Abstract
Breathlessness is common in the general population. Existing data were obtained primarily with the uni-dimensional modified Medical Research Council breathlessness scale (mMRC) that does not assess intensities of unpleasantness nor physical, emotional and affective dimensions. The aim of this research was to determine the prevalence and intensity of these dimensions of breathlessness in elderly males and any associations with their duration, change over time and mMRC grade.
We conducted a population-based, cross-sectional study of 73-year-old males in a county in southern Sweden. Breathlessness was self-reported at one time point using a postal survey including the Dyspnea-12 (D-12), the Multidimensional Dyspnea Profile (MDP) and the mMRC. Presence of an increased dimension score was defined as a score ≥minimal clinically important difference for each dimension scale. Association with the mMRC, recalled change since age 65, and duration of breathlessness were analysed with linear regression.
Among 907 men, an increased dimension score was present in 17% (D-12 total score), 33% (MDP A1 unpleasantness), 19% (D-12 physical), 17% (MDP immediate perception), 10% (D-12 affective) and 17% (MDP emotional response). The unpleasantness and affective dimensions were strongly associated with mMRC≥3. Higher MDP and D-12 scores were associated with worsening of breathlessness since age 65, and higher MDP A1 unpleasantness was associated with breathlessness of less than 1 year duration.
Increased scores of several dimensions of breathlessness are prevalent in 73-year-old males and are positively correlated with mMRC scores, worsening of breathlessness after age 65, and duration of less than 1 year.
Short abstract
This first epidemiological study of multidimensional breathlessness shows that unpleasant, physical, affective and emotional experiences of breathlessness are common among elderly males, and are strongly associated with mMRC ≥3 https://bit.ly/3EThp5a
Introduction
Breathlessness is prevalent in the general population [1, 2] and is a dominant symptom of cardio-respiratory disease [2]. Approximately 10–25% of the middle-aged and older population experience breathlessness in their daily activities [2, 3]. Chronic breathlessness is persistent and disabling even after optimal treatment of underlying condition(s) [4]. It is an independent risk factor for morbidity and mortality among older adults [5] and is associated with reduced physical capacity, anxiety and depression, and impaired health-related quality of life [6–8].
Breathlessness is defined by the American Thoracic Society as “a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity” [9]. Breathlessness should therefore be seen as a multidimensional condition, comprising overall intensity, intensity of sensory qualities such as air hunger and chest tightness, level of unpleasantness and discomfort, with emotional responses such as fear and anxiety, and impacts on physical activity and function [6]. Research from the past decade has shown that different physiological conditions lead to differences in qualitative breathlessness experience, and these experiences may be relevant when assessing causes and symptomatic treatment of breathlessness [9]. The goal of developing new therapies for the alleviation of symptomatic chronic breathlessness requires knowledge of the prevalence of the known dimensions of breathlessness, as well as how these are related to the duration of the symptom and changes over time.
Previous epidemiological studies [10–12] have assessed the prevalence of breathlessness chiefly through the use of the modified Medical Research Council breathlessness scale (mMRC) [9, 13]. The mMRC scale is a uni-dimensional, ordinal scale assessing the degree of exertion before breathlessness limits the person. It is scored from zero to four but does not assess the intensity or severity of symptoms per se or their affective and emotional impacts [9]. To measure multiple dimensions of breathlessness, Dyspnea-12 (D-12) [14] and Multidimensional Dyspnea Profile [15] (MDP) are frequently used [16], both of which can summarise scores relating to specific dimensions of breathlessness. A common definition of clinically relevant breathlessness in population studies is an mMRC score ≥2 [17]. When using instruments which summarise domain scores in population studies, a common threshold to define a clinically relevant increased score often do not exist. Minimal clinically important difference (MCID) scores have emerged as a method of establishing a threshold at which a change in a symptom score becomes clinically relevant for the individual, which can be used as a treatment outcome in clinical trials. Recent studies have defined MCID scores for the D-12 and MDP [18, 19], but MCID scores have not been used as a threshold for defining clinically relevant increased breathlessness in population studies before. The prevalence of dimensions of breathlessness and their intensities in the older population are unknown, as is their impact on physical limitations, how these change over time and their duration.
The primary aim of this study was to describe the prevalence of the dimensions of breathlessness and their intensities among elderly males measured using the D-12 and MDP. We also analysed these dimensions of breathlessness in relation to the mMRC score, duration of the symptom, and self-reported change since the age of 65.
Methods
Study design and participants
Older males from Blekinge, Sweden were recruited in 2010 to the longitudinal VAScular and Chronic Obstructive Lung disease (VASCOL) study [20]. In 2019, when participants were approximately 73 years old, a postal survey focusing on breathlessness was sent to participants with a known address. Data collection and the VASCOL study has been described previously [20]. Inclusion criteria for the present study were participants in the 2019 VASCOL study that completed the D-12, MDP and mMRC breathlessness scales and responded to additional questions about the unpleasantness of breathlessness, its duration and any changes over time.
