Abstract
A PCR that amplifies a recently discovered Vibrio cholerae RTX (repeat in toxin) toxin gene was developed. Among 166 clinical and environmental isolates of V. cholerae causing epidemics and sporadic cases of cholera in various parts of the world, all were found to be toxigenic by both PCR and HEp-2 cell cytotoxicity assay. Standard strains of the classical biotype containing a deletion within the gene cluster exhibited negative results by both assays. This is the first rapid genotyping method for differentiation of V. cholerae O1 classical biotype strains from El Tor biotype strains as well as strains of other non-O1 serogroups including serogroup O139. The PCR assay that was developed also specifically detects RTX toxin genes in V. cholerae, as clinical isolates of Vibrio parahaemolyticus, diarrheagenic Escherichia coli, Aeromonas species, and Plesiomonas species were all negative by the RTX toxin-specific PCR as well as the HEp-2 cytotoxicity assay. These findings highlight the characteristics of the RTX toxins in V. cholerae. Their role in the pathogenicity of the bacterium requires further investigation.
Vibrio cholerae is an important cause of diarrheal disease in many parts of Asia and Africa. It is the only enteric pathogen that has the potential to produce pandemics of disease and is of immense public health importance. Cholera is caused by V. cholerae serogroup O1, which has been highly prevalent in Southeast Asia in the past 20 years (2, 5, 13). V. cholerae O1 biotype El Tor was responsible for the cholera epidemic in Hong Kong between 1986 and 1997 (7, 22, 23). Although cholera toxin-producing V. cholerae O139 has been sweeping across the Indian subcontinent since 1992, epidemic spread of this novel strain of V. cholerae has not occurred in Hong Kong since the first imported case was detected in May 1993 (21, 27).
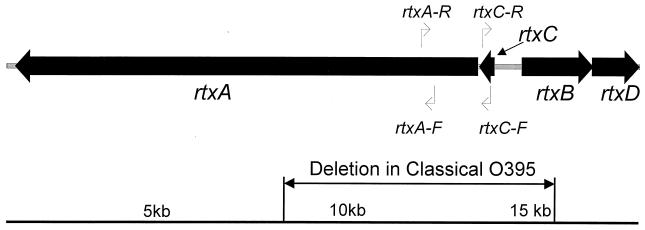
Traditionally, life-threatening diarrhea associated with the cholera syndrome is attributed to massive luminal secretion of electrolytes and water from enterocytes, with elevated cyclic AMP levels induced by the cholera toxin (CT). CT is encoded by the ctxA and ctxB genes of the core element (8). A novel toxin in V. cholerae that belongs to the RTX (repeat in toxin) family of toxins, which are generally produced by several pathogenic gram-negative bacteria (10), was recently discovered. The RTX toxins represent a family of important virulence factors that have disseminated widely among gram-negative bacteria (1). The RTX toxin gene cluster in V. cholerae encodes the presumptive cytotoxin (rtxA), an acyltransferase (rtxC), and an associated ATP-binding cassette transporter system (RtxB and RtxD, two proteins for toxin transportation) (Fig. 1). It is physically linked to the core element in the V. cholerae genome, although its activity is independent of the core element (10). Phenotypically, these genes are proven to be associated with cytotoxicity in HEp-2 cells. In the study described here, a highly specific PCR was developed to identify this toxin gene in more than 100 clinical and environmental isolates of V. cholerae causing sporadic and epidemic cases of cholera in various parts of the world.
FIG. 1.
Genomic structure of RTX toxin element in V. cholerae. Adapted from reference 10, with permission from the National Academy of Sciences, U.S.A.
(This work was done in partial fulfillment of the M.Phil. degree at the University of Hong Kong by K. H. Chow.).
MATERIALS AND METHODS
Sources of strains.
