Summary
As a major social and economic burden for the healthcare system, kidney diseases contribute to the constant increase of worldwide deaths. A deeper understanding of the underlying mechanisms governing the etiology, development and progression of kidney diseases may help to identify potential therapeutic targets. As a superfamily of ligand-dependent transcription factors, nuclear receptors (NRs) are critical for the maintenance of normal renal function and their dysfunction is associated with a variety of kidney diseases. Increasing evidence suggests that ligands for NRs protect patients from renal ischemia/reperfusion (I/R) injury, drug-induced acute kidney injury (AKI), diabetic nephropathy (DN), renal fibrosis and kidney cancers. In the past decade, some breakthroughs have been made for the translation of NR ligands into clinical use. This review summarizes the current understanding of several important NRs in renal physiology and pathophysiology and discusses recent findings and applications of NR ligands in the management of kidney diseases.
Keywords: Kidney, PPAR, MR, LXR, FXR
Introduction
The kidney is an important excretory and endocrine organ and plays an essential role in the maintenance of body homeostasis by controlling fluid and electrolyte balance and the pressure, composition, and volume of blood. The mammalian renal functions depend on the coordinated development and well-organized renal glomeruli and tubule system composed of distinct cell populations. Damage of the glomeruli and renal tubules leads to the deterioration of renal function and results in substantial morbidity and mortality. Kidney diseases represent a considerable burden in the healthcare system worldwide. As a rapid decline in renal function, acute kidney injury (AKI) is a common and devastating pathological condition with high in-hospital mortality. Patients who survive AKI may subsequently develop to chronic kidney disease (CKD) and end-stage renal disease (ESRD).1 In addition, CKD secondary to diabetes, obesity and hypertension has become globally recognized major causes of ESRD.2 Unfortunately, there is no specific treatment for AKI and CKD and the only efficient therapies for ESRD are kidney transplantation and dialysis. Therefore, understanding the underlying mechanisms associated with renal physiological regulation and the pathogenesis of kidney diseases is of great importance for developing novel effective therapeutic agents.
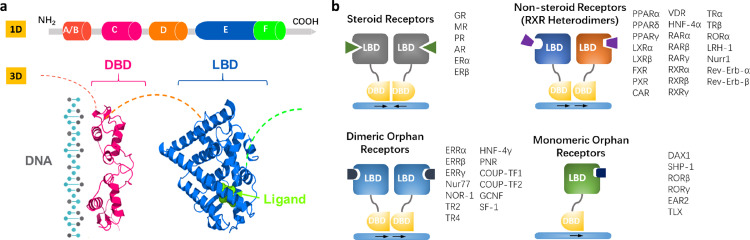
In the past decades, increasing evidence has shown that nuclear receptors (NRs) play a critical role in renal physiology and pathophysiology. NRs comprise a superfamily of evolutionally conserved transcription factors and are mostly activated by chemically diverse small lipophilic ligands including hormones, endogenous metabolites, and exogenous xenobiotics.3 Ligand binding induces NR protein conformational change and the recruitment of co-regulators, leading to the translocation of the NR into the nucleus where it binds to the response element in the promoter region of its target gene throughout the genome. Since estrogen receptors (ERs) were first reported in l960s, total 48 members of the NR family have been identified in human (Supplementary Table 1), which share a common structure composed of five or six domains (Figure 1a), including N-terminal domain (A/B), DNA-binding domain (DBD, C), hinge region (D), and ligand-binding domain (LBD, E/F). The NRs can be divided into four sub-classes based on their dimerization and DNA-binding properties (Figure 1b), which was extensively reviewed elsewhere.4,5 A large body of evidence clearly demonstrates that NRs play a critical role in maintaining body homeostasis and aberrant expression and function of NRs are involved in the pathogenesis of various human diseases. Therefore, NRs represent as ideal therapeutic targets for the treatment of numerous diseases including kidney diseases.
Figure 1.
Structure and classification of NRs.
(a) Structural characteristics of NRs. A/B, a variable and intrinsically unfolded N-terminal domain harboring transcriptional activation function 1 (AF-1); C, a conserved DNA-binding domain (DBD); D, a relatively conservative hinge domain; E, C-terminal ligand-binding domain (LBD); F, a C-terminal activation function 2 connected to LBD. Hydrophobic pocket enclosed in the LBD has the ability to accommodate suitable ligands which are mostly lipophilic endo- or exogenous compounds.
(b) Four groups of NRs classified by their dimerization and DNA-binding properties. The 48 NRs in human have been listed in these four groups.
Most NRs show a constitutive expression pattern in the kidney, suggesting NRs widely participate in the regulation of renal physiology. Recent single cell RNA-sequencing studies have defined the precise expression and localization of NRs in the kidney, which provide very meaningful information regarding the potential role of each nuclear receptor in renal tissue (Table 1). In this review, we provide an overview of the latest advances in the study of NRs in renal physiology and pathophysiology, with a specific focus on PPARs, LXRs, MR, FXR, PXR, ERs, GR and VDR. We also summarize the role of these NRs in different kidney diseases and discuss the potential renoprotective effects of their ligands.
Table 1.
Expression of NRs in the glomerulus and along renal tubules in mouse.
 |
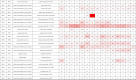 |
The expression of each NR is extracted from the database providing the mean gene expression values (transcripts per million, TPM) for micro-dissected mouse renal glomeruli (https://esbl.nhlbi.nih.gov/MRECA/G/) and tubule segments (https://esbl.nhlbi.nih.gov/MRECA/Nephron/).67 The gradient of the red color indicates the expression level in different micro-dissected segments. GlOM, glomerulus; PTS1, the initial segment of the proximal convoluted tubule; PTS2, the proximal straight tubule in cortical medullary rays; PTS3, the last segment of the proximal straight tubule in the outer stripe of outer medulla; DTL1, the short descending limb of the loop of Henle; DTL2, the long descending limb of the loop of Henle in the outer medulla; DTL3, the long descending limb of the loop of Henle in the inner medulla; ATL, the thin ascending limb of the loop of Henle; MTAL, medullary thick ascending limb of the loop of Henle; CTAL, cortical thick ascending limb of the loop of Henle; DCT, the distal convoluted tubule; CNT, the connecting tubule; CCD, cortical collecting duct; OMCD, outer medullary collecting duct; IMCD, inner medullary collecting duct.
Peroxisome proliferator-activated receptors (PPARs)
Since their identification, three subtypes of PPARs have been discovered in many mammalian species, and designated as PPARα (NR1C1), PPARβ/δ (NR1C2), and PPARγ (NR1C3). Each PPAR isoform is encoded by its own gene and has unique tissue distribution, ligand specificity, and metabolic regulatory activities.6 PPARs can interact with a variety of PPAR cofactors to regulate multiple metabolic processes especially glucose and lipid metabolism.7 In general, PPARs control various physiological processes including the regulation of lipid and lipoprotein metabolism, glucose homeostasis, cell proliferation and differentiation, cell cycle progression, inflammation and extracellular matrix remodeling.8 Therefore, PPARs attract an enormous attention and are considered as potential therapeutic targets in many human diseases.6 The different intrarenal distribution and their biological roles in renal physiology and pathophysiology have been intensively investigated and reviewed in the literature.8,9
PPARα
Natural ligands for PPARα include unsaturated fatty acids, phospholipids, leukotriene B4 and 8 (S)-hydroxyeicosatetraenoic acid. Synthetic activators of PPARα have also been developed such as fibrates, pemafibrate and LY518674. PPARα is highly expressed in metabolically active tissues such as brown adipose tissue, liver, heart, skeletal muscle, and kidney, where it promotes fatty acid β-oxidation. The fibrate class of PPARα agonists has been clinically used to treat hypertriglyceridemia (Table 2). In the kidney, PPARα is predominantly expressed in the proximal tubules and the medullary thick ascending limbs with much less expression in the glomeruli (Table 1).10 Although expressed at low level in glomerular mesangial cells, it has been reported that PPARα can reduce inflammatory response in LPS-treated mesangial cells,11 suggesting it may play a role in glomerular diseases. PPARα agonist clofibrate can regulate renal cortical mitochondrial and peroxisomal β-oxidation enzyme gene expression in the developing kidneys of rats receiving a high-fat diet.12 In an acute renal injury model, PPARα activation was repeatedly found to exert renoprotective effect on renal ischemia/reperfusion (I/R) injury possibly via stimulating fatty acid β-oxidation (FAO).13,14 However, the role of PPARα in acute cisplatin nephrotoxicity remains debated, since protective effect was observed in both PPARα agonist-treated and PPARα gene-deficient animals.15,16 In an adriamycin-associated renal injury model, PPARα agonist fibrate significantly reduced proteinuria and renal damage.17 In alcohol-associated kidney injury, increased methylation of PPARα m6A by FTO-mediated YTHDF2 epigenetic modification was found to be associated with alcohol-induced activation of Nod-like receptor (NLR) family and pyrin domain containing 3 (NLRP3) inflammasomes and NF-κB-driven renal inflammation in the kidney.18
Table 2.
A partial list of clinically used drugs based on NR modulation and their renal benefits.
