Abstract
Background
There is conflicting evidence for vitamin D supplementation in childhood asthma. We aimed to systematically synthesise the evidence on the efficacy and safety of vitamin D supplementation in childhood asthma.
Methods
We searched electronic databases (Medline, Embase and Web of Science) and a register (CENTRAL) for randomised controlled trials (RCTs) published until 30 September 2021. RCTs enrolling asthmatic children (1–18 years old) and comparing vitamin D against placebo/routine care were included if they met at least one of the endpoints of interest (asthma attacks, emergency visits or hospitalisation). We used the Risk of Bias 2 tool for risk of bias assessment. Random-effects meta-analysis with RevMan 5.3 software was performed. The Grading of Recommendations Assessment, Development and Evaluation approach was used to assess the level of certainty of the evidence.
Results
18 RCTs (1579 participants) were included. The pooled meta-analysis did not find a significant effect of vitamin D supplementation on asthma attacks requiring rescue systemic corticosteroids (six studies with 445 participants; risk ratio (RR) 1.13, 95% CI 0.86–1.48; I2=0%) (moderate-certainty evidence). In addition, there was no significant difference in the proportion of children with asthma attacks of any severity (11 trials with 1132 participants; RR 0.84, 95% CI 0.65–1.09; I2=58%) (very low-certainty evidence). Vitamin D does not reduce the need for emergency visits (three studies with 361 participants; RR 0.97, 95% CI 0.89–1.07; I2=0%) and hospitalisation (RR: 1.38, 95% CI 0.52–3.66; I2=0%) (low-certainty evidence).
Conclusion
Very low- to moderate-certainty evidence suggests that vitamin D supplementation might not have any protective effect in childhood asthma.
Short abstract
Very low to moderate certainty evidence suggests that adjuvant vitamin D supplementation might not have any protective effect in childhood asthma. Therefore, routine vitamin D supplementation in asthmatic children should be avoided. https://bit.ly/3xQVitV
Introduction
Asthma is the most common chronic disease, affecting 5–30% of children [1–4]. Almost 50% of asthmatic children experience one or more acute attacks in a year, making it the third leading cause of hospitalisation and the most common reason for missing school in children [2–4]. Asthma attacks are mediated by proinflammatory cytokines such as interleukin (IL)-13, IL-17A and interferon-γ [5–7]. Vitamin D has immunomodulatory properties; therefore, it might have a role in asthma control [5, 7].
Observational studies showed an association between a low 25-hydroxyvitamin D (25(OH)D) and an increased risk for asthma attacks in children [5]. These findings paved the way for randomised controlled trials (RCTs) to assess the therapeutic potential of vitamin D supplementation. Initial RCTs showed a favourable response with vitamin D supplementation [8–11]. Riverin et al. [12] found low-quality evidence favouring vitamin D supplementation; however, they suggested further studies before its routine use. Subsequent meta-analyses of adults and children suggested potential benefits with vitamin D supplementation in asthmatic patients [7, 13]. However, recent RCTs did not find a significant advantage in children [14–17]. Because of these conflicting results, there is a need to review and update the existing evidence systematically.
We aimed to evaluate the benefits and risks of vitamin D supplementation as adjunct therapy on acute asthma attacks requiring rescue systemic corticosteroids, emergency visits, hospitalisation, and pulmonary function, and adverse effects of vitamin D supplementation in asthmatic children and adolescents (≤18 years old).
Methods
Search strategy and selection criteria
This review was performed following the guidance from the Cochrane Handbook for Systematic Reviews of Interventions [18] and is reported in compliance with Preferred Reporting Items for Systematic Reviews and Meta-analyses 2020 guidelines [19]. The review was prospectively registered with PROSPERO (CRD42021229450). We included RCTs meeting all the following criteria. 1) Population: children aged 1–18 years diagnosed with bronchial asthma; 2) intervention: vitamin D supplementation as an adjunct to asthma-specific therapy; 3) comparison: either placebo or control group. The control group should not have received vitamin D above the maintenance dose (400 IU·day−1) recommended for healthy children [20, 21]. We allowed maintenance of 400 IU·day−1 in the control group because some authors consider it unethical to withhold maintenance vitamin D in children with known vitamin D deficiency or whose vitamin D status is not known at enrolment. As vitamin D is fat-soluble and has a long half-life in tissue, a washout of ≥4 weeks is desirable [22, 23]. Therefore, crossover trials with a short washout period were excluded.