Ethical considerations
The study was approved by the Swedish Ethical Review Authority (ref. 2019-00134). Written informed consent was obtained from all participants.
Assessments
Self-reported questionnaire data included height, weight, smoking history, and physician-diagnosed condition(s) which were categorised as lung disease (chronic obstructive pulmonary disease (COPD), asthma, tuberculosis, sleep apnoea or other lung disease), cardiovascular disease (myocardial infarction, angina, atrial fibrillation, heart failure, valvular disease, bypass, aortic aneurysm, carotid artery stenosis, stroke) and diabetes.
Breathlessness was self-reported using the D-12, MDP and mMRC instruments, with the focal period being during the previous 2 weeks. The D-12 tool consists of 12 items with descriptors of breathlessness. The physical dimension scale has a maximum score of 21 and the affective dimension scale has a maximum of 15 [14], for a possible total score of 36. The MDP consists of 11 items divided into three scales: A1 unpleasantness (range 0–10), immediate perception (range 0–60) and emotional response (range 0–50). Higher scores indicate increased severity. This study used validated Swedish versions of the D-12 and MDP [21, 22]. The mMRC version used in this study was translated from Mahler and Wells [23]. The D-12 total scores were imputed for 20 participants as recommended in the original paper [14]. No other imputations were made. The participants were asked to grade the level of unpleasantness or troublesome breathlessness as none, mild, moderate or severe. Recalled change in breathlessness since age 65 was measured with a seven-point ordinal Global Impression of Change scale: very much better (1), much better (2), minimally better (3), no difference (4), minimally worse (5), much worse (6) or very much worse (7). Responses were categorised as better (1–3), no different (4) or worse (5–7). The participants were also asked to state how long they had experienced breathlessness in the number of years, less than 1 year or “I do not remember”. The duration was categorised as <1 year, 1–5 years or >5 years.
The prevalence of clinically relevant increased breathlessness dimension scores was reported by a score ≥MCID for each scale of D-12 and MDP. The MCID scores of the MDP and D-12 scales used in this study have been defined by previous studies [18, 19]. As an example, the MCID score for the D-12 total (range 0–36) is a score of 2.83. MCID is usually used in treatment studies for defining a clinically significant change on a symptom scale, in comparison with a statistically significant change. Use of MCID increases the comparability between scales. Hence reporting the prevalence of an increased breathlessness dimension score using the MCID scores of D-12 and MDP can be considered as a valuable complement to reporting crude scores.
Statistical analysis
Characteristics of the participants and the prevalence of scores ≥MCID and non-zero scores of the D-12 and MDP dimension scales were tabulated using descriptive statistics. The percentage of participants with mMRC ≥1 and scores ≥MCID of D-12 and MDP, as well as mMRC ≥1 and non-zero scores of D-12 and MDP scales, were tabulated. The mean of the MDP item scores among participants reporting scores ≥MCID of MDP A1 unpleasantness were plotted. Differences in the D-12 and MDP dimension scales among the total study population relative to mMRC score (each mMRC score 1–4 separately compared to the reference mMRC of 0), perceived change in breathlessness since age 65 (worse or no difference compared to the reference better), and duration of breathlessness (<1 year or >5 years compared to the reference 1–5 years) were analysed using linear regression. β coefficients and 95% confidence intervals (CIs) were presented in forest plots. To facilitate comparison between dimension scales of different ranges, the β coefficient as a percentage of the scale's range was also presented in the forest plots. For example, an MDP A1 β coefficient of 2 will represent 20% of its range, since the maximum score of MDP A1 is 10. The D-12 and MDP dimension scale medians and interquartile ranges of each category of mMRC, as well as the duration of and change in breathlessness, are presented in boxplots for comparison of linear regression estimates. In the linear regressions and boxplots including change and duration of breathlessness, participants who had not experienced breathlessness after the age of 65 years were excluded, as indicated by reporting mMRC grade 0 and that their breathlessness was unchanged since age 65. Statistical analysis was conducted with R 4.0.2 (R Foundation for Statistical Computing, Austria).
Results
Patient characteristics
Of the 1193 men invited, 907 (76%) participated. Mean body mass index was 27.1 (±3.8) and mean years of smoking was 9.5 (16.2). At least one respiratory disease was reported by 17%, and at least one cardiovascular disease by 37% (table 1).
TABLE 1.