| NR | Drugs | Description & function | Targeted diseases | Potential renal benefits |
|---|---|---|---|---|
| PPARα | Gemfibrozil | promotes lipid metabolism and decreases serum triglyceride levels | hypertriglyceridemia (particularly in type IV and V hyperlipidemia) | reduces glomerular injury and lipid accumulation and improves renal function, prevents the progression of chronic kidney disease in obese Dahl salt-sensitive rats |
| Ciprofibrate | decreases total cholesterol (TC), triglycerides, apolipoprotein B (apo B) and low-density lipoprotein (LDL) cholesterol, apolipoproteins, and increases high-density lipoprotein (HDL) cholesterol and apolipoprotein AI (apo A-I) | type IIa hypercholesterolaemia, type IIb combined hyperlipidaemia, and type IV hypertriglyceridaemia | inhibits d-limonene nephrotoxicity | |
| Bezafibrate | reduces plasma triglyceride and cholesterol concentrations; ameliorates deranged bile acid homoeostasis and attenuates raised concentration of liver enzymes |
hypertriglyceridaemia, hypercholesterolaemia; primary biliary cholangitis | improves renal function of patients with chronic kidney disease, increases glomerular filtration rate (eGFR); decreases blood urea and serum creatinine levels | |
| Pemafibrate | reduces triglyceride and elevates high-density lipoprotein-cholesterol (HDL-C) | dyslipidemia,metabolic disease, diabetic complications and liver diseases. | protects against fatty acid-induced nephropathy by maintaining renal fatty acid metabolism | |
| Fenofibrate | ameliorates retinal vascular leakage and leukostasis, downregulates vascular endothelial growth factor, and reduces endothelial cell and pericyte loss | dyslipidemia, type 2 diabetes and diabetic retinopathy (DR) | attenuates ischemia reperfusion-induced acute kidney injury | |
| PPARγ | Pioglitazone | thiazolidinedione; increases insulin sensitivity, reduces insulin resistance, promotes adipogenesis | type 2 diabetes mellitus | inhibits renal fibrosis and protects renal function in type 2 diabetes |
| Rosiglitazone | thiazolidinedione; increases insulin sensitivity, reduces insulin resistance, promotes adipogenesis | type 2 diabetes mellitus | protects against renal tubular epithelial cells damage by hydrogen peroxide; protects the kidneys by reducing renal inflammation and renal injury | |
| MR | Spironolactone | a synthetic steroidal mineralocorticoid receptor antagonist (MRA); increases urine sodium excretion, reduces urine potassium output | hypertension, primary hyperaldosteronism and peripheral oedema associated with cardiac failure and other pathologies associated with aldosteronism | reduces proteinuria and retards renal progression in chronic kidney disease patients; prevents AKI-induced CKD |
| Eplerenone | a synthetic steroidal mineralocorticoid receptor antagonist (MRA); increases urine sodium excretion, reduces urine potassium output, decreases blood pressure, and reduces left ventricular ejection fraction | arterial hypertension and heart failure | abrogates renal injury, including ischemia-induced damage | |
| Finerenone | a non-steroidal mineralocorticoid receptor antagonist (MRA); possesses anti-inflammatory and anti-fibrotic properties | heart failure and diabetic nephropathy | reduces kidney failure and disease progression in diabetic kidney disease | |
| Esaxerenone | a non-steroidal mineralocorticoid receptor antagonist (MRA); elicits a strong blood pressure-lowering effect in hypertensive animals | hypertension | reduces the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and microalbuminuria | |
| FXR | Obeticholic acid | a synthetically modified bile acid and potent agonist of FXR; improves primary biliary cholangitis and the histological features of NASH | non-alcoholic steatohepatitis; primary biliary cholangitis; primary sclerosing cholangitis | renoprotective roles in diabetes- and obesity-related kidney disease; inhibits renal inflammation and oxidative stress during lipopolysaccharide-induced acute kidney injury |
| VDR | Calcitriol | an active metabolite of 1,25-dihydroxyvitamin D3; induces an inhibitory effect on the growth of various cell types, and the expression of different markers of cell differentiation | osteoporosis, corticosteroid-induced osteoporosis, hypocalcemia due to chronic renal failure, hypoparathyroidism and pseudohypoparathyroidism | ameliorates kidney injury through reducing podocyte and tubular injury, inflammation and fibrosis |
| Paricalcitol (Zemplar) | a synthetic vitamin D2 analogue, agonist of the VDR; inhibits the secretion of parathyroid hormone (PTH) through binding to the vitamin D receptor | secondary hyperparathyroidism | attenuates contrast-induced acute kidney injury; attenuates albuminuria and renal interstitial fibrosis |
|
| Alfacalcidol | a 1,25-dihydroxyvitamin D3 analog; anti-bone loss and anti-fracture efficacies in postmenopausal osteoporosis | osteoporosis | may reduce cardiovascular disease events in patients with predialysis chronic kidney disease | |
| Ergocalciferol | Vitamin D2; helps promote calcium absorption in the gut, maintains adequate serum calcium and phosphate levels, and promotes bone growth, modulates cell growth | osteoporosis | improves chronic kidney disease – mineral and bone disorder | |
| Cholecalciferol | Vitamin D3; helps promote calcium absorption in the gut, maintains adequate serum calcium and phosphate levels, promotes bone growth, modulates cell growth | osteoporosis | improves chronic kidney disease – mineral and bone disorder | |
| Dihydrotachysterol | a synthetic analogue of vitamin D2; promotes the absorption of intestinal calcium, and regulates the calcification of bone | hypoparathyroidism, renal dystrophy in hemodialysis patients, osteodystrophy and hypoparathyroidism | promotes linear growth or causing hypercalcemia in children with chronic renal insufficiency | |
| Doxercalciferol | 1α-hydroxyvitamin D2; inhibits the secretion of whole parathyroid hormone | secondary hyperparathyroidism | prevents renal diseases, especially for dietary fat-induced renal disease and renal lipid metabolism |
PPARα is also involved in the pathogenesis of many chronic kidney diseases. It has been shown that PPARα agonists are effective in improving diabetic nephropathy (DN) in both type 1 and type 2 diabetes as reflected by reduced albuminuria and renal fibrosis.19,20 In other chronic kidney diseases, tubulointerstitial fibrosis is a strong predictor of disease progression in patients, which is often accompanied by lipid accumulation in renal tubules. Suppressed PPARα expression by ATF6α was found to be associated with reduced activity of fatty acid β-oxidation and increased cytosolic accumulation of lipid droplets, contributing to lipotoxicity-induced tubulointerstitial fibrosis.21 In addition, in an aging-associated CKD model, impaired renal PPARα signaling accelerated lipid accumulation, decreased activity of FAO pathway and aggravated renal fibrosis development.22 Collectively, these findings demonstrate that PPARα may represent as an attractive target for the treatment of many renal diseases especially AKI and DN.
PPARβ/δ
Natural ligands for PPARβ/δ include unsaturated fatty acids, components of very low-density lipoprtoein (VLDL), 13-S-hydroxyostadecadienoic acid and prostacyclin (PGI2). Synthetic activators of PPARβ/δ have also been developed such as GW501516, GW610742, GW0742, L-165041, and MBX-8025.7,23 PPARβ/δ is ubiquitously expressed at a low level in almost every tissue examined. In the kidney, PPARβ/δ is diffusely expressed along the nephron and collecting ducts (Table 1). Detectable expression is also found in medullary interstitial cells, where it is acivated by PGI2 to protect the cells from hypertonicity-induced injury.10 Recent studies have further revealed that PPARβ/δ may play a critical role in controlling renal blood flow. PPARβ/δ was found to mediate renal vasodilation through a COX-2/PGI2/PPARβ/δ axis.24
PPARβ/δ agonist pretreatment can protect mouse kidney against renal I/R injury in a model of a 30-min renal ischemia followed by a 36 h reperfusion through reducing medullary necrosis and inflammation by activating the antiapoptotic Akt signaling pathway.25 In addition, PPARβ/δ agonist GW0742 attenuates, while its antagonist GSK0660 aggravates, I/R injury in diabetic rats through a mechanism of reducing local inflammatory response.26 Furthermore, PPARβ/δ agonist GW0742 is capable of improving renal fibrosis in type 2 db/db mice.27 Altogether, these results indicate that PPARβ/δ plays a critical role in renal physiology and pathphysiology and may serve as a novel target for the treatment of both acute and chronic kidney disorders.
PPARγ
Two isoforms, G1 (γ1) and G2 (γ2), exist in mammalian PPARγ, as derived from a single gene and transcribed using different promoters. PPARγ2 contains additional 30 amino acids at the N-terminal of PPARγ1 and is exclusively expressed in white adipose tissue (WAT), whereas PPARγ1 is expressed at a relatively low level in non-WAT tissues such as intestine, kidney, spleen, adrenal, heart, liver, lung, and brain.28,29 Natural ligands for PPARγ include unsaturated fatty acids, 15-hydroxyelcosatetraenoic acid, 9-S-hydroxyostadecadienoic acid, 13-S-hydroxyostadecadienoic acid and 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2). Synthetic activators of PPARγ have also been developed such as thiazolidnediones (TZDs) including rosiglitazone and pioglitazone.23 TZDs possess a promising insulin-sensitizing effect and have been clinically used for the treatment of type 2 diabetes mellitus (Table 2). PPARγ coordinates a variety of biological processes including adipose differentiation, lipid uptake and storage, thermogenesis, lipogenesis, glucose uptake, insulin signaling, oxidative stress, and endoplasmic reticulum (ER) stress.6 Both human and mouse studies clearly demonstrate that PPARγ is a critical transcription factor in insulin sensitivity and adipogenesis.30 The P115Q PPARγ gain-of-function mutations result in higher insulin sensitivity and morbid obesity, while the P12A PPARγ partial loss-of-function mutation leads to impaired insulin sensitivity with lower body mass in human.31 Mouse genetically deficient for PPARγ exhibits a lipodystrophy phenotype, while wild-type mouse receiving TZD PPARγ agonist treatment frequently develops obesity.32
In the kidney, PPARγ is primarily expressed in medullary collecting ducts, with lower expression in the glomeruli and renal microvasculature. In renal collecting ducts, PPARγ promotes sodium and fluid reabsorption via increasing epithelial sodium channel (ENaC) expression and its overactivation contributes to TZD-associated fluid and sodium retention and cardiovascular adverse events in type 2 diabetic patients.33 A large body of studies in human and rodents indicates that TZD PPARγ agonists exert renoprotective effect on DN in both type 1 and type 2 diabetes,34 suggesting systemic and kidney-specific actions of PPARγ are involved in its beneficial renal effect. As a synthetic ligand of PPARγ, pioglitazone hydrochloride, an clinically used TZD class insulin-sensitizing agent, was found to ameliorate renal damage by inhibiting the NF-κB signaling pathway and inflammatory response in a rat model of renal I/R injury.35 In a sepsis-induced kidney injury model, treatment with the PPARγ agonist geniposide significantly ameliorated acute kidney damage.36 It is also found that the PPARγ agonist GW1929 improves, while the PPARγ antagonist GW9662 aggravates, inorganic mercury-induced nephrotoxicity through modulating NLRP3 inflammasome activation and apoptosis.37 PPARγ agonists, rosiglitazone and 15d-PGJ2, also showed protective effects in chronic experimental cyclosporine A-induced nephrotoxicity in male Wistar rats.38 A recent study has demonstrated that PPARγ agonist rosiglitazone can attenuate hyperuricemic nephropathy in rat through effectively preserving renal function, decreasing urine microalbumin, and inhibiting interstitial fibrosis and macrophage infiltration.39 PPARγ has been repeatedly shown to have anti-fibrotic effect in CKD. The asiatic acid (a triterpenoid compound from Chinese medicine Centella Asiatica) can alleviate renal fibrosis in unilateral ureteral obstruction (UUO) rats through promoting production of 15d-PGJ2. However, the protective effect of asiatic acid was completely abolished by the PPARγ antagonist GW9662, suggesting PPARγ might be a promising target for renal fibrosis.40 In support, a recent study further showed that treatment of UUO rats with rosiglitazone-loaded nanoparticles significantly reduced collagen deposition and successfully attenuated renal fibrosis.41 Altogether, convincing data demonstrate that PPARγ plays a critical role in renal physiology and its agonists may have great potential in treating many renal diseases including AKI and CKD.
