Abstract
Neuronal dysfunction and subsequent apoptosis under high glucose conditions during diabetes contribute majorly to the manifestation of diabetic peripheral neuropathy (DPN). PERK (protein kinase RNA (PKR)-like ER kinase) one among the three canonical arms of unfolded protein response (UPR), is believed to play a crucial role in determining the cell fate during endoplasmic reticulum stress (ERS/ER stress) conditions. We evaluated the role of PERK inhibitor GSK2606414 in high glucose (30 mM) treated neuroblastoma (N2A) cells. High glucose resulted in disruption of ER proteostasis by activation of UPR which is evident through increased (p < 0.001) expression of GRP78, p-PERK, p-eIF2α, ATF-4 and CHOP when compared to normal cells. It is accompanied with enhanced GRP78 localization in Endoplasmic Reticulum (ER) lumen evident from ER labeling Immunofluorescence (IF) staining. PERK activation resulted in altered mitochondrial function evident by increased mitochondrial superoxide production and compromised mitochondrial homeostasis with decrease in Mfn-2 levels. Additionally, ER stress induced neuronal apoptosis was attenuated by GSK2606414 treatment via inhibiting the PERK-eIF2α-ATF4-CHOP axis that not only curtailed the levels of apoptotic proteins like Bax and caspase 3 but also elevated the levels of anti-apoptotic Bcl-2. Collectively, our findings revealed the neuroprotective potential of GSK2606414 against high glucose induced neurotoxicity in N2A cells.
Keywords: GSK2606414, High glucose, PERK, ER stress, Mitochondrial function
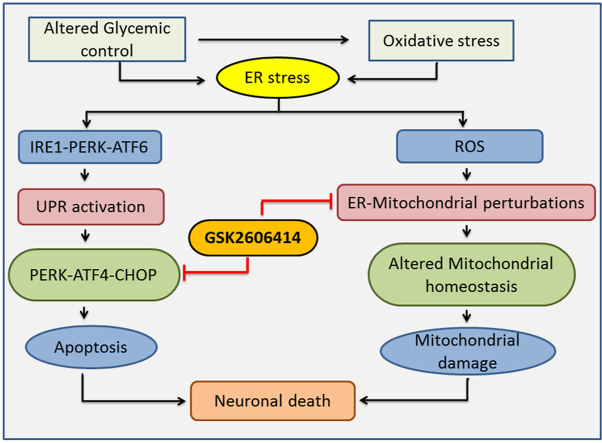
Graphical abstract
Schematic diagram representing the possible effects of GSK2606414 against high glucose induced neurotoxicity.
Highlights
-
•
Unregulated ER stress drives neuronal (N2A) apoptosis following high glucose (HG) exposure (30 mM).
-
•
Mitochondrial dysfunction aggravated by ER stress under hyperglycemic conditions.
-
•
PERK/p-eIF2α/ATF4/CHOP axis underlies the apoptosis of N2A cells upon HG exposure.
-
•
GSK2606414 attenuates PERK/p-eIF2α/ATF4/CHOP axis to mitigate HG induced neurotoxicity in N2A cells.
1. Introduction
Endoplasmic reticulum (ER) is a major site for the biosynthesis of lipids as well as proper synthesis, maturation and folding of proteins. Various pathophysiological perturbations, including hypoxia, oxidative stress, glucose deprivation, viral infection entail a specific stress response by accumulation of misfolded and unfolded proteins in the ER leading to development of ER stress (ERS) which underlies the development of a variety of neurological disorders (Lindholm et al., 2006). To combat against the ERS, an adaptive cellular response termed as unfolded protein response (UPR) is activated. UPR is a signaling network that facilitates cellular repair by sensing the imbalance between protein synthesis, quality control, and degradation in the ER. In response to various stimuli, UPR exhibits dual role as pro-adaptive and pro-apoptotic signaling cascade based on the intensity and duration of the ER exposure to stress (Hetz, 2012). The UPR is orchestrated by three main sensors PERK (protein kinase RNA (PKR)-like ER kinase), IRE1α (inositol-requiring enzyme-1 alpha) and ATF6 (activating transcription factor-6), that reside in the ER membrane. These transmembrane proteins bind to the chaperone Glucose regulated protein of 78 kDa or binding immunoglobulin protein (GRP78/BiP) in their inactive states in ER lumen (Iurlaro and Muñoz-Pinedo, 2016).
Hyperglycemia activates various metabolic pathways like polyol pathway, advanced glycation end products (AGE) pathway, and hexosamine pathway which contributes to hyperglycemia induced cell death (Rolo and Palmeira, 2006). In brief, the enzymatic conversion of glucose to sorbitol, with simultaneous diminution of NADPH and glutathione is accelerated. The resulting depletion of antioxidant reducing equivalents worsens the sensitivity to oxidative stress associated with enhanced intracellular ROS (Ponugoti et al., 2012). In turn, the overproduction of superoxides by hyperglycemia hampers the mitochondrial ETC capacity to regulate ATP leading to maladaptive responses (Rochette et al., 2014). High glucose induced Reactive Oxygen Species (ROS) generation triggers oxidative stress by accumulating unfolded and misfolded proteins in the ER lumen, subsequently activating unfolded protein response (UPR) (Lelkes et al., 2001). Given the critical role of PERK, IRE1α and ATF6 in UPR signaling, unregulated activation of these proteins could contribute significantly to ER stress-induced apoptosis (Cao et al., 2012; Bhardwaj et al., 2019). The existing literature highlights that neurons require tremendous amount of energy in the form of ATP to perform vital cellular operations and thus proper mitochondrial functioning is essential for typical neuronal activity (Nakamura et al., 2020). Hyperglycemia-induced ROS generation not only causes oxidative stress, but also hampers antioxidant defense system in neuronal cells as antioxidants have been shown to protect neurons following hyperglycemic conditions (Moghaddam et al., 2014). It is not surprising that the increased oxidative stress also elevates the level of mitochondrial ROS above the threshold, leading to neuronal death via apoptotic events (Singh and Devasahayam, 2020). Perturbations in ER-mitochondrial tethering induced by ROS are detrimental for cellular homeostasis in terms of calcium regulation, protein folding machinery and mitochondrial dynamics (Naon et al., 2016; Veeresh et al., 2019). Certainly, the mitochondrial ROS debilitate ER function by virtue of its ability to rise ER stress, so is implicated as a major risk factor in the onset and progression of neuronal damage. Of note, disturbances in mitochondria-associated ER membranes (MAMs) have been proposed to be linked with manifestation of neurodegenerative diseases (Murphy, 2013; Paillusson et al., 2016).
The aim of the current study is to focus on one of the three arms of unfolded protein response (UPR) mediated by protein kinase RNA (PKR)-like ER kinase (PERK). PERK monomer is localized on the ER membrane, that plays a vital role in translational control of ER stress by detecting the accumulated chunk of unfolded proteins in the ER lumen. An ER transmembrane molecular chaperone protein termed BiP (GRP78; glucose regulated protein of 78 kDa) holds PERK, along with IRE-1 and ATF6 making them inactive under normal conditions (Halliday et al., 2017). Under physiological conditions, GRP78 is expressed at a low level and is confined to ER transmembrane receptors. On the other side, when ER stress is triggered, the unfolded or misfolded proteins bind with free GRP78 causing its dissociation from IRE1α, PERK and ATF6 facilitating their activation (Logue et al., 2013). Once activated the three proteins undergo auto-phosphorylation and are engaged in their respective downstream signaling mechanisms that under acute conditions restores cellular homeostasis while under chronic conditions lead to apoptosis of neuronal cells via activating various transcriptional mediators (Walter and Ron, 2011).
