Abstract
Lignocellulosic biomass generated from different sectors (agriculture, forestry, industrial) act as biorefinery precursor for production of second-generation (2G) bioethanol and other biochemicals. The integration of various conversion techniques on a single platform under biorefinery approach for production of biofuel and industrially important chemicals from LCB is gaining interest worldwide. The waste generated on utilization of bio-resources is almost negligible or zero in a biorefinery along with reduced greenhouse gas emissions, which supports the circular bioeconomy concept. The economic viability of a lignocellulosic biorefinery depends upon the efficient utilization of three major components of LCB—cellulose, hemicellulose and lignin. The heterogeneous structure and recalcitrant nature of LCB is main obstacle in its valorization into bioethanol and other value-added products. The success of bioconversion process depends upon methods used during pre-treatment, hydrolysis and fermentation processes. The cost involved in each step of the bioconversion process affects the viability of cellulosic ethanol. The lignocellulose biorefinery has ample scope, but much-focused research is required to fully utilize major parts of lignocellulosic biomass with zero wastage. The present review entails lignocellulosic biomass valorization for ethanol production, along with different steps involved in its production. Various value-added products produced from LCB components were also discussed. Recent technological advances and significant challenges in bioethanol production are also highlighted in addition to future perspectives.
Graphical abstract
Keywords: Biorefinery, Sustainable, Feedstock, Value-added Products, Waste Management
Introduction
Globally, the mankind is witnessing the most significant challenges with the utilization of fossil fuels in a more sustained way and the generation of energy from alternate and renewable sources. With a growing population and rising living standards, the energy demand is ever-increasing worldwide. The energy and chemicals produced from fossil-based resources are used universally for development; however, fossil fuel burning aggravates environmental challenges like global warming, climate change and pollution load. Therefore, finding alternative sources for energy and chemical generation in a clean and green way is need-of-the-hour [1]. One of the best alternatives to fossil-reliant resources is lignocellulosic biomass (LCB), the copious raw material to produce energy in a viable way. It minimizes dependency on petroleum-based fossil fuels [2]. LCB is obtained from plants and can be used as a substrate for biofuel production. It is an inexpensive, renewable resource that is abundantly available and acts as a resource for the extensive and economical production of bioenergy and other compounds [3]. LCB is a complex material made of three prime parts—cellulose, hemicellulose and lignin—and other components like pectin, protein, extractives and inorganic compounds minerals, which are also present in it a minimal quantity [4]. Cellulose and hemicellulose constitute 70–80% by dry weight, while lignin constitutes about 10–25% [5]. In the LCB complex, lignin being a recalcitrant requires specific pre-treatment to hydrolyze the cellulose and hemicellulose part into their respective sugars [2]. It can transform LCB components’ dissociation into simpler compounds that can be transformed into several value-added bioproducts and biofuels. Along with LCB, by-products from numerous industrial and human sectors (e.g. food industry, pulp and paper industry, municipal solid waste and bioethanol production) can be utilized as renewable resources for biorefinery and circular economy (CE) actualization [6]. According to many studies, the LCB has excellent potential for generating chemicals and fuels in a sustainable way [7, 8]. Being a bio-resource for energy production, LCB can support enormous possibilities after an intensive evaluation of production cost, availability and market demand [9]. The plant biomass, which contains 90% lignocellulosic materials, amount to 200 × 109 t/year, out of which only 8–20 × 109 tons is used potentially [10].
Moreover, the cost of lignocellulosic feedstock is much lesser than sugar and starch-based feedstocks [11, 12]. Since the production rate of LCB is high and the cost of feedstock is low, it is a competent source for value-added products and energy production. In several developing countries, the agricultural waste is not entirely discarded, and its disposal becomes a source of pollution. The waste biomass can be utilized as a feedstock for fuel generation, which reduces the problem of their management and provides a good option for bio-based processes [13]. To get various products from LCB, it should undergo several process steps. Pre-treatment breaks down LCB into reducing sugars, which can be utilized to produce biofuels like bioethanol, bio-hydrogen and biogas and different organic acids, phenols and aldehydes. Pre-treatment is followed by enzymatic breakdown of cellulose by cellulase enzyme for its conversion to bioethanol [3]. Therefore, there is a massive demand for cellulase enzymes, which can be further utilized in industries and research and development. The primary aim of bioethanol production from LCB is to reduce the cost of conversion technologies and to realize the potential of biorefineries in meeting our energy requirements. Bioethanol appears as a good and cheap replacement to the petrochemical fuels and received immense attention, considering its efficient conversion technology. Different LCB feedstocks are used in a biorefinery to recover bioenergy through relevant conversion technologies. Waste biomass can be used as feedstock in biorefinery, closing the loop of circular bioeconomy (CE) [14]. Agricultural and industrial lignocellulosic waste is recycled in second-generation biorefinery by various integrated processes and transformed into biomaterials (food, feed, chemicals) and bioenergy (biofuels, power and heat) in a sustainable way [15]. The primary goal of CE, depending on the reuse, reduce and recycling of waste, is to achieve a closed-loop system to obtain maximum value-added materials from waste.
The literature has witnessed different review articles over the years covering various aspects of lignocellulosic bioethanol production, lignocellulose biorefinery, CE, etc. Ferreira et al. [16] elaborated different combination methods for generating products from LCB and the leftover from the first-generation bioethanol on aiding co-generation techniques and conversion techniques from microbes. Awasthi et al. [17] conducted a critical evaluative review in organic manure recycling for circular bioeconomy that produce sustainable bioproducts like biogas and fertilizers. Zabaniotou and Kamaterou [18] assessed various possibilities and objections to change single process to a combined spent coffee grounds biorefinery. A study based on different waste biorefineries that lead to circular economies in developing countries was reviewed and performed by Nizami et al. [19]. Garlapati et al. [20] focused on value-added products from lignin under circular economy and lignocellulose biorefinery approach. Pant et al. [21] formulated research on the present growth of the bio-based economy among European countries and India. Yaashikaa et al. [22] discussed various sources of agro-industrial waste and different methodologies for its valorization along with life cycle analysis in agricultural circular bioeconomy. Technological improvements in bioethanol production along with its blending mandates and policies worldwide were reviewed by Raj et al. [23].
The production of bioethanol from LCB has some constraints like recalcitrant nature of biomass, cost of the production process and feasibility of the production technologies, so multidisciplinary developmental research is essential for minimizing the process’s total costs and reducing the environmental impacts of these technologies. Therefore, this review paper details the lignocellulosic sources and their compositional by-products along with value-added products production from each part. It also elucidates the circular bioeconomy concept in the bioethanol production process from LCB to reduce the total cost by co-generating other valuable products. The current review also highlights the challenges, technological advances and techno-economic analysis of bioethanol production process.
Biorefineries in Circular Bioeconomy
Circular Economy Action Plan (CEAP) [24] delineates CE as the preservation of products, specific resources and materials in the economy for a long time having minimum production of wastes. The aim of the circular economy and environment (CEE) unit is to achieve a sustainable approach by changing the current society to a sustainable one. The circular bioeconomy is the integration of CE and bioeconomy. The circular bioeconomy is focused on sustainable and resource-effective valorization of biomass in biorefineries along with utilization of wastes and residues through cascading [25]. Various raw materials such as minerals, metals, fossil carbons and biomass are converted to products, and their waste left are shared, reused, redistributed and recycled [26]. European Commission Action Plan [24] has also defined their key priorities, which are connected with the economy, including waste derived from food and transformation of biomass. Food waste is one of the significant areas in the CE and can be recognized as an essential part at various levels. Some of the food materials which are first digested and then excreted play a significant role in waste recycling, retrieval of energy or landfill disposal. Biodegradable products can include organic recycling and trap and utilize carbon (i.e. carbon recycling) [26]. The idea of CE emerged from reviewing the production process in the 1970s to 1980s and was built on industrial ecology [27]. In 1990, CE was much familiar and an opposing viewpoint regarding the linear economy, where the industrial effects on the environment were minimized [28]. The primary aim of CE is to re-shape the development of a product to minimize the negative impacts on the environment.
The concept of CE has gained much appreciation and importance in the last few years among various scholars, managers of public and private areas that showed an immediate increase in the publication number on this topic [29]. In 2014, articles published on CE were just 27 in the count, which rose to 371 in 2017, which showed an increase in the interest of academicians and researchers by 1275% in just 3 years [30]. Alhawari et al. [31] revealed that the total articles on CE were 1408 in 2020 as per the Scopus database. CE can lead to the sustainable growth of the economy by safeguarding the environment and preventing pollution. It becomes an integral part of an economic plan by saving the materials to facilitate innovative methods to change linear consumption methods to circular ones. CE is a collective term that encloses all the activities related to reducing, reusing and recycling in production and consumption procedures. Additionally, it may be described as a production and consumption process in which the loss is minimized by using reuse, recycling and recovery technology often. The implementation of CE principles brings many benefits for the environment and society, such as reduced use of resources, less production of waste and limited energy consumption, which directly led to sustainable growth [32].
Biorefinery is elucidated by the International Energy Agency (IEA) as ‘the processing of different biomass to various biomass-based materials such as food, chemicals, feed and bioenergy such as biofuels and heat in a sustainable way’ [33]. Biomass is a replenishable carbon source and has several benefits: carbon sequestration, bioenergy generation and bioproducts. Biomass can be used as a valuable raw material in a biorefinery only if well planned and systematic techniques of conversion and valorization are available. All types of biomass generated from agriculture, aquaculture, industries and households can be used in biorefinery. Biomass is a good energy source with an abundant supply but low energy density. Low calorific value, seasonality and geographic location are barriers to biomass utility [34]. However, using multi-criteria analysis could select suitable locations for the instalment of biorefineries. The integrated biorefinery method transforms the various conversion wastes into valuable bioproducts. A biorefinery is a basic framework provision designed for biomass raw feed such as LCB, algae and other wastes, where all the conversion techniques are integrated in a systematic way which leads to the production of sustainable bioproducts such as bioenergy, biofuels, biochemicals and other valued-added bioproducts [16, 35]. Although biorefinery is a renewable method used to transform biomass by different treatment methods to value-added products using different technologies, its execution and application mainly depend on its availability, characteristics and economic value.
A literature review by Clark and Deswarte [36] showed various generations of biorefinery being mentioned in the study:
Stage I biorefinery (single raw material, one procedure and one primary product)
Stage II biorefinery (single raw material, many procedures and many significant products)
Stage III biorefinery (many raw materials, many procedures and many significant products)
A good biorefinery must be the one that leaves minimum or no waste during a process. A zero-waste biorefinery must follow the proper steps of fractionation and extraction with a combined biochemical and thermal processes approach where recycling of energy and waste streams should be continuous. Zero-waste biorefinery needs the skills and proficiency from different interdisciplinary fields such as chemistry, biochemistry, chemical engineering, biology, biomolecular engineering and materials sciences. Zero waste biorefinery is a combined substructure of different techniques joined in sequential steps to transform bio-based material into valuable bio-based products such as biofuels, certain chemicals and power, similar to that of petro-refinery. Various ‘waste cycles’ are similar to the natural cycles operating in the ecosystem. These waste cycles keep circulating different materials in a controlled manner to nourish the ecosystem. Various bio-processes like retrieval of minerals, acidogenesis, bioelectrogenesis, methanogenesis, photosynthesis and thermocatalytic processes work in a well-controlled system, where the waste generated from one process becomes raw material for another [37]. Combining the various procedures mentioned above in a particular format can increase the possibility of getting resources out of a residue, thus giving rise to a circular bioeconomy. Effective combined photosynthetic equipment such as algal mass and photosynthesizing bacteria and techniques as microbial decarbonization are employed, providing a good opportunity to develop the bioeconomy required for gaseous residues [38]. Improved unification of biological and technical nutrient patterns gives extra profits and substitute nutrients for the environment and industry. Different waste biorefinery concepts may use various feedstocks from wastes. The waste biorefinery system efficiently gives rise to various current utilization of fossil-based stocks to the renewable or ‘green’ resources with additional sustainability benefits [37].
Lignocellulosic Biorefinery
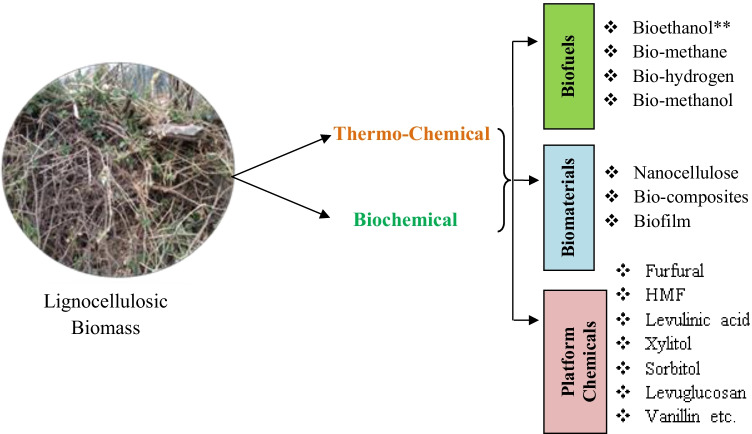
LCB comes under second-generation biomass feedstocks and provides a better option than first-generation feedstocks that need a separate area for their growth. As depicted through the yearly index, the global annual production of LCB is around 1.3 billion tons, out of which bio-based chemicals, bio-based energy and non-food bioproducts accounts for only 3% [39]. LCB is a recalcitrant structure than starch-based feedstocks poses a challenge at industrial level. LCB biorefinery is a profitable approach to produce multiple products by LCB fractionation [12]. Figure 1 elucidates various routes for the conversion of LCB into different value-added products. Among all the feedstocks, the primary source of LCB is rice straw, sugarcane bagasse, barley straw, rice straw, coconut husk, corn stalk, sorghum, wheat straw, fruit bunch and wood, etc. According to De Bhowmick et al. [40], lignocellulosic biorefinery can be employed as a platform to highlight the manufacturing of value-added products and various biofuels sustainably concerning sustainable development. Lignin valorization is also an attractive approach in circular bioeconomy concept of LCB to increase the economy of the biorefinery. Out of 224 biofuel biorefinery, 43 were based on LCB in Europe.