Mineralocorticoid receptor (MR)
MR (NR3C2) is classically known as the receptor for aldosterone, a circulatory mineralocorticoid hormone, which is essential in regulating ENaC activity. In the kidney, MR is mainly expressed in the distal nephrons and collecting ducts serving as a master regulator of sodium balance, body fluid, and blood pressure (Table 1). Beyond the distal tubular cells, MR is also distributed in the endothelial cells, smooth muscle, mesangial and inflammatory cells, podocytes, and fibroblasts.42 MR antagonists (MRAs) exert vital effect on renal pathophysiology which has been summarized in a recent elegant review.43 Multiple MRAs are currently being tested in preclinical studies and clinical trials to confirm their therapeutic actions in CKD.43 In brief, MRAs have been found to attenuate inflammation via suppressing pro-inflammatory cytokines, chemo-attractants, and pro-oxidants, and increasing anti-inflammatory cytokine production in renal tissue.44,45 In I/R-related renal injury, MRAs are able to improve renal function by reducing proteinuria, increasing glomerular filtration and renal blood flow, improving inflammation and oxidative stress.46 Also, MRAs have been found to be beneficial for renal injury caused by nephrotoxic drugs including antibiotics (e.g., gentamicin, amikacin), anticancer drugs (e.g., cisplatin, and carboplatin), ifosfamide, radiocontrast media, nonsteroidal anti-inflammatory drugs, and folic acid, mainly via reducing oxidative stress, renal inflammation, and renal fibrosis.47, 48, 49, 50 MRAs can prevent the transition from AKI to CKD by reducing inflammation and oxidative stress.51,52 In streptozotocin (STZ)-induced type-1 diabetes mellitus, MRAs were reported to significantly diminish glomerular, tubulointerstitial, and perivascular collagen deposition and podocyte apoptosis, with a beneficial effect on albuminuria and histopathological abnormalities.53, 54, 55 In type 2 diabetic db/db mouse, MRAs were very effective in lowering serum glucose and insulin levels, improving insulin resistance, reducing albuminuria, glomerular hypertrophy, mesangial expansion and tubulointerstitial injury.56,57 MRAs have been shown to attenuate renal fibrosis in animals with UUO and adenine diet feeding, possibly by reducing renal inflammation, interstitial cell proliferation and oxidative stress.58, 59, 60, 61 In addition to direct renal action, systemic antihypertensive effect of MRAs may also contribute to their renoprotection in patients with chronic kidney disease. Moreover, increasing evidence suggests some of MRAs significantly improve renal and cardiovascular-endpoints in patients with type 2 diabetes and CKD, opening a new avenue for effective cardiorenal protection.
Approved steroidal MRAs include spironolactone, canrenone, potassium canrenoate, and eplerenone, which are prone to cause hyperkalemia, frequently resulting in therapy discontinuation.62 A group of non-steroidal MRAs with an improved safety profile is currently in the preclinical development, including esaxerenone, finerenone, aparenone, PF-03882845, SM-368229, DSR-71167, DSR-30192, LY2623091, AZD9977, KBP-5074, and BR-4628.43 Finerenone, as the first selective nonsteroidal MRA with favorable effects on cardiorenal outcomes in CKD patients with severely elevated albuminuria and type 2 diabetes,63,64 has been approved for medical use in the United States, and under-reviewed by the European Union and China (Table 2). In addition, esaxerenone, another selective nonsteroidal MRA, has recently approved in Japan for clinical treatment of hypertension. Increasing evidence demonstrates that esaxerenone also has renal beneficial effect in patients with type 2 diabetes mellitus and microalbuminuria, supporting future clinical development of esaxerenone for renal disease treatment.65,66 Taken together, MR represents a promising target for the treatment of CKD with various etiologies.
Liver X receptors (LXRs)
There are two subtypes of LXRs, LXRα (NR1H3) and LXRβ (NR1H2). Despite of sharing more than 78% homology in amino acid sequence, LXRα and LXRβ have different tissue distribution patterns. LXRα is highly expressed in metabolically active tissues, including the liver, intestine, kidney, adipose and macrophages, while LXRβ is ubiquitously expressed. LXRs are activated by the binding of either endogenous ligands including oxysterols and bridge sterols, or synthetic agonists such as GW3965, LXR623 and TO901317. To date, it remains unclear where LXRα and LXRβ are localized in the kidney. A recent study using single-cell RNA-sequencing technique has reported that LXRα is predominantly expressed in the proximal tubules, while LXRβ is ubiquitously present in all renal tubules (Table 1).67 It has also been reported that activation of LXRs in renal proximal straight tubules regulates fatty acid desaturation by increasing stearoyl-CoA desaturase (SCD1) expression, contributing to lipid homeostasis in this nephron segment.68 As an intracellular sterol sensor controlling the genes involved in cholesterol absorption, excretion, catabolism, and efflux, LXRα promotes cholesterol efflux via the ATP binding cassette subfamily A member 1 (ABCA1) in glomerular mesangial cells, serving as a potential therapeutic target for lipid-laden renal glomerular diseases.69 LXRβ has been reported as a key regulator of body water homeostasis. Deletion of LXRβ gene led to impaired capacity of urine concentration, reduced renal aquaporin 1 (AQP1) expression, decreased hypothalamic vasopressin-positive neurons, and down-regulated urinary AVP excretion in mice.70 Moreover, LXRβ increases aquaporin 2 (AQP2) protein levels in the kidney by the suppression of AQP2 ubiquitination.71 Consistently, LXRβ is associated with water homeostasis in other tissues and cell types by modulating the expression of water channels.72,73 In addition, LXRs have also been found to regulate the expression of sodium-phosphate (NaPi) transporter, organic anion transporter 1 (OAT1) and epithelial sodium channel (ENaC).74, 75, 76 These findings demonstrate that LXRα is essential for renal lipid metabolism, while LXRβ is critical in water and electrolyte homeostasis.
In addition to their important role in renal physiology, LXRs are also involved in the pathogenesis of multiple kidney diseases. It has been reported that the LXR agonist TO901317 protects against acute kidney injury (AKI) induced by cisplatin via suppressing inflammation and oxidative stress in mice.77 Although it remains unknown whether LXR activation is beneficial in renal ischemic AKI, LXR agonists showed protective role in I/R injury in central nervous system and myocardium.78 In mice, knockout of LXRα and LXRβ (LXRα/β−/−) led to a ten-fold increase of the albumin/creatinine ratio and a 40-fold increase in glomerular lipid accumulation compared to the wild-type mice. After the induction of diabetes by STZ, LXRα/β−/− mice exhibited accelerated mesangial matrix expansion and glomerular lipid accumulation with upregulated markers of inflammation and oxidative stress.79 In addition, the LXR agonist N, N-dimethyl-hydroxycholenamide (DMHCA) also significantly decreased albumin and nephrin excretion, glomerular lipids and plasma triacylglycerol and cholesterol, and alleviated renal inflammation and oxidative stress in STZ-induced diabetic mice.79 LXR agonist GW3965 has been shown to protect against DN by down-regulating proinflammatory and profibrotic cytokines, inhibiting renal lipid accumulation and reactive oxygen species in the LDLR-deficient hyperlipidemic and STZ-induced diabetic mice.80 Paradoxically, LXR activation by TO901317 has also been reported to accelerate podocyte loss and worsen albuminuria with a significant reduction in autophagy activity of renal podocytes in STZ-induced diabetic mice, suggesting LXR activation may be harmful for DN in type 1 diabetes.81 Collectively, LXRs play an important role in the regulation of renal lipid metabolism and the maintenance of water and electrolyte homeostasis. Although activation of LXRs may have beneficial effect on cisplatin-associated AKI, its therapeutic implication in DN is uncertain and requires further investigation.
Farnesoid X receptor (FXR)
FXR (NR1H4) is a major regulator in the synthesis, transport and reabsorption of bile acid metabolism, and also involved in the metabolism of carbohydrates and lipids.82 Although FXR is mainly distributed in the liver and small intestine, the kidney also has abundant FXR expression in most of renal tubule segments.83 The natural agonists for FXR are bile acids (BAs), of which the most potent one is the primary BA chenodeoxycholic acid (CDCA).84 Due to the unspecific binding of most bile acids to FXR, a lot of efforts have been undertaken to discover and develop more specific FXR agonists. Obeticholic acid (OCA, INT-747), a synthetic derivative of CDCA, is one of the most famous FXR specific modulator which has already been approved as a drug for primary biliary cholangitis (PBC) and is currently being investigated in clinical trials for other diseases especially nonalcoholic fatty liver disease (NAFLD).85 The most potent synthetic FXR agonist is GW4064 which contains a stilbene structure harboring the risk for toxic side effects, and the bioavailability of GW4064 is very low with a very short half-life.86 Our previous study reported that FXR exerts a vital effect on the regulation of urinary concentrating capacity mainly via transcriptionally up-regulating AQP2 in the renal medullary collecting ducts (MCDs).83 FXR is also critical for the survival of MCDs under hypertonic stress via increasing the expression and nuclear translocation of tonicity response enhancer binding protein (TonEBP).87 Moreover, crystallin zeta might be another factor mediating the attenuation of hypertonicity-induced cell death by FXR activation.88
FXR also plays a critical role in protecting the kidney from acute and chronic injuries. OCA has been shown to significantly improve the severity of ischemic renal damage and delay the progression of AKI to CKD in mice with renal I/R injury by regulating renal autophagy and apoptosis.89,90 OCA also showed inhibitory effects on renal inflammation and oxidative stress in LPS-induced AKI via blocking the nuclear translocation of NF-κB p65 and p50 subunits in tubular epithelial cells of renal cortex, attenuating LPS-induced renal glutathione depletion, lipid peroxidation and protein nitration, and reducing the up-regulated NADPH oxidase and iNOS gene expression.91 In a STZ-induced type 1 diabetes mellitus animal model, FXR gene knockout markedly worsened DN, while treatment with OCA attenuated proteinuria, glomerulosclerosis, and tubulointerstitial fibrosis, by lowering renal lipid accumulation, macrophage infiltration, and renal expression of sterol-regulatory element binding proteins (SREBPs), pro-fibrotic growth factors, and oxidative stress enzymes.92 In animal models of type 2 diabetes, OCA and GW4064 were effective in preventing progression of DN by ameliorating proteinuria, podocyte injury, pro-fibrotic and pro-inflammatory gene expression, and the proliferation of mesangial cells.93, 94, 95 In addition, OCA treatment has been reported to have favorable effects on age-related kidney disease through preventing the up-regulation of proinflammatory tumor necrosis factor-α (TNF-α), toll-like receptor 2 (TLR2), and TLR4. FXR activation by CDCA was also found to alleviate renal fibrosis and down-regulated the SMAD family member 3 (Smad3) expression in UUO mice.96 Consistently, a recent study has reported that a novel FXR agonist EDP-305 can reduce UUO-induced interstitial renal fibrosis by decreasing transforming growth factor beta 1 (TGF-β1)-induced nuclear localization of yes-associated protein (YAP) via increasing inhibitory YAP phosphorylation.97 Collectively, these data demonstrate a critical role of FXR in renal physiology and a therapeutic potential of FXR agonists in the treatment of acute and chronic renal diseases with different etiology.
