Abstract
Background
Neutrophils and albumin had been shown to be independent predictors of mortality from various diseases. Purpose of this study was to investigate the effect of neutrophil-to-albumin ratio (NPAR) as an independent predictor of mortality in heart failure (HF) patients.
Methods
Data were extracted from Medical Information Mart for Intensive Care-III database. Primary outcome was 30-day mortality, secondary outcomes were in-hospital, 90-day, 365-day mortality, length of stay (LOS) in hospital. Cox proportional hazards regression model and receiver operating characteristic (ROC) curve analysis and Pearson correlation analysis were used.
Results
The HR (95% CI) values of the mid-tertile and the upper tertile were 1.27 (1.01 to 1.59) and 2.29 (1.87 to 2.81) in 30-day mortality compared with the reference. The trend continued after adjusted for demographic and clinical variables. In the secondary outcomes were the same trends. The data of the Second Affiliated Hospital of Wenzhou Medical University showed the correlation coefficient between hospital LOS with NPAR.
Conclusion
NPAR was an independent factor of mortality in HF patients, which was correlated with hospital LOS. Our results need to be verified by prospective studies.
Keywords: neutrophils, albumin, neutrophil-to-albumin ratio, all-cause mortality, heart failure
Introduction
Heart failure (HF) was a clinical syndrome characterized by symptoms and signs caused by cardiac abnormality that resulting in a reduced cardiac output and/or elevated intracardiac pressure at rest or during stress.1 It was a common disease among the elderly and a common cause of hospitalization in the elderly population aged 65 and above.2 It was currently a major public health problem in the world.3 Heart failure affected more than 26 million people worldwide, and its prevalence was increasing.4 It was estimated that the prevalence of heart failure worldwide was between 0.1% and 6.7%.5
The determination of peripheral leukocytes, mainly neutrophils, and counting was a cheap and widely available method to assess the presence of any inflammation. Studies had shown that patients with heart failure had higher circulating levels of pro-inflammatory cytokines, including non-cellular and cellular components, compared with healthy people.6 Albumin was a medium-sized protein with a molecular weight of 66–69 kDa, accounting for more than half of the whole serum body’s composition.7 Albumin had many functions, including osmoregulation, antioxidation and anti inflammation.8,9 Hypoalbuminemia was common in patients with HF, and was associated with the mortality of patients with heart failure in both acute and chronic conditions.10,11 In elderly patients without heart failure, hypoalbuminemia was found to be significantly related to the incidence of heart failure.12,13 Recently, several studies had combined these two markers and found that neutrophil-to-albumin ratio (NPAR) could be used as an inflammation based prognostic predictor in patients with STEMI, acute kidney injury, septic shock, rectal cancer, mucinous colorectal adenocarcinoma, or cardiogenic shock.14–19
However, to our knowledge, no previous studies had explored the prognostic value of NPAR in HF patients. The purpose of this study was to investigate the association between admission level of NPAR and mortality in HF patients by using the Medical Information Mart for Intensive Care III database version 1.4 (MIMIC III v1.4) and the data of the Second Affiliated Hospital of Wenzhou Medical University.
Materials and Methods
Data Source
Our research used the data from Medical Information Mart for Intensive Care III database version 1.4 (MIMIC III v1.4) and the Second Affiliated Hospital of Wenzhou Medical University. MIMIC-III was a publicly available single-center critical care database, which was approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center (BIDMC, Boston, MA, USA) and the Massachusetts Institute of Technology (MIT, Cambridge, MA, USA), included information on more than 40,000 patients who were admitted to various ICUs of BIDMC in Boston, Massachusetts from 2001 to 2012.20 The database included demographic, vital signs, laboratory tests, vital status and other chart events; documents International Classification of Diseases and Ninth Revision (ICD-9) codes; the physiological data of each hour were recorded and confirmed by ICU nurses; and stored written evaluations of radiologic films by specialists covering in the corresponding time period. We completed the National Institutes of Health online course and passed the Examination for Protecting Human Research Participants and applied for access. Then the data was extracted from the database for research purposes, and the patient’s identity information was hidden to protect their privacy. Regarding patients data from the Second Affiliated Hospital of Wenzhou Medical University, the Institutional Research and Ethics Institute of the Second Affiliated Hospital of Wenzhou Medical University approved this study involving human subjects. Since the data was anonymous, no informed consent was required. (Ethical Lot Number: 2021-K-71-01)
Population Selection Criteria
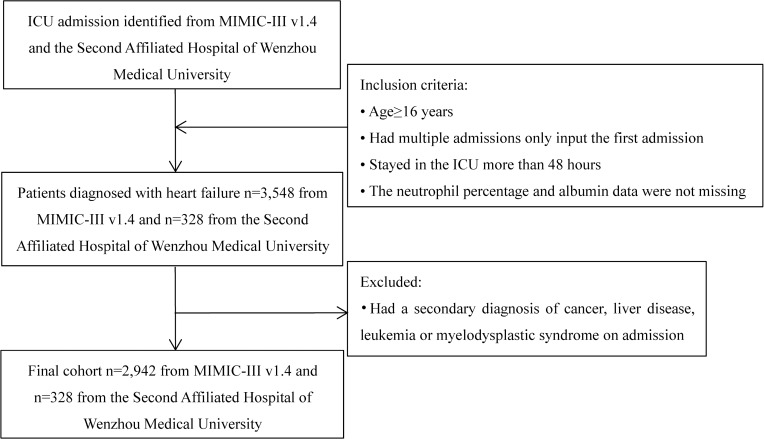
We included all ICU patients (aged ≥16 years and stayed in the hospital > 48 hours) with the primary diagnosis of HF using International Classification of Diseases (ICD)-9 diagnosis codes (ICD-9 codes for HF) in the MIMIC-III database (Additional file S1). Patients were excluded if they had [1] a secondary diagnosis of cancer, leukemia, myelodysplastic syndrome, or liver disease on admission; or [2] incomplete or unobtainable documented neutrophil percentage, albumin, or other important data records. 2942 patients were finally included in the MIMIC III database. We collected 328 patients admitted from June 2020 to May 2021 in the Second Affiliated Hospital of Wenzhou Medical University, subjects enrolled should also meet the above criteria. For multiple admissions, only the data of the first admission was included.