Fig. 1.
Different routes of formation of value-added products from lignocellulosic biomass for circular economy
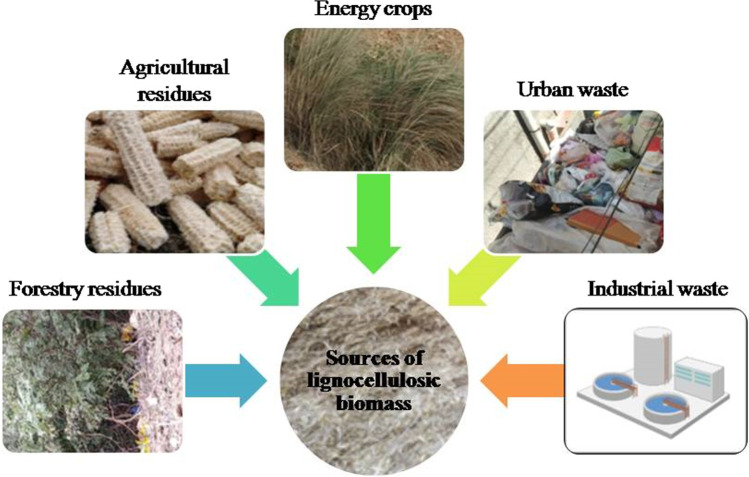
LCB is a complex structure with a significant portion of three polymers cellulose, hemicellulose and lignin and a minor amount of pectin, protein, extractives, inorganic compounds minerals and phenolic substituents. However, each polymeric component of LCB has a different composition. LCB, either retrieved from cultivated plants or in residue, is mainly collected from abundantly available perennial herbaceous plant species, woody crops and other plant constituents. Hemicellulose acts as a matrix covering the cellulose, while the layer of lignin encrusts them and protects both the other components. All three components are cross-linked by covalent bonds and form a composite structure. It is widely available in the forest, urban refuse, rural farms and even organic waste generated from agro-based industries. Different sources of LCB which can be used as feedstock for ethanol production are shown in Fig. 2. The majority of sources for LCB are the following:
Forestry residues: Forests, whether natural or cultivated, are rich sources of fuel, timber, wood and charcoal. Residues that remained after removing stem wood during harvesting, such as roots, branches and foliage, can be used as a source of lignocellulosic biomass. The annual production of woody biomass is about 4.6 Gt worldwide [41].
Agricultural residues: Crops are available in abundance as the natural source, and it is even easy to collect and store. Some residues include rice husks, wheat straw, corn cobs, cotton sticks, sugarcane bagasse, groundnut and coconut shells. These can be applied to produce second-generation biofuels. In 2018, about 683 million tons of crop residues from different crop harvesting were produced in India, which were used for different purposes like fuel, fodder and industrial processes [42].
Energy crops: Energy farming is done to produce energy crops. These are the fast-growing plants that can be used to produce gaseous and liquid biofuels. Such crops are grown to produce biomass, which can be further used in biorefinery areas.
Animal waste: It is an organic material and has a combustible nature and a rich fuel source. Dung cakes used for cooking are substrates for biogas, especially in rural areas.
Urban waste: The waste material includes two primary forms—(1) municipal solid waste from household garbage, kitchen and garden waste and (2) liquid waste that emerges from domestic sewage and effluents. Sewage is mainly processed for the production of biogas. In India, the approximate potential for energy generation from urban solid waste and liquid waste was 1247 MW and 375 MW, respectively (https://www.mnre.gov.in/waste-to-energy/current-status, Retrieved on 10 February 2021).
Industrial waste: The waste material produced from various industrial units, including pulp and paper industry effluent, starch and glucose industry waste, palm oil industry and distillery and tannery, can be a source for the production of biofuels. The primary feedstock used in the pulp and paper industry is LCB. The industrial units mainly emphasize on separation of lignin and cellulose of the biomass. The total potential for energy production from urban and industrial organic waste was 5690 MW approximately in India (https://www.mnre.gov.in/waste-to-energy/current-status, Retrieved on 10 February 2021).
Biowaste streams: It includes household waste, municipal solid waste, market waste, packaging waste, food processing waste, etc.
Fig. 2.
Various sources of lignocellulosic biomass
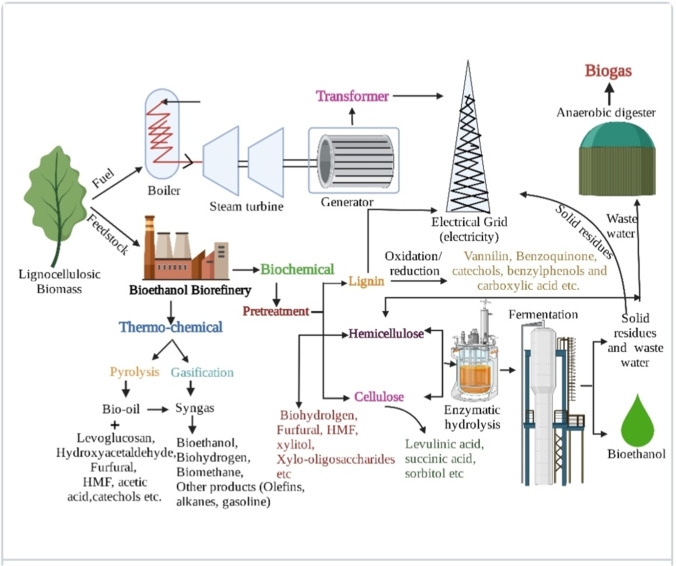
By employing thermochemical, biochemical and mechanical techniques on the above-mentioned LCB sources, different value-added products like biomethane, bio-hydrogen, bioethanol, bio-char and organic acids have been generated (Fig. 1). Pyrolysis and gasification are the thermo-chemical methods that decompose the biomass at high temperature and resulted into syn gas (CO + H2), bio-char and bio-oil which on further processing produce methanol, ethanol, bio-hydrogen and other chemicals (aromatics, phenols, olefins, alcohols, etc.). Biochemical processing of LCB generates bioethanol through fermentation, biomethane and bio-hydrogen through anaerobic digestion. All these products of biochemical conversion can be co-generated by following process integration (discussed in the next sections). The mechanical method includes extraction, fractionation and pelletization through which other value-added products can be obtained like furfural, hesperidin, nanocellulose, lignin residues and organic acids [43].
Value-Added Products from LCB Components
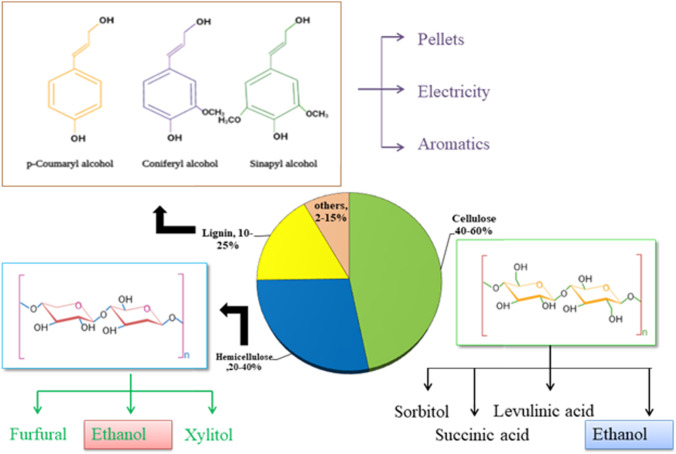
LCB is a potent feedstock, but its degradation is tricky because of its complex, non-uniform and three-dimensional structure. The compositional content of LCB and the value-added products produced from the biomass are shown in Fig. 3. LCB is mainly made of two types of polymers: (a) carbohydrate polymer (cellulose and hemicellulose) and (b) aromatic polymer (lignin). The valuable products obtained from different components of LCB are the following:
Fig. 3.
Generation of different value-added products from the constituents of the lignocellulosic biomass along with their composition
Cellulose
Cellulose is the significant biomass component and constitutes about 30–50% of the total dry matter of lignocellulose. The repeating unit in cellulose is disaccharide cellobiose, consisting of two glucose molecules. Cellulose is a C6 sugar mainly made of glucopyranosyl monomeric units connected by 1–4 β glycosidic bonds. The structure of cellulose appears to be flat sheet type with glucose monomer units. Furthermore, many cellulose strands are packed into crystalline fibrils. Cellulose structure consists of many intra-molecular and intermolecular hydrogen bonds with which the glucose unit is bound tightly [44]. The main forces of interaction between cellulose sheets are Vander Waal forces. At the same time, the cellulose polymeric units have two different types of linkages, including glycosidic linkage (considered as ether bond) and hydrogen bond linkage (between two hydroxyl groups) [45]. The glycosidic bond connects the glucose units, while the hydrogen bond connects the straight chains of the polymer. Cellulose has a high degree of polymerization, due to which it has low flexibility and is primarily insoluble in water and most of the solvents [46].
The value-added products from cellulose were ethanol, sorbitol, levulinic acid (LA), succinic acid (SA), levoglucosan, hydroxyacetaldehyde, etc. LA is produced by acid hydrolysis of C6 sugars. Pileidis and Titirici [47] demonstrated the LA production at a large scale. La has potential applications in pharmaceuticals, fragrances, cosmetics, plasticizers, etc. Several companies like Maine Biochemicals, Avantium and Segetis demonstrated and investigated the production of LA from cellulosic biomass. SA is also produced by glucose fermentation by Escherichia coli by Roquette/DSM and BioAmber at a commercial scale [48]. The market value of SA is USD 147.42 million in 2020 and is expected to reach USD 268.8 million in 2028 at 8%CAGR from 2021 to 2028 [49]. Sorbitol is produced by transition metal-catalyzed hydrogenation of glucose and has been used in the production of cosmetics, confectionaries, ascorbic acid, industrial surfactants, pharmaceuticals, healthcare products, etc. Roquette, a French-based company, is the largest producer of sorbitol [50].
Hemicellulose
Hemicellulose is the second primary polymer of LCB. Its structure is amorphous and composed of various heteropolymers, including xylan, galactomannan, arabinoxylan and xyloglucan [51]. Hemicellulose appears branched in structure with the availability of functional groups like acetyl, methyl, glucuronic acid and galacturonic acid. Hardwood hemicellulose contains xylan, and softwood hemicellulose mainly contains glucomannans. The main forces of interaction in hemicellulose are non-covalent. It is attached to cellulose fibrils’ surface, thus appearing as an amorphous matrix material. It forms a complex of bonds in the plant cell wall, which provides robustness by associating cellulose fibrils to microfibrils and cross-links with lignin [52].
Compared to cellulose, hemicellulose is easily hydrolyzed due to the lesser degree of polymerization. Hemicellulose has short chains of sugars, which distinguishes it from cellulose. Therefore, it can be extracted quickly and used to produce bioethanol [45]. Xylan is the most known polymer belonging to the hemicellulose polysaccharide family. Xylan molecule has 1–4 xylopyranosyl linked to α-4-O-methyl-D-glucuronopyranosyl units and is a branched-chain polymer having a 5-carbon sugar monomer [53]. Most of the hemicellulose is soluble in alkaline solvents.
Various value-added products, such as lactic acid, xylitol, xylo-oligosaccharides, furfural, hydroxymethylfurfural (HMF) and polyhydroxyalkanoates, were obtained from extracted hemicellulose or its hydrolysate. Lactic acid is one of the industrially known organic acids and has many applications in manufacturing cosmetics, pharmaceuticals and biopolymers. Furfural and HMF is the dehydration product of hemicellulose. Furfural can be generated from 5-HMF through cracking reactions or by splitting the hemiacetal bonds in the xylan depolymerization [54]. Xylitol is also a popular sweetener (low calorie) produced from xylose bioconversion and used in toothpaste, chewing gums, diabetic products and dental preventive products. Xylo-oligosaccharides are non-digestible dietary fibres with potential prebiotic activity and health benefits [55].
Lignin
It is a three-dimensional, non-carbohydrate phenolic polymer of phenylpropanoid units and constitutes about 15–20% of feedstock matter. It is found in the secondary cell wall and acts as a cellular adhesive. Lignin further provides toughness to the plant tissue and fibres and binds them together. The presence of lignin made the cell wall stiff and resistant to insects and pathogens [51]. Lignin does not take part in the fermentation process; instead, it is used to extract other chemicals, which are majorly used as a part of biorefinery [20]. The lignin polymer consists of three phenylpropanoid monomeric subunits: guaiacyl (G), syringyl (S) and p-hydroxyphenyl (H) [46]. The presence of these subunits distinguishes its composition from others, such as softwood or hardwood, and significantly impacts the delignification process. Different monomers in layers of lignin are due to secondary cell wall deposition, when oxidative coupling of 4-hydrophenylpropanoids takes place, leading to the high heterogeneity of lignin. LCB is linked by four different types of linkages, carbon to carbon bonds, ether bonds, ester bonds and hydrogen bonds [52]. They form intra-polymer linkage (individual components) and inter-polymer linkage (different components) to form complexity in lignocellulose [20].
Lignin is used for energy purposes, mainly as fuel to boilers for pulp production. It is of great interest for biorefineries to produce lignin-derived value-added products. Lignol Innovations Company used wood and agricultural residues for bioethanol and lignin (high purity) production in their pilot plant. It has great potential on a large scale in substituting the petroleum-based products utilized to manufacture industrial coatings, gels and emulsifiers, etc. [56]. Vanillin is also generated from lignin by treating its aqueous solution with oxidants at basic pH and high pressure and temperature. Vanillin is a flavouring agent in the pharmaceutical and food industries. In addition to the flavouring property of vanillin, it also possesses antioxidant, antimicrobial, anticarcinogenic and anti-slicking properties made it an attractive intermediate for the production of polymers and fine chemicals.
Furthermore, benzoquinone and carboxylic acid are also produced from the oxidation of lignin. The former has application in dye manufacturing, a prime component in the biologically active compound, supercapacitors electrolytes, etc. The latter is used as a precursor in the food, polymer and pharmaceutical factories. The thermal depolymerization process also converts lignin to bio-oil, methanol and syngas. Alkozyphenols, benzyl phenols, catechols and methoxy phenols are also formed from lignin by the reductive depolymerization process [54, 57].
