Graphical abstract
Keywords: Cadmium, Wild boars, Environmental contamination, Monocyte-derived macrophages, Cytokines, TLRs, Antimicrobial molecules
Abbreviations: Cd2+, cadmium; moMФ, monocyte-derived macrophages; IFN, Interferon; TLR, Toll-like receptor; IL, Interleukin; TNF, tumor necrosis factor; LDH, lactate dehydrogenase; Arg-1, arginase 1; NOS2, nitric oxide synthase 2; MyD88, myeloid differentiation factor 88; MD2, myeloid differentiation protein 2; BD, beta defensin; LPS, lipopolysaccharide; PAMPs, pathogen associated molecular patterns; iNOS, inducible nitric oxide synthase
Highlights
-
•
Wild boar represents useful bioindicator for Cadmium environmental exposure.
-
•
Cadmium can be absorbed by wild boar moMФ with subsequent cell viability decrease.
-
•
Moderate cadmium concentration down-regulated IL-12p40, TNF-α expression in moMФ.
-
•
Moderate cadmium concentration decreased antimicrobial molecules expression in moMФ.
-
•
Moderate cadmium concentration down-regulated expression of several TLRs in moMФ.
Abstract
Cadmium (Cd2+) is regarded as one of the most toxic heavy metals, which can enter the food chain through environmental contamination and be bioaccumulated. Its exposure in Ligurian wild boars was monitored between 2016–2020 and revealed high level of this heavy metal in different provinces. In one of these polluted area, 21 wild boars were additionally sampled and the relationship between hepatic and renal Cd2+ concentration suggested that majority of these animals presented chronic intoxication. Cd2+ exposure of wild boar might lead to an immunosuppression status, thus in vitro experiments on wild boar monocyte-derived macrophages (moMФ) were carried out. Effects of Cd2+ scalar doses were evaluated through viability and adsorption assays, ELISA, qPCR. Moderate doses of this environmental pollutant (20 μM) were absorbed by moMФ, with subsequent reduction of their viability. This heavy metal did not trigger release of either IFN- β, anti-inflammatory or pro-inflammatory cytokines by moMФ, instead 24 h treatment with 20 μM of Cd2+ resulted in down-regulated expression of TNF-α, IL-12p40, several TLRs, CD14, MD2, BD2, MyD88, p65, and NOS2. The results of our monitoring activity suggested that wild boar can be useful to monitor environmental exposure of this heavy metal and can help in understanding the type of contamination. In addition, in vitro experiments on wild boar moMФ revealed that Cd2+ exposure negatively affected the immune function of these cells, likely leading to increased susceptibility to infection.
1. Introduction
Cadmium (Cd2+) is one of the most toxic environmental and industrial heavy metals; many human activities lead to Cd2+ production, such as the combustion of fossil fuels, run-off from agricultural land, leachate from landfill sites, electroplating to protect steel from corrosion, and the manufacture of Nickel–Cd batteries, pigments, stabilizers and alloys. This heavy metal has been reviewed by the International Register of Potentially Toxic Chemicals of the United Nations Environment Program and included on the list of chemical substances considered to be potentially dangerous at the global level, indeed it has been reported to be carcinogenic and mutagenic [[1], [2], [3]]. Several studies highlighted a link between cadmium exposure and cancer in humans, and the main affected organs are liver, prostate, breast, lungs, kidney, skin and pancreas [[3], [4], [5]]. This toxic heavy metal can modulate the activity of cellular enzymes, initiate oxidative stress, suppress mitochondrial functions, disrupt calcium, homeostasis, negatively modulate the immune response and act as an endocrine disruptor, in particular of the thyroid and nervous system [[6], [7], [8]]. A link between cadmium exposure and cancer was highlighted by several in vivo studies using rodents as animal model [4].
Cadmium is not eliminated from ecosystems and, because of its long half-life (15–30 years), it can enter the food chain through environmental contamination of soil, enhancing the bioaccumulation along all the trophic levels of the ecological pyramid. In cows and ewes, effects on various systems have been reported due to the ingestion of this metal present in feed and water. Physiological concentrations cadmium in blood from cattle reared around different industrial/urban areas have been reported to range from 0.03 to 0.12 μg/mL; in several species, long-term exposure to this environmental pollutant causes organ functional deficiency and in female mammals can affect ovarian function both directly and indirectly [9]. Due to its ability to bioaccumulate, it is recommended to monitor its level in the environment, in order to prevent food contamination and subsequent cadmium poisoning [5].
Wild boars might be the most suitable bioindicator for organic pollutants, because they are exposed to contaminants by both water and soil due to innate habits of omnivorous scavengers, both abundant and widely diffuse, other than taking soil due to digging during foraging [10,11]. They can also move long distances through the day, incorporating the contamination of broad areas. They were also frequently used to monitor Cd2+ and other heavy metal pollutants in different areas [[12], [13], [14]]. Thus, in the first phase of study, liver samples from 1271 wild boars from the four provinces of the Liguria Region were examined, to monitor environmental contamination and to identify polluted area.
Heavy metals can negatively affect the host immune system, compromising the response to infection [15]. Previous studies showed that this environmental pollutant induced strong immunosuppression, with decreased phagocytic activity of peritoneal macrophages, reduced activity of natural killer (NK) cells, and decreased resistance to infections [[16], [17], [18]]. Cd exposure can induce oxidative stress and downregulate of T cell-specific cytokines, leading to T cell apoptosis [53]. Thus in the second part of the study in vitro experiments on wild boar immune cells were carried out. In the past, we investigated the impact of 2 or 20 μM of Cd2+ on porcine enterocytes (IPEC-J2 cells), using doses previously defined as ‘low’ (2 μM) or ‘moderate’ (20 μM) [19]. IPEC-J2 cells were able to absorb Cd2+, with subsequent decreased in their viability and modulation several pro-inflammatory molecules [20]. In this study, we instead tested the effects of this heavy metal on wild boar macrophages, in order to better understand any risks associated with exposure to this environmental contaminant. Macrophages are professional phagocytic cells that detect, internalize, and clear foreign bodies [21]. They are key cells of the innate immune system, able to respond to both infection and non-infectious stressors, and that can drive the development of an antigen-specific acquired immune responses [21]. In addition, humans and pigs share many physiological and immunological characteristics [22]. In particular, pig macrophages resemble human macrophages more than rodent’s [[23], [24], [25]], thus discoveries in this wild species should well reflect the effects of this environmental pollutant on human innate immune system.
The effects of scalar doses of Cd2+ on this wild boar immune cells were investigated through an integrative analytic approach, spanning from viability assay, adsorption test, microscopy, multiplex ELISA and qPCR.
2. Materials and methods
2.1. Wild boar sampling
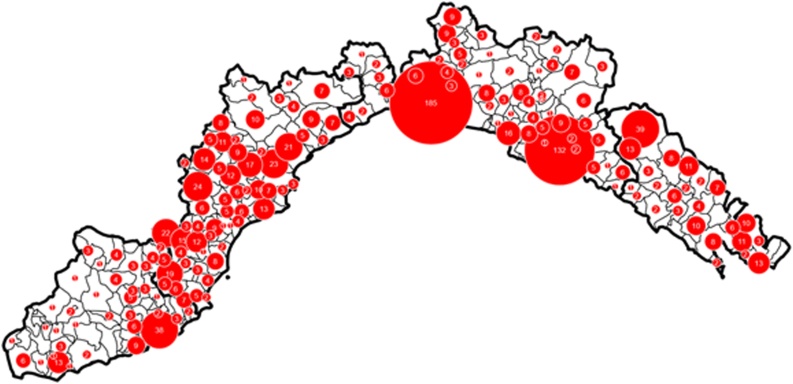
During the hunting season from 2016 to 2020, 1271 wild boars (Sus scrofa) were killed by hunters or found dead in the different areas of Liguria Region (Fig. 1). After death, livers were dissected by hunters and immediately refrigerated. Samples were transported to the laboratory by hunters; occasionally kidney samples were also collected. At the laboratory, samples were immediately stored at – 20 °C until analyzed.
