Abstract
Aim
Physiological tests to assess systemic vascular function are not included in the risk score for atrial fibrillation (AF). We aimed to examine whether cardio-ankle vascular index (CAVI), a systemic arterial stiffness parameter, is associated with the presence of AF in Japanese general population.
Methods
A cross-sectional study (N = 47,687) and a cohort study (N = 5418, four consecutive years) in Japanese urban residents who participated in annual health screening were conducted.
Results
A total of 164 subjects (0.34%) had AF in the cross-sectional data. After propensity score matching for age and gender, logistic regression analysis revealed that CAVI is independently associated with AF, as are body mass index and estimated glomerular filtration rate. In a 4-year cohort study, 22 subjects (0.41%) with new-appearance of AF showed higher CAVI at baseline than those without. In the receiver-operating-characteristic curve analysis, the area under the curve, which is a measure of predictability, of CAVI for the new-appearance of AF was 0.747, and the cut-off value of CAVI was 8.0. Kaplan–Meier analysis revealed that the cumulative incidence of new-appearance of AF was higher in subjects with CAVI ≥8.0 compared to those with CAVI <8.0. In Cox-proportional hazards analysis, CAVI ≥8.0 as well as gender were identified as independent predictors for the new-appearance of AF, whereas age ≥65 years was not.
Conclusion
Increased CAVI may represent a major modifiable risk factor for the development of AF. Studies are needed to confirm that CAVI is a predictor of AF independent of various AF risk factors and that CAVI-lowering interventions can prevent new-appearance or recurrence of AF.
Keywords: atrial fibrillation, cardio-ankle vascular index, CAVI, arterial stiffness, health screening
Introduction
Atrial fibrillation (AF) is the most common chronic arrhythmia requiring therapeutic intervention and is associated with increased risk of frailty and dementia, as well as chronic heart failure and cerebral thromboembolism.1 The prevalence of AF increases markedly after the age of 60 years and is more than 20% in those over 85 years.2 The presence of AF in the middle-aged population is also a major mortality risk, although the incidence is relatively low.3,4 In the Multi-Ethnic Study of Atherosclerosis, the incidence of AF was 46% to 65% lower in Hispanics, Asians, and blacks aged 65 years and older than in non-Hispanic whites.5 In addition, in a study of Veterans Affairs patients, the age-adjusted prevalence of AF in whites was about twice as high as in other races.6 From the data of 630,138 Japanese subjects aged 40 years or more, the AF prevalence of men was three times that of women (1.35 vs 0.43%, p < 0.0001), and the overall prevalence was estimated to be 0.56%.7
Recently, the risk score for AF incidence using traditional risk factors that are easily obtained in routine health screenings has been established in Japan.8 However, physiological tests to assess systemic vascular function are not included in the risk score.
Cardio-ankle vascular index (CAVI) is known to reflect the stiffness of the arterial tree from the origin of the aorta to the ankle, independent of blood pressure (BP) at the time of measurement,9 and has been reported to be associated positively with cardiovascular disease (CVD) risks,10–12 severity of CVD,13 and future CVD events.14 Several cross-sectional studies have shown that CAVI reflects cardiac structural and functional changes associated with AF.15,16 Furthermore, increased CAVI is significantly associated with AF prevalence even after adjusting CVD risks,17 indicating the bidirectional interaction between systemic arterial stiffening and AF. However, the predictability of CAVI for the new-appearance of AF has not yet been explored, especially in general population receiving health screening.
With the above background, this real-world retrospective study aimed to investigate the relationship of clinical variables including CAVI with AF in real-world Japanese population. From the results obtained, the causality between systemic circulation and arrhythmia as well as how CAVI can be used in the management of AF in routine clinical practice will be discussed.
Materials and Methods
Ethics Approval and Consent to Participate
The protocol of the study was prepared in accordance with the Declaration of Helsinki, and this study was approved by the Institutional Review Board and Ethics Committee of Sakura Hospital, School of Medicine, Toho University (No. S20091). Written informed consent for the examinations was obtained from the participants, and informed consent to participate in this study was obtained by opt-out method.
Design, Subjects and Data Collection
We conducted a retrospective study of Japanese urban residents who underwent annual health screening between January 2013 and December 2018. The population-based sample with sufficient data, including CAVI, for the study used in this analysis consisted of 47,687 Japanese subjects living in major cities across Japan who participated in an annual CVD and cancer screening program organized by the Japan Health Promotion Foundation. We analyzed archival data retrospectively, collected from existing records. The participants were unpaid volunteers and were not recruited for the study (as opposed to subjects in a clinical trial).
In the first phase of the present study, a cross-sectional analysis was conducted, focusing on the presence of AF. This was followed in the second phase by a cohort analysis of 5418 subjects without AF at baseline, with data collected over 4 consecutive years.
All parameters in the health screening were assessed using standardized methods. Height and body weight (BW) were measured, and body mass index (BMI) was calculated as follows: BW (kg) divided by square of height (m). Blood samples were collected from the antecubital vein in the morning after 12 hours of fasting to measure fasting plasma glucose (FPG), triglycerides (TG) and high-density lipoprotein cholesterol (HDL-C). Low-density lipoprotein-cholesterol (LDL-C) was calculated using Friedewald formula: LDL-C = total cholesterol (TC) – (HDL-C) – (TG/5). Since this formula is not valid for patients with TG ≥ 400 mg/dL,18 subjects with baseline TG ≥ 400 mg/dL (0.6% of all participants) were excluded from the analysis of LDL-C.