Pregnane X receptor (PXR)
PXR (NR1I2) has been generally considered as an important xenobiotic receptor regulating the expression of genes encoding drug-metabolizing enzymes and drug transporters to detoxify and eliminate xenobiotics and endotoxins from the body. Both endobiotic and xenobiotic chemical compounds can activate PXR. In addition to pregnane, steroid, bile acids and other endobiotic chemicals, various clinically used drugs and environmental pollutants are able to activate PXR.98 It is worth mentioning that several compounds and drugs have been recently identified as selective PXR agonists, including lathyrane diterpenoids, garcinoic acid, calcium channel blocker felodipine, anti-cancer drug dabrafenib, atypical antipsychotics quetiapine and etc.99,100 Unlike most of other NRs, PXR activation is species-specific due to the distinction of its LBD. In scientific field, pregnenolone-16a-carbonitrile (PCN) is a specific agonist used for activating mouse PXR, while rifampicin represents a selective agonist for human PXR. In spite of high expression in the liver and gastrointestinal tract, PXR is broadly distributed at a low level in many other tissues including the kidney, hypothalamus, spinal cord, heart, bone marrow, and adrenal gland.101 Within mouse kidney, PXR protein is located in almost all the segments of renal tubules, with the highest expression in the proximal tubules and thick ascending limbs (TAL) of the loop of Henle.102 The intrarenal distribution pattern of PXR indicates a vital role of PXR in the regulation of renal physiological processes and the development of kidney diseases. A recent study has reported that PXR targets aldo-keto reductase family 1, member B7 (AKR1B7) to preserve mitochondrial metabolism and renal function in rat models of cisplatin-induced AKI and I/R-associated AKI.103 The protective effect of PXR in AKI was also confirmed in a mouse model of cisplatin-induced AKI, in which the beneficial renal effect of the PXR activation is achieved via a PI3K/AKT-dependent manner, leading to amelioration of renal tubular apoptosis, oxidative and ER stress, and inflammatory gene expression.102 Importantly, it has been recently reported that PXR is robustly down-regulated and negatively correlated with renal dysfunction in human kidneys with AKI and is significantly up-regulated in renal biopsy samples from CKD patients (including two patients with DN).104 These observations suggest that PXR may also participate in the development of human AKI and CKD and might represent as a target for potential novel therapies.
Vitamin D receptor (VDR)
As a central regulator in controlling serum calcium and phosphorus homeostasis, vitamin D (VD) exerts vital functions by binding to the nuclear receptor VDR (NR1I1). Recent studies have revealed that, other than VD, polyunsaturated fatty acids, lithocholic acid, curcumin, and gamma-tocotrienol can also activate VDR.105 VDR is widely expressed in diverse organs and tissues. Immunohistochemical studies have shown that VDR is localized in several renal regions including glomerular parietal epithelial cells, podocytes, mesangial cells, proximal and distal tubular epithelial cells, and collecting duct cells.106 Dysfunction of VDR is closely associated with a variety of kidney diseases, including AKI and DN. VDR activation by paricalcitol, an active low-calcemic third-generation VD analog (Table 2), exerts renoprotective effect on AKI induced by I/R, cisplatin, LPS, gentamicin and contrast.107, 108, 109, 110
The VD/VDR signaling pathway has also been recognized as an important modulator in the progression of DN. In a mouse model of STZ-induced type 1 diabetes, VDR-null mice exhibited significantly increased albuminuria and glomerular podocyte loss compared to WT mice possibly due to the activation of renin-angiotensin system (RAS),111 suggesting VDR activation may protect DN in diabetic animals. In line with this finding, in both type 1 and type 2 diabetic rodents, paricalcitol or calcitriol in combination with the ARB losartan or renin inhibitor aliskiren effectively ameliorated albuminuria and renal fibrosis.112, 113, 114 In diabetic patients, increasing evidences have demonstrated that VD and multiple VDR activators possess a variety of renoprotective effects via their antiproteinuria, antifibrosis and anti-inflammatory actions and preventing podocyte damage.115 In patients with DN, VD supplements including calcitriol, alfacalcidol and 1,25 (OH)2VitD3 showed beneficial effects on 24 h urine protein and inflammation indexes.116 It has also been reported that high-dose VD supplementation is associated with albuminuria reduction and amelioration of renal injury in type 1 diabetes. In a large-scale randomized controlled study, patients with both type 2 diabetes mellitus and DN treated with paricalcitol in combination with the angiotensin-converting enzyme inhibitors or angiotensin receptor blockers achieved a sustained reduction in residual albuminuria excretion.117 In the kidneys of patients with IgA nephropathy (IgAN), decreased calcitriol production and increased VD degradation were both evident. It has been reported that low VD level is remarkably associated with increased risk of renal progression and poorer outcome in IgAN.118 In mice with diet-induced obesity, the VDR agonist doxercalciferol (1α-hydroxyvitamin D2) improved renal manifestations including proteinuria, podocyte injury, mesangial expansion, and extracellular matrix protein accumulation.119 In a rat UUO model, paricalcitol showed a renoprotective effect by activating VDR, preventing fibrosis and decreasing renal cell apoptosis in the proximal tubules and collecting ducts.120 Altogether, VD, VD analogues and VDR agonists possess promising therapeutic actions against multiple kidney diseases especially DN.
Glucocorticoid receptor (GR)
GR (NR3C1) has two main variants: α and β. Unlike GRα, the GRβ splice variant does not bind to glucocorticoid, but is constitutively present in the nucleus where it acts as a natural dominant negative inhibitor of the GRα subtype at many glucocorticoid response target genes. GR is involved in many key physiological processes including development, cell growth and differentiation, glucose and lipid metabolism and inflammation via binding to its internal ligands glucocorticoids (GCs) secreted from the adrenal gland. Synthetic GCs include steroid hormones and non-steroid hormones such as dexamethasone (DEX), betamethasone, prednisolone and mifepristone (RU486) to activate or block GR.121 GR is expressed along the whole nephron and collecting duct system especially in the medullary loop (Table 1), and widely found in most renal cell types. The physiology and pharmacology of GCs and the GRs have been reviewed extensively elsewhere.122 GCs have long been used to treat glomerulonephritis and idiopathic nephrotic syndrome based on their immunosuppressive actions. They are usually effective in preserving podocyte function and reducing proteinuria.123 It has been reported that the key components of GR complex including the GR, heat shock protein 90, and the immunophilins FKBP51 and FKBP52 are expressed in rat glomerular podocytes, where the signaling pathway of GR can be activated by DEX treatment.124 GCs, as a mainstay of therapy in podocytopathy, were found to perform its cytoprotective action via stabilizing actin filament in podocytes.125 It was reported that the podocyte-specific GR gene knockout mice had comparable renal function to wild type. However, DEX treatment can recover damaged podocyte cytoskeleton in wild type mice caused by LPS but not in podocyte-specific GR-null mice.126 A recent study, integrating data from transcriptome, transcription factor binding, histone modification, and genome topology, has revealed that GR in podocyte was enriched at transcriptional interaction hubs and super-enhancers.127
GR in glomerular endothelial cells also possesses a protective role in DN. In a STZ-induced DN model, compared to control mice, mice lacking endothelial glucocorticoid receptor (GR) had more severe renal fibrosis via stimulating Wnt signaling-dependent epithelial-to-mesenchymal transition (EMT) in tubular epithelial cells,128 suggesting endothelial GR helps prevent the development of DN. In contrast, compared to control mice, the Pax8-Cre/GRfl/fl mice with inactivation of GR specifically in renal epithelial cells attenuated albuminuria and cellular crescent formation after the induction of crescentic nephritis.129 Renal GR can also activate the circadian rhythm of sodium chloride cotransporter (NCC) and prevent its phosphorylation, which has a profound impact on the diurnal dynamic balance of blood pressure.130 Hydrocortisone can protect the kidneys and inhibit the activity of NF-κB by increasing the expression of GRα in the kidneys of rats with sepsis.131 In addition, GR blockers were capable of restoring the renal antioxidant barrier by inhibiting the activities of adenosine deaminase and xanthine oxidase, implicating the involvements of GR in renal dysfunctions caused by combined oral contraceptive via disrupted glutathione antioxidative barrier.
Currently, glucocorticoid drugs are widely used in clinical practice for the treatment of various renal diseases such as nephrotic syndrome, IgA nephropathy, membranoproliferative glomerulonephritis, membranous nephropathy and anti-glomerular basement membrane disease. Both agonistic and antagonistic effects of GRs may be beneficial in certain renal disorders. Therefore, cell type specific role of GR needs to be further clarified and targeted delivery of glucocorticoid drugs may provide a new approach to clinical practice.
Estrogen receptors (ERs)
The cellular effects of estrogen are mainly mediated by estrogen receptors (ERs), ERα (NR3A1) and ERβ (NR3A2). Abnormal estrogen signaling can lead to different types of pathological conditions, such as cancer and metabolic diseases. ERα and ERβ are both expressed in the kidney. Estrogen contributes to blood pressure regulation, electrolyte balance and water homeostasis in the kidney.132 Numerous studies have revealed that ERs participate in the development of kidney diseases such as AKI, IgAN, and CKD, which were elegantly reviewed recently.133 Although estrogen exhibits renal protection in animal models of AKI, the dose required for renoprotective effect is beyond the physiological level.134 In diabetic patients and animals, ER activation reduces the progression of DN by alleviating glomerulosclerosis and fibrosis, promoting cell survival and preventing podocyte loss.135,136 On contrary, increased serum phytoestrogens have been reported in DN patients, which may contribute to negative effect on the development of diabetic renal and cardiovascular complications.137 In an UUO-induced tubulointerstitial fibrosis mouse model, tamoxifen was found to improve renal injury by the suppression of renal fibroblast activation via the modulation of ERα-mediated renal TGF-β1/Smad signaling pathway.138
Conclusion
As a major social health problem, kidney disease has high morbidity and mortality, and also leads to a substantial increased risk of other diseases especially cardiovascular disease. Coordination of scientific and public health efforts for renal disease appears to be the most efficient strategy for the prevention and treatment of this devastating disease. A large body of evidence has clearly demonstrated that modulation of the NRs discussed in this review contributes to the alleviation or even prevention of kidney diseases. Numerous pharmacological approaches specifically targeting these NRs have been proved to be beneficial in slowing renal functional decline in preclinical studies or clinical trials, with some of the NR agonists and antagonists successfully used for the treatment of kidney diseases (Table 2). The increased understanding of the role of each NR and the NR network in renal health and disease will enable us to identify novel therapeutic targets for the development of more efficient medicine, ultimately curing both acute and chronic kidney diseases.