Fig. 1.
The Liguria region is divided into provinces, from left to right: Imperia, Savona, Genoa and La Spezia. The numbers represent samples taken in monitored municipalities.
In a selected area (Chiavari municipality, within Genova province), additional sampling was carried out: 21 wild boars from a polluted area in that municipality were examined.
Animals were sampled after being hunted or found dead. No wild boar was sacrificed for this study in order to determine Cd2+ contents in its selected organs; thus, approval of the ethics committee was not required to monitor Cd2+ environmental contamination in the Liguria Region. Wild boars were preserved in suitable conditions and transported to the laboratories of IZS of Piemonte, Liguria and Valle d’Aosta. Necroscopies were carried out, during which samples of kidney and liver were immediately frozen and stored at – 20 °C.
2.2. Chemical analysis of wild boar organs
An aliquot (approximately 1 g) of tissue samples was homogenized and then was transferred to a Teflon® microwave vessel and mixed with 5 mL of 65 % nitric acid (Sigma-Aldrich S.r.l., Milano, cat. V001338) and 1.5 mL of hydrogen peroxide (Merck Millipore, Germany, cat. 1,086,001,000). The samples then were digested using a laboratory microwave oven. The extract was filtered and diluted to 25 mL with ultrapure water. Determination of Cd2+ contents was carried out using Analytical Yena 650 Plus Atomic Absorption Spectrometer with graphite furnace, at 228.8 nm with a current of 4 mA. Quantification was obtained by standard addition method. In brief, calibration was carried out by scalar addition of standards (certified standard solution at 10 mg/L by Ultra Scientific) to the matrix solution. The data were plotted as absorbance versus the amount of the standard added. The least squares line intersects the x-axis at the negative of the concentration of the sample. The quantification limit (LOQ) was 0.020 mg/kg. For testing the purity of the reagents and possible contamination, “blanks” was analysed for each run, using the same procedure.
2.3. Generation of wild boar monocyte-derived macrophages and cadmium treatment
Five healthy wild boars, 9–12 months of age, were used as blood donors for in vitro experiments. Animals were kept at the University of Sassari, Faculty of Veterinary Medicine (Sassari, Italy). EDTA blood was collected by puncture of the cranial vena cava; blood sampling was approved by the local ethics committee, in accordance with the Guide of Use of Laboratory Animals issued by the Italian Ministry of Health, as we previously described [26].
Wild boar peripheral blood mononuclear cells (PBMCs) were prepared by diluting 20 mL of EDTA blood in 10 mL of phosphate buffered saline (PBS), layering it over 20 mL of Histopaque-1077 (Sigma-Aldrich, Saint Louis, MO, USA), and centrifuging it at 1400 x g for 30 min at room temperature (RT), in a rotating bucket centrifuge, without braking. PBMCs were aspirated from the plasma-Histopaque interface and washed three times in PBS by centrifugation at 1000 x g for 5 min at 4 °C [27]. MoMФ cultures were obtained from PBMCs, using methods previously described with slight modifications [26,28]. In brief, PBMCs were cultured for 7 days in RPMI-1640 (Sigma-Aldrich, Saint Louis, MO, USA) supplemented with 10 % fetal bovine serum (FBS; Sigma-Aldrich), 100 U/mL streptomycin, and 100 μg/mL penicillin (complete RPMI, cRPMI), and with 50 ng/mL of recombinant human M-CSF (hM-CSF) (Thermo Fisher Scientific, Waltham, MA, USA), using Petri dishes. Wild boar MoMΦ were then harvested, washed, re-suspended in cRPMI and seeded in 12-well plates (Greiner CELLSTAR, Sigma-Aldrich) (8–10 × 105 live cells per well) or 8-well chamber slide (Thermo Fisher Scientific, Waltham, MA, USA) (1 × 105 live cells per well). Cells were incubated at 37 °C 5% CO2 for further 24 h before treatment. Different Cd2+ concentrations (Carlo Erba reagents srl, Milano, cat 505,548) were tested: 2 μM or 20 μM, as previously used on swine epithelial cells [20]. In selected experiment, two additional Cd2+ concentrations were investigated: 0.02 or 0.2 μM.
2.4. Cadmium uptake
MoMΦ ability to adsorb Cd2+ was investigated by atomic absorption spectroscopy. Cells were left untreated (control) or treated with scalar doses of cadmium (0.02, 0.2, 2, 20 μM). 24 h later, the intracellular concentration of Cd2+ was checked using a graphite furnace atomic absorption spectroscopy (model ZEEnit 650 P, Analytik-Jena, Germany) with inverse Zeeman-effect background correction system, as we previously described [20]. In brief, culture supernatants were removed, and cells were lysed in 400 μL/well of tissue lysis buffer ATL (Qiagen, Milan, Italy); then the cell lysate was digested with 600 μL of a solution of nitric acid 69 % and hydrogen peroxide 30 % 5:1 ratio, filtered through a 0.20 μm paper filter, finally diluted in 5 mL with ultrapure water. Intracellular Cd2+ concentration was expressed as μg Cd2+/106 cells.
2.5. Cd2+ impact on moMФ morphology
Wild boar moMΦ were cultured in 8-well chamber slides at 1 × 105 live cells per well. Cells were exposed to scalar doses of Cd2+ (0, 0.02, 0.2, 2, 20 μM) and 24 h later moMΦ were fixed with 4% paraformaldehyde, washed in PBS, and labeled with DAPI (Roche Diagnostics GmbH, Mannheim, Germany) to visualize nuclei [28]. Microscopy was performed using a Leica SP5 Confocal Microscope (Leica Microsystem, Wetzlar, Germany) equipped with a HCX PL APO lambda blue 63 × 1.40 OIL UV objective. Nuclei and DIC image were acquired using simultaneous UV-diode (405 nm) for DAPI signal and argon laser (488 nm) as light source. Images were acquired on a format of 1024 × 1024 pixels and were processed with LAS AF Lite software (Leica Microsystem) as previously described [28].
2.6. Viability assay
Impact of cadmium on wild boar moMΦ viability was evaluated using a non-radioactive cytotoxicity assay, as previously described [29]. Cells were seeded in 12 well plate, left untreated (control) or cultured 24 h in the presence of scalar doses of cadmium (0.02, 0.2, 2, 20 μM). LDH (lactate dehydrogenase) levels in culture supernatants were determined using a Cytotox 96® Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI, USA) following manufacturer’s instructions; a lysis solution provided by the manufacturer used as positive control. Absorbance was read at 492 nm, using an Epoch microplate reader (BioTek, Winoosky, USA).
2.7. Detection of cytokine levels in culture supernatants
Wild boar moMΦ were cultured in 12 well plates at 8−10 × 105 live cells per well. Cells were exposed to scalar doses of Cd2+ (0, 2, 20 μM) for 24 h, then cytokine levels in culture supernatants were determined as previously described [28,30]. The simultaneous measurement of IL-1α, IL-1 β, IL-6, IL-10, IL-12, and TNF-α in culture supernatants were performed using Porcine Cytokine/Chemokine Magnetic Bead Panel Quantikine assay (Merck Millipore, Darmstadt, Germany) and a Bioplex MAGPIX Multiplex Reader (Bio-Rad, Hercules, USA), according to the manufacturer’s instructions. The measurement of IFN-β was instead performed using a sandwich enzyme immunoassay (porcine IFN-β ELISA kit, MyBiosource, San Diego, CA, USA), according to manufacturer’s directions. Absorbance was read with an Epoch microplate reader (BioTek).