Data Extraction
The data on the first day of ICU admission were extracted from MIMIC III using Structured Query Language (SQL) with Navicat Premium including demographic data, basic vital signs, comorbidities, basic laboratory parameters and scoring system before treatment. If the missing variable was greater than 10%, the variable would not be included. Demographic information included age, gender and ethnicity. Vital signs on admission included systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MBP), heart rate, respiratory rate (RR), temperature and pulse oximetry-derived oxygen saturation (SpO2) during oxygen therapy. Comorbidities included congestive heart failure (CHF), cardiac arrhythmias, infected, valvular disease, pulmonary circulation, chronic pulmonary, hypertension and renal failure. Laboratory findings included albumin, neutrophil percentage, NPAR, serum creatinine, glucose, serum urea nitrogen, hematocrit, hemoglobin, white blood cell (WBC) count, platelet, red blood cell distribution width (RDW), sodium, potassium, bicarbonate, anion gap, activated partial thromboplastin time (APTT), international normalized ratio (INR) and prothrombin time (PT). If the patient had undergone multiple laboratory tests during hospitalization, only the initial test results were used for analysis. Two scoring systems (the Simplified Acute Physiology Score II [SAPS II] and the Acute Physiology Score III [APS III]) were calculated within the first 48 hours after admission using the values associated with the greatest severity of illness. In-hospital management data included vasopressin use, renal replacement treatment (RRT) and mechanical ventilation administration. The collection of patients data from the Second Affiliated Hospital of Wenzhou Medical University was the same as above.
The neutrophil percentage was defined as the percentage of neutrophils in white blood cells. The NPAR was calculated as the neutrophil percentage as the numerator divided by albumin using the same blood samples drawn on admission according to the formula: (Neutrophil percentage (%) * 100/Albumin (g/dl)).
The start date for follow-up was the date of the patient’s admission. The date of death was obtained from Social Security Death Index records from the US government. The primary outcome of our study was all-cause 30-day mortality. We selected all-cause in-hospital, 90-day, 365-day mortality and hospital LOS as secondary outcomes. In the data of the Second Affiliated Hospital of Wenzhou Medical University, we calculated the hospital LOS through the patients’ admission date and discharge date, and obtained the outcome of in-hospital mortality.
Statistical Analysis
Baseline characteristics were divided into three groups according to the NPAR and were presented as frequency (percent) for categorical data and as mean (SD) or IQR for continuous data. We did comparisons between groups by the χ2 test or Fisher’s exact test for categorical variables and the variance analysis or the Kruskal–Wallis test for continuous ones.
Used the Cox proportional hazards model to examine the association between NPAR and the results. The results were analyzed according to the tertiles of the NPAR level. The first tertile group was regarded as the reference group. The results were expressed as HR with 95% CI or β with 95% CI. And used two adjusted models for multivariate analysis. The confounders selected were based on their relevance to the outcome or changes in effect estimates of more than 10% or were considered meaningful by clinicians in our model. In adjust I, we adjusted covariates for age, gender and ethnicity. In adjust II, covariates were adjusted for age, gender, ethnicity, anion gap, bicarbonate, APTT, INR, PT, serum urea nitrogen, RDW, heart rate, SBP, DBP, RR, temperature, SpO2, cardiac arrhythmias, hypertension, vasopressin use, infected, SAPSII and APSIII. Performed trend testing to check differences between groups.
In addition, we performed stratification analysis to confirm whether the effect of NPAR differs in each of the subgroups that were classified by demographic information (eg, age, gender and ethnicity), vital signs (eg, MAP, heart rate, RR), comorbidities (eg CHF, valvular disease, hypertension, renal failure), laboratory parameters (eg, serum creatinine, glucose, serum urea nitrogen, RDW, anion gap, APTT, INR, PT), scoring systems (SAPSII and APSIII).
In order to further evaluate the predictive value of NPAR, we performed receiver operating characteristic (ROC) curve analysis on the 30-day mortality rate based on SPASII score and SPASII score plus NPAR.
According to the data of patients in the Second Affiliated Hospital of Wenzhou Medical University, we also divided the patients into tertiles according to NPAR value and described the baseline characteristics of the patients. Then the NPAR value was analyzed by Pearson correlation analysis with hospital LOS.
A two-tailed p value<0.05 was deemed statistically significant. All the analyses were conducted with the R software (Version 3.6.1, http://www.r-project.org).
Results
Subject Characteristics
After excluding patients who did not meet the inclusion criteria, we included a total of 2942 patients in the MIMIC III database. (Figure 1) According to the tertiles of NPAR, we divided the patients into three groups. Baseline characteristics classified by NPAR tertiles were presented in Table 1. 980 (33.31%) patients were in the low NPAR group (NPAR<22.56), 980 (33.31%) patients were in the medium NPAR group (22.56–27.64), and 982 (33.38%) patients were in the high NPAR group (NPAR>27.64). Participants with higher calibrated NPAR (NPAR>27.64) were more likely to be white and to report a history of infected; they also had higher levels of serum urea nitrogen, WBC, neutrophil percentage, platelet, RDW, and PT and were more likely to use vasopressin and mechanical ventilation than those with lower NPAR (<22.56). Their scoring systems (SAPS II and APS III) were also higher than those with lower NPAR (<22.56). The baseline characteristics of patients in the Second Affiliated Hospital of Wenzhou Medical University were shown in additional file Table S2. The group with higher NPAR had lower DBP, higher temperature and higher risk of acute coronary syndrome, heart valve disease, chronic kidney disease and pneumonia. In addition, the data from the Second Affiliated Hospital of Wenzhou Medical University also showed that for those patients with higher NPAR, their hospital LOS was longer.
Figure 1.
Flow chart of cohort selection.
Abbreviations: ICU, intensive care unit; MIMIC-III, Medical Information Mart for Intensive Care-III.
Table 1.