Outstanding questions
As the largest group of transcription factors, NRs serve as drug targets for various diseases, and multiple modulators of NRs including GR, MR, ER, VDR, PPARα, PPARg, and FXR have been successfully developed as therapeutic agents. Accumulating evidence has clearly indicated that some other NRs such as LXRα and LXRb discussed in this review also contribute to the kidney homeostasis and disease development. However, due to the length limitation of the paper and the lack of solid experimental evidence, many other NRs such as estrogen related receptors (ERRs), retinoid X receptors (RXRs), RAR-related orphan receptors (RORs) and Rev-Erb-α/b, which are constitutively expressed at high level in the kidney, were not discussed in this review (Table 1). More intensive basic research is required for characterizing the roles of these NRs in renal physiology and pathophysiology. The agonists or antagonists of these receptors might also have the potential to protect against renal diseases. Furthermore, it is of great significance to identify and develop novel agonists and antagonists of NRs with fewer side-effects, which may further benefit the prevention, treatment and prognosis of kidney diseases.
Declaration of interests
The authors declare that they have no competing interests.
Acknowledgments
Search strategy and selection criteria
Data for this review were identified through searches in PubMed using the following search terms: “Nuclear receptors”, “Kidney”, “Peroxisome proliferator-activated receptors (PPARs)”, “Mineralocorticoid receptor (MR)”, “Liver X receptors (LXRs)”, “Farnesoid X receptor (FXR)”, “Pregnane X receptor (PXR)”, “Vitamin D receptor (VDR)”, “Glucocorticoid receptor (GR)”, and “Estrogen receptors (ERs)”. They were selected based on their relevance to the topic. Only articles published in English between 1995 and 2021 were included.
Contributors
Z. L.: literature search, writing - original draft, writing – review & editing
C. Z.: literature search, writing - original draft, writing – review & editing
W. M.: literature search, writing - original draft
Y. H.: literature search, writing - original draft
X. Z.: conceptualization, writing - review & editing
Y. G.: conceptualization, literature search, writing - review & editing
All authors have read and approved the final version of the manuscript.
Acknowledgment
This work was supported by the National Key R&D Program of China 2020YFC2005000, National Natural Science Foundation of China Grants 81970606 (to X.Z.), 81970595 (to Y.G.), Education Department of Liaoning Province, China LZ2020023 (to Z.L.), and the Dalian Young Star of Science and Technology 2019RQ116 (to Z.L.). We are also grateful for the support from Liaoning BaiQianWan Talents Program. The funders had no roles in paper design, data collection, data analysis, interpretation, and writing of the paper.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.103855.
Contributor Information
You-Fei Guan, Email: guanyf@dmu.edu.cn.
Xiao-Yan Zhang, Email: xyzhang@hsc.ecnu.edu.cn.
Appendix. Supplementary materials
Reference
- 1.Kurzhagen J.T., Dellepiane S., Cantaluppi V., Rabb H. AKI: an increasingly recognized risk factor for CKD development and progression. J Nephrol. 2020;33(6):1171–1187. doi: 10.1007/s40620-020-00793-2. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz-Ortega M., Rayego-Mateos S., Lamas S., Ortiz A., Rodrigues-Diez R.R. Targeting the progression of chronic kidney disease. Nat Rev Nephrol. 2020;16(5):269–288. doi: 10.1038/s41581-019-0248-y. [DOI] [PubMed] [Google Scholar]
- 3.Gustafsson JA. Historical overview of nuclear receptors. J Steroid Biochem Mol Biol. 2016;157:3–6. doi: 10.1016/j.jsbmb.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Mangelsdorf D.J., Thummel C., Beato M., et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weikum E.R., Liu X., Ortlund EA. The nuclear receptor superfamily: a structural perspective. Protein Sci. 2018;27(11):1876–1892. doi: 10.1002/pro.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubois V., Eeckhoute J., Lefebvre P., Staels B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J Clin Invest. 2017;127(4):1202–1214. doi: 10.1172/JCI88894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libby A.E., Jones B., Lopez-Santiago I., Rowland E., Levi M. Nuclear receptors in the kidney during health and disease. Mol Aspects Med. 2021;78 doi: 10.1016/j.mam.2020.100935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan Y., Breyer M.D. Peroxisome proliferator-activated receptors (PPARs): novel therapeutic targets in renal disease. Kidney Int. 2001;60(1):14–30. doi: 10.1046/j.1523-1755.2001.00766.x. [DOI] [PubMed] [Google Scholar]
- 9.Ruan X., Zheng F., Guan Y. PPARs and the kidney in metabolic syndrome. Am J Physiol Ren Physiol. 2008;294(5):F1032–F1047. doi: 10.1152/ajprenal.00152.2007. [DOI] [PubMed] [Google Scholar]
- 10.Guan Y., Zhang Y., Davis L., Breyer M.D. Expression of peroxisome proliferator-activated receptors in urinary tract of rabbits and humans. Am J Physiol. 1997;273(6):F1013–F1022. doi: 10.1152/ajprenal.1997.273.6.F1013. [DOI] [PubMed] [Google Scholar]
- 11.Kono K., Kamijo Y., Hora K., et al. PPAR{alpha} attenuates the proinflammatory response in activated mesangial cells. Am J Physiol Ren Physiol. 2009;296(2):F328–F336. doi: 10.1152/ajprenal.00484.2007. [DOI] [PubMed] [Google Scholar]
- 12.Ouali F., Djouadi F., Merlet-Bénichou C., Bastin J. Dietary lipids regulate beta-oxidation enzyme gene expression in the developing rat kidney. Am J Physiol. 1998;275(5):F777–F784. doi: 10.1152/ajprenal.1998.275.5.F777. [DOI] [PubMed] [Google Scholar]
- 13.Portilla D., Dai G., Peters J.M., Gonzalez F.J., Crew M.D., Proia A.D. Etomoxir-induced PPARalpha-modulated enzymes protect during acute renal failure. Am J Physiol Ren Physiol. 2000;278(4):F667–F675. doi: 10.1152/ajprenal.2000.278.4.F667. [DOI] [PubMed] [Google Scholar]
- 14.Sivarajah A., Chatterjee P.K., Hattori Y., et al. Agonists of peroxisome-proliferator activated receptor-alpha (clofibrate and WY14643) reduce renal ischemia/reperfusion injury in the rat. Med Sci Monit Int Med J Exp Clin Res. 2002;8(12):Br532–Br539. [PubMed] [Google Scholar]
- 15.Abdel-Razek E.A., Abo-Youssef A.M., Azouz A.A. Benzbromarone mitigates cisplatin nephrotoxicity involving enhanced peroxisome proliferator-activated receptor-alpha (PPAR-α) expression. Life Sci. 2020;243 doi: 10.1016/j.lfs.2020.117272. [DOI] [PubMed] [Google Scholar]
- 16.Freitas-Lima L.C., Budu A., Arruda A.C., et al. PPAR-α deletion attenuates cisplatin nephrotoxicity by modulating renal organic transporters MATE-1 and OCT-2. Int J Mol Sci. 2020;21(19) doi: 10.3390/ijms21197416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y., Kong X., Zhao P., et al. Peroxisome proliferator-activated receptor-alpha is renoprotective in doxorubicin-induced glomerular injury. Kidney Int. 2011;79(12):1302–1311. doi: 10.1038/ki.2011.17. [DOI] [PubMed] [Google Scholar]
- 18.Yu J.T., Hu X.W., Chen H.Y., et al. DNA methylation of FTO promotes renal inflammation by enhancing m(6)A of PPAR-α in alcohol-induced kidney injury. Pharmacol Res. 2021;163 doi: 10.1016/j.phrs.2020.105286. [DOI] [PubMed] [Google Scholar]
- 19.Park C.W., Zhang Y., Zhang X., et al. PPARalpha agonist fenofibrate improves diabetic nephropathy in db/db mice. Kidney Int. 2006;69(9):1511–1517. doi: 10.1038/sj.ki.5000209. [DOI] [PubMed] [Google Scholar]
- 20.Feng X., Gao X., Wang S., et al. PPAR-α agonist fenofibrate prevented diabetic nephropathy by inhibiting M1 macrophages via improving endothelial cell function in db/db mice. Front Med. 2021;8 doi: 10.3389/fmed.2021.652558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jao T.M., Nangaku M., Wu C.H., et al. ATF6α downregulation of PPARα promotes lipotoxicity-induced tubulointerstitial fibrosis. Kidney Int. 2019;95(3):577–589. doi: 10.1016/j.kint.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 22.Chung K.W., Lee E.K., Lee M.K., Oh G.T., Yu B.P., Chung H.Y. Impairment of PPARα and the fatty acid oxidation pathway aggravates renal fibrosis during aging. J Am Soc Nephrol JASN. 2018;29(4):1223–1237. doi: 10.1681/ASN.2017070802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gross B., Pawlak M., Lefebvre P., Staels B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat Rev Endocrinol. 2017;13(1):36–49. doi: 10.1038/nrendo.2016.135. [DOI] [PubMed] [Google Scholar]
- 24.Kirkby N.S., Sampaio W., Etelvino G. Cyclooxygenase-2 selectively controls renal blood flow through a novel PPARbeta/delta-dependent vasodilator pathway. Hypertension. 2018;71(2):297–305. doi: 10.1161/HYPERTENSIONAHA.117.09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Letavernier E., Perez J., Joye E., et al. Peroxisome proliferator-activated receptor beta/delta exerts a strong protection from ischemic acute renal failure. J Am Soc Nephrol JASN. 2005;16(8):2395–2402. doi: 10.1681/ASN.2004090802. [DOI] [PubMed] [Google Scholar]
- 26.Collino M., Benetti E., Miglio G., et al. Peroxisome proliferator-activated receptor β/δ agonism protects the kidney against ischemia/reperfusion injury in diabetic rats. Free Radic Biol Med. 2011;50(2):345–353. doi: 10.1016/j.freeradbiomed.2010.10.710. [DOI] [PubMed] [Google Scholar]
