Abstract
Introduction
Burn injuries are frequently encountered in emergency cases and often become the port of entry for pathogens. More than 450,000 burn injuries occur annually causing nearly 3,400 deaths in the United States. The prevalence of burn injury in Indonesia is 0.7% in 2013. More than half of these According to several studies on the use of patients were treated for bacterial infections, some of which were resistant to certain antibiotics. Using hyperbaric oxygen therapy (HBOT) to treat burns has several positive effects including managing bacterial infections, as well as accelerating the wound healing process. Therefore, this study aims to prove the effectiveness of HBOT in inhibiting bacterial growth.
Methods
This is an experimental research study in rabbits using a post-test control group design. 38 rabbits were given second-degree burns on the shoulder region with a metal iron plate that has been previously heated for 3 min. Bacterial cultures were taken on days 5 and 10 after exposure to the burns. The samples were divided into two groups, HBOT and control. Statistical analyses were performed using the Mann-Whitney U method.
Results
Gram-negative bacteria were the most frequently found pathogen in both groups. Citrobacter freundi was the most common Gram-negative bacteria (34%) found in the culture results of both groups.
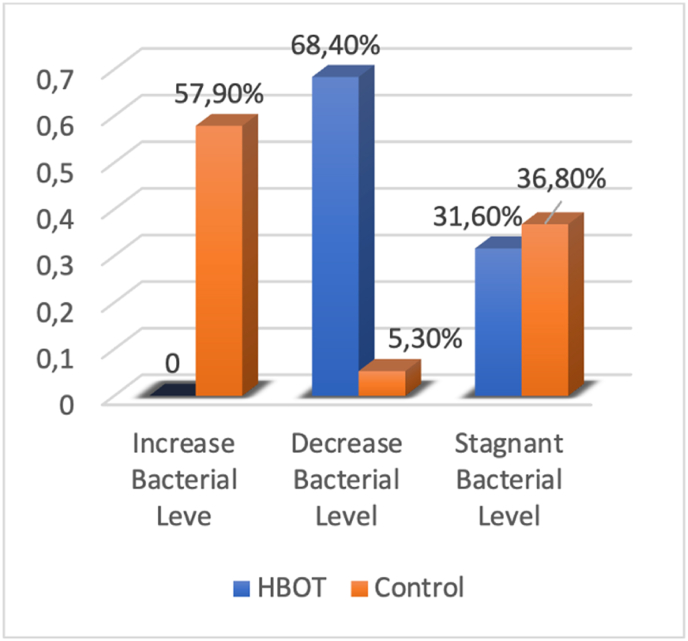
In contrast to the control group, there was no bacterial growth found in the HBOT group's culture results, (0%) vs (58%). A significant reduction of bacterial growth was observed in the HBOT group (69%) compared with the control group (5%). Bacterial levels stagnated in 6 rabbits (31%) in the HBOT group and 7 rabbits (37%) in the control group. Overall, there was significantly less bacterial growth in the HBOT treatment group compared with the control group (p < 0.001).
Conclusion
HBOT administration can significantly reduce bacterial growth in burn injuries.
Keywords: Hyperbaric oxygen therapy, Infection, Gram-negative bacteria, Burn injury, Bacterial infection
Highlights
-
•
Hyperbaric oxygen therapy (HBOT) to treat burns has several positive effects including managing bacterial infections.
-
•
HBOT influence the extent of a bacterial infection based on reduced bacterial counts after receiving HBOT.
-
•
The bactericidal effects of HBOT are obtained by inducing the release of reactive oxygen species.
1. Introduction
Burn injuries are frequently encountered in many emergency cases [1]. In the United States, more than 450,000 burn injuries occur annually with nearly 3,400 deaths [2]. In a developing country such as Indonesia, the prevalence of burn injury is 0.7% [3]. In Poland, cases of burn injuries in March 2020 were three times higher than in February 2020. From 2016 to 2019 hospitalization rates caused by burn had never exceeded 5% of incoming patients, but in 2020 the rate increased to 15.28% cases [4].