Baseline Characteristics of the Study Population
| Characteristics | The Level of NPAR | P value | ||
|---|---|---|---|---|
| <22.56 (n=980) | 22.56–27.64 (n=980) | >27.64 (n=982) | ||
| NPAR, dl/g | 18.55 ± 4.19 | 25.03 ± 1.40 | 33.82 ± 6.40 | <0.001 |
| Clinical parameters | ||||
| Age, years | 72.13 ± 13.95 | 72.99 ± 13.52 | 73.11 ± 14.13 | 0.237 |
| Gender, n(%) | 0.420 | |||
| Male | 520 (53.06) | 497 (50.71) | 494 (50.31) | |
| Female | 460 (46.94) | 483 (49.29) | 488 (49.69) | |
| Ethnicity, n(%) | <0.001 | |||
| White | 687 (70.10) | 712 (72.65) | 724 (73.73) | |
| Black | 153 (15.61) | 107 (10.92) | 88 (8.96) | |
| Other | 140 (14.29) | 161 (16.43) | 170 (17.31) | |
| Vital signs | ||||
| SBP, mmHg | 118.54 ± 18.36 | 117.26 ± 17.13 | 113.45 ± 16.82 | <0.001 |
| DBP, mmHg | 60.11 ± 11.80 | 58.02 ± 10.17 | 55.95 ± 10.38 | <0.001 |
| MAP, mmHg | 77.31 ± 11.89 | 75.65 ± 10.49 | 73.38 ± 11.01 | <0.001 |
| Heart rate, beats/minute | 83.32 ± 16.61 | 84.45 ± 16.19 | 87.92 ± 16.11 | <0.001 |
| RR, times/minute | 19.79 ± 3.92 | 20.16 ± 4.07 | 20.34 ± 4.16 | 0.009 |
| Temperature, °C | 36.75 ± 0.65 | 36.78 ± 0.67 | 36.74 ± 0.70 | 0.461 |
| SpO2, % | 96.89 ± 2.07 | 96.77 ± 2.63 | 96.99 ± 2.83 | 0.170 |
| Comorbidities, n(%) | ||||
| CHF | 942 (96.12) | 944 (96.33) | 956 (97.35) | 0.273 |
| Cardiac arrhythmias | 526 (53.67) | 571 (58.27) | 559 (56.92) | 0.109 |
| Infected | 412 (42.04) | 547 (55.82) | 691 (70.37) | <0.001 |
| Valvular disease | 245 (25.00) | 246 (25.10) | 195 (19.86) | 0.007 |
| Pulmonary circulation | 134 (13.67) | 137 (13.98) | 109 (11.10) | 0.113 |
| Chronic pulmonary | 324 (33.06) | 354 (36.12) | 306 (31.16) | 0.063 |
| Hypertension | 636 (64.90) | 634 (64.69) | 528 (53.77) | <0.001 |
| Renal failure | 330 (33.67) | 341 (34.80) | 325 (33.10) | 0.721 |
| Laboratory parameters | ||||
| Albumin, g/dl | 3.73 ± 0.48 | 3.31 ± 0.34 | 2.62 ± 0.41 | <0.001 |
| Serum creatinine, mg/dl | 2.14 ± 2.11 | 2.10 ± 1.91 | 2.10 ± 1.75 | 0.838 |
| Glucose, mg/dl | 160.28 ± 93.25 | 168.67 ± 96.08 | 155.49 ± 98.02 | 0.009 |
| Serum urea nitrogen, mg/dl | 38.63 ± 27.12 | 40.65 ± 27.80 | 44.10 ± 29.20 | <0.001 |
| Hematocrit, % | 35.18 ± 6.73 | 33.29 ± 6.57 | 31.49 ± 5.71 | <0.001 |
| Hemoglobin, g/dl | 11.66 ± 2.28 | 10.98 ± 2.23 | 10.30 ± 1.94 | <0.001 |
| WBC, 109/l | 11.90 ± 13.77 | 13.10 ± 7.37 | 15.25 ± 8.63 | <0.001 |
| Neutrophil percentage, % | 69.53 ± 17.54 | 82.78 ± 8.00 | 86.53 ± 6.97 | <0.001 |
| Platelet, 109/l | 234.15 ± 119.42 | 251.99 ± 118.31 | 266.00 ± 140.54 | <0.001 |
| RDW, fl | 15.50 ± 2.18 | 15.65 ± 2.18 | 16.26 ± 2.30 | <0.001 |
| Sodium, mmol/l | 137.87 ± 5.28 | 138.04 ± 5.44 | 138.36 ± 5.90 | 0.135 |
| Potassium, mmol/l | 4.42 ± 0.88 | 4.38 ± 0.78 | 4.29 ± 0.78 | 0.002 |
| Bicarbonate, mmol/l | 24.68 ± 5.83 | 24.20 ± 5.89 | 23.60 ± 5.90 | <0.001 |
| Anion gap, mmol/l | 16.92 ± 4.63 | 16.52 ± 4.50 | 15.78 ± 4.45 | <0.001 |
| APTT, second | 38.58 ± 25.17 | 38.91 ± 24.87 | 40.37 ± 25.11 | 0.256 |
| INR | 1.76 ± 1.46 | 1.92 ± 1.94 | 1.87 ± 1.74 | 0.126 |
| PT, second | 17.52 ± 9.58 | 18.74 ± 13.45 | 19.03 ± 15.55 | 0.029 |
| Scoring systems | ||||
| SAPSII | 40.07 ± 12.80 | 40.88 ± 11.57 | 45.41 ± 14.03 | <0.001 |
| APSIII | 49.55 ± 18.08 | 50.97 ± 16.63 | 59.91 ± 20.80 | <0.001 |
| In-hospital management, n(%) | ||||
| Vasopressin use | 336 (34.29) | 368 (37.55) | 470 (47.86) | <0.001 |
| RRT | 71 (7.24) | 69 (7.04) | 89 (9.06) | 0.184 |
| Mechanical ventilation | 382 (38.98) | 424 (43.27) | 486 (49.49) | <0.001 |
| Primary outcome, n(%) | ||||
| 30-day mortality | 137 (13.98) | 170 (17.35) | 291 (29.63) | <0.001 |
| Secondary outcomes | ||||
| In-hospital mortality, n(%) | 115 (11.73) | 126 (12.86) | 228 (23.22) | <0.001 |
| 90-day mortality, n(%) | 212 (21.63) | 263 (26.84) | 404 (41.14) | <0.001 |
| 365-day mortality, n(%) | 347 (35.41) | 408 (41.63) | 566 (57.64) | <0.001 |
| Hospital LOS, day | 10.79 ± 10.92 | 11.53 ± 9.30 | 14.66 ± 12.68 | <0.001 |
Note: The data came from patients in MIMIC III database.
Abbreviations: NPAR, ratio of neutrophil to albumin; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; RR, respiratory rate; SpO2, pulse oximetry-derived oxygen saturation; CHF, congestive heart failure; WBC, white blood cell count; RDW, red blood cell distribution width; APTT, activated partial thromboplastin time; INR, international normalized ratio; PT, prothrombin time; SAPSII, simple acute physiology score II; APSIII, acute physiology score III; RRT, renal replacement treatment; LOS, length of stay.