GSK2606414 is a first in class oral PERK inhibitor developed by GlaxoSmithKline in 2012 (Axten et al., 2012). Since its inception, tremendous research has been carried out using this compound in various preclinical models of diseases. Of note, neuroprotective effect of GSK2606414 is well studied in rodent models of parkinson's (Mercado et al., 2018), alzheimer's disease (Yang et al., 2016), down syndrome (Lanzillotta et al., 2020) and prion diseases (Moreno et al., 2013). However, its role in diabetic neuropathy is yet unidentified. Hence, in the current study we aimed to i) investigate the role of PERK/p-eIF2α/ATF4/CHOP signaling in high glucose induced neurotoxicity in neuro2a cells (N2A), ii) identify the neuroprotective effect of PERK inhibitor GSK2606414 in high glucose treated N2A cells and iii) delineate the possibility of inhibiting PERK in strengthening mitochondrial function and inhibiting apoptotic module of chronic ER stress mediated by PERK/p-eIF2α/ATF4/CHOP signaling axis.
Owing to its PERK inhibition, GSK2606414 unveiled beneficial in several diseases other than neuroprotection. A few of them are highlighted here, GSK2606414 is reported to have suppressed RIPK1 (Receptor-interacting serine/threonine-protein kinase 1) mediated cell death dependent on Tumor necrosis factor (TNF). It is interesting to know that this activity is independent of PERK inhibition (Rojas-Rivera et al., 2017). Further studies on GSK2606414 revealed that, it exhibited protective effect against human ovarian cancer cells A2780 and A2780CP from Evodiamine (EVO) -induced apoptosis where EVO is reported to activate PERK (Chen et al., 2016). Additionally, GSK2606414 also displayed anti-parasitic activity in toxoplasmosis and human cutaneous leishmaniasis by targeting PERK branch of ER stress response (Dias-Teixeira et al., 2017; Augusto et al., 2018). A recent study highlighted the ability of reovirus to provoke ER stress and postulated that targeting UPR may sensitize cancer cells to reovirus. Surprisingly, PERK inhibition by GSK2606414 enhanced the efficacy of reovirus in treating head and neck squamous cell cancer. In contrast to the canonical ER stress response, GSK2606414 induced eIF2a-ATF4 signaling in the circumstances of reovirus infection, and the authors claim that this occurs only in the combination of reovirus and GSK2606414. This was evident from the studies performed by Martin McLaughlin and co-workers. (McLaughlin et al., 2020).
2. Materials and methods
2.1. Materials
GSK2606414 and most of the other chemicals are reagent grade and obtained from Sigma Aldrich, USA. All the antibodies were purchased from Santa Cruz Biotechnology, USA unless mentioned. The chemicals and solvents used were of analytical grade.
2.2. Methods
2.2.1. Experimental design
Mouse N2A neuroblastoma cells obtained from NCCS, Pune were cultured in Minimum Essential media (MEM containing 5.5 mM glucose) supplemented with 10% FBS, streptomycin/penicillin (1%), glutamine (2 mM) and grown at 37 °C, in a humidified atmosphere of 95% air and 5% CO2. 25 mM glucose is added to the medium to simulate high glucose conditions (final concentration in medium is 30 mM). When confluent, the cells were seeded into T25 or multi well plates as per the experimental requirement. Experiments were carried out 24 h after cells were seeded. Treatment was given in 5% FBS culture medium and the cells were divided into following groups, NC: normal N2A cells, GSK 1: normal N2A cells treated with GSK260414 (1 μM), HG: hyperglycemic N2A cells (30 mM β-D glucose), HG+ GSK 0.5: HG cells treated with GSK260414 (0.5 μM), HG+ GSK1: HG cells treated with GSK260414 (1 μM). Biochemical and molecular evaluations are performed 24 h post treatment. Concentrations and time intervals were chosen with respect to the method sensibility and specificity of the experiments. All assays were conducted in triplicate, and each experiment was repeated at least thrice.
2.2.2. Cell viability assay
Cell viability was detected using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, N2A cells were seeded in 96 well plates at a density of 5000 cells/well and incubated for 24 h. After incubation, cells were treated with different concentrations of GSK2606414 (0.01–200 μM) and co-treated with high glucose (30 mM) then incubated for 24 h. Then MTT solution (3-[4, 5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide, 5 mg/10 ml in MEM) was added to the 96-well plate and incubated at 37 °C for 4 h. Media from 96 well plate was removed and 200 μL of Dimethyl sulfoxide (DMSO) was added to solubilize the crystals. Absorbance was measured at 570 nm using a multimode reader (Spectramax M4, USA) (Kalvala et al., 2020b).
2.2.3. ROS estimation by 2′,7′- dichlorofluorescin diacetate (DCFDA) staining
Cellular Reactive Oxygen Species (ROS) was measured in Neuro2a cells seeded in 6-well plate at a density of 5000 cells/well. The assay uses reagent 2′,7′-dichlorofluorescin diacetate (DCFDA), a fluorogenic dye that measures hydroxyl, peroxyl & other reactive ROS activity within the cell at 5 μM concentration. Fluorescence is detected at an excitation/emission of 485 nm/535 nm using fluorescence microscopy (Bachewal et al., 2018).
2.2.4. Western blotting
N2A cells were treated with GSK2606414, post 24 h of treatment the cells were washed with ice-cold Phosphate Buffered Saline (PBS) and were suspended in Radioimmunoprecipitation assay buffer (RIPA) containing protease and phosphatase inhibitor. Each lysate sample was centrifuged at 4°C at 10000 rpm for 10 min, after which the supernatant was collected as whole cell extract. Protein levels were estimated by Bradford's assay. A total of 30–40 μg/20 μl of protein extract from N2A cells was heated for 5 min in SDS sample buffer [2% sodium dodecyl sulfate (SDS), 62.5 mM Tris-HCL (pH 6.8), 10% glycerol and 0.001% bromophenol blue]. Protein expression study was performed using western blotting method. Equal amount of proteins were separated by SDS-PAGE (10% unless specified, for low molecular weight proteins 16% gel is used), electrophoresis is run for 90 min at 80 V in running buffer (25 mM Tris, 192 mM glycine, 0.1% (w/v) SDS, pH 8.3) and then transferred to a PVDF (Polyvinylidene difluoride) or Nitrocellulose (NC) membrane in transfer buffer (25 mM Tris, 192 mM glycine, 20% (v/v) methanol, pH 8.3). After blocking with 3% bovine serum albumin, the membranes were incubated with primary antibodies at 4 °C overnight; GRP78 (Cat#: sc-13539; 1:1000), PERK (Cat#: sc-377400; 1:1000), ATF4 (Cat#: sc-390063; 1:1000), eIF2α (Cat#: sc-133132; 1:1000), CHOP (Cat#: sc-7351; 1:1000), TFAM (Cat#: sc-166965; 1:1000), NRF1 (Cat#: sc-515360; 1:1000), β-Actin (Cat#: sc-47778; 1:1000), (Santa Cruz Biotechnology, Inc, USA), Bax (Cat#: 5023; 1:1000), Bcl2 (Cat#: 3498; 1:1000), Caspase 3 (Cat#: 9662; 1:1000) (CST, USA), Complex I (Cat#: (ab110245); 1:1000), Complex II (Cat#: (ab110410); 1:1000) and ATP synthase C (Cat#: ab181243; 1:1000) (Abcam, USA), p-PERK (Cat#: ITP07576; 1:1000), Mfn-2 (Cat#: ITT2740; 1:1000), p-eIF2α (Cat#: ITP03087; 1:1000), (G-biosciences, USA) at 1:1000 dilution in TBST. Then the membranes were incubated with Horseradish peroxidase (HRP) tagged secondary antibodies for 2 h at room temperature. Chemiluminescence signal was captured using a Fusion-FX imager (VilberLourmat, Germany) and relative band intensities were quantified by densitometry using Image-J software (version 1.48, NIH, USA) (Arruri et al., 2017).
