Abstract
Although diabetes normally causes an elevation of cholesterol biosynthesis and induces hypercholesterolemia in animals and human, the mechanism linking diabetes to the dysregulation of cholesterol biosynthesis in the liver is not fully understood. As liver peroxisomal β-oxidation is induced in the diabetic state and peroxisomal oxidation of fatty acids generates free acetate, we hypothesized that peroxisomal β-oxidation might play a role in liver cholesterol biosynthesis in diabetes. Here, we used erucic acid, a specific substrate for peroxisomal β-oxidation, and 10,12-tricosadiynoic acid, a specific inhibitor for peroxisomal β-oxidation, to specifically induce and suppress peroxisomal β-oxidation. Our results suggested that induction of peroxisomal β-oxidation increased liver cholesterol biosynthesis in streptozotocin-induced diabetic mice. We found that excessive oxidation of fatty acids by peroxisomes generated considerable free acetate in the liver, which was used as a precursor for cholesterol biosynthesis. In addition, we show that specific inhibition of peroxisomal β-oxidation decreased cholesterol biosynthesis by reducing acetate formation in the liver in diabetic mice, demonstrating a crosstalk between peroxisomal β-oxidation and cholesterol biosynthesis. Based on these results, we propose that induction of peroxisomal β-oxidation serves as a mechanism for a fatty acid-induced upregulation in cholesterol biosynthesis and also plays a role in diabetes-induced hypercholesterolemia.
Keywords: fatty acid oxidation, peroxisome, cholesterol, biosynthesis, diabetes
Abbreviations: AcAc-CoA, acetoacetyl-CoA; ACOT12, acetyl-CoA hydrolase; CAT, carnitine acyltransferase; FAO, fatty acid oxidation system; FFA, free fatty acid; HMG-CoA, 3-hydroxy-3-methyl glutaryl coenzyme A; HRO, high erucic acid rapeseed oil; LC-acyl-CoA, long-chain acyl-CoA; MVA, mevalonic acid; OO, olive oil; STZ, streptozotocin; TAG, triacylglycerol; TDYA, 10,12-tricosadiynoic acid; VLDL-C, very low density lipoprotein-cholesterol
Numerous reports demonstrated that uncontrolled diabetes is associated with hypercholesterolemia, which greatly increases the risk of atherosclerosis and related cardiovascular diseases (1, 2, 3). However, the mechanisms by which diabetes cause elevation in plasma cholesterol level are not fully demonstrated. As liver cholesterol biosynthesis is enhanced in the diabetic animals (4, 5), the dysregulated cholesterol biosynthesis might play a role in diabetes-induced hypercholesterolemia. It is well-known that free fatty acid (FFA) stimulates hepatic cholesterol biosynthesis (6, 7, 8), and liver-FFAs increased significantly under the condition of diabetes. Therefore, the increased supply of fatty acids might lead to elevated cholesterol synthesis and very low density lipoprotein-cholesterol (VLDL-C) secretion in the diabetic animals.
To explore the potential roles of fatty acids in cholesterol biosynthesis and hyperlipidemia in diabetes, we focused on peroxisomal β-oxidation, a fatty acid oxidation (FAO) system that acted on long-chain fatty acids (9). Although the crosstalk between peroxisomal β-oxidation and cholesterol biosynthesis is not established so far, we noted that peroxisomal FAO is induced in the liver of diabetic animals (10, 11, 12, 13), and peroxisomal β-oxidation generated free acetate as the ultimate product (14, 15, 16). It was reported that cholesterol biosynthesis from acetate is increased in the liver of diabetic animals (17, 18, 19), and [1-14C]-acetate incorporation into cholesterol by liver slices was much greater in animals fed erucic acid than in those fed palmitic, stearic, oleic, or linoleic acid (20). Furthermore, direct measurement of cholesterol synthesis from [1-14C]-lignoceric acid suggested that the acetyl-CoA generated from peroxisomal β-oxidation was preferentially used for biosynthesis of cholesterol (21). We hypothesized that peroxisomal oxidation of endogenous fatty acids might play a role in liver cholesterol biosynthesis and induce elevation in plasma cholesterol in the diabetic animals. Erucic acid (C22:1) as a specific substrate for peroxisomal β-oxidation was used to induce peroxisomal β-oxidation flux (16, 22), and 10,12-tricosadiynoic acid (TDYA), a specific inhibitor for peroxisomal β-oxidation, was applied to suppress peroxisomal β-oxidation (23).
This study investigated the role and potential mechanism of peroxisomal β-oxidation in liver de novo cholesterol biosynthesis in streptozotocin (STZ)-induced diabetic mice.
Results
Peroxisomal β-oxidation was induced in liver of the STZ-induced diabetic mice
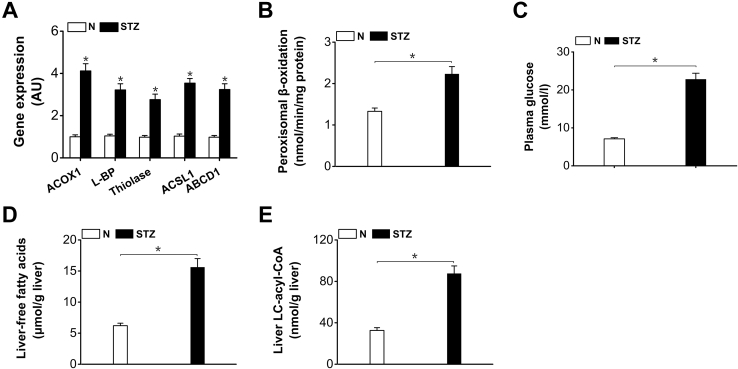
The gene expressions of the enzymes involved in peroxisomal β-oxidation were upregulated in livers of the STZ-induced diabetic mice (Fig. 1A). Peroxisomal β-oxidation increased significantly in the liver of the diabetic mice (increased by 69% versus normal control) (Fig. 1B). Plasma glucose was elevated remarkably in the STZ-treated mice (Fig. 1C). Liver-FFAs content increased significantly in the diabetic mice (by 152% versus normal control) (Fig. 1D). Long-chain fatty acids are identified to be endogenous ligands for peroxisome proliferator activator receptor α isoform; excessive hepatic uptake of fatty acids in diabetic mice resulted in activation of peroxisome proliferator activator receptor α isoform and triggered downstream transcription of the genes involved in peroxisomal β-oxidation (9, 24). Liver long-chain acyl-CoA (LC-acyl-CoA) increased considerably in the diabetic mice (by 168% versus normal control) (Fig. 1E), which provided sufficient substrates for peroxisomal β-oxidation.
Figure 1.
Peroxisomal β-oxidation was enhanced in livers of the STZ-induced diabetic mice.A, gene expressions of enzymes in peroxisomal β-oxidation were upregulated in livers of the STZ-induced diabetic mice. B, peroxisomal β-oxidation was enhanced in liver of the STZ-induced diabetic mice. C, plasma glucose was elevated remarkably in the STZ-induced diabetic mice. D, liver-free fatty acids increased significantly in the STZ-induced diabetic mice. E, liver LC-acyl-CoA increased significantly in the STZ-induced diabetic mice. ABCD1, peroxisomal ATP-binding cassette transporter D; ACOX1, acyl-CoA oxidase-1; ACS, acyl-CoA synthetase; ACSL1, long-chain acyl-CoA synthetase-1; L-BP, L-bifunctional protein; STZ, streptozotocin. Mean ± SEM, n = 6, ∗p < 0.05 by t test between paired conditions.