Bioethanol Production from LCB
Bioethanol is a colourless and flammable liquid that is an inexhaustible source and an alternative to petroleum fuels. Developing bioethanol as fuel is because ethanol is produced from biomass which is both renewable and sequesters carbon dioxide during production, resulting in no net release of carbon dioxide into the atmosphere. It is also used to enhance octane numbers in unleaded gasoline. Moreover, it is also used to mix with other fuels to clean-burn gasoline with oxygen additives. Therefore, these biofuels help reduce pollution and improve the quality of air [58, 59]. For many years, it has been well known that alcohol has been produced from natural and agricultural products rich in starch and sugar content. Microorganisms were allowed to grow in the carbohydrate feedstock for transformation to ethanol. The polysaccharide is a polymer of monomer units, including fructose and glucose, which are first hydrolyzed by enzymes and then fermentation to generate ethanol. The annual production of fuel ethanol at the global level has enhanced from 25,700 million gallons in 2015 to 26,059 million gallons in 2020 (ethanolrfa.org), with a bit of dip of about 10% that might be due to the COVID-19 pandemic. Most of the ethanol supply (80%) comes from Brazil and USA, using corn and sugarcane as feedstock. However, in developing countries, the material used for bioethanol production comes from non-food materials like cassava and sweet sorghum.
LCB is a competent feedstock for second-generation bioethanol production and is an excellent substitute for first-generation feedstock. LCB is widely available in large amounts worldwide and is found in the form of peels, leaves and branches. Hence, bioethanol produced from LCB is a promising fuel, especially for the countries that generate a lot of agricultural and forestry waste. Bioethanol is produced from lignocellulosic feedstock through two main processes including the following:
The thermochemical process
includes processes like pyrolysis or gasification that produce synthesis gas (CO + H2), as shown in Fig. 1. The gas produced is assorted with a catalyst and employed to generate ethanol and other liquid co-products. At temperatures 500–600 °C, biomass pyrolysis produces bio-oil whose hydroprocessing converted it to the precursors for drop-in fuels. At 700 °C, biomass gasification resulted in syngas that converted to bioethanol by metallic catalyst (aluminium, cobalt, etc.) through Fischer–Tropsch synthesis or microorganisms. Methanol production was first noticed in some cases, which on catalytic shift produce ethanol and provides 80% yield of it. Syngas solubility and gas to liquid mass transfer are barriers to the commercialization of syngas fermentation technology. Ethanol recovery cost is also very high, which will become cost-effective if the ethanol concentration is 15% (v/v) [60].
Biochemical process
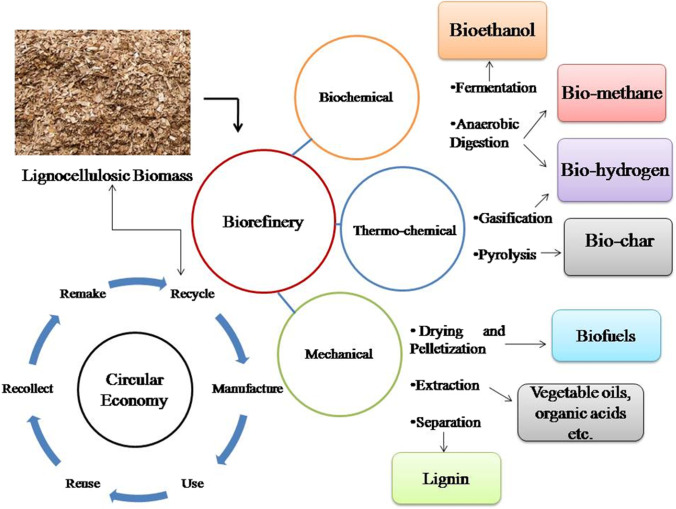
In this technique, specialized microorganisms and enzymes convert lignocellulosic components to sugars and then ferment them to produce ethanol. The schematic diagram of the biochemical process of ethanol production has been mentioned in Fig. 4.
Fig. 4.
Schematic diagram of the biochemical process of bioethanol production
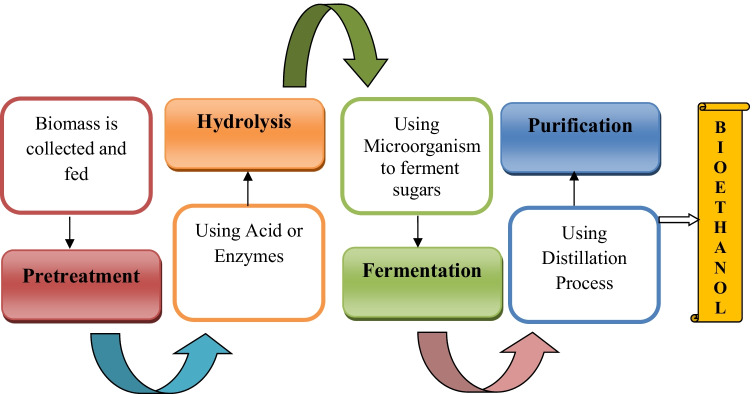
The biochemical process is employed for the conversion of LCB into ethanol via four significant steps which are the following:
1. Pre-treatment where biomass is treated to make it suitable for hydrolysis.
2. Enzymatic hydrolysis to break down cellulose into simpler sugars.
3. Fermentation is which various microbes are used to ferment sugars to produce ethanol.
The following steps are discussed in detail:
Pre-treatment
It is a significant step in bioethanol production from LCB as the biomass structure is complex, which needs specific treatment to open it. There are four types of pre-treatment methods, i.e. physical, chemical, physicochemical and biological, used to disrupt the biomass structure and enhance its accessibility. The ideal pre-treatment method is the one that provides good sugar yield with less inhibitor production in an economical way.
Physical Pre-treatment
In this pre-treatment method, the surface area of biomass increased due to reduction in size and reduction in crystallinity index of the cellulose by techniques like mechanical comminution, extrusion and freezing. Each technique needs some optimum conditions, and a specific tool for biomass degradation like comminution needs milling tools, freezing needs low-temperature apparatus and extrusion needs extruder. Overall, physical methods are efficient, but the expense and power requirement is much higher than causes their restricted use. Zhang et al. [61] applied the extrusion pre-treatment on rice hull for enhanced hydrolysis yield. The optimum extrusion conditions used for the pre-treatment were 143 °C temperature, screw speed of 350 rpm and material diameter and moisture of 60 mesh and 29%, respectively. Huang et al. [62] applied ball milling on LCB (bagasse and Pennisetum) along with a small amount of alkali. The results revealed that the small alkali dosage could enhance the saccharification of bagasse and Pennisetum. Meng and Wang [63] used vacuum freeze-drying to pre-treat biomass, i.e. switchgrass, wheat straw and poplar. This study blended the samples with distilled water and frozen at –20 °C, followed by drying samples at − 52.1 °C. The results showed an enhanced pore structure of biomass through the water to ice transformation.
Chemical Pre-treatment
The chemical pre-treatment method includes the application of chemicals like acids, alkalis, solvents and oxidants. Different chemicals react with the biomass and disrupt it according to their characteristics. The chemical agents like acids (H2SO4, HCl, H3PO4, HNO3, etc.) break the hemicellulose linkages under mild temperature conditions, increasing biomass porosity. Junior et al. [64] pre-treated the sugarcane biomass with phosphoric acid, resulting in fermentable sugars with 98% glucose in the hemicellulose hydrolysate. Similarly, alkali pre-treatment led to the rupturing of the lignin bonds and enhanced the accessible surface area of the biomass. Goshadrou et al. [65] investigated the effect of alkali on cogongrass and found that it improved enzymatic hydrolysis from 24.8 up to 90.8%. Organosolv method includes the organic solvents, i.e. acetone, methanol and ethanol, employed along with the organic acid catalysts for lignin elimination and increased solubilization. Tsegaye et al. [66] used organosolv pre-treatment to remove lignin and solubilization of the polysaccharides that resulted in the release of 74.09% of cellulose and 73.17% and 46.62% of the lignin and hemicellulose solubilization, respectively. The acid, alkali and solvent pre-treatment methods are effective but have a drawback of corrosion, neutralization and solvent removal. However, both ozonolysis and oxidative delignification depend on the oxidants. Ozonolysis is a specific and selective method and utilizes ozone as an oxidant to remove lignin without affecting cellulose and hemicellulose [1]. However, oxidative delignification is not selective and uses ozone, air and hydrogen peroxide for lignin elimination and affects cellulose and hemicellulose. Perrone et al. [67] employed the ozonolysis on sugarcane biomass and found that it removed 25% of the lignin after pre-treatment. Hernández-Guzmán et al. [68] pre-treated wheat straw with NaOH (2% v/v) and H2O2 (2% v/v) and obtained the cellulose content of 19–26% after pre-treatment and efficacy of 57 ± 3.74% for lignin removal. Therefore, ionic liquids are also used for biomass pre-treatment and break the non-covalent linkages between the biomass. This method is gaining attention due to its advantages; however, the cost of solvents is one of its drawbacks. Semerci and Ersan [69] employed the ionic liquids (triethylammonium hydrogen sulphate, TEAHSO4; 1-butylimidazolium hydrogen sulphate, HBIMHSO4; and 4-methylmorpholinium hydrogen sulphate, HMMorpHSO4) on hornbeam for its pre-treatment. The results obtained from the pre-treatment showed that the cellulose content was increased up to 81%, while removed 91% of lignin was removed when treated with HBIMHSO4. This study showed that at 30% of biomass loading, 70% of the lignin was extracted with TEAHSO4 and HBIMHSO4.
Physicochemical Pre-treatment
This method pre-treats the biomass both physically and chemically with the help of techniques like a steam explosion, carbon dioxide (CO2) explosion, ammonia method, wet oxidation (WO) and liquid hot water (LHW). Steam explosion and ammonia pre-treatment both involve biomass exposure at high pressure; however, in the ammonia method, it occurred in the presence of ammonia. Both methods increases the accessible area by opening the biomass structure. The steam explosion method produces inhibitors that are toxic for the microbial species involved. The ammonia method also lacks efficiency in the case of woody biomass [70]. Semwal et al. [71] pre-treated the different fractions of rice straw (5–20 mm) with the steam of 1–1.5 MPa. They obtained the glucans conversion of 53.6 ± 1.02% (5 mm), 61.1 ± 1.52% (10 mm) and 27.9 ± 0.82% (20 mm) with water impregnated steam explosion, respectively. Sugarcane bagasse was pre-treated with different concentrations of liquid ammonia and found the optimized conditions as ammonia concentration 15.64% (v/v) with solid loading of 10.51% w/v, temperature of 84.9 °C and residence time of 23.95 h and obtained 545.57 ± 7.1 g/kg total sugar [72]. In the CO2 explosion method, biomass is subjected to supercritical CO2 at high pressure and mild temperature. In the presence of steam, carbon dioxide causes the swelling of biomass and forms carbonic acid that leads to biomass breakdown. This method is economical and non-toxic. Zhao et al. [73] treated the biomass with supercritical carbon dioxide with process conditions of 50–80 °C temperature and pressure of17.5–25.0 Mpa for an incubation period of 12–60 h. The surface of the biomass was increased and made the biomass accessible for enzymatic hydrolysis. The authors observed 3–fourfold enhancement in sugar yield compared to the untreated biomass. The conditions enhanced sugar yield from the hydrolysis by 3–fourfold compared to the untreated biomass. The wet oxidization (WO) method involves utilizing water, oxygen and hydrogen peroxide at high temperatures for biomass rupturing. Water acts as an acid at high temperatures and releases hydrogen ions that hydrolyze the hemicellulose and oxidize the lignin. The cost involved with the method is relatively high, which restricts its commercial use [74]. Liquid hot water (LHW) pre-treatment uses water in its liquid form at high temperatures and pressure. The method solubilizes the cellulose and hemicellulose fractions. Though the LHW method is inexpensive, inhibitors are formed at high temperatures. Kang et al. [75] conducted an experiment in which liquid hot water was used to pre-treat hybrid Pennisetum and found that the LHW can change the biomass structure and effective solubilization of hemicellulose resulted in 100% xylan removal from the hybrid Pennisetum.
Biological Pre-treatment
This method is done with the help of microorganisms (bacteria and fungi) and macro-organisms (insects, worms and gastropods). Microorganisms degrade the lignocellulose structure by releasing cellulolytic, hemicellulolytic and ligninolytic enzymes and producing bioethanol following fermentation [39]. Macro-organism possesses different mechanisms such as mechanical and enzymatic in combination with physiological functions and break the LCB structure. The macro-organisms that could digest biomass include litter, leaf and wood. The earthworm feeds on detritus and digests organic matter more efficiently. These detritivores can even digest cellulose, starch, lignin and carbohydrates [76]. The factors influencing the biodegradation of biomass include physical factors (temperature, pressure, aeration, surface areas), chemical factors (pH, composition, carbon and nitrogen source, organic and inorganic compounds), enzymes and biological factors (microbial species, their interaction and competition) [42]. The biological pre-treatment methods have advantages over other methods which include fewer energy inputs and needless harsh chemicals. However, time consumption in the process is the major drawback of the biological method, which acts as a barrier to its use on a commercial scale [77].
Hydrolysis
During this step, polysaccharides present in feedstocks are hydrolyzed to monosaccharide sugars with the help of acid/enzymes. The cellulose released is transformed to glucose, catalyzed by acid (dilute or concentrated) or cellulase.
Acid Hydrolysis
The advantage of acid hydrolysis is that it can pierce the lignin in LCB without pre-treatment. The process dissociates the cellulose and hemicelluloses into individual sugar molecules. The commonly used acid for hydrolysis is hydrochloric acid and sulphuric acid. The conversion of lignocellulosic material is done by hydrolysis with either acid with higher concentration at mild temperature or dilute acid at elevated temperature. The concentrated acid hydrolysis has a high hydrolysis yield of cellulose; however, concentrated acid leads to corrosion of equipment [78]. At high temperatures, hydrolysis with dilute acid enhances the rate of hemicellulosic sugars solubilization and inhibitory compounds formation like furfural [78]. Therefore, two-step dilute acid hydrolysis is employed to eliminate these drawbacks. The process is carried out at moderate temperature conditions (170–190 °C) to solubilize amorphous hemicellulose in the 1st stage. In contrast, in the 2nd stage, used harsh conditions (200–230 °C) to hydrolyze more crystalline and resistant cellulose [79]. The utility of acid in the hydrolysis process has some drawbacks, including corrosion of the equipment with acid, utilization of unsafe chemicals and addition of neutralization step [80].