- 27.Matsushita Y., Ogawa D., Wada J., et al. Activation of peroxisome proliferator-activated receptor delta inhibits streptozotocin-induced diabetic nephropathy through anti-inflammatory mechanisms in mice. Diabetes. 2011;60(3):960–968. doi: 10.2337/db10-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu Y., Qi C., Korenberg J.R., et al. Structural organization of mouse peroxisome proliferator-activated receptor gamma (mPPAR gamma) gene: alternative promoter use and different splicing yield two mPPAR gamma isoforms. Proc Natl Acad Sci USA. 1995;92(17):7921–7925. doi: 10.1073/pnas.92.17.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tontonoz P., Hu E., Graves R.A., Budavari A.I., Spiegelman B.M. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8(10):1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 30.Wafer R., Tandon P., Minchin JEN. The role of peroxisome proliferator-activated receptor gamma (PPARG) in adipogenesis: applying Knowledge from the fish aquaculture industry to biomedical research. Front Endocrinol. 2017;8:102. doi: 10.3389/fendo.2017.00102. (Lausanne) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ristow M., Muller-Wieland D., Pfeiffer A., Krone W., Kahn C.R. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. N Engl J Med. 1998;339(14):953–959. doi: 10.1056/NEJM199810013391403. [DOI] [PubMed] [Google Scholar]
- 32.Gray S.L., Dalla Nora E., Vidal-Puig A.J. Mouse models of PPAR-gamma deficiency: dissecting PPAR-gamma's role in metabolic homoeostasis. Biochem Soc Trans. 2005;33(Pt 5):1053–1058. doi: 10.1042/BST0331053. [DOI] [PubMed] [Google Scholar]
- 33.Guan Y., Hao C., Cha D.R., et al. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med. 2005;11(8):861–866. doi: 10.1038/nm1278. [DOI] [PubMed] [Google Scholar]
- 34.Yang J., Zhou Y., Guan Y. PPARgamma as a therapeutic target in diabetic nephropathy and other renal diseases. Curr Opin Nephrol Hypertens. 2012;21(1):97–105. doi: 10.1097/MNH.0b013e32834de526. [DOI] [PubMed] [Google Scholar]
- 35.Zou G., Zhou Z., Xi X., Huang R., Hu H. Pioglitazone ameliorates renal ischemia-reperfusion injury via inhibition of NF-κB Activation and Inflammation in Rats. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.707344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J., Zhao N., Shi G., Wang H. Geniposide ameliorated sepsis-induced acute kidney injury by activating PPARγ. Aging. 2020;12(22):22744–22758. doi: 10.18632/aging.103902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li S., Shi M., Wan Y., et al. Inflammasome/NF-κB translocation inhibition via PPARγ agonist mitigates inorganic mercury induced nephrotoxicity. Ecotoxicol Environ Saf. 2020;201 doi: 10.1016/j.ecoenv.2020.110801. [DOI] [PubMed] [Google Scholar]
- 38.Korolczuk A., Maciejewski M., Smolen A., Dudka J., Czechowska G., Widelska I. The role of peroxisome-proliferator-activating receptor gamma agonists: rosiglitazone and 15-deoxy-delta12,14-prostaglandin J2 in chronic experimental cyclosporine A-induced nephrotoxicity. J Physiol Pharmacol. 2014;65(6):867–876. an official journal of the Polish physiological society. [PubMed] [Google Scholar]
- 39.Wang X., Deng J., Xiong C., et al. Treatment with a PPAR-γ agonist protects against hyperuricemic nephropathy in a rat model. Drug Des Dev Ther. 2020;14:2221–2233. doi: 10.2147/DDDT.S247091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z.H., He J.Q., Zhao Y.Y., Chen H.C., Tan NH. Asiatic acid prevents renal fibrosis in UUO rats via promoting the production of 15d-PGJ2, an endogenous ligand of PPAR-γ. Acta Pharmacol Sin. 2020;41(3):373–382. doi: 10.1038/s41401-019-0319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei S., Xu C., Zhang Y., Shi Z., Wu M., Yang B. Ultrasound assisted a peroxisome proliferator-activated receptor (PPAR)γ agonist-loaded nanoparticle-microbubble complex to attenuate renal interstitial fibrosis. Int J Nanomed. 2020;15:7315–7327. doi: 10.2147/IJN.S262052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barrera-Chimal J., Jaisser F. Vascular and inflammatory mineralocorticoid receptors in kidney disease. Acta Physiol. 2020;228(2):e13390. doi: 10.1111/apha.13390. (Oxford, England) [DOI] [PubMed] [Google Scholar]
- 43.Patel V., Joharapurkar A., Jain M. Role of mineralocorticoid receptor antagonists in kidney diseases. Drug Dev Res. 2021;82(3):341–363. doi: 10.1002/ddr.21760. [DOI] [PubMed] [Google Scholar]
- 44.Martín-Fernández B., Rubio-Navarro A., Cortegano I., et al. Aldosterone induces renal fibrosis and inflammatory M1-macrophage subtype via mineralocorticoid receptor in rats. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0145946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zitt E., Eller K., Huber J.M., et al. The selective mineralocorticoid receptor antagonist eplerenone is protective in mild anti-GBM glomeru-lonephritis. Int J Clin Exp Pathol. 2011;4(6):606–615. [PMC free article] [PubMed] [Google Scholar]
- 46.Barrera-Chimal J., Prince S., Fadel F., et al. Sulfenic acid modification of endothelin B receptor is responsible for the benefit of a nonsteroidal mineralocorticoid receptor antagonist in renal ischemia. J Am Soc Nephrol JASN. 2016;27(2):398–404. doi: 10.1681/ASN.2014121216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okui S., Yamamoto H., Li W., et al. Cisplatin-induced acute renal failure in mice is mediated by chymase-activated angiotensin-aldosterone system and interleukin-18. Eur J Pharmacol. 2012;685(1-3):149–155. doi: 10.1016/j.ejphar.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 48.Elseweidy M.M., Askar M.E., Elswefy S.E., Shawky M. Nephrotoxicity induced by cisplatin intake in experimental rats and therapeutic approach of using mesenchymal stem cells and spironolactone. Appl Biochem Biotechnol. 2018;184(4):1390–1403. doi: 10.1007/s12010-017-2631-0. [DOI] [PubMed] [Google Scholar]
- 49.Nielsen F.T., Jensen B.L., Hansen P.B., Marcussen N., Bie P. The mineralocorticoid receptor antagonist eplerenone reduces renal interstitial fibrosis after long-term cyclosporine treatment in rat: antagonizing cyclosporine nephrotoxicity. BMC Nephrol. 2013;14:42. doi: 10.1186/1471-2369-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ali B.H., Al-Qarawi A.A., Mahmoud O.M., Hashad M. Influence of spironolactone treatment on gentamicin-induced nephrotoxicity in rats. Basic Clin Pharmacol Toxicol. 2004;95(1):20–23. doi: 10.1111/j.1742-7843.2004.pto950105.x. [DOI] [PubMed] [Google Scholar]
- 51.Lattenist L., Lechner S.M., Messaoudi S., et al. Nonsteroidal mineralocorticoid receptor antagonist finerenone protects against acute kidney injury-mediated chronic kidney disease: role of oxidative stress. Hypertension. 2017;69(5):870–878. doi: 10.1161/HYPERTENSIONAHA.116.08526. (Dallas, Tex : 1979) [DOI] [PubMed] [Google Scholar]
- 52.Barrera-Chimal J., Rocha L., Amador-Martínez I., et al. Delayed spironolactone administration prevents the transition from acute kidney injury to chronic kidney disease through improving renal inflammation. Nephrol Dial Transplant. 2019;34(5):794–801. doi: 10.1093/ndt/gfy246. official publication of the European dialysis and transplant association - European renal association. [DOI] [PubMed] [Google Scholar]
- 53.Lian M., Hewitson T.D., Wigg B., Samuel C.S., Chow F., Becker GJ. Long-term mineralocorticoid receptor blockade ameliorates progression of experimental diabetic renal disease. Nephrol Dial Transplant. 2012;27(3):906–912. doi: 10.1093/ndt/gfr495. official publication of the European dialysis and transplant association - European renal association. [DOI] [PubMed] [Google Scholar]
- 54.Molina-Jijón E., Rodríguez-Muñoz R., González-Ramírez R., Namorado-Tónix C., Pedraza-Chaverri J., Reyes J.L. Aldosterone signaling regulates the over-expression of claudin-4 and -8 at the distal nephron from type 1 diabetic rats. PLoS One. 2017;12(5) doi: 10.1371/journal.pone.0177362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin S., Li D., Jia J., Zheng Z., Jia Z., Shang W. Spironolactone ameliorates podocytic adhesive capacity via restoring integrin alpha 3 expression in streptozotocin-induced diabetic rats. J Renin Angiotensin Aldosterone Syst JRAAS. 2010;11(3):149–157. doi: 10.1177/1470320310369603. [DOI] [PubMed] [Google Scholar]
- 56.Katayama S., Yamada D., Nakayama M., et al. A randomized controlled study of finerenone versus placebo in Japanese patients with type 2 diabetes mellitus and diabetic nephropathy. J Diabetes Complications. 2017;31(4):758–765. doi: 10.1016/j.jdiacomp.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 57.Ferreira N.S., Bruder-Nascimento T., Pereira C.A., et al. NLRP3 inflammasome and mineralocorticoid receptors are associated with vascular dysfunction in type 2 diabetes mellitus. Cells. 2019;8(12) doi: 10.3390/cells8121595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brem A.S., Morris D.J., Ge Y., Dworkin L., Tolbert E., Gong R. Direct fibrogenic effects of aldosterone on normotensive kidney: an effect modified by 11β-HSD activity. Am J Physiol Ren Physiol. 2010;298(5):F1178–F1187. doi: 10.1152/ajprenal.00532.2009. [DOI] [PubMed] [Google Scholar]
- 59.Kadoya H., Satoh M., Sasaki T., Taniguchi S., Takahashi M., Kashihara N. Excess aldosterone is a critical danger signal for inflammasome activation in the development of renal fibrosis in mice. FASEB J. 2015;29(9):3899–3910. doi: 10.1096/fj.15-271734. official publication of the Federation of American Societies for Experimental Biology. [DOI] [PubMed] [Google Scholar]
- 60.Leader C.J., Kelly D.J., Sammut I.A., Wilkins G.T., Walker R.J. Spironolactone mitigates, but does not reverse, the progression of renal fibrosis in a transgenic hypertensive rat. Physiol Rep. 2020;8(10):e14448. doi: 10.14814/phy2.14448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen H., Sun F., Zhong X., Shao Y., Yoshimura A., Liu Y. Eplerenone-mediated aldosterone blockade prevents renal fibrosis by reducing renal inflammation, interstitial cell proliferation and oxidative stress. Kidney Blood Press Res. 2013;37(6):557–566. doi: 10.1159/000355736. [DOI] [PubMed] [Google Scholar]