Excessive fatty acid flux through peroxisomal β-oxidation stimulated cholesterol biosynthesis in the diabetic mice
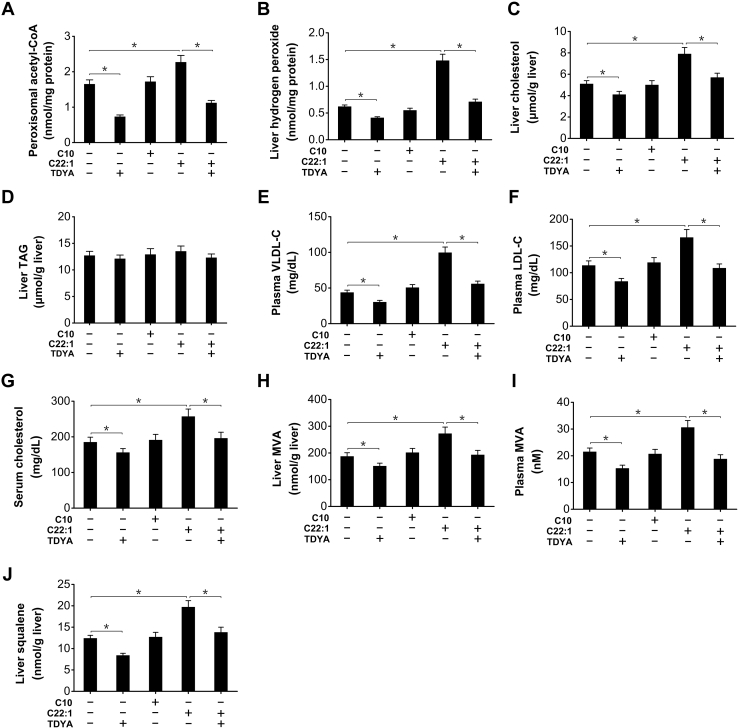
To address the role of peroxisomal β-oxidation in cholesterol biosynthesis, erucic acid (C22:1) was acutely administered to STZ-induced diabetic mice to increase fatty acid flux through peroxisomal β-oxidation system, and decanoic acid (C10), a medium-chain fatty acid that is preferentially metabolized in mitochondria was used as a control to determine whether mitochondrial FAO was also involved in cholesterol biosynthesis. The results indicated that peroxisomal content of acetyl-CoA, final products of β-oxidation, was increased by the administration of C22:1 (by 49% versus STZ control) and reduced by TDYA, whereas there was no alteration for C10-treated group (Fig. 2A). Hydrogen peroxide, a byproduct for peroxisomal β-oxidation, was also significantly increased in liver after C22:1 treatment (by 139% versus STZ control), as reduced by TDYA (Fig. 2B). Liver cholesterol content increased significantly by administration of C22:1 (by 55% versus STZ control), as reduced by TDYA, whereas administration of C10 had no significant effect on cholesterol level (Fig. 2C). Liver triacylglycerol (TAG) content was not significantly affected after treatment of C22:1 or C10 (Fig. 2D). Plasma VLDL-C and low density lipoprotein-cholesterol increased by the treatment of C22:1 (by 128% and 46% versus STZ control, respectively) (Fig. 2, E and F). Serum cholesterol level also increased after treatment of C22:1 (by 39% versus STZ control), and reduced by TDYA, whereas there was no alteration for C10 (Fig. 2G). Liver content of mevalonic acid (MVA), a key intermediate in cholesterol biosynthesis, increased by 45% in C22:1 treated group versus STZ control group, as reduced by TDYA, and there was no alteration in C10 treated group (Fig. 2H). Plasma MVA as a measure of liver cholesterol biosynthesis was also significantly elevated in C22:1 treated mice (by 42% versus STZ control), which was reduced by TDYA and C10 treatment showed no significant change (Fig. 2I). Liver content of squalene, another key intermediate in cholesterol synthesis, increased by 59% in C22:1 treated mice versus STZ control group (Fig. 2J), whereas there was no alteration in C10 treated group. The results provided evidence that peroxisomal β-oxidation rather than mitochondrial FAO stimulated cholesterol biosynthesis in the liver of the STZ-induced diabetic mice, which resulted in increased VLDL-C secretion and might play a role in diabetes-induced hypercholesterolemia.
Figure 2.
Increased substrate flux through peroxisomal β-oxidation stimulated biosynthesis of cholesterol in livers of the STZ-induced diabetic mice.A, changes in peroxisomal acetyl-CoA content after treatment of C10, C22:1, and TDYA in the diabetic mice. B, C22:1 treatment significantly increased hydrogen peroxide content in liver of the STZ-induced diabetic mice, which was reduced by pretreatment of TDYA. C, C22:1 treatment increased hepatic cholesterol content in the diabetic mice, as reduced by TDYA. D, C22:1 treatment showed no significant increase in liver TAG in the diabetic mice. E–G, C22:1 treatment significantly elevated plasma levels of VLDL-C (E), LDL-C (F), and serum cholesterol (G) in the STZ-induced diabetic mice, as abolished by pretreatment with TDYA. H and I, C22:1 treatment significantly increased liver (H) and plasma (I) MVA level, as reduced by pretreatment with TDYA. J, C22:1 treatment significantly increased liver squalene content in the diabetic mice, as reduced by TDYA. Mean ± SEM, n = 8, ∗p < 0.05 by t test between paired conditions. LDL-C, low density lipoprotein-cholesterol; MVA, mevalonic acid; STZ, streptozotocin; TAG, triacylglycerol; TDYA, 10,12-tricosadiynoic acid; VLDL-C, very low density lipoprotein-cholesterol.
Chronic feeding of HRO increased cholesterol biosynthesis in the diabetic mice
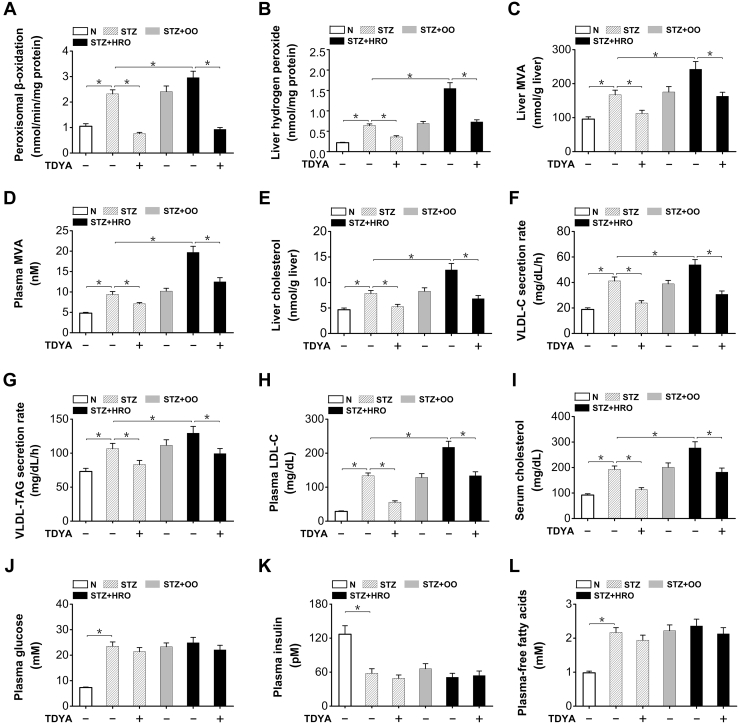
To further determine whether chronic alteration of peroxisomal FAO might affect cholesterol biosynthesis, the diabetic mice were fed a diet with high erucic acid rapeseed oil (HRO), and a diet with high olive oil (OO) was used as a control. 10,12-tricosadiynoic acid was administered to specifically suppress peroxisomal β-oxidation. Peroxisomal β-oxidation was induced in livers of the STZ-induced diabetic mice compared to the normal group, as further enhanced by HRO feeding (by 28% versus STZ control) and strongly suppressed by TDYA, whereas there was no alteration by OO feeding (Fig. 3A). Liver hydrogen peroxide increased significantly in the diabetic mice fed HRO, as reduced by the treatment of TDYA (by 53% versus STZ + HRO control) and no significant changes in the mice feeding OO diet (Fig. 3B). The results suggested that chronic HRO feeding increased substrate flux through peroxisomal β-oxidation in the diabetic mice. Liver and plasma levels of MVA increased by HRO feeding, as significantly reduced by TDYA whereas there were no significant alterations in the OO feeding group (Fig. 3, C and D). Liver cholesterol content was significantly higher in the diabetic mice (by 67% versus normal control), and further increased by HRO feeding (by 59% versus diabetic control), as reduced by the treatment with TDYA (Fig. 3E), whereas OO feeding showed no significant effect on liver cholesterol level in the diabetic mice. Very low density lipoprotein-cholesterol secretion rate was also measured, and the results indicated that VLDL-C secretion rate increased significantly in diabetic mice (by 119% versus normal control), as further enhanced by HRO feeding (by 31% versus STZ control), and reduced by TDYA (Fig. 3F). In the meantime, HRO feeding significantly increased VLDL-TAG secretion rate in STZ-induced diabetic mice, which was suppressed by the treatment of TDYA (Fig. 3G). The results suggested that the increase in cholesterol biosynthesis as induced by peroxisomal β-oxidation stimulated VLDL secretion in the diabetic mice, whereas suppression of peroxisomal β-oxidation lowered cholesterol synthesis and VLDL secretion, which was in well agreement with previous reports that the suppression of cholesterol biosynthesis by HMG-CoA reductase inhibitors reduced VLDL secretion in animals and man (25, 26). Plasma level of low density lipoprotein-cholesterol increased remarkably in the STZ-induced diabetic mice fed HRO (by 63% versus STZ control), as decreased by TDYA (Fig. 3H). Serum cholesterol was significantly higher in the STZ-induced diabetic mice fed HRO compared to the STZ control, whereas administration of TDYA decreased serum cholesterol level in the diabetic mice (Fig. 3I). Blood glucose, plasma-FFA, and plasma insulin were measured, and administration of erucic acid or TDYA did not cause significant alteration of the body weight, plasma blood glucose, FFA, or insulin in the diabetic mice, as shown in Figure 3, J–L. Therefore, all the results suggested that feeding HRO to the diabetic mice enhanced peroxisomal FAO and significantly increased liver cholesterol biosynthesis, which resulted in increased VLDL-C secretion and elevated serum cholesterol level, as abolished by administration of a specific inhibitor for peroxisomal β-oxidation.