Enzymatic Hydrolysis
In the case of enzymatic hydrolysis, a mixture of different enzymes extracted from microorganisms are used to hydrolyze cellulose and hemicellulose. It can hydrolyze hemicellulose efficiently as compared to cellulose. Cellulose is more stable and has a crystalline structure; therefore, it cannot be easily depolymerized. The cellulase complex is used to hydrolyze cellulose. The complex of cellulase contains three main enzymes that act on cellulose and hydrolyze it into reducing sugar [81]:
Endo-1, 4-β-glucanases: It breaks the 1, 4-β-glucan bonds randomly.
Exo-1, 4-β-D-glucanases: The function of this enzyme is to free up the D-glucose and cellobiose and hydrolyze cellobiose gradually.
β-D-glucosidase: To form D-glucose from cellobiose.
Along with these three main enzymes, other enzymes are also used to degrade the hemicellulose polymers, including xylanases, galactomannase, acetylesterase and glucomannase. Bacteria and fungi are two chief sources for the aforementioned enzymes that possess cellulolytic and hemicellulolytic capabilities [81]. The activity of various enzymes from different sources is shown in Table 1. Cellulose and hemicellulose conversion is provided in reactions (i) and (ii) in which glucan (for hexoses) and xylan (for pentose) reacts with water:
| 1 |
| 2 |
Table 1.
Activity of various enzymes from different sources used for degradation of biomass
| S. no | Enzyme | Source | Enzyme activity | References |
|---|---|---|---|---|
| 1 | Cellulase | Aspergillus niger | 484.3 U/mg | [82] |
| 2 | Cellulase | Trichoderma reesei RUT C30 | 19.85 FPU/ml | [83] |
| 3 | Cellulase | Bacillus velezensis | 20.20 ± 0.74 U/ml | [84] |
| 4 | Cellulase | A. niger | 10.2 U/ml | [85] |
| 5 | Xylanases | Beauveria bassiana SAN01 | 304.48 ± 13.25 U/ml | [86] |
| 6 | Endoglucanase | B. bassiana SAN01 | 17.16 ± 0.41 U/ml | [86] |
| 7 | Xylanase | Aspergillus terreus | 474 U/ml | [87] |
| 8 | Xylanase | A. niger (wild strain) | 4.124 U/ml | [88] |
| 9 | Xylanase | A. niger (UV-mutated strain) | 9.3641 U/ml | [88] |
| 10 | Xylanase | Bacillus tequilensis UD-3 | 8.54 IU/ml | [89] |
| 11 | Endoglucanase | Myceliophthora thermophila M.7.7 | 2.22 U/ml | [90] |
| 12 | Endoglucanase | Tricholoma matsutake | 414.6 U/mg | [91] |
| 13 | Cellulase | Bacillus paranthracis | 1.0 U/ml | [92] |
| 14 | Xylanases | Bacillus nitratireducens | 9.2 U/ml | [92] |
Fermentation
Fermentation is the biochemical process by which glucose (a hexose sugar) and xylose (a pentose sugar) are converted into ethanol. Depending upon the composition of the hydrolyzed material, a specific bacteria or yeast is used to perform the process.
The reactions (iii) and (iv) shows the glucose and xylose conversion as given below:
| 3 |
| 4 |
The yeast majorly employed for ethanol production is Saccharomyces cerevisiae; however, it cannot metabolize xylose. The microorganisms usually taken for this process metabolize the monomer sugars and convert them into bioethanol. There are three methods used for the fermentation, including separate hydrolysis and fermentation (SHF), simultaneous hydrolysis and fermentation (SSF) and consolidated bioprocessing (CBP). Various fermentation technologies used for bioethanol production, yield and concentration from various substrates are shown in Table 2.
Table 2.
Different fermentation technologies used for bioethanol yield/concentration from different substrates (SHF, SSF and CBP)
| S. no | Method | Substrate | Bioethanol yield/concentration | References |
|---|---|---|---|---|
| 1 | SSF | Wheat straw (WS) | 15.3 g ethanol/100 g WS | [93] |
| 2 | Fed batch SSF | Sweet potato peels | 0.355 g ethanol/g sugar | [94] |
| 3 | Fed batch SHF | Beta vulgaris | 0.479 g ethanol/g sugar | [94] |
| 4 | SHF | Cynara cardunculus | 13.17 g ethanol/100 g of biomass | [95] |
| 5 | Semi-SSF | C. cardunculus | 13.64 g ethanol/100 g of biomass | [95] |
| 6 | CBP | Parthenium hysterophorus | 81.5 mg/g biomass | [96] |
| 7 | CBP | Pennisetum species | 0.55 g/g of reducing sugar | [92] |
| 8 | CBP | Rice straw | 1.8 g/l | [97] |
| 9 | SHF | Waste bamboo | 83.1% | [98] |
| 10 | SSF | Corn cob | 23.69 g/l | [99] |
| 11 | SHF | Corn cob | 17.4 g/l | [99] |
| 12 | PSSF(SSF with prehydrolysis) | Corn cob | 20.12 g/l | [99] |
| 13 | SHF | Rice straw | 83.5% | [100] |
Separate Hydrolysis and Fermentation
In this method, two separate reactors are used for hydrolysis and fermentation. The cellulose liberated is treated inside a separate reactor for hydrolysis, and then the released sugars are transferred to the fermentation reactor along with the fermenting microorganisms. Although it uses two reactors and sugar apt microbes in the fermentation, inhibition of glucose, and concentration to fermenting microbes is still a barrier. Moreover, SHF has some drawbacks like higher investment cost, contamination, higher incubation time, inhibitor formation and requirement of extra equipment [101]. Jin et al. [101] conducted an experiment that generated bioethanol from NaOH pre-treated rice straw by utilizing Saccharomyces tanninophilus through SHF with an enzyme loading of 200 FPU/ml for 20 h and obtained an ethanol concentration and yield of 9.45 g/l and 83.5%, respectively. To make the process more efficient, another method, i.e. separate hydrolysis and co-fermentation (SHCF), was developed by a slight variation in the SHF process in which 5-carbon and 6-carbon sugars were produced in the hydrolysis of cellulose and hemicellulose and were fermented together in the process.
Simultaneous Hydrolysis and Fermentation
The SSF method involves degradation of cellulose under the influence of enzyme in combination with fermentation method, resulting in released sugars from the hydrolysis process and followed with fermented consecutively. This method has advantages over SHF as it generates less inhibitory effect with sugar accumulation; moreover, only a single reactor is required, and therefore, the operation is much easier [102]. SSF technique is the most suitable method to convert LCB to bioethanol; however, the method has the drawback of optimizing the conditions for different processes, including hydrolysis and fermentation. Qui et al. [93] produced the bioethanol from wheat straw by SSF process at solid loading rates of 10%, 15% and 20% (w/w). They revealed that ethanol conversion decrease with an increase in solid loadings, i.e. 99.4%, 95.4% and 75.7% with 10, 15 and 20%, respectively. A new technique for process integration has also been developed named simultaneous Saccharification, filtration and fermentation (SSFF). This process integrates both SHF and SSF. In this method, pre-treated biomass is solubilized in the reactor, while the suspension is repeatedly pumped through the cross-flow membrane. The sugar filtrate obtained from it is subjected to fermentation, and the retentate is again fed back to the hydrolysis vessel [103] (Zabed et al., 2016).
Consolidated Bioprocessing
The conversion of biomass to sugars and then to bioethanol is a multistep process. The cost involved in each step makes the process expensive. It can be reduced by developing a structure or process mode that can convert biomass directly into ethanol in a single step. Various researchers used the consolidated bioprocessing approach for the fermentation of sugars. CBP is still developing, and much effort is required to explore [104]. Various studies found CBP as a competent approach. The total expense of the process can be decreased using only one microbe or consortium for enzyme production, saccharification, and fermentation in a single step [100]. Singh et al. [97] experimented with bioethanol production from rice straw using CBP and observed 29.4% solubilization of rice husk and 1.8 g/l of bioethanol concentration.
Bioreactors and Their Operating Modes Involved in the Process
The mode of the bioreactor applied depends on the feeding of the substrate into the bioreactor. It can be classified into three main types: batch, fed-batch and continuously based on the discontinuous, continuous or semi-continuous mode of substrate feeding in a bioreactor. Table 3 illustrates the different studies related to bioreactor modes along with the substrate used for fermentation.
Table 3.
Different mode of bioreactors applied for ethanol production using different substrates and microorganism
| S. no | Type of bioreactor | Substrate | Microorganism involved | Ethanol concentration/productivity | References |
|---|---|---|---|---|---|
| 1 | Pervaporation membrane bioreactor | Glucose | S. cerevisiae | Maximum ethanol concentration was 22.085 g/l in feed side and 435.47 g/l in permeate side after 44 h | [105] |
| 2 | Batch reactor | Sugarcane bagasse | Scheffersomyces stipis | Maximum ethanol production after 72 h was 7.34 g/l | [106] |
| 3 | Batch reactor | Sugarcane bagasse | Scheffersomyces shehatae | Maximum ethanol production after 72 h was 18 g/l | [106] |
| 4 | Fed batch | Corn stover | S. cerevisiae SyBE005 | Maximum ethanol concentration was 48.2 g/l | [107] |
| 5 | Continuous mode bioreactor | Sugarcane bagasse | S. cerevisiae | Maximum ethanol concentration in the permeate was 43.2 g/l after 19 h | [108] |
| 6 | Packed bed Biofilm reactor (repeated batch) | Rice straw hydrolysate | Zymomonas mobilis | Ethanol yield after 3 days was 0.36 to 0.38 g/g | [109] |
| 7 | Stirred tank bioreactor (single step batch) | Cassava starch | Kluyveromyces marxianus SS106 | Ethanol concentration after 72 h was 7.91% (v/v) | [110] |
| 8 | Fed-batch bioreactor | Glycerol | Klebsiella pneumoniae Kp17 | Final ethanol production after 30 h was 17.30 g/l | [111] |
| 9 | Fed-batch bioreactor | Sugarcane juice and molasses mix | S. cerevisiae | Ethanol concentration of 135.0 g/l after 30 h | [112] |
| 10 | Continuous mode bioreactor | Glucose | Immobilized Z. mobilis ATCC 29,191 cells | Ethanol productivity was 31.09 g/l/h | [113] |
Discontinuous or Batch Type
The operation is a simple and most commonly studied process with bioreactors. The substrate is filled in the bioreactor initially, and volume remains constant throughout reaction time, without any additional nutrients in the reaction. In this process, productivity is less due to inhibition by the product, long lag phase, an interruption due to sterilizing, cleaning and filling [114]. Phukoetphim et al. [115] evaluated sweet sorghum for bioethanol production by Saccharomyces cerevisiae NP01 in repeated batch fermentation for five cycles to facilitate the efficiency of the process. The experiment’s outcomes revealed that the average ethanol concentration was 112.3 g/l for the five cycles. Zhang et al. [116] experimented on batch fermentation (SSF) of the pre-treated corncob for ethanol production and observed an ethanol concentration of 69.2 g/l.
Fed-Batch Type or Semi-continuous Type
In this type, nutrients are supplied intermittently during the process to reduce the substrate associated growth inhibition. It encourages the uniformity of the living system and gives more yield than batch type. It is also a substitute for repressing mixing problems associated with immense solid load, as excessive substrate viscosity is prevented [117]. In this type, inhibition problems are also reduced due to reduced unproductive enzyme binding. Lu et al. [118] produced bioethanol from the pre-treated liquid hot water reed using fed-batch (S-SSSF) fermentation. The optimal conditions for the experiment were 36 °C temperature for fermentation of pre-treated reed; 18-h pre-hydrolysis was carried out at a temperature of 50 °C and 4.8 pH. The inclusion of fed-batch substrate (6.4%) was done after 6th h of the 18-h enzymatic pre-hydrolysis. Zhang et al. [116] investigated pre-treated corncob (acid–alkali: H2SO4-NaOH) for ethanol production using the fed-batch SSF process. The fed-batch substrate (6%) was supplemented with the process for the first 24 h and obtained an ethanol concentration of 84.7 g/l after 96 h. The overall yield and cellulose conversion during the experiment were 79.6% and 82.3%, respectively.
Continuous Type Operation Mode
In this type of operation, the substrate is added to the bioreactor continuously, and the product is removed frequently with a similar flow rate. Heat rate and temperature control is a continuous and straightforward type. Typical bioreactors belonging to this category are (i) suspended (free) cell, (ii) membrane cell recycle and (iii) immobilized cell.
(i) Suspended (Free) Cell
In this bioreactor, agitation is provided to the cells by a mechanical agitator to move freely within the fermentation broth. The reactor initiated at batch mode and growth of cells is permitted up to an exponential phase, and prepared media is continuously introduced to the reaction tank. The precaution related to cell inhibition for entry in the stationary phase must be taken in the bioreactor [119]. The system can be in single, double and multi-stages. In a continuous type of single-stage fermentation, higher reactor productivity can be achieved at the cost of low product concentration, in contrast to the productivity acquired through the batch process. In double stage fermentation, the inhibitory compound production decreases by allowing the process to occur in two different containers. In a multi-stage system, 6–8 tanks are used to carry out the process.
Compared to batch fermentation, the continuous fermentation process is more productive; however, low cell concentration is the barrier to higher productivity in the free cell continuous process. In this process, there is no alternate way to keep cells in the bioreactor, and the washout occurs at a tremendous dilution rate [120].