2.2.5. Detection of mitochondrial superoxides using MitoSOX staining
MitoSOX (Mitochondrial superoxide) Red reagent is a fluorogenic color explicitly intended to target the mitochondria in cells under live condition. Oxidation of MitoSOX reagent by superoxide turns outs to red fluorescence. In this way, the intensity of red fluorescence produced directly indicates the level of mitochondrial superoxide. Briefly, 6-well plate was cultivated with Neuro2a cells (4000 cells/well) with MEM for 24 h. These cells were then exposed to 30 mM glucose, and 0.5 and 1 μM of GSK2606414 for 6 h followed by incubation with 5 μM MitoSOX for 10 min at 37 °C. The cells were then washed twice with phosphate buffer saline (PBS) and processed for imaging under a fluorescence microscope at absorption/emission of ∼510/580 nm (Bachewal et al., 2018).
2.2.6. Immunocytochemistry (ICC)
Post 24 h of treatment, N2A cells were washed with PBS for three times and fixed in 4% paraformaldehyde solution followed by washing with PBS. Then cells were blocked with 3% BSA solution and incubated with PDI (protein disulfide isomerase; an ER resident enzyme) and primary antibody; GRP78 (Santa Cruz Biotechnology), (1:200) or CHOP (1:50) in 3% BSA (Bovine serum albumin) at 4 °C temperature for 12 h. Followed by incubation with secondary anti-rat antibody conjugated with rhodamine (Santa Cruz Biotechnology Inc., CA, USA) or FITC (Fluorescein isothiocyanate) Sigma) for 2 h in dark at room temperature. After washing and mounting with Fluoroshield DAPI, then cells are subjected to visualization using confocal microscopy (Arruri et al., 2021).
Cells were grown on coverslips in a 6 well plate, then exposed to 30 mM glucose and 0.5 and 1 μM of GSK2606414. MitoTracker® Deep Red FM dye (200 nM; Molecular Probes, Invitrogen) was utilized to stain the cells for 30–45 min. Cells were washed with PBS and fixed in 4% paraformaldehyde (prepared in PBS) for 5 min at room temperature. Cells were then permeabilized using 0.5% Triton- × 100 for 10 min. Thereafter, cells were washed and subjected to blocking with 3% BSA for a span of 1 h at room temperature. Later on cells were incubated with primary antibody solution (1:100) of anti-Mfn2 antibody (rabbit polyclonal, G-biosciences) in PBS. After 12 h of incubation cells were washed thrice with PBS, and then subjected to FITC conjugated anti-rabbit secondary antibody solution (1:100) incubation for 2 h in the dark. Post which cells were washed with PBS, air-dried, and mounted with DAPI (Invitrogen) on a glass slide. At last imaging the slides with SP8 version of Leica confocal microscope absorption/emission of ∼581/644 nm (Germany) is performed (Bheereddy et al., 2020).
2.2.7. Statistical analysis
Data are represented as mean ± SEM (Standard Error of Mean) . The intergroup variation was measured by one-way analysis of variance (ANOVA) followed by “Bonferroni's multiple comparison post-hoc test” using the Graph Pad Prism (version-5.0). Results with p values < 0.05 were considered to be statistically significant.
3. Results
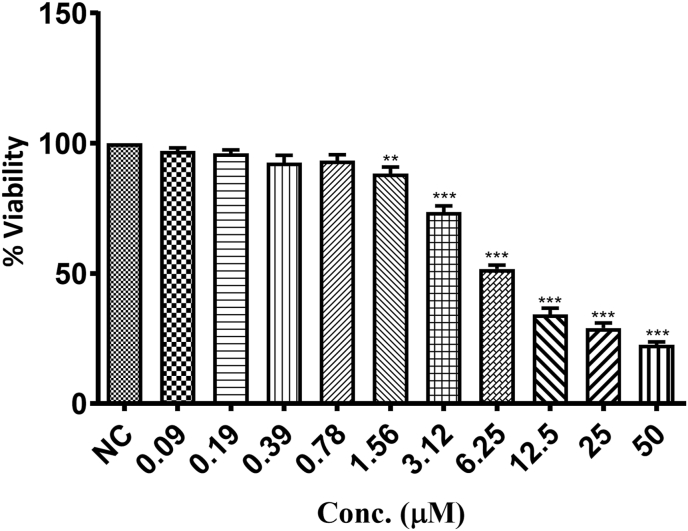
3.1. Effect of GSK2606414 on cell viability
Based on MTT results obtained, we have chosen 0.5 μM and 1 μM of GSK2606414 (IC50: 5.3 μM) as sub maximal doses to assess its neuroprotective potential in HG induced neurotoxicity in N2A cells (Fig. 1).
Fig. 1.
Effect of GSK2606414 on cell viability. Graphical representation of cell viability of N2A cells treated with different concentrations (0.1–100 μM) of GSK2606414 with high glucose (30 mM). Values are expressed as mean ± SEM (n = 3). ∗∗P < 0.01 and ∗∗∗P < 0.001 Vs Normal N2A cells (NC).
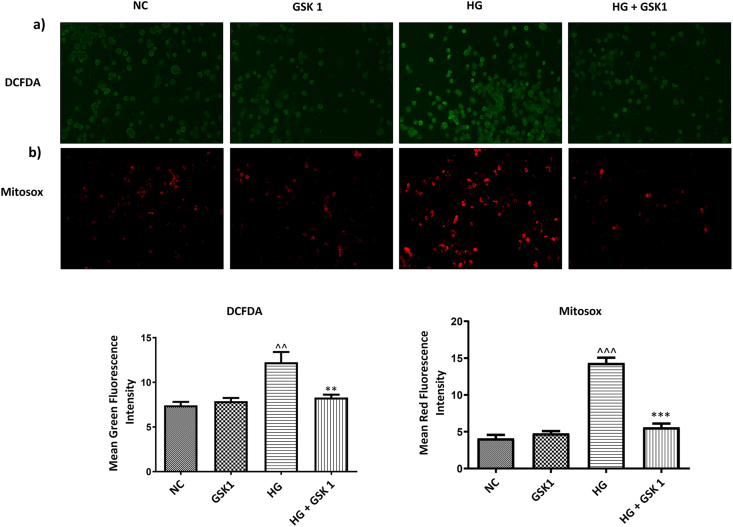
3.2. GSK2606414 decreased ROS production in N2A cells following high glucose exposure
Induction of reactive oxygen species is considered to be one of the main deleterious effects in diabetic condition and ROS induced perturbations in neuronal homeostasis has been widely implicated in several neurodegenerative disorders. HG exposure of N2A cells resulted in increased ROS production when compared to normal cells which was measured using DCFDA staining technique. However, treatment with GSK2606414 (1 μM) markedly decreased the ROS levels which was evident from decreased DCFDA fluorescence intensity. These results indicate the anti-oxidant potential of GSK2606414 (Fig. 3 (a)).
Fig. 3.
Effect of GSK2606414 on ROS and mitochondrial function. Representative images and corresponding bar graphs of (a) DCFDA staining (b) Mitosox staining. Photographs were taken at 200× magnification (scale: 100 μM). Values are expressed as mean ± SEM (n = 3). NC: normal N2A cells, GSK 1: normal N2A cells treated with GSK260414 (1 μM), HG: hyperglycemic N2A cells (30 mM β-D glucose), HG+ GSK 0.5: HG cells treated with GSK260414 (0.5 μM), HG+ GSK1: HG cells treated with GSK260414 (1 μM), ^^P < 0.01, ^^^P < 0.001 v/s NC and ∗∗P < 0.01, ∗∗∗P < 0.001 v/s HG.