Characteristics of 907 men aged 73 years
| Variable (non-missing observations) | Value |
| Age, years | 73.2 (0.67) |
| Body mass index, kg·m−2 (n= 895) | 27.1 (3.8) |
| Smoking status (n=892) | |
| Daily | 41 (6%) |
| Sometimes | 11 (1%) |
| Former smoker | 530 (59%) |
| Never smoker | 310 (35%) |
| Pack-years of smoking (ever smokers) (n=522) | 9.1 (6.08–18.2)# |
| Respiratory disease (n=861) | 143 (17%) |
| Asthma | 47 (5%) |
| COPD | 32 (4%) |
| Sleep apnoea | 79 (9%) |
| Tuberculosis | 3 (1%) |
| Cardiovascular disease (n=861) | 318 (37%) |
| Angina pectoris | 62 (7%) |
| Atrial fibrillation | 135 (16%) |
| Carotid artery stenosis | 24 (3%) |
| Heart failure | 35 (4%) |
| Myocardial infarction | 79 (9%) |
| Stroke | 66 (8%) |
| Valvular heart disease | 43 (5%) |
| Diabetes mellitus | 146 (17%) |
Data are mean (sd) or frequency (percentage). #: medians (first and third quartile). COPD: chronic obstructive pulmonary disease.
Prevalence of increased breathlessness dimension scores
The prevalence of mMRC ≥2 was 17% (table 2). The prevalence of an increased breathlessness dimension score ≥MCID was 17% based on the D-12 total, and 33% based on the MDP A1 unpleasantness. The prevalence of an increased breathlessness dimension scale with non-zero scores was 29% based on the D-12 total, and 33% based on the MDP A1 unpleasantness (table 3). The prevalence of scores ≥MCID and non-zero scores of all D-12 and MDP dimension scales are shown in table 3. The mean intensities of MDP item scores in participants reporting an MDP A1 unpleasantness score ≥MCID are shown in figure 1.
TABLE 2.
Distribution of breathlessness scores in 907 men aged 73 years
| Item (non-missing observations) | Mean (sd) or frequency (%) |
| Dyspnea-12 scales | |
| Total (n=858) | 1.62 (4.0) |
| Physical (n=846) | 1.08 (2.5) |
| Affective (n=850) | 0.54 (1.7) |
| Multidimensional Dyspnea Profile scales | |
| A1 unpleasantness (n=830) | 0.71 (1.4) |
| Immediate perception (n=721) | 2.62 (6.4) |
| Emotional response (n=809) | 1.77 (5.1) |
| mMRC (n=880) | |
| 0 | 606 (67%) |
| 1 | 120 (13%) |
| 2 | 71 (8%) |
| 3 | 44 (5%) |
| 4 | 39 (4%) |
| Breathlessness severity (n=844) | |
| None | 555 (66%) |
| Mild | 186 (22%) |
| Moderate | 98 (12%) |
| Severe | 5 (1%) |
| Recalled change in breathlessness since age 65 years (n=900) | |
| Better | 97 (11%) |
| No difference | 543 (60%) |
| Worse | 260 (29%) |
| Recalled duration of experienced breathlessness (n=167) | |
| Less than 1 year | 48 (29%) |
| 1–5 years | 72 (43%) |
| More 5 years | 47 (28%) |
Data are means (sd) or frequency (percentage). A higher score on the Dyspnea-12 or Multidimensional Dyspnea Profile scales signify worse breathlessness. Recalled change in breathlessness since age 65 years was measured through global impression of change. mMRC: Medical Research Council breathlessness scale.
TABLE 3.
Prevalence and distribution of breathlessness based on the Dyspnea-12 (D-12) and Multidimensional Dyspnea Profile (MDP) scales
| D-12 scales | MDP scales | |||||
| Total (0–36) | Physical (0–21) | Affective (0–15) | A1 unpleasantness (0–10) | Immediate perception (0–60) | Emotional response (0–50) | |
| Score >0, n (%) | 249 (29) | 244 (29) | 119 (14) | 271 (33) | 285 (40) | 194 (24) |
| mMRC ≥1 and score >0, n (%) | 162 (19) | 160 (19) | 93(11) | 169 (21) | 157 (22) | 106 (13) |
| Mean | 5.6 (5.9) | 3.7 (3.5) | 3.8 (3) | 2.2 (1.6) | 6.6 (8.7) | 7.4 (8.2) |
| Median | 3 (1–8) | 2 (1–5) | 3 (1–5) | 2 (1–3) | 3 (1–8) | 4 (2–9) |
| Score ≥MCID, n (%)# | 144 (17) | 162 (19) | 89 (10) | 271 (33) | 120 (17) | 134 (17) |
| mMRC ≥1 and score ≥MCID, n (%) | 114 (14) | 119 (14) | 71 (9) | 169 (21) | 80 (11) | 80 (10) |
| Mean | 8.7 (6.1) | 5.1 (3.5) | 4.8 (2.9) | 2.2 (1.6) | 13.2 (10.3) | 10 (8.7) |
| Median | 7 (4–11) | 4 (2–7) | 4 (3–6) | 2 (1–3) | 9 (6–16) | 6.5 (4–14) |
Data are presented as mean (sd), median (first and third quartile) or frequency (percentage). A higher score on D-12 or MDP scales signify worse breathlessness. #: minimal clinically important difference (MCID) values used for stratification were based on previous research [18] as follows: D-12, total=2.83; physical subdomain=1.81; affective subdomain=1.07. MDP A1=0.82; MDP immediate perception=4.63, MDP emotional response=2.37.
mMRC: Medical Research Council breathlessness scale.