(ii) Membrane Cell Recycle Bioreactor
The bioreactor is characterized by high cell accumulation, which leads to high product concentration and substrate conversion. It is a hybrid system in which a conventional fermentor is attached to a membrane filtration system [121]. In this system design, a bioreactor was connected to a filter where cells are retained while the product is recovered through an in-line flash tank. The cost of the membrane is relatively high, and broth in the membrane cell recycle bioreactor may result in begriming of the membrane, which imposes a drawback to use the system [122].
(iii) Immobilized Cell Bioreactor
The advantages of immobilized cell bioreactor are high cell accumulation, operation of a reactor at the high flow rate, more prolonged continuous operation and no cell washout is needed, resulting in high productivity and yield [123]. In this bioreactor type, the feed is provided from the base (lower side), and the product is withdrawn from the uppermost portion of the bioreactor. This method is also called a ‘non-mixing reactor’ in which product inhibition is significantly reduced. For the immobilization of the cells, one can use any one of the three methods, i.e. entrapment, adsorption and covalent bonding [124].
The bioreactor may be a fluidized bed and packed bed. In fluidized bed types, microorganisms are bound to the particles, which remain suspended with high upward flow rate feed and oxygen-free gas, i.e. nitrogen. While in a packed bed type bioreactor, cells are immobilized on large stationary particles [120].
Circular Economy/Biorefinery Approach in Bioethanol Process
During ethanol production, other value-added products have also been produced like furfural, hydroxylmethyfurfural (HMF) and lignin residues. The circular bioeconomy approach for bioethanol production and other value-added products production from LCB has been illustrated in Fig. 5. Furfural and HMF are mainly produced during the pre-treatment process and act as an inhibitor for ethanol fermenting microorganisms. Furfural is a product of xylose sugar and has several applications, i.e. making inks, plastics, fertilizers, antacids, nematicides, adhesives, fungicides and flavouring compounds. Its extraction during ethanol production provides benefits like increased ethanol yield and its utilization for producing other valuable products. In recent times, pyrrole and D-proline production has been reported from furfural. These compounds are directly or indirectly used as a precursor in pharmaceuticals [125]. Ntimbani et al.[126] produced ethanol and furfural from sugarcane bagasse. In this study, furfural was extracted during sulphuric acid pre-treatment of sugarcane bagasse in a heated 2-l Büchiglasuster® pressure reactor, and ethanol is produced from non-detoxified furfural residues. The results reported a maximum furfural yield of 69% at 170 °C and 0.5 wt.% sulphuric acid and ethanol yields of 77 to 95%. Similarly, ethanol, hesperidin (antimicrobial and antioxidant) and nanocellulose were obtained from orange juice industry waste. Citrus Pulp of Floater (CPF), a waste product of the orange juice industry, on enzymatic hydrolysis provides sugar-rich liquid utilized for ethanol production and on extraction (Soxhlet extractor) provides hesperidin. The remaining solid residues from both processes were used for nanocellulose production. The yield of hesperidin and nanocellulose obtained were 1.2% and 1.4%, respectively, and 27.5% hydrolysis yield was obtained after 6 h [127]. After pre-treatment, water washing is the most common method applied to remove inhibitory compounds. So, this wastewater recycled and integrated the ethanol production process with anaerobic digestion. Yuan et al. [128] proposed the integrated approach for a lignocellulosic biorefinery. The pre-treatment of the biomass with high solid loadings omits lots of wastewater utilized in the anaerobic digestion unit as substrate resulted in biogas production and bioethanol (from pre-treated biomass). Conesa et al. [129] also indicated in their study that Persimmon is an efficient substrate for ethanol production and other valuable products like carotenoids. As a circular biorefinery approach, Chatterjee and Mohan [130] simultaneously produced bio-hydrogen and bioethanol from separated streams of sugarcane bagasse hydrolysate. They treated the biomass with 2% sulphuric acid, resulting in the xylose-rich hydrolysate. The hydrolysate is used as two streams, i.e. one is fed in dark fermentation for bio-hydrogen production and the other for SSF for ethanol production. In dark fermentation, acidogenic effluent generated during the bio-hydrogen production was utilized as a phosphate solubilizing organic fertilizer for chickpea cultivation. Their study showed the feasibility and maximum potential for zero waste biorefinery. Khaire et al. [131] also overviewed bioethanol production, xylo-oligosaccharides and lignin from sugarcane tops. For adding value to the process, xylan and lignin separation from biomass before enzymatic hydrolysis is also a beneficial approach. Sugar-rich hydrolysate produced ethanol through fermentation; xylan used to produce xylo-oligosaccharides, food coatings, etc. Lignin residues on modification have a varied application like polyolefin, rubber intensifier and rubber packing. In totality, it has been observed from the above-mentioned studies that the circular bioeconomy approach in bioethanol process enhanced the overall efficacy of the production process and was also beneficial in reducing the capital cost of the bioethanol process.
Fig. 5.
Circular bioeconomy/biorefinery approach in bioethanol production process from LCB
Techno-economic Analysis
The techno-economic analysis of ethanol production from lignocellulosic biomass includes the capital investment, operational cost and the minimum selling price (MSP) of the bioethanol. The economic input in each production process, like lignocellulosic, pre-treatment and production costs, is equally important. The biomass cost ranges from $22 to 85 per ton, depending on its variability and accessibility [132]. Its cost also depends on inflation, labour and transportation costs. An increase in these factors leads to an increase in biomass cost. Likewise, pre-treatment and production cost depend on the capital and operation cost.
Some studies on techno-economic analysis of the bioethanol process have been mentioned. A case study on ethanol biorefinery was optimized in Maharashtra state by Vikash and Shastri [133] using 14 substrates, i.e. wheat pod, rice straw, rice husk, wheat stalk, bagasse, maize cob, cotton husk, cotton boll shell, jowar cob, jowar husk, jowar husk, sugarcane stalk, maize stalk and cotton stalk following four bioconversion methods (SHF, SHCF, SSF and SSCF). The study results showed that maximum ethanol production cost, fixed operating cost and variable operating expense are Rs. 96.79, 19.11 and 35.76 per litre, respectively. It was reported that the maximum cost related to feedstock is because of transportation involved (Rs. 5.08/L), and the ionic liquid method is the expensive method among the other pre-treatment techniques involved. Solarte-Toro et al. [134] also reported that the pre-treatment conditions impacted the ethanol yield and suggested the production of co-products to diminish the utilities of the process. Okolie et al. [135] assessed the economic feasibility of bioethanol and biomethane biorefinery through glycerol valorization (co-product of the diesel production process). They made three scenarios (S), i.e. S1 made of hydrothermal gasification (HG) and syngas fermentation (SF) and not involve the carbon dioxide capture; S2 involved the bioethanol production through HG and SF along with CO2 capture and storage; and S3 is somewhat similar to S2 but includes the biomethane production from the captured CO2. The minimum selling price (MSP) of bioethanol production was utilized to assess the economic viability of the process that shows the trend like this—S1 (USD $1.4/L) > S2 (USD $1.32/L) > S3 ((USD $0.31). The lowest value of S3 was ascribed due to the production of value-added products (biomethane and oxygen) along with bioethanol. Likewise, Khounani et al. [136] produced bioethanol from safflower straw, a waste product of the biodiesel production process from its seed. The extracted straw and the residual cake (obtained from oil extraction) were subjected to pre-treatment, hydrolysis and fermentation. Wastewater from solid–liquid separation unit is used for biogas production in an anaerobic digester unit. The process simulations of both scenarios A (fermentation by Zymomonas mobilis) and B (S. cerevisiae) were done by Aspen Plus v.10 software. The profitability index of scenarios (S) A and B was 1.14 and 0.81, respectively, showing the cost-effectiveness of SA but not economically sustainable for the B scenario. The utilization of Z. mobilis reduced the cost of bioethanol production from 0.12 to 0.09 $/L, suggesting that Z. mobilis is a preferred biocatalyst for bioethanol production from a techno-economic point of view. It has been deduced from the studies mentioned above that the cost of the bioethanol production process can be reduced by the production of other value-added products along with it.
Recent Trends, Challenges and Future Prospects
In the last few years, there has been a massive development of various biorefineries to produce value-added products like hydrogen, methane, ethanol, butanol and other biochemicals such as alcohols, furfurals and organic acids [48, 137, 138]. To increase the sustainable bioeconomy, one should implement bio-based techniques. There will be a change of economy from linear to CE if the bioeconomy has more sustainability and circularity. According to the WBA (World Bioenergy Association), the demand for bioenergy worldwide will increase significantly (https://worldbioenergy.org/uploads/201210%20WBA%20GBS%202020.pdf). Due to this reason, there should be a need for efficient usage of biomass resources (such as crops, algae and wastes). LCB biorefinery for bioethanol has some challenges like feedstock choice and its complex recalcitrant structure. The choice of feedstock is based on its cost, availability, biological utilization, composition, processing, harvesting, storage and transportation. So, efficient ethanol production in a biorefinery will need the detailed study of the composition, characterization of the substrate and its feasibility with the processing technology and the optimum conditions of the same. Disruption of LCB structure required pre-treatment, which adds to the total processing cost. Modification in the lignin content reported in several studies makes the pre-treatment inexpensive through metabolic engineering [60]. Developing the new bio-cascading and circular techniques is indispensable to fulfil bioenergy and biochemical needs [139]. Integration of hydrothermal and biological techniques may further overcome the recalcitrant nature of biomass, which could easily lead to biofuels and biochemical production. This integration technique can be up-and-coming for biomass valorization. The sustainability of lignocellulose biorefineries for a long time depends on how lignin is being utilized [21]. The chemicals obtained from lignin can be a sustainable replacement of fossil fuel-based chemicals, considering high energy content, renewable nature and reduced carbon footprints [140]. Recent development in the research revealed that the bioconversion of lignin to synthesize higher lipid with the utilization of oleaginous microbes is an essential step in developing different combined biofuels. Research is also focused on the over-expression of cellulolytic and hemicellulolytic enzymes for enhanced polysaccharide production.
Now, the interest is shifting in the area of consolidated bioprocessing (CBP). One microorganism is engineered with genes or the consortia of microbes, reducing the cost involved with the processes. Another challenge is the microorganism survival and the co-utilization of pentose and hexose sugars completely and simultaneously by the fermenting microorganism. Now, the research is focused on the generation of modified strains, which can disrupt the polysaccharides and use sugars even in the presence of toxic compounds generated during pre-treatment [141]. Several studies have been executed on processing LCB waste obtained from domestic waste, restaurant kitchens, and food processing plants through enzymatic treatment.
Furthermore, few studies highlighted towards pavement of sustainable way on utilizing the biomass completely with adopting zero-waste plan [18, 142]. To enhance the monetary benefits, the products obtained from primary biorefinery processes should be processed again to generate other value-added products instead of converting them to minor valued products, including compost or fuel. Future aspects of utilization of LCB bioconversion should be more systematic, with enhanced microorganism and enzyme activities and imposing a particular focus on economic viability and environmental impacts. The accomplishment of the biorefinery concept requires techno-economic viability and some major renovations like coproduction strategies in one process, reutilization of LCB for maximum resource utilization and value addition in the generated waste during the process [143]. Further, it has been observed that many efforts and research are required to make lignocellulosic biorefinery an economically and sustainably viable option.
Conclusion
Global environmental issues are shifting the nations towards development of biofuels and bio-based economy. Circular economy and biorefineries concepts also advocate the utilization of sustainable and renewable feedstock like LCB. The usage of LCB for biofuel production and other value-added products provides an opportunity to increase the economy of biorefineries on generating energy and reduce the burden of waste management. Lignocellulosic biomass abundance makes this an appropriate source for valuable products production. The success of a biorefinery depends upon the utilization of hemicellulose and lignin part for production of value-added products along with generation of 2G ethanol from cellulose and hemicellulose part. Balanced and focused research is required for its efficient conversion of LCB into bioethanol and other value-added products. The technical and economic barriers involved in its availability, processing, pre-treatment, hydrolysis and saccharification steps must be addressed for its successful utilization.
Acknowledgements
The author (Anita Singh) would like to acknowledge SERB grant ( ECR/2018/000672) for providing financial support to carry out this work.
Author Contribution
AD, SB, HK and AS designed the outline of the manuscript. AD, SB and HK performed the literature research and prepared the manuscript. AS, RK and DP critically revised the manuscript.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Ethics Approval and Consent to Participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for Publication
All authors give consent for publication.
Competing Interest
The authors declare no competing interests.
