-
•
Several lorlatinib-resistant ALK compound mutations and ALK-independent resistance genetic alterations were identified.
Introduction
Anaplastic lymphoma kinase (ALK) gene rearrangements are found in ∼3%-7% of non-small-cell lung cancers (NSCLCs).1 ALK-rearranged lung cancer is more common in young, nonsmoking, and adenocarcinoma (ADC) patients.2 Sequential treatment with crizotinib and second- and third-generation ALK tyrosine kinase inhibitors (TKIs) such as alectinib, brigatinib, ceritinib, ensartinib, and lorlatinib have been widely applied to patients with ALK-positive lung cancer and have significantly extended the survival time of these patients.3, 4, 5 However, acquired resistance driven by secondary ALK mutations often develops during the course of treatment.6 Multiple molecular mechanisms can cause resistance to second-generation ALK inhibitors. ALK kinase domain mutations (i.e. on-target resistance) were identified in ∼50% and 70% of biopsies in patients relapsing on second-generation ALK TKIs and lorlatinib, respectively.7,8 By contrast, those resistance diseases without identifiable ALK resistance mutations were ALK independent with resistance mediated by off-target mechanisms such as bypass signaling or lineage changes.6
Plasma-based next-generation sequencing (NGS) provides a feasible way for dynamically monitoring tumor genomic evolution. It has been widely used in analyzing TKI resistance in patients with NSCLC receiving targeted therapies including ALK TKIs.7,9 Currently, NGS assays are increasingly used in routine clinical practice for NSCLC,10 which can provide sufficient clinical-genomic data for a comprehensive understanding of the molecular mechanism of resistance and clonal evolution. In this study, we retrospectively analyzed longitudinal plasma circulating tumor DNA (ctDNA) from 116 ALK-positive lung ADC patients who underwent real-world ALK TKI treatment, aiming to investigate the ALK resistance mechanisms and tumor evolution during sequential treatment using a plasma-based NGS test.
Patients and methods
We performed a retrospective cohort study of 24 468 lung cancer patients from a clinical-genomic database from November 2013 to April 2020. All patients enrolled in the study gave written consent for genetic testing and research. The study methodologies conformed to the Declaration of Helsinki and were approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University. Lung ADC patients were included based on the following criteria (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100337): (i) available plasma samples for analysis; (ii) identified ALK rearrangement in plasma or tumor tissue from each patient; and (iii) treated with ALK inhibitors. Overall, a total of 76 tumor tissue specimens and 263 plasma specimens from 116 patients who progressed on first-, second-, or third-generation ALK inhibitors were subjected for further analysis.
Targeted NGS was performed in tumor tissue and plasma samples with a gene panel of 139 genes (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100337). All samples were matched to a whole-blood sample from the same patient as a control. DNA extraction, library preparation, hybridization-based targeted enrichment, and sequencing and data analysis were performed as previously described.11 All exons (including flanking intronic regions) and fusion-related introns of ALK were covered by the targeted panel. Different types of genetic alterations were called using an internally validated bioinformatics analysis pipeline.12 In brief, Trimmomatic13 was used for FASTQ file quality control. Leading/trailing low-quality (quality reading below 20) or N bases were removed. Pair-end reads were then aligned to the human reference genome (hg19) using Burrows–Wheeler Aligner14 with default parameters. PCR deduplication was performed using Picard version 2.9.4 (Broad Institute, MA). Local realignment around indels and base quality score recalibration were performed with the Genome Analysis Toolkit (GATK 3.4.0). Somatic single-nucleotide variants and insertions/deletions (indels) were identified using MuTect15 and Scalpel,16 respectively. The cut-off for mutation detection was 0.5% variant allele frequency and five reads in plasma samples; 1% variant allele frequency and six reads in tissue samples. For patients with multiple plasma samples, if a mutation meets the above cut-off in at least one sample, the detection cut-off for the same mutation was dropped in other samples to reduce false negatives. For calling of copy number variations (CNVs), we used an in-house developed bioinformatics pipeline to analyze CNV. A fold change of ≥1.6 and ≥2.0 is used to detect CNV gain in liquid biopsy samples and tumor tissues, respectively, whereas a fold change ratio ≤0.6 is used to detect CNV loss in both sample types.