Association Between Neutrophil-to-Albumin Ratios and Mortality
In-hospital deaths and during the 30-day, 90-day and 365-day follow-up period, 469, 598, 879 and 1321 deaths were recorded respectively in the MIMIC III database. The results of the relationship between NPAR and mortality and hospital LOS in HF patients were shown in Table 2.
Table 2.
HR (95% CI) or β (95% CI) for Outcomes Across Groups of Neutrophil-to-Albumin Ratios
| Non-Adjusted | Adjust I | Adjust II | ||||
|---|---|---|---|---|---|---|
| Mortality | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value |
| 30-Day all-cause mortality | ||||||
| NPAR | 1.04 (1.03, 1.05) | <0.001 | 1.04 (1.03, 1.05) | <0.001 | 1.02 (1.01, 1.03) | <0.001 |
| Tertiles | ||||||
| <22.56 | 1.00 | 1.00 | 1.00 | |||
| 22.56–27.64 | 1.27 (1.01, 1.59) | 0.037 | 1.22 (0.97, 1.53) | 0.085 | 1.18 (0.93, 1.49) | 0.171 |
| >27.64 | 2.29 (1.87, 2.81) | <0.001 | 2.18 (1.78, 2.67) | <0.001 | 1.52 (1.22, 1.90) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | |||
| In-hospital all-cause mortality | ||||||
| NPAR | 1.02 (1.00, 1.03) | 0.004 | 1.01 (1.00, 1.02) | 0.017 | 1.00 (0.99, 1.02) | 0.416 |
| Tertiles | ||||||
| <22.56 | 1.00 | 1.00 | 1.00 | |||
| 22.56–27.64 | 1.01 (0.78, 1.30) | 0.968 | 0.96 (0.74, 1.24) | 0.752 | 0.99 (0.76, 1.29) | 0.962 |
| >27.64 | 1.37 (1.10, 1.72) | 0.006 | 1.31 (1.04, 1.64) | 0.020 | 1.09 (0.85, 1.40) | 0.483 |
| P for trend | 0.002 | 0.007 | 0.436 | |||
| 90-Day all-cause mortality | ||||||
| NPAR | 1.04 (1.03, 1.04) | <0.001 | 1.04 (1.03, 1.05) | <0.001 | 1.02 (1.01, 1.03) | <0.001 |
| Tertiles | ||||||
| <22.56 | 1.00 | 1.00 | 1.00 | |||
| 22.56–27.64 | 1.28 (1.07, 1.53) | 0.007 | 1.24 (1.03, 1.48) | 0.022 | 1.19 (0.99, 1.43) | 0.069 |
| >27.64 | 2.17 (1.84, 2.56) | <0.001 | 2.09 (1.77, 2.47) | <0.001 | 1.46 (1.22, 1.75) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | |||
| 365-Day all-cause mortality | ||||||
| NPAR | 1.03 (1.03, 1.04) | <0.001 | 1.04 (1.03, 1.04) | <0.001 | 1.02 (1.01, 1.03) | <0.001 |
| Tertiles | ||||||
| <22.56 | 1.00 | 1.00 | 1.00 | |||
| 22.56–27.64 | 1.24 (1.07, 1.43) | 0.004 | 1.20 (1.04, 1.39) | 0.012 | 1.14 (0.99, 1.33) | 0.075 |
| >27.64 | 2.01 (1.76, 2.30) | <0.001 | 1.97 (1.72, 2.26) | <0.001 | 1.43 (1.24, 1.66) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | |||
| Hospital LOS, day | β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value |
| NPAR | 0.19 (0.14, 0.24) | <0.001 | 0.19 (0.14, 0.24) | <0.001 | 0.07 (0.01, 0.12) | 0.017 |
| Tertiles | ||||||
| <22.56 | 0.00 | 0.00 | 0.00 | |||
| 22.56–27.64 | 0.74 (−0.24, 1.72) | 0.139 | 0.80 (−0.18, 1.77) | 0.109 | −0.23 (−1.18, 0.73) | 0.643 |
| >27.64 | 3.87 (2.89, 4.85) | <0.001 | 3.93 (2.95, 4.90) | <0.001 | 1.38 (0.35, 2.40) | 0.008 |
| P for trend | <0.001 | <0.001 | 0.006 | |||
Notes: Non-adjusted and Adjust I and II were derived from Cox proportional hazards regression models: Adjust I covariates were adjusted for age, gender and ethnicity; Adjust II covariates were adjusted for age, gender, ethnicity, anion gap, bicarbonate, APTT, INR, PT, serum urea nitrogen, RDW, heart rate, SBP, DBP, RR, temperature, SpO2, cardiac arrhythmias, hypertension, vasopressin use, mechanical ventilation, infected, SAPSII, APSIII. The data came from patients in MIMIC III database.
Abbreviations: NPAR, ratio of neutrophil to albumin; HR, hazard ratio; CI, confidence interval; OR, odds ratio; RRT, renal replacement treatment; APTT, activated partial thromboplastin time; INR, international normalized ratio; PT, prothrombin time; RDW, red blood cell distribution width; SBP, systolic blood pressure; DBP, diastolic blood pressure; RR, respiratory rate; SpO2, pulse oximetry-derived oxygen saturation; SAPSII, simple acute physiology score II; APSIII, acute physiology score III; LOS, length of stay.
For the primary outcome of 30-day mortality, we found that higher NPAR was related to increased risk of mortality. The HR (95% CI) values of the mid-tertile (NPAR=22.56–27.64) and the upper tertile (NPAR>27.64) were 1.27 (1.01 to 1.59) and 2.29 (1.87 to 2.81), respectively, when compared with the reference (NPAR<22.56). After adjusted for age, gender and ethnicity in adjust I, an increasing trend was also observed in the upper tertile (NPAR>27.64) was 2.18 (1.78 to 2.67). After further adjusted for potential confounders in adjust II, the upward trend remained statistically significant in the upper tertile: 1.52 (1.22 to 1.90). The similar trends were also observed for the secondary outcomes of 90-day and 365-day mortality and hospital LOS. Among the secondary results of in-hospital all-cause mortality, only the non-adjusted and adjust I observed an increasing trend in the upper tertile (NPAR>27.64) were 1.37 (1.10 to 1.72) and 1.31 (1.04 to 1.64).