2.8. Gene expression
Gene expression in moMФ after Cd2+ exposure was also monitored. Changes in mRNA expression profiles of IL-1β, IL-6, IL-10, IL-12p40, TNF-α, IFN-β, IFN- α1, TLR3, TLR4, TLR5, TLR7, TLR8, TRL9, MyD88, p65, CD14, MD2, BD1, BD2, Arg-1, NOS2 in wild boar moMФ stimulated with two different Cd2+ concentrations were evaluated as previously described [20,28,31]. Wild boar moMΦ were cultured in 12 well plates at 8−10 × 105 live cells per well. Cells were left untreated (control) or treated with two different doses of cadmium (2 or 20 μM). 0, 3, 6, 24 h post-treatment, culture supernatants were removed, and cells were lysed with buffer RTL (Qiagen). Then, total RNA was extracted using the RNeasy Mini Kit (Qiagen) and eluted in 100 μL of ultrapure RNase-free water (Sigma). 250 ng of purified RNA was used as template for cDNA synthesis, as previously described [32]. Gene expression was evaluated by RT-qPCR, using primer sets reported in Table S1 [28,31,47,[49], [50], [51], [52]]. Real-time PCR amplification was performed in a CFX96™ Real-Time System after the reverse transcription step, with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a reference gene [20]. In each sample, the relative expression of the test genes was calculated using the widely adopted 2−ΔΔCq method, with Cq acronym of quantification cycle [20].
2.9. Data analysis and statistics
In vitro experiments were performed in technical duplicate (multiplex ELISA, sandwich ELISA, qPCR) or triplicate (cytotoxic test). In vitro experiments repeated at least three times with different blood donors (at least three biological replicates). Adsorption test was repeated using five diverse blood donors. Data were presented as median with interquartile range or means with standard deviations (SD) quoted to indicate the uncertainty around the estimate of the group mean. Graphical and statistical analysis was performed using GraphPad Prism 7.02 (GraphPad Software Inc, La Jolla, USA) and Minitab (Minitab Inc., Coventry, UK). All data were checked for normality using the Anderson Darling test and analysed by the parametric one-way ANOVA followed by Dunnett’s multiple comparison test or the non-parametric Mann-Whitney test or Kruskal-Wallis followed by Dunn’s multiple comparison test.
3. Results
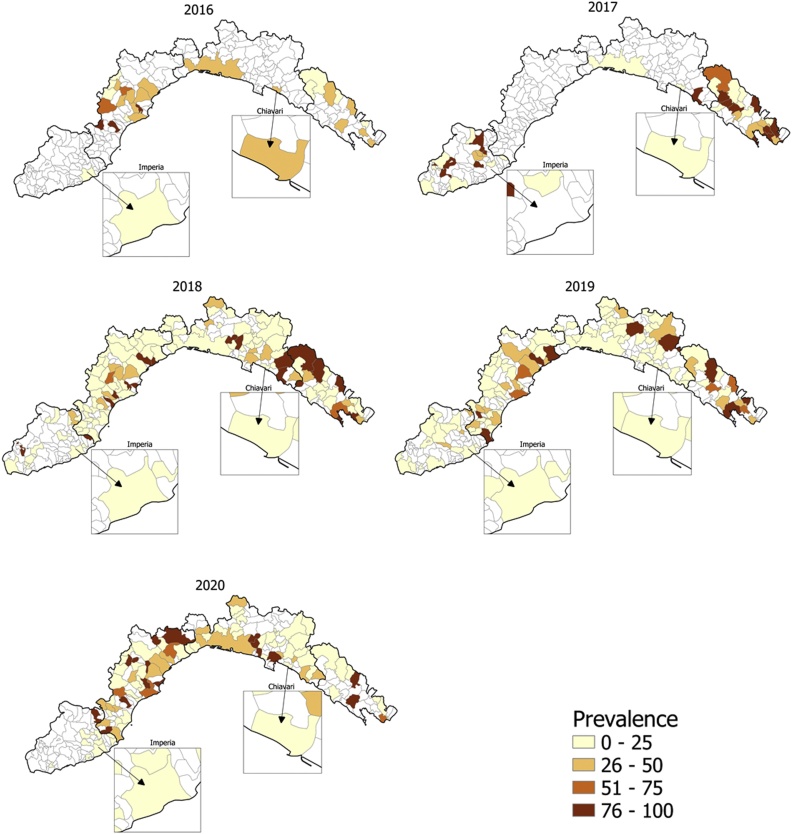
Samples with a cadmium concentration higher than 0.50 mg/kg were considered contaminated (as reported in EC Reg. 1881/2006 for liver of cattle, sheep, pigs, poultry and horses) and the total prevalence attested to 25 %. As shown by Fig. 2, over the years, the number of samples tested increased, revealing a greater prevalence of positivity in eastern Liguria. Imperia was the least polluted province by Cd2+, with an average positivity rate of 16 % (24 out of 152), the highest concentration detected was 1.28 mg/kg in 2017. La Spezia was instead the area with the highest average positivity rate of 38 % (65 out of 172), but the highest measured concentration was 6.48 mg/kg in 2020; Genoa and Savona, despite having a positivity rate of 20 % (103 out of 511) and 29 % (125 out of 436) respectively, were the provinces most exposed to Cd2+ contamination, with maximum concentrations of 13.56 mg/kg for Genoa in 2020 and 17.32 mg/kg for Savona in 2016, higher than those observed in 2015.
Fig. 2.
Prevalence of samples with Cadmium concentration > 0.5 mg/kg over the years. Highlighted on the right the municipality of Chiavari where a further 21 samples were collected (polluted area) and on the left the area in Imperia municipality where control samples were taken (control area).
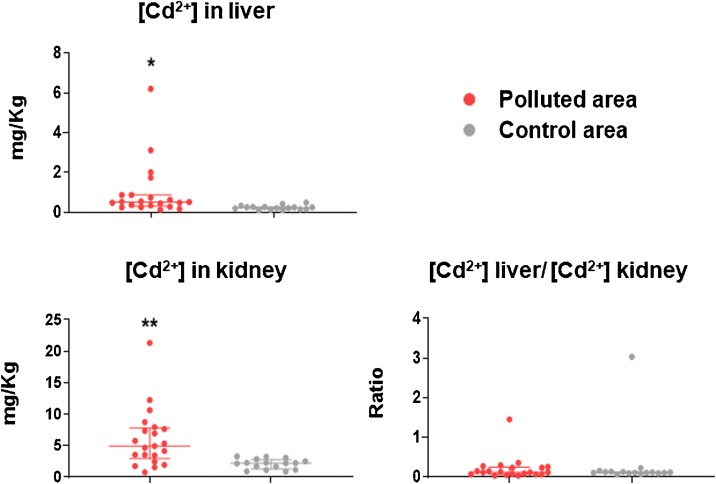
In one of the polluted areas (within Chiavari municipality) several hunters notified that wild boar, belonging to different age classes, presented poor body condition with anaemia, cachexia and weakness (personal communication), thus further sampling was carried out from that area. Most of the tested subjects (11 out of 21) presented Cd2+ levels which exceeded the limits of current legislation (Reg. EC 1881/2006 Cd2+: 0.50 mg/Kg for liver and 1 mg/kg for kidney), as reported in Table S1. The relationship between hepatic and renal concentration was also monitored because it is considered a valid index of intoxication degree (Chronic <1 / Acute> 1) [33] and our data suggested that most animals presented chronic intoxication of this heavy metal (Fig. 3, Table S2). Indeed, the toxicity index in animals tested from adjacent contaminated areas (Chiavari municipality) showed a value of 0.21, while the average toxicity index of the control group (animals from the Imperia area), was around 0.11.
Fig. 3.
Cd2+ levels in wild boar’s liver and kidney from a polluted area of Liguria. Cd2+ concentration (mg/kg) in liver (a), kidney (b) of wild boar collected from a polluted area (within Chiavari municipality) and a control area (within Imperia province) of Liguria. (c) Cd intoxication index, determined as the ratio between cadmium concentration in liver and kidney. Values are presented as median with interquartile range. Data were compared using a Mann-Whitney test; ** p < 0.01, * p < 0.05.
Chronic exposure of this environmental pollutant might negatively affect wild boar immune system. Thus, in the second part of this study, we investigated Cd2+ impact on wild boar moMФ, which are phagocytic cells at the frontline of defence against foreign invaders [21].