Footnotes
Arti Devi and Somvir Bajar are equal contribution as first authors
Highlights
1. Circular bioeconomy, a green approach, is elucidated using LCB.
2. Biorefinery is an emerging and sustainable way of using waste.
3. Current perspective of bioethanol production from LCB discussed in detail.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singh A, Srivastava S, Rathore D (2021) Environmental microbiology and biotechnology: volume 2: bioenergy and environmental health. Springer, Singapore. https:// 103.7.177.7:80/handle/123456789/208333
- 2.Nanda S, Mohanty P, Pant KK, Naik S, Kozinski JA, Dalai AK. Characterization of North American lignocellulosic biomass and biochars in terms of their candidacy for alternate renewable fuels. Bioenergy Res. 2013;6:663–677. doi: 10.1007/s12155-012-9281-4. [DOI] [Google Scholar]
- 3.Shahzadi T, Mehmood S, Irshad M, Anwar Z, Afroz A, Zeeshan N, Rashid U, Sughra K. Advances in lignocellulosic biotechnology: a brief review on lignocellulosic biomass and cellulases. Adv Biosci Biotechnol. 2014;5:246–251. doi: 10.4236/abb.2014.53031. [DOI] [Google Scholar]
- 4.Kavitha S, Kannah RY, Kasthuri S, Gunasekaran M, Pugazhendi A, Rene ER, Pant D, Kumar G, Banu JR. Profitable biomethane production from delignified rice straw biomass: the effect of lignin, energy and economic analysis. Green Chem. 2020;22:8024–8035. doi: 10.1039/D0GC02738C. [DOI] [Google Scholar]
- 5.Liguori R, Faraco V. Biological processes for advancing lignocellulosic waste biorefinery by advocating circular economy. Bioresour Technol. 2016;215:13–20. doi: 10.1016/j.biortech.2016.04.054. [DOI] [PubMed] [Google Scholar]
- 6.Anwar Z, Gulfraz M, Irshad M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: a brief review. J Radiat Res Appl Sci. 2014;7:163–173. doi: 10.1016/j.jrras.2014.02.003. [DOI] [Google Scholar]
- 7.Somerville C, Youngs H, Taylor C, Davis SC, Long SP. Feedstocks for lignocellulosic biofuels. Science. 2010;329:790–792. doi: 10.1126/science.1189268. [DOI] [PubMed] [Google Scholar]
- 8.Taarning E, Osmundsen CM, Yang X, Voss B, Andersen SI, Christensen CH. Zeolite-catalyzed biomass conversion to fuels and chemicals. Energy Environ Sci. 2011;4:793–804. doi: 10.1039/C004518G. [DOI] [Google Scholar]
- 9.Zhu JY, Pan XJ. Woody biomass pretreatment for cellulosic ethanol production: technology and energy consumption evaluation. Bioresour Technol. 2010;101:4992–5002. doi: 10.1016/j.biortech.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Al-Battashi HS, Annamalai N, Sivakumar N, Al-Bahry S, Tripathi BN, Nguyen QD, Gupta VK. Lignocellulosic biomass (LCB): a potential alternative biorefinery feedstock for polyhydroxyalkanoates production. Rev Environ Sci Biotechnol. 2019;18:183–205. doi: 10.1007/s11157-018-09488-4. [DOI] [Google Scholar]
- 11.Patrick MF, Champagne P, Cunninghama MF, Whitney RA. A biorefinery processing perspective: treatment of lignocellulosic materials for the production of value-added products. Bioresour Technol. 2010;101:8915–8922. doi: 10.1016/j.biortech.2010.06.125. [DOI] [PubMed] [Google Scholar]
- 12.Zhang YP. Reviving the carbohydrate economy via multi-product lignocellulose biorefineries. J Ind Microbial Biotechnol. 2008;35:367–375. doi: 10.1007/s10295-007-0293-6. [DOI] [PubMed] [Google Scholar]
- 13.Kapoor R, Ghosh P, Kumar M, Sengupta S, Gupta A, Kumar SS, Vijay V, Kumar V, Vijay VK, Pant D. Valorization of agricultural waste for biogas based circular economy in India: a research outlook. Bioresour Technol. 2020;304:123036. doi: 10.1016/j.biortech.2020.123036. [DOI] [PubMed] [Google Scholar]
- 14.Mohan SV, Varjani S, Pant D, Sauer M, Chang JS. Circular bioeconomy approaches for sustainability. Bioresour Technol. 2020;318:124084. doi: 10.1016/j.biortech.2020.124084. [DOI] [PubMed] [Google Scholar]
- 15.Liguori R, Amore A, Faraco V. Waste valorization by biotechnological conversion into added value products. Appl Microbiol Biotechnol. 2013;97:6129–6147. doi: 10.1007/s00253-013-5014-7. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira AF (2017) Biorefinery Concept. In: Rabaçal M, Ferreira AF, Silva CAM, Costa M (ed) Biorefineries: targeting energy, high value products and waste valorisation. Springer, Switzerland, pp 1–20. 10.1007/978-3-319-48288-0
- 17.Awasthi MK, Sarsaiya S, Wainaina S, Rajendran K, Kumar S, Quan W, Duan Y, Awasthi SK, Chen H, Pandey A, Zhang Z, Jain A, Taherzadeh MJ. A critical review of organic manure biorefinery models toward sustainable circular bioeconomy: technological challenges, advancements, innovations, and future perspectives. Renew Sust Energ Rev. 2019;111:115–131. doi: 10.1016/j.rser.2019.05.017. [DOI] [Google Scholar]
- 18.Zabaniotou A, Kamaterou P. Food waste valorization advocating circular bioeconomy-a critical review of potentialities and perspectives of spent coffee grounds biorefinery. J Clean Prod. 2019;211:1553–1566. doi: 10.1016/J.JCLEPRO.2018.11.230. [DOI] [Google Scholar]
- 19.Nizami AS, Rehan M, Waqas M, Naqvi M, Ouda OK, Shahzad K, Miandad R, Khan MZ, Syamsiro M, Ismail IM, Pant D. Waste biorefineries: enabling circular economies in developing countries. Bioresour Technol. 2017;24:1101–1117. doi: 10.1016/j.biortech.2017.05.097. [DOI] [PubMed] [Google Scholar]
- 20.Garlapati VK, Chandel AK, Kumar SJ, Sharma S, Sevda S, Ingle AP, Pant D. Circular economy aspects of lignin: towards a lignocellulose biorefinery. Renew Sust Energy Rev. 2020;130:109977. doi: 10.1016/j.rser.2020.109977. [DOI] [Google Scholar]
- 21.Pant D, Misra S, Nizami AS, Rehan M, van Leeuwen R, Tabacchioni S, Goel R, Sarma P, Bakker R, Sharma N, Kwant K, Diels L, Elst K. Towards the development of a biobased economy in Europe and India. Crit Rev Biotechnol. 2019;39:779–799. doi: 10.1080/07388551.2019.1618787. [DOI] [PubMed] [Google Scholar]
- 22.Yaashikaa PR, Kumar PS, Varjani S. Valorization of agro-industrial wastes for biorefinery process and circular bioeconomy: a critical review. Bioresour Technol. 2022;343:126126. doi: 10.1016/j.biortech.2021.126126. [DOI] [PubMed] [Google Scholar]
- 23.Raj T, Chandrasekhar K, Kumar AN, Banu JR, Yoon JJ, Bhatia SK, Yang YH, Varjani S, Kim SH. Recent advances in commercial biorefineries for lignocellulosic ethanol production: current status, challenges and future perspectives. Bioresour Technol. 2022;344:126292. doi: 10.1016/j.biortech.2021.126292. [DOI] [PubMed] [Google Scholar]
- 24.Circular Economy Action Plan (CEAP)- For a cleaner and more competitive Europe (European Commission, 2015). https://ec.europa.eu/environment/pdf/circular-economy/new_circular_economy_action_plan.pdf
- 25.Stegmann P, Londo M, Junginger M. The circular bioeconomy: its elements and role in European bioeconomy clusters. Resour Conserv Recycl X. 2020;6:100029. doi: 10.1016/j.rcrx.2019.100029. [DOI] [Google Scholar]
- 26.Carus M, Dammer L. The circular bioeconomy—concepts, opportunities, and limitations. Ind Biotechnol. 2018;14:83–91. doi: 10.1089/ind.2018.29121.mca. [DOI] [Google Scholar]
- 27.Frosch RA, Gallopoulos NE. Strategies for manufacturing. Sci Am. 1989;261:144–153. doi: 10.1038/scientificamerican0989-144. [DOI] [Google Scholar]
- 28.Pearce DW, Ker R. Economics of natural resources and the environment. Land Econ. 1991;67:272–276. doi: 10.2307/3146419. [DOI] [Google Scholar]
- 29.Leipold S, Petit-Boix A, Luo A, Helander H, Simoens M, Ashton W, Babbitt C, Bala A, Bening C, Birkved M, Blomsma F et al (2021) Lessons, narratives and research directions for a sustainable circular economy. PREPRINT available at Research Square.10.21203/rs.3.rs-429660/v1. Accessed 10 March 2021
- 30.Ruiz-Real JL, Uribe-Toril J, De Pablo VJ, Gázquez-Abad JC. Worldwide research on circular economy and environment: a bibliometric analysis. Int J Environ Res Public Health. 2018;15:2699. doi: 10.3390/ijerph15122699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alhawari O, Awan U, Bhutta MKS, Ülkü MA. Insights from circular economy literature: a review of extant definitions and unravelling paths to future research. Sustainability. 2021;13:859. doi: 10.3390/su13020859. [DOI] [Google Scholar]
- 32.Abad-Segura E, González-Zamar MD, Belmonte-Ureña LJ. Effects of circular economy policies on the environment and sustainable growth: worldwide research. Sustainability. 2020;12:5792. doi: 10.3390/su12145792. [DOI] [Google Scholar]
- 33.IEA (2017) Bioenergy and biofuels. http://www.iea.org/topics/renewables/bioenergy/2017. Accessed 25 January 2021.
- 34.Basu P. Biomass gasification, pyrolysis and torrefaction: practical design and theory. London: Academic Press; 2018. [Google Scholar]
- 35.Cherubini F. The biorefinery concept: using biomass instead of oil for producing energy and chemicals. Energy Convers Manag. 2010;51:1412–1421. doi: 10.1016/j.enconman.2010.01.015. [DOI] [Google Scholar]
- 36.Clark JH, Deswarte FE (2015) The biorefinery concept—an integrated approach. In: Clark JH, Deswarte FE (ed) Introduction to chemicals from biomass, 2nd edn. John Wiley & Sons, United Kingdom,pp1–29. 10.1002/9781118714478.ch1
- 37.Chiranjeevi P, Dahiya S, Kumar N. Waste derived bioeconomy in India: a perspective. N Biotechnol. 2018;40:60–69. doi: 10.1016/j.nbt.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Kondaveeti S, Abu-Reesh IM, Mohanakrishna G, Bulut M, Pant D. Advanced routes of biological and bio-electrocatalytic carbon dioxide (CO2) mitigation toward carbon neutrality. Front Energy Res. 2020;8:94. doi: 10.3389/fenrg.2020.00094. [DOI] [Google Scholar]
- 39.Baruah J, Nath BK, Sharma R, Kumar S, Deka RC, Baruah DC, Kalita E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front Energy Res. 2018;6:141. doi: 10.3389/fenrg.2018.00141. [DOI] [Google Scholar]
- 40.De Bhowmick G, Sarmah AK, Sen R. Lignocellulosic biorefinery as a model for sustainable development of biofuels and value added products. Bioresour Technol. 2018;247:1144–1154. doi: 10.1016/j.biortech.2017.09.163. [DOI] [PubMed] [Google Scholar]
- 41.Tripathi N, Hills CD, Singh RS, Atkinson CJ. Biomass waste utilisation in low-carbon products: harnessing a major potential resource. NPJ Clim Atmos Sci. 2019;2:1–10. doi: 10.1038/s41612-019-0093-5. [DOI] [Google Scholar]
- 42.Usmani Z, Sharma M, Awasthi AK, Lukk T, Tuohy MG, Gong L, Nguyen-Tri P, Goddard AD, Bill RM, Nayak SC, Gupta VK. Lignocellulosic biorefineries: the current state of challenges and strategies for efficient commercialization. Renew Sustain Energy Rev. 2021;148:111258. doi: 10.1016/j.rser.2021.111258. [DOI] [Google Scholar]
- 43.Konwar LJ, Mikkola JP, Bordoloi N, Saikia R, Chutia RS, Kataki R (2018) Sidestreams from bioenergy and biorefinery complexes as a resource for circular bioeconomy. In: Bhaskar T, Pandey A, Mohan SV, Lee DJ, Khanal SK (ed) Waste biorefinery: potential and perspectives. Elsevier, Amsterdam, pp 85–125 10.1016/B978-0-444-63992-9.00003-3
- 44.Niju S, Swathika M, Balajii M (2020) Pretreatment of lignocellulosic sugarcane leaves and tops for bioethanol production. In: Yousuf A, Sannino F,Pirozzi D (ed) Lignocellulosic biomass to liquid biofuels. Academic Press, Cambridge, pp 301–324. 10.1016/B978-0-12-815936-1.00010-1
- 45.Ginni G, Kavitha S, Kannah Y, Bhatia SK, Kumar A, Rajkumar M, Kumar G, Pugazhendhi A, Chi NTL. Valorization of agricultural residues: different bio refinery routes. J Environ Chem Eng. 2021;9:105435. doi: 10.1016/j.jece.2021.105435. [DOI] [Google Scholar]
- 46.Devi A, Singh A, Bajar S, Pant D. Ethanol from lignocellulosic biomass: an in-depth analysis of pre-treatment methods, fermentation approaches and detoxification processes. J Environ Chem Eng. 2021;9:105798. doi: 10.1016/j.jece.2021.105798. [DOI] [Google Scholar]
- 47.Pileidis FD, Titirici MM. Levulinic acid biorefineries: new challenges for efficient utilization of biomass. Chem Sus Chem. 2016;9:1–22. doi: 10.1002/cssc.201501405. [DOI] [PubMed] [Google Scholar]
- 48.Takkellapati S, Li T, Gonzalez MA. An overview of biorefinery-derived platform chemicals from a cellulose and hemicellulose biorefinery. Clean Technol Environ Policy. 2018;20:1615–1630. doi: 10.1007/s10098-018-1568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.https://www.verifiedmarketresearch.com/product/succinic-acid-market/#:~:text=Succinic%20Acid%20Market%3F,Succinic%20Acid%20Market%20was%20valued%20at%20USD%20147.42%20Million%20in,8.0%25%20from%202021%20to%202028