A total of 166 V. cholerae isolates from five different regions were included in our study (Table 1). The majority of the isolates were V. cholerae O1 El Tor, O139, and non-O1 serogroup strains collected from patients or the environment over a decade in Hong Kong (7, 21, 22, 23, 27). The remaining 51 clinical isolates of O1 El Tor and O139 were isolated from Hong Kong, China; Shenzhen, China; Singapore; Thailand; and Ukraine from 1991 to 1999. In addition, five strains each of diarrheagenic Escherichia coli (three isolates of verocytotoxigenic E. coli and two isolates of enteropathogenic E. coli), Vibrio parahaemolyticus, Aeromonas species, and Plesiomonas species isolated from patients suffering from diarrhea in Hong Kong were also included for comparative study (18, 19, 20).
TABLE 1.
Characteristics of clinical and environmental isolates of V. cholerae
| Strain | Yr of isolation | Origin | Source | No. of isolates | PCR result for:
|
HEp-2 cell cytotoxicity assay result | Reference(s)a | ||
|---|---|---|---|---|---|---|---|---|---|
| rtxA | rtxC | ctxB | |||||||
| V. cholerae | |||||||||
| O1 EI Tor | NAb | NEQ AS4043 | Standard strain | 1 | + | + | + | + | NEQ |
| O1 classical | NA | ATCC 9458 | Standard strain | 1 | − | − | + | − | ATCC |
| O1 classical | NA | ATCC 11628 | Standard strain | 1 | − | − | + | − | ATCC |
| O1 EI Tor | 1986–1999 | Hong Kong SAR | Patient | 81 | + | + | + | + | 7, 22, 23 |
| 1989–1998 | Hong Kong SAR | Environment | 3 | + | + | + | + | 7, 22, 23 | |
| 1999 | Shenzhen, China | Patient | 7 | + | + | + | + | This study | |
| 1996 | Singapore | Patient | 1 | + | + | + | + | This study | |
| 1995 | Thailand | Patient | 29 | + | + | + | + | This study | |
| 1991–1994 | Ukraine | Patient | 3 | + | + | + | + | This study | |
| 1994–1995 | Ukraine | Environment | 3 | + | + | + | + | This study | |
| O139 | 1994–1999 | Hong Kong SAR | Patient | 10 | + | + | + | + | 21, 27, this study |
| 1999 | Shenzhen, China | Patient | 1 | + | + | + | + | This study | |
| Non-O1 | 1993–1999 | Hong Kong SAR | Patient | 5 | + | + | − | + | 19, this study |
| 1993–1999 | Hong Kong SAR | Environment | 23 | + | + | − | + | 19, this study | |
| V. parahaemolyticus | 1993 | Hong Kong SAR | Patient | 5 | − | − | − | − | 19 |
| Diarrheagenic E. coli | 1993–1998 | Hong Kong SAR | Patient | 5 | − | − | − | − | 18, 19, 20 |
| Aeromonas species | 1998–1999 | Hong Kong SAR | Patient | 5 | − | − | − | − | This study |
| Plesiomonas species | 1998–1999 | Hong Kong SAR | Patient | 5 | − | − | − | − | This study |
NEQ, National External Quality Assessment Scheme for Microbiology, Colindale, United Kingdom; ATCC, American Type Culture Collection, Manassas, Va.
NA, not available.
PCR for rtxA, rtxC, and ctxB.
DNA from all purified strains was prepared from overnight liquid cultures grown in 5 ml of brain heart infusion broth at 37°C. Cell pellets from 1 ml of culture were washed with normal saline, centrifuged, and resuspended in 100 μl of sterile Milli-Q H2O. After the suspension was heated in a dry bath at 85°C for 15 min, the pellet was spun down at 15,900 × g for 15 min at 4°C, and the supernatant was saved for use as the DNA template in the PCR.