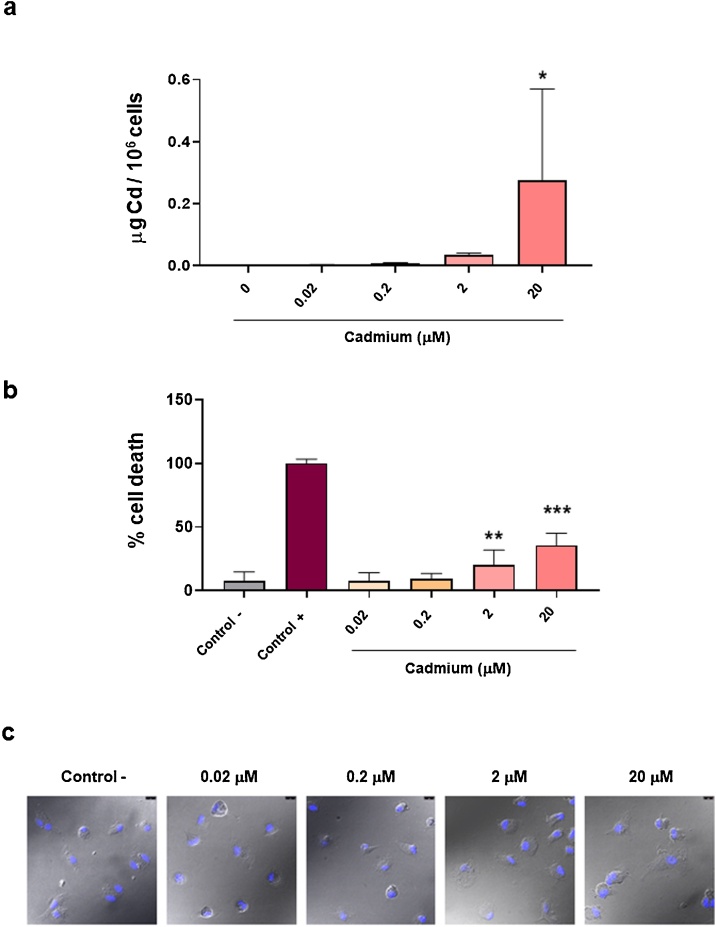
First, ability of these cells to uptake this heavy metal and the subsequent impact on moMФ viability were evaluated, using doses we previously tested on IPEC-J2 cells [20], which were defined as ‘low’ (2 μM) or ‘moderate’ (20 μM) by Luevano and Demodaran [19]. Nevertheless, moMØ are key players of innate immunity and highly responsive to environmental cues [34], thus initially other two lower concentrations were included in our in vitro experiments: 0.2 μM and 0.02 μM. Our results revealed a significant (P < 0.05) increase of Cd2+ intracellular levels after 24 h (P < 0.05) of exposure at 20 μM of Cd2+ concentrations (Fig. 4a, Figure S1). Then Cd2+ impact on wild boar moMФ viability was investigated using a non-radiolabelled immunoassay. Cells were exposed to Cd2+ (0, 0.02, 0.2, 2 or 20 μM) and 24 h later LDH levels in culture supernatants were quantified using a cytotoxicity non-radioactive assay. Cell viability decreased when Cd2+ was added at 2 or 20 μM (Fig. 4b). Morphology was next investigated using confocal microscopy: MoMФ presented with a spherical shape with short hairy protrusions on their surface, as observed in our previous study [28], irrespective of Cd2+ treatment (Fig. 4c).
Fig. 4.
Cd2+ adsorption by wild boar moMФ, its impact on cellular viability and morphology. Wild boar moMФ were left untreated (0) or treated with different Cd2+ concentration (0.02, 0.2, 2, 20 μM). (a) Ability of these cells to adsorb Cd2+ was investigated by atomic absorption spectroscopy. (b) 24 h post-treatment, viability was assessed using a non-radioactive cytotoxic assay. A lysis solution provided by the manufacturer was used as positive control (Control +). Mean data and SD from five (a) or three (b) independent experiments using different wild boar are presented; values for Cd2+-stimulated samples were compared to untreated control (Control -) using a Kruskal–Wallis multiple comparison test (a) or a one-way ANOVA followed by Dunnett’s multiple comparison test (b); *** p < 0.001, ** p < 0.01, * p < 0.05. (c) 24 h post-treatment, moMФ were morphologically evaluated though confocal microscopy. Images of representative moMФ, one from each condition are presented. Scale bar, 10 μm.
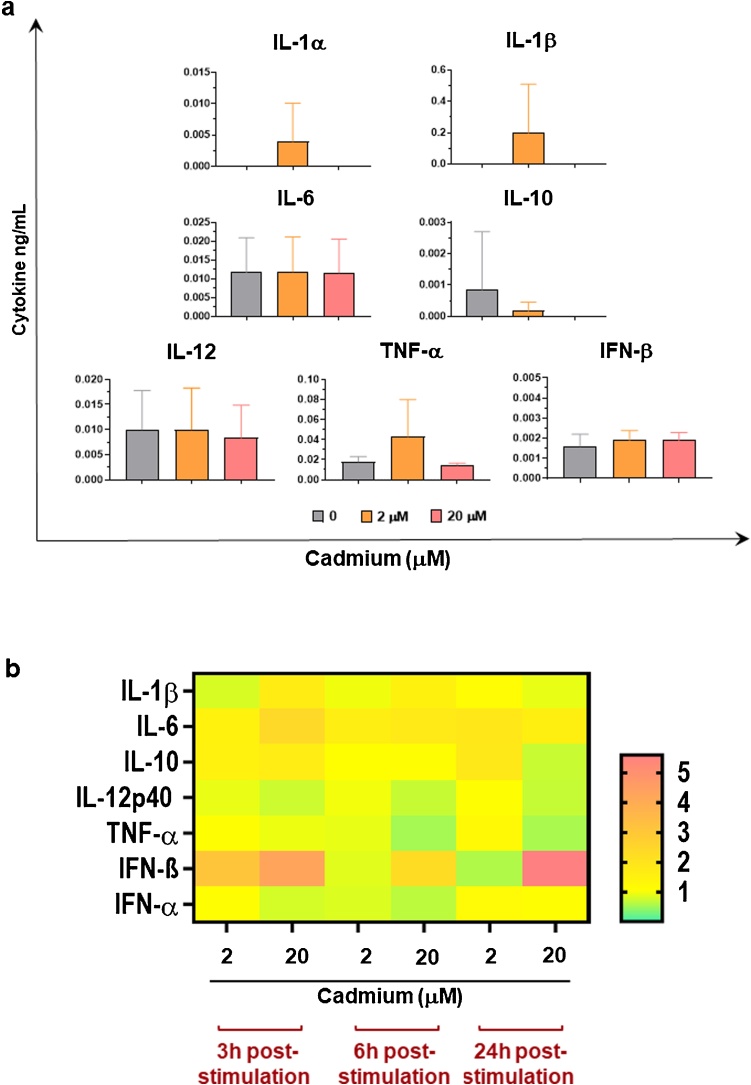
Then, Cd2+ immunological impact on wild boar moMФ was assessed. We opted to test only low (2 μM) and moderate Cd2+ (20 μM) doses, considering that at lower concentration Cd2+ was neither adsorbed or affected moMФ’s viability. Pro-inflammatory or anti-inflammatory cytokines levels in culture supernatants of moMФ were determined using multiplex ELISA. A small increase in IL-1α, IL-1 β and TNF-α were observed in moMФ treated with low but not moderate doses of Cd2+ (Fig. 5a, Figure S2). No changes in IL-6, IL-10, IL-12 levels were appreciated 24 h post stimulation with either low (2 μM) or moderate (20 μM) doses of this heavy metals.
Fig. 5.