Estimated glomerular filtration rate (eGFR) was calculated by the following equation from the Japanese Society of Nephrology:19
eGFR (mL/min/1.73m2) = 194 × creatinine−1.094 × age−0.287 (× 0.739 if female).
In addition, renal dysfunction was defined as eGFR <60 mL/min/1.73m2, corresponding to GFR category G3a or worse.20
Current smoking and habitual alcohol consumption status were determined by a questionnaire. Habitual alcohol consumption was defined as daily drinking. The existence of current treatment for each metabolic disorder (hypertension, diabetes mellitus and dyslipidemia) was investigated as well.
Measurement of CAVI and Blood Pressure
CAVI was calculated automatically from blood pressure (BP) and pulse wave velocity (PWV) measured with a VaSera VS-1500 (Fukuda Denshi Co Ltd, Tokyo, Japan), with simultaneous monitoring of electrocardiogram and heart sounds.9 First, BP [systolic BP (SBP) and diastolic BP (DBP)] was measured from the cuff at the upper arm. PWV was calculated by dividing the distance from the aortic valve to the ankle artery by the sum of the time from aortic valve closure to brachial pulse wave notch and the time from brachial pulse wave rise to ankle pulse wave. CAVI was then calculated by the following equation: CAVI = a{(2ρ/ΔP)×ln(Ps/Pd)PWV2}+b, where Ps is SBP; Pd is DBP; ΔP is Ps - Pd; ρ is blood density, and a and b are constants. The higher value of the left and right CAVIs was adopted. Since CAVI may be falsely low in patients with severe arterial occlusive disease, we excluded subjects with an ankle-brachial index lower than 0.90. Elevated BP was diagnosed by SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg.21
Definition of Atrial Fibrillation
Participants were diagnosed with AF if an electrocardiogram obtained during a physical examination showed atrial fibrillation or flutter. Various findings in electrocardiogram, such as left ventricular hypertrophy, P-wave indices, ST-T abnormality and QTc interval, have been reported to independently predict the new-appearance of AF.22 However, since this study aimed to investigate how CAVI and clinical parameters are associated with AF, these findings in electrocardiogram at baseline were not evaluated.
Statistical Analysis
The SPSS software (version 27.0.1, Chicago, IL, USA) was used for statistical analyses. All data are expressed as median [interquartile range (IQR)]. When comparing clinical characteristics of participants in two groups with and without AF, it was necessary to correct for imbalance related to age and gender. Therefore, propensity score matching (PSM) with a 1:4 ratio was performed to match the cases in two groups using the nearest matching method with a caliper width equal to 0.2. Standardized difference <0.1 of the absolute value was considered to be a relatively small imbalance. Mann–Whitney U-test or Fisher’s exact test was performed as post-hoc test to determine whether between-group differences were statistically significant. Logistic regression analysis was used to identify contributors to the presence of AF and expressed as odds ratio with 95% confidence interval (CI). Kaplan–Meier survival analysis was employed to estimate the time to end point, and Log rank test was used to compare between the four groups. Sensitivity and specificity with respect to new-appearance of AF were analyzed using conventional receiver-operating-characteristic (ROC) curve, and cut-off value of CAVI was estimated using Youden’s J index (J = sensitivity + specificity ‒ 1), as the point on the ROC curve where the Youden’s index is maximum.23 The predictability of CAVI in ROC curve analysis was expressed as area under the ROC curve (AUC) and 95% confidence interval (CI). Cox-proportional hazards analysis was performed to identify predictors for new-appearance of AF, and the result is expressed as hazard ratio with 95% CI. In all comparisons, two-sided p values less than 0.05 were considered statistically significant.
Results
Comparison of Demographic and Clinical Characteristics in Subjects with or without Atrial Fibrillation (Cross-Sectional Study)
In the first cross-sectional analysis, all subjects (19,868 men and 27,819 women; median age 47 years, median BMI 22 kg/m2) were divided into groups with and without AF, and demographic and clinical characteristics were compared before and after PSM (Table 1).
Table 1.