Results
Study population
Between November 2013 and April 2020, plasma-based liquid biopsies were performed in 116 ALK-positive patients after first- (crizotinib), second- (ceritinib, brigatinib, alectinib, ensartinib, and SAF-189s), or third-generation (lorlatinib) ALK TKI treatment. Baseline clinical characteristics of these patients are summarized in Table 1. The median age of patients at the time of diagnosis was 51.5 years (range, 20-79 years). Forty-four (38%) patients underwent two lines of ALK TKIs and 21 (18%) underwent three or more lines of ALK TKIs, while 41 patients were treated with only one ALK TKI (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100337). A total of 146 plasma samples were obtained after patients progressed on ALK TKI treatments [progressive disease (PD) samples]. Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100337 summarized types of ALK TKIs used and lines of treatment of PD samples.
Table 1.
Clinicopathological characteristics of all patients
| Characteristics | Values |
|---|---|
| Patients, n | 116 |
| Age (years) | |
| Median (range) | 51.5 (20-79) |
| Sex, n (%) | |
| Male | 59 (51) |
| Female | 57 (49) |
| Histology, n (%) | |
| Adenocarcinoma | 116 (100) |
| ALK fusion type, n (%) | |
| EML4-ALK v1 | 37 (32) |
| EML4-ALK v3 | 49 (42) |
| Other EML4-ALK | 20 (17) |
| Non EML4-ALK | 10 (9) |
| Number of ALK TKI treatment, n (%) | |
| 1 | 51 (44) |
| 2 | 44 (38) |
| ≥3 | 21 (18) |
| ALK TKI, n (%) | |
| Crizotinib | 103 (89) |
| Ceritinib | 13 (11) |
| Brigatinib | 26 (22) |
| Alectinib | 30 (26) |
| Ensartinib | 6 (5) |
| SAF-189s | 3 (3) |
| Conteltinib | 2 (2) |
| Lorlatinib | 25 (22) |
ALK, anaplastic lymphoma kinase; EML4, echinoderm microtubule-associated protein-like 4; TKI, tyrosine kinase inhibitor.
Concordance between tissue and plasma genotypes
We first evaluated concordance between baseline tissue and plasma genotyping. A pair of tissue and plasma biopsies was considered contemporaneous if performed within 1 week and with no therapeutic interevent in-between. A total of 13 pairs of tissue and plasma samples were available. As shown in Supplementary Figure S3A and B, available at https://doi.org/10.1016/j.esmoop.2021.100337, mutations in paired tissue and plasma samples showed a high concordance. About 72% (31/43) of mutations in tissue samples were detectable in paired plasma samples and 69% of mutations in plasma samples were detectable in paired tissue samples. We further investigated the impact of the normal blood control on the comparison of tumor/ctDNA NGS results and identified a median of 2.5 (range, 1-9) mutations in 6 out of the 13 plasma samples filtered out by normal controls which were not detectable in the corresponding