Subgroup Analyses
The subgroup analysis was performed to determine the consistency of the association between NPAR and 30-day mortality in HF patients in the MIMIC III database. In the subgroup analyses, the association between the NPAR and the risk of 30-day mortality was similar for most strata (P=0.065–0.942) (Table 3). All subgroup factors showed low significance with 30-day mortality.
Table 3.
Subgroup Analysis of the Association Between NPAR and 30-Day Mortality
| Variables | No. of Patients | HR | Lower Limit of 95% CI | Upper Limit of 95% CI | P for Interaction |
|---|---|---|---|---|---|
| Age, years | 0.942 | ||||
| <75 | 1471 | 1.04 | 1.02 | 1.05 | |
| ≥75 | 1471 | 1.04 | 1.03 | 1.05 | |
| Gender | 0.381 | ||||
| Male | 1511 | 1.04 | 1.03 | 1.06 | |
| Female | 1431 | 1.04 | 1.02 | 1.05 | |
| Ethnicity | 0.491 | ||||
| White | 2123 | 1.04 | 1.03 | 1.05 | |
| Black | 348 | 1.04 | 1.02 | 1.06 | |
| Other | 471 | 1.03 | 1.00 | 1.05 | |
| MAP, mmHg | 0.165 | ||||
| <74 | 1468 | 1.03 | 1.02 | 1.04 | |
| ≥74 | 1469 | 1.04 | 1.03 | 1.06 | |
| Heart rate, beats/minute | 0.233 | ||||
| <84 | 1468 | 1.05 | 1.04 | 1.07 | |
| ≥84 | 1469 | 1.03 | 1.02 | 1.04 | |
| RR, times/minute | 0.118 | ||||
| <20 | 1468 | 1.04 | 1.03 | 1.05 | |
| ≥20 | 1469 | 1.04 | 1.03 | 1.05 | |
| CHF | 0.632 | ||||
| Yes | 2842 | 1.04 | 1.03 | 1.05 | |
| No | 100 | 1.02 | 0.95 | 1.10 | |
| Valvular disease | 0.292 | ||||
| Yes | 686 | 1.05 | 1.03 | 1.08 | |
| No | 2256 | 1.04 | 1.03 | 1.05 | |
| Hypertension | 0.447 | ||||
| Yes | 1798 | 1.04 | 1.03 | 1.05 | |
| No | 1144 | 1.03 | 1.02 | 1.05 | |
| Renal failure | 0.558 | ||||
| Yes | 996 | 1.04 | 1.03 | 1.06 | |
| No | 1946 | 1.04 | 1.03 | 1.05 | |
| Serum creatinine, mg/dl | 0.768 | ||||
| ≤1.4 | 1455 | 1.04 | 1.03 | 1.05 | |
| ≥1.5 | 1487 | 1.04 | 1.03 | 1.05 | |
| Glucose, mg/dl | 0.761 | ||||
| ≤134 | 1458 | 1.03 | 1.02 | 1.05 | |
| ≥135 | 1482 | 1.05 | 1.03 | 1.06 | |
| Serum urea nitrogen, mg/dl | 0.815 | ||||
| ≤32 | 1454 | 1.04 | 1.03 | 1.05 | |
| ≥33 | 1488 | 1.04 | 1.02 | 1.05 | |
| RDW, fl | 0.636 | ||||
| ≤15.2 | 1421 | 1.04 | 1.02 | 1.06 | |
| ≥15.3 | 1518 | 1.03 | 1.02 | 1.04 | |
| Anion gap, mmol/l | 0.065 | ||||
| ≤15 | 1379 | 1.04 | 1.03 | 1.05 | |
| ≥16 | 1563 | 1.05 | 1.03 | 1.06 | |
| APTT, second | 0.756 | ||||
| ≤31 | 1421 | 1.04 | 1.02 | 1.06 | |
| >31 | 1428 | 1.04 | 1.03 | 1.05 | |
| INR | 0.114 | ||||
| ≤1.2 | 1147 | 1.04 | 1.02 | 1.06 | |
| ≥1.3 | 1699 | 1.03 | 1.02 | 1.04 | |
| PT, second | 0.103 | ||||
| ≤14.5 | 1416 | 1.03 | 1.01 | 1.05 | |
| ≥14.6 | 1430 | 1.04 | 1.03 | 1.05 | |
| SAPSII | 0.526 | ||||
| ≤40 | 1464 | 1.04 | 1.02 | 1.06 | |
| ≥41 | 1478 | 1.03 | 1.02 | 1.04 | |
| APSIII | 0.614 | ||||
| ≤50 | 1419 | 1.03 | 1.01 | 1.05 | |
| ≥51 | 1523 | 1.03 | 1.02 | 1.04 |
Notes: HR (95% CI) were derived from Cox proportional hazards regression models. The data came from patients in MIMIC III database.
Abbreviations: NPAR, ratio of neutrophil to albumin; HR, hazard ratio; CI, confidence interval; MAP, mean arterial pressure; RR, respiratory rate; CHF, congestive heart failure; RDW, red blood cell distribution width; APTT, activated partial thromboplastin time; INR, international normalized ratio; PT, prothrombin time; SAPSII, simple acute physiology score II; APSIII, acute physiology score III.