Figure 3.
Chronic feeding of HRO increased liver cholesterol biosynthesis and elevated serum cholesterol level in the STZ-induced diabetic mice.A, peroxisomal β-oxidation was enhanced in livers of the diabetic mice fed HRO and suppressed by the treatment with TDYA. B, hydrogen peroxide content was increased in liver of the diabetic mice, as further elevated after HRO feeding and reduced by TDYA. C and D, liver (C) and plasma MVA (D) levels were elevated in diabetic mice, as further increased after HRO feeding and reduced by TDYA. E, liver cholesterol was significantly higher in the diabetic mice, as further increased by HRO feeding and reduced by TDYA. F, very low density lipoprotein-cholesterol secretion rate was upregulated in STZ-induced diabetic mice, as further elevated after HRO feeding and reduced by TDYA. G, high erucic acid rapeseed oil feeding significantly increased VLDL-TAG secretion rate in STZ-induced diabetic mice, which was suppressed by the treatment of TDYA. H, plasma LDL-C was significantly higher in the diabetic mice compared to the normal control, which was further elevated after HRO feeding and lowered by TDYA. I, serum cholesterol level was significantly higher in the diabetic mice compared to normal group, as further elevated by HRO feeding and reduced by the treatment of TDYA. J–L, plasma glucose (J), plasma insulin (K), and plasma-free fatty acids (L) were not significantly altered in the STZ-induced diabetic mice treated with HRO or TDYA. Mean ± SEM, n = 8, ∗p < 0.05 by t test between paired conditions. HRO, high erucic acid rapeseed oil; LDL-C, low density lipoprotein-cholesterol; MVA, mevalonic acid; OO, olive oil; STZ, streptozotocin; TAG, triacylglycerol; TDYA, 10,12-tricosadiynoic acid.
The enzymes involved in cholesterol biosynthesis were not altered after induction of peroxisomal β-oxidation
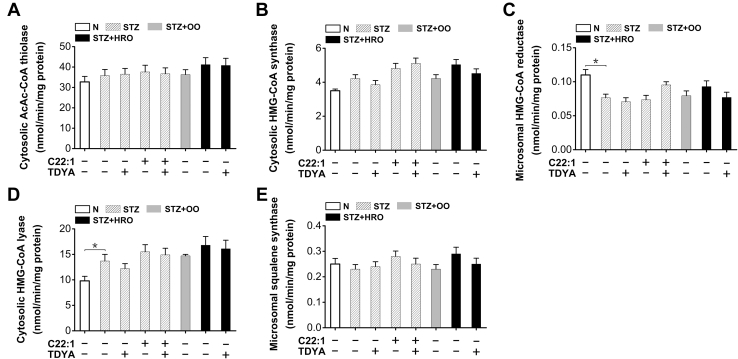
The enzyme activities involved in cholesterol biosynthesis were analyzed to determine whether the enhanced cholesterol biosynthesis as caused by C22:1 or HRO was attributed to alteration in the enzymatic turnover rate. Liver AcAc-CoA (acetoacetyl-CoA) thiolase and HMG-CoA (3-hydroxy-3-methyl glutaryl coenzyme A) synthase were not significantly altered among all the groups (Fig. 4, A and B). Moreover, the activity of HMG-CoA reductase, the rate-limiting enzyme in cholesterol synthesis, was also no significantly changed in the diabetic mice treated with C22:1 or HRO (Fig. 4C). Liver HMG-CoA lyase activity was slightly increased in the STZ-induced diabetic mice compared to the normal group, whereas there was no significant alteration after treatment with C22:1, OO, HRO, or TDYA (Fig. 4D). Furthermore, liver squalene synthase activity was not significantly changed among all the groups (Fig. 4E). The results indicated that the increased biosynthesis of cholesterol in liver as induced by peroxisomal β-oxidation as was not attributed to alterations in the enzyme activities involved in cholesterol biosynthesis.
Figure 4.
The enzyme activities involved in cholesterol biosynthesis were not significantly altered in livers of the diabetic mice treated with C22:1 or HRO.A–C, liver cytosolic AcAc-CoA thiolase (A), cytosolic HMG-CoA synthase (B), and microsomal HMG-CoA reductase (C) activities were not significantly affected in the STZ-induced diabetic mice after the treatment with OO, HRO, C22:1 or TDYA. D, liver cytosolic HMG-CoA lyase activity was elevated in the diabetic mice compared to the normal control, whereas there was no alteration in the diabetic mice treated with C22:1, OO, HRO, or TDYA. E, liver microsomal squalene synthase activity was not statistically different among all the groups. Mean ± SEM, n = 8, ∗p < 0.05 by t test between paired conditions. AcAc-CoA, acetoacetyl-CoA; HRO, high erucic acid rapeseed oil; OO, olive oil; STZ, streptozotocin; TDYA, 10,12-tricosadiynoic acid.
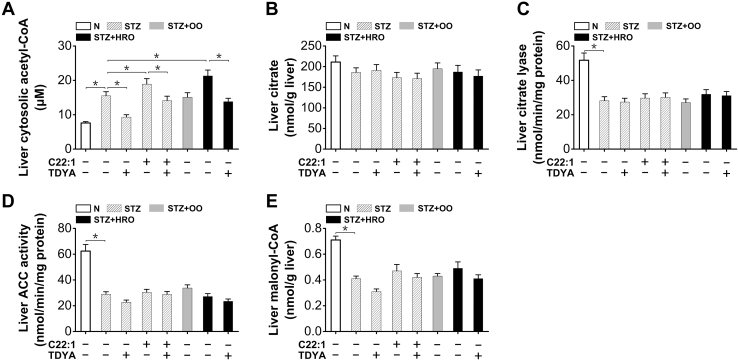
Peroxisomal β-oxidation increased cytosolic formation of acetyl-CoA
Cytosolic acetyl-CoA in liver increased in the STZ-induced diabetic mice (by 104% versus normal control) could be further elevated by the treatment of C22:1 or HRO and reduced by TDYA (Fig. 5A). Liver citrate content was not significantly changed among all the groups (Fig. 5B). Liver citrate lyase activity was reduced in STZ induced-diabetic mice, whereas no significant changes in the diabetic mice treated with C22:1, OO, HRO, or TDYA (Fig. 5C). Liver acetyl-CoA carboxylase activity decreased significantly in the STZ-induced diabetic mice (by 54% versus normal control), and no significant changes in the diabetic mice treated with C22:1, HRO or TDYA (Fig. 5D). Moreover, liver malonyl-CoA was reduced in the STZ-induced diabetic mice (by 42% versus normal control), whereas no significant change after treatment of C22:1, HRO, or TDYA (Fig. 5E). The results suggested that the increased formation of cytosolic acetyl-CoA as caused by the enhanced peroxisomal β-oxidation was not because of alteration in citrate cleavage or reduced malonyl-CoA formation in the liver of the diabetic mice.
Figure 5.
Peroxisomal β-oxidation increased cytosolic acetyl-CoA formation in the STZ-induced diabetic mice.A, liver cytosolic acetyl-CoA was significantly higher in diabetic mice compared to the normal group, which was further elevated after treatment with C22:1 or HRO and reduced by pretreatment of TDYA. B, liver citrate content was significantly lower in the STZ-induced diabetic mice compared to the normal group, whereas there was no alteration after treatment with C22:1, OO, HRO, or TDYA. C and D, citrate lyase (C) and acetyl-CoA carboxylase (ACC) activities (D) in liver homogenate were reduced in the STZ-induced diabetic mice compared to the normal group, whereas there was no alteration after the treatment with OO, HRO, C22:1, or TDYA. E, liver malonyl-CoA was significantly lower in diabetic mice compared to the normal group, while no significant changes in the diabetic mice treated with C22:1, OO, HRO, or TDYA. Mean ± SEM, n = 8, ∗p < 0.05 by t test between paired conditions. HRO, high erucic acid rapeseed oil; OO, olive oil; STZ, streptozotocin; TDYA, 10,12-tricosadiynoic acid.