tissue samples, including mutations from DNMT3A. Without the control of normal blood samples, the concordance of mutations in plasma with tissue samples was only 46% (31/67) (Supplementary Figure S3C, available at https://doi.org/10.1016/j.esmoop.2021.100337). These results indicated that normal blood controls can significantly control the false-positive mutations from clonal hematopoiesis or sequencing errors.
ALK resistance mutations in TKI-resistant specimens
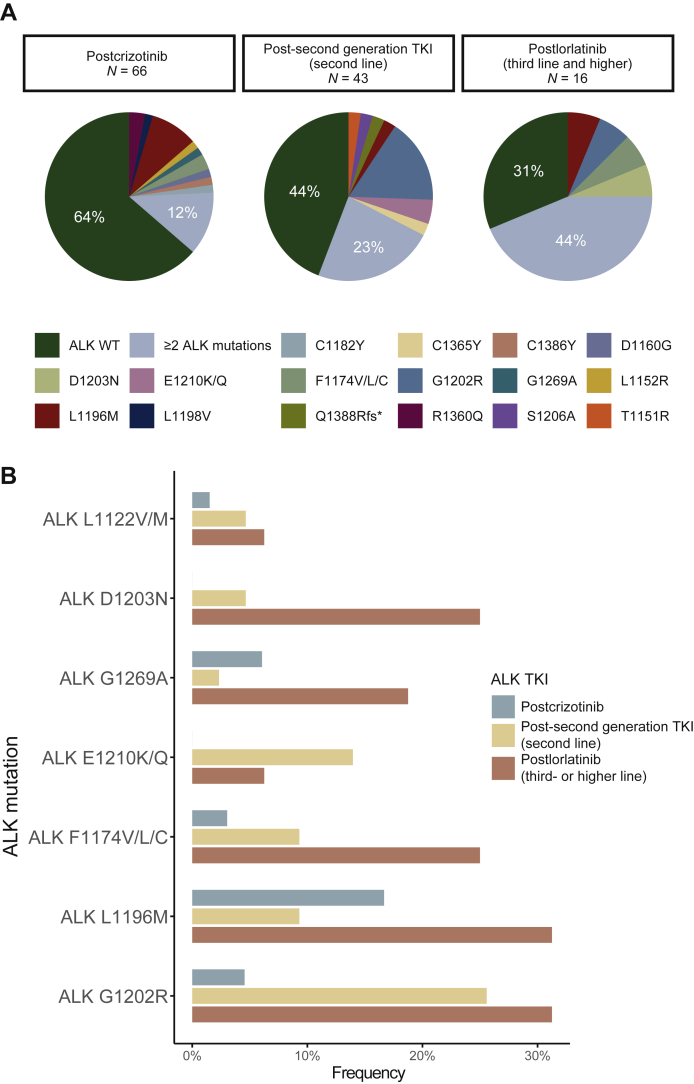
In this cohort, crizotinib (n = 66) was used as first-line ALK TKI treatment. Among the 66 patients, the median duration of crizotinib treatment was 9.2 months (range, 2.1-48.6 months). We detected an ALK resistance mutation in plasma from 24 (36%) of 66 patients relapsing on crizotinib. The most common ALK resistance mutations were L1196M and G1269A (Figure 2A and B). The second-generation ALK TKI alectinib was also used for first-line ALK TKI treatment in 11 patients. Their median duration of treatment was also 9.2 months (range, 2.0-24.3 months) but only one of them harbored ALK resistance mutations (Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2021.100337).
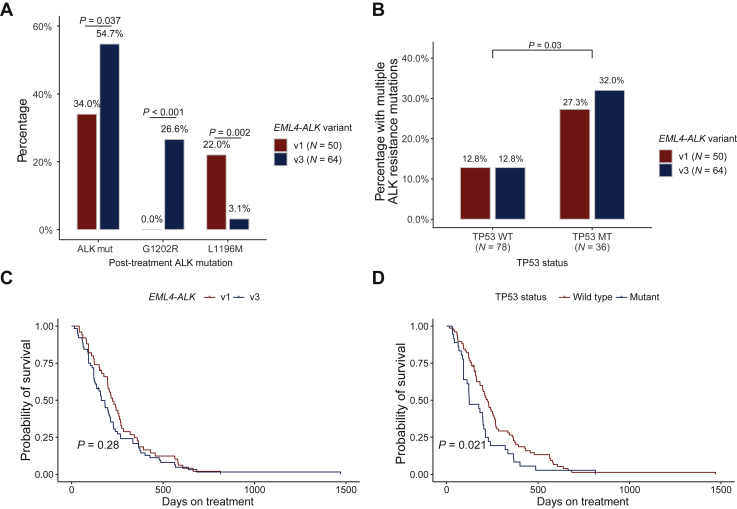
Figure 2.
(A) ALK resistance mutations in plasma samples after progression on an ALK TKI according to EML4-ALK variant. (B) Multiple frequencies of ALK resistance mutations in plasma samples after progression on an ALK TKI according to EML4-ALK variants and TP53 status. (C) Kaplan–Meier curve of RFS in patients stratified by EML4-ALK variants. (D) Kaplan–Meier curve of RFS in patients stratified by TP53 status.
ALK, anaplastic lymphoma kinase; EML4, echinoderm microtubule-associated protein-like 4; RFS, recurrence-free survival; TKI, tyrosine kinase inhibitor.
Second-generation ALK TKIs were mostly used as second-line ALK TKI after crizotinib (Supplementary Figure S5, available at https://doi.org/10.1016/j.esmoop.2021.100337). The median duration of second-generation ALK TKIs as second-line ALK TKI was 6.6 months (range, 0.5-22.5 months). We detected an ALK resistance mutation in plasma from 24 (56%) of 43 patients relapsing on a second-generation ALK TKI after crizotinib (Figure 1A). The most frequently observed ALK mutation was G1202R and E1210K/Q, detected in 11 (26%) samples and 6 (14%) samples, respectively (Figure 1B). Ten (23%) plasma specimens contained ≥2 ALK mutations (Figure 1A).
Figure 1.
(A) Number of ALK resistance mutation identified in samples from patients progressing on different ALK TKIs (B) Number of ALK resistance mutation identified in samples from patients progressing on different ALK TKIs.
ALK, anaplastic lymphoma kinase; TKI, tyrosine kinase inhibitor; WT, wild type.