A study in South Africa showed that more than half (52.5%) of burn patients were treated for infections. These infections derive from Gram-negative bacteria such as A. baumanni, P. aeruginosa, and K. pneumoniae, which are resistant to certain types of antibiotics [5]. Two surveillance studies in North America found that 73% and 88% cases were resistant to Oxacillin, 55% and 66% resistant to levofloxacin, and 70%–73% resistant to erythromycin. Similar results were also obtained in a study conducted in the United Kingdom [6]. Some studies reveal that the release of reactive oxygen species (ROS) is induced by HBOT and equally being destructive to pathogen DNA and other biology molecules [7].
According to several studies on the use of hyperbaric oxygen therapy (HBOT) for burn injuries, it has many benefits ranging from treating bacterial infection to accelerating the wound healing process. Therefore, this study aims to establish the effectiveness of HBOT in inhibiting bacterial cell growth.
2. Materials and methods
2.1. Sample examination
This was an experimental research study using rabbits with a post-test control group design comprised of a control group and an experimental group. This study was approved by the Ethics Commission of Kandou Hospital Manado (No.: 067/EC-UPKT/III/2016). The work was carried out in line with the ARRIVE Guidelines for Reporting Animal Research [8].
2.2. Population and sample
Total thirty-eight rabbits (Federer formula) were quarantined in individual cages at the research laboratory of the Faculty of Medicine, Sam Ratulangi University, Manado. These experimental animals were fed with carrots, kale, sweet potatoes, cucumbers, and sweet corns without additional feeding for approximately 1 week for the adaptation period. Feeding took place daily, with adequate water provided to drink. The cage was of standard size, cleaned regularly, and kept at 24 ± 2 °C.
2.3. Second-degree burn model
The second-degree burn model was prepared as described by Guo et al. [9] with modifications. The rabbits were shaved on their shoulder, then diazepam at 1 mg/kg body weight (BW) was administered intramuscularly as premedication for the rabbits. Intramuscular ketamine hydrochloride 40 mg of BW/day was injected as an anesthetic.
The affected area was disinfected with povidone-iodine 1%. After that, a 2 × 1 cm second-degree burn was generated using a metal iron plate that had been previously heated in hot water at 100 °C for 3 min on the shoulder of each rabbit for 6 s. This method obtained deep dermal burns, which were confirmed by the results of the histopathological examination [10]. Immediately after the procedure, analgesia with paracetamol 200 mg/kg and amoxicillin 50 mg/kg antibiotics were given orally.
2.4. Bacterial cultures
Samples were taken on days 5 and 10 after the burn using a swabbing procedure on the wounded area by rubbing the cotton bud in a circular motion so that the entire surface of the cotton bud is in contact with the wound surface. The bacterial culture was carried out on a Petri dish filled with layers of agar with blood agar plates (BAPs) as the culture media since these are generally suitable for both Gram-positive and -negative bacterial cultures [11]. The resulting colonies were isolated using tweezers for Gram-positive and -negative coloring. In addition to the difference in color (Gram-negative colored pink and Gram-positive purple), we can also evaluate or name the bacteria based on the shape seen under the microscope. To determine the bacterial species (more specific), we used the agglutination examination method or PCR through gene analysis of 16SrRNA, without the Gram-coloring method anymore.
The samples were then divided into two groups, HBOT and control. The treatment group received HBOT (directly after the swab) for 6 consecutive days. In both groups, bacterial cultures were obtained by wound swab and examined on the 10th day. Finally, data were analyzed using SPSS.
2.5. Statistical analysis
The sample size was determined using the Kirk formula [12]. The data were subjected to statistical analysis using SPSS version 25 (IBM Corp.). From the collected data, the type and numbers of bacteria were compared between the two groups using the following tests: the difference was tested using non-parametrical Mann-Whitney test and Kolmogorov-Smirnov test was used to indicate the normality of the data.