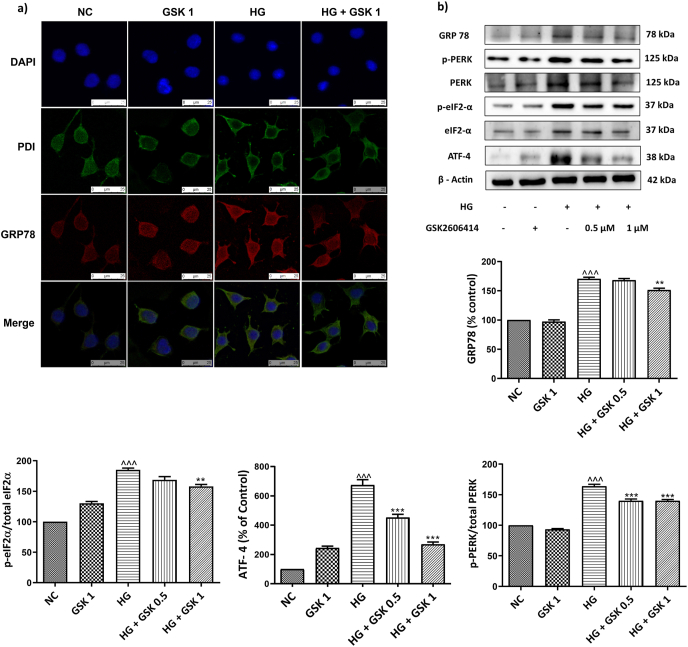
3.3. GSK2606414 ameliorated ERS markers in HG induced neuro2a cells
High glucose (30 μM) induction triggers ER stress which was evident through the western blotting analysis. It was observed that the key markers of ERS such as GRP78, phosphorylated forms of PERK and eIF2α were increased after high glucose induction. Intriguingly, treatment with PERK inhibitor, GSK2606414 ameliorated the expression of these ERS markers suggesting the possible ERS inhibitory activity of GSK2606414 (Fig. 2).
Fig. 2.
Effect of GSK2606414 on ER stress markers. (a) Immunofluorescence staining of N2A cells, representing co-expression of PDI & GRP78. Photographs were taken at 400× magnification (scale: 25 μM) (b) Representative immunoblots of ER stress markers. Values are expressed as mean ± SEM (n = 3). NC: normal N2A cells, GSK 1: normal N2A cells treated with GSK260414 (1 μM), HG: hyperglycemic N2A cells (30 mM β-D glucose), HG+ GSK 0.5: HG cells treated with GSK260414 (0.5 μM), HG+ GSK1: HG cells treated with GSK260414 (1 μM). ^^^P < 0.001 v/s NC and ∗∗P < 0.01, ∗∗∗P < 0.001 v/s HG.
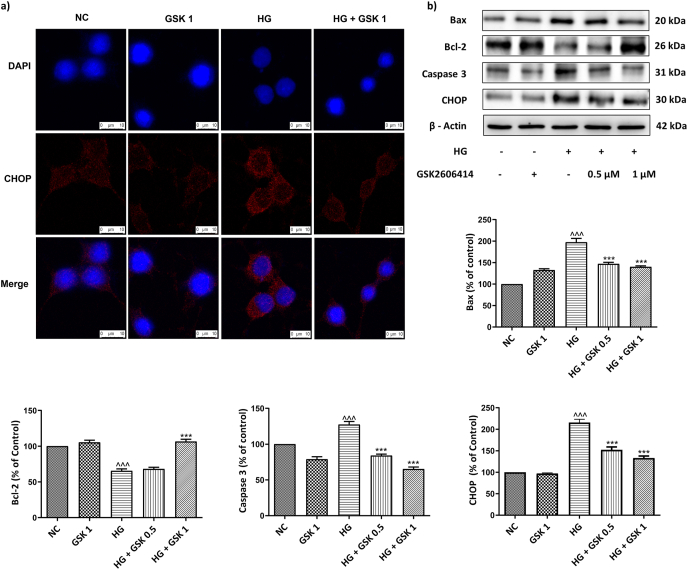
3.3.1. Effect of GSK2606414 on glucose regulator protein of 78 kDa (GRP78)
Under normal physiological conditions GRP78 is expressed at a low level but the high glucose exposure triggered the increase in levels of GRP78. In line with this, our findings have shown that GRP78 levels were markedly upregulated (p < 0.001) in HG insulted N2A cells compared to that of normal cells. However, treatment with GSK2606414 (1 μM) significantly reduced (P < 0.001) the levels of GRP78, a master regulator of ERS (Fig. 2 (b)).
3.3.2. GSK2606414 treatment decreased expression of GRP78 in ER
Further, to confirm the upregulation of GRP78 levels in ER, we performed immunolocalization assay of GRP78 with ER labeling kit (SELECTFX ALEXA FLUOR 488 ENDOP 1 KIT, Santacruz biotechnologies, USA). As shown in the Fig. 2 (a), the Immunofluorescence staining images depict the localization of GRP78 in ER is increased in high glucose insulted N2A cells (evident from the presence of PDI). Whereas, treatment with GSK2606414 markedly decreased the levels of GRP78 within ER.
3.4. GSK2606414 curtails PERK/p-eIF2α/ATF4/CHOP signaling axis
Immunoblotting analysis was performed in different groups of N2A cells post 24 h exposure to high glucose (30 mM) and treatment with GSK2606414. High glucose exposure to N2A cells resulted in upregulation of PERK and its downstream signaling proteins such as eIF2α, ATF-4 and CHOP when compared to the normal cells. However, treatment with GSK2606414 significantly reduced the levels of p-PERK in a dose dependent manner (GSK2606414; 0.5 μM (p < 0.001), 1 μM (p < 0.001)) there by reducing the phosphorylation of eIF2α (p < 0.001) (Fig. 2). GSK2606414 administration also ameliorated CHOP levels by virtue of its action on PERK downstream signalling (Fig. 5 (b)).
Fig. 5.
Effect of GSK2606414 on apoptosis. (a) Immunofluorescence staining of CHOP (red) (b) Representative immunoblots of proteins involved in apoptosis. Photographs were taken at 400× magnification (scale: 10 μM). Values are expressed as mean ± SEM (n = 3). NC: normal N2A cells, GSK 1: normal N2A cells treated with GSK260414 (1 μM), HG: hyperglycemic N2A cells (30 mM β-D glucose), HG+ GSK 0.5: HG cells treated with GSK260414 (0.5 μM), HG+ GSK1: HG cells treated with GSK260414 (1 μM). ^^^P < 0.001 v/s NC and ∗∗∗P < 0.001 v/s HG. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Further to emphasize the role of CHOP in neuronal apoptosis, we performed Immunofluorescence staining of N2A cells, as shown in the Fig. 5 high immunolocalization of CHOP levels indicate that apoptosis triggered by chronic ER stress is significantly high in HG insulted N2A cells when compared to the normoglycemic cells. However, treatment with GSK2606414 restored the CHOP levels indicating possible anti-apoptotic effect of GSK2606414 (Fig. 5 (a)).