Six patients received lorlatinib after progressing on their first ALK TKI treatment (four with crizotinib and 2 with alectinib) and only two had ALK resistance mutations. Another 16 patients received lorlatinib as third- or higher-line ALK TKI treatment. ALK resistance mutations were identified in 11 (69%) specimens (Figure 1A) among these 16 postlorlatinib treatment plasma samples. The most recurrently seen ALK resistance mutations were G1202R (31%), L1196M (31%), F1174V/L/C (25%), D1203N (25%), and G1269A (19%; Figure 1B). Moreover, seven specimens contained two or more ALK resistance mutations, including compound mutations ALK D1203N/L1196M, ALK G1202R/L1196M, and ALK G1202R/F1174C (Supplementary Figure S6, available at https://doi.org/10.1016/j.esmoop.2021.100337).
Furthermore, the frequencies of concurrent (≥2) ALK resistance mutations in the postprogression specimens exhibited a significant increase along with the sequential use of first-, second-, and third-generation ALK TKIs (P = 0.0009, Cochran Armitage test; Figure 1B), which were 12% (7/66), 23% (10/43), and 44% (7/16), respectively.
ALK resistance mutations by ALK rearrangement variants and TP53 mutant status
Among the 116 patients, 106 (91%) had an echinoderm microtubule-associated protein-like 4 (EML4)-ALK fusion. The most frequent EML4-ALK variant was variant 3 (v3) [(E6; A20)], which was identified in 49 patients (42%), whereas variant 1 (v1) [(E13; A20)] was found in 37 patients (32%; Table 1). Overall, the v1 form was identified in 50 PD samples, whereas v3 was found in 64 PD samples. ALK resistance mutations were identified in 17 patients (v1 34.0% versus v3 54.7%; P = 0.037; Figure 2A). Consistent with prior reports,17 ALK G1202R was significantly more common in patients with v3 variants than in v1 (27% versus 0%; P < 0.001). Moreover, we identified that ALK L1196M, by contrast, was more common in v1 than in v3 with statistical significance (22% versus 3%; P = 0.005). Of note, the frequency of v1 or v3 was balanced among PD samples from different types or different lines of ALK TKIs treatment (Supplementary Figure S7, available at https://doi.org/10.1016/j.esmoop.2021.100337).
Prior studies indicated a differential response of EML4-ALK v1 versus v3 to ALK TKIs18,19 as well as TP53 mutations as a poor prognostic factor for progression-free survival (PFS) and overall survival.20 Further studies also showed that EML4-ALK v3/TP53mt patients had even shorter median PFS.21 These studies mainly investigated the impact of EML4-ALK variants and concurrent TP53 mutations on the aspect of clinical outcome, but we extended to investigate the resistance mutations in the setting of TP53 status and EML4-ALK variants. Although TP53 mutant patients showed no significant higher rate of harboring ALK resistance mutations in either v1 or v3 (P = 0.28 and 0.72; Fisher’s exact test, one-sided; Supplementary Figure S8, available at https://doi.org/10.1016/j.esmoop.2021.100337), we noticed that TP53 mutant patients were significantly associated with a higher rate of multiple (≥2) ALK resistance mutations (P = 0.03; Fisher’s exact test; Figure 2B), and v3+/TP53 mutant patients had the highest rate of harboring multiple ALK resistance mutations (32.5%, 8/25). Furthermore, consistent with prior studies, we found that TP53 mutant patients had significantly shorter PFS than TP53 wild-type patients (Figure 2C). However, no significant difference was found between EML4-ALK v1 and v3 patients (Figure 2D).
Dynamic changes in plasma ALK mutations during sequential ALK TKI therapy
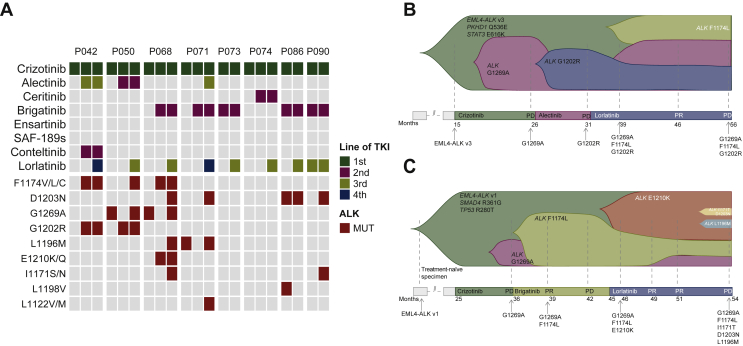
Serial plasma analysis has been shown to reveal the dynamic change in the secondary ALK resistance mutations spectrum.7 Eight patients which received sequential use of three generations of ALK TKIs had paired prelorlatinib and postlorlatinib treatment samples. Four of them developed at least one new ALK mutation (Figure 3A). Two representative cases of molecular evolution in plasma (P050 and P068) are shown in Figure 3B and C. G1269A emerged as the resistant mechanism to crizotinib and then disappeared after treatment with second-generation TKIs in both cases. P050 then developed G1202R after 5 months of alectinib treatment. During treatment with brigatinib, P068 initially developed F1174L and then E1210K at the progression of disease. Tumors eventually progressed in both patients after lorlatinib treatment with the accumulation of multiple ALK resistance mutations, whereas G1269A, which was sensitive to second-generation ALK TKI in both cases, came back after lorlatinib treatment.