3. Results
38 rabbits were equally distributed between both the treatment and control groups. No rabbits were excluded or dropped out because all rabbits managed to survive until the 10th day and did not suffer from other injuries.
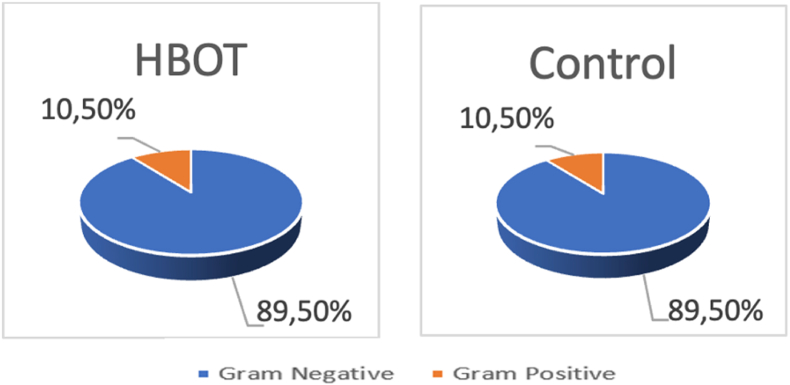
After 10 days, Gram-negative bacteria outnumbered their Gram-positive counterparts in both groups (Fig. 1).
Fig. 1.
Distribution of Gram-negative and -positive counts in both treatments.
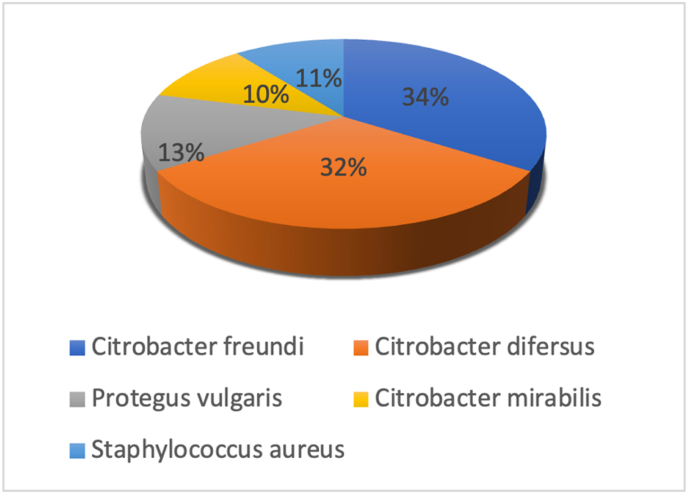
Citrobacter freundi (34%), Citrobacter difersus (32%), Proteus vulgaris (13%), Citrobacter mirabilis (10,5%), and Gram-positive bacteria - Staphylococcus aureus (10.5%). No significant differences in the types of bacteria were found between the two groups (Fig. 2).
Fig. 2.
Bacterial distribution.
The results from days 5–10 indicate that the increasing level of bacteria in HBOT was lower than the control group, otherwise, the decreasing levels of bacteria in HBOT groups are higher than the control group (Fig. 3).
Fig. 3.
Comparison of the bacterial development from day 5 to day 10 in the HBOT and control groups.