3.5. GSK2606414 attenuated HG induced mitochondrial dysfunction and neuronal apoptosis
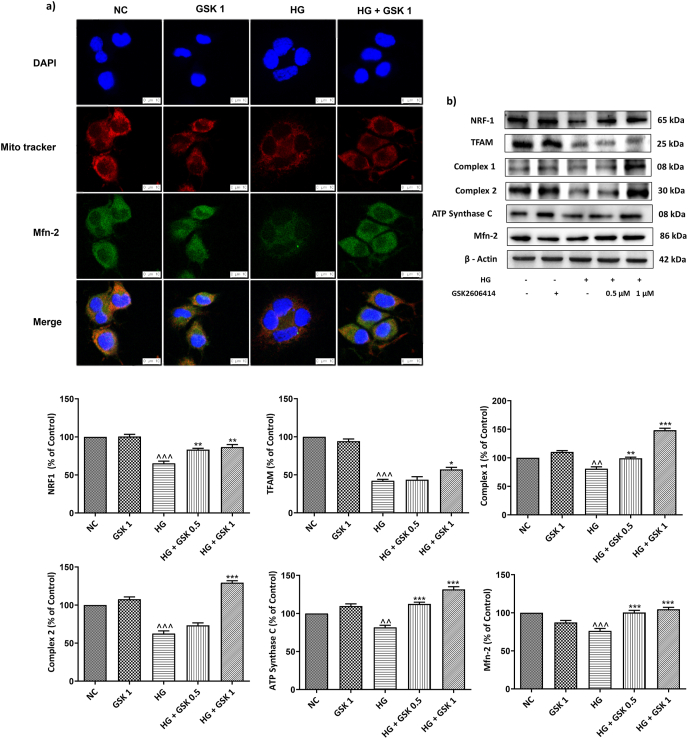
High glucose exposure of N2A cells triggers mitochondrial dysfunction mediated through enhanced PERK/p-eIF2α/ATF4/CHOP signalling which was evident through raised mitochondrial superoxide levels (Fig. 3 (b)) and significant decrease in expression of TFAM, NRF1, complex 1, complex 2 and ATP synthase levels when compared to the normal N2A cells. Treatment with GSK2606414 augmented the levels of these proteins in a significant manner suggesting the role of PERK inhibition in maintaining mitochondrial homeostasis (Fig. 4 (b)). Moreover, to explain the crucial role of enhanced PERK/p-eIF2α/ATF4/CHOP signaling in disruption of ER-mitochondrial tethering we have assessed the levels of Mfn-2 in fixed N2A cells and cellular homogenates following high glucose exposure. It was observed that amplified PERK/p-eIF2α/ATF4/CHOP signaling under high glucose conditions disrupted ER-mitochondrial juxtaposition and results in altered mitochondrial homeostasis as evident from decreased Mfn-2 levels (Fig. 4 (a) and (b)).
Fig. 4.
(a) Immunofluorescence staining of Mfn-2 (green) along with mitotracker (red) and (b) Representative immunoblots of proteins depicting mitochondrial function. Photographs were taken at 400× magnification (scale: 10 μM). Values are expressed as mean ± SEM (n = 3). NC: normal N2A cells, GSK 1: normal N2A cells treated with GSK260414 (1 μM), HG: hyperglycemic N2A cells (30 mM β-D glucose), HG+ GSK 0.5: HG cells treated with GSK260414 (0.5 μM), HG+ GSK1: HG cells treated with GSK260414 (1 μM), ^^P < 0.01, ^^^P < 0.001 v/s NC and ∗ P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 v/s HG. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Additionally, chronic ER stress induced by high glucose exposure triggers apoptosis via upregulation of apoptotic markers like Bax, Caspase-3 and CHOP. Treatment with GSK2606414 prevented the high glucose induced protein expression changes by markedly decreasing the levels of these apoptotic markers. Similarly, anti-apoptotic protein Bcl-2 expression was significantly low in HG treated cells whereas GSK2606414 administration reversed this effect. These results suggest that anti-apoptotic effect of GSK2606414 may be due to attenuation of PERK/p-eIF2α/ATF4/CHOP signaling (Fig. 5 (b)).
4. Discussion
The present study findings revealed that prolonged hyperglycemia is a major causative factor for ROS accumulation in ER and mitochondria thereby leading to Endoplasmic Reticulum Stress (ERS) and mitochondrial dysfunction. Accumulating evidence suggests that exposure of N2A cells to high glucose leads to metabolic perturbations via generation of advanced glycation of proteins and oxidative stress which causes ERS (Rashid et al., 2017). It has been reported that compounds that attenuate ERS response confers neuroprotection upon exposure to toxic compounds (Yuan et al., 2021). The increased ROS production and compromised endogenous antioxidant defenses under high glucose conditions can disturb ER protein folding which eventually leads to aggregation of unfolded and misfolded proteins, this further escalates ER protein overload (O'Brien et al., 2014). Indeed, it is well established that the crosstalk between ER stress and oxidative stress are closely linked to cell homeostasis and apoptosis which is evident from the altered redox homeostasis resulting in ERS (Cao and Kaufman, 2014). Our study findings indicated that GSK2606414 ameliorated the effect of ROS by reducing ERS markers namely GRP78, p-PERK, p-eIF2α, ATF4 and CHOP in HG exposed N2A cells. As oxidative stress and ER stress potentiates each other, curtailing ER stress response by GSK26060414 offers anti-oxidant response. Moreover, mitochondrial function is also improved via attenuating the intracellular ROS levels.
Unfolded protein response armed with several key players which regulate the cell fate against ER stress. Among which GRP78 (glucose regulated protein of 78 kDa) an ER resident chaperone plays a pivotal role in activation of downstream signals upon ER stress and usually referred as an established biomarker to detect the presence of cellular ER stress (Lee, 2005). In healthy cells, GRP78 is bound to three different ER transmembrane proteins; IRE1α (Ionositol requiring kinase/endoribonuclease1 alpha), PERK (protein kinase activated by double stranded RNA-like ER kinase) and ATF-6 (activating transcription factor-6). Activity of these transmembrane proteins is inhibited by coupling with GRP78. However, ER stress stimulates GRP78 and makes it segregated from attached transmembrane proteins, thus leading to activation of these proteins, there by unraveling the three distinct branches of ER stress response system. All these signaling events contribute to additional chaperone capacity, degradation of terminally misfolded proteins through ERAD (ER associated degradation) and induction of autophagy, etc. These signaling mechanisms will be returned to their inactive modes once the ER homeostasis has been re-established (Hetz, 2012; Adams et al., 2019). In line with these reports we have observed that HG exposure triggered GRP78 activation and thereby unravels the activation of PERK leading to activation of its downstream signaling through eIF2α. Conversely, GSK2606414 treatment significantly reduced the activation of these proteins as evident from decrease in phosphorylated forms of PERK and eIF2α, suggesting the potential of GSK2606414 in intervening PERK/p-eIF2α/ATF4/CHOP signaling following hyperglycemic conditions.
Converging lines of evidence indicate that ER has an intricate quality of restoring cellular homeostasis by activating unfolded protein response (UPR). Under acute stress, the conserved machinery gets enacted by recruiting chaperones to bring the cells to their normal state and attempt proper protein folding (Wu and Kaufman, 2006). However, under chronic conditions, ER fails to restore protein homeostasis and thus engage apoptosis. The current study focuses on one of the three ER transmembrane proteins PERK, which is a key ER stress sensor that exhibits a unique role in ER-mitochondrial interaction in maintaining neuronal health (Wen et al., 2017; Meng et al., 2018; Kumar and Maity, 2021). In response to high glucose treatment, ROS provokes PERK activation followed by its homodimerization and autophosphorylation, which is followed by phosphorylation of its downstream molecule eIF2α (eukaryotic initiation factor 2 alpha) (Lei et al., 2018). Further, the activated eIF2α regulates the expression of specific genes associated with ER stress through activating transcription factor-4 (ATF-4). ATF-4 predominantly monitors the cellular response to stress by promoting cell survival under acute stress whereas during chronic ER stress, it induces apoptosis (Fels and Koumenis, 2006; Huang et al., 2017; Wortel et al., 2017). The persistent activation of ATF-4 results in enhanced expression of CHOP which initiates a coordinated transcriptional profile in the cell that favors cell death via apoptosis under prolonged ER stress conditions.
Our study findings revealed that treatment with GSK2606414 reduced the expression of ERS markers viz., GRP78, p-PERK, p-eIF2α, ATF-4 levels in HG insulted N2A cells and as a result reducing the ER overload.
The emerging evidence indicates the important role of hyperglycemia induced ROS as a connecting link between UPR and mitochondrial dysfunction in neuronal cells (O'Brien et al., 2014). Thus, to understand the connection between ER and mitochondria we further elucidated the role of ERS in promoting mitochondrial damage. In this connection, we observed that hyperglycaemia is found to tamper the mitochondrial homeostasis through enhanced superoxide production which eventually compromises mitochondrial integrity via UPR.