Figure 3.
(A) ALK resistance mutations of serial plasma samples during sequential treatment with crizotinib, one second-generation ALK TKI, and lorlatinib. ALK-activating mutations detected along the disease course of (B) Patient P050 and (C) Patient P068.
ALK, anaplastic lymphoma kinase; MUT, mutation; TKI, tyrosine kinase inhibitor.
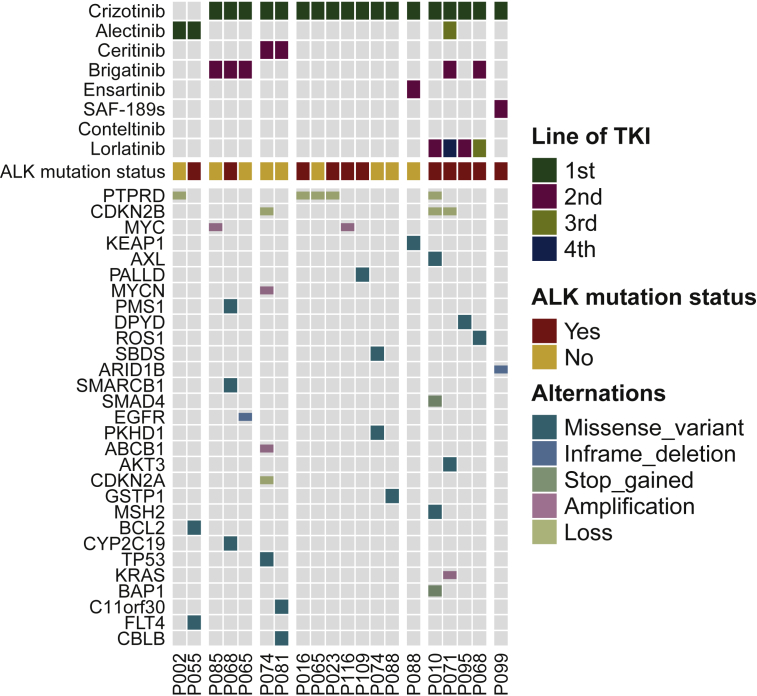
Figure 4.
Non-ALK acquired mutations detected using paired pretreatment and post-treatment plasma samples.
ALK, anaplastic lymphoma kinase.
ALK-independent mechanisms of resistance
Although ALK resistance mutations were the predominant mechanism of resistance to ALK TKIs, we observed that 55% of postprogression ALK TKI specimens were negative for ALK resistance mutations. To investigate the potential role of off-target mechanisms of resistance, we evaluated the spectrum of non-ALK acquired mutations in paired pre- and post-TKI plasma samples (Figure 4).
Activation of other bypass kinases or downstream signaling pathways is frequently the crucial off-target mechanism that confers resistance to ALK inhibitions.6,22 PTPRD and CDKN2A/B genes were common tumor suppressors. Among our patients, PTPRD loss was identified acquired in five patients, including two samples lacking ALK mutations. Loss of two CDKN2 genes, CDKN2A and CDKN2B, were found in three patients. MYC amplification was reported as a potential mechanism of primary resistance to crizotinib.23 We found that two MYC transcription factor family genes, MYC and MYCN, were amplified after ALK TKI treatment in three patients. Other acquired alterations were also identified that may confer resistance, including a KRAS amplification and a EGFR19del.
Discussion
With advancements in genomics, there has been great progress in the treatment of lung cancer patients, especially in the ALK-positive population. In the recent Crown trial (NCT03052608), the third-generation ALK TKI lorlatinib has demonstrated superior PFS and a higher frequency of intracranial response in treatment-naïve advanced ALK-positive NSCLC patients compared with crizotinib.24 By contrast, lorlatinib has a cognitive effect and mood side-effects, which are distinct from other ALK TKIs. Although these are mostly low-grade, they can still impact the life quality of the patients. Therefore, the management of ALK TKI therapy is important for optimal outcomes in ALK-positive lung cancer.