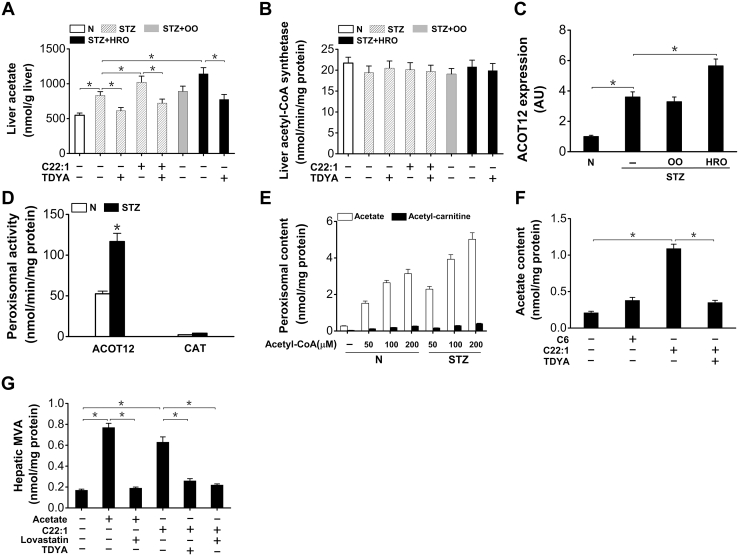
Peroxisomal β-oxidation generated acetate as a precursor for cholesterol biosynthesis
As peroxisomal β-oxidation generates free acetate as the ultimate product (14, 15, 16), the elevated acetyl-CoA in the cytosol was most likely from the enhanced release of free acetate from peroxisomal β-oxidation in the diabetic mice treated with C22:1 or HRO. Liver acetate was significantly higher in the diabetic mice (by 52% versus normal mice), as further elevated in the diabetic mice treated with C22:1 and HRO (by 23% and 38% versus diabetic control, respectively) and reduced by the treatment with TDYA (Fig. 6A), suggesting that peroxisomal metabolism of fatty acids stimulated acetate formation. On the other hand, liver acetyl-CoA synthetase activity was not significantly affected by the STZ-induced diabetic mice treated with C22:1, OO, HRO, or TDYA (Fig. 6B). Gene expression of peroxisomal carnitine acetyl-CoA hydrolase (ACOT12) was upregulated in livers of the diabetic mice (by 260% versus normal mice), as further elevated by HRO feeding (by 58% versus diabetic control) (Fig. 6C). Peroxisomal carnitine acetyl transferase (CAT) and ACOT12 activities were assayed, and the results indicated that ACOT12 activity was markedly induced in the diabetic mice (by 122% versus normal mice) and the activity of ACOT12 was much higher than that of CAT (Fig. 6D). Incubation of purified liver peroxisomes from STZ-induced diabetic mice with acetyl-CoA led to release of acetate dose dependently, whereas the generation of acetyl-carnitine was marginal (Fig. 6E), suggesting that acetate rather than acetyl-carnitine was the predominant ultimate product of fatty acids subjected to peroxisomal β-oxidation in the liver of diabetic mice. Incubation of isolated hepatocyte with C22:1 remarkably increased cellular acetate content, as reduced by pretreatment with TDYA (Fig. 6F). Incubation of isolated hepatocyte with acetate as well as C22:1 increased MVA formation, as reduced by pretreatment of TDYA or lovastatin, a specific inhibitor for HMG-CoA reductase (Fig. 6G). Therefore, due to the high ACOT12 activity in liver peroxisomes of the diabetic mice, most of the acetyl-CoA generated in peroxisomal β-oxidation was hydrolyzed to free acetate and directed to cytosolic acetyl-CoA formation and cholesterol biosynthesis.
Figure 6.
Peroxisomal β-oxidation generated free acetate that used as a precursor for cholesterol biosynthesis in the STZ-induced diabetic mice.A, liver acetate was significantly higher in the diabetic mice, as further increased after treatment with C22:1 or HRO and reduced by TDYA. B, liver acetyl-CoA synthetase activity was not significantly affected in the STZ-induced diabetic mice treated with C22:1, OO, HRO, or TDYA. C, mRNA expression level of ACOT12 was upregulated in livers of the diabetic mice and strongly induced by HRO feeding. D, peroxisomal ACOT12 activity increased significantly in livers of the diabetic mice compared to the normal control, whereas peroxisomal CAT activity was very low in livers of the diabetic mice. Mean ± SEM, n = 8, ∗p < 0.05 by t test between paired conditions. E, generation of acetate and acetyl-carnitine with acetyl-CoA as a substrate by isolated peroxisome lysate. The isolated peroxisomes were lysed with addition of 0.025% (v/v) Triton-X-100, and the lysate was warmed at 37 °C for 30 min before addition of acetyl-CoA and 1 mM L-carnitine to the incubation medium. F, oxidation of C22:1 by isolated hepatocytes released acetate dose dependently, as reduced by pretreatment with TDYA. G, generation of MVA by isolated hepatocytes was stimulated in the presence of acetate or C22:1 and suppressed by pretreatment of lovastatin or TDYA. Mean ± SEM, n = 6, ∗p < 0.05 by t test between paired conditions. ACOT12, acetyl-CoA hydrolase; CAT, carnitine acyltransferase; HRO, high erucic acid rapeseed oil; MVA, mevalonic acid; OO, olive oil; STZ, streptozotocin; TDYA, 10,12-tricosadiynoic acid.
Discussion
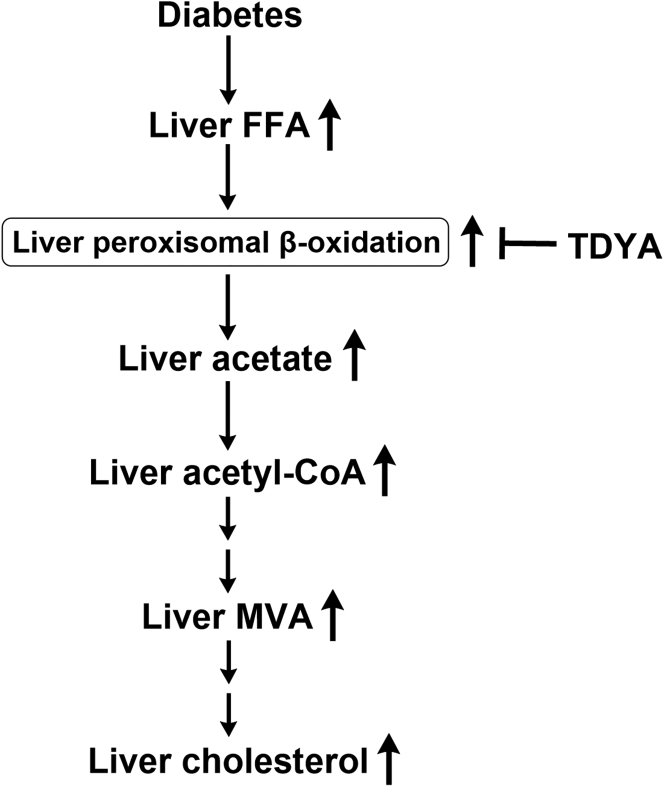
Diabetes has been reported to induce cholesterol biosynthesis in animals (4, 5), whereas the precise mechanism by which dysregulated glucose and fatty acid metabolism in diabetes leads to increased cholesterol biosynthesis is not clear. This study demonstrated an important role of peroxisomal β-oxidation in cholesterol biosynthesis. The proposed mechanism was shown in Figure 7. Elevation in plasma FFA in diabetes results in increased uptake of fatty acids in liver and induces peroxisomal β-oxidation. Accelerated peroxisomal metabolism of fatty acids stimulates acetate release into the cytosol and increased cytosolic content of acetyl-CoA, a precursor for cholesterol biosynthesis.
Figure 7.
Proposed mechanism by which peroxisomal β-oxidation stimulates cholesterol biosynthesis in the liver of diabetic animals. FFA, free fatty acids; MVA, mevalonic acid; TDYA, 10,12-tricosadiynoic acid.
This research demonstrated a cross-talk between peroxisomal β-oxidation and cholesterol biosynthesis. Peroxisomal β-oxidation system has been discovered for more than 40 years (27), whereas the physiological roles of peroxisomal FAO in liver lipid homeostasis are not fully illustrated. Our previous study identified a cross-talk between peroxisomal β-oxidation and mitochondrial fatty acid oxidation and suggested that induction of peroxisomal β-oxidation negatively regulated mitochondrial FAO by stimulating liver malonyl-CoA formation in mice fed a high-fat diet, which caused accumulation of TAG and insulin resistance (16). However, whether peroxisomal FAO might play a role in regulating cholesterol biosynthesis is not known. In this study, our results suggested that accelerated peroxisomal β-oxidation stimulated cholesterol biosynthesis and elevated plasma cholesterol level under the condition of diabetes, as abolished by specific inhibition of peroxisomal β-oxidation. As peroxisomal β-oxidation plays a role in metabolism of long-chain fatty acids (9), the bulk of endogenous fatty acids in liver and the contribution of peroxisomes to liver FAO has been studied by several groups (28, 29, 30). It was estimated that peroxisomal β-oxidation accounted for about 30% that of overall FAO under normal condition, although this ratio might be significantly elevated under diabetic condition because liver peroxisomal β-oxidation is induced in diabetes and much more LC-acyl-CoAs (>50%) might undergo β-oxidation in peroxisomes; most of the acetyl-CoA generated in peroxisomal β-oxidation will then be used for cholesterol biosynthesis. Therefore, we propose that one of the physiological functions of peroxisomal β-oxidation is to stimulate biosynthesis of cholesterol under the condition of diabetes and possibly in obesity when peroxisomal β-oxidation is induced and fatty acid synthesis is suppressed. Future studies with genetic approaches disrupting peroxisomal β-oxidation in diabetic or obese animals or specific inhibitors for mitochondrial FAO would help to pin down the proposed role of peroxisomal β-oxidation in cholesterol biosynthesis.
