Abstract
Background:
This research was designed to probe into the role of miRNA-21 in the pathogenesis of childhood asthma and its correlation with the severity.
Methods:
Fifty-four children with bronchial asthma admitted to the Third Affiliated Hospital of Qiqihar Medical University from Jun 2018 to Dec 2019 were included. Forty nine healthy children underwent physical examination at this time period were also enrolled. The miR-21 expression in peripheral blood serum was analyzed by qRT-PCR. The relationship between the expression and severity of asthma in children was explored by Spearman correlation analysis and ROC curve. Bronchial epithelial cell lines were cultured in vitro and divided into blank control group, negative control group and miR-21 inhibition and activation group. The changes of cell proliferation after treatment were detected by CCK-8 test in different groups. The expression of TGF-β1/Smad signaling pathway protein in cells was assessed by Western blot (WB).
Results:
Compared with that of healthy children, the miR-21 expression in peripheral blood serum of asthmatic children was higher (P<0.001). MiR-21 expression was positively correlated with the severity of illness (r=0.853, P<0.001). The results of cell experiments in vitro signified that miR-21 can promote the proliferation of bronchial epithelial cells, and may be involved in regulating the expression of TGF-β1/Smad3 signaling pathway, thus affecting cell proliferation.
Conclusion:
miRNA-21 regulates the proliferation of bronchial epithelial cells by activating TGFβ1/Smad signaling pathway. And it is positively correlated with the severity of asthma in children.
Keywords: Childhood asthma, MicroRNA, Proliferation, Transforming growth factor β1
Introduction
Asthma is a chronic airway inflammation state of respiratory tract, which is mainly characterized by airway hyper responsiveness, bronchospasm, increased mucus secretion and airflow restriction. The clinical manifestations of the disease are cough, wheezing, shortness of breath, chest tightness and other respiratory symptoms (1). However, due to the heterogeneity of symptoms of the disease, some patients only have mild respiratory symptoms, which can also lead to severe respiratory distress (2). In recent years, the morbidity of asthma in children and adolescents in the world has been increasing year by year. In China in 2010, the prevalence of asthma in children under 14 yr was 3.02%, nearly double that that in 2000 (3,4). Childhood asthma has a lasting impact on health. About 47% of 50-yr asthma patients have status asthmatics before the age of 6, and 15% have suffered from severe asthma in childhood (5). The risk of developing chronic obstructive pulmonary disease at the age of 50 is 75% higher in patients who had severe asthma in childhood than that of normal people (6). Asthma is the most common chronic disease in children, and it is of great significance to explore the mechanism of disease occurrence and progression for early diagnosis and treatment.
Chronic airway inflammatory diseases mainly include epithelial cell injury and necrosis, infiltration of inflammatory cells in mucosa and submucosa, hypertrophy and proliferation of goblet cells, exuberant secretion of glands, thickening and fibrosis of basement membrane, etc. Among them, the proliferation and apoptosis of airway epithelial cells can lead to the damage of airway barrier function, and its severity is related to the severity of bronchial asthma (7). Therefore, it is vital to explore the influence of bronchial epithelial cells on the pathogenesis of asthma.
At the moment, microRNAs play a key role in the pathogenesis of asthma. Many microRNAs have been confirmed to be highly expressed in mouse asthma models, including miR-21, miRNA-181a, miR-155, miR-3162-3p, miR-150, miRNA-221, miR-106a, etc. (8, 9). miRNA-21 was highly expressed in asthma mouse model induced by IL-13 in lung (10). miRNA-21 is increased in peripheral blood serum of asthmatic children. It may be used as a brand-new marker for diagnosis and treatment prognosis (11, 12). miR-21 is a crucial regulator of cell proliferation, migration and apoptosis, which is often highly expressed in various tumors, cardiovascular and immune-related diseases. miR-21 regulates tumor cell proliferation, apoptosis and migration through PTEN/PI3K/AKT, TIMP3 and tumor necrosis factor-α (TNF-α) (13–15). However, the mechanism of miR-21 in bronchial epithelial cells and its relationship with the severity of asthma in children are still vague.