ROC Curve Analysis
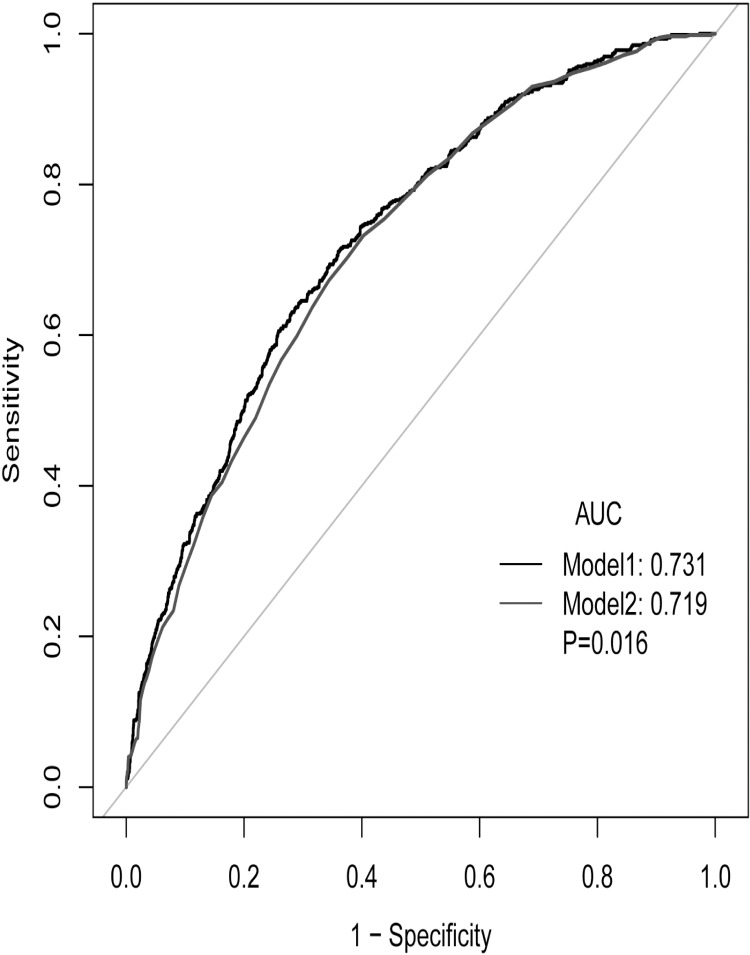
ROC curve analysis was performed to further test the potential prognostic value of NPAR in predicting the survival of HF patients in the MIMIC III database. As shown in Figure 2, when ROC curve analysis was performed combining NPAR and SAPSII score, the C statistic was higher than that of SAPSII scores (0.731 vs 0.719, 0.016).
Figure 2.
ROC curve for combining SAPSII and NPAR. (Model 1: SAPSII+NPAR; model 2: SAPSII).
Note: The data came from patients in MIMIC III database.
Abbreviations: AUC, area under the curve; SAPSII, simple acute physiology score II; APSIII, acute physiology score III; NPAR, ratio of neutrophil to albumin; ROC, receiver operator characteristic curve.
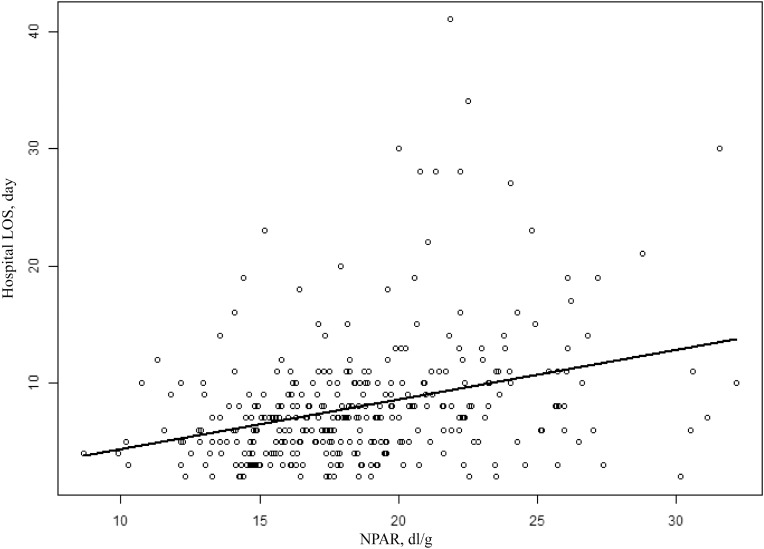
Association between neutrophil-to-albumin ratios and hospital LOS For the data of patients in the Second Affiliated Hospital of Wenzhou Medical University, we plotted the scatter plot of hospital LOS, and the results showed that hospital LOS was positively correlated with NPAR (Figure 3). In addition, Pearson correlation analysis was performed for hospital LOS with NPAR, and the results were shown in additional file Table S3. The correlation coefficient between hospital LOS and NPAR was 0.325, which was better than that of neutrophil percentage and albumin alone.
Figure 3.
Pearson correlation analysis of the NPAR and hospital LOS.
Note: The data came from patients in the Second Affiliated Hospital of Wenzhou Medical University.
Abbreviations: NPAR, ratio of neutrophil to albumin; LOS, length of stay.
Discussion
According to our research findings, it could be summarized as follows. First of all, it could be observed that hospital admission NPAR was closely related to the patient’s main outcome 30-day mortality rate, and high-value NPAR was related to the patient’s increased risk of death. Second, higher NPAR was associated with increased risks of hospitalization, 90-day and 365-day mortality and hospital LOS in HF patients. Third, after adjusted for potential confounding factors, hospital admission NPAR was determined to be an independent predictor of the clinical outcome of HF patients. Fourth, NPAR combined with SAPSII proved to have better outcome predictors than SAPSII alone. Fifth, consistent with the results in MIMIC-III database, hospital LOS and in-hospital all-cause mortality of patients with heart failure were closely related to NPAR, high NPAR might increase hospital LOS, as the data from the Second Affiliated Hospital of Wenzhou Medical University.