FIGURE 1.
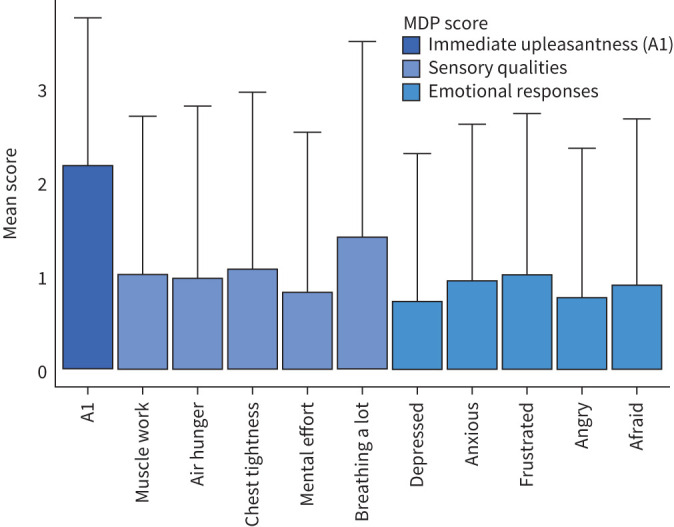
Dyspnoea profiles among 271 men experiencing unpleasant breathlessness. Intensity of Multidimensional Dyspnea Profile (MDP) A1 unpleasantness, sensory qualities, and emotional response. Participants with an MDP A1 unpleasantness score >minimal clinically important difference. Standard deviation is marked by the error bars.
Associations with mMRC, and duration and change of breathlessness
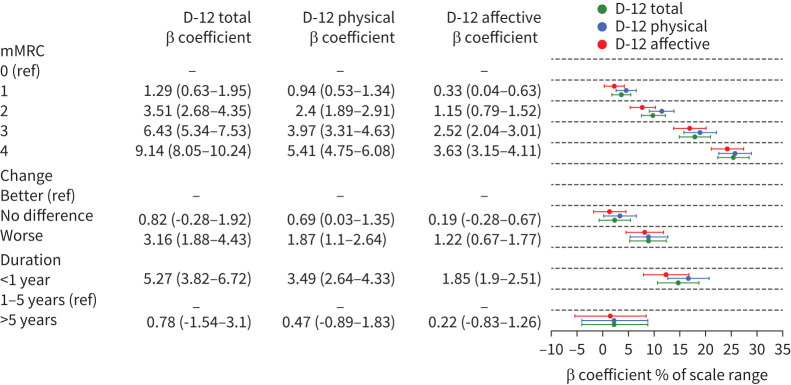
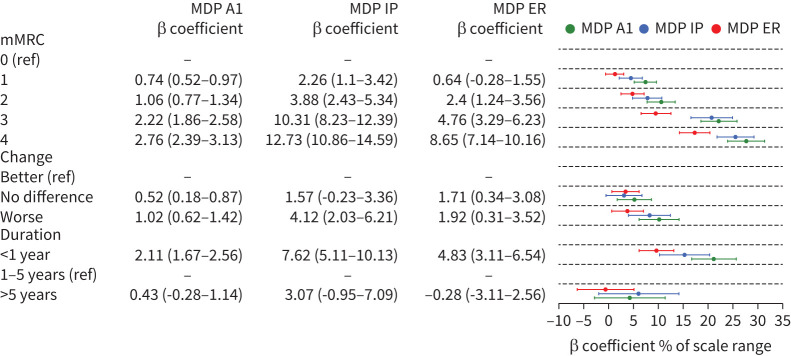
Higher D-12 and MDP estimates were associated with higher mMRC scores with the reported unpleasantness and affective dimensions markedly greater in participants with mMRC ≥3 scores (figures 2, 3, 4), as well as with the reported change category worse (figures 2, 3, Supplemental Materials Figure S1). Higher D-12 and MDP scores were associated with breathlessness of less than 1 year duration. The MDP A1 unpleasantness dimension was markedly increased in participants experiencing breathlessness of less than 1 year duration. The D-12 and MDP ratings were slightly higher in participants experiencing breathlessness for more than 5 years (figures 2, 3). The median D-12 and MDP total scores were lower in participants experiencing breathlessness of less than 1 year duration. (Supplemental Materials figure S2). Overall, there were larger differences in-between the MDP subdomain scores compared to in-between the D-12 subdomain scores (figures 2, 3).
FIGURE 2.
Dyspnea-12 (D-12) score relative to modified Medical Research Council breathlessness scale (mMRC) score, change and duration of breathlessness. Table shows β coefficients (CI) from simple linear regression. To facilitate comparison between scales of different ranges, the points represent the β coefficient percentage of a scale range with 95% CI. ref: reference.
FIGURE 3.