Comparison of Demographic and Clinical Characteristics in Subjects with or without Atrial Fibrillation (Cross-Sectional Study)
| Variables | Before PSM | After PSM | ||
|---|---|---|---|---|
| AF | Non-AF | AF | Non-AF | |
| (N = 164) | (N = 47,523) | (N = 163) | (N = 652) | |
| Male (%) | 139 (84.8) | 19,729 (41.5)* | 138 (84.7) | 560 (85.9) |
| Age (years) | 68 (61–74) | 47 (37–59)* | 68 (61–74) | 68 (61–74) |
| BMI (kg/m2) | 24.3 (22.6–26.1) | 22.0 (19.0–24.4)* | 24.3 (22.6–26.2) | 23.1 (21.5–25.0)* |
| SBP (mmHg) | 131 (121–140) | 122 (112–132)* | 131 (121–140) | 134 (124–144)* |
| DBP (mmHg) | 78 (72–86) | 72 (64–80)* | 78 (72–86) | 78 (71–84) |
| CAVI | 9.3 (8.8–9.8) | 7.5 (7.0–8.3)* | 9.3 (8.8–9.8) | 9.0 (8.4–9.5)* |
| FPG (mg/dL) | 92 (86–100) | 84 (79–90)* | 92 (86–100) | 91 (85–100) |
| LDL-C (mg/dL) | 117 (103–142) | 122 (101–145) | 117 (103–142) | 128 (106–147) |
| HDL-C (mg/dL) | 57 (50–69) | 67 (55–79)* | 57 (50–69) | 60 (49–70) |
| TG (mg/dL) | 88 (72–126) | 79 (56–118)* | 98 (72–139) | 88 (72–126) |
| Creatinine (mg/dL) | 0.91 (0.79–1.04) | 0.70 (0.60–0.83)* | 0.91 (0.79–1.04) | 0.84 (0.75–0.94)* |
| eGFR (mL/min/1.73m2) | 64.1 (51.6–74.0) | 79.8 (70.6–90.2)* | 64.0 (51.5–74.1) | 69.1 (60.3–51.5)* |
| Current smoking (%) | 24 (14.6) | 7433 (15.6) | 24 (14.7) | 80 (12.3) |
| Habitual alcohol drinking (%) | 37 (22.6) | 5796 (12.2)* | 37 (22.7) | 138 (21.1) |
| BMI ≥ 25 kg/m2 (%) | 64 (39.0) | 9925 (20.9)* | 64 (39.3) | 167 (25.6)* |
| SBP ≥140 and/or 90 mmHg (%) | 55 (33.5) | 7548 (15.9) | 55 (33.7) | 230 (35.3) |
| Treatment of hypertension (%) | 66 (40.2) | 4262 (9.0)* | 66 (40.5) | 195 (29.9)* |
| Treatment of diabetes (%) | 11 (6.7) | 897 (1.9)* | 11 (6.7) | 51 (7.8) |
| Treatment of dyslipidemia (%) | 25 (15.2) | 2862 (6.0)* | 138 (84.7) | 93 (14.3) |
| eGFR <60 mL/min/1.73m2 (%) | 71 (43.3) | 3204 (6.7)* | 71 (43.6) | 160 (24.5)* |
Notes: Data are presented as median (interquartile range) or percent of subjects. *p < 0.05; Mann–Whitney U-test for continuous variables, or Fisher’s exact test for dichotomous variables among two groups.
Abbreviations: AF, atrial fibrillation; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; CAVI, cardio-ankle vascular index; FPG, fasting plasma glucose; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; eGFR, estimated glomerular filtration rate.
Before PSM, a total of 164 subjects (0.34%) had AF in this cross-sectional data. The prevalence of AF by age group was 0.0% in the 20s and 30s, 0.1% in the 40s, 0.2% in the 50s, 0.7% in the 60s, 1.7% in the 70s, and 4.0% in the 80s and older. AF group showed higher age, BP, CAVI, FPG, TG, creatinine, rates of male sex, habitual alcohol drinking, obesity, elevated BP and treatment for metabolic disorders, and lower HDL-C and eGFR compared to non-AF group. LDL-C and current smoking rate did not differ between the two groups. After PSM, small imbalances were obtained for gender (standardized difference: 1.003 to 0.039) and age (standardized difference: 1.528 to 0.026), and the differences in BMI, CAVI, renal function, and treatment of hypertension frequency remained significant.
Logistic Regression Model for the Presence of Atrial Fibrillation After Propensity Score Matching (Cross-Sectional Study)
Next, we examined the factors associated with AF using multivariate logistic regression model as shown in Table 2. The parameters showing significant differences between the two-group after PSM (Table 1) were mainly adopted as confounding variables.
Table 2.
Logistic Regression Model for the Presence of Atrial Fibrillation After Propensity Score Matching (Cross-Sectional Study)
| Variables | Odds Ratio | 95% Confidence Interval | p value |
|---|---|---|---|
| BMI (every 1 kg/m2) | 1.15 | 1.08–1.22 | <0.001 |
| CAVI (every 1 index) | 1.37 | 1.08–1.22 | 0.008 |
| eGFR (every 10 mL/min/1.73m2) | 0.807 | 0.705–0.924 | 0.002 |
| Hypertension treatment (Yes; 1, No; 0) | 1.14 | 0.781–1.66 | 0.497 |
Note: Akaike’s information criterion: 777.2, residual deviance: 767.2, p < 0.001.
Abbreviations: BMI, body mass index; CAVI, cardio-ankle vascular index; eGFR, estimated glomerular filtration rate.
Resultantly, logistic regression analysis revealed that BMI, CAVI and eGFR were significantly associated with the presence of AF. On the other hand, treatment of hypertension was not extracted as significant confounders.
Comparison of Demographic and Clinical Characteristics in Subjects with or without the New-Appearance of Atrial Fibrillation During the 4-Year Period (Cohort Study)
Subsequent analyses included a total of 5438 Japanese urban residents (2368 men and 3070 women; median age 48 years, median BMI 21.9 kg/m2) who had undergone four consecutive annual health check programs and were free of AF at baseline. Table 3 compares the demographic and baseline clinical characteristics in subjects with (N = 22) and without (N = 5396) new-appearance of AF during the 4-year period.
Table 3.