HF was a complex clinical syndrome involving multiple pathogenesis, including myocardial hypertrophy, neurohormonal disorders, balance disorders between sympathetic and parasympathetic tension, and the destruction of the system of renin-angiotensin-aldosterone.21–24 Inflammation played an important role in the development of heart failure and was related to the pathway of cardiac remodeling. The relationship between the two was complex and bidirectional, which had not been fully clarified.25 HF patients had elevated levels of pro-inflammatory circulating factors, such as IL-6, TNF-α, GDF15 and galectin-4.26 The activity of neutrophils was regulated systemically by a variety of mediators, including cytokines, “classical” neuroendocrine hormones and bioactive lipids, neutrophils also release a variety of inflammatory factors at the same time.27 The inflammatory process could lead to myocardial damage, and inflammatory factors could promote the worsening and progression of heart failure.28 Neutrophils were the main type of leukocytes in acute inflammation. New evidence that neutrophils contributed to the clinical manifestations of cardiovascular disease had been fully discussed, including the pathogenesis and repair processes of heart failure, myocardial infarction and neointima formation.29 A recent study showed that neutrophil transcription activation was associated with systemic inflammation and functional damage in patients with heart failure, which may indicate that neutrophils play a pathogenic role in the pathogenesis of the disease.30
Albumin was a protein synthesized by the liver, and its plasma concentration was affected by its ratio of synthesis, loss and dilution of exogenous albumin.8,31–33 When in a state of inflammation, including infection, trauma, and surgery, plasma albumin levels would also decrease.11 Various reasons were known to cause the reduction of plasma albumin levels in patients with heart failure, including malnutrition, blood dilution, decreased synthesis caused by liver congestion, inflammation, increased metabolic activity, and proteinuria.34 Studies had found that patients with hypoalbuminemia were more likely to have a history of chronic renal failure than patients without hypoalbuminemia, suggesting that renal insufficiency was one of the causes of patients with hypoalbuminemia.35 There was evidence that severe hypoalbuminemia promotes fluid retention and edema by reducing plasma osmotic pressure, which may further aggravate heart failure and renal failure.36 An investigation found that human serum albumin may be a marker of protein metabolism disorder and low-grade catabolism inflammation in patients with chronic heart failure.37
Our findings were consistent with studies evaluating the prognostic value of NPAR in other clinical settings including STEMI, acute kidney injury, septic shock, rectal cancer, mucinous colorectal adenocarcinoma, and cardiogenic shock.14–19 Our study found that NPAR was an independent predictor of all-cause mortality in patients with heart failure. Combined with neutrophils and albumin, NPAR was better than neutrophils or albumin alone in evaluating inflammatory process. Moreover, ROC showed that the combination of NPAR and SAPSII can enhance the prediction ability of SAPSII. The data from the Second Affiliated Hospital of Wenzhou Medical University showed that NPAR was also positively correlated with hospital LOS. A previous retrospective study on this index and cardiogenic shock showed that this index was associated with mortality in patients with cardiogenic shock.19 This meant that NPAR might be one of the effective indicators to predict the prognosis of patients with inflammatory related heart disease. As a potential new biomarker, NPAR could be quickly and easily read from the admission laboratory, and had certain advantages in simplicity. Given its low cost, availability, and ability to predict mortality, NPAR had clinical value for patients with heart failure and could help clinicians make quick judgments.
Our study had several advantages. Our analysis adjusted for potential confounders that might influence the relationship between NPAR and all-cause mortality in patients with failure, and we repeatedly validated our results with multiple models. However, there were some limitations to our study. Firstly, we used a single-center retrospective study design, therefore, our study may have potential bias. Future prospective studies were needed to address this issue. Secondly, NPAR was readily available in clinical practice, but the missing of etiology, exacerbation factors of heart failure, NT-proBNP, LV ejection, LV volume and diastolic dysfunction grade in the database were still prevalent, which might lead to selection bias. Third, these preliminary data suggested that NPAR might be a risk adjusted tool with prognostic significance for HF. To establish NPAR as a prognostic marker, researchers must further verify its clinical significance. Fourth, the history and use of useless drugs were the most important factors affecting the long-term efficacy, especially the appropriate drugs affecting the survival rate of heart failure (RAS inhibitors, beta-blockers, ect.), so there was a big deviation in the study of the relationship between NPAR and mortality in patients with heart failure. In conclusion, future studies need to further validate the results of this study.
Conclusions
NPAR was an independent related factor of all-cause mortality in patients with heart failure. ROC analysis showed that NPAR combined with SAPSII score can improve the predictive ability of SAPSII score. Our results need to be verified by prospective studies.
Funding Statement
The authors did not receive support from any organization for the submitted work.
Abbreviations
HF, heart failure; NPAR, neutrophil-to-albumin ratio; MIMIC-III, Multiparameter Intelligent Monitoring in Intensive Care III database; ICD-9, International Classification of Diseases and Ninth Revision codes; SQL, Structured Query Language; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean arterial pressure; RR, respiratory rate; SpO2, pulse oximetry-derived oxygen saturation; CHF, congestive heart failure; WBC, white blood cell; RDW, red blood cell distribution width; APTT, activated partial thromboplastin time; INR, international normalized ratio; PT, prothrombin time; SAPS II, Simplified Acute Physiology Score II; APS III, Acute Physiology Score III; RRT, renal replacement treatment; ROC, receiver operating characteristic; LOS, length of stay.
Data Sharing Statement
Our research used the Medical Information Mart for Intensive Care III database version 1.4 (MIMIC III v1.4). MIMIC-III was a publicly available single-center critical care database, which was approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center (BIDMC, Boston, MA, USA) and the Massachusetts Institute of Technology (MIT, Cambridge, MA, USA).
Ethical approval and consent to participate
The Institutional Research and Ethics Institute of the Second Affiliated Hospital of Wenzhou Medical University approved this study involving human subjects. Since the data was anonymous, no informed consent was required. (Ethical Lot Number: 2021-K-71-01)
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflict of interest.
References