Multidimensional Dyspnea Profile (MDP) score relative to modified Medical Research Council breathlessness scale (mMRC) score, change and duration of breathlessness. Table shows β coefficients (CI) from simple linear regression. To facilitate comparison between scales of different ranges, the points represent the coefficient percentage of a scale range with 95% CI. ER: emotional response; IP: immediate perception; ref: reference.
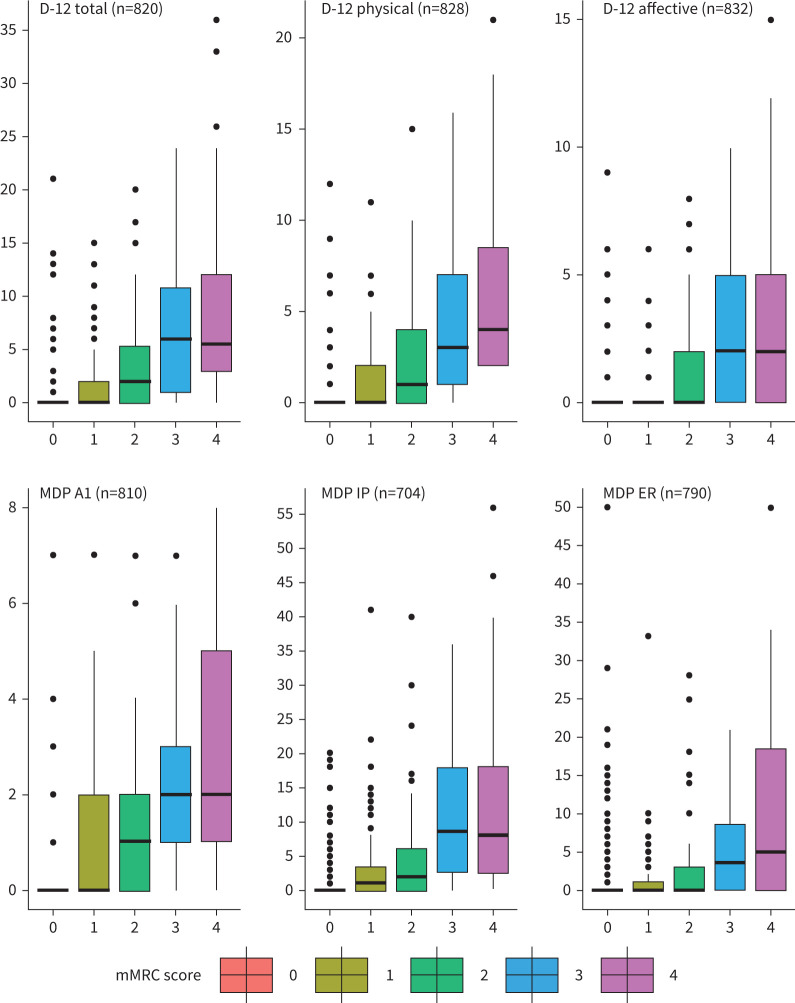
FIGURE 4.
Median scores of Dyspnea-12 (D-12) and Multidimensional Dyspnea Profile (MDP) relative to modified Medical Research Council breathlessness scale (mMRC) categories in 73-year-old males. Whiskers represent interquartile range×1.5 with outliers represented by dots. ER: emotional response; IP: immediate perception.
Discussion
This is the first epidemiological study of breathlessness from a multi-dimensional perspective in a sample from the general population. Multiple impacts and experiences of breathlessness were common in the studied elderly male population. A score ≥MCID by MDP A1 was reported by approximately one third of the subjects completing the MDP scales, while score ≥MCID as indicated by the D-12 total, D-12 physical and MDP emotional response were present in approximately 20%. In the studied population, breathlessness was not dominated by a single dimension, but included multiple dimensions that impact overall well-being. Level of perceived dimensions of breathlessness increased stepwise in relation to increasing mMRC scores. Unpleasant and affective dimensions (consequences of physical limitations due to breathlessness) increased in people with mMRC ≥3.
Among the patients reporting a score ≥MCID by MDP A1, the mean values are similar across the MDP sensory qualities and emotional response scores, which is similar to a study of patients admitted to hospital and reporting breathing discomfort [24]. However, our results differed from a study of COPD patients [25] that reported greater differences across MDP mean values in comparison to our study. Our study and that of hospitalised patients [24] included participants with multiple health conditions, possibly explaining the greater similarities across the MDP scores. This suggests that the profile may be disease-dependant and supports previous research showing that qualitative experiences of breathlessness differ with pathophysiological mechanisms [9].
This study is the first evaluating the relationship between manifestations of breathlessness and mMRC scores at a population level. The MDP A1 and D-12 affective scales correlated with mMRC 3, suggesting the unpleasant and affective dimensions of breathlessness are worse in subjects in whom minimal exertion induces breathlessness. The severity of all dimension scales of breathlessness in individuals with an mMRC score ≥3 suggests that focus in clinical practice should be on patients’ experiences of breathlessness and not only on limits to activity, as previously suggested [26]. This is especially true if the aim is to improve patient overall well-being by treating symptoms of severe breathlessness. Some participants reported increased breathlessness dimension scores not detected by the mMRC, as seen in figure 4 and reinforced in the lower overlap percentages in table 3. This further suggests that the mMRC only evaluates some aspects of breathlessness given that it is uni-dimensional. The mMRC is shorter and more suitable than the D-12 and MDP as a screening tool in clinical practice. However, the D-12 and MDP can be used as additional tools for evaluate individuals with an mMRC score ≥2.