Two authors (J. Kumar and J.P. Goyal) developed a search strategy using database-specific index terms/subject headings and free words. The search strategy comprised of terms related to the study population (children aged 1–18 years with bronchial asthma), intervention (vitamin D) and study design (RCT). We used variable keywords, entry terms, word variations and synonyms to improve the sensitivity (e-table 1). Two authors (J.P. Goyal and J. Meena) reviewed the search strategy using the Peer Review of Electronic Search Strategies checklist.
Two investigators (J. Kumar and J. Meena) independently performed a literature search in Medline (via PubMed), Embase, Web of Science and CENTRAL for RCTs published until 30 September 2021. The electronic search was supplemented by a manual search of the bibliography of relevant reports to identify additional studies. We also searched various registries (until 30 September 2021), namely ClinicalTrials.gov (https://clinicaltrials.gov/ct2/home), the Clinical Trial Registry of India (http://ctri.nic.in/), the Australian and New Zealand Clinical Trials Registry (www.anzctr.org.au/) and the European Union Clinical Trials Register (https://www.clinicaltrialsregister.eu/). We did not use any language restrictions or filters.
Initially, two researchers (J.P. Goyal and C. Thakur) independently screened the titles and/or abstracts to identify potentially eligible reports. Later, two researchers (C. Thakur and P. Kumar) thoroughly examined the full text of these reports and identified reports meeting all the inclusion criteria. If a study had more than two arms but each component tested one drug only, we used the arms comparing vitamin D and placebo/control, whereas for studies using a combination of active interventions (e.g. vitamin D plus immunotherapy versus immunotherapy versus placebo), we used data from the arms with similar interventions except for vitamin D (e.g. vitamin D plus immunotherapy versus immunotherapy alone in the above example). We excluded studies with an additional active intervention (other than vitamin D and standard pharmacological management of asthma) in the treatment arm (like immunotherapy or probiotics), which is not used in the placebo arm because the effects cannot be attributed to vitamin D alone.
Outcomes
Our primary outcome was the proportion of children requiring rescue systemic (intravenous or oral) corticosteroids for asthma attacks. We chose this primary outcome as it is the most robust and clinically meaningful outcome, representing moderate to severe asthma attacks, and is widely used [7, 13]. Secondary outcomes included the proportion of patients with at least one asthma attack of any severity, asthma attacks requiring unscheduled/emergency visits, hospitalisation, need for rescue therapy (β2-agonists), asthma control as assessed by scores and treatment steps such as the Childhood Asthma Control Test (C-ACT), Asthma Control Test (ACT) and Global Initiative for Asthma (GINA), improvement in pulmonary function, and adverse effects. Since there was wide variability in defining asthma attacks (e-table 2), we used the authors’ reported outcome (irrespective of definition or severity) [24].
Data analysis
Two researchers (P. Choudhary and C. Thakur) independently extracted data from the eligible reports. The data comprised first author name, year of publication, study design, setting, methodology, participant characteristics, inclusion and exclusion criteria, intervention and control group details, follow-up schedule, and outcomes (as mentioned above). Disagreement was resolved through discussion with an expert (J.P. Goyal). Two researchers (J.Kumar and P. Kumar) independently rechecked the accuracy and completeness of the extracted data. We came across an individual participant data meta-analysis (IPD-MA) [7] with five studies [8, 11, 21, 25, 26] in common with our review. To improve robustness, we used some of the data (not provided in original reports) from this IPD-MA.
Two researchers (J. Kumar and J.P. Goyal) independently assessed the risk of bias with the Risk of Bias 2 (RoB2) tool, and generated traffic plots and summary plots using the online robvis visualisation tool [27]. Any discrepancy among them was resolved through mutual discussion.
We provided a quantitative and qualitative synthesis of primary and secondary outcomes. We performed the quantitative synthesis for the outcomes reported in at least two trials in the desired format. Median (interquartile range or 95% CI) was converted to mean±sd using appropriate conversion formulas and the RevMan calculator [18]. The dichotomous outcomes are reported as risk ratio (RR) with 95% confidence intervals and continuous data as mean difference (MD) with 95% confidence intervals. We used RevMan version 5.4 and STATA version 14.2 (StataCorp, College Station, TX, USA) software for statistical analysis. Considering inherent heterogeneity among trials, we used a random-effects model for quantitative synthesis. Heterogeneity among studies was assessed by Chi-squared test on Cochrane's Q statistics and quantified using I2 statistics. Egger's test and funnel plots were used to evaluate publication bias. As decided a priori, we performed sensitivity analysis for risk of bias. We also performed random-effects meta-regression analysis for sample size, cumulative vitamin D dosage (which takes care of both dose and duration), active treatment use in the control group (some used maintenance dose of vitamin D) and co-treatments. We followed Grading of Recommendations Assessment, Development and Evaluation recommendations for assessing the level of certainty of the evidence [28].