Thus, the purpose of this research was to explore the role of miRNA-21 in bronchial epithelial cell proliferation in asthma, and to clarify the correlation between miRNA-21 and the severity of illness, so as to provide reliable basis for diagnosis and treatment.
Materials and Methods
Research objects
This research was approved by the Ethics Committee of the Third Affiliated Hospital of Qiqihar Medical University.
Fifty-four bronchial asthma children were collected who were admitted to the Third Affiliated Hospital of Qiqihar Medical University from Jun 2018 to Dec 2019. There were 30 males and 24 females, with an average age of (9.26±0.40) yr. There were 49 children in the healthy control group who had physical examination in our hospital at the same time period, including 26 males and 23 females, with an average age of (9.61±0.41) yr. No marked difference was found in gender and age between the two groups (P=0.54).
The inclusion criteria for children with asthma were: 1) patients who were diagnosed as bronchial asthma according to the 2016 edition of the Pediatric Branch Respiratory Group of the Chinese Medical Association Pediatric Branch; 2) after asthma control, the symptoms were relatively stable; 3) patients’ guardians were fully aware of the purpose and content of this research and sign informed consent forms. According to the attack frequency and degree of children with bronchial asthma and lung function status (FEV1 accounts for less than 60% of normal estimated value), they were divided into intermittent state (grade I), mild state (grade II), moderate persistent state (grade III) and severe asthma (grade IV), including 15 cases of intermittent state and 19 of mild state.
The exclusion criteria were: 1) patients complicated with other respiratory diseases, such as congenital malformation of respiratory tract, pneumonia, bronchiectasis, etc.; 2) patients complicated with other serious systemic diseases, such as liver and kidney insufficiency, heart diseases, blood system diseases and nervous system diseases, etc.
Peripheral blood sample collection
Five ml peripheral venous blood was collected from all subjects using blood collection tube without anticoagulant, and then centrifuged at 4 °C for 10 min at 3000 r/min to obtain peripheral blood serum. The samples were stored in a refrigerator at −80 °C for miRNA extraction.
qRT-PCR detection
The miRNeasy Mini Kit (217004, Qiagen) was purchased from Qiagen. MiRNA was extracted according to the instructions of the kit, and cDNA was synthesized in light of the instructions of the reverse transcription kit (218161, Qiagen). The obtained cDNA template was amplified by real-time fluorescence quantitative PCR using a kit (204143, Qiagen), and the primers of miR-21 and U6 were synthesized by Invitrogen. PCR amplification conditions were: 95 °C for 15 min, 94 °C for 15 s after 40 cycles, 55 °C for 30 s, 70 °C for 34 s. The experiment was repeated three times, and U6 was used as an internal reference. The miR-21 relative expression was calculated by 2−ΔΔCT method (Table 1).
Table 1:
Primer sequences of fluorescence quantitative PCR amplification
| Amplified genes | Primer sequences | |
|---|---|---|
| miR-21 | Forward | 5’-CGGGATCCAGCCACTACCAAGGCATGTT -3’ |
| Reverse | 5’-CGGAATTCAACCACGACTAGAGGCTGAC-3’ | |
| U6 | Forward | 5’-GCGCGT CGTGAAGCGTTC-3’ |
| Reverse | 5’-GTGCAGGGTCCGAGGT-3’ |
Cell culture
Bronchial epithelial cell line 16HBE (ZQ-0001) was purchased from Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd., and the cells were cultured in complete epithelial cell culture medium (ZQ-1303). It was placed in a constant temperature incubator at 37 °C with 5% CO2 and 95% air. When the cell density was increased to a suitable level, 200 mg/L Smad7 agonist Asiaticoside (HY-N0439, MCE) was added to the medium and cultured for 24 h.