- 50.https://www.globenewswire.com/news-release/2019/06/26/1874725/0/en/The-Worldwide-Sorbitol-Market-to-2024-Dominated-by-Roquette-Freres.html
- 51.Sharma D, Saini A (2020) Cellulosic ethanol feedstock: diversity and potential. In: Sharma D, Saini A, (ed) Lignocellulosic Ethanol Production from a Biorefinery Perspective. Springer, Singapore, pp 23–63 10.1007/978-981-15-4573-3_2
- 52.Aftab MN, Iqbal I, Riaz F, Karadag A, Tabatabaei M (2019) Different pretreatment methods of lignocellulosic biomass for use in biofuel production. In: Abomohra AEF (ed) Biomass for bioenergy-recent trends and future challenges. Intechopen, London, pp 15–38. 10.5772/intechopen.84995
- 53.Najjarzadeh N, Matsakas L, Rova U, Christakopoulos P. Effect of oligosaccharide degree of polymerization on the induction of xylan-degrading enzymes by Fusarium oxysporum f. sp. Lycopersici. Molecules. 2020;25:5849. doi: 10.3390/molecules25245849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yogalakshmi KN, Sivashanmugam P, Kavitha S, Kannah Y, Varjani S, AdishKumar S, Kumar G. Lignocellulosic biomass-based pyrolysis: a comprehensive review. Chemosphere. 2022;286(2):131824. doi: 10.1016/j.chemosphere.2021.131824. [DOI] [PubMed] [Google Scholar]
- 55.Patel A, Shah A. Integrated lignocellulosic biorefinery: Gateway for production of second generation ethanol and value added products. J Bioresources Bioprod. 2021;6:108–128. doi: 10.1016/j.jobab.2021.02.001. [DOI] [Google Scholar]
- 56.De Corato U, De Bari I, Viola E, Pugliese M. Assessing the main opportunities of integrated biorefining from agro-bioenergy co/by-products and agroindustrial residues into high-value added products associated to some emerging markets: a review. Renew Sust Energ Rev. 2018;88:326–346. doi: 10.1016/j.rser.2018.02.041. [DOI] [Google Scholar]
- 57.Wong SS, Shu R, Zhang J, Liu H, Yan N. Downstream processing of lignin derived feedstock into end products. Chem Soc Rev. 2020;49:5510–5560. doi: 10.1039/D0CS00134A. [DOI] [PubMed] [Google Scholar]
- 58.Tsai JH, Ko YL, Huang CM, Chiang HL. Effects of blending ethanol with gasoline on the performance of motorcycle catalysts and airborne pollutant emissions. Aerosol Air Qual Res. 2019;19:2781–2792. doi: 10.4209/aaqr.2019.10.0539. [DOI] [Google Scholar]
- 59.Yao YC, Tsai JH, Chiang HL. Effects of ethanol-blended gasoline on air pollutant emissions from motorcycle. Sci Total Environ. 2009;407:5257–5262. doi: 10.1016/j.scitotenv.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 60.Kumar SJ, Kumar NS, Chintagunta AD. Bioethanol production from cereal crops and lignocelluloses rich agro-residues: prospects and challenges. SN Appl Sci. 2020;2:1–11. doi: 10.1007/s42452-020-03471-x. [DOI] [Google Scholar]
- 61.Zhang Y, Li T, Shen Y, Wang L, Zhang H, Qian H, Qi X. Extrusion followed by ultrasound as a chemical-free pretreatment method to enhance enzymatic hydrolysis of rice hull for fermentable sugars production. Ind Crops Prod. 2020;149:112356. doi: 10.1016/j.indcrop.2020.112356. [DOI] [Google Scholar]
- 62.Huang J, Zhu Y, Liu T, Sun S, Ren J, Wu A, Li H. A novel wet-mechanochemical pretreatment for the efficient enzymatic saccharification of lignocelluloses: small dosage dilute alkali assisted ball milling. Energy Convers Manag. 2019;194:46–54. doi: 10.1016/j.enconman.2019.04.078. [DOI] [Google Scholar]
- 63.Meng F, Wang D. Effects of vacuum freeze drying pretreatment on biomass and biochar properties. Renew Energ. 2020;155:1–9. doi: 10.1016/j.renene.2020.03.113. [DOI] [Google Scholar]
- 64.Junior WGM, Pacheco TF, Corrêa PS, Martins AA, Mata TM, Caetano NS. Acid pretreatment of sugarcane biomass to obtain hemicellulosichydrolisate rich in fermentable sugar. Energy Rep. 2020;6:18–23. doi: 10.1016/j.egyr.2020.10.015. [DOI] [Google Scholar]
- 65.Goshadrou A. Bioethanol production from cogongrass by sequential recycling of black liquor and wastewater in a mild-alkali pretreatment. Fuel. 2019;258:116141. doi: 10.1016/j.fuel.2019.116141. [DOI] [Google Scholar]
- 66.Tsegaye B, Balomajumder C, Roy P. Organosolv pretreatments of rice straw followed by microbial hydrolysis for efficient biofuel production. Renew Energ. 2020;148:923–934. doi: 10.1016/j.renene.2019.10.176. [DOI] [Google Scholar]
- 67.Perrone OM, de Souza Moretti MM, Bordignon SE, de Cassia PJ, da Silva R, Gomes E, Boscolo M. Improving cellulosic ethanol production using ozonolysis and acid as a sugarcane biomass pretreatment in mild conditions. Bioresour Technol Rep. 2021;13:100628. doi: 10.1016/j.biteb.2021.100628. [DOI] [Google Scholar]
- 68.Hernández-Guzmán A, Navarro-Gutiérrez IM, Meléndez-Hernández PA, Hernández-Beltrán JU, Hernández-Escoto H. Enhancement of alkaline-oxidative delignification of wheat straw by semi-batch operation in a stirred tank reactor. Bioresour Technol. 2020;312:123589. doi: 10.1016/j.biortech.2020.123589. [DOI] [PubMed] [Google Scholar]
- 69.Semerci I, Ersan G. Hornbeam pretreatment with protic ionic liquids: Cation, particle size, biomass loading and recycling effects. Ind Crops Prods. 2021;159:113021. doi: 10.1016/j.indcrop.2020.113021. [DOI] [Google Scholar]
- 70.Menon V, Rao M. Trends in bioconversion of lignocellulose: biofuels, platform chemicals & biorefinery concept. Prog Energy Combust Sci. 2012;38:522–550. doi: 10.1016/j.pecs.2012.02.002. [DOI] [Google Scholar]
- 71.Semwal S, Raj T, Kumar R, Christopher J, Gupta RP, Puri SK, Kumar R, Ramakumar SSV. Process optimization and mass balance studies of pilot scale steam explosion pretreatment of rice straw for higher sugar release. Biomass Bioenergy. 2019;130:105390. doi: 10.1016/j.biombioe.2019.105390. [DOI] [Google Scholar]
- 72.Hans M, Garg S, Pellegrini VO, Filgueiras JG, de Azevedo ER, Guimaraes FE, Chandel AK, Polikarpov I, Chadha BS, Kumar S. Liquid ammonia pretreatment optimization for improved release of fermentable sugars from sugarcane bagasse. J Clean Prod. 2021;281:123922. doi: 10.1016/j.jclepro.2020.123922. [DOI] [Google Scholar]
- 73.Zhao MJ, Xu QQ, Li GM, Zhang QZ, Zhou D, Yin JZ, Zhan HS. Pretreatment of agricultural residues by supercritical CO2 at 50–80°C to enhance enzymatic hydrolysis. J Energy Chem. 2019;31:39–45. doi: 10.1016/j.jechem.2018.05.003. [DOI] [Google Scholar]
- 74.Kumari D, Singh R. Pretreatment of lignocellulosic wastes for biofuel production: a critical review. Renew Sust Energy Rev. 2018;90:877–891. doi: 10.1016/j.rser.2018.03.111. [DOI] [Google Scholar]
- 75.Kang X, Zhang Y, Lin R, Li L, Zhen F, Kong X, Sun Y, Yuan Z. Optimization of liquid hot water pretreatment on Hybrid Pennisetum anaerobic digestion and its effect on energy efficiency. Energy Convers Manag. 2020;210:112718. doi: 10.1016/j.enconman.2020.112718. [DOI] [Google Scholar]
- 76.Devi J, Deb U, Barman S, Das S, Bhattacharya SS, Tsang YF, Lee JH, Kim KH. Appraisal of lignocellusoic biomass degrading potential of three earthworm species using vermireactor mediated with spent mushroom substrate: compost quality, crystallinity, and microbial community structural analysis. Sci Total Environ. 2020;716:135215. doi: 10.1016/j.scitotenv.2019.135215. [DOI] [PubMed] [Google Scholar]
- 77.Cheah WY, Sankaran R, Show PL, Ibrahim TNBT, Chew KW, Culaba A, Jo-Shu C. Pretreatment methods for lignocellulosic biofuels production: current advances, challenges and future prospects. Biofuel Res J. 2020;7:1115. doi: 10.18331/BRJ2020.7.1.4. [DOI] [Google Scholar]
- 78.Verardi A, De Bari I, Ricca E, Calabrò V (2012) Hydrolysis of lignocellulosic biomass: current status of processes and technologies and future perspectives. In: Lima MAP, Natalense APP (ed) Bioethanol. InTech, Rijeka,pp95–122
- 79.Wyman CE (1999) Production of low cost sugars from biomass: progress, opportunities, and challenges. In: Overerd RP, Cornet E(ed) Biomass- a growth opportunity in green energy and value-added products. Proocedings of the 4th Biomass conference of the Americas. Pergamon, Oxford, pp 867–872
- 80.Katzen R, Schell DJ (2005) Lignocellulosic feedstock biorefinery: history and plant development for biomass hydrolysis. In: Kamm B, Gruber PR, Kamm M (ed) Biorefineries-Industrial Processes and Products: Status Quo and Future Directions. Wiley–VCG, Germany,pp 129–138 10.1002/9783527619849.ch6
- 81.Singh A, Bajar S, Devi A, Pant D (2021) An overview on the recent developments in fungal cellulase production and their industrial applications. Bioresour Technol Rep 100652. 10.1016/j.biteb.2021.100652
- 82.Sulyman AO, Igunnu A, Malomo SO. Isolation, purification and characterization of cellulase produced by Aspergillus niger cultured on Arachis hypogaea shells. Heliyon. 2020;6:e05668. doi: 10.1016/j.heliyon.2020.e05668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xiang J, Wang X, Sang T (2020) Cellulase production from Trichoderma reesei RUT C30 induced by continuous feeding of steam-exploded Miscanthus lutarioriparius. Ind Crops Prod 11312910.1016/j.indcrop.2020.113129
- 84.Li F, Xie Y, Gao X, Shan M, Sun C, Niu YD, Shan A. Screening of cellulose degradation bacteria from Min pigs and optimization of its cellulase production. Electron J Biotechnol. 2020;48:29–35. doi: 10.1016/j.ejbt.2020.09.001. [DOI] [Google Scholar]
- 85.Akula S, Golla N (2018) Optimization of cellulase production by Aspergillu sniger isolated from forest soil. Open Biotechnol J 1210.2174/1874070701812010256
- 86.Amobonye A, Bhagwat P, Singh S, Pillai S. Enhanced xylanase and endoglucanase production from Beauveria bassiana SAN01, an entomopathogenic fungal endophyte. Fungal Biol. 2021;125:39–48. doi: 10.1016/j.funbio.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 87.Bakri Y, Akeed Y, Jawhar M, Arabi MIE. Evaluation of xylanase production from filamentous fungi with different lifestyles. Acta Aliment. 2020;49:197–203. doi: 10.1556/066.2020.49.2.9. [DOI] [Google Scholar]
- 88.Ire FS, Chima IJ, Ezebuiro V. Enhanced xylanase production from UV-mutated Aspergillus niger grown on corn cob and sawdust. Biocatal Agric Biotechnol. 2021;31:101869. doi: 10.1016/j.bcab.2020.101869. [DOI] [Google Scholar]
- 89.Patel K, Dudhagara P. Optimization of xylanase production by Bacillus tequilensis strain UD-3 using economical agricultural substrate and its application in rice straw pulp bleaching. Biocatal Agric Biotechnol. 2020;30:101846. doi: 10.1016/j.bcab.2020.101846. [DOI] [Google Scholar]
- 90.Massarente VS, de Araujo ZJ, Gomes E, Bonilla-Rodriguez GO. Biochemical characterization of endoglucanases produced by Myceliophthora thermophila M. 7.7 in solid-state culture. Biocatal Agric Biotechnol. 2020;27:101684. doi: 10.1016/j.bcab.2020.101684. [DOI] [Google Scholar]
- 91.Onuma H, Hara K, Sugita K, Kano A, Fukuta Y, Shirasaka N. Purification and characterization of a glycoside hydrolase family 5 endoglucanase from Tricholoma matsutake grown on barley based solid-state medium. J Biosci Bioeng. 2019;128:669–676. doi: 10.1016/j.jbiosc.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 92.Mohapatra S, Jena S, Jena PK, Badhai J, Acharya AN, Thatoi H. Partial consolidated bioprocessing of pretreated Pennisetum sp. by anaerobic thermophiles for enhanced bioethanol production. Chemosphere. 2020;256:127126. doi: 10.1016/j.chemosphere.2020.127126. [DOI] [PubMed] [Google Scholar]
- 93.Qiu J, Tian D, Shen F, Hu J, Zeng Y, Yang G, Zhang Y, Deng S, Zhang J. Bioethanol production from wheat straw by phosphoric acid plus hydrogen peroxide (PHP) pretreatment via simultaneous saccharification and fermentation (SSF) at high solid loadings. Bioresour Technol. 2018;268:355–362. doi: 10.1016/j.biortech.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 94.Mithra MG, Jeeva ML, Sajeev MS, Padmaja G. Comparison of ethanol yield from pretreated lignocellulo-starch biomass under fed-batch SHF or SSF modes. Heliyon. 2018;4:e00885. doi: 10.1016/j.heliyon.2018.e00885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cotana F, Cavalaglio G, Gelosia M, Coccia V, Petrozzi A, Ingles D, Pompili E. A comparison between SHF and SSSF processes from cardoon for ethanol production. Ind Crops Prod. 2015;69:424–432. doi: 10.1016/j.indcrop.2015.02.064. [DOI] [Google Scholar]
- 96.Nargotra P, Sharma V, Bajaj BK. Consolidated bioprocessing of surfactant-assisted ionic liquid-pretreated Parthenium hysterophorus L. biomass for bioethanol production. Bioresour Technol. 2019;289:121611. doi: 10.1016/j.biortech.2019.121611. [DOI] [PubMed] [Google Scholar]