Results
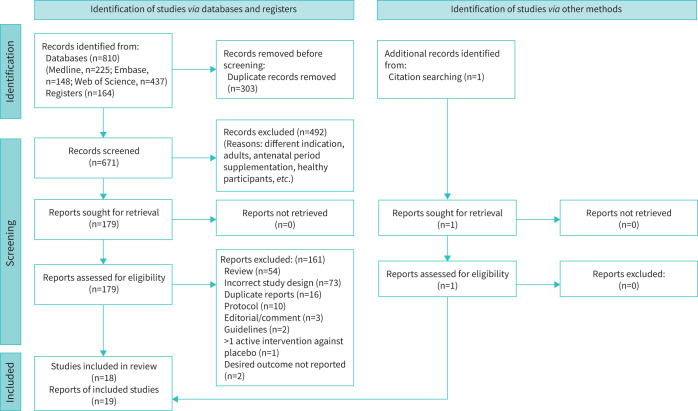
We identified 974 records, of which 303 were duplicates (figure 1). The remaining 671 records were screened through title and/or abstract and 179 reports were considered for full-text retrieval. After reading the complete text, we excluded 161 reports. The foremost reasons for excluding full-text reports were incorrect study design (case–control, cohort or cross-over), reviews (narrative or systematic), duplicate reports (most were conference abstracts) and study protocols (supplementary material). We identified one additional eligible study [29] through citation searching. One study had two reports; therefore, it was considered a single study and we summarised the findings under the main study [16, 30]. Finally, we included 18 trials [8–11, 14–17, 21, 25, 26, 29, 31–36] (1579 participants), of which one is published as abstract only [10]. We excluded one cross-over trial with a shorter washout period [37].
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 flow chart.
Study characteristics
15 RCTs were blinded controlled parallel-group trials, two [17, 29] were open-label, and one [10] (published as abstract only) did not provide any information. 13 out of 18 were performed in the outpatient setting [9–11, 14–16, 21, 25, 29, 31, 32, 34, 36]. Six trials [14, 16, 17, 29, 34, 35] enrolled only vitamin D-deficient (VDD) or vitamin D-insufficient participants. The remaining 12 did not prespecify vitamin D deficiency as an entry criterion, although many participants were VDD. Studies enrolling VDD children used variable cut-offs to define vitamin D deficiency/insufficiency. Recent guidelines consider a level ≥20 ng·mL−1 as sufficient and <12 ng·mL−1 (some consider <10 ng·mL−1) as deficient [38]. None of the trials enrolled children exclusively in the range of 25(OH)D <12 ng·mL−1. Therefore, we considered the authors' defined threshold for classifying deficient/insufficient. The dosing schedule, disease severity and follow-up period varied considerably (table 1).
TABLE 1.
Characteristics of included studies (n=18)
| First author [ref.] | Study | Population | Vitamin D intervention | Comparison | Primary outcome | Follow-up timepoints # | |||||||||
| Year | RCT design | Setting | Age range, years | Sample size | VDD children | Asthma severity | Baseline 25(OH)D, ng·mL−1, mean±sd | Dose | Duration | Cumulative dose | Therapy | Baseline 25(OH)D levels, ng·mL−1, mean±sd | |||
| Majak [ 36 ] | 2009 | Double-blind parallel | OPD | 6–12 | 54 | No | All severity | 31.3±3.4 | 1000 IU weekly +inhaled prednisone 20 mg +SCIT | 3 months | 90 000 IU | Inhaled prednisone 20 mg + SCIT | 32.0±3.1 | ICS dose reduction | 3, 12 |
| Urashima [ 8 ] | 2010 | Double-blind parallel | Multicentric | 6–15 | 110 | No | All severity | − | 1200 IU daily | 4 months | 144 000 IU | Placebo | − | Rate of influenza infection | 4 |
| Majak [ 11 ] | 2011 | Double-blind, parallel | OPD | 5–18 | 48 | No | Newly diagnosed | 35.1±16.9 | 500 IU daily +budesonide 800 μg·day−1 | 6 months | 90 000 IU | Budesonide 800 μg·day−1 | 36.1±13.9 | ATAQ score | 1, 2, 3, 4, 5, 6 |
| Lewis [ 33 ] | 2012 | Double blind, parallel | Hospital | 6–17 | 30 | No | Chronic persistent asthma | − | 1000 IU daily | 12 months | 360 000 IU | Placebo | − | ACT | 6, 12 |
| Darabi [ 10 ] | 2013 | Parallel group | OPD | 6–14 | 63 | No | Newly diagnosed | − | 500 IU daily +fluticasone 500 μg·day−1 | 6 months | 90 000 IU | Fluticasone 500 μg·day−1 | − | Asthma attacks, FEV1 | 6 |