Comparison of Demographic and Clinical Characteristics in Subjects with or without the New-Appearance of Atrial Fibrillation During the 4-Year Period (Cohort Study)
| Variables | Subjects with New-Appearance of AF (N=22) | Subjects without New-Appearance of AF (N=5396) | p value |
|---|---|---|---|
| Male sex (%) | 16 (72.7) | 2331 (43.2) | <0.001* |
| Age (years) | 62.5 (58–74.3) | 48 (40–58) | <0.001 |
| BMI (kg/m2) | 23.0 (20.9–25.2) | 21.9 (20.0–23.9) | 0.152 |
| SBP (mmHg) | 127 (117–139) | 115 (107–126) | 0.005 |
| DBP (mmHg) | 76 (72–86) | 72 (66–80) | 0.058 |
| CAVI | 8.7 (8.0–9.4) | 7.6 (7.1–8.3) | <0.001 |
| FPG (mg/dL) | 89 (85–100) | 85 (80–90) | 0.011 |
| LDL-C (mg/dL) | 124 (114–142) | 125 (105–147) | 0.929 |
| HDL-C (mg/dL) | 66 (51–75) | 70 (58–83) | 0.114 |
| TG (mg/dL) | 94 (69–128) | 77 (56–111) | 0.090 |
| Creatinine (mg/dL) | 0.78 (0.69–0.90) | 0.71 (0.60–0.84) | 0.030 |
| eGFR (mL/min/1.73m2) | 69.2 (62.2–81.5) | 78.3 (69.6–87.9) | 0.014 |
| Age ≥ 65 years (%) | 10 (45.5) | 741 (13.7) | <0.001* |
| Current smoking (%) | 5 (22.7) | 726 (13.5) | 0.207* |
| Habitual alcohol consumption (%) | 4 (18.2) | 835 (15.5) | 0.766* |
| Treatment of hypertension (%) | 10 (45.5) | 453 (8.4) | <0.001* |
| Treatment of diabetes (%) | 1 (4.5) | 58 (1.1) | 0.214* |
| Treatment of dyslipidemia (%) | 1 (4.5) | 104 (1.9) | 0.073* |
Notes: Data are presented as median (interquartile range) or percent of subjects. Mann–Whitney U-test or *Fisher’s exact test was used to compare subjects in two groups.
Abbreviations: AF, atrial fibrillation; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; CAVI, cardio-ankle vascular index; FPG, fasting plasma glucose; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; eGFR, estimated glomerular filtration rate.
Subjects with new-appearance of AF showed higher age, SBP, CAVI, FPG, creatinine, and rates of male sex and hypertension treatment at baseline. On the other hand, there were no differences in BMI, DBP, lipid parameters, rates of current smoking, habitual alcohol drinking, receiving treatments for diabetes and dyslipidemia in both groups.
Discriminatory Power of CAVI for Prediction of the New-Appearance of Atrial Fibrillation (Cohort Study)
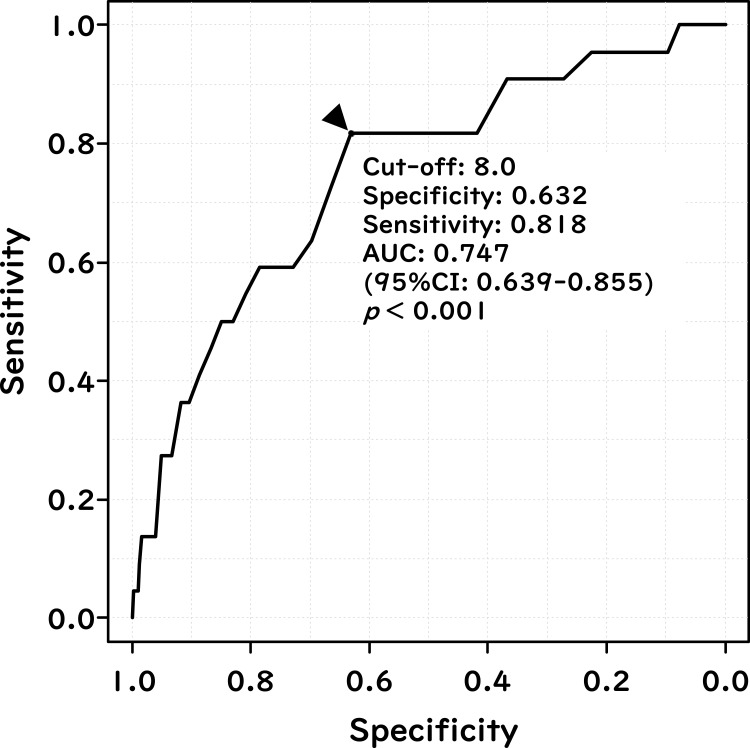
A ROC curve was generated to evaluate the discriminatory power and cut-off of CAVI for predicting new-appearance of AF, as shown in Figure 1. The AUC, which is a measure of predictability of CAVI for the new-appearance of AF, was 0.747 (95% CI: 0.639–0.855, p < 0.001). Additionally, from the ROC curve, the cut-off value of 8.0 for CAVI to predict the new-appearance of AF was decided and used as a covariate in the subsequent Kaplan–Meier survival analysis (Figure 2) and Cox-proportional hazards analysis (Table 4).
Figure 1.
Discriminatory power of CAVI for the prediction of the new-appearance of atrial fibrillation (cohort study). The receiver-operating-characteristic curve shows the performance of CAVI to discriminate new-appearance of AF. The Youden Index was used in to select the optimal cut-off point of CAVI.
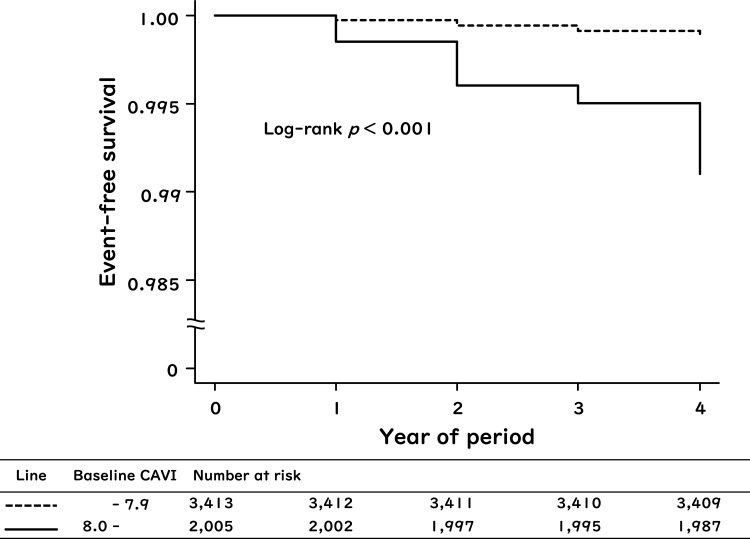
Figure 2.