Induction and release of a key cytokines by moMФ in response to Cd2+. Wild boar moMФ were left untreated (0) or treated with different Cd2+ concentration. a) At 24 h post-stimulation, levels of IL-1α, IL-1β, IL-6, IL-10, IL-12, TNF-α in culture supernatants were quantified using a multiplex ELISA, whereas levels of IFN-β were determined using a sandwich enzyme immunoassay. Mean data and SD from three independent experiments using different wild boar are presented. Values for Cd2+-stimulated samples were compared to untreated control (0) using or a one-way ANOVA followed by Dunnett’s multiple comparison test or a Kruskal–Wallis multiple comparison test. (b) At 3, 6, and 24 h post-stimulation, gene expression levels of IL-1β, IL-6, IL-10, IL-12p40, TNF-α, IFN- β, and IFN-α1 genes were determined using RT-qPCR. At each time point, data were normalized on the values of untreated control and expressed as 2−ΔΔCq, with ΔCq = Cq (target gene) —Cq (reference gene), and ΔΔCq = ΔCq (Cd2+-treated samples) —ΔCq (untreated sample, moMΦ). Heatmap displays mean data from five independent experiments using different blood donor wild boar. The colors in the cells represent the relative magnitude of gene expression. The yellow color represents the average magnitude of gene expression. The green color represents the smallest value, and the brightest orange represents the highest value.
Cd2+ modulation of key cytokines gene expression was also quantified using qPCR. This heavy metal did not alter expression of pro-inflammatory IL-1 β or IL-6 at any tested time points, whereas a small increase of IL-1 β was observed 3 h post-exposure (Fig. 5, Figure S3). On the contrary, 20 μM of Cd2+ induced small down-regulation of IL-12p40 and TNF-α 24 h post-treatment (Fig. 5, Figure S3). A small increase in IL-10 gene expression was observed 3 h post-treatment with high doses of this environmental pollutant, although no modulations were appreciated at later time points (Fig. 5, Figure S3).
Type I IFNs play a crucial role in the fight against viral infections [35], and in this study we assessed whether this heavy metal affect its induction/synthesis. Release of IFN- β was investigated using a sandwich enzyme immunoassay, whereas induction of both IFN-α1 and IFN- β gene expression was monitored over time through qPCR. No statistically significant differences were observed between untreated and Cd2+-treated moMФ, with the exception of a small down-regulation of IFN-α1 expression 6 h post-treatment with moderate doses of this heavy metal (Fig. 5, Figure S3).
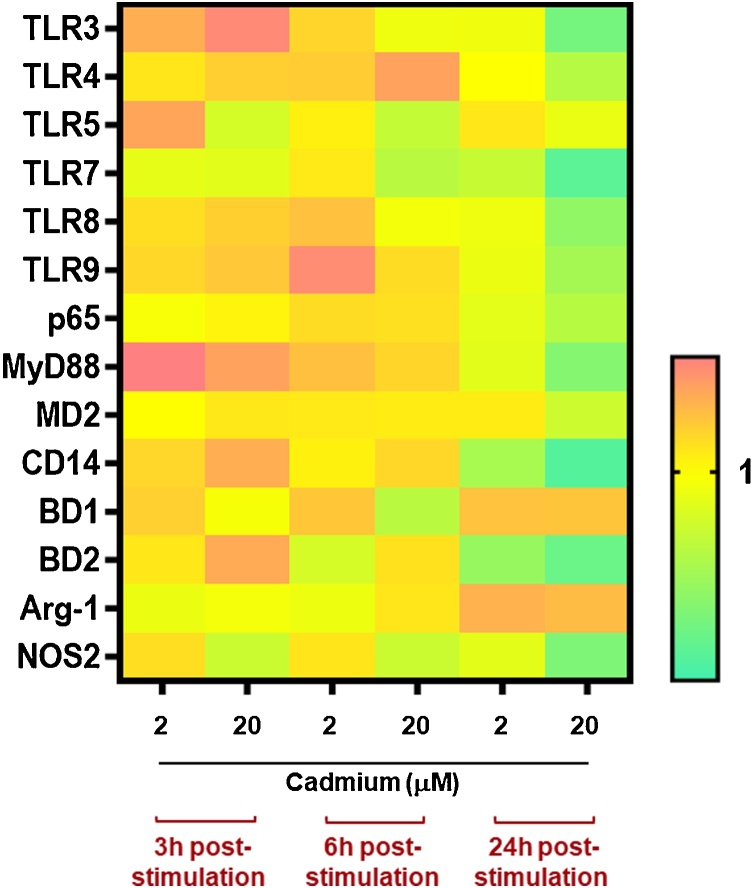
Cd2+ modulation of Toll like receptors (TLRs) expression was next investigated. These receptors are expressed by different immune cells and can recognize a broad range of pathogen associated molecular patterns (PAMPs). TLR3, TLR7, TLR8, TLR9 are intracellular TLRs and recognize nucleic acids derived from bacteria and viruses, whereas TLR4 and TLR5 are located on the cell-surface and recognize bacterial lipopolysaccharide (LPS) or flagellin, respectively [36,37]. Treatment with 20 μM of this heavy metal induced a statistically significant downregulation of TLR3 (at 24 h), TLR4 (at 24 h), TLR7 (at 6, and 24 h), TLR8 (at 24 h), TLR9 (at 24 h) (Fig. 6, Figure S4). Low doses of this heavy metal did not result in modulation of any of the tested TLRs (Fig. 6, Figure S4).
Fig. 6.
Expression of 14 key genes of the innate immunity in moMФ exposed to scalar doses of Cd2+. Heatmap of 14 key genes of innate immunity differentially expressed in moMФ. Wild boar moMФ were left untreated or stimulated with scalar doses of Cd2+ (2 or 20 μM). At 3, 6, and 24 h post-stimulation, gene expression levels of six TLRs (TLR3, TLR4, TLR5, TLR7, TLR8, TLR9), p65, MYD88, MD2, CD14, BD1, BD2, Arg1, NOS2 genes were determined using qPCR. At each time point, data were normalized on the values of untreated control and expressed as 2−ΔΔCq, with ΔCq = Cq (target gene) —Cq (reference gene), and ΔΔCq = ΔCq (Cd2+-treated samples) —ΔCq (untreated sample, moMΦ). Heatmap displays mean data from five independent experiments using different blood donor wild boar. The colors in the cells represent the relative magnitude of gene expression. The yellow color represents the average magnitude of gene expression. The green color represents the smallest value, and the brightest orange represents the highest value.
We subsequently investigated Cd2+ ability to modulate gene expression of other key innate immunity molecules, such as the adaptor protein myeloid differentiation factor 88 (MyD88), p65 (a subunit of transcription factor NF-κB) [38], and molecules with antimicrobial activities: CD14 and myeloid differentiation protein 2 (MD2) (both involved in LPS recognition by TLR4 [39]), and the host antimicrobial peptides beta defensin 1 (BD1) and 2 (BD2) [40]. Treatment with 20 μM of Cd2+ reduced expression of MyD88 (24 h), p65 (24 h), MD2 (24 h), CD14 (24 h), and BD2 (24 h) (Fig. 6, Figure S5). Reduced expressions of BD2 and CD14 were also triggered by low doses of this heavy metal (24 h) (Fig. 6, Figure S5). Enhanced expression of BD1 was induced by low doses of Cd2+ after 6 h of treatment, although no differences between treated and untreated moMФ were detected at later time points (Fig. 6, Figure S5).
Finally, we investigated the impact of this heavy metal on two enzymes involved in macrophage arginine metabolism: induction of both arginase 1 (Arg-1) and nitric oxide synthase 2 (NOS2) were monitored over time [54]. NOS2 encodes for the enzyme inducible nitric oxide synthase (iNOS), which generates nitic oxide (NO) from arginine, whereas Arg-1 is an enzyme that hydrolyses arginine to ornithine and urea. Arg-1 expression was not affected by treatment with either low (2 μM) or moderate doses (20 μM) of this heavy metal, whereas NOS2 expression was down-regulated at both 6 h and 24 h post-treatment with moderate Cd2+ doses (Fig. 6, Figure S5).