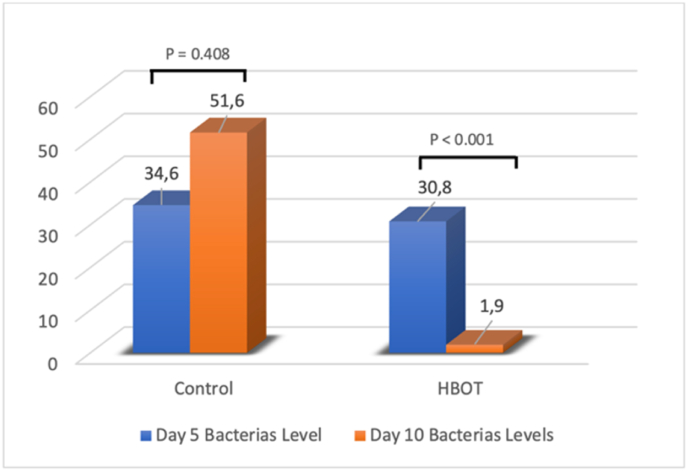
Normality data testing of the number of bacteria found on days 5 and 10 were analyzed using the Kolmogorov-Smirnov test. The test result shows that the number of bacteria in both treatments did not spread normally (p < 0.05). Due to the abnormality of data dissemination, the Mann-Whitney-U test was used as a further testing method. There was no meaningful difference in the bacterial count between both groups on day 5 (p = 0.408) (Fig. 4). However, on day 10, the bacterial counts in the HBOT group plummeted, leaving a statistically significant difference between the two groups (p < 0.001) (Table 1).
Fig. 4.
Descriptive statistical value for the number of bacteria on days 5 and 10.
Table 1.
Mann-Whitney U test results on day 10.
| Treatment | Average | Mann-Whitney U Test |
|---|---|---|
| HBOT | 11.57 | P < 0.001 |
| Control | 27.47 |
4. Discussion
The main objectives of burn management are to reduce the occurrence of edema, maintain the viability of tissues in the static zone, protect microvascular circulation and aid the immune system [13,14]. When burns heal, wound regeneration cannot occur without a balance between its management, the patient, the severity and location of the wound itself. Without such a balance, extensive scarring could be one of the consequences [15].
This study showed that aerobic bacteria are the most common type found on burn wounds. For example, citrobacter are pathogenic bacteria that can cause intraabdominal and respiratory tract infections [16]. Similarly, proteus and staphylococcus infect the digestive and respiratory tract. Staphylococcus are Gram-positive bacteria and have become one of the common causes of purulent skin and soft tissues infections [17,18].
Excessive amounts of infecting bacteria could be indicative of cases of antibiotics resistance [19]. Infections that occur in severe burns are characterized by persistent hypermetabolic responses such as catabolism occurring throughout the body, degradation of muscles and proteins, growth delays, insulin resistance, and multiorgan dysfunction [20]. A study on the relationship between HBOT and burns confirmed the positive interactions between HBOT, the mechanism, and the healing of burn injury [21].
HBOT enables increasing the partial pressure of oxygen of the inhalation to 100% oxygen in a pressurized chamber [22,23]. The oxygen then dissolves in the blood serum under Henry's law, where the amount of the ideal gas dissolved in a solution becomes proportional to its partial pressure. During treatment, arterial oxygen tension reaches 2000 mmHg, and oxygen levels increase from 200 to 400 mmHg in tissues [24,25]. During tissue repair and wound healing, the demand for oxygen increases to prevent hypoxia-induced tissue death [26,27]. Chronic hypoxia that occurs in wounds is associated with delayed or absent wound healing. This suggests that the increased oxygen supply in hypoxic tissues provides many advantages, particularly in wound healing [28].