Two pairs of primers were derived from the rtxA and rtxC genes of V. cholerae N16961. The sequences of the primers extended within the deletion region of the RTX gene cluster in classical biotype strain O395 (Fig. 1). PCR amplified a 417-bp product of the rtxA gene (primers rtxA-F [5′-CTG AAT ATG AGT GGG TGA CTT ACG-3′] and rtxA-R [5′GTG TAT TGT TCG ATA TCC GCT ACG-3′]) and a 263-bp product of the rtxC gene (primers rtxC-F [5′-CGA CGA AGA TCA TTG ACG AC-3′] and rtxC-R [5′-CAT CGT CGT TAT GTG GTT GC-3′]). The total reaction volume was 25 μl, which contained 3 μl of DNA template and 2.5 μl of 10× PCR buffer (final concentrations, 1.5 mM MgCl2 and 0.4 mg of bovine serum albumin per ml [Applied Biosystems, Foster City, Calif.]), 1 μM primers, deoxynucleoside triphosphates at a concentration of 0.2 mM, 1 U of AmpliTaq Gold polymerase (Perkin-Elmer), and 1 drop of mineral oil. After pretreatment by heating of the mixture at 94°C for 12 min to activate the enzyme polymerase prior to the cycling reaction, DNA amplification was carried out for 30 cycles at 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. An extension period of 72°C for 10 min was added at the end of the cycling reaction. For product detection, 5 μl of the PCR mixture was subjected to electrophoresis in a 2% agarose gel.
The amplification of a 460-bp ctxB gene (primers ctxB2 [5′-GAT ACA CAT AAT AGA ATT AAG GAT G-3′] and ctxB3 [5′-GGT TGC TTC TCA TCA TCG AAC CAC-3′]) from the sample strains was performed as described previously (3, 12).
Cytotoxicity and CT assays.
All bacterial strains were tested to determine whether they had a cytopathic effect on HEp-2 cells, as described previously (10). Briefly, HEp-2 cells were cultured in RPMI 1640 medium (Gibco, Grand Island, N.Y.) with 10% fetal calf serum and supplement (without antibiotics) and were seeded onto dishes for assay. Washed bacterial cultures were added to the HEp-2 cells, and the dishes were incubated at 37°C in 5% CO2 for 1 h. A positive result was indicated by rounding up and detachment of monolayer HEp-2 cells from the culture dishes.
Active CT production was also performed by the VET-RPLA (Oxoid Ltd., Basingstoke, United Kingdom) assay, with modifications, as described previously (24). Briefly, polymyxin B was added to the overnight bacterial cultures, followed by incubation at 37°C for 4 h with shaking. After centrifugation, the supernatants were diluted in V-type microtiter plates in duplicate until the last well contained diluent only. Latex suspensions sensitized with antibodies to CT were added to each well and were left undisturbed at room temperature for 20 to 24 h. Agglutination was examined macroscopically and recorded.
RESULTS
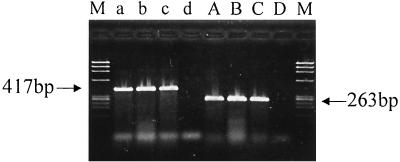
All 166 clinical and environmental strains of V. cholerae were collected from 1986 to 1999 in Hong Kong, China, Singapore, Thailand, and Ukraine (Table 1). V. cholerae isolates of the O1 El Tor, O139, and non-O1 serogroups exhibited positive PCR results for both rtxA and rtxC genes (Fig. 2). Concurrently, all these isolates were also positive by the HEp-2 cytotoxicity assay. Complete concordance was found between the genotypic and the phenotypic expressions of the RTX toxins in all strains tested, indicating the integrity of the RTX toxin gene cluster among the strains. Although no clinical strain of the V. cholerae classical biotype was collected during the period studied, the rtxA and rtxC genes were not amplified from standard V. cholerae classical biotype strains, strains ATCC 9458 and ATCC 11628. These two classical strains also exhibited negative results by the HEp-2 cytotoxicity assay. Except for the non-O1 serogroups, all V. cholerae O1 and O139 isolates were positive for CT by VET-RPLA and PCR assay. Five strains each of V. parahaemolyticus, diarrheagenic E. coli, Aeromonas species, and Plesiomonas species included in the present study exhibited negative results by all PCR and toxin assays.
FIG. 2.