4. Discussion
Cd2+ is a non-infectious stressor, a toxic pollutant that can be bioaccumulated, thus it is recommended to monitor its level in the environment [5]. Wildlife species accumulate environmental pollutants, thus are frequently used as bioindicators for habitat contamination [10,11]. Liguria region is a highly urbanized area, where human–wildlife interface increased over the last years, facilitating the spread of infectious diseases from wildlife to humans [41]. The data collected during the monitoring activity (2016–2020) showed a prevalence of contaminated samples (Cd2+ concentration higher than 0.50 mg/kg) in the eastern part of Liguria. Additional samplings were carried out in one polluted area to better understand the type of contamination. The relationship between hepatic and renal Cd2+ concentration of the 21 additionally sampled wild boar was determined: the [Cd2+] liver/ [Cd2+] kidney ratio was lower than 1 in 20 out of 21 tested subjects. These results support the thesis of a cadmium chronic exposure, since the liver is the first site of cadmium absorption [33]. The results of our monitoring activity suggest that wild boar can be useful to monitor Cd2+ environmental exposure in highly urbanized area, such as Liguria, and can help in understanding Cd2+ type of contamination (chronic or acute). These monitor activity can be of crucial importance to guarantee food products safety and prevent cadmium poisoning. Previous studies in humans and rodents showed that this heavy metal triggered macrophage immune dysfunctions [16,42], whereas other studies reported that cadmium polarize lung macrophages toward a pro-inflammatory phenotype, with subsequent exacerbation of lung injury [43].
Thus, in the second part of this study, we investigated Cd2+ effects on wild boar moMФ, with the aim to further elucidate Cd2+ modulation of the immune system. Macrophages are key elements of the innate immune system, at first line of defence to foreign invaders [21]. Humans and pigs share many physiological and immunological characteristics [22]. In particular, pig macrophages resemble human macrophages more than rodent’s [[23], [24], [25]], thus discoveries in this wild species should well reflect the effects of this environmental pollutant on human innate immune system.
Wild boar moMФ were able to adsorb Cd2+, with subsequent decrease in percentages of live cells. Similar findings were reported in our previous study on IPEC-J2 cells, where adsorption of this heavy metal led rapidly to cell death [20]. Treatment of porcine intestinal cells with 20 μM of Cd2+ resulted in complete detachment of cell monolayer [20], whereas we observed that 24 h post treatment with moderate doses more than 50 % of wild boar moMФ were still alive. This difference is probably linked to higher ability of IPEC-J2 to absorb that metal compared to moMФ.
In the above-mentioned study, we observed that absorption of this heavy metal by porcine intestinal cells was related to a significant modulation of key pro-inflammatory genes [20], thus we investigated Cd2+ modulation of pro-inflammatory and anti-inflammatory cytokines in moMФ. Proinflammatory properties of subtoxic doses of this heavy metal have been reported not only in our previous study on porcine intestinal cells [20], but also in several murine and human cell lines or primary cells (reviewed in [44]). In particular, Cd2+ doses similar to those used in our study (10 μM) lead to increased secretion of IL-1 β by murine macrophages (RAW 264.7 macrophages) [45]. We observed that 3 h post-exposure to this heavy metal induced a slight increased expression of two pro-inflammatory cytokines (IL-1 β and IL-6), although without statistical significance. Nevertheless, no statistically significant increase in any pro-inflammatory cytokines was observed in culture supernatants of Cd2+-treated moMФ and 24 h post-exposure to moderate Cd2+ doses lead to down-regulation of TNF-α and IL-12p40 expression. In addition, moderate dose of this heavy metal induced down-regulated expression of IFN-α. We might speculate that wild boar moMФ initially respond to this foreign invader through induction of pro-inflammatory cytokines, but then cadmium affect macrophage’s viability and negatively modulate the ability of this phagocytic cells to combat invading microbes.
Cadmium-mediated macrophage immune disfunction is also supported by our observation on TLRs expression: 24 h exposure to moderate doses of this heavy metal resulted in down-regulated expression of TLR3, TLR4, TLR7, TLR8, TLR9. These data suggest that this environmental pollutant decrease macrophage ability to detect PAMPs, with subsequent lower ability to fight foreign invaders.
We next evaluated Cd2+ impact on expression of four molecules with antimicrobial activities (CD14, MD2, BD1, and BD2) and two genes involved in TLR intracellular signaling (the adaptor protein MyD88 and p65), which promote induction of inflammatory cytokine genes [38]. We observed that moderate dose of this heavy metal induced a statistically significant decrease in CD14, BD2, MyD88, p65 expression, indicating that Cd2+ adsorption likely promoted a decrease of macrophage antimicrobial activities. These results are in accordance with those of Cox and collaborators (2016), which described that cadmium treatment decreased p65 activity in human moMФ; inhibition of the NF-k β pathway resulted in macrophage immune disfunction, likely increasing susceptibility to infection in vivo [42].
Finally, modulation of two genes involved in arginine metabolism was investigated: NOS2 encodes for the enzyme iNOS, which generates nitic oxide (NO) from arginine, whereas Arg-1 encode for an enzyme which catalyzes the hydrolysis of arginine to ornithine, with subsequent increase of polyamine synthesis, promoting tissue repair and remodeling [54]. iNOS and Arg-1 are regarded as hallmark of classical (M1) or alternative (M2) polarization, respectively [54]. M1 macrophages are mainly associated with pro-inflammatory and antimicrobial activities, on the other hand M2 macrophages are mostly involved in immunosuppression and wound healing functions [46]. A previous study showed that exposure to high doses of cadmium (50 μM CdCl2) for 3 h triggered up-regulation of TNF-α, NOS2, and down-regulation of IL-10, and Arg-1 in THP-1 macrophages, suggesting that this heavy metal polarize macrophages toward a pro-inflammatory classically activated phenotype [43]. On the contrary, we observed that exposure to this environmental pollutant resulted in null modulation of Arg-1 and instead decrease NOS2 expression (6 h and 24 h post-treatment). Differences are maybe linked to the type of macrophages used in the two different studies: THP-1 [43] and wild boar moMФ (our study). Our data suggest that wild boar moMФ exposed to Cd2+ did not polarize toward a pro-inflammatory M1 phenotype, on the contrary moderate dose of this heavy metal decreased macrophage’s antimicrobial defenses, supporting the other data generated in this study.
In conclusion, this wild species can help understanding how environmental factors, such heavy metal, shape immunity and can be a valid model to study the effects of this environmental pollutant on human innate immune system. Overall our data revealed that the exposure of macrophages to moderate doses (20 μM) of Cd2+ affected their viability and lead to down-regulated expression of pro-inflammatory cytokines, several TLRs, p65, NOS2 and other molecules with microbicidal activities, indicating that exposure to this heavy metal negatively affect macrophage’s immune functions, which potentially increased susceptibility to infection.
Author statement
Giulia Franzoni: Writing - Review & Editing; Conceptualization; Methodology; Formal analysis; investigation; data curation
Valentina Ciccotelli: Writing - Review & Editing; Conceptualization; Methodology; Formal analysis; investigation; data curation
Masiello Lucia: Review & Editing; Methodology; Formal analysis
De Ciucis Chiara Grazia: Review & Editing; Methodology; Formal analysis
Antonio Giovanni Anfossi: Review & Editing; Methodology; Formal analysis; data curation
Vivaldi Barbara: Resources; Data curaction; Editing
Ledda Mauro: Methodology; Formal analysis
Zinellu Susanna: Methodology; Formal analysis
Silvia dei Giudici: Data curaction; Investigation; Methodology
Berio Enrica: Review & Editing; Methodology; Formal analysis
Andreoli Tiziana: Review & Editing; Methodology; Formal analysis
Dellepiane Monica: Resources; Data curaction; Editing
Zoppi Simona: Data curation, Resources, Formal analysis; Editing
Masotti Chiara: Investigation, Methodology; Formal analysis
Crescio Maria Ines: Data curation, Resources, Formal analysis; Editing
Annalisa Oggiano: Review & Editing; Valisation; Resources;
Ercolini Carlo: Funding acquisition; Project administration; Editing
Razzuoli Elisabetta: Funding acquisition; Project administration; Supervision; Writing - Review & Editing; Conceptualization; Methodology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors want to thank Dr Giulia Mignone, Dr Fabrizio Lazzara and Dr Monica Ferraris (IZS of Piemonte, Liguria e Valle d’Aosta) for the skilful technical assistance; her work is gratefully acknowledged. This study was supported by Liguria Region, Italy, grant 12ALA.