Here we studied a cohort of 116 ALK TKI-treated lung ADC patients diagnosed over 7 years, during which time crizotinib was commonly used as first-line therapy. Most ALK-positive lung cancer patients were administrated with three generations of ALK TKIs across multiple lines of therapies. Several works have already highlighted the potential clinical utility of NGS of ctDNA for molecular profiling of acquired resistance and guiding selection of next-line therapy in ALK-positive patients.25, 26, 27 In this study, we performed our analysis on a relatively large cohort with post-treatment samples of three generations of ALK TKIs. In this sequential ALK TKI setting, ALK G1202 resistance mechanism was inclined to cases with EML4-ALK v3 fusion, which was also mentioned in a study of ALK-positive lung cancer in a US population.17 In addition, we identified the enrichment of ALK L1196M resistance in cases with EML4-ALK v1 fusion. TP53 mutations, as a poor prognostic factor for PFS and overall survival, were also found to be associated with harboring multiple ALK resistance, which emphasized the association between variants and clinical outcomes and might be considered in the optimal selection of ALK TKIs for patients. Along with the increasing use of plasma NGS in routine clinical practice, a large and detailed real-world clinical-genomic dataset became available. These real-world genomic data can provide valuable insights into the analysis of drug resistance and the variety of longitudinal treatments, allowing for guiding drug development and precision oncology.
Yoda et al.28 previously confirmed the stepwise accumulation of ALK mutations during sequential treatment through whole-exome sequencing of patients’ serial plasma samples. In the current study we highlighted that in the real-world clinical setting, accumulation of compound mutations with sequential TKI treatment can limit the therapeutic choices for lung cancer patients. Several studies reported a compound mutations rate of ∼30%-35% on treatment with multiple lines of ALK TKI.28,29 Here, we identified three compound mutations, ALK D1203N/L1196M, ALK G1202R/L1196M, and ALK G1202R/F1174C, after patients progressed on later-line lorlatinib. G1202R-based compound mutations including ALK G1202R/L1196M, ALK G1202R/F1174C, ALK G1202R/l1198F, ALK G1202R/G1269A have been shown to be potentially lorlatinib resistant through steric hindrances in the ALK kinase domain.30,31 G1202R/L1196M and G1202R/F1174 were also identified in two patients without lorlatinib treatment (Supplementary Figure S6, available at https://doi.org/10.1016/j.esmoop.2021.100337). Both cases received more than two lines of ALK TKIs and may be primary resistant to lorlatinib.
Previous clinical trials have shown that alectinib as first-line treatment can help achieve a median PFS of almost 3 years.32,33 However, in our cohort, most patients had complicated therapy history and variant disease stages. Among the 11 patients who received alectinib as first-line ALK TKI, 9 were previously treated with one or more lines of other therapies, including chemotherapy and other kinds of TKI. The two patients with the shortest PFS were previously treated with chemotherapy and gefitinib followed by chemotherapy, respectively. These may explain the relatively shorter PFS following alectinib as first-line ALK TKI in this study. Furthermore, the relatively lower rate of harboring ALK resistance mutation may still indicate a broader therapeutic opportunity to subsequent ALK TKIs compared with crizotinib.
Our studies on serial plasma samples allowed to further explore off-target mechanisms of resistance to ALK TKIs. In this study and other cohort studies,7,8 ∼70% and 50% of patients do not harbor ALK resistance mutations following first- and second/third-generation TKI, respectively. To date, a variety of different bypass signaling pathways conferring resistance to ALK inhibitors have been reported, including EGFR, MET, SHP2, MYC, YAP, and RAS/MAPK,23,34, 35, 36, 37, 38 and therefore combining other inhibitors such as MET and EGFR inhibitors can be an effective strategy to overcome this mechanism of resistance. Prior studies using postprogression biopsies mainly focused on on-target resistance mechanism.7,8 In this study, however, we investigated off-target genomic bypass mechanisms using paired pretreatment and post-treatment samples and identified several potential resistance-related alterations, such as PTPRD and CNKN2A/B loss, MYC, MYCN and KRAS amplification, and EGFR19del. However, these altered genes need further examination of their function by experiments.
Our study has several important limitations. First, as a retrospective analysis, clinical information was provided on sample submission. Therefore, complete treatment history and clinical follow-up were not available (and cannot be verified) for all patients. Furthermore, paired pretreatment and post-treatment samples were only available for some patients and as a result, we only investigated acquired non-ALK mutations in a small subset of patients. Another limitation of this analysis is that the sample sizes of several second-generation TKI-biopsy cohorts were relatively small and we analyzed them as a whole.