Regulation of miR-21 expression
After the density proliferated by 40%–60%, the cells were divided into blank control group, inhibitor control group, miR-21 inhibitor group, mimics control group and miR-21 mimics group. Thereinto, 1 μl PBS buffer and 1 μl DMSO were added into the culture medium of blank control group. Afterwards, 100 μl Opti-MEM was added into 1.5 ml EP tube. Then 50 mM inhibitor control, miR-21 inhibitor, mimics control and miR-21 mimics (Shanghai GenePharma Co., Ltd) were added. Finally 5 μl Lipo2000 (11668027, Invitrogen) transfection reagent was added. They were mixed evenly and incubated at room temperature for 20 min. Next, the mixed droplets were added into the medium for cell culture.
CCK-8 experiment
16HBE cells were digested into suspension, and 100 μl cell suspension was inoculated into 96-well plate, and cultured in an incubator at 37 °C for 12 h. Next, 10 μl diluted CCK-8 (CA1210-500T, Solarbio) solution was added to each well, and then cultured at constant temperature for 6 h. The absorbance values at 450 nm and 600 nm were measured by microplate reader, and cell activity was calculated according to standard curve.
Western blot experiment
Totally 1 mM PMSF (P0100, Solarbio) was added to RIPA protein lysate (P0013C, Beyotime), and 100 μl mixed solution was added to each well of six-well plate for lysis. After 30 min, cell lysate was collected and centrifuged at 15000 g at 4 °C for 10 min to extract total protein. The total protein concentration was detected by BCA protein quantitative kit (P0012, Beyotime). After SDS-PAGE gel electrophoresis, the protein was transferred to PVDF membrane (88585, Thermo Scientific) by semi-wet transfer method. The 5% skimmed milk powder was incubated for 1 h, then TGF-β1 (1:1000, ab92486, Abcam), Smad7 (1: 1000, ab216428, Abcam), Smad2/3 (1:1000, ab217553, Abcam), p-Smad2/3 (1:1000, ab272332, Abcam) and GAPDH (1:10000, ab181602, Abcam) Primary Antibody Dilution Buffer was added and cultivated at 4 °C all night. The sample was washed three times with 0.1% PBST, and then the corresponding secondary antibody diluent (1:4000, ab205718, abcam) was added and incubated for 1 h at indoor temperature. Subsequently, it was cleaned three times with 0.1% PBST again, developed with ECL chemiluminescence reagent (P0018FS, Beyotime), fixed and photographed by chemiluminescence imaging system (BioSpectrum 510, UVP), and analyzed by ImageJ 1.52.
Prediction results of miR-21 target gene
The binding site of miR-21 and TGF-β1 target gene was predicted by Target gene prediction software (Targetscan).
Detection of dual luciferase reporter gene
MiR-21 dual luciferase reporter gene test was carried out with 293 T competent cells and dual luciferase reporter gene detection kit (RG027, Beyotime). The miR-21 mimics, miR-21 NC and TGF-β1 3’ non-coding region dual luciferase pmirGLO vector was constructed by Wuhan Genecreate bioengineering Co., Ltd, regarding sea kidney luciferase as an internal reference. The relative light unit values of different samples were measured by BioSpectrum 510 chemiluminescence analyzer, and the activation degree of the target reporter gene was judged by dividing the relative light unit values of marine luciferase.
Statistical methods
The data results were analyzed by SPSS 23.0 (Chicago, IL, USA), and the measurement data such as miR-21 expression in peripheral blood serum of asthmatic children were expressed by mean±standard deviation (x̄±s). The miRNA expression in peripheral blood between healthy and asthmatic children was compared by independent-samples t test, and the proliferation of bronchial epithelial cells under different miR-21 treatments was analyzed by one-way ANOVA. The results of dual luciferase reporter gene experiment were assessed by two-way ANOVA, and the correlation between miR-21 and severity of asthma in children was analyzed by Spearman. The difference was statistically remarkable with P<0.05.