Mitochondria are known to be the principal source of ROS which results from imperfectly coupled electron transport with the influence of altered glucose. Furthermore, accumulating literature suggests that HG manifests a compromise in mitochondrial complex activities. (Dassanayaka et al., 2015; Kalvala et al., 2020a). The ROS mediated activation of UPR especially PERK signaling branch caused alteration in mitochondrial proteostasis like ETC (Complexes I-IV), ATP synthase (Complex V) and degradation of mitochondrial transcription factor (TFAM). Blockade of PERK/p-eIF2α/ATF4/CHOP axis by GSK2606414 improved mitochondrial function by enhancing the levels of TFAM, complex activities of I, II and ATP synthase C. This effect could be possibly due to the induction of protease LON whose effect is dependent on PERK-regulated transcription factor ATF4. The upregulated TFAM transcription regulates the mitochondrial biogenesis via enhancing NRF1 levels (Rainbolt et al., 2014). These findings indicate the unprecedented role of PERK in ERS mediated mitochondrial damage.
Another mitochondrial protein Mfn2, which is a critical component of MAM function, helps in maintaining the juxtaposition of ER and mitochondria (Verfaillie et al., 2012). ER and mitochondria though separate organelles, they establish a link via ERMES complex (ER-mitochondria encounter structures) where Mfn2 is one of the key tethering forces that ensure communication between these two organelles (Delmotte and Sieck, 2020). Gladys A. Ngoh and his team elucidated the role of mitochondrial fusion protein Mfn2 in ER stress response and proved that ablation of Mfn2 leads to ER stress-induced cell death by caspase activation and induction of CHOP levels (Ngoh et al., 2012). Based on the inference from similar studies, we sought to confirm whether high glucose mediated ERS alters the mitochondrial fusion. Intriguingly, in line with these results, our study findings depicted the beneficial role of GSK2606414 treatment in upregulating the levels of Mfn2 in HG induced N2A cells by targeting PERK. Various published reports suggest the role of CHOP in execution of apoptotic events following ER stress (Enogieru et al., 2019). PERK activation induces phosphorylation of eIF2α that in turn promotes transcriptional induction of ATF-4 whose target genes include CHOP, GADD 34 and ATF3, etc. (Han et al., 2013). Notably, eIF2α (Ser51Ala) knock-in cells (resistant to phosphorylation by eIF2α kinases such as PERK) and cells that lack PERK (PERK−/−) and ATF-4 (ATF4−/−) failed to induce CHOP under ER stress conditions, suggesting the key role of PERK/peIF2α/ATF4/CHOP signaling axis in directing the cell fate towards apoptosis during ER stress (Harding et al., 2000; Novoa et al., 2001; Scheuner et al., 2001). In consistent with data, we observed that ER stress induced apoptotic events were attenuated by GSK2606414 via inhibiting the PERK/p-eIF2α/ATF4/CHOP axis that not only reduced the levels of apoptotic proteins like Bax and caspase 3 but also elevated the levels of anti-apoptotic Bcl-2.
5. Conclusion
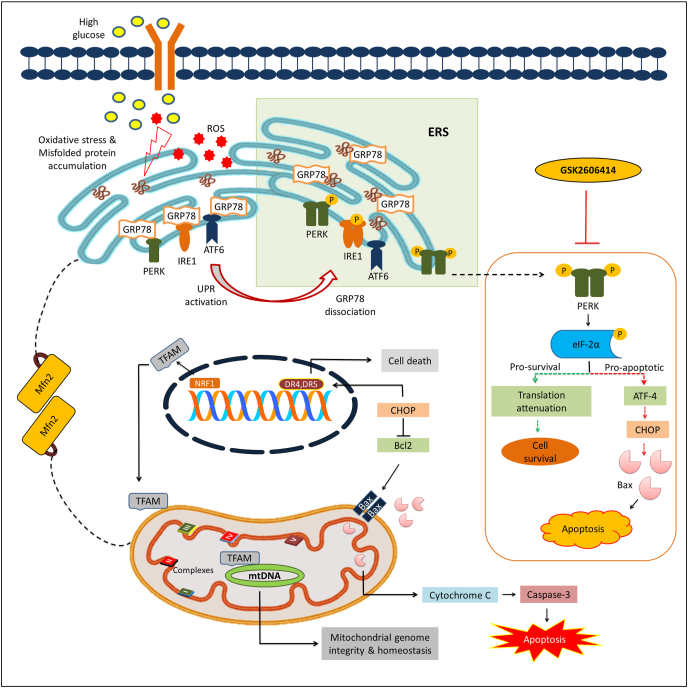
In summary, ER stress response displays dual modules where initial mild or acute stress favors the activation of pro-survival module that results in either amelioration of initial stress or adaptation to it. However, chronic stress induces cell death by activating pro-apoptotic module. In both cases, the final phenotypic outcome either survival/adaptation or apoptosis depends upon gene expression patterns governed by initial stress signals (Sano and Reed, 2013). GSK2606414, a synthetic inhibitor of PERK, ameliorated high glucose induced neurotoxicity in N2A cells via attenuating PERK/p-eIF2α/ATF4/CHOP axis and augmenting mitochondrial function (Fig. 6). Thus, the obtained results provide an avenue for further studies which may be helpful to develop GSK2606414 as a novel therapeutic candidate against high glucose induced neurotoxicity which is prevalent in neurological complications of diabetes.
Fig. 6.
Probable mechanistic basis of GSK2606414 against high glucose induced neurotoxicity. High glucose exposure to N2A cells causes oxidative stress and alters protein folding capacity of ER by accumulation of misfolded/unfolded proteins that triggers UPR. UPR is executed by activation of three transmembrane proteins and their respective downstream signalling events by which ERS response exhibits a dichotomic module. PERK plays a critical role in UPR which decides the cell fate where initial mild or acute stress favors the activation of pro-survival module (represented in green dotted line) that results in either amelioration of initial stress or adaptation to it. However, chronic stress induces cell death by activating pro-apoptotic events (represented in red dotted line). Further ERS potentiates mitochondrial dysfunction by multiple mechanisms including excess generation of ROS, CHOP/Bax/Cytochrome C release and altering ER-mitochondria tethering via modulation of Mfn2. GSK2606414, a synthetic PERK inhibitor, modulates the PERK/p-eIF2α/ATF4/CHOP signalling axis and augments mitochondrial function to mitigate high glucose induced neurotoxicity in N2A cells. GRP78: glucose-regulated protein of 78 kDa, ROS: Reactive Oxygen Species, PERK: protein kinase activated by double stranded RNA-like ER kinase, ATF-6: Activating transcription factor 6, ATF-4: Activating transcription factor 4, eIF2α: Eukaryotic Initiation Factor 2 α, IRE1α: Inositol-requiring enzyme 1 alpha, UPR: Unfolded Protein Response, CHOP: C/EBP homologous protein, Mfn2: Mitofusin 2, Bax: BCL2-associated X protein, Bcl2: B-cell lymphoma 2, DR4: Death receptor 4, DR5: Death receptor 5, TFAM: Mitochondrial transcription factor A, NRF1: Nuclear respiratory factor 1, mtDNA: Mitochondrial DNA. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Funding
The authors are grateful for the financial support of Department of Science and Technology (DST), Women Scientist Scheme-A (WOS-A) (grant number: SR/WOS-A/LS-485/2017 to Gundu Chayanika) to carry out this study and NIPER-Hyderabad, India, in the preparation of this paper.
Availability of data and material
The data that support the findings of this study are available on request from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Chayanika Gundu: Data curation, Conceptualization, Methodology, Writing – original draft. Vijay Kumar Arruri: Conceptualization, Methodology, Writing – review & editing. Bhoomika Sherkhane: Conceptualization, Methodology. Dharmendra Kumar Khatri: Conceptualization, Supervision, Writing – review & editing. Shashi Bala Singh: Conceptualization, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Dharmendra Kumar Khatri, Email: dkkhatri10@gmail.com.
Shashi Bala Singh, Email: sbsingh.dipas@gmail.com.