In conclusion, our data depicted the changes of TKI-resistance landscape during sequential ALK TKI treatment in lung ADC patients. The association between resistant mutations and specific ALK rearrangement variants/TP53 status as well as accumulation of multiple ALK resistant mutations with lines of therapies should be considered for better patient management and disease outcomes.
Acknowledgments
Funding
None declared.
Disclosure
XC, RY, HB, JL, XW, and YS are employees of Nanjing Geneseeq Technology Inc. All other authors have declared no conflicts of interest.
Contributor Information
B. Liang, Email: 105778271@qq.com.
K. Lu, Email: 13605179453@126.com.
Supplementary data
References
- 1.Rosas G., Ruiz R., Araujo J.M., Pinto J.A., Luis Mas L. ALK rearrangements: biology, detection and opportunities of therapy in non-small cell lung cancer. Crit Rev Oncol Hematol. 2019;136:48–55. doi: 10.1016/j.critrevonc.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Shaw A.T., Yeap B.Y., Mino-Kenudson M., et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoang T., Myung S.K., Pham T.T., Park B. Efficacy of crizotinib, ceritinib, and alectinib in ALK-positive non-small cell lung cancer treatment: a meta-analysis of clinical trials. Cancers (Basel) 2020;12:526. doi: 10.3390/cancers12030526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon B.J., Besse B., Bauer T.M., et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19:1654–1667. doi: 10.1016/S1470-2045(18)30649-1. [DOI] [PubMed] [Google Scholar]
- 5.Kim D.W., Tiseo M., Ahn M.J., et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017;35:2490–2498. doi: 10.1200/JCO.2016.71.5904. [DOI] [PubMed] [Google Scholar]
- 6.Lin J.J., Riely G.J., Shaw A.T. Targeting ALK: precision medicine takes on drug resistance. Cancer Discov. 2017;7:137–155. doi: 10.1158/2159-8290.CD-16-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagogo-Jack I., Rooney M., Lin J.J., et al. Treatment with next-generation ALK inhibitors fuels plasma ALK mutation diversity. Clin Cancer Res. 2019;25:6662–6670. doi: 10.1158/1078-0432.CCR-19-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gainor J.F., Dardaei L., Yoda S., et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6:1118–1133. doi: 10.1158/2159-8290.CD-16-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Z., Yang N., Ou Q., et al. Investigating novel resistance mechanisms to third-generation EGFR tyrosine kinase inhibitor osimertinib in non–small cell lung cancer patients. Clin Cancer Res. 2018;24:3097–3107. doi: 10.1158/1078-0432.CCR-17-2310. [DOI] [PubMed] [Google Scholar]
- 10.Rolfo C., Mack P.C., Scagliotti G.V., et al. Liquid biopsy for advanced non-small cell lung cancer (NSCLC): a statement paper from the IASLC. J Thorac Oncol. 2018;13:1248–1268. doi: 10.1016/j.jtho.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 11.Shu Y., Wu X., Tong X., et al. Circulating tumor DNA mutation profiling by targeted next generation sequencing provides guidance for personalized treatments in multiple cancer types. Sci Rep. 2017;7:583. doi: 10.1038/s41598-017-00520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong L., Ding N., Tong X., et al. Tumor-derived DNA from pleural effusion supernatant as a promising alternative to tumor tissue in genomic profiling of advanced lung cancer. Theranostics. 2019;9:5532. doi: 10.7150/thno.34070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cibulskis K., Lawrence M.S., Carter S.L., et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31:213–219. doi: 10.1038/nbt.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang H., Bergmann E.A., Arora K., et al. Indel variant analysis of short-read sequencing data with Scalpel. Nat Protoc. 2016;11:2529–2548. doi: 10.1038/nprot.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin J.J., Zhu V.W., Yoda S., et al. Impact of EML4-ALK variant on resistance mechanisms and clinical outcomes in ALK-positive lung cancer. J Clin Oncol. 2018;36:1199. doi: 10.1200/JCO.2017.76.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christopoulos P., Endris V., Bozorgmehr F., et al. EML4-ALK fusion variant V3 is a high-risk feature conferring accelerated metastatic spread, early treatment failure and worse overall survival in ALK+ non-small cell lung cancer. Int J Cancer. 2018;142:2589–2598. doi: 10.1002/ijc.31275. [DOI] [PubMed] [Google Scholar]