Results
Expression of miR-21 in peripheral blood serum of children with asthma increases
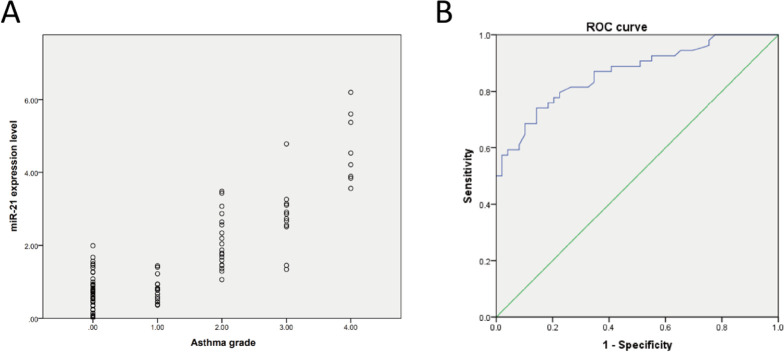
MiRNA was extracted from peripheral blood of healthy and asthmatic children, and the expression of miR-21 in serum was tested by qRT-PCR. The expression of miR-21 in serum of asthmatic children increased (Fig. 1A), and it gradually increased with the severity of the disease. The level in peripheral blood of mild, moderate and severe asthma was higher than that of intermittent children (Fig. 1B).
Fig. 1:
Expression of miR-21 in peripheral blood serum of children with asthma increases
Note: A: expression of miR-21 in peripheral blood serum of healthy and asthmatic children; B: expression of miR-21 in peripheral blood of children with different asthma grades. P<0.01 means **, and P<0.001 means ***
Expression of miR-21 in peripheral blood serum of children with asthma is positively correlated with severity of illness
The relationship between serum miR-21 level and children’s illness was explored by Spearman correlation analysis. The miR-21 level was positively correlated with illness (r=0.853, P<0.001) (Fig. 2A). ROC curve sub-line showed that miR-21 could be used as a marker for diagnosing asthma in children (95%CI: 0.791–0.934, P<0.001) (Fig. 2B).
Fig. 2:
Relationship between miR-21 expression in peripheral blood serum and severity of asthma
Note: A: the relationship between miR-21 expression in peripheral blood serum of healthy and asthma children and disease classification is assessed by Spearman correlation analysis; B: the specificity and sensitivity of miR-21 in diagnosing childhood asthma are investigated by ROC curve
MiR-21 can promote proliferation of bronchial epithelial cells
In order to explore the role of miR-21 in childhood asthma, the bronchial epithelial cell line 16HBE were divided into five groups: blank control group, inhibitor control group, miR-21 inhibitor group, and mimics control group and miR-21 mimics group. The changes of cell proliferation in different treatment groups were detected by CCK-8 test. The high expression of miR-21 could enhance cell proliferation, while the inhibition of miR-21 could decrease cell proliferation (Fig. 3).