| Yadav [ 9 ] | 2014 | Double-blind, parallel | OPD | 5–13 | 100 | No | Moderate to severe | − | 60 000 IU monthly | 6 months | 360 000 IU | Placebo | − | Asthma control by GINA | 1, 2, 3, 4, 5, 6 |
| Baris [ 31 ] | 2014 | Double blind parallel | OPD | 5–15 | 50 | No | Mild to moderate persistent | 19±9 | 650 IU daily + SCIT | 12 months | 234 000 IU | SCIT alone | 20±12 | Symptom and medication score | 6, 12 |
| Bar Yoseph [ 34 ] | 2015 | Double-blind, parallel | OPD | 6–18 | 39 | Yes (<30 ng·mL−1) | Mild | 20.8±6.5 | 14 000 IU weekly | 6 weeks | 84 000 IU | Placebo | 20.0±7.1 | FEV1 | 6 weeks |
| Jensen [ 21 ] | 2016 | Double-blind parallel | OPD | 1–5 | 22 | No | Moderate to severe | − | 100 000 IU statim followed by 400 IU·day−1 | 6 months | 172 000 IU | 400 IU vitamin D daily × 6 months, cumulative 72 000 IU | − | Severe exacerbations | 3, 6 |
| Kerley [ 25 ] | 2016 | Double-blind, parallel | OPD | 6–16 | 44 | No | Moderate to severe | 23.2±8.9 | 2000 IU daily | 15 weeks | 210 000 IU | Placebo | 20.4±7.4 | Pulmonary functions | 15 weeks |
| Tachimoto [ 26 ] | 2016 | Double-blind, parallel | Multicentric | 6–15 | 89 | No | All severity | 28.5±7.4 | 800 IU daily | 2 months | 32 000 IU | Placebo | 29±7.4 | Asthma control by GINA | 2, 6 |
| Alansari [ 17 ] | 2017 | Open-label, parallel | Emergency | 2–14 | 231 | Yes (<25 ng·mL−1) | Moderate to severe | 15.1±5.4 | <5 years: 300 000 IU statim followed by 400 IU·day−1 >5 years: 600 000 IU statim followed by 400 IU·day−1 |
12 months | <5 years: 446 000 IU >5 years: 746 000 IU |
400 IU vitamin D daily × 12 months, cumulative dose 146 000 IU |
15.8±5.2 | Asthma exacerbation | 3, 6, 9, 12 |
| Najmuddin [ 29 ] | 2017 | Open label, parallel | OPD | 6–12 | 66 | Yes (<20 ng·mL−1) | All severity | − | 60 000 IU weekly | 10 weeks | 600 000 IU | None | − | Pulmonary function | 10 weeks |
| Ducharme [ 32 ] | 2019 | Triple blind parallel | OPD | 1–5 | 47 | No | Moderate to Severe | 28.2±5.3 | 100 000 IU × 2 doses, 14 weeks apart ± daily ICS | 7 months | 200 000 IU | Placebo ± daily ICS | 27.4±10.4 | Asthma exacerbation | 3.5, 7 |
| Swangtrakul [ 35 ] | 2019 | Double blind, parallel | Hospital | 3–18 | 84 | Yes (<20 ng·mL−1) | Mild to moderate | 16.5±2.2 | <30 kg: 300 000 IU >30 kg: 600 000 IU |
3 months | <30 kg: 420 000 IU >30 kg: 840 000 IU |
Placebo | 16.2±2.3 | Asthma control, FOT | 1, 3 |
| Forno [ 16 ] | 2020 | Double-blind parallel | OPD | 6–16 | 192 | Yes (10–30 ng·mL−1) | Moderate to severe | 22.5±4.6 | 4000 IU daily + inhaled fluticasone | 12 months | 1440 000 IU | Placebo + inhaled fluticasone | 22.8±4.6 | Severe asthma exacerbations | 4, 8, 12 |
| Jat [ 14 ] | 2021 | Double-blind, parallel | OPD | 4–12 | 250 | Yes (<20 ng·mL−1) | Persistent asthma of all severity | 11.6±4.6 | 1000 IU daily | 9 months | 270 000 IU | Placebo | 10.8±4.4 | C-ACT | 1, 3, 6, 9 |
| Thakur [15] | 2021 | Double blind parallel | OPD | 6–11 | 60 | No | Moderate | 15.8±8.2 | 2000 IU daily+inhaled steroids | 3 months | 180 000 IU | Placebo + inhaled steroids | 16.5±9.9 | Improvement in C-ACT | 1, 2, 3 |
RCT: randomised controlled trial; VDD: vitamin D-deficient; 25(OH)D: 25-hydroxyvitamin D; OPD: outpatient department; −: either 25(OH)D was not measured at baseline or the levels were not clearly presented; IU: international unit; SCIT: subcutaneous immunotherapy; ICS: inhaled corticosteroid; ATAQ: Asthma Therapy Assessment Questionnaire; ACT: Asthma Control Test; FEV1: forced expiratory volume in 1 s; GINA: Global Initiative for Asthma; FOT: forced oscillation technique; C-ACT: Childhood Asthma Control Test. #: in months unless otherwise stated.