References
- Adams C.J., Kopp M.C., Larburu N., Nowak P.R., Ali M.M. Structure and molecular mechanism of ER stress signaling by the unfolded protein response signal activator IRE1. Front. Mol. Biosci. 2019;6:11. doi: 10.3389/fmolb.2019.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruri V., Komirishetty P., Areti A., Dungavath S.K.N., Kumar A. Nrf2 and NF-κB modulation by Plumbagin attenuates functional, behavioural and biochemical deficits in rat model of neuropathic pain. Pharmacol. Rep. 2017;69:625–632. doi: 10.1016/j.pharep.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Arruri V.K., Gundu C., Kalvala A.K., Sherkhane B., Khatri D.K., Singh S.B. Carvacrol abates NLRP3 inflammasome activation by augmenting Keap1/Nrf-2/p62 directed autophagy and mitochondrial quality control in neuropathic pain. Nutr. Neurosci. 2021:1–16. doi: 10.1080/1028415X.2021.1892985. [DOI] [PubMed] [Google Scholar]
- Augusto L., Martynowicz J., Staschke K.A., Wek R.C., Sullivan W.J., Jr. Effects of PERK eIF2α kinase inhibitor against Toxoplasma gondii. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.01442-18. e01442-01418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axten J.M., Medina J.s.R., Feng Y., Shu A., Romeril S.P., Grant S.W., Li W.H.H., Heerding D.A., Minthorn E., Mencken T. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl) phenyl] acetyl}-2, 3-dihydro-1 H-indol-5-yl)-7 H-pyrrolo [2, 3-d] pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) J. Med. Chem. 2012;55:7193–7207. doi: 10.1021/jm300713s. [DOI] [PubMed] [Google Scholar]
- Bachewal P., Gundu C., Yerra V.G., Kalvala A.K., Areti A., Kumar A. Morin exerts neuroprotection via attenuation of ROS induced oxidative damage and neuroinflammation in experimental diabetic neuropathy. Biofactors. 2018;44:109–122. doi: 10.1002/biof.1397. [DOI] [PubMed] [Google Scholar]
- Bhardwaj A., Bhardwaj R., Dhawan D.K., Kaur T. Exploring the effect of endoplasmic reticulum stress inhibition by 4-phenylbutyric acid on AMPA-induced hippocampal excitotoxicity in rat brain. Neurotox. Res. 2019;35:83–91. doi: 10.1007/s12640-018-9932-0. [DOI] [PubMed] [Google Scholar]
- Bheereddy P., Yerra V.G., Kalvala A.K., Sherkhane B., Kumar A. SIRT1 activation by polydatin alleviates oxidative damage and elevates mitochondrial biogenesis in experimental diabetic neuropathy. Cell. Mol. Neurobiol. 2020;41(7):1563–1577. doi: 10.1007/s10571-020-00923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Dai D.-L., Yao L., Yu H.-H., Ning B., Zhang Q., Chen J., Cheng W.-H., Shen W., Yang Z.-X. Saturated fatty acid induction of endoplasmic reticulum stress and apoptosis in human liver cells via the PERK/ATF4/CHOP signaling pathway. Mol. Cell. Biochem. 2012;364:115–129. doi: 10.1007/s11010-011-1211-9. [DOI] [PubMed] [Google Scholar]
- Cao S.S., Kaufman R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxidants Redox Signal. 2014;21:396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.-C., Chien C.-C., Wu M.-S., Chen Y.-C. Evodiamine from Evodia rutaecarpa induces apoptosis via activation of JNK and PERK in human ovarian cancer cells. Phytomedicine. 2016;23:68–78. doi: 10.1016/j.phymed.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Dassanayaka S., Readnower R.D., Salabei J.K., Long B.W., Aird A.L., Zheng Y.-T., Muthusamy S., Facundo H.T., Hill B.G., Jones S.P. High glucose induces mitochondrial dysfunction independently of protein O-GlcNAcylation. Biochem. J. 2015;467:115. doi: 10.1042/BJ20141018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmotte P., Sieck G.C. Endoplasmic reticulum stress and mitochondrial function in airway smooth muscle. Front. Cell Dev. Biol. 2020;7:374. doi: 10.3389/fcell.2019.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Teixeira K.L., Calegari-Silva T.C., Medina J.M., Vivarini Á.C., Cavalcanti Á., Teteo N., Santana A.K.M., Real F., Gomes C.M., Pereira R.M.S. Emerging role for the PERK/eIF2α/ATF4 in human cutaneous leishmaniasis. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-17252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enogieru A.B., Haylett W.L., Miller H.C., van der Westhuizen F.H., Hiss D.C., Ekpo O.E. Attenuation of endoplasmic reticulum stress, impaired calcium homeostasis, and altered bioenergetic functions in MPP+-exposed SH-SY5Y cells pretreated with rutin. Neurotox. Res. 2019;36:764–776. doi: 10.1007/s12640-019-00048-4. [DOI] [PubMed] [Google Scholar]
- Fels D.R., Koumenis C. The PERK/eIF2α/ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biol. Ther. 2006;5:723–728. doi: 10.4161/cbt.5.7.2967. [DOI] [PubMed] [Google Scholar]
- Halliday M., Hughes D., Mallucci G.R. Fine-tuning PERK signaling for neuroprotection. J. Neurochem. 2017;142:812–826. doi: 10.1111/jnc.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Back S.H., Hur J., Lin Y.-H., Gildersleeve R., Shan J., Yuan C.L., Krokowski D., Wang S., Hatzoglou M. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding H.P., Novoa I., Zhang Y., Zeng H., Wek R., Schapira M., Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- Huang H., Miao L., Liang F., Liu X., Xu L., Teng X., Wang Q., Ridder W.H., Shindler K.S., Sun Y. Neuroprotection by eIF2 α-CHOP inhibition and XBP-1 activation in EAE/optic neuritiss. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2017.329. e2936-e2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iurlaro R., Muñoz-Pinedo C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016;283:2640–2652. doi: 10.1111/febs.13598. [DOI] [PubMed] [Google Scholar]
- Kalvala A.K., Kumar R., Sherkhane B., Gundu C., Arruri V.K., Kumar A. Bardoxolone methyl ameliorates hyperglycemia induced mitochondrial dysfunction by activating the keap1-Nrf2-ARE pathway in experimental diabetic neuropathy. Mol. Neurobiol. 2020;57:3616–3631. doi: 10.1007/s12035-020-01989-0. [DOI] [PubMed] [Google Scholar]
- Kalvala A.K., Yerra V.G., Kumar A. LONP1 induction by SRT1720 attenuates mitochondrial dysfunction against high glucose induced neurotoxicity in PC12 cells. Toxicol. Vitro. 2020;62:104695. doi: 10.1016/j.tiv.2019.104695. [DOI] [PubMed] [Google Scholar]
- Kumar V., Maity S. ER stress-sensor proteins and ER-mitochondrial crosstalk—signaling beyond (ER) stress response. Biomolecules. 2021;11:173. doi: 10.3390/biom11020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzillotta C., Zuliani I., Tramutola A., Abisambra J.F., Barone E., Perluigi M., Domenico F.D. PERK inhibition promotes the rescue of protein translation and Nrf2-related antioxidant response: molecular and cell biology/oxidative stress. Alzheimer's Dementia. 2020;16 [Google Scholar]
- Lee A.S. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35:373–381. doi: 10.1016/j.ymeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Lei J., Zhao L., Zhang Y., Wu Y., Liu Y. High glucose-induced podocyte injury involves activation of mammalian target of rapamycin (mTOR)-induced endoplasmic reticulum (ER) stress. Cell. Physiol. Biochem. 2018;45:2431–2443. doi: 10.1159/000488231. [DOI] [PubMed] [Google Scholar]
- Lelkes E., Unsworth B.R., Lelkes P.I. Reactive oxygen species, apoptosis and alte1red NGF-induced signaling in PC12 pheochromocytoma cells cultured in elevated glucose: an in vitro cellular model for diabetic neuropathy. Neurotox. Res. 2001;3:189–203. doi: 10.1007/BF03033191. [DOI] [PubMed] [Google Scholar]