Fig. 3:
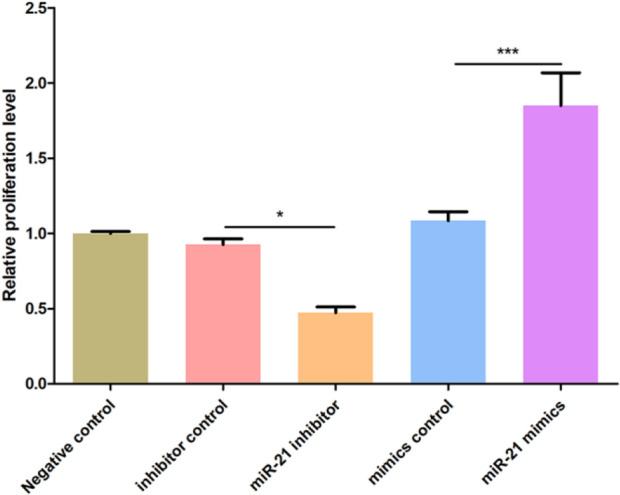
Effect of miR-21 over-expression and knockout on proliferation in bronchial epithelial cells
Note: the effect of miR-21 inhibition and over-expression on proliferation of bronchial epithelial cells 16HBE is assessed by CCK-8 proliferation test
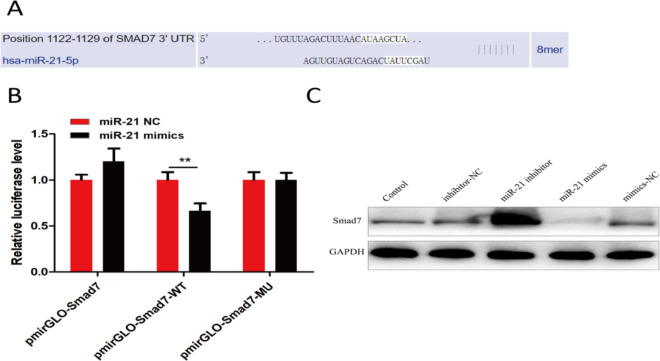
MiR-21 can directly regulate expression of Smad7, an inhibitor of Smad signaling pathway
To further explore the specific mechanism of miR-21 affecting the proliferation of bronchial epithelial cells, miRNA target gene prediction software TargetScan analysis was carried out. Smad7 might be the target gene of miR-21 (Fig. 4A), and the experimental results of dual luciferase reporter gene also verified the regulatory relationship between them (Fig. 4B). Hence, the effect of miR-21 over-expression or inhibition on Smad 7 protein expression was verified by Western blot (WB). It showed that miR-21 inhibition could up-regulate Smad7 protein expression, while miR-21 over-expression could reduce Smad7 protein expression (Fig. 4C).
Fig. 4:
miR-21 can directly regulate expression of Smad signal pathway inhibitor Smad7
Note: A: the binding sites of miR-21 and Smad7 are analyzed by using Targetscan software; B: Dual luciferase reporter gene experiment verifies that there is a regulatory relationship between miR-21 and Smad7; C: the effect of miR-21 inhibition or over-expression on Smad7 protein expression is explored by WB experiment
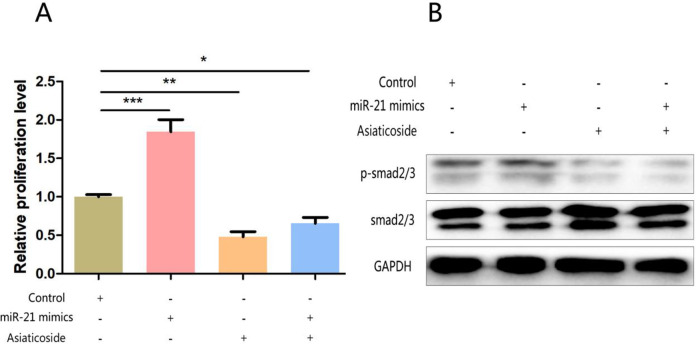
MiR-21 can activate Smad signaling pathway and promote cell proliferation by affecting Smad7 expression
The mechanism of miR-21 in regulating the proliferation of bronchial epithelial cells was verified. The promotion of miR-21 knockout on cell proliferation could be counteracted by Smad7 agonist (Fig. 5A). In addition, a large number of studies have shown that Smad 7 is an inhibitor of Smad 7 signaling pathway, so its down-regulation can activate the pathway. WB results showed that miR-21 over-expression can promote the activation of Smad7 signaling pathway, and this effect can also be antagonized by Smad7 agonists (Fig. 5B).