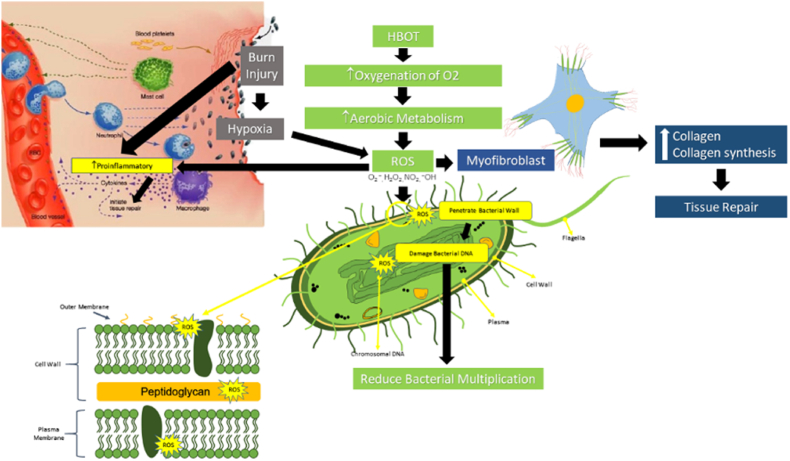
In a study by Memar et al., the patients with burn injuries who were treated with HBOT had lower fluid requirements compared with controls [29]. Administration of HBOT increases oxygenation of, which affects the increase in aerobic metabolism thus stimulating ROS activation (Fig. 5) [29]. ROS activation triggers the process of myofibroblast formation, which affects the synthesis of collagen central to the repair of injured tissues [30]. In addition, myofibroblast ROS will also stimulate a pro-inflammatory response in tissue repair. Of note, injuries due to burns in addition to stimulating the pro-inflammatory response, also provide a hypoxia effect on the network so that the activation of the ROS signal will be strongly supported by the activation of ROS caused by the administration of HBOT [31]. Among HBOT, the most crucial work is the bactericidal effect on the bacteria directly. ROS penetrate through the cell walls of the bacteria and cause damage to the bacterial DNA, thus limiting the multiplication of the bacteria itself [29,32,33].
Fig. 5.
Suggested pathway: HBOT's bactericidal role in burn injuries and its relationship with ROS [[29], [30], [31], [32], [33]].
Regarding the present study, the number of aerobic bacteria decreased after the administration of HBOT. HBOT increases oxygen perfusion to the wound area, in addition to its bactericidal function. Moreover, HBOT also enhances the function of PMN cells since oxygen is necessary for phagocytosis and bacterial destruction. Another possible mechanism underlying HBOT's efficacy is antimicrobial by triggering the formation of ROS. ROS are reactive radicals that includes, superoxidation anions (O2--), peroxides (O2-2), hydrogen peroxide (H2O2), hydroxyl radicals (OH'), and hydroxyl ions (OH--) [30].
The present study highlights that HBOT can influence the extent of a bacterial infection based on reduced bacterial counts after receiving HBOT. Therefore, HBOT represents one of the factors that plays a role in reducing infection after burns injury because if the wound contains more bacteria, the risk of infection is greater.
5. Conclusions
HBOT can contribute to reducing infection in burns because when the wound is contaminated with a large number of bacteria, the risk of infection is greater.
Provenance and peer review
Not commissioned, externally peer reviewed.
Ethical approval
All procedure for Animal experiment has been approved by Ethics Commission of Kandou Hospital Manado Number: 067/EC-UPKT/III/2016.
sources of funding
No funding or sponsorship.
Author contribution
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Registration of research studies
None.
Guarantor
Mendy Hatibie Oley.
Consent
This manuscript does not involve human participants, human data, or human tissue.
Declaration of competing interest
The authors declare that they have no conflict of interests.
Acknowledgment
We do acknowledge Leo Sagay, MD for his help in providing us the statistical and linguistic assistance for this research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.103314.
Contributor Information
Mendy Hatibie Oley, Email: mendy.hatibie@unsrat.ac.id.
Maximillian Christian Oley, Email: max_oley@unsrat.ac.id.
Louise A.J. Waworuntu Wewengkang, Email: wisye.wawo@gmail.com.
Billy Johnson Kepel, Email: billy.kepel@unsrat.ac.id.
Fima Lanra Fredrik G. Langi, Email: flangi2@unsrat.ac.id.
Taat Setiadi, Email: taatse@gmail.com.
Deanette Michelle R. Aling, Email: aling.michelle@gmail.com.
Deborah Florencia Gunawan, Email: deborahgunawan011@student.unsrat.ac.id.
Marcella Tirsa Tulong, Email: tirsatulong@yahoo.com.