The mechanism by which elevated fatty acids oxidation in diabetes causes an increase in cholesterol biosynthesis is unclear. As fatty acid synthesis is suppressed in the liver of the diabetic animals, although the enzymes involved in liver cholesterol biosynthesis is not significantly affected (18, 19). Therefore, the supply of cytosolic acetyl-CoA will be a critical factor in regulating cholesterol biosynthesis. It is well known that there are two major sources of the acetyl-CoA for biosynthesis of cholesterol, one is through citrate cleavage, and another is from acetate generated by FAO (31, 32). As liver citrate level and citrate lyase are diminished in diabetes, the acetyl-CoA that used for cholesterol biosynthesis is not likely from citrate cleavage, and most possibly from the released acetate in FAO as liver FAO is enhanced in the diabetic animals (33). Indeed, acetate is increased in liver of fasting or diabetic animals (34, 35), which well supports that the acetate from FAO can be used for cholesterol biosynthesis.
To identify the source of acetate, we noted the well-known fact that the acetyl-CoA generated in mitochondrial FAO is used for ketone body formation or completely oxidized in tricarboxylic acid cycle (33). It was reported that mitochondrial FAO did not generate free acetate (16, 35), as confirmed in our study that C6 treatment to isolated hepatocyte showed no significant increase in acetate level (Fig. 6G), indicating that the increased acetate as caused by FAO was generated extramitochondrially. It was reported that peroxisomal β-oxidation generated free acetate as the ultimate product (14, 15); our previous results confirmed that peroxisomal oxidation of erucic acid as well as LC-acyl-CoA generated free acetate as the predominant product, as reduced by the treatment of a specific inhibitor for peroxisomal β-oxidation (16). Therefore, the increased formation of acetate in the diabetic liver was attributed to the enhanced peroxisomal FAO (Fig. 3A). As peroxisomes are not permeable to acetyl-CoA (36), there are two pathways for acetyl-group transfer from peroxisome to the cytosol, one way is to hydrolyze acetyl-CoA to acetate via peroxisomal ACOT12, the other way is to transform the acetyl-CoA to acetyl-carnitine via CAT (37). It is proposed that in liver and kidney, most of the acetyl-CoA generated in peroxisomal β-oxidation is hydrolyzed to acetate, whereas in heart and muscle, the acetyl-CoA is transformed to acetyl-carnitine for burning in mitochondria (38, 39). The formation of acetate from acetyl-CoA was attributed to high level expression and activity of ACOT12 in rat liver. On the other hand, the activity of CAT was very low in rat liver, whereas its expression and activity were high in heart and muscle (36). Cytosolic free acetate can barely be metabolized in liver due to lack of a specific acetyl-CoA synthetase in liver mitochondria (40). The acetate that released from peroxisomal β-oxidation of long-chain fatty acids can then be used for biosynthesis of cholesterol. Moreover, direct measurement of cholesterol synthesis from 14C-labeled substrate for peroxisomal β-oxidation suggested that the acetyl group (acetyl-CoA) generated from peroxisomal β-oxidation was preferentially used for biosynthesis of cholesterol (21), which was in well agreement with our results. It was reported the metabolism of ethanol in liver generated acetate and stimulated cholesterol synthesis and led to elevation in liver and plasma cholesterol level (41).
This study demonstrated a novel pathogenic mechanism for diabetes-induced increase in cholesterol biosynthesis and hypercholesterolemia in rodents and possibly in man. The results suggested that induction of peroxisomal β-oxidation enhanced liver cholesterol biosynthesis in the diabetic mice, whereas specific inhibition of peroxisomal β-oxidation suppressed cholesterol biosynthesis and lowered plasma cholesterol level. However, it should be admitted that in this study, the role of peroxisomal β-oxidation in cholesterol biosynthesis was investigated using a specific inhibitor for peroxisomal β-oxidation, whereas the possibility of off-target effects could not be fully eliminated. Therefore, further experiments using genetic approaches to disrupt liver peroxisomal β-oxidation in diabetic animals are warranted to confirm our results and the proposed mechanism because liver-specific acyl-CoA oxidase-1 knockout mice have been generated recently (42).
The results also indicated potential pathogenic role of HRO. As erucic acid is preferentially metabolized by peroxisomal β-oxidation system (16), excessive intake of HRO in diabetic patients might increase metabolism of this very long-chain fatty acid by peroxisomes, which further enhance cholesterol biosynthesis and secretion, and leads to hypercholesterolemia. High erucic acid rapeseed oil as edible oil is widely consumed in South China and India (43), and the people live in diabetes are more than 109 million in China (44). Although the clinical relevance between HRO intake and hypercholesterolemia is not established so far, we suggest that chronic intake of HRO might increase the risk of hypercholesterolemia in the diabetic patients and strongly suggest low erucic acid rapeseed oil or OO as the edible oil instead of HRO in our daily life, which might benefit our liver and heart by reducing the harmful metabolites released from peroxisomal β-oxidation and reducing cholesterol biosynthesis.
Experimental procedures
Materials
Acetyl-carnitine, cholesterol, STZ, lovastatin, decanoic acid (C10), hexanoic acid (C6), acetyl-CoA, AcAc-CoA , squalene, mevalonic acid, L-carnitine, coenzyme A sodium salt, Percoll, 5,5-dithio-bis(2-nitrobenzoic acid) (DTNB), PEG1500, and defatted bovine serum albumin were purchased from Sigma. 10,12-Tricosadiynoic acid and erucic acid (C22:1) were from Tokyo Chemical Industry. The mono-CoA thioesters of dodecanedioic acid were enzymatically prepared by a microsomal acyl-CoA synthetase and purified by high-performance liquid chromatography as previously described (45). All other chemical reagents used were of analytical grade or better.
Animal studies
Male C57BL/6J mice were purchased from Slac Laboratory Animal Co Ltd Standard rodent diet (12% fat by calories), HRO diet containing 15% (w/w) rapeseed oil (55% fat by calories), and high OO diet containing 15% (w/w) OO (55% fat by calories) were supplied by Slac Laboratory Animal Co Ltd. The rapeseed oil used in HRO diet contained 35% (w/w) erucic acid. All animals were housed in single cage with free access to food and water under controlled temperature (22 °C) and light (12 h of light and 12 h of dark).
Diabetic mellitus was induced by administration of STZ (80 mg/kg, i.p.) to male C57BL/6J mice at the age of 8 weeks. Two weeks after the injection, the blood glucose level was determined by tail vein bleeding using a glucometer (Lifescan, Johnson and Johnson), and the mice with overt hyperglycemia (plasma glucose > 400 mg/dl) were chosen for the experiments. For acute study of peroxisomal β-oxidation on cholesterol biosynthesis, erucic acid or decanoic acid at 4 g/kg were administered by gavage to 12 h-fasted diabetic mice for consecutive two times, every 3 h per dosage. For the purpose of suppressing peroxisomal β-oxidation, TDYA at 100 mg/kg was administered to the diabetic mice 60 min before administration of erucic acid. For the study of chronic induction of peroxisomal β-oxidation on cholesterol biosynthesis, the diabetic mice were exposed to either HRO diet or OO diet for 4 weeks, TDYA at 100 mg/kg/d was administered orally to suppress peroxisomal β-oxidation. Normal group (N) and diabetic control (STZ) were fed standard rodent diet. After the experiments, all the mice were bled from the eyes and then sacrificed. Livers were removed quickly and stored in liquid nitrogen immediately.
All the animal studies were approved by the Animal Care Committee of Hunan University of Science and Technology.