Fig. 5:
miR-21 can activate Smad signaling pathway and promote cell proliferation by affecting Smad7 expression
Note: A: CCK8 experiment explores the effects of miR-21 over-expression and Smad7 agonist on bronchial epithelial cell proliferation; B: WB experiment verifies the over-expression of miR-21 and the activation of Smad signaling pathway by Smad7 agonist
Discussion
Childhood asthma is a chronic inflammatory state of respiratory tract, and its pathogenesis is influenced by both genetic and environmental factors. At present, the mechanism leading to pathophysiological changes of this disease is ambiguous, thus there is no completely effective treatment so far. miRNA plays a vital role in developing and maintaining the physiological function of lung (16). The expression of miRNA-21 in peripheral blood of asthmatic children is higher than that of healthy control group (11–17). Besides, the expression of miRNA-21 in steroid-resistant children is higher than that in steroid-sensitive children. The expression in serum is negatively correlated with IL-12p35 and FEV1 in serum, and positively correlated with the number of eosinophils in sputum and blood (18). Serum miR-21 not only can be used as a diagnostic marker of asthma in children, but also can be used to distinguish steroid hormone inhalation sensitive and resistant patients. Thus, this research was dedicated to verifying the diagnostic marker role of miR-21 in asthma in children and exploring its influence in the pathogenesis of asthma.
In this research, the correlation between miRNA-21 and the severity of asthma in children was clarified, and the effect of miRNA-21 on the proliferation of bronchial epithelial cells in asthma was discussed, which provided a solid theoretical basis for diagnosis and treatment. We collected the peripheral blood of healthy and asthmatic children, and quantitatively analyzed the expression of miR-21 in the serum. Combined with StatIX online sample size estimation website, it was verified that the data were statistically valid. It revealed that miR-21 in the serum of asthmatic children increased, and the expression of miR-21 was positively correlated with the severity of asthma in children, which was consistent with previous research conclusions. To further explore the role of miR-21 in the pathogenesis of asthma, bronchial epithelial cells were treated with miR-21 activation and inhibition in this study. CCK-8 proliferation experiment showed that miR-21 activation could promote the proliferation of epithelial cells. On this basis, we found that miR-21 could bind Smad7 gene and promote the activation of TGF-β1/Smad3 signaling pathway, thereby leading to the enhancement of proliferation of bronchial epithelial cells.
MiR-21 gene is located on human chromosome 17, which is a highly conserved microRNA. It has been found that miR-21 is up-regulated in various solid tumors, including breast cancer, lung cancer, gastric cancer and colorectal cancer (19–22). miR-21 mainly leads to post-transcriptional silencing of target genes by binding to the 3’-UTR region of target genes, mediating the expression changes of many oncogenes and tumor suppressor genes, including PTEN, Bcl-1, Smad7 and TGF-β, and promoting the proliferation, invasion and migration of tumor cells (23,24). What’s more, miR-21 has been related to pathophysiological processes such as inflammation, fibrosis, cardiac hypertrophy and ventricular remodeling (25). The level of circulating miR-21 in patients with hypertensive heart disease was higher than that in the control group, and was positively correlated with the expression of serum myocardial fibrosis markers (26). The results of mouse model experiment manifested that the expression of miR-21 in mouse heart tissue increased after infusion of angiotensin II or abdominal aortic coarctation. Furthermore, the expression of programmed cell death 4 (PDCD4) decreased. PDCD4, as a major target gene of miR-21, its expression could be regulated by miR-21. Besides, the results of in vitro experiments in neonatal mouse cardiomyocytes also verified that miR-21 inhibitor could abolish the decrease of PDCD4 expression mediated by angiotensin II, and lead to the inactivation of its downstream activator of transcription-1/TGF-β1 signaling pathway, thus preventing ventricular remodeling mediated by hypertension in mouse heart. In this research, the bronchial epithelial cells were activated and inhibited by miR-21 in vitro, and it was found that miR-21 could promote cell proliferation, thus mediating airway tissue remodeling in asthma.