Our findings suggest that the breathlessness dimension scores may take the form of a U-shaped curve related to its duration. The severity was lower in the group experiencing breathlessness for 1–5 years, compared to the groups experiencing breathlessness for <1 year or 5 years or more. People affected by breathlessness may adapt and learn to cope with the symptoms over time. This relates to concept described by Hutchinson et al. [26] as “breathing space”, which suggests that personal limitations depend on how the individual is engaged in coping with and seeking help for breathlessness, along with the healthcare professional's responsiveness [26]. This finding of a U-shaped relationship between the breathlessness dimension scores and its duration is also mirrored by a previously reported U-shaped relationship between breathlessness and quality of life; postulated to be due to a similar mechanism of adaptation over time [7, 27].
This study included the largest sample to date of participants reporting breathlessness-related data using validated instruments. The population characteristics were similar to those of the age-matched male general population in Sweden [20]. The prevalence of non-zero scores was higher compared to scores ≥MCID. Using scores ≥MCID to report prevalence of increased breathlessness dimensions could be seen as a strength as it reports experiences that are of clinical relevance to the participant as opposed to non-zero scores. MCID can therefore be seen as less “noisy” than non-zero scores as MCID can filter out temporary worsening. Also, MCID simplifies the comparison between scales of different ranges, which was very important in this study. However, this is the first use of MCID as a threshold to define the prevalence of clinically relevant dimension scores of breathlessness, and the validity of this approach is therefore uncertain. The usage of MCID in population studies of breathlessness and other symptoms needs to be further evaluated in future studies; for example, by exploring the association to future health events and mortality. The linear regression models used in this study should be seen as robust, as the results were reinforced by the calculated medians, which showed a similar pattern in most analyses.
The study was restricted to 73-year-old males, effectively eliminating sex- or age-related bias in the analyses. However, the findings may not be generalisable to females and other age groups. The VASCOL study is an ongoing study and is planning to recruit females and younger age groups in the next follow-up [20]. The prevalence of COPD and asthma was slightly lower than previously reported among males and females of the same age [28, 29]. The prevalence of breathlessness as established by mMRC among males was similar to that found in previous studies, but the prevalence of breathlessness indicated by mMRC has been showed to be higher among females [30]. Another limitation is that the duration of, and change in, breathlessness was not based on objective data, but on recall, as breathlessness was not measured among the participants at the age of 65 years. Among other symptoms, the highest and final experienced intensity has been shown to be what the individual recalls, the so called “peak-end-rule” [31]. If the same is true with breathlessness, this could impact the result in our study to overestimate the breathlessness intensities, especially as the final experiences of breathlessness among the participants might be more severe at the age of 73 than 65. However, the knowledge of recalled breathlessness is lacking and needs to be further investigated [31]. Breathlessness can also be avoided by the individual until the symptom gets sufficiently severe to interfere with the basic instrumental activities of daily living, as suggested previously [9]. We used a postal survey to examine recalled breathlessness in the past 2 weeks, and not breathlessness during a standardised recalled activity. This will lead to a systematic (and unquantifiable) underestimate of the prevalence of increased breathlessness dimensions in our study. We did not explore how breathlessness dimension scores differ between conditions and how individual items relate with duration. Future studies should therefore explore breathlessness profiles at an item level in relation to conditions and duration. Follow-up studies within the VASCOL study are planned to measure and validate the change and duration of multiple dimensions of breathlessness longitudinally.
Conclusions
This study demonstrates that multiple increased dimension scores of breathlessness are common in a population of elderly males. More severe impacts are associated with a higher mMRC score, with worsening breathlessness after 65 years and with duration less than 1 year. Increased breathlessness is a public health issue in elderly males with a high prevalence of increased dimension scores and unpleasantness manifestations. Increased awareness of the multiple aspects of the condition could enhance the health of older men affected by severe breathlessness.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
FIGURE S1 Median score of D-12 and MDP relative to experience of change in breathlessness categories in 73-year-old males with available data experiencing breathlessness since age 65 years. Whiskers represent IQR times 1.5. D-12: Dyspnoea-12; ER: emotional response; MDP: Multidimensional Dyspnea Profile. 00553-2021.figureS1 (312.6KB, jpeg)
FIGURE S2 Median score of D-12 and MDP in duration of breathlessness categories in 73-year-old males with available data experiencing breathlessness since age 65 years. Whiskers represent IQR times 1.5 with outliers represented by dots. D-12: Dyspnoea-12; ER: emotional response; MDP: Multidimensional Dyspnea Profile. 00553-2021.figureS2 (311.6KB, jpeg)
Footnotes
Author contributions: Conception and design: M. Ekström; data collection: M. Olsson and M. Ekström; analysis: M. Olsson and M. Ekström; interpretation: all authors; first draft: M. Olsson and M. Ekström; revisions and approval of the version to publish: all authors.