Kaplan–Meier curves for the rates of the new-appearance of atrial fibrillation when subjects were stratified by baseline CAVI (cohort study). Participants were divided into two groups: those with baseline CAVI ≥ 8.0 and those with baseline CAVI < 8.0.
Table 4.
Cox-Proportional Hazard Model for the Association of the New-Appearance of Atrial Fibrillation with Clinical Variables (Cohort Study)
| Variables | Hazard Ratio | 95% Confidence Interval | p value |
|---|---|---|---|
| Sex (male; 1, female; 0) | 3.29 | 1.29–8.40 | 0.013 |
| Older age (age ≥ 65 y; 1, < 65 y; 0) | 2.17 | 0.863–5.46 | 0.099 |
| CAVI ≥ 8.0 (Yes; 1, No; 0) | 5.27 | 1.60–17.3 | 0.006 |
Abbreviation: CAVI, cardio-ankle vascular index.
Additionally, the predictability of CAVI for the presence of AF in cross-sectional data (N = 47,687), expressed as AUC, was 0.875 (95% CI: 0.850–0.899, p < 0.001) (data not shown).
Kaplan–Meier Curves of the New-Appearance of Atrial Fibrillation Rate According to Baseline CAVI (Cohort Study)
The total incidence of new-appearance of AF during the 4-year period was 0.40% (22/5438). Kaplan–Meier survival analysis of the relationship between baseline CAVI and the rate of new-appearance of AF is shown in Figure 2. The cumulative incidence of new-appearance of AF was higher in subjects with baseline CAVI ≥ 8.0 compared to those with baseline CAVI < 8.0 (Log rank test p < 0.001).
Cox-Proportional Hazard Model for Association of the New-Appearance of Atrial Fibrillation with Clinical Variables (Cohort Study)
Finally, we examined the predictability of increased CAVI for the new-appearance of AF using Cox-proportional hazards analysis, as shown in Table 4. Elderly people over the age of 65 years are at high risk of AF, and guidance from the National Institute for Health and Care Excellence recommends that elderly people with AF should be anticoagulated.24 Additionally, since there were only 22 new-appearance of AF cases in this study, the number of confounding factors used in Cox-proportional hazards analysis was limited. From these backgrounds, two major unmodifiable factors that contribute remarkably to AF, age ≥ 65 years and gender, were used as adjusting confounders.
Resultantly, male sex and high CAVI (CAVI ≥ 8.0) were identified as independent predictors for new-appearance of AF, whereas age ≥ 65 years was not.
Discussion
We conducted the cross-sectional and cohort study on the possible association between CAVI and the presence of AF in Japanese urban residents. The prevalence of AF by age group was similar to that previously reported in Japan.7 Subjects with AF at baseline showed relatively higher CAVI than those without. Even among subjects without AF at baseline, those who developed new-appearance of AF during the 4-year period had significantly higher CAVI than those who did not. In the cross-sectional study after PSM for age and sex, in addition to CAVI, BMI, eGFR were also extracted as independent factors associated with AF. Baseline CAVI was also associated with the new-appearance of AF in the cohort study, and ROC curve analysis decided CAVI 8.0 as the optimal cut-off for predicting new-appearance of AF. Cox-proportional hazards analysis revealed that CAVI ≥ 8.0, as well as male sex, were independent predictors for the new-appearance of AF. This cut-off value of CAVI is almost consistent with previous reports on the relationship of CAVI with coronary artery stenosis, calcifications and CVD events.12,25,26 These findings may suggest the need to emphasize the importance of CAVI in addition to CVD risks for early detection of AF in real-world Japanese population.
The CAVI cut-off of 8.0, decided from ROC analysis in this study, should be interpreted with caution, even though it was an independent predictor for new-appearance of AF. In this study, the cumulative incidence for the new-appearance of AF during the 4-year period was only 0.12% in subjects with CAVI < 8.0, and 0.90% in subjects with CAVI ≥ 8.0 (Figure 2). Therefore, it is not valid to conclude that CAVI of 8.0 or higher is a risk factor for the development of AF in middle-aged Japanese receiving health screening. In the future, it is needed to verify how well CAVI can predict the appearance of AF in elderly high-risk outpatients, independent of existing risk scores for AF.
There are two possible explanations for the ability of CAVI in predicting new-appearance of AF. First, the development of AF is closely related to atherosclerosis, and CAVI can be regarded as a correlating factor reflecting this pathophysiology. Risk factors of AF including aging, male sex, diabetes, hypertension, alcohol consumption and smoking are also related to endothelial dysfunction in the early phase of atherosclerosis.27,28 In a multi-ethnic large-scale population study, decrease in flow-mediated dilation was found to reflect endothelial dysfunction with inflammation, oxidative stress, increased adhesion molecules and decreased nitric oxide, and also predicted the development of AF.29 Similarly, systemic inflammation indicated by elevated C-reactive protein was observed in patients with AF.30 In addition, Lee et al31 also reported that atrial fibrosis with increased cytokine expression was the main pathophysiology of arrhythmogenic atrial remodeling in a canine congestive heart failure model. Taking into account that CAVI reflects inflammation and oxidative stress in systemic circulation,32–34 CAVI may correlate positively with the risk factors of AF.