Previously, TGF-β1 in activated state binds with TGF-Br2 receptor on cell membrane, and mediates phosphorylation of R-Smad in cytoplasm. Phosphorylated R-Smad protein can combine with co-Smad to form a complex, and then transfer to nucleus and combine with transcription factors to form a transcriptional activity regulation complex. This complex participates in the expression regulation of proliferation-related genes in cells (27), in which inhibitory protein Smad7 plays an important role in activating TGF-β signaling pathway. In addition, TGF-β1/Smad3 signaling pathway not only promotes cell proliferation, but also mediates the extensive role of cell differentiation, immune response and extra-cellular matrix deposition in asthma (28). The activation of TGF-β1/Smad signaling pathway in airway tissue of asthma patients can mediate the high expression of collagen, α-smooth muscle actin, connective tissue growth factor and plasminogen activated graft, thus inducing fibroblast proliferation, fibroblast differentiation and synthesis of extracellular matrix components. It also participates in mediating pathological changes such as basement membrane thickening, collagen deposition and tissue fibrosis in airway of asthma, resulting in airway tissue remodeling (29). Hence, we made further plans to carry out in vivo experiments in mouse models in future studies to explore the effects of miR-21 on bronchial epithelial cell proliferation, extracellular matrix deposition and tissue fibrosis. In this research, the expression of miR-21 in peripheral blood of children with asthma was verified, and it was positively correlated with the severity of asthma in children. On this basis, it was found that miR-21 might be used as a marker for diagnosis. In vitro experiments showed that miR-21 might regulate the proliferation of bronchial epithelial cells by activating TGFβ1/Smad signaling pathway, which provided solid clinical and theoretical evidence for miR-21 as a marker for diagnosing childhood asthma.
Conclusion
MiRNA-21 regulates the proliferation of bronchial epithelial cells by activating TGFβ1/Smad signaling pathway. It is positively correlated with the severity of asthma in children.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
This study is funded by the scientific research project of basic scientific research business expenses of colleges and universities in Heilongjiang Province, 2020 (2020-KYYWF-0020).
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Barnes PJ. (2018). Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nat Rev Immunol, 18(7): 454–466. [DOI] [PubMed] [Google Scholar]
- 2.Tarlo SM, Malo JL. (2006). An ATS/ERS report: 100 key questions and needs in occupational asthma. Eur Respir J, 27(3): 607–614. [DOI] [PubMed] [Google Scholar]
- 3.National Cooperative Group on Childhood Asthma; Institute of Environmental Health and Related Product Safety, Chinese Center for Disease Control and Prevention. Chinese Center for Disease Control and Prevention (2013). Third nationwide survey of childhood asthma in urban areas of China. Zhonghua Er Ke Za Zhi, 51(10): 729–735. [PubMed] [Google Scholar]
- 4.GBD 2015 Chronic Respiratory Disease Collaborators (2017). Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med, 5(9): 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tai A, Tran H, Roberts M, et al. (2014). Outcomes of childhood asthma to the age of 50 years. J Allergy Clin Immunol, 133(6): 1572–8.e3. [DOI] [PubMed] [Google Scholar]
- 6.Tai A. (2015). Childhood asthma and chronic obstructive pulmonary disease: outcomes until the age of 50. Curr Opin Allergy Clin Immunol, 15(2): 169–174. [DOI] [PubMed] [Google Scholar]
- 7.Iosifidis T, Sutanto EN, Buckley AG, et al. (2020). Aberrant cell migration contributes to defective airway epithelial repair in childhood wheeze. JCI Insight, 5(7): e133125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perry MM, Adcock IM, Chung KF. (2015). Role of microRNAs in allergic asthma: present and future. Curr Opin Allergy Clin Immunol, 15(2): 156–162. [DOI] [PubMed] [Google Scholar]