Isolation of liver subcellular fractions
For the isolation of peroxisomes, light mitochondrial fraction after differential centrifugation was further isolated by a Percoll gradient according to the method described before (46, 47). Three milliliter light mitochondrial fraction sample containing ∼15 mg proteins was layered on 5 ml of a 50% (v/v) solution of Percoll containing 250 mM sucrose, 12% (wt/vol) PEG1500, 2 mM Mops, 1 mM EGTA, and 0.1% (v/v) ethanol at pH 7.2. After centrifugation at 85,000g for 30 min on a Beckman Optima MAX-XP ultracentrifuge with a MLN80 rotor, the fractions were collected for catalase activity assay. The pooled peak fractions were diluted with 250 mM sucrose and centrifuged at 35,000g for 15 min to recover sediment containing purified peroxisomes. For the preparation of cytosolic fraction, the post-light mitochondrial supernatant was firstly centrifuged at 27,000g for 15 min to pellet residual large organelles and finally centrifuged at 100,000g for 60 min to obtain cytosolic (supernatant) and microsomal (pellet) fraction for metabolites and enzyme assays. All the isolation procedures were kept at 2 °C.
Mevalonate synthesis from acetate by isolated hepatocytes
Hepatocytes of treated mice were prepared, as described before (48). Cell viability was assessed by trypan blue exclusion and estimated to be more than 90%. Hepatocytes (1 × 106) were incubated under an atmosphere of O2/CO2 (19:1) in Krebs-Henseleit bicarbonate medium, pH 7.4. To inhibit cholesterol synthesis, 50 μM lovastatin or 100 μM TDYA-CoA were firstly added to the reaction mixture respectively. Then, 0.1 mM erucic acid or 0.4 mM hexanoic acid or 0.8 mM acetate, 0.34 mM defatted bovine serum albumin, and 1 mM carnitine were added to start the reaction. After incubation at 37 °C for 30 min, the reaction was terminated by the addition of ice cold perchloric acid.
Characteristics of the enzymes involved in cholesterol synthesis
The activity of cytosolic AcAc-CoA thiolase was determined spectrophotometrically by following the decrease in 300 nm due to AcAc-CoA cleavage (49). The reaction mixture contained 100 mM Tris–HCl (pH 8.0), 0.1 mM EDTA, 0.12 mM AcAc-CoA, and 90 μM CoA. Cytosolic HMG-CoA synthase activity was assayed according to the previous method by following the decrease in 300 nm due to AcAc-CoA consumption (50). The reaction system contained 100 mM Tris–HCl (pH 8.0), 0.1 mM EDTA, 20 mM MgCl2, 50 μM AcAc-CoA, and 0.2 mM acetyl-CoA. Liver microsomal HMG-CoA reductase activity was assayed by a commercial kit from Sigma. Cytosolic HMG-CoA lyase activity was measured by incubation of the enzyme sample with HMG-CoA and subsequent determination of the acetoacetate formed by a hydroxybutyrate dehydrogenase method (51). The incubation mixture consisted of 50 mM Tris–HCl buffer (pH 8.5), 5 mM MgCl2, and 0.4 mM HMG-CoA. The assay of microsomal squalene synthase activity was conducted, as described previously (49). The lipids were extracted with hexane and separated by thin-layer chromatography developed with hexane. The spots were scraped and measured by liquid scintillation counting.
Measurement of peroxisomal β-oxidation
Peroxisomal β-oxidation was assayed in liver homogenate by acyl-CoA dependent NAD+ reduction in the presence of KCN as developed by Lazarow PB (52), with 100 μM palmitoyl-CoA as the substrate.
Measurement of plasma and liver MVA and squalene
The plasma and liver MVA concentrations were quantified using previously described method (53). Free MVA was converted to its lactone and extracted in hexane. The mevalonolactone was extracted into CH2Cl2-PrOH (9:1 v/v) and purified by silica cartridge, then reconverted to free MVA and its 3,5-bis(trifluoromethyl)benzyl ester. The trimethylsilyl ether derivate was assayed by GC-MS.
Liver squalene was determined by HPLC-PAD method with minor modification (54). Briefly, the saponification of squalene was carried at 75 °C in 1 M KOH for 30 min. After that, the squalene was extracted by petroleum ether and separated on C18 column and then detected using photodiode array detection.
Measurement of total and free cholesterol
Lipoprotein classes were separated using discontinuous density gradient ultracentrifugation (55). After a slow start, speed was increased to 100,000g for 19 h. The LDL and VLDL fractions were collected, subjected to exhaustive dialysis against 0.09% NaCl and 0.01% EDTA (24 h), and checked for purity on agarose gel. Cholesterol was determined using commercial kits according to the manufacturer’s instructions (Boehringer Mannheim GmbH).
Quantitative real time PCR
Total RNA was extracted from liver tissues with TRIzol reagent (Life Technologies Corporation). RNA was reverse-transcribed with standard reagents (High Capacity Reverse Transcription Kits, Applied Biosystems) using random primers. Complementary DNA was amplified in a 7500 Fast Real-time PCR System using 2 × SYBR Green Supermix (Applied Biosystems). The following primers were used: acyl-CoA oxidase-1, 5′-TGGAGAGCCCTCAGC TATGG-3′ (F) and 5′-CGTTTCACCGCCTC GTAAG-3′ (R); LC-acyl-CoA synthetases-1, 5′-GCAAGAACAGCTGAAGCCC-3′ (F) and 5′-AGGTGCCATTTGGCAGCCA-3′ (R); L-BP, 5′-AAATACAGAGATACCAGA AGCCG-3′ (F) and 5′-AAGAAT CCCC AGTGTGACTTC-3′ (R); thiolase, 5′- CCTGACATCATGGG CATCG-3′ (F) and 5′-A GTCAGCCCTGCTTTCTGCA-3′ (R); peroxisomal ATP-binding cassette transporter D, 5′-GGGCCTAAAGCAAC AGTCTCA-3′ (F) and 5′-GGGCAAC ATACACAGACAGGAA-3′ (R); ACOT12, 5′-GGAGATTAC CACCACC TTGG-3′ (F) and 5′-TTCAACCTTAACAG ATA TGGCATC3′ (R); 18S rRNA, 5′-GTTATGGTCTTTGGTCG C-3′ (F) and 5′-CGTCTGCCCTATCAACTTTC-3′ (R). mRNA expression levels normalized to 18S rRNA were expressed using the comparative delta CT method.
Biochemical analysis
Liver-FFA concentration was determined using a colorimetric kit based on ACS-ACOX method (Wako Pure Chemical Corporation). Plasma and liver total cholesterol were determined by a commercial kit from Sigma and performed according to the manufacturer’s instructions. Acyl-CoA esters from whole tissue samples or the preparations from subcellular fractionation were extracted and determined according to the method of Tubbs and Garland (56), the content of acetyl-CoA and LC-acyl-CoA were analyzed from the acid-soluble and acid-insoluble fractions respectively. Liver TAG was extracted by the method of Bligh and Dyer (57) and determined using a commercial kit (Wako). Liver VLDL-C and VLDL-TAG secretion rate was determined by the method, as described previously (58). Briefly, mice were injected intravenously with Triton WR1339 at 500 mg/kg, then blood samples were taken at 0, 1, 2, 3, and 4 h after injection and VLDL was isolated from serum by density gradient ultracentrifugation, liver VLDL-C and VLDL-TAG secretion rate were calculated from the slope of the curve and expressed as mg/dl/h. Liver hydrogen peroxide, acetate, and citrate were determined using commercial kits according to the manufacturer’s instructions (Sigma). Liver malonyl-CoA was analyzed by HPLC, as described previously (59). Liver and peroxisomal acetyl-CoA was determined enzymatically using commercial assay kit (Sigma). Liver acetyl-CoA carboxylase and citrate lyase was determined using commercial kits (Solarbio). Acetyl-CoA synthetase activity was determined spectrophotometrically, as described previously (40). Liver and peroxisomal acetyl-carnitine was assayed by a combined reaction catalyzed by carnitine acetyl transferase, L-malate dehydrogenase, and citrate synthase (60). Protein concentration was measured by Bio-Rad DC protein assay kit (Hercules). The samples for peroxisomal acetyl-CoA thioesterase assays were prepared according to the method of Nakanishi (61). The isolated peroxisomes were lysed with addition of 0.025% (v/v) Triton X-100, and the incubation was warmed at 37 °C for 30 min and after that, the activity was assayed. Peroxisomal ACOT12 was measured spectrophotometrically by determining the rate of formation of CoASH from acetyl-CoA using DTNB (61, 62). Peroxisomal CAT activity was assayed as described before with acetyl-carnitine as a substrate (63).
Statistical analysis
Data are presented as mean ± SEM. n = 6 to 8 for all the groups. The significance of differences was evaluated using one way ANOVA with Dunnett’s T3 test or Student's test by SPSS 18.0. p < 0.05 was considered statistically significant.
Data availability
All data are contained within the article.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
This work was supported by the National Natural Science Fund of P. R. China (30900024), Distinguished Professor Funds from Hunan University of Science and Technology, and Project of graduate innovation in Hunan province (CX20190814).
Authors contributions
X. Z., Y. W., H. Y., S. D., T. G., and L. S. investigation; X. Z., Y. W., H. Y., Xiaocui Chen, Xiaojuan Cui, and J. Z. methodology; X. Z., Y. W., and S. D., formal analysis; J. Z. conceptualization; J. Z. supervision; J. Z. writing–original draft; J. Z. writing–review and editing; J. Z. funding acquisition; J. Z. project administration.