Provenance: Submitted article, peer reviewed.
Conflict of interest: M. Olsson has nothing to disclose.
Conflict of interest: D. Currow reports receiving consulting fees from Specialised Therapeutics Australia Pty Ltd and Mayne Pharma, outside the submitted work; participation on a Helsinn Pharmaceuticals advisory board outside the submitted work; and intellectual property payment received from Helsinn Pharmaceuticals, outside the submitted work.
Conflict of interest: M.J. Johnson has nothing to disclose.
Conflict of interest: J. Sandberg has nothing to disclose.
Conflict of interest: G. Engström has nothing to disclose.
Conflict of interest: M. Ekström has nothing to disclose.
Support Statement: The VASCOL baseline study was funded by the Research Council of Blekinge. M. Olsson and M. Ekström was supported by an unrestricted grant from the Swedish Research Council (ref. 2019-02081). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Smith AK, Currow DC, Abernethy AP, et al. Prevalence and outcomes of breathlessness in older adults: a national population study. J Am Geriatr Soc 2016; 64: 2035–2041. doi: 10.1111/jgs.14313 [DOI] [PubMed] [Google Scholar]
- 2.Gronseth R, Vollmer WM, Hardie JA, et al. Predictors of dyspnoea prevalence: results from the BOLD study. Eur Respir J 2014; 43: 1610–1620. doi: 10.1183/09031936.00036813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandberg J, Ekstrom M, Borjesson M, et al. Underlying contributing conditions to breathlessness among middle-aged individuals in the general population: a cross-sectional study. BMJ Open Respir Res 2020; 7: e000643. doi: 10.1136/bmjresp-2020-000643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson MJ, Yorke J, Hansen-Flaschen J, et al. Towards an expert consensus to delineate a clinical syndrome of chronic breathlessness. Eur Respir J 2017; 49: 1602277. doi: 10.1183/13993003.02277-2016 [DOI] [PubMed] [Google Scholar]
- 5.Sandberg J, Engström G, Ekström Met al. Breathlessness and incidence of COPD, cardiac events and all-cause mortality: A 44-year follow-up from middle age throughout life. PLoS ONE. 2019; 14: e0214083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laviolette L, Laveneziana P, ERS Research Seminar Faculty. Dyspnoea: a multidimensional and multidisciplinary approach. Eur Respir J 2014; 43: 1750–1762. [DOI] [PubMed] [Google Scholar]
- 7.Currow DC, Dal Grande E, Ferreira D, et al. Chronic breathlessness associated with poorer physical and mental health-related quality of life (SF-12) across all adult age groups. Thorax. 2017; 72: 1151–1153. [DOI] [PubMed] [Google Scholar]
- 8.Currow DC, Chang S, Reddel HK, et al. Breathlessness, anxiety, depression, and function–the BAD-F study: a cross-sectional and population prevalence study in adults. J Pain Symptom Manage 2020; 59: 197–205.e2. doi: 10.1016/j.jpainsymman.2019.09.021 [DOI] [PubMed] [Google Scholar]
- 9.Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 2012; 185: 435–452. doi: 10.1164/rccm.201111-2042ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007; 370: 741–750. doi: 10.1016/S0140-6736(07)61377-4 [DOI] [PubMed] [Google Scholar]
- 11.Menezes AM, Perez-Padilla R, Jardim JR, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet 2005; 366: 1875–1881. doi: 10.1016/S0140-6736(05)67632-5 [DOI] [PubMed] [Google Scholar]
- 12.Heinrich J, Richter K, Frye C, et al. European Community Respiratory Health Survey in Adults (ECRHS). Pneumologie 2002; 56: 297–303. doi: 10.1055/s-2002-30699 [DOI] [PubMed] [Google Scholar]
- 13.Antoniu SA. Descriptors of dyspnea in obstructive lung diseases. Multidiscip Respir Med 2010; 5: 216–219. doi: 10.1186/2049-6958-5-3-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yorke J, Moosavi SH, Shuldham C, et al. Quantification of dyspnoea using descriptors: development and initial testing of the Dyspnoea-12. Thorax 2010; 65: 21–26. doi: 10.1136/thx.2009.118521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banzett RB, O'Donnell CR, Guilfoyle TE, et al. Multidimensional Dyspnea Profile: an instrument for clinical and laboratory research. Eur Respir J 2015; 45: 1681–1691. doi: 10.1183/09031936.00038914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams MT, Johnston KN. Multidimensional measurement of breathlessness: recent advances. Curr Opin Support Palliat Care 2019; 13: 184–192. doi: 10.1097/SPC.0000000000000436 [DOI] [PubMed] [Google Scholar]