Handling Editor: Dr. Aristidis Tsatsakis
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2022.01.009.
Contributor Information
Giulia Franzoni, Email: giulia.franzoni@izs-sardegna.it.
Valentina Ciccotelli, Email: valentina.ciccotelli@izsto.it.
Lucia Masiello, Email: lucia.masiello@izsto.it.
Chiara Grazia De Ciucis, Email: chiaragrazia.deciucis@izsto.it.
Antonio Giovanni Anfossi, Email: aanfossi@uniss.it.
Barbara Vivaldi, Email: barbara.vivaldi@izsto.it.
Mauro Ledda, Email: vetleddamauro@gmail.com.
Susanna Zinellu, Email: Zinellu@izs-sardegna.it.
Silvia Dei Giudici, Email: Silvia.DeiGiudici@izs-sardegna.it.
Enrica Berio, Email: enrica.berio@izsto.it.
Andreoli Tiziana, Email: tiziana.andreoli@izsto.it.
Monica Dellepiane, Email: monica.dellepiane@izsto.it.
Simona Zoppi, Email: simona.zoppi@izsto.it.
Chiara Masotti, Email: chiara.masotti@izsto.it.
Maria Ines Crescio, Email: mariaines.crescio@izsto.it.
Annalisa Oggiano, Email: annalisa.oggiano@izs-sardegna.it.
Carlo Ercolini, Email: carlo.ercolini@izsto.it.
Elisabetta Razzuoli, Email: elisabetta.razzuoli@izsto.it.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Thompson J., Bannigan J. Cadmium: toxic effects on the reproductive system and the embryo. Reprod. Toxicol. 2008;25:304–331. doi: 10.1016/j.reprotox.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Cabral M., Toure A., Garçon G., Diop C., Bouhsina S., Dewaele D., Cazier F., Courcot D., Tall-Dia A., Shirali P., Diouf A., Fall M., Verdian A. Effects of environmental cadmium and lead exposure on adults neighboring a discharge: evidences of adverse health effects. Environ. Pollut. 2015;206:247–255. doi: 10.1016/j.envpol.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 3.Genchi G., Sinicropi M.S., Lauria G., Carocci A., Catalano A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health. 2020;17:3782. doi: 10.3390/ijerph17113782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waalkes M. Cadmium carcinogenesis. Mutat. Res. 2003;533:107–120. doi: 10.1016/j.mrfmmm.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Rafati-Rahimzadeh M., Rafati-Rahimzadeh M., Kazemi S., Moghadamnia A.A. Cadmium toxicity and treatment: an update. Caspian J. Intern. Med. 2017;8:135–145. doi: 10.22088/cjim.8.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rusanov A.L., Smirnova A.V., Poromov A.A., Fomicheva K.A., Luzgina N.G., Majouga A.G. Effects of cadmium chloride on the functional state of human intestinal cells. Toxicol. In Vitro. 2015;29:1006–1011. doi: 10.1016/j.tiv.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Tchounwou P.B., Yedjou C.G., Patlolla A.K., Sutton D.J. In: Luch A., editor. vol 101. Springer; Basel: 2012. Heavy metal toxicity and the environment. (Molecular, Clinical and Environmental Toxicology. Experientia Supplementum). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seralini G.E., Jungers G. Endocrine disruptors also function as nervous disruptors and can be renamed endocrine and nervous disruptors (ENDs) Toxicol. Program Tech. Rep. Ser. 2021;8(1538-):1557. doi: 10.1016/j.toxrep.2021.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akara Y., Ahmadb N., Khalidc M. The effect of cadmium on the bovine in vitro oocyte maturation and early embryo development. Int. J. Vet. Sci. Med. 2018;6:S73–S77. doi: 10.1016/j.ijvsm.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kowalczyk J., Numata J., Zimmermann B., Klinger R., Habedank F., Just P., Schafft H., Lahrssen-Wiederholt M. Suitability of Wild Boar (Sus scrofa) as a bioindicator for environmental pollution with perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) Arch. Environ. Contam. Toxicol. 2018;75:594–606. doi: 10.1007/s00244-018-0552-8. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Gomez X., Cambeiro-Perez N., Figueiredo-Gonzalez M., Martínez-Carballo E. Wild boar (Sus scrofa) as bioindicator for environmental exposure to organic pollutants. Chemosphere. 2020;268 doi: 10.1016/j.chemosphere.2020.128848. [DOI] [PubMed] [Google Scholar]
- 12.Chiari M., Cortinovis C., Bertoletti M., Alborali L., Zanoni M., Ferretti E., Caloni F. Lead, cadmium and organochlorine pesticide residues in hunted red deer and wild boar from northern Italy. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015;32:1867–1874. doi: 10.1080/19440049.2015.1087058. [DOI] [PubMed] [Google Scholar]
- 13.Mulero R., Cano-Manuel J., Ráez-Bravo A., Pérez J.M., Espinosa J., Soriguer R., Fandos P., Granados J.E., Romero D. Lead and cadmium in wild boar (Sus scrofa) in the Sierra Nevada natural Space (southern Spain) Environ. Sci. Pollut. Res. 2016;23:16598–16608. doi: 10.1007/s11356-016-6845-4. [DOI] [PubMed] [Google Scholar]
- 14.Bilandizić N., Sedak M., Vratarić D., Perić T., Simić B. Lead and cadmium in red deer and wild boar from different hunting grounds in Croatia. Sci. Total Environ. 2009;407:4243–4247. doi: 10.1016/j.scitotenv.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Lehmann I., Sack U., Lehmann J. Metal ions affecting the immune system. Metal ions in life science. 2011;8:157–185. [PubMed] [Google Scholar]
- 16.So K.Y., Lee B.H., Oh S.H. The critical role of autophagy in cadmium-induced immunosuppression regulated by endoplasmic reticulum stress-mediated calpain activation in RAW264.7 mouse monocytes. Toxicology. 2017;27:15–25. doi: 10.1016/j.tox.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Hurtenbach U., Oberbarnscheidt J., Gleichmann E. Modulation of murine T and B cell reactivity after short-term cadmium exposure in vivo. Arch. Toxicol. 1988;62:22–28. doi: 10.1007/BF00316252. [DOI] [PubMed] [Google Scholar]
- 18.Simonet M., Berche P., Fauchere J.L., Veron M. Impaired resistance to Listeria monocytogenes in mice chronically exposed to cadmium. Immunology. 1984;53:155–163. [PMC free article] [PubMed] [Google Scholar]
- 19.Luevano J., Damodaran C. A review of molecular events of cadmium-induced carcinogenesis. J. Environ. Pathol. Toxicol. Oncol. 2014;33:183–194. doi: 10.1615/jenvironpatholtoxicoloncol.2014011075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razzuoli E., Mignone G., Lazzara F., Vencia W., Ferraris M., Masiello L., Vivaldi B., Ferrari A., Bozzetta E., Amadori M. Impact of cadmium exposure on swine enterocytes. Toxicol. Lett. 2018;287:92–99. doi: 10.1016/j.toxlet.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Hume D.A. The many alternative faces of macrophage activation. Front. Immunol. 2015;6:370. doi: 10.3389/fimmu.2015.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pabst R. The pig as a model for immunology research. Cell Tissue Res. 2020;380:287–304. doi: 10.1007/s00441-020-03206-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapentanovic R., Fairbairn L., Beraldi D., Sester D.P., Archibald A.L., Tuggle C.K., Hume D.A. Pig bone marrow-derived macrophages resemble human macrophages in their response to bacterial lipopolysaccharide. J. Immunol. 2012;18:3382–3394. doi: 10.4049/jimmunol.1102649. [DOI] [PubMed] [Google Scholar]
- 24.Kapentanovic R., Fairbairn L., Downing A., Beraldi D., Sester D.P., Freeman T.C., Tuggle C.K., Archibald A.L., Hume D.A. The impact of breed and tissue compartment on the response of pig macrophages to lipopolysaccharide. BMC Genom. 2013;14:581. doi: 10.1186/1471-2164-14-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fairbairn L., Kapetanovic R., Sester D.P., Hume D.A. The mononuclear phagocyte system of the pig as a model for understanding human innate immunity and disease. J. Leukoc. Biol. 2011;8:855–871. doi: 10.1189/jlb.1110607. [DOI] [PubMed] [Google Scholar]