Agarose gel electrophoresis of PCR products of rtxA (lanes a to d) and rtxC (lanes A to D). Lanes a and A, V. cholerae O1 El Tor; lanes b and B, V. cholerae O139; lanes c and C, V. cholerae non-O1; lanes d and D, V. cholerae classical strain ATCC 9458; lanes M, molecular mass markers (BsuRI-digested φX174 DNA).
DISCUSSION
On the basis of the discovery of RTX toxins in V. cholerae, we developed two assays specific for rtxA and rtxC and screened for the presence of functional RTX toxin genes in our collection of strains obtained from various parts of the world from 1986 to 1999. Our findings for chronologically and geographically disparate strains indicate that the presence of an intact RTX toxin gene cluster is consistent with the phenotypic expression of cytotoxic activity in all these isolates. Standard strains of the classical biotype exhibited negative results by both PCR and cytotoxicity assays, which was explained by the deletion of the gene cluster. In addition, the PCR detection assay described here was highly specific for V. cholerae, as all clinical isolates of V. parahaemolyticus, diarrheagenic E. coli, Aeromonas species, and Plesiomonas species exhibited negative results.
Although genotypic methods like PCR of the ctx gene are available for the rapid identification of toxigenic V. cholerae, definitive identification of the classical and El Tor biotypes within the O1 serogroup relies on conventional biochemical methods, which are tedious and time-consuming. Although only two V. cholerae classical strains were included in the present study, the PCR assays developed for rtxA or rtxC, when used in combination with PCR for CT, not only identify CT-producing V. cholerae but also differentiate the biotypes of strains within the V. cholerae O1 serogroup.
While CT is a principal virulence factor for V. cholerae, the contribution of the RTX toxins to its pathogenesis requires further investigation. At present, the cytolytic RTX toxins represent a family of important virulence factors for organisms that produce the toxin. Good evidence of the role of the hemolysin of E. coli as a cause of extraintestinal infections is available (17). In our study, the rtx gene cluster was absent only from the V. cholerae classical O1 serogroup strain, which has greater epidemic potential than strains of the other serogroups, despite its displacement by the El Tor biotype since the seventh pandemic. Nonepidemic V. cholerae non-O1 serogroup strains, which cause only sporadic, milder cases of diarrhea, do secrete the RTX cytotoxins but do not secrete CT. Several groups proposed that virulence factors account for the clinical manifestations of diarrhea caused by non-O1 serogroup strains (6, 15, 25, 26). One distinct finding indicated that V. cholerae non-O1 serogroup strains caused necrosis of the luminal epithelium in the colon and mild inflammatory cell infiltration in the adjacent lamina propria (14). Evidence of the inflammatory response due to a V. cholerae O1 El Tor strain from which all known toxin genes excluding the rtx gene cluster have been deleted has also been reported (16). The vacuolating activity of the V. cholerae El Tor hemolysin in nucleated mammalian cells may be associated with gastrointestinal symptoms caused by nontoxigenic V. cholerae (11). A recent investigation (4) demonstrated that the RTX toxins of V. cholerae caused actin depolymerization and cross-linking in HEp-2 cells. Similar actin rearrangement or condensation was observed in HEp-2 cells and was caused by a protein encoded by the eaeA gene of enteropathogenic and hemorrhagic E. coli, leading to the effacement of microvilli, with subsequent hemorrhagic colitis and bloody diarrhea (9). Our findings highlight the occurrence of RTX toxins in strains of V. cholerae except those exhibiting the classical biotype. Further investigation is required to determine the role of RTX in the pathogenicity of V. cholerae.
ACKNOWLEDGMENTS
We thank the following persons for providing bacterial strains: Clifford Clark of the National Laboratory for Enteric Pathogens, Bureau of Microbiology, Laboratory Centre for Disease Control, Ottawa, Ontario, Canada; T. Kuyyakanond of the Department of Microbiology, Khon Kaen University, Khon Kaen, Thailand; and Ling Moi Lin of the Department of Pathology and Laboratory Medicine, Tan Tock Seng Hospital, Singapore. We also thank K. W. Wong for excellent technical assistance.