- 62.Kintscher U., Bakris G.L., Kolkhof P. Novel non-steroidal mineralocorticoid receptor antagonists in cardiorenal disease. Br J Pharmacol. 2021 doi: 10.1111/bph.15747. [DOI] [PubMed] [Google Scholar]
- 63.Pitt B., Filippatos G., Agarwal R., et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021 doi: 10.1056/NEJMoa2110956. [DOI] [PubMed] [Google Scholar]
- 64.Bakris G.L., Agarwal R., Anker S.D., et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 65.Duggan S. Esaxerenone: first global approval. Drugs. 2019;79(4):477–481. doi: 10.1007/s40265-019-01073-5. [DOI] [PubMed] [Google Scholar]
- 66.Ito S., Kashihara N., Shikata K., et al. Esaxerenone (CS-3150) in patients with type 2 diabetes and microalbuminuria (ESAX-DN): phase 3 randomized controlled clinical trial. Clin J Am Soc Nephrol. 2020;15(12):1715–1727. doi: 10.2215/CJN.06870520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen L., Chou C.L., Knepper M.A. A comprehensive map of mRNAs and their isoforms across all 14 renal tubule segments of mouse. J Am Soc Nephrol. 2021 doi: 10.1681/ASN.2020101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y., Zhang X., Chen L., et al. Liver X receptor agonist TO-901317 upregulates SCD1 expression in renal proximal straight tubule. Am J Physiol Ren Physiol. 2006;290(5):F1065–F1073. doi: 10.1152/ajprenal.00131.2005. [DOI] [PubMed] [Google Scholar]
- 69.Wu J., Zhang Y., Wang N., et al. Liver X receptor-alpha mediates cholesterol efflux in glomerular mesangial cells. Am J Physiol Ren Physiol. 2004;287(5):F886–F895. doi: 10.1152/ajprenal.00123.2004. [DOI] [PubMed] [Google Scholar]
- 70.Gabbi C., Kong X., Suzuki H., et al. Central diabetes insipidus associated with impaired renal aquaporin-1 expression in mice lacking liver X receptor beta. Proc Natl Acad Sci U S A. 2012;109(8):3030–3034. doi: 10.1073/pnas.1200588109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Su W., Huang S.Z., Gao M., et al. Liver X receptor beta increases aquaporin 2 protein level via a posttranscriptional mechanism in renal collecting ducts. Am J Physiol Ren Physiol. 2017;312(4):F619–FF28. doi: 10.1152/ajprenal.00564.2016. [DOI] [PubMed] [Google Scholar]
- 72.Gabbi C., Kim H.J., Hultenby K., et al. Pancreatic exocrine insufficiency in LXRbeta-/- mice is associated with a reduction in aquaporin-1 expression. Proc Natl Acad Sci U S A. 2008;105(39):15052–15057. doi: 10.1073/pnas.0808097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang Y.J., Kim P., Lu Y.F., Feingold KR. PPARgamma activators stimulate aquaporin 3 expression in keratinocytes/epidermis. Exp Dermatol. 2011;20(7):595–599. doi: 10.1111/j.1600-0625.2011.01269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caldas Y.A., Giral H., Cortazar M.A., et al. Liver X receptor-activating ligands modulate renal and intestinal sodium-phosphate transporters. Kidney Int. 2011;80(5):535–544. doi: 10.1038/ki.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kittayaruksakul S., Soodvilai S., Asavapanumas N., Muanprasat C., Chatsudthipong V. Liver X receptor activation downregulates organic anion transporter 1 (OAT1) in the renal proximal tubule. Am J Physiol Ren Physiol. 2012;302(5):F552–F560. doi: 10.1152/ajprenal.00341.2011. [DOI] [PubMed] [Google Scholar]
- 76.Soodvilai S., Jia Z., Fongsupa S., Chatsudthipong V., Yang T. Liver X receptor agonists decrease ENaC-mediated sodium transport in collecting duct cells. Am J Physiol Ren Physiol. 2012;303(12):F1610–F1616. doi: 10.1152/ajprenal.00283.2012. [DOI] [PubMed] [Google Scholar]
- 77.Yang M., Wang R., Sun J., et al. The liver X receptor agonist TO901317 protects mice against cisplatin-induced kidney injury. Exp Biol Med. 2015;240(12):1717–1727. doi: 10.1177/1535370215589906. (Maywood) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen L., Song D., Chen B., Yang X., Cheng O. Activation of liver X receptor promotes hippocampal neurogenesis and improves long-term cognitive function recovery in acute cerebral ischemia-reperfusion mice. J Neurochem. 2020;154(2):205–217. doi: 10.1111/jnc.14890. [DOI] [PubMed] [Google Scholar]
- 79.Patel M., Wang X.X., Magomedova L., et al. Liver X receptors preserve renal glomerular integrity under normoglycaemia and in diabetes in mice. Diabetologia. 2014;57(2):435–446. doi: 10.1007/s00125-013-3095-6. [DOI] [PubMed] [Google Scholar]
- 80.Kiss E., Kranzlin B., Wagenblabeta K., et al. Lipid droplet accumulation is associated with an increase in hyperglycemia-induced renal damage: prevention by liver X receptors. Am J Pathol. 2013;182(3):727–741. doi: 10.1016/j.ajpath.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Z., Tang S., Gui W., et al. Liver X receptor activation induces podocyte injury via inhibiting autophagic activity. J Physiol Biochem. 2020;76(2):317–328. doi: 10.1007/s13105-020-00737-1. [DOI] [PubMed] [Google Scholar]
- 82.Jiang L., Zhang H., Xiao D., Wei H., Chen Y. Farnesoid X receptor (FXR): structures and ligands. Comput Struct Biotechnol J. 2021;19:2148–2159. doi: 10.1016/j.csbj.2021.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang X., Huang S., Gao M., et al. Farnesoid X receptor (FXR) gene deficiency impairs urine concentration in mice. Proc Natl Acad Sci U S A. 2014;111(6):2277–2282. doi: 10.1073/pnas.1323977111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Makishima M., Okamoto A.Y., Repa J.J., et al. Identification of a nuclear receptor for bile acids. Science. 1999;284(5418):1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 85.Nevens F., Andreone P., Mazzella G., et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med. 2016;375(7):631–643. doi: 10.1056/NEJMoa1509840. [DOI] [PubMed] [Google Scholar]
- 86.Maloney P.R., Parks D.J., Haffner C.D., et al. Identification of a chemical tool for the orphan nuclear receptor FXR. J Med Chem. 2000;43(16):2971–2974. doi: 10.1021/jm0002127. [DOI] [PubMed] [Google Scholar]
- 87.Xu S., Huang S., Luan Z., et al. Farnesoid X receptor is essential for the survival of renal medullary collecting duct cells under hypertonic stress. Proc Natl Acad Sci U S A. 2018;115(21):5600–5605. doi: 10.1073/pnas.1803945115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alam G., Luan Z., Gul A., et al. Activation of farnesoid X receptor (FXR) induces crystallin zeta expression in mouse medullary collecting duct cells. Pflug Arch Eur J Physiol. 2020;472(11):1631–1641. doi: 10.1007/s00424-020-02456-4. [DOI] [PubMed] [Google Scholar]
- 89.Gai Z., Chu L., Xu Z., Song X., Sun D., Kullak-Ublick GA. Farnesoid X receptor activation protects the kidney from ischemia-reperfusion damage. Sci Rep. 2017;7(1):9815. doi: 10.1038/s41598-017-10168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim D.H., Park J.S., Choi H.I., et al. The critical role of FXR is associated with the regulation of autophagy and apoptosis in the progression of AKI to CKD. Cell Death Dis. 2021;12(4):320. doi: 10.1038/s41419-021-03620-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu J.B., Xu S., Li J., et al. Farnesoid X receptor agonist obeticholic acid inhibits renal inflammation and oxidative stress during lipopolysaccharide-induced acute kidney injury. Eur J Pharmacol. 2018;838:60–68. doi: 10.1016/j.ejphar.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 92.Wang X.X., Jiang T., Shen Y., et al. Diabetic nephropathy is accelerated by farnesoid X receptor deficiency and inhibited by farnesoid X receptor activation in a type 1 diabetes model. Diabetes. 2010;59(11):2916–2927. doi: 10.2337/db10-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Han S.Y., Song H.K., Cha J.J., Han J.Y., Kang Y.S., Cha DR. Farnesoid X receptor (FXR) agonist ameliorates systemic insulin resistance, dysregulation of lipid metabolism, and alterations of various organs in a type 2 diabetic kidney animal model. Acta Diabetol. 2021;58(4):495–503. doi: 10.1007/s00592-020-01652-z. [DOI] [PubMed] [Google Scholar]
- 94.Wang X.X., Wang D., Luo Y., et al. FXR/TGR5 dual agonist prevents progression of nephropathy in diabetes and obesity. J Am Soc Nephrol JASN. 2018;29(1):118–137. doi: 10.1681/ASN.2017020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou B., Feng B., Qin Z., et al. Activation of farnesoid X receptor downregulates visfatin and attenuates diabetic nephropathy. Mol Cell Endocrinol. 2016;419:72–82. doi: 10.1016/j.mce.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 96.Zhao K., He J., Zhang Y., et al. Activation of FXR protects against renal fibrosis via suppressing Smad3 expression. Sci Rep. 2016;6:37234. doi: 10.1038/srep37234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li S., Ghoshal S., Sojoodi M., et al. The farnesoid X receptor agonist EDP-305 reduces interstitial renal fibrosis in a mouse model of unilateral ureteral obstruction. FASEB J. 2019;33(6):7103–7112. doi: 10.1096/fj.201801699R. official publication of the Federation of American Societies for Experimental Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xing Y., Yan J., Niu Y. PXR: a center of transcriptional regulation in cancer. Acta Pharm Sin B. 2020;10(2):197–206. doi: 10.1016/j.apsb.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang D., Wang R.M., Li W., et al. Lathyrane diterpenoids as novel hPXR agonists: isolation, structural modification, and structure-activity relationships. ACS Med Chem Lett. 2021;12(7):1159–1165. doi: 10.1021/acsmedchemlett.1c00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reddy R.T., Nyunoya T. Identification of novel pregnane X receptor (PXR) agonists by In silico and biological activity analyses and reversal of cigarette smoke-induced PXR downregulation. Biochem Biophys Res Commun. 2021;555:1–6. doi: 10.1016/j.bbrc.2021.02.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carnahan V.E., Redinbo M.R. Structure and function of the human nuclear xenobiotic receptor PXR. Curr Drug Metab. 2005;6(4):357–367. doi: 10.2174/1389200054633844. [DOI] [PubMed] [Google Scholar]