Edited by Dennis Voelker
References
- 1.Cassader M., Ruiu G., Gambino R., Alemanno N., Veglia F., Pagano G. Hypercholesterolemia in non-insulin-dependent diabetes mellitus: Different effect of simvastatin on VLDL and LDL cholesterol level. Atherosclerosis. 1993;99:47–53. doi: 10.1016/0021-9150(93)90049-z. [DOI] [PubMed] [Google Scholar]
- 2.Haffner S.M. Diabetes, hyperlipidemia, and coronary artery disease. Am. J. Cardiol. 1999;83:17F–21F. doi: 10.1016/s0002-9149(99)00213-1. [DOI] [PubMed] [Google Scholar]
- 3.Grant G.R., Wakabayashi S., Yamamoto R., Matsutomo R., Takebayashi K., Inukai T. Comparison of hyperglycemia, hypertension, and hypercholesterolemia management in patients with type 2 diabetes. Am. J. Med. 2002;112:603–609. doi: 10.1016/s0002-9343(02)01103-8. [DOI] [PubMed] [Google Scholar]
- 4.Hotta S., Chaikoff I.L. Mechanism of increased hepatic cholesterogenesis in diabetes: Its relation to carbohydrate utilization. J. Biol. Chem. 1954;206:835–844. [PubMed] [Google Scholar]
- 5.Kwong L.K., Feingold K.R., Peric-Golia L., Le T., Karkas J.D., Alberts A.W., Wilson D.E. Intestinal and hepatic cholesterogenesis in hypercholesterolemic dyslipidemia of experimental diabetes in dogs. Diabetes. 1991;40:1630–1639. doi: 10.2337/diab.40.12.1630. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson A., Sundler R., Akesson B. Effect of different albumin-bound fatty acids on fatty acid and cholesterol biosynthesis in rat hepatocytes. FEBS Lett. 1974;45:282–285. doi: 10.1016/0014-5793(74)80862-8. [DOI] [PubMed] [Google Scholar]
- 7.Gu Y., Yin J. Saturated fatty acids promote cholesterol biosynthesis: Effects and mechanisms. Obes. Med. 2020;18:100201. [Google Scholar]
- 8.Schafer H.F., Kattermann R. Fatty acid modulation of HepG2 cell cholesterol biosynthesis and esterification. Clin. Biochem. 1992;25:325–330. doi: 10.1016/0009-9120(92)80008-5. [DOI] [PubMed] [Google Scholar]
- 9.Reddy J.K., Hashimoto T. Peroxisomal β-oxidation and peroxisome proliferator–activated receptor α: An adaptive metabolic system. Annu. Rev. Nutr. 2001;21:193–230. doi: 10.1146/annurev.nutr.21.1.193. [DOI] [PubMed] [Google Scholar]
- 10.Asayama K., Sandhir R., Sheikh F.G., Hayashibe H., Nakane T., Singh I. Increased peroxisomal fatty acid beta-oxidation and enhanced expression of peroxisome proliferator-activated receptor-alpha in diabetic rat liver. Mol. Cell. Biochem. 1999;194:227–234. doi: 10.1023/a:1006930513476. [DOI] [PubMed] [Google Scholar]
- 11.Horie S., Ishii H., Suga T. Changes in peroxisomal fatty acid oxidation in the diabetic rat liver. J. Biochem. 1981;90:1691–1696. doi: 10.1093/oxfordjournals.jbchem.a133645. [DOI] [PubMed] [Google Scholar]
- 12.Thomas H., Schladt L., Knehr M., Oesch F. Effect of diabetes and starvation on the activity of rat liver epoxide hydrolases, glutathione S-transferases and peroxisomal β-oxidation. Biochem. Pharmacol. 1989;38:4291–4297. doi: 10.1016/0006-2952(89)90528-5. [DOI] [PubMed] [Google Scholar]
- 13.Asayama K., Yokota S., Kato K. Peroxisomal oxidases in various tissues of diabetic rats. Diabetes Res. Clin. Pract. 1991;11:89–94. doi: 10.1016/0168-8227(91)90096-v. [DOI] [PubMed] [Google Scholar]
- 14.Leighton F., Bergseth S., Rørtveit T., Christiansen E.N., Bremer J. Free acetate production by rat hepatocytes during peroxisomal fatty acid and dicarboxylic acid oxidation. J. Biol. Chem. 1989;264:10347–10350. [PubMed] [Google Scholar]
- 15.Hovik R., Brodal B., Bartlett K., Osmundsen H. Metabolism of acetyl-CoA by isolated peroxisomal fractions: Formation of acetate and acetoacetyl-CoA. J. Lipid Res. 1991;32:993–999. [PubMed] [Google Scholar]
- 16.Chen X., Shang L., Deng S., Li P., Chen K., Gao T., Zhang X., Chen Z., Zeng J. Peroxisomal oxidation of erucic acid suppresses mitochondrial fatty acid oxidation by stimulating malonyl-CoA formation in the rat liver. J. Biol. Chem. 2020;295:10168–10179. doi: 10.1074/jbc.RA120.013583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brady R.O., Gurin S. Biosynthesis of labeled fatty acids and cholesterol in experimental diabetes. J. Biol. Chem. 1950;187:589–596. [PubMed] [Google Scholar]
- 18.Hotta S., Chaikoff I.L. Cholesterol synthesis from acetate in the diabetic liver. J. Biol. Chem. 1952;198:895–899. [PubMed] [Google Scholar]
- 19.Van Bruggen J.T., Yamada P., Hutchens T.T., West E.S. Lipogenesis of the intact alloxan-diabetic rat. J. Biol. Chem. 1954;209:635–640. [PubMed] [Google Scholar]
- 20.Carroll K.K. Effect of erucic acid on incorporation of acetate-1-C14 into cholesterol and faty acids. Can. J. Biochem. Physiol. 1959;37:803–810. [PubMed] [Google Scholar]
- 21.Hashimoto F., Ishikawa T., Hamada S., Hayashi H. Effect of gemfibrozil on lipid biosynthesis from acetyl-CoA derived from peroxisomal beta-oxidation. Biochem. Pharmacol. 1995;49:1213–1221. doi: 10.1016/0006-2952(95)00041-w. [DOI] [PubMed] [Google Scholar]
- 22.Bremer J., Norum K.R. Metabolism of very long-chain monounsaturated fatty acids (22:1) and the adaptation to their presence in the diet. J. Lipid Res. 1982;196:149–159. [PubMed] [Google Scholar]
- 23.Zeng J., Deng S., Wang Y., Li P., Tang L., Pang Y. Specific inhibition of acyl-CoA oxidase-1 by an acetylenic acid improves hepatic lipid and reactive oxygen species (ROS) metabolism in mice fed a high fat diet. J. Biol. Chem. 2017;292:3800–3809. doi: 10.1074/jbc.M116.763532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomassen M.S., Christiansen E.N., Norum K.R. Characterization of the stimulatory effect of high-fat diets on peroxisomal β-oxidation in rat liver. Biochem. J. 1982;206:195–202. doi: 10.1042/bj2060195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshino G., Kazumi T., Kasama T., Iwai M., Iwatani I., Matsuba K., Matsushita M., Baba S. Effect of CS-514 (pravastatin) on VLDL-triglyceride kinetics in rats. Atherosclerosis. 1988;73:191–195. doi: 10.1016/0021-9150(88)90041-x. [DOI] [PubMed] [Google Scholar]
- 26.Ginsberg H.N. Effects of statins on triglyceride metabolism. Am. J. Cardiol. 1998;81:32B–35B. doi: 10.1016/s0002-9149(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 27.Lazarow P.B., De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc. Natl. Acad. Sci. U. S. A. 1976;73:2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kondrup J., Lazarow P.B. Flux of palmitate through the peroxisomal and mitochondrial beta-oxidation systems in isolated rat hepatocytes. Biochim. Biophys. Acta. 1985;835:147–153. doi: 10.1016/0005-2760(85)90041-4. [DOI] [PubMed] [Google Scholar]
- 29.Rognstad R. Estimation of peroxisomal and mitochondrial fatty acid oxidation in rat hepatocytes using tritiated substrates. Biochem. J. 1991;279:147–150. doi: 10.1042/bj2790147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Handler J.A., Thurman R.G. Catalase-dependent ethanol oxidation in perfused rat liver. Requirement for fatty-acid-stimulated H2O2 production by peroxisomes. Eur. J. Biochem. 1988;176:477–484. doi: 10.1111/j.1432-1033.1988.tb14305.x. [DOI] [PubMed] [Google Scholar]
- 31.Dupont J. Fatty acid oxidation in relation to cholesterol biosynthesis in mice. Lipids. 1966;1:415–421. doi: 10.1007/BF02532545. [DOI] [PubMed] [Google Scholar]
- 32.Cenedella R.J., Allen A. Differences between the metabolism of linoleic and palmitic acid: Utilization for cholesterol synthesis and oxidation to respiratory CO2. Lipids. 1969;4:155–158. doi: 10.1007/BF02531936. [DOI] [PubMed] [Google Scholar]