- 1.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 2.Tognini S, Pasqualetti G, Calsolaro V, Polini A, Caraccio N, Monzani F. Cardiovascular risk and quality of life in elderly people with mild thyroid hormone deficiency. Front Endocrinol (Lausanne). 2014;5:153. doi: 10.3389/fendo.2014.00153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He T, Mischak M, Clark AL, et al. Urinary peptides in heart failure: a link to molecular pathophysiology. Eur J Heart Fail. 2021;23:1875–1887. doi: 10.1002/ejhf.2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3(1):7–11. doi: 10.15420/cfr.2016:25:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park JJ, Choi DJ. Current status of heart failure: global and Korea. Korean J Intern Med. 2020;35(3):487–497. doi: 10.3904/kjim.2020.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adamo L, Rocha-Resende C, Prabhu SD, Mann DL. Publisher Correction: reappraising the role of inflammation in heart failure. Nat Rev Cardiol. 2021;18:735. doi: 10.1038/s41569-021-00534-3 [DOI] [PubMed] [Google Scholar]
- 7.Arques S. Human serum albumin in cardiovascular diseases. Eur J Intern Med. 2018;52:8–12. doi: 10.1016/j.ejim.2018.04.014 [DOI] [PubMed] [Google Scholar]
- 8.Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17(6):432–437. doi: 10.1111/j.0894-0959.2004.17603.x [DOI] [PubMed] [Google Scholar]
- 9.Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. 2008;582(13):1783–1787. doi: 10.1016/j.febslet.2008.04.057 [DOI] [PubMed] [Google Scholar]
- 10.Uthamalingam S, Kandala J, Daley M, et al. Serum albumin and mortality in acutely decompensated heart failure. Am Heart J. 2010;160(6):1149–1155. doi: 10.1016/j.ahj.2010.09.004 [DOI] [PubMed] [Google Scholar]
- 11.Horwich TB, Kalantar-Zadeh K, MacLellan RW, Fonarow GC. Albumin levels predict survival in patients with systolic heart failure. Am Heart J. 2008;155(5):883–889. doi: 10.1016/j.ahj.2007.11.043 [DOI] [PubMed] [Google Scholar]
- 12.Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, et al. Serum albumin concentration and heart failure risk the health, aging, and body composition study. Am Heart J. 2010;160(2):279–285. doi: 10.1016/j.ahj.2010.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filippatos GS, Desai RV, Ahmed MI, et al. Hypoalbuminaemia and incident heart failure in older adults. Eur J Heart Fail. 2011;13(10):1078–1086. doi: 10.1093/eurjhf/hfr088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui H, Ding X, Li W, Chen H, Li H. The neutrophil percentage to albumin ratio as a new predictor of in-hospital mortality in patients with ST-Segment elevation myocardial infarction. Med Sci Monit. 2019;25:7845–7852. doi: 10.12659/MSM.917987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang B, Li D, Cheng B, Ying B, Gong Y. The neutrophil percentage-to-albumin ratio is associated with all-cause mortality in critically ill patients with acute kidney injury. Biomed Res Int. 2020;2020:5687672. doi: 10.1155/2020/5687672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong Y, Li D, Cheng B, Ying B, Wang B. Increased neutrophil percentage-to-albumin ratio is associated with all-cause mortality in patients with severe sepsis or septic shock. Epidemiol Infect. 2020;148:e87. doi: 10.1017/S0950268820000771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tawfik B, Mokdad AA, Patel PM, Li HC, Huerta S. The neutrophil to albumin ratio as a predictor of pathological complete response in rectal cancer patients following neoadjuvant chemoradiation. Anticancer Drugs. 2016;27(9):879–883. doi: 10.1097/CAD.0000000000000411 [DOI] [PubMed] [Google Scholar]
- 18.Liao YC, Ying HQ, Huang Y, et al. Role of chronic inflammatory ratios in predicting recurrence of resected patients with Stage I-III mucinous colorectal adenocarcinoma. Cancer Manag Res. 2021;13:3455–3464. doi: 10.2147/CMAR.S303758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng Y, Xue Y, Wang J, et al. Association between neutrophil-to-albumin ratio and mortality in patients with cardiogenic shock: a retrospective cohort study. BMJ Open. 2020;10(10):e039860. doi: 10.1136/bmjopen-2020-039860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035. doi: 10.1038/sdata.2016.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mann DL, Deswal A, Bozkurt B, Torre-Amione G. New therapeutics for chronic heart failure. Annu Rev Med. 2002;53:59–74. doi: 10.1146/annurev.med.53.082901.104004 [DOI] [PubMed] [Google Scholar]
- 22.Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334(21):1349–1355. doi: 10.1056/NEJM199605233342101 [DOI] [PubMed] [Google Scholar]
- 23.Merit HF; International Steering Committee. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure. Lancet. 1999;353(9169):2001–2007. doi: 10.1016/S0140-6736(99)04440-2 [DOI] [PubMed] [Google Scholar]
- 24.FaBbender K, Mielke O. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353(9146):9–13. doi: 10.1016/S0140-6736(98)11181-9 [DOI] [PubMed] [Google Scholar]
- 25.Van Linthout S, Tschope C. Inflammation - cause or consequence of heart failure or both? Curr Heart Fail Rep. 2017;14(4):251–265. doi: 10.1007/s11897-017-0337-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rullman E, Melin M, Mandic M, Gonon A, Fernandez-Gonzalo R, Gustafsson T. Circulatory factors associated with function and prognosis in patients with severe heart failure. Clin Res Cardiol. 2020;109(6):655–672. doi: 10.1007/s00392-019-01554-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994;56(6):672–686. doi: 10.1002/jlb.56.6.672 [DOI] [PubMed] [Google Scholar]
- 28.Niessner A, Hohensinner PJ, Rychli K, et al. Prognostic value of apoptosis markers in advanced heart failure patients. Eur Heart J. 2009;30(7):789–796. doi: 10.1093/eurheartj/ehp004 [DOI] [PubMed] [Google Scholar]
- 29.Silvestre-Roig C, Braster Q, Ortega-Gomez A, Soehnlein O. Neutrophils as regulators of cardiovascular inflammation. Nat Rev Cardiol. 2020;17(6):327–340. doi: 10.1038/s41569-019-0326-7 [DOI] [PubMed] [Google Scholar]
- 30.Bai B, Cheng M, Jiang L, Xu J, Chen H, Xu Y. High neutrophil to lymphocyte ratio and its gene signatures correlate with diastolic dysfunction in heart failure with preserved ejection fraction. Front Cardiovasc Med. 2021;8:614757. doi: 10.3389/fcvm.2021.614757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuhrman MP, Charney P, Mueller CM. Hepatic proteins and nutrition assessment. J Am Diet Assoc. 2004;104(8):1258–1264. doi: 10.1016/j.jada.2004.05.213 [DOI] [PubMed] [Google Scholar]
- 32.De Feo P, Lucidi P. Liver protein synthesis in physiology and in disease states. Curr Opin Clin Nutr Metab Care. 2002;5(1):47–50. doi: 10.1097/00075197-200201000-00009 [DOI] [PubMed] [Google Scholar]
- 33.Mitch WE. Malnutrition: a frequent misdiagnosis for hemodialysis patients. J Clin Invest. 2002;110(4):437–439. doi: 10.1172/JCI16494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasini E, Aquilani R, Gheorghiade M, Dioguardi FS. Malnutrition, muscle wasting and cachexia in chronic heart failure: the nutritional approach. Ital Heart J. 2003;4(4):232–235. [PubMed] [Google Scholar]
- 35.Liu M, Chan CP, Yan BP, et al. Albumin levels predict survival in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2012;14(1):39–44. doi: 10.1093/eurjhf/hfr154 [DOI] [PubMed] [Google Scholar]
- 36.Arques S, Ambrosi P. Human serum albumin in the clinical syndrome of heart failure. J Card Fail. 2011;17(6):451–458. doi: 10.1016/j.cardfail.2011.02.010 [DOI] [PubMed] [Google Scholar]
- 37.Pasini E, Comini L, Dioguardi FS, et al. Hypoalbuminemia as a marker of protein metabolism disarrangement in patients with stable chronic heart failure. Minerva Med. 2020;111(3):226–238. doi: 10.23736/S0026-4806.20.06244-8 [DOI] [PubMed] [Google Scholar]