Muhammad Faruk, Email: faroex8283@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wittels K.A. Ninth Edit, Elsevier Inc.; 2012. Thermal Burns. [DOI] [Google Scholar]
- 2.Phillips T., Lee M. Anesth. Secrets. sixth ed. Elsevier Inc.; 2021. The burned patient; pp. 376–380. [DOI] [Google Scholar]
- 3.Kawalec A.M. The changes in the number of patients admissions due to burns in Paediatric Trauma Centre in Wroclaw (Poland) in March 2020. Burns. 2020;46:1713–1714. doi: 10.1016/j.burns.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahemia I.A., Muganza A., Moore R., Sahid F., Menezes C.N. Microbiology and antibiotic resistance in severe burns patients: a 5 year review in an adult burns unit. Burns. 2015;41:1536–1542. doi: 10.1016/j.burns.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Toltzis P. Princ. Pract. Pediatr. Infect. Dis. Elsevier; 2018. Staphylococcus epidermidis and other coagulase-negative staphylococci; pp. 706–712. e4. [DOI] [Google Scholar]
- 6.Van Acker H., Coenye T. The role of reactive oxygen species in antibiotic-mediated killing of bacteria. Trends Microbiol. 2017;25:456–466. doi: 10.1016/j.tim.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Memar M.Y., Ghotaslou R., Samiei M., Adibkia K. Antimicrobial use of reactive oxygen therapy: current insights. Infect. Drug Resist. 2018;11:567–576. doi: 10.2147/IDR.S142397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo H.-F., Ali R.M., Hamid R.A., Zaini A.A., Khaza’ai H. A new model for studying deep partial-thickness burns in rats. Int. J. Burns Trauma. 2017;7:107–114. [PMC free article] [PubMed] [Google Scholar]
- 10.dos D., Tavares Pereira S., Lima-Ribeiro M.H.M., de Pontes-Filho N.T., dos A.M., Carneiro-Leão A., dos M.T., Correia S. Development of animal model for studying deep second-degree thermal burns. J. Biomed. Biotechnol. 2012:460841. doi: 10.1155/2012/460841. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prihantono P., Ardi Syamsu S., Smaradhania N., Ahmad M., Siagian N.A., Lubis K., Umrah A.S. Application of Scaevola taccada (Gaertn.) roxb. Reduce pro-inflammatory cytokines interleukin-1β in sprague dawley mice suffering from mastitis, open access maced. J. Med. Sci. 2020;8:423–427. doi: 10.3889/oamjms.2020.4363. [DOI] [Google Scholar]
- 12.Kirk R.E. In: Exp. Des. Proced. Behav. Sci. fourth ed. Kirk R.E., editor. SAGE Publications, Inc., 2455 Teller Road; Thousand Oaks California 91320 United States: 2013. Experimental designs: an overview; pp. 30–76. [DOI] [Google Scholar]
- 13.Strudwick X.L., Cowin A.J. Hot Top. Burn Inj. InTech; 2018. The role of the inflammatory response in burn injury. [DOI] [Google Scholar]
- 14.Nielson C.B., Duethman N.C., Howard J.M., Moncure M., Wood J.G. Burns: pathophysiology of systemic complications and current management. J. Burn Care Res. 2017;38 doi: 10.1097/BCR.0000000000000355. e469–e481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eming S.A., Martin P., Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3009337. 265sr6-265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatlen T.J., Miller L.G. Staphylococcal skin and soft tissue infections. Infect. Dis. Clin. 2021;35:81–105. doi: 10.1016/j.idc.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Tong S.Y.C., Davis J.S., Eichenberger E., Holland T.L., Fowler V.G. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015;28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee A.S., de Lencastre H., Garau J., Kluytmans J., Malhotra-Kumar S., Peschel A., Harbarth S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Prim. 2018;4:18033. doi: 10.1038/nrdp.2018.33. [DOI] [PubMed] [Google Scholar]