- 97.Singh N, Gupta A, Mathur AS, Barrow C, Puri M. Integrated consolidated bioprocessing for simultaneous production of Omega-3 fatty acids and bioethanol. Biomass Bioenergy. 2020;137:105555. doi: 10.1016/j.biombioe.2020.105555. [DOI] [Google Scholar]
- 98.Song Y, Lee YG, Cho EJ, Bae HJ. Production of xylose, xylulose, xylitol, and bioethanol from waste bamboo using hydrogen peroxicde-acetic acid pretreatment. Fuel. 2020;278:118247. doi: 10.1016/j.fuel.2020.118247. [DOI] [Google Scholar]
- 99.David AN, Sewsynker-Sukai Y, Sithole B, Kana EG. Development of a green liquor dregs pretreatment for enhanced glucose recovery from corn cobs and kinetic assessment on various bioethanol fermentation types. Fuel. 2020;274:117797. doi: 10.1016/j.fuel.2020.117797. [DOI] [Google Scholar]
- 100.Toor M, Kumar SS, Malyan SK, Bishnoi NR, Mathimani T, Rajendran K, Pugazhendhi A. An overview on bioethanol production from lignocellulosic feedstocks. Chemosphere. 2020;242:125080. doi: 10.1016/j.chemosphere.2019.125080. [DOI] [PubMed] [Google Scholar]
- 101.Jin X, Song J, Liu GQ. Bioethanol production from rice straw through an enzymatic route mediated by enzymes developed in-house from Aspergillus fumigatus. Energy. 2020;190:116395. doi: 10.1016/j.energy.2019.116395. [DOI] [Google Scholar]
- 102.Marulanda VA, Gutierrez CDB, Alzate CAC (2019) Thermochemical, biological, biochemical, and hybrid conversion methods of bio-derived molecules into renewable fuels. In: Hosseini M (ed)Advanced bioprocessing for alternative fuels, biobased chemicals, and bioproducts. Woodhead Publishing, United Kingdom, pp 59–81 10.1016/B978-0-12-817941-3.00004-8
- 103.Zabed H, Sahu JN, Boyce AN, Faruq G. Fuel ethanol production from lignocellulosic biomass: an overview on feedstocks and technological approaches. Renew Sust Energ Rev. 2016;66:751–774. doi: 10.1016/j.rser.2016.08.038. [DOI] [Google Scholar]
- 104.Bajpai P (2021) Production of bioethanol. In: Bajpai P(ed) Developments in Bioethanol. Springer, Singapore, pp 41–110 10.1007/978-981-15-8779-5_5
- 105.Shafiei Amrei S, Asghari M, Esfahanian M, Zahraei Z. Highly selective carbon nanotube-coupled graphene oxide-incorporated polydimethylsiloxane membrane for pervaporative membrane bioreactor ethanol production. J Chem Technol Biotechnol. 2020;95:1604–1613. doi: 10.1002/jctb.6355. [DOI] [Google Scholar]
- 106.Dussán KJ, Silva DD, Perez VH, da Silva SS. Evaluation of oxygen availability on ethanol production from sugarcane bagasse hydrolysate in a batch bioreactor using two strains of xylose-fermenting yeast. Renew Energ. 2016;87:703–710. doi: 10.1016/j.renene.2015.10.065. [DOI] [Google Scholar]
- 107.Li WC, Li X, Zhu JQ, Qin L, Li BZ, Yuan YJ. Improving xylose utilization and ethanol production from dry dilute acid pretreated corn stover by two-step and fed-batch fermentation. Energy. 2018;157:877–885. doi: 10.1016/j.energy.2018.06.002. [DOI] [Google Scholar]
- 108.Saha K, Maharana A, Sikder J, Chakraborty S, Curcio S, Drioli E. Continuous production of bioethanol from sugarcane bagasse and downstream purification using membrane integrated bioreactor. Catal Today. 2019;331:68–77. doi: 10.1016/j.cattod.2017.11.031. [DOI] [Google Scholar]
- 109.Todhanakasem T, Salangsing OL, Koomphongse P, Kaewket S, Kanokratana P, Champreda V. Zymomonas mobilis biofilm reactor for ethanol production using rice straw hydrolysate under continuous and repeated batch processes. Front Microbiol. 2019;10:1777. doi: 10.3389/fmicb.2019.01777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Malairuang K, Krajang M, Rotsattarat R, Chamsart S. Intensive multiple sequential batch simultaneous saccharification and cultivation of Kluyveromyces marxianus SS106 thermotolerant yeast strain for single-step ethanol fermentation from raw cassava starch. Processes. 2020;8:898. doi: 10.3390/pr8080898. [DOI] [Google Scholar]
- 111.da Silva VZ, OuriqueLJ, de David C, Ayub MAZ, Construction of recombinant Klebsiella pneumoniae to increase ethanol production on residual glycerol fed-batch cultivations. Appl Biochem Biotechnol. 2020;192:1147–1162. doi: 10.1007/s12010-020-03397-5. [DOI] [PubMed] [Google Scholar]
- 112.Cruz ML, de Resende MM, Ribeiro EJ. Improvement of ethanol production in fed-batch fermentation using a mixture of sugarcane juice and molasse under very high-gravity conditions. Bioprocess Biosyst Eng. 2021;44:617–625. doi: 10.1007/s00449-020-02462-x. [DOI] [PubMed] [Google Scholar]
- 113.Nurhayati CCL, Nagarajan D, Chang JS. Immobilization of Zymomonas mobilis with Fe2O3-modified polyvinyl alcohol for continuous ethanol fermentation. Biochem Eng J. 2016;114:298–306. doi: 10.1016/j.bej.2016.07.021. [DOI] [Google Scholar]
- 114.Ingledew, WMM, Lin YH (2011) Ethanol from starch-based feedstocks. In: Johnston-Monje D, Raizada MN (ed) Comprehensive biotechnology. Elsevier, Amsterdam, pp 37–49. 10.1016/B978-0-08-088504-9.00457-8
- 115.Phukoetphim N, Khongsay N, Laopaiboon P, Laopaiboon L. A novel aeration strategy in repeated-batch fermentation for efficient ethanol production from sweet sorghum juice. Chin J Chem Eng. 2019;27:1651–1658. doi: 10.1016/j.cjche.2018.11.010. [DOI] [Google Scholar]
- 116.Zhang M, Wang F, Su R, Qi W, He Z. Ethanol production from high dry matter corncob using fed-batch simultaneous saccharification and fermentation after combined pretreatment. Bioresour Technol. 2010;101:4959–4964. doi: 10.1016/j.biortech.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 117.Pino MS, Rodríguez-Jasso RM, Michelin M, Flores-Gallegos AC, Morales-Rodriguez R, Teixeira JA, Ruiz HA. Bioreactor design for enzymatic hydrolysis of biomass under the biorefinery concept. Chem Eng J. 2018;347:119–136. doi: 10.1016/j.cej.2018.04.057. [DOI] [Google Scholar]
- 118.Lu J, Li X, Yang R, Yang L, Zhao J, Liu Y, Qu Y. Fed-batch semi-simultaneous saccharification and fermentation of reed pretreated with liquid hot water for bio-ethanol production using Saccharomyces cerevisiae. Bioresour Technol. 2013;144:539–547. doi: 10.1016/j.biortech.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 119.Kumar M, Gayen K. Developments in biobutanol production: new insights. Appl Energy. 2011;88:1999–2012. doi: 10.1016/j.apenergy.2010.12.055. [DOI] [Google Scholar]
- 120.Buyondo JP, Liu S. Processes and bioreactor designs for butanol production from lignocellulosic biomass. J Bioprocess Eng Biorefinery. 2012;1:53–68. doi: 10.1166/jbeb.2012.1009. [DOI] [Google Scholar]
- 121.Giorno L (2015)Cell Recycle Membrane Fermentor. In: Drioli E, Giorno L(ed) Encyclopedia of Membranes. Springer, Berlin, Heidelberg 10.1007/978-3-642-40872-4_1474-1
- 122.Mariano AP, Costa CBB, De Angelis DDF, MaugeriFilho F, Atala DIP, Maciel MRW, MacielFilho R. Optimisation of a continuous flash fermentation for butanol production using the response surface methodology. Chem Eng Res Des. 2010;88:562–571. doi: 10.1016/j.cherd.2009.11.002. [DOI] [Google Scholar]
- 123.Karagoz P, Bill RM, Ozkan M. Lignocellulosic ethanol production: evaluation of new approaches, cell immobilization and reactor configurations. Renew Energ. 2019;143:741–752. doi: 10.1016/j.renene.2019.05.045. [DOI] [Google Scholar]
- 124.Nemati M, Webb C (2011) Immobilized cell bioreactors. In: Moo-Young, Webb M, Colin (ed) Comprehensive Biotechnology.Academic Press, Burlington,pp 331–346. 10.1016/B978-0-08-088504-9.00100-8
- 125.Song S, Fung Kin Yuen V, Di L, Sun Q, Zhou K, Yan N. Integrating biomass into the organonitrogen chemical supply chain: production of pyrrole and d-proline from furfural. Angew Chem. 2020;132:20018–20022. doi: 10.1002/ange.202006315. [DOI] [PubMed] [Google Scholar]
- 126.Ntimbani RN, Farzad S, Görgens JF (2021) Furfural production from sugarcane bagasse along with co-production of ethanol from furfural residues. Biomass Convers Biorefin 1-1110.1007/s13399-021-01313-3
- 127.Cypriano DZ, da Silva LL, Tasic L. High value-added products from the orange juice industry waste. Waste Manage. 2018;79:71–78. doi: 10.1016/j.wasman.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 128.Yuan HW, Tan L, Kida K, Morimura S, Sun ZY, Tang YQ. Potential for reduced water consumption in biorefining of lignocellulosic biomass to bioethanol and biogas. J Biosci Bioeng. 2021;131:461–468. doi: 10.1016/j.jbiosc.2020.12.015. [DOI] [PubMed] [Google Scholar]
- 129.Conesa C, Laguarda-Miro N, Fito P, Seguí L. Evaluation of Persimmon (Diospyros kaki Thunb. cv. Rojo Brillante) industrial residue as a source for value added products. Waste Biomass Valorization. 2020;11:3749–3760. doi: 10.1007/s12649-019-00621-0. [DOI] [Google Scholar]
- 130.Chatterjee S, Mohan SV. Simultaneous production of green hydrogen and bioethanol from segregated sugarcane bagasse hydrolysate streams with circular biorefinery design. Chem Eng J. 2021;425:130386. doi: 10.1016/j.cej.2021.130386. [DOI] [Google Scholar]
- 131.Khaire KC, Moholkar VS, Goyal A. Bioconversion of sugarcane tops to bioethanol and other value added products: an overview. Mater Sci Energ Technol. 2021;4:54–68. doi: 10.1016/j.mset.2020.12.004. [DOI] [Google Scholar]
- 132.Karkee A (2016) Optimization and cost analysis of lignocellulosic biomass feedstocks supply chains for biorefineries. Doctoral dissertation, Iowa State University. https://lib.dr.iastate.edu/etd/14996
- 133.Vikash PV, Shastri Y (2017) Economic optimization of integrated lignocellulosic biorefinery. In: Espuña A, Graells M, Puigjaner L (ed) Computer Aided Chemical Engineering. Elsevier, Amsterdam, pp 2503–2508 10.1016/B978-0-444-63965-3.50419-0
- 134.Solarte-Toro JC, Romero-García JM, Martínez-Patiño JC, Ruiz-Ramos E, Castro-Galiano E, Cardona-Alzate CA. Acid pretreatment of lignocellulosic biomass for energy vectors production: a review focused on operational conditions and techno-economic assessment for bioethanol production. Renew Sust Energ Rev. 2019;107:587–601. doi: 10.1016/j.rser.2019.02.024. [DOI] [Google Scholar]
- 135.Okolie JA, Tabat ME, Gunes B, Epelle EI, Mukherjee A, Nanda S, Dalai AK. A techno-economic assessment of biomethane and bioethanol production from crude glycerol through integrated hydrothermal gasification, syngas fermentation and biomethanation. Energ Convers Manage: X. 2021;12:100131. doi: 10.1016/j.ecmx.2021.100131. [DOI] [Google Scholar]
- 136.Khounani Z, Nazemi F, Shafiei M, Aghbashlo M, Tabatabaei M. Techno-economic aspects of a safflower-based biorefinery plant co-producing bioethanol and biodiesel. Energ Convers Manage. 2019;201:112184. doi: 10.1016/j.enconman.2019.112184. [DOI] [Google Scholar]
- 137.Nagappan S, Nakkeeran E (2020) Biorefinery: a concept for co-producing biofuel with value-added products. In: Gothandam KM, Ranjan S, Dasgupta N, Lichtfouse E (ed) Environmental Biotechnology Vol. 2. Springer, Singapore, pp 23–52 10.1007/978-3-030-38196-7_2
- 138.Vedovato V, Vanbroekhoven K, Pant D, Helsen J. Electrosynthesis of biobased chemicals using carbohydrate bioethanol production from cereal crops s as a Feedstock. Molecules. 2020;25(16):3712. doi: 10.3390/molecules25163712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA, Frederick WJ, Hallett JP, Leak DJ, Liotta CL, Mielenz JR. The path forward for biofuels and biomaterials. Science. 2006;311:484–489. doi: 10.1126/science.1114736. [DOI] [PubMed] [Google Scholar]
- 140.Liao Y, Koelewijn SF, Van den Bossche G, Van Aelst J, Van den Bosch S, Renders T, Navare K, Nicolaï T, Van Aelst K, Maesen M, Matsushima H. A sustainable wood biorefinery for low–carbon footprint chemicals production. Science. 2020;367:1385–1390. doi: 10.1126/science.aau1567. [DOI] [PubMed] [Google Scholar]
- 141.Kondo A, Ishii J, Harac KY, Hasunuma T, Matsuda F. Development of microbial cell factories for bio-refinery through synthetic bioengineering. J Biotechnol. 2013;163:204–216. doi: 10.1016/j.jbiotec.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 142.Dahiya S, Kumar AN, Sravan JS, Chatterjee S, Sarkar O, Mohan SV. Food waste biorefinery: Sustainable strategy for circular bioeconomy. Bioresour Technol. 2018;248:2–12. doi: 10.1016/j.biortech.2017.07.176. [DOI] [PubMed] [Google Scholar]
- 143.Diwan B, Mukhopadhyay D, Gupta P (2020) Recent trends in biorefinery-based valorisation of lignocellulosic biomass. In: Rathinam NK, Sani R (ed) Biovalorisation of Wastes to Renewable Chemicals and Biofuels. Elsevier, Amsterdam, pp. 219–242 10.1016/B978-0-12-817951-2.00011-0
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.