REFERENCES
- 1.Coote J G. Structural and functional relationships among the RTX toxin determinants of gram-negative bacteria. FEMS Microbiol Rev. 1992;8:137–161. doi: 10.1111/j.1574-6968.1992.tb04961.x. [DOI] [PubMed] [Google Scholar]
- 2.Dalsgaard A, Forslund A, Tam N V, Vinh D X, Cam P D. Cholera in Vietnam: changes in genotypes and emergence of class I integrons containing aminoglycoside resistance gene cassettes in Vibrio cholerae O1 strains isolated from 1979 to 1996. J Clin Microbiol. 1999;37:734–741. doi: 10.1128/jcm.37.3.734-741.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fields P I, Popovic T, Wachsmuth K, Olsvik O. Use of polymerase chain reaction for detection of toxigenic Vibrio cholerae O1 strains from the Latin American cholera epidemic. J Clin Microbiol. 1992;30:2118–2121. doi: 10.1128/jcm.30.8.2118-2121.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fullner K J, Mekalanos J J. In vivo covalent cross-linking of cellular actin by the Vibrio cholerae RTX toxin. EMBO J. 2000;19:5315–5323. doi: 10.1093/emboj/19.20.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoge C W, Bodhidatta L, Echeverria P, Deesuwan M, Kitporka P. Epidemiologic study of Vibrio cholerae O1 and O139 in Thailand: at the advancing edge of the eighth pandemic. Am J Epidemiol. 1996;143:263–268. doi: 10.1093/oxfordjournals.aje.a008737. [DOI] [PubMed] [Google Scholar]
- 6.Ichinose Y, Yamamoto K, Nakasone N, Tanabe M J, Takeda T, Miwatani T, Iwanaga M. Enterotoxicity of El Tor-like hemolysin of non-O1 Vibrio cholerae. Infect Immun. 1987;55:1090–1093. doi: 10.1128/iai.55.5.1090-1093.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kam K M, Leung T H, Ho Y Y, Ho N K, Saw T A. Outbreak of Vibrio cholerae O1 in Hong Kong related to contaminated fish tank water. Public Health. 1995;109:389–395. doi: 10.1016/s0033-3506(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 8.Kaper J B, Fasano A, Trucksis M. Toxins of Vibrio cholerae. In: Wachsmuth I K, Blake P A, Olsvik I, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D. C.: American Society for Microbiology; 1994. pp. 145–176. [Google Scholar]
- 9.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin W, Fullner K J, Clayton R, Sexton J A, Rogers M B, Calia K E, Calderwood S B, Fraser C, Mekalanos J J. Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc Natl Acad Sci USA. 1999;96:1071–1076. doi: 10.1073/pnas.96.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitra R, Figueroa P, Mukhopadhyay A K, Shimada T, Takeda Y, Berg D E, Nair G B. Cell vacuolation, a manifestation of the El Tor hemolysin of Vibrio cholerae. Infect Immun. 2000;68:1928–1933. doi: 10.1128/iai.68.4.1928-1933.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olsvik O, Wahlberg J, Petterson B, Uhlen M, Popovic T, Wachsmuth I K, Fields P I. Use of automated sequencing of polymerase chain reaction-generated amplicons to identify three types of cholera toxin subunit B in Vibrio cholerae O1 strains. J Clin Microbiol. 1993;31:22–25. doi: 10.1128/jcm.31.1.22-25.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radu S, Ho Y K, Lihan S, Yuherman, Rusul G, Yasin R M, Khair J, Elhadi N. Molecular characterization of Vibrio cholerae O1 and non-O1 from human and environmental sources in Malaysia. Epidemiol Infect. 1999;123:225–232. doi: 10.1017/s0950268899002769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russel R G, Tall B D, Morris J G., Jr Non-O1 Vibrio cholerae intestinal pathology and invasion in the removable intestinal tie adult rabbit diarrhea model. Infect Immun. 