- Lindholm D., Wootz H., Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13:385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- Logue S.E., Cleary P., Saveljeva S., Samali A. New directions in ER stress-induced cell death. Apoptosis. 2013;18:537–546. doi: 10.1007/s10495-013-0818-6. [DOI] [PubMed] [Google Scholar]
- McLaughlin M., Pedersen M., Roulstone V., Bergerhoff K.F., Smith H.G., Whittock H., Kyula J.N., Dillon M.T., Pandha H.S., Vile R. The PERK inhibitor GSK2606414 enhances reovirus infection in head and neck squamous cell carcinoma via an ATF4-dependent mechanism. Mol. Ther. Oncol. 2020;16:238–249. doi: 10.1016/j.omto.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng C., Zhang J., Dang B., Li H., Shen H., Li X., Wang Z. PERK pathway activation promotes intracerebral hemorrhage induced secondary brain injury by inducing neuronal apoptosis both in vivo and in vitro. Front. Neurosci. 2018;12:111. doi: 10.3389/fnins.2018.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado G., Castillo V., Soto P., López N., Axten J.M., Sardi S.P., Hoozemans J.J., Hetz C. Targeting PERK signaling with the small molecule GSK2606414 prevents neurodegeneration in a model of Parkinson's disease. Neurobiol. Dis. 2018;112:136–148. doi: 10.1016/j.nbd.2018.01.004. [DOI] [PubMed] [Google Scholar]
- Moghaddam H.K., Baluchnejadmojarad T., Roghani M., Khaksari M., Norouzi P., Ahooie M., Mahboobi F. Berberine ameliorate oxidative stress and astrogliosis in the hippocampus of STZ-induced diabetic rats. Mol. Neurobiol. 2014;49:820–826. doi: 10.1007/s12035-013-8559-7. [DOI] [PubMed] [Google Scholar]
- Moreno J.A., Halliday M., Molloy C., Radford H., Verity N., Axten J.M., Ortori C.A., Willis A.E., Fischer P.M., Barrett D.A. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3006767. 206ra138-206ra138. [DOI] [PubMed] [Google Scholar]
- Murphy M.P. Mitochondrial dysfunction indirectly elevates ROS production by the endoplasmic reticulum. Cell Metabol. 2013;18:145–146. doi: 10.1016/j.cmet.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Park J.-H., Hayakawa K. Therapeutic use of extracellular mitochondria in CNS injury and disease. Exp. Neurol. 2020;324:113114. doi: 10.1016/j.expneurol.2019.113114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naon D., Zaninello M., Giacomello M., Varanita T., Grespi F., Lakshminaranayan S., Serafini A., Semenzato M., Herkenne S., Hernández-Alvarez M.I. Critical reappraisal confirms that Mitofusin 2 is an endoplasmic reticulum–mitochondria tether. Proc. Natl. Acad. Sci. Unit. States Am. 2016;113:11249–11254. doi: 10.1073/pnas.1606786113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngoh G.A., Papanicolaou K.N., Walsh K. Loss of mitofusin 2 promotes endoplasmic reticulum stress. J. Biol. Chem. 2012;287:20321–20332. doi: 10.1074/jbc.M112.359174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoa I., Zeng H., Harding H.P., Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien P.D., Hinder L.M., Sakowski S.A., Feldman E.L. ER stress in diabetic peripheral neuropathy: a new therapeutic target. Antioxidants Redox Signal. 2014;21:621–633. doi: 10.1089/ars.2013.5807. [DOI] [PubMed] [Google Scholar]
- Paillusson S., Stoica R., Gomez-Suaga P., Lau D.H., Mueller S., Miller T., Miller C.C. There's something wrong with my MAM; the ER–mitochondria axis and neurodegenerative diseases. Trends Neurosci. 2016;39:146–157. doi: 10.1016/j.tins.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponugoti B., Dong G., Graves D.T. Role of forkhead transcription factors in diabetes-induced oxidative stress. Exp. Diabetes Res. 2012;2012 doi: 10.1155/2012/939751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbolt T.K., Saunders J.M., Wiseman R.L. Stress-responsive regulation of mitochondria through the ER unfolded protein response. Trends Endocrinol. Metabol. 2014;25:528–537. doi: 10.1016/j.tem.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Rashid K., Chowdhury S., Ghosh S., Sil P.C. Curcumin attenuates oxidative stress induced NFκB mediated inflammation and endoplasmic reticulum dependent apoptosis of splenocytes in diabetes. Biochem. Pharmacol. 2017;143:140–155. doi: 10.1016/j.bcp.2017.07.009. [DOI] [PubMed] [Google Scholar]
- Rochette L., Zeller M., Cottin Y., Vergely C. Diabetes, oxidative stress and therapeutic strategies. Biochim. Biophys. Acta Gen. Subj. 2014;1840:2709–2729. doi: 10.1016/j.bbagen.2014.05.017. [DOI] [PubMed] [Google Scholar]
- Rojas-Rivera D., Delvaeye T., Roelandt R., Nerinckx W., Augustyns K., Vandenabeele P., Bertrand M.J. When PERK inhibitors turn out to be new potent RIPK1 inhibitors: critical issues on the specificity and use of GSK2606414 and GSK2656157. Cell Death Differ. 2017;24:1100–1110. doi: 10.1038/cdd.2017.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol. Appl. Pharmacol.. 15;212(2):167-178. [DOI] [PubMed]
- Sano R., Reed J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta Mol. Cell Res. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D., Song B., McEwen E., Liu C., Laybutt R., Gillespie P., Saunders T., Bonner-Weir S., Kaufman R.J. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- Singh E., Devasahayam G. Neurodegeneration by oxidative stress: a review on prospective use of small molecules for neuroprotection. Mol. Biol. Rep. 2020;47:3133–3140. doi: 10.1007/s11033-020-05354-1. [DOI] [PubMed] [Google Scholar]
- Veeresh P., Kaur H., Sarmah D., Mounica L., Verma G., Kotian V., Kesharwani R., Kalia K., Borah A., Wang X. Endoplasmic reticulum–mitochondria crosstalk: from junction to function across neurological disorders. Ann. N. Y. Acad. Sci. 2019;1457:41–60. doi: 10.1111/nyas.14212. [DOI] [PubMed] [Google Scholar]
- Verfaillie T., Rubio N., Garg A., Bultynck G., Rizzuto R., Decuypere J., Piette J., Linehan C., Gupta S., Samali A. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 2012;19:1880–1891. doi: 10.1038/cdd.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Wen L., Xiao B., Shi Y., Han F. PERK signalling pathway mediates single prolonged stress-induced dysfunction of medial prefrontal cortex neurons. Apoptosis. 2017;22:753–768. doi: 10.1007/s10495-017-1371-5. [DOI] [PubMed] [Google Scholar]
- Wortel I.M., van der Meer L.T., Kilberg M.S., van Leeuwen F.N. Surviving stress: modulation of ATF4-mediated stress responses in normal and malignant cells. Trends Endocrinol. Metabol. 2017;28:794–806. doi: 10.1016/j.tem.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Kaufman R. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 2006;13:374–384. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- Yang W., Zhou X., Zimmermann H.R., Cavener D.R., Klann E., Ma T. Repression of the eIF2α kinase PERK alleviates mGluR-LTD impairments in a mouse model of Alzheimer's disease. Neurobiol. Aging. 2016;41:19–24. doi: 10.1016/j.neurobiolaging.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Zhao S.W., Wen S.Q., Zhu Q.P., Wang L., Zou H., Gu J.H., Liu X.Z., Bian J.C., Liu Z.P. Alpha-lipoic acid attenuates cadmium-and lead-induced neurotoxicity by inhibiting both endoplasmic-reticulum stress and activation of Fas/FasL and mitochondrial apoptotic pathways in rat cerebral cortex. Neurotox. Res. 2021;39:1103–1115. doi: 10.1007/s12640-021-00348-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author upon reasonable request.