- 19.Zhang S.S., Nagasaka M., Zhu V.W., Ou S.I. Going beneath the tip of the iceberg. Identifying and understanding EML4-ALK variants and TP53 mutations to optimize treatment of ALK fusion positive (ALK+) NSCLC. Lung Cancer. 2021;158:126–136. doi: 10.1016/j.lungcan.2021.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Qin K., Hou H., Liang Y., Zhang X. Prognostic value of TP53 concurrent mutations for EGFR-TKIs and ALK-TKIs based targeted therapy in advanced non-small cell lung cancer: a meta-analysis. BMC Cancer. 2020;20:1–16. doi: 10.1186/s12885-020-06805-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christopoulos P., Kirchner M., Bozorgmehr F., et al. Identification of a highly lethal V3+ TP53+ subset in ALK+ lung adenocarcinoma. Int J Cancer. 2019;144:190–199. doi: 10.1002/ijc.31893. [DOI] [PubMed] [Google Scholar]
- 22.Tabbò F., Reale M.L., Bironzo P., Scagliotti G.V. Resistance to anaplastic lymphoma kinase inhibitors: knowing the enemy is half the battle won. Transl Lung Cancer Res. 2020;9:2545. doi: 10.21037/tlcr-20-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rihawi K., Alfieri R., Fiorentino M., et al. MYC amplification as a potential mechanism of primary resistance to crizotinib in ALK-rearranged non-small cell lung cancer: a brief report. Transl Oncol. 2019;12:116–121. doi: 10.1016/j.tranon.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw A.T., Bauer T.M., de Marinis F., et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383:2018–2029. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
- 25.McCoach C.E., Blakely C.M., Banks K.C., et al. Clinical utility of cell-free DNA for the detection of ALK fusions and genomic mechanisms of ALK inhibitor resistance in non–small cell lung cancer. Clin Cancer Res. 2018;24:2758–2770. doi: 10.1158/1078-0432.CCR-17-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dagogo-Jack I., Brannon A.R., Ferris L.A., et al. Tracking the evolution of resistance to ALK tyrosine kinase inhibitors through longitudinal analysis of circulating tumor DNA. JCO Precis Oncol. 2018;2:1–14. doi: 10.1200/PO.17.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dietz S., Christopoulos P., Yuan Z., et al. Longitudinal therapy monitoring of ALK-positive lung cancer by combined copy number and targeted mutation profiling of cell-free DNA. EBioMedicine. 2020;62:103103. doi: 10.1016/j.ebiom.2020.103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoda S., Lin J.J., Lawrence M.S., et al. Sequential ALK inhibitors can select for lorlatinib-resistant compound ALK mutations in ALK-positive lung cancer. Cancer Discov. 2018;8:714–729. doi: 10.1158/2159-8290.CD-17-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christopoulos P., Dietz S., Angeles A.K., et al. Earlier extracranial progression and shorter survival in ALK-rearranged lung cancer with positive liquid rebiopsies. Transl Lung Cancer Res. 2021;10:2118–2131. doi: 10.21037/tlcr-21-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma G.G., Cortinovis D., Agustoni F., et al. A Compound L1196M/G1202R ALK mutation in a patient with ALK-positive lung cancer with acquired resistance to brigatinib also confers primary resistance to lorlatinib. J Thorac Oncol. 2019;14:e257–e259. doi: 10.1016/j.jtho.2019.06.028. [DOI] [PubMed] [Google Scholar]
- 31.Zhu V.W., Nagasaka M., Madison R., Schrock A.B., Cui J., Ignatius S.H. A novel sequentially evolved EML4-ALK variant 3 G1202R/S1206Y double mutation in cis confers resistance to lorlatinib: a brief report and literature review. JTO Clin Res Rep. 2021;2:100116. doi: 10.1016/j.jtocrr.2020.100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters S., Camidge D.R., Shaw A.T., et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 33.Mok T., Camidge D., Gadgeel S., et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol. 2020;31:1056–1064. doi: 10.1016/j.annonc.2020.04.478. [DOI] [PubMed] [Google Scholar]
- 34.Engelman J.A., Zejnullahu K., Mitsudomi T., et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki T., Koivunen J., Ogino A., et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 2011;71:6051–6060. doi: 10.1158/0008-5472.CAN-11-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dardaei L., Wang H.Q., Singh M., et al. SHP2 inhibition restores sensitivity in ALK-rearranged non-small-cell lung cancer resistant to ALK inhibitors. Nat Med. 2018;24:512–517. doi: 10.1038/nm.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yun M.R., Choi H.M., Lee Y.W., et al. Targeting YAP to overcome acquired resistance to ALK inhibitors in ALK-rearranged lung cancer. EMBO Mol Med. 2019;11:e10581. doi: 10.15252/emmm.201910581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crystal A.S., Shaw A.T., Sequist L.V., et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science. 2014;346:1480–1486. doi: 10.1126/science.1254721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.