- 33.McGarry J.D., Foster D.W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu. Rev. Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- 34.Murthy V.K., Steiner G. Hepatic acetate levels in relation to altered lipid metabolism. Metabolism. 1973;21:81–84. doi: 10.1016/0026-0495(73)90032-2. [DOI] [PubMed] [Google Scholar]
- 35.Knowles S.E., Jarrett I.G., Filsell O.H., Ballard F.J. Production and utilization of acetate in mammals. Biochem. J. 1974;142:401–411. doi: 10.1042/bj1420401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antonenkov V.D., Hiltunen J.K. Peroxisomal membrane permeability and solute transfer. Biochem. Biophys. Acta. 2006;1763:1697–1706. doi: 10.1016/j.bbamcr.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 37.Hunt M.C., Alexson S.E.H. Novel functions of acyl-CoA thioesterases and acyltransferases as auxiliary enzymes in peroxisomal lipid metabolism. Prog. Lipid Res. 2008;47:405–421. doi: 10.1016/j.plipres.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Westin M.A.K., Hunt M.C., Alexson S.E.H. Short-and medium-chain carnitine acyltransferases and acyl-CoA thioesterases in mouse provide complementary systems for transport of β-oxidation products out of peroxisomes. Cell. Mol. Life Sci. 2008;65:982–990. doi: 10.1007/s00018-008-7576-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi Y.R., Fogle P.J., Clarke P.R., Bieber L.L. Quantitation of water-soluble acylcarnitines and carnitine acyltransferases in rat tissues. J. Biol. Chem. 1977;252:7930–7931. [PubMed] [Google Scholar]
- 40.Fujino T., Kondo J., Ishikawa M., Morikawa K., Yamamoto T.T. Acetyl-CoA synthetase 2, a mitochondrial matrix enzyme involved in the oxidation of acetate. J. Biol. Chem. 2001;276:11420–11426. doi: 10.1074/jbc.M008782200. [DOI] [PubMed] [Google Scholar]
- 41.Reitz R.C. The effects of ethanol ingestion on lipid metabolism. Prog. Lipid Res. 1979;18:87–115. doi: 10.1016/0163-7827(79)90007-9. [DOI] [PubMed] [Google Scholar]
- 42.He A., Chen X., Tan M., Chen Y., Lu D., Zhang X., Dean J.M., Razani B., Lodhi I.J. Acetyl-CoA derived from hepatic peroxisomal β-oxidation inhibits autophagy and promotes steatosis via mTORC1 activation. Mol. Cell. 2020;79:30–42. doi: 10.1016/j.molcel.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carré P., Pouzet A. Rapeseed market, worldwide and in Europe. OCL. 2014;21 [Google Scholar]
- 44.Hu C., Jia W. Diabetes in China: Epidemiology and genetic risk factors and their clinical utility in personalized medication. Diabetes. 2018;67:3–11. doi: 10.2337/dbi17-0013. [DOI] [PubMed] [Google Scholar]
- 45.Vamecq J., Draye J.P. Interactions between the ω-and β-oxidations of fatty acids. J. Biochem. 1987;102:225–234. doi: 10.1093/oxfordjournals.jbchem.a122035. [DOI] [PubMed] [Google Scholar]
- 46.Neat C.E., Thomassen M.S., Osmundsen H. Induction of peroxisomal β-oxidation in rat liver by high-fat diets. Biochem. J. 1980;186:369–371. doi: 10.1042/bj1860369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antonenkov E.D., Sormunen R.T., Hiltunen J.K. The behavior of peroxisomes in vitro: Mammalian peroxisomes are osmotically sensitive particles. Am. J. Physiol. Cell Physiol. 2004;287:C1623–C1635. doi: 10.1152/ajpcell.00142.2004. [DOI] [PubMed] [Google Scholar]
- 48.Ferré P., Satabin P., Decaux J.F., Escriva F., Girard J. Development and regulation of ketogenesis in hepatocytes isolated from newborn mice. Biochem. J. 1983;214:937–942. doi: 10.1042/bj2140937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Honda A., Salen G., Nguyen L.B., Tint G.S., Batta A.K., Shefer S. Down-regulation of cholesterol biosynthesis in sitosterolemia: Diminished activities of acetoacetyl-CoA thiolase, 3-hydroxy-3-methylglutaryl-CoA synthase, reductase, squalene synthase, and 7-dehydrocholesterol Δ7-reductase in liver and mononuclear leukocytes. J. Lipid Res. 1998;39:44–50. [PubMed] [Google Scholar]
- 50.Clinkenbeard K.D., Reed W.D., Mooney R.A., Lane M.D. Intracellular localization of the 3-hydroxy-3-methylglutaryl coenzme A cycle enzymes in liver. Separate cytoplasmic and mitochondrial 3-hydroxy-3-methylglutaryl coenzyme A generating systems for cholesterogenesis and ketogenesis. J. Biol. Chem. 1975;250:3108–3116. [PubMed] [Google Scholar]
- 51.Williamson D.H., Bates M.W., Krebs H.A. Activity and intracellular distribution of enzymes of ketone-body metabolism in rat liver. Biochem. J. 1968;108:353–361. doi: 10.1042/bj1080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lazarow P.B. Assay of peroxisomal β-oxidation of fatty acids. Methods Enzymol. 1981;72:315–319. doi: 10.1016/s0076-6879(81)72021-4. [DOI] [PubMed] [Google Scholar]
- 53.Scoppola A., Montecchi F.R., Menzinger G., Lala A. Urinary mevalonate excretion rate in type 2 diabetes: Role of metabolic control. Atherosclerosis. 2001;156:357–361. doi: 10.1016/s0021-9150(00)00660-2. [DOI] [PubMed] [Google Scholar]
- 54.Yuan C., Ju Y., Jin R., Ren L., Liu X. Simultaneous HPLC–DAD analysis of tocopherols, phytosterols, and squalene in vegetable oil deodorizer distillates. Chromatographia. 2015;78:273–278. [Google Scholar]
- 55.Chung B.H., Wilkinson T., Geer J.C., Segrest J.P. Preparative and quantitative isolation of plasma lipoproteins: Rapid, single discontinuous density gradient ultracentrifugation in a vertical rotor. J. Lipid Res. 1980;21:284–291. [PubMed] [Google Scholar]
- 56.Tubbs P.K., Garland P.B. Variations in tissue contents of coenzyme A thio esters and possible metabolic implications. Biochem. J. 1964;93:550. doi: 10.1042/bj0930550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Physiol. Pharm. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 58.Kuipers F., Jong M.C., Lin Y., Eck M.V., Havinga R., Bloks V., Verkade H.J., Hofker M.H., Moshage H., Berkel T.J., Vonk R.J., Havekes L.M. Impaired secretion of very low density lipoprotein-triglycerides by apolipoprotein E-deficient mouse hepatocytes. J. Clin. Invest. 1997;100:2915–2922. doi: 10.1172/JCI119841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.King M.T., Reiss P.,D., Cornell N.W. Determination of short-chain coenzyme A compounds by reversed-phase high-performance liquid chromatography. Methods Enzymol. 1988;166:70–79. doi: 10.1016/s0076-6879(88)66012-5. [DOI] [PubMed] [Google Scholar]
- 60.Pearson D.J., Tubbs P.K. Acetyl-carnitine in heart and liver. Nature. 1964;202:91. doi: 10.1038/202091a0. [DOI] [PubMed] [Google Scholar]
- 61.Nakanishi Y., Okamoto K., Isohashi F. Subcellular distribution of ATP-stimulated and ADP-inhibited acetyl-CoA hydrolase in livers from control and clofibrate-treated rats: Comparison of the cytosolic and peroxisomal enzyme. J. Biochem. 1994;115:328–332. doi: 10.1093/oxfordjournals.jbchem.a124337. [DOI] [PubMed] [Google Scholar]
- 62.Hunt M.C., Solaas K., Kase B.F., Alexson S.E. Characterization of an acyl-CoA thioesterase that functions as a major regulator of peroxisomal lipid metabolism. J. Biol. Chem. 2002;277:1128–1138. doi: 10.1074/jbc.M106458200. [DOI] [PubMed] [Google Scholar]
- 63.Bieber L.L., Markwell M.A. Peroxisomal and microsomal carnitine acetyltransferases. Methods Enzymol. 1981;71:351–358. doi: 10.1016/0076-6879(81)71044-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are contained within the article.