- 17.Hess MW. The 2017 Global Initiative for Chronic Obstructive Lung Disease report and practice implications for the respiratory therapist. Respir Care 2017: 62: 1492–1500. doi: 10.4187/respcare.05402 [DOI] [PubMed] [Google Scholar]
- 18.Ekstrom MP, Bornefalk H, Skold CM, et al. Minimal clinically important differences and feasibility of Dyspnea-12 and the Multidimensional Dyspnea Profile in cardiorespiratory disease. J Pain Symptom Manage 2020; 60: 968–975.e1. doi: 10.1016/j.jpainsymman.2020.05.028 [DOI] [PubMed] [Google Scholar]
- 19.Ekstrom M, Bornefalk H, Skold CM, et al. Minimal clinically important differences for Dyspnea-12 and MDP scores are similar at 2 weeks and 6 months: follow-up of a longitudinal clinical study. Eur Respir J 2020; 57: 2002823. doi: 10.1183/13993003.02823-2020 [DOI] [PubMed] [Google Scholar]
- 20.Olsson M, Engström G, Currow DC, et al. VAScular and Chronic Obstructive Lung disease (VASCOL): a longitudinal study on morbidity, symptoms and quality of life among older men in Blekinge county, Sweden. BMJ Open 2021; 11: e046473. doi: 10.1136/bmjopen-2020-046473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sundh J, Bornefalk H, Skold CM, et al. Clinical validation of the Swedish version of Dyspnoea-12 instrument in outpatients with cardiorespiratory disease. BMJ Open Respir Res 2019; 6: e000418. doi: 10.1136/bmjresp-2019-000418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ekstrom M, Bornefalk H, Skold M, et al. Validation of the Swedish Multidimensional Dyspnea Profile (MDP) in outpatients with cardiorespiratory disease. BMJ Open Respir Res 2019; 6: e000381. doi: 10.1136/bmjresp-2018-000381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988; 93: 580–586. doi: 10.1378/chest.93.3.580 [DOI] [PubMed] [Google Scholar]
- 24.Stevens JP, Sheridan AR, Bernstein HB, et al. A multidimensional profile of dyspnea in hospitalised patients. Chest 2019; 156: 507–517. doi: 10.1016/j.chest.2019.04.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morelot-Panzini C, Gilet H, Aguilaniu B, et al. Real-life assessment of the multidimensional nature of dyspnoea in COPD outpatients. Eur Respir J 2016; 47: 1668–1679. doi: 10.1183/13993003.01998-2015 [DOI] [PubMed] [Google Scholar]
- 26.Hutchinson A, Barclay-Klingle N, Galvin K, et al. Living with breathlessness: a systematic literature review and qualitative synthesis. Eur Respir J 2018; 51: 1701477. doi: 10.1183/13993003.01477-2017 [DOI] [PubMed] [Google Scholar]
- 27.Currow DC, Chang S, Grande ED, et al. Quality of life changes with duration of chronic breathlessness: a random sample of community-dwelling people. J Pain Symptom Manage 2020; 60: 818–827.e4. doi: 10.1016/j.jpainsymman.2020.05.015 [DOI] [PubMed] [Google Scholar]
- 28.Backman H, Vanfleteren L, Lindberg A, et al. Decreased COPD prevalence in Sweden after decades of decrease in smoking. Respir Res 2020; 21: 283. doi: 10.1186/s12931-020-01536-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borna E, Nwaru BI, Bjerg A, et al. Changes in the prevalence of asthma and respiratory symptoms in western Sweden between 2008 and 2016. Allergy 2019; 74: 1703–1715. doi: 10.1111/all.13840 [DOI] [PubMed] [Google Scholar]
- 30.Ekstrom M, Sundh J, Schioler L, et al. Absolute lung size and the sex difference in breathlessness in the general population. PLoS ONE 2018; 13: e0190876. doi: 10.1371/journal.pone.0190876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandberg J, Lansing R, Anderberg P, et al. Relating Experienced To Recalled breathlessness Observational (RETRO) study: a prospective study using a mobile phone application. BMJ Open Respir Res 2019; 6: e000370. doi: 10.1136/bmjresp-2018-000370 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
FIGURE S1 Median score of D-12 and MDP relative to experience of change in breathlessness categories in 73-year-old males with available data experiencing breathlessness since age 65 years. Whiskers represent IQR times 1.5. D-12: Dyspnoea-12; ER: emotional response; MDP: Multidimensional Dyspnea Profile. 00553-2021.figureS1 (312.6KB, jpeg)
FIGURE S2 Median score of D-12 and MDP in duration of breathlessness categories in 73-year-old males with available data experiencing breathlessness since age 65 years. Whiskers represent IQR times 1.5 with outliers represented by dots. D-12: Dyspnoea-12; ER: emotional response; MDP: Multidimensional Dyspnea Profile. 00553-2021.figureS2 (311.6KB, jpeg)