The second possible explanation is that systemic arterial stiffening directly induces AF through elevated left ventricular filling pressure and atrial enlargement. A cross-sectional study suggests that left ventricular concentric remodeling/hypertrophy correlates positively with increased CAVI.35 Moreover, in the treatment of congestive heart failure, increased left ventricular ejection fraction was associated with decreased CAVI.36 In addition to left ventricular function, left atrial reservoir function evaluated by Speckle-tracking echocardiography was also inversely related to CAVI in a community-based cohort.37 Indeed, Nakamura et al16 reported that CAVI was closely associated with atrial structural and electrical remodeling in patients with AF. These findings may indicate a causality that impaired left atrial reservoir function is caused by high CAVI-mediated left ventricular overload, and this relationship is consistent with the hypothesis that systemic arterial stiffening is located upstream of the pathogenesis for AF. Longitudinal studies are needed to confirm that elevated LV filling pressures associated with high CAVI predict the new-appearance of AF.
As mentioned earlier, traditional CVD risk factors are strongly associated with the pathogenesis of AF. Therefore, patients who develop AF are more likely to have prior organ damage such as ischemic heart disease and chronic kidney disease. Additionally, such organ damage and AF form a vicious cycle in which they aggravate each other.38–40 On the other hand, previous study has shown the possibility that appropriate treatments and behavior modification may decrease CAVI.12,36,41 In addition to the management of metabolic disorders, other approaches including smoking cessation,42 treatment of periodontitis,43 improvement of sleep duration,44 and continuous positive airway pressure for obstructive sleep apnea45 may contribute to the improvement of CAVI. Therefore, CAVI-lowering interventions are expected to play a role in preventing new-appearance of and recurrence of AF by breaking the bidirectional interaction between organ damage and AF.
The Framingham Heart Study has shown that carotid-femoral pulse wave velocity (cfPWV), an indicator of vascular function, predicts the development of AF.27 However, unlike CAVI, PWV is essentially affected by BP at the time of measurement,8,46 and thus may underestimate the degree of vascular dysfunction caused by CVD risk factors other than hypertension. In fact, a multicenter longitudinal study performed in a large European population revealed that cfPWV was unnaturally more associated with hypertension than glucose intolerance and dyslipidemia.47 Therefore, this critical disadvantage of cfPWV as an indicator of vascular function makes it difficult to evaluate the pathophysiology that bridges AF with systemic arterial stiffening occurring without hypertension. On the other hand, in the cross-sectional analysis of the present study, BP and hypertension treatment did not show a greater involvement in AF than CAVI. Unfortunately, the longitudinal analysis to compare BP and CAVI was not possible because of the small number of the new-appearance of AF. However, it is necessary to investigate whether CAVI can be added to BP in daily clinical practice to enhance the predictive ability of the new-appearance of AF.
A limitation of this study with a study population consisting of mainly non-elderly subjects is that the absolute number of new-appearance of AF as an endpoint was small, and confounders may have been inadequately extracted. It is also curious that older age was not extracted as a significant contributor in the longitudinal analysis, but this may be because the number of the new-appearance of AF was too small to create a Cox model with high accuracy. Furthermore, as mentioned earlier, appropriate therapeutic interventions may prevent the new-appearance of AF through the reduction of CAVI. However, due to the limited number of applicable subjects and information in the current study, we were not able to clarify the association between treatment of metabolic disorders and AF. Next, the BP employed in the current study was automatically measured during supine rest, and may have been lower than normal sitting BP. In addition, this study did not examine persistent and long-standing AF separately. Therefore, unrecognized AF may have been overlooked. Finally, CAVI measured in subjects with AF is generally not validated because of the characteristic beat-to-beat variability. However, Nakamura et al reported that there is a strong correlation between CAVI values during AF and those during sinus rhythm,16 suggesting the reproducibility and usefulness of CAVI during AF.
Conclusion
Increased CAVI may represent a major modifiable risk factor for the development of AF. Studies are needed to confirm that CAVI is a predictor of AF independent of various AF risk factors, and that CAVI-lowering interventions can prevent new-appearance or recurrence of AF.
Acknowledgments
We would like to thank all the staff members in our departments who contributed to this study.
Funding Statement
No funding was provided for this study.