- 26.Dei Giudici S., Franzoni G., Bonelli P., Bacciu D., Sanna G., Angioi P.P., Ledda M., Pilo G., Nicolussi P., Oggiano A. Interaction of historical and modern Sardinian African swine fever viruses with porcine and wild-boar monocytes and monocyte-derived macrophages. Arch. Virol. 2019;164:739–745. doi: 10.1007/s00705-018-04140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franzoni G., Kurkure N.K., Edgar D.S., Everett H.E., Gerner W., Bodman-Smith K.B., Crooke H.R., Graham S.P. Assessment of the phenotype and functionality of porcine CD2+8 t cell responses following vaccination with live attenuated classical swine fever virus (CSFV) and virulent CSFV challenge. Clin. Vaccine Immunol. 2013;20:1604–1616. doi: 10.1128/CVI.00415-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franzoni G., Anfossi A., De Ciucis C.G., Mecocci S., Carta T., Dei Giudici S., Fruscione F., Zinellu S., Vito G., Graham S.P., Oggiano A., Chessa B., Razzuoli E. Targeting toll-like receptor 2: polarization of porcine macrophages by a mycoplasma-derived Pam2cys lipopeptide. Vaccines. 2021;9:692. doi: 10.3390/vaccines9070692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franzoni G., Dei Giudici S., Loi F., Sanna D., Floris M., Fiori M., Sanna M.L., Madrau P., Scarpa F., Zinellu S., Giammarioli M., Cappai S., De Mia G.M., Laddomada A., Rolesu S., Oggiano A. African swine fever circulation among free-ranging pigs in Sardinia: data from the eradication program. Vaccines. 2020;8:549. doi: 10.3390/vaccines8030549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Razzuoli E., Franzoni G., Carta T., Zinellu S., Amadori M., Modesto P., Oggiano A. Modulation of type I interferon system by african swine fever virus. Pathogens. 2020;9:361. doi: 10.3390/pathogens9050361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carta T., Razzuoli E., Fruscione F., Zinellu S., Meloni D., Anfossi A., Chessa B., Dei Giudici S., Graham S.P., Oggiano A., Franzoni G. Comparative phenotypic and functional analyses of the effects of IL-10 or TGF-β on porcine macrophages. Animals. 2021;11:1098. doi: 10.3390/ani11041098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mecocci S., Porcellato I., Armando F., Mechell L., Brachelente C., Pepe M., Gialletti R., Passeri B., Modesto P., Ghelardi A., Capelli K., Razzuoli E. Equine genital squamous cell carcinoma associated with EcPV2 infection: RANKL pathway correlated to inflammation and wnt signaling activation. Biology. 2021;10:244. doi: 10.3390/biology10030244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheuhammer A.M. The chronic toxicity of aluminum, cadmium, mercury and lead in birds: a review. Environ. Pollut. 1987;46:263–296. doi: 10.1016/0269-7491(87)90173-4. [DOI] [PubMed] [Google Scholar]
- 34.Oishi Y., Manabe H. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018;30:511–528. doi: 10.1093/intimm/dxy054. [DOI] [PubMed] [Google Scholar]
- 35.Isaacs A., Lindenmann J. Virus interference. I. The interferon. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1957;147:258–267. [PubMed] [Google Scholar]
- 36.Miyake K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin. Immunol. 2007;19:3–10. doi: 10.1016/j.smim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Luchner M., Reinke S., Milicic A. TLR agonists as vaccine adjuvants targeting Cancer and infectious diseases. Pharmaceutics. 2021;22:142. doi: 10.3390/pharmaceutics13020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilmore T.D. Introduction to NF-κB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 39.Petersen C.B., Nygård A.B., Fredholm M., Aasted B., Salomonsen J. Cloning, characterization and mapping of porcine CD14 reveals a high conservation of mammalian CD14 structure, expression and locus organization. Devel. Comp. Immunol. 2006;31:729–737. doi: 10.1016/j.dci.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 40.Veldhuizen E.J., Rijnders M., Claassen E.A., van Dijk A., Haagsman H.P. Porcine beta-defensin 2 displays broad antimicrobial activity against pathogenic intestinal bacteria. Mol. Immunol. 2008;45:386–394. doi: 10.1016/j.molimm.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Modesto P., De Ciucis C.G., Vencia W., Pugliano M.C., Mignone W., Berio E., Masotti C., Ercolini C., Serracca L., Andreoli T., Dellepiane M., Adriano D., Zoppi S., Meloni D., Razzuoli E. Evidence of antimicrobial resistance and presence of pathogenicity genes in Yersinia enterocolitica isolate from wild boars. Pathogens. 2021;10:398. doi: 10.3390/pathogens10040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cox J.N., Rahman M.A., Bao S., Liu M., Wheeler S.E., Knoell D.L. Cadmium attenuates the macrophage response to LPS through inhibition of the NF-κB pathway. Am. J. Physiol. Lung Cell Mol. Physiol. 2016;311:L754–L765. doi: 10.1152/ajplung.00022.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larson-Casey J.L., Gu L., Fiehn O., Carter A.B. Cadmium-mediated lung injury is exacerbated by the persistence of classically activated macrophages. J. Biol. Chem. 2020;295:15754–15766. doi: 10.1074/jbc.RA120.013632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olszowski T., Baranowska-Bosiacka I., Gutowska I., Chlubek D. Pro-inflammatory properties of cadmium. Acta Biochim. Pol. 2012;59:475–482. [PubMed] [Google Scholar]
- 45.Riemschneider S., Herzberg M., Lehmann J. Subtoxic doses of cadmium modulate inflammatory properties of murine RAW 264.7 macrophages. Biomed Res. Int. 2015 doi: 10.1155/2015/295303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mosser D.M. The many faces of macrophage activation. J. Leukoc. Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 47.Cheng G., Chen W., Li Z., Yan W., Zhao X., Xie J., Liu M., Zhang H., Zhong Y., Zheng Z. Characterization of the porcine alpha interferon multigene family. Gene. 2006;382:28–38. doi: 10.1016/j.gene.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 49.Razzuoli E., Villa R., Sossi E., Amadori M. Reverse transcription real‐time PCR for detection of porcine interferon α and β genes. Scand. J. Immunol. 2011;74:412–418. doi: 10.1111/j.1365-3083.2011.02586.x. [DOI] [PubMed] [Google Scholar]
- 50.Razzuoli E., Villa R., Amadori M. IPEC-J2 cells as reporter system of the anti-inflammatory control actions of interferon-alpha. J. Interferon Cytokine Res. 2013;33:597–605. doi: 10.1089/jir.2012.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Razzuoli E., Amadori M., Lazzara F., Bilato D., Ferraris M., Vito G., Ferrari A. Salmonella serovar-specific interaction with jejunal epithelial cells. Vet. Microbiol. 2017;207:219–225. doi: 10.1016/j.vetmic.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Yoo I., Han J., Lee S., Jung W., Kim J.H., Kim Y.W., Kim H.J., Hong M., Ka H. Analysis of stage-specific expression of the toll-like receptor family in the porcine endometrium throughout the estrous cycle and pregnancy. Theriogenology. 2019;125:173–183. doi: 10.1016/j.theriogenology.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 53.Ebrahimi M., Khalili N., Razi S., Keshavarz-Fathi N., Khalili N., Rezaei N. Effects of lead and cadmium on the immune system and cancer progression. Journal of Environmental Health Science and Engineering. 2020;18:335–343. doi: 10.1007/s40201-020-00455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rath M., Muller I., Kropf P., Closs E., Munder M. Metabolism via arginase or nitric oxide synthase: Two competing arginine pathways in macrophages. Front. Immunol. 2014;5:532. doi: 10.3389/fimmu.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.