- 9.LuL F, Liston A. (2009). MicroRNA in the immune system, microRNA as an immune system. Immunology, 127(3): 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HY, Lee HY, Choi JY, et al. (2017). Inhibition of MicroRNA-21 by an antagomir ameliorates allergic inflammation in a mouse model of asthma. Exp Lung Res, 43(3): 109–119. [DOI] [PubMed] [Google Scholar]
- 11.Sawant DV, Yao W, Wright Z, et al. (2015). Serum MicroRNA-21 as a Biomarker for Allergic Inflammatory Disease in Children. Microrna, 4(1): 36–40. [DOI] [PubMed] [Google Scholar]
- 12.Hammad Mahmoud Hammad R, Hamed DHED, Eldosoky MAER, et al. (2018). Plasma microRNA-21, microRNA-146a and IL-13 expression in asthmatic children. Innate Immun, 24(3): 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Liu C, Li H, et al. (2020). Effects of miR-21 on proliferation and apoptosis of WT cells via PTEN/Akt pathway. Exp Ther Med, 19(3): 2155–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X, Xiong J, Lu S, et al. (2019). Inhibitory Effect of Corilagin on miR-21-Regulated Hepatic Fibrosis Signaling Pathway. Am J Chin Med, 47(7): 1541–1569. [DOI] [PubMed] [Google Scholar]
- 15.Yang K, Hu X. (2019). Research progress on miR-21 in heart diseases. Zhejiang Da Xue Xue Bao Yi Xue Ban, 48(2): 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis CC, Yang JY, Huang X, et al. (2008). Disease-specifific gene expression profifiling in multiple models of lung disease. Am J Respir Crit Care Med, 177(4): 376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang C, Yu H, Sun Q, et al. (2016). Extracellular microRNA-21 and microRNA-26a increase in body fluids from rats with antigen induced pulmonary inflammation and children with recurrent wheezing. BMC Pulm Med, 16: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elbehidy RM, Youssef DM, El-Shal AS, et al. (2016). MicroRNA-21 as a novel biomarker in diagnosis and response to therapy in asthmatic children. Mol Immunol, 71: 107–114. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Tan Z, Hu H, et al. (2019). MicroRNA-21 promotes breast cancer proliferation and metastasis by targeting LZTFL1. BMC Cancer, 19(1): 738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang HY. (2013). MicroRNA-21 regulates stemness in cancer cells. Stem Cell Res Ther, 4(5): 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng W, Zhao J, Tao Y, et al. (2018). MicroRNA-21: A promising biomarker for the prognosis and diagnosis of non-small cell lung cancer. Oncol Lett, 16(3): 2777–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, Wan X, Ruan Q. (2016). The MicroRNA-21 in Autoimmune Diseases. Int J Mol Sci, 17(6): 864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sims EK, Lakhter AJ, Anderson-Baucum E, et al. (2017). MicroRNA 21 targets BCL2 mRNA to increase apoptosis in rat and human beta cells. Diabetologia, 60(6): 1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang LB, Tian L, Zhang CG. (2018). Bone marrow stem cells-derived exosomes extracted from osteoporosis patients inhibit osteogenesis via microRNA-21/SMAD7. Eur Rev Med Pharmacol Sci, 22(19): 6221–6229. [DOI] [PubMed] [Google Scholar]
- 25.Liu RH, Ning B, Ma XE, et al. (2016). Regulatory roles of microRNA-21 in fibrosis through interaction with diverse pathways. Mol Med Rep, 13(3): 2359–2366. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe K, Narumi T, Watanabe T, et al. (2020). The association between microRNA-21 and hypertension-induced cardiac remodeling. PLoS One, 15(2): e0226053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grainger DJ, Mosedale DE, Metcalfe JC. (2000). TGF-beta in blood: a complex problem. Cytokine Growth Factor Rev, 11(1–2): 133–145. [DOI] [PubMed] [Google Scholar]
- 28.Ojiaku CA, Yoo EJ, Panettieri RA., Jr (2017). Transforming Growth Factor β1 Function in Airway Remodeling and Hyperresponsiveness. The Missing Link? Am J Respir Cell Mol Biol, 56(4): 432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang YC, Zhang N, Van Crombruggen K, et al. (2012). Transforming growth factor-beta1 in inflammatory airway disease: a key for understanding inflammation and remodeling. Allergy, 67(10): 1193–1202. [DOI] [PubMed] [Google Scholar]