Disclosure
The authors have no conflicts of interest for this work to declare.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al.; American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–360. doi: 10.1161/CIR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–125. doi: 10.1161/CIRCULATIONAHA.105.595140 [DOI] [PubMed] [Google Scholar]
- 3.Ohsawa M, Okayama A, Okamura T, et al. Mortality risk attributable to atrial fibrillation in middle-aged and elderly people in the Japanese general population: nineteen-year follow-up in NIPPON DATA80. Circ J. 2007;71(6):814–819. doi: 10.1253/circj.71.814 [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Abbott RD, Savage DD, McNamara PM. Coronary heart disease and atrial fibrillation: the Framingham Study. Am Heart J. 1983;106(2):389–396. doi: 10.1016/0002-8703(83)90208-9 [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez CJ, Soliman EZ, Alonso A, et al. Atrial fibrillation incidence and risk factors in relation to race-ethnicity and the population attributable fraction of atrial fibrillation risk factors: the Multi-Ethnic Study of Atherosclerosis. Ann Epidemiol. 2015;25:71–76, 76.e1. doi: 10.1016/j.annepidem.2014.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borzecki AM, Bridgers DK, Liebschutz JM, Kader B, Kazis LE, Berlowitz DR. Racial differences in the prevalence of atrial fibrillation among males.J. Natl Med Assoc. 2008;100:237–245. doi: 10.1016/s0027-9684(15)31212-8 [DOI] [PubMed] [Google Scholar]
- 7.Inoue H, Fujiki A, Origasa H, et al. Prevalence of atrial fibrillation in the general population of Japan: an analysis based on periodic health examination. Int J Cardiol. 2009;137(2):102–107. doi: 10.1016/j.ijcard.2008.06.029 [DOI] [PubMed] [Google Scholar]
- 8.Kokubo Y, Watanabe M, Higashiyama A, Nakao YM, Kusano K, Miyamoto Y. Development of a basic risk score for incident atrial fibrillation in a Japanese general population - the Suita Study. Circ J. 2017;81(11):1580–1588. doi: 10.1253/circj.CJ-17-0277 [DOI] [PubMed] [Google Scholar]
- 9.Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb. 2006;13:101–107. doi: 10.5551/jat.13.101 [DOI] [PubMed] [Google Scholar]
- 10.Nagayama D, Watanabe Y, Saiki A, Shirai K, Tatsuno I. Lipid parameters are independently associated with cardio-ankle vascular index (CAVI) in healthy Japanese subjects. J Atheroscler Thromb. 2018;25:621–633. doi: 10.5551/jat.42291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagayama D, Watanabe Y, Saiki A, Shirai K, Tatsuno I. Difference in positive relation between cardio-ankle vascular index (CAVI) and each of four blood pressure indices in real-world Japanese population. J Hum Hypertens. 2019;33:210–217. doi: 10.1038/s41371-019-0167-1 [DOI] [PubMed] [Google Scholar]
- 12.Saiki A, Ohira M, Yamaguchi T, et al. New horizons of arterial stiffness developed using cardio-ankle vascular index (CAVI). J Atheroscler Thromb. 2020;27(8):732–748. doi: 10.5551/jat.RV17043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura K, Tomaru T, Yamamura S, Miyashita Y, Shirai K, Noike H. Cardio-ankle vascular index is a candidate predictor of coronary atherosclerosis. Circ J. 2008;72:598–604. doi: 10.1253/circj.72.598 [DOI] [PubMed] [Google Scholar]
- 14.Sato Y, Nagayama D, Saiki A, et al. Cardio-ankle vascular index is independently associated with future cardiovascular events in outpatients with metabolic disorders. J Atheroscler Thromb. 2016;23:596–605. doi: 10.5551/jat.31385 [DOI] [PubMed] [Google Scholar]
- 15.Miyoshi T, Doi M, Noda Y, et al. Arterial stiffness determined according to the cardio-ankle vascular index is associated with paroxysmal atrial fibrillation: a cross-sectional study. Heart Asia. 2014;6(1):59–63. doi: 10.1136/heartasia-2014-010503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura K, Takagi T, Kogame N, et al. The association of cardio-ankle vascular Index (CAVI) with biatrial remodeling in atrial fibrillation. J Atheroscler Thromb. 2021;28(6):590–603. doi: 10.5551/jat.57737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung GE, Park HE, Lee H, Choi SY. Clinical significance of increased arterial stiffness associated with atrial fibrillation, according to Framingham risk score. Sci Rep. 2021;11(1):4955. doi: 10.1038/s41598-021-84311-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts WC. The Friedewald-Levy-Fredrickson formula for calculating low-density lipoprotein cholesterol, the basis for lipid-lowering therapy. Am J Cardiol. 1988;62:345–346. doi: 10.1016/0002-9149(88)90248-2 [DOI] [PubMed] [Google Scholar]
- 19.Matsuo S, Imai E, Horio M, et al.; Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992. doi: 10.1053/j.ajkd.2008.12.034 [DOI] [PubMed] [Google Scholar]
- 20.Kidney Disease| Improving Global Outcomes. Chapter 1: definition and classification of CKD. Kidney Int Suppl. 2013;3(1):19–62. doi: 10.1038/kisup.2012.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carretero OA, Oparil S. Essential hypertension. Part I: definition and etiology. Circulation. 2000;101(3):329–335. doi: 10.1161/01.CIR.101.3.329 [DOI] [PubMed] [Google Scholar]
- 22.Aizawa Y, Watanabe H, Okumura K. Electrocardiogram (ECG) for the prediction of incident atrial fibrillation: an overview. J Atr Fibrillation. 2017;10(4):1724. doi: 10.4022/jafib.1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: [DOI] [PubMed] [Google Scholar]