1992;60:435–442. doi: 10.1128/iai.60.2.435-442.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saha P K, Koley H, Nair G B. Purification and characterization of an extracellular secretogenic non-membrane-damaging cytotoxin produced by clinical strains of Vibrio cholerae non-O1. Infect Immun. 1996;64:3101–3108. doi: 10.1128/iai.64.8.3101-3108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva T M J, Schleupner M A, Tacket C O, Steiner T S, Kaper J B, Edelman R, Guerrant R L. New evidence for an inflammatory component in diarrhea caused by selected new, live attenuated cholera vaccines and by El Tor and O139 Vibrio cholerae. Infect Immun. 1996;64:2362–2364. doi: 10.1128/iai.64.6.2362-2364.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welch R A, Forestier C, Lobo A, Pellett S, Thomas W, Jr, Rowe G. The synthesis and function of the Escherichia coli hemolysin and related RTX exotoxins. FEMS Microbiol Immunol. 1992;5:29–36. doi: 10.1111/j.1574-6968.1992.tb05883.x. [DOI] [PubMed] [Google Scholar]
- 18.Wong S S Y, Yam W C, Leung P M H, Woo P C Y, Yuen K Y. Verocytotoxin-producing Escherichia coli infection: the Hong Kong Experience. J Gastroenterol Hepatol. 1998;13(Suppl.):S289–S293. doi: 10.1111/j.1440-1746.1998.tb01895.x. [DOI] [PubMed] [Google Scholar]
- 19.Yam W C, Chan C Y, Ho B S W, Tam T Y, Kueh C, Lee T. Abundance of clinical enteric bacterial pathogens in coastal waters and shellfish. Water Res. 1999;34:51–56. [Google Scholar]
- 20.Yam W C, Tsang D N C, Que T L, Peiris M, Seto W H, Yuen K Y. Unique strain of E. coli O157:H7 excreting low level of verocytotoxin missed by commercial EIA kit. Clin Infect Dis. 1998;27:905–906. doi: 10.1086/517167. [DOI] [PubMed] [Google Scholar]
- 21.Yam W C, Yuen K Y, Wong S S, Que T L. Vibrio cholerae O139 susceptible to vibriostatic agent O/129 and co-trimoxazole. Lancet. 1994;344:404–405. [PubMed] [Google Scholar]
- 22.Yam W C, Lung M L, Ng K Y, Ng M H. Molecular epidemiology of Vibrio cholerae in Hong Kong. J Clin Microbiol. 1989;27:1900–1902. doi: 10.1128/jcm.27.8.1900-1902.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yam W C, Lung M L, Ng K Y, Ng M H. Restriction fragment length polymorphism analysis of Vibrio cholerae strains associated with a cholera outbreak in Hong Kong. J Clin Microbiol. 1991;29:1058–1059. doi: 10.1128/jcm.29.5.1058-1059.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yam W C, Lung M L, Ng K Y, Ng M H. Evaluation and optimization of a latex agglutination assay for the detection of cholera toxin and Escherichia coli heat-labile toxin. J Clin Microbiol. 1992;30:2518–2520. doi: 10.1128/jcm.30.9.2518-2520.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto K, Al-Omani M, Honda T, Takeda Y, Miwatani T. Non-O1 Vibrio cholerae hemolysin: purification, partial characterization, and immunological relatedness to El Tor hemolysin. Infect Immun. 1984;45:192–196. doi: 10.1128/iai.45.1.192-196.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto K, Ichinose Y, Nakasone N, Tanabe M, Nagahama M, Sakurai J, Iwanaga M. Identity of hemolysins produced by Vibrio cholerae non-O1 and V. cholerae O1, biotype El Tor. Infect Immun. 1986;51:927–931. doi: 10.1128/iai.51.3.927-931.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuen K Y, Yam W C, Wong S S. V. cholerae O139 synonym Bengal in Hong Kong. Clin Infect Dis. 1994;19:553–554. doi: 10.1093/clinids/19.3.553. [DOI] [PubMed] [Google Scholar]