- 102.Luan Z., Wei Y., Huo X., et al. Pregnane X receptor (PXR) protects against cisplatin-induced acute kidney injury in mice. Biochim et Biophys Acta Mol Basis Dis. 2021;1867(3) doi: 10.1016/j.bbadis.2020.165996. [DOI] [PubMed] [Google Scholar]
- 103.Yu X., Xu M., Meng X., et al. Nuclear receptor PXR targets AKR1B7 to protect mitochondrial metabolism and renal function in AKI. Sci Transl Med. 2020;12(543) doi: 10.1126/scitranslmed.aay7591. [DOI] [PubMed] [Google Scholar]
- 104.Velenosi T.J., Feere D.A., Sohi G., Hardy D.B., Urquhart BL. Decreased nuclear receptor activity and epigenetic modulation associates with down-regulation of hepatic drug-metabolizing enzymes in chronic kidney disease. FASEB J. 2014;28(12):5388–5397. doi: 10.1096/fj.14-258780. official publication of the Federation of American Societies for Experimental Biology. [DOI] [PubMed] [Google Scholar]
- 105.Haussler M.R., Whitfield G.K., Kaneko I., et al. Molecular mechanisms of vitamin D action. Calcif Tissue Int. 2013;92(2):77–98. doi: 10.1007/s00223-012-9619-0. [DOI] [PubMed] [Google Scholar]
- 106.Wang X.X., Jiang T., Levi M. Nuclear hormone receptors in diabetic nephropathy. Nat Rev Nephrol. 2010;6(6):342–351. doi: 10.1038/nrneph.2010.56. [DOI] [PubMed] [Google Scholar]
- 107.Lee J.W., Kim S.C., Ko Y.S., et al. Renoprotective effect of paricalcitol via a modulation of the TLR4-NF-kappaB pathway in ischemia/reperfusion-induced acute kidney injury. Biochem Biophys Res Commun. 2014;444(2):121–127. doi: 10.1016/j.bbrc.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 108.Bae E., Kim J.H., Jung M.H., et al. Paricalcitol attenuates contrast-induced acute kidney injury by regulating mitophagy and senescence. Oxid Med Cell Longev. 2020;2020 doi: 10.1155/2020/7627934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hu Z., Zhang H., Yi B., et al. VDR activation attenuate cisplatin induced AKI by inhibiting ferroptosis. Cell Death Dis. 2020;11(1):73. doi: 10.1038/s41419-020-2256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Du J., Jiang S., Hu Z., et al. Vitamin D receptor activation protects against lipopolysaccharide-induced acute kidney injury through suppression of tubular cell apoptosis. Am J Physiol Ren Physiol. 2019;316(5):F1068–F1f77. doi: 10.1152/ajprenal.00332.2018. [DOI] [PubMed] [Google Scholar]
- 111.Zhang Z., Sun L., Wang Y., et al. Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int. 2008;73(2):163–171. doi: 10.1038/sj.ki.5002572. [DOI] [PubMed] [Google Scholar]
- 112.Ohara I., Tanimoto M., Gohda T., et al. Effect of combination therapy with angiotensin receptor blocker and 1,25-dihydroxyvitamin D(3) in type 2 diabetic nephropathy in KK-A(y)/Ta mice. Nephron Exp Nephrol. 2011;117(4):e124–e132. doi: 10.1159/000320284. [DOI] [PubMed] [Google Scholar]
- 113.Zhang Z., Zhang Y., Ning G., Deb D.K., Kong J., Li YC. Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: blockade of compensatory renin increase. Proc Natl Acad Sci USA. 2008;105(41):15896–15901. doi: 10.1073/pnas.0803751105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eren Z., Gunal M.Y., Bakir E.A., et al. Effects of paricalcitol and aliskiren combination therapy on experimental diabetic nephropathy model in rats. Kidney Blood Press Res. 2014;39(6):581–590. doi: 10.1159/000368471. [DOI] [PubMed] [Google Scholar]
- 115.Lei M., Liu Z., Guo J. The emerging role of vitamin D and vitamin D receptor in diabetic nephropathy. Biomed Res Int. 2020;2020 doi: 10.1155/2020/4137268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang Y., Yang S., Zhou Q., Zhang H., Yi B. Effects of vitamin D supplementation on renal function, inflammation and glycemic control in patients with diabetic nephropathy: a systematic review and meta-analysis. Kidney Blood Press Res. 2019;44(1):72–87. doi: 10.1159/000498838. [DOI] [PubMed] [Google Scholar]
- 117.de Zeeuw D., Agarwal R., Amdahl M., et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376(9752):1543–1551. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]
- 118.Li X.H., Huang X.P., Pan L., et al. Vitamin D deficiency may predict a poorer outcome of IgA nephropathy. BMC Nephrol. 2016;17(1):164. doi: 10.1186/s12882-016-0378-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang X.X., Jiang T., Shen Y., et al. Vitamin D receptor agonist doxercalciferol modulates dietary fat-induced renal disease and renal lipid metabolism. Am J Physiol Ren Physiol. 2011;300(3):F801–F810. doi: 10.1152/ajprenal.00338.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Garcia I.M., Altamirano L., Mazzei L., et al. Role of mitochondria in paricalcitol-mediated cytoprotection during obstructive nephropathy. Am J Physiol Ren Physiol. 2012;302(12):F1595–F1605. doi: 10.1152/ajprenal.00617.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang T., Liang Y., Zhang J. Natural and synthetic compounds as dissociated agonists of glucocorticoid receptor. Pharmacol Res. 2020;156 doi: 10.1016/j.phrs.2020.104802. [DOI] [PubMed] [Google Scholar]
- 122.Caratti G., Matthews L., Poolman T., Kershaw S., Baxter M., Ray D. Glucocorticoid receptor function in health and disease. Clin Endocrinol. 2015;83(4):441–448. doi: 10.1111/cen.12728. (Oxf) [DOI] [PubMed] [Google Scholar]
- 123.Ponticelli C., Locatelli F. Glucocorticoids in the treatment of glomerular diseases: pitfalls and pearls. Clin J Am Soc Nephrol. 2018;13(5):815–822. doi: 10.2215/CJN.12991117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guess A., Agrawal S., Wei C.C., Ransom R.F., Benndorf R., Smoyer WE. Dose- and time-dependent glucocorticoid receptor signaling in podocytes. Am J Physiol Ren Physiol. 2010;299(4):F845–F853. doi: 10.1152/ajprenal.00161.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ransom R.F., Lam N.G., Hallett M.A., Atkinson S.J. Smoyer WE. Glucocorticoids protect and enhance recovery of cultured murine podocytes via actin filament stabilization. Kidney Int. 2005;68(6):2473–2483. doi: 10.1111/j.1523-1755.2005.00723.x. [DOI] [PubMed] [Google Scholar]
- 126.Zhou H., Tian X., Tufro A., Moeckel G., Ishibe S., Goodwin J. Loss of the podocyte glucocorticoid receptor exacerbates proteinuria after injury. Sci Rep. 2017;7(1):9833. doi: 10.1038/s41598-017-10490-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang H., Duan A., Zhang J., et al. Glucocorticoid receptor wields chromatin interactions to tune transcription for cytoskeleton stabilization in podocytes. Commun Biol. 2021;4(1):675. doi: 10.1038/s42003-021-02209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Srivastava S.P., Zhou H., Setia O., et al. Loss of endothelial glucocorticoid receptor accelerates diabetic nephropathy. Nat Commun. 2021;12(1):2368. doi: 10.1038/s41467-021-22617-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kuppe C., van Roeyen C., Leuchtle K., et al. investigations of glucocorticoid action in GN. J Am Soc Nephrol. 2017;28(5):1408–1420. doi: 10.1681/ASN.2016010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ivy J.R., Jones N.K., Costello H.M., et al. Glucocorticoid receptor activation stimulates the sodium-chloride cotransporter and influences the diurnal rhythm of its phosphorylation. Am J Physiol Ren Physiol. 2019;317(6):F1536–F1f48. doi: 10.1152/ajprenal.00372.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu Y.X., Wu D.W., Peng M.M., Chen C., Lu H.N., Zhao L.K. [The effect of low-dose hydrocortisone on the expression of glucocorticoid receptor alpha of the septic kidney and its protective effect on kidney in rat] Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2011;23(7):426–429. [PubMed] [Google Scholar]
- 132.Burris D., Webster R., Sheriff S., et al. Estrogen directly and specifically downregulates NaPi-IIa through the activation of both estrogen receptor isoforms (ERalpha and ERbeta) in rat kidney proximal tubule. Am J Physiol Ren Physiol. 2015;308(6):F522–F534. doi: 10.1152/ajprenal.00386.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ma H.Y., Chen S., Du Y. Estrogen and estrogen receptors in kidney diseases. Ren Fail. 2021;43(1):619–642. doi: 10.1080/0886022X.2021.1901739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Darvishzadeh Mahani F., Khaksari M., Raji-Amirhasani A. Renoprotective effects of estrogen on acute kidney injury: the role of SIRT1. Int Urol Nephrol. 2021;53(11):2299–2310. doi: 10.1007/s11255-020-02761-y. [DOI] [PubMed] [Google Scholar]
- 135.Maric-Bilkan C. Sex differences in diabetic kidney disease. Mayo Clin Proc. 2020;95(3):587–599. doi: 10.1016/j.mayocp.2019.08.026. [DOI] [PubMed] [Google Scholar]
- 136.Doublier S., Lupia E., Catanuto P., Elliot SJ. Estrogens and progression of diabetic kidney damage. Curr Diabetes Rev. 2011;7(1):28–34. doi: 10.2174/157339911794273982. [DOI] [PubMed] [Google Scholar]
- 137.von Hertzen L., Forsblom C., Stumpf K., et al. Highly elevated serum phyto-oestrogen concentrations in patients with diabetic nephropathy. J Intern Med. 2004;255(5):602–609. doi: 10.1111/j.1365-2796.2004.01330.x. [DOI] [PubMed] [Google Scholar]
- 138.Kim D., Lee A.S., Jung Y.J., et al. Tamoxifen ameliorates renal tubulointerstitial fibrosis by modulation of estrogen receptor alpha-mediated transforming growth factor-beta1/Smad signaling pathway. Nephrol Dial Transplant. 2014;29(11):2043–2053. doi: 10.1093/ndt/gfu240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.