- 24.Lund J, Saunders CL, Edwards D, Mant J. Anticoagulation trends in adults aged 65 years and over with atrial fibrillation: a cohort study. Open Heart. 2021;8(2):e001737. doi: 10.1136/openhrt-2021-001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park HE, Choi SY, Kim MK, Oh BH. Cardio-ankle vascular index reflects coronary atherosclerosis in patients with abnormal glucose metabolism: assessment with 256 slice multi-detector computed tomography. J Cardiol. 2012;60(5):372–376. doi: 10.1016/j.jjcc.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 26.Kirigaya J, Iwahashi N, Tahakashi H, et al. Impact of cardio-ankle vascular index on long-term outcome in patients with acute coronary syndrome. J Atheroscler Thromb. 2020;27(7):657–668. doi: 10.5551/jat.51409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaikh AY, Wang N, Yin X, et al. Relations of arterial stiffness and brachial flow-mediated dilation with new-onset atrial fibrillation: the Framingham Heart Study. Hypertension. 2016;68(3):590–596. doi: 10.1161/HYPERTENSIONAHA.116.07650 [DOI] [PubMed] [Google Scholar]
- 28.Börschel CS, Rübsamen N, Ojeda FM, et al. Noninvasive peripheral vascular function and atrial fibrillation in the general population. J Hypertens. 2019;37(5):928–934. doi: 10.1097/HJH.0000000000002000 [DOI] [PubMed] [Google Scholar]
- 29.O’Neal WT, Efird JT, Yeboah J, et al. Brachial flow-mediated dilation and incident atrial fibrillation: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:2717–2720. doi: 10.1161/ATVBAHA.114.304560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104(24):2886–2891. doi: 10.1161/hc4901.101760 [DOI] [PubMed] [Google Scholar]
- 31.Lee KW, Everett TH, Rahmutula D, et al. Pirfenidone prevents the development of a vulnerable substrate for atrial fibrillation in a canine model of heart failure. Circulation. 2006;114(16):1703–1712. doi: 10.1161/CIRCULATIONAHA.106.624320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagayama D, Saiki A, Endo K, et al. Improvement of cardio-ankle vascular index by glimepiride in type 2 diabetic patients. Int J Clin Pract. 2010;64:1796–1801. doi: 10.1111/j.1742-1241.2010.02399.x [DOI] [PubMed] [Google Scholar]
- 33.Carlucci PM, Purmalek MM, Dey AK, et al. Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus. JCI Insight. 2018;3:e99276. doi: 10.1172/jci.insight.99276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaguchi T, Shirai K, Nagayama D, et al. Bezafibrate ameliorates arterial stiffness assessed by cardio-ankle vascular index in hypertriglyceridemic patients with type 2 diabetes mellitus. J Atheroscler Thromb. 2019;26:659–669. doi: 10.5551/jat.45799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimizu K, Tabata T, Sasaki T, et al. The relationship between various left ventricular geometries and the cardio ankle vascular index. J Cardio Vasc Med. 2020;8:1–11. doi: 10.17303/jcvm.2020.6.201 [DOI] [Google Scholar]
- 36.Zhang C, Ohira M, Iizuka T, et al. Cardio-ankle vascular index relates to left ventricular ejection fraction in patients with heart failure. A retrospective study. Int Heart J. 2013;54(4):216–221. doi: 10.1536/ihj.54.216 [DOI] [PubMed] [Google Scholar]
- 37.Yoshida Y, Nakanishi K, Daimon M, et al. Association of arterial stiffness with left atrial structure and phasic function: a community-based cohort study. J Hypertens. 2020;38(6):1140–1148. doi: 10.1097/HJH.0000000000002367 [DOI] [PubMed] [Google Scholar]
- 38.Carrero JJ, Trevisan M, Evans M, Svennberg E, Szummer K. Kidney function and the risk of heart failure in patients with new-onset atrial fibrillation. Int J Cardiol. 2020;320:101–105. doi: 10.1016/j.ijcard.2020.08.003 [DOI] [PubMed] [Google Scholar]
- 39.Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ. 2016;354:i4482. doi: 10.1136/bmj.i4482 [DOI] [PubMed] [Google Scholar]
- 40.Liang F, Wang Y. Coronary heart disease and atrial fibrillation: a vicious cycle. Am J Physiol Heart Circ Physiol. 2021;320(1):H1–H12. doi: 10.1152/ajpheart.00702.2020.36 [DOI] [PubMed] [Google Scholar]
- 41.Nakamura K, Iizuka T, Takahashi M, et al. Association between cardio-ankle vascular index and serum cystatin C levels in patients with cardiovascular risk factor. J Atheroscler Thromb. 2009;16(4):371–379. doi: 10.5551/jat.no687 [DOI] [PubMed] [Google Scholar]
- 42.Noike H, Nakamura K, Sugiyama Y, et al. Changes in cardio-ankle vascular index in smoking cessation. J Atheroscler Thromb. 2010;17(5):517–525. doi: 10.5551/jat.3707 [DOI] [PubMed] [Google Scholar]
- 43.Hayashida H, Saito T, Kawasaki K, et al. Association of periodontitis with carotid artery intima-media thickness and arterial stiffness in community-dwelling people in Japan: the Nagasaki Islands study. Atherosclerosis. 2013;229(1):186–191. doi: 10.1016/j.atherosclerosis.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 44.Morita N, Kambayashi I, Okuda T, et al. Inverse relationship between sleep duration and cardio-ankle vascular index in children. J Atheroscler Thromb. 2017;24(8):819–826. doi: 10.5551/jat.36517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomita Y, Kasai T. Relationship between cardio-ankle vascular index and obstructive sleep apnea. Rev Cardiovasc Med. 2020;21(3):353–363. doi: 10.31083/j.rcm.2020.03.67 [DOI] [PubMed] [Google Scholar]
- 46.Shirai K, Song M, Suzuki J, et al. Contradictory effects of β1- and α1- aderenergic receptor blockers on cardio-ankle vascular stiffness index (CAVI)–CAVI independent of blood pressure. J Atheroscler Thromb. 2011;18(1):49–55. doi: 10.5551/jat.3582 [DOI] [PubMed] [Google Scholar]
- 47.Topouchian J, Labat C, Gautier S, et al. Effects of metabolic syndrome on arterial function in different age groups: the Advanced Approach to Arterial Stiffness study. J Hypertens. 2018;36(4):824–833. doi: 10.1097/HJH.0000000000001631 [DOI] [PMC free article] [PubMed] [Google Scholar]