- 19.Blyth D.M., Yun H.C., Tribble D.R., Murray C.K. Lessons of war. J. Trauma Acute Care Surg. 2015;79:S227–S235. doi: 10.1097/TA.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smolle C., Lindenmann J., Kamolz L., Smolle-Juettner F.-M.M. The history and development of hyperbaric oxygenation (HBO) in thermal burn injury. Medicina (B. Aires) 2021;57:49. doi: 10.3390/medicina57010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam Gretl B., Fontaine Rocky C., Loss F.A.M., Chiu E.S.M. vol. 25. 2012. pp. 38–44. (Hyperb. Oxyen Ther. Explor. Clin. Evid). Clinical Management Extra. [Google Scholar]
- 22.Eggleton P., Bishop A., Smerdon G. Safety and efficacy of hyperbaric oxygen therapy in chronic wound management: current evidence. Chron. Wound Care Manag. Res. 2015;2:81–93. doi: 10.2147/CWCMR.S60319. [DOI] [Google Scholar]
- 23.Oley M.H., Oley M.C., Langi F.L.F.G., Langi Y.A., Keppel B.J., Tangkilisan A.N., Lampus H.F., Sipayung E.F., Aling D.M.R., Faruk M. Predicting hyperbaric oxygen therapy success using the decision tree approach. Ann. Med. Surg. 2021;69:102725. doi: 10.1016/j.amsu.2021.102725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heyboer M., Sharma D., Santiago W., McCulloch N. Hyperbaric oxygen therapy: side effects defined and quantified. Adv. Wound Care. 2017;6:210–224. doi: 10.1089/wound.2016.0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hadanny A., Efrati S. The hyperoxic-hypoxic paradox. Biomolecules. 2020;10 doi: 10.3390/biom10060958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nauta T.D., van Hinsbergh V.W.M., Koolwijk P. Hypoxic signaling during tissue repair and regenerative medicine. Int. J. Mol. Sci. 2014;15:19791–19815. doi: 10.3390/ijms151119791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong W., Hu M., Esquivel M., Liang G., Rennert R., McArdle A., Paik K., Duscher D., Gurtner G., Lorenz H., Longaker M. The role of hypoxia-inducible factor in wound healing. Adv. Wound Care. 2014;3:390–399. doi: 10.1089/wound.2013.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen P.Ø., Møller S.A., Lerche C.J., Moser C., Bjarnsholt T., Ciofu O., Faurholt-Jepsen D., Høiby N., Kolpen M. Improving antibiotic treatment of bacterial biofilm by hyperbaric oxygen therapy: not just hot air. Biofilms. 2019;1:100008. doi: 10.1016/j.bioflm.2019.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akamatsu T., Arai Y., Kosugi I., Kawasaki H., Meguro S., Sakao M., Shibata K., Suda T., Chida K., Iwashita T. Direct isolation of myofibroblasts and fibroblasts from bleomycin-injured lungs reveals their functional similarities and differences. Fibrogenesis Tissue Repair. 2013;6:15. doi: 10.1186/1755-1536-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fazal N. T cell suppression in burn and septic injuries. Immunosuppression - Role Health Dis. 2012 doi: 10.5772/26523. [DOI] [Google Scholar]
- 31.Poff A.M., Kernagis D., D'Agostino D.P. Hyperbaric environment: oxygen and cellular damage versus protection. Compr. Physiol. 2017;7:213–234. doi: 10.1002/cphy.c150032. [DOI] [PubMed] [Google Scholar]
- 32.Bogdan J., Zarzyńska J., Pławińska-Czarnak J., Zarzynska J.M., Bogdan J., Zarzyńska J., Pławińska-Czarnak J., Lam G., Fontaine R., Ross F., Chiu E., Fazal N. Comparison of infectious agents susceptibility to photocatalytic effects of nanosized titanium and zinc oxides: a practical approach. Nanoscale Res. Lett. 2015;10:309. doi: 10.1186/s11671-015-1023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Memar M.Y., Yekani M., Alizadeh N., Baghi H.B. Hyperbaric oxygen therapy: antimicrobial mechanisms and clinical application for infections. Biomed. Pharmacother. 2019;109:440–447. doi: 10.1016/j.biopha.2018.10.142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.