Risk of bias
We used the RoB2 tool for the risk of bias assessment (e-figure 1). Six trials had some bias arising from the randomisation process [9, 10, 17, 29, 33, 35]. Another two had some concerns in handling missing data [8, 9]. Two were open-label and had some concerns in multiple domains; therefore, they were considered at high risk of bias [17, 29]. The trial by Yadav and Mittal [9] was at risk of bias in two domains (randomisation process and handling missing data) and therefore, considered at high risk of bias. Overall, four trials were at high risk of bias, three had some concerns in one or another domain and the remaining 11 were considered at low risk of bias in all domains. The clinical outcomes, measurement scales and assessment time varied considerably across studies (table 2).
TABLE 2.
Summary of clinical parameters studied among trials and their outcomes
| First author [ref.] | Year | Asthma exacerbations | ED visit | Steroid use | Asthma control | Pulmonary function test | Post-intervention vitamin D levels | ||||||
| GINA | C-ACT/ACT | ATAQ | Other scores | FEV1 | PEFR | F ENO | FOT | ||||||
| Majak [ 36 ] | 2009 | - | - | ns | - | - | - | ns # | ns | - | - | - | ↑ |
| Urashima [ 8 ] | 2010 | ↓ | - | - | - | - | - | - | - | - | - | - | - |
| Majak [ 11 ] | 2011 | ↓ | - | - | - | - | ns | - | ns | - | - | - | ns |
| Lewis [ 33 ] | 2012 | - | - | - | - | ns | - | - | ns | - | - | - | ns |
| Darabi [ 10 ] | 2013 | ↓ | - | - | - | ns ¶ | ns | - | - | - | ↑ | ||
| Yadav [ 9 ] | 2014 | ↓ | ↓ | ↓ | ↓ | - | - | - | - | ↑ | - | - | - |
| Baris [ 31 ] | 2014 | ns | - | ns | - | - | - | ns + | ns | ns | - | - | ↑ |
| Bar Yoseph [ 34 ] | 2015 | - | - | - | - | - | - | - | ns | - | ns | - | ↑ |
| Jensen [ 21 ] | 2016 | ns | ns | ns | - | - | - | - | - | - | - | - | ↑ |
| Kerley [ 25 ] | 2016 | - | - | ns | ns | ns | - | - | ns | - | - | - | ↑ |
| Tachimoto [ 26 ] | 2016 | ns | ns | ns | ↓ | ↓ | - | - | - | ns | - | - | ↑ |
| Alansari [ 17 ] | 2017 | ns | ns | - | - | - | - | - | - | - | - | - | ↑ |
| Najmuddin [ 29 ] | 2017 | - | - | - | - | - | - | - | ↑ | ↑ | - | - | - |
| Ducharme [ 32 ] | 2019 | ns | ns | ns | - | - | - | - | - | - | - | - | ↑ |
| Swangtrakul [ 35 ] | 2019 | - | - | - | - | ns | - | - | - | - | - | ns | - |
| Forno [ 16 ] | 2020 | ns | ns | ns | - | - | - | - | - | - | - | - | ↑ |
| Jat [ 14 ] | 2020 | ns | ns | - | ns | ns | - | - | ns | ns | - | - | ↑ |
| Thakur [ 15 ] | 2021 | ns | ns | ns | - | ns | - | - | ns | - | ns | - | ↑ |
ED: emergency department; GINA: Global Initiative for Asthma; C-ACT: Childhood Asthma Control Test; ACT: Asthma Control Test; ATAQ: Asthma Therapy Assessment Questionnaire; FEV1: forced expiratory volume in 1 s; PEFR: peak expiratory flow rate; FENO: exhaled nitric oxide fraction; FOT: forced oscillation technique; ns: nonsignificant; -: not reported. #: asthma symptom diary; ¶: Asthma Control Questionnaire score; +: total asthma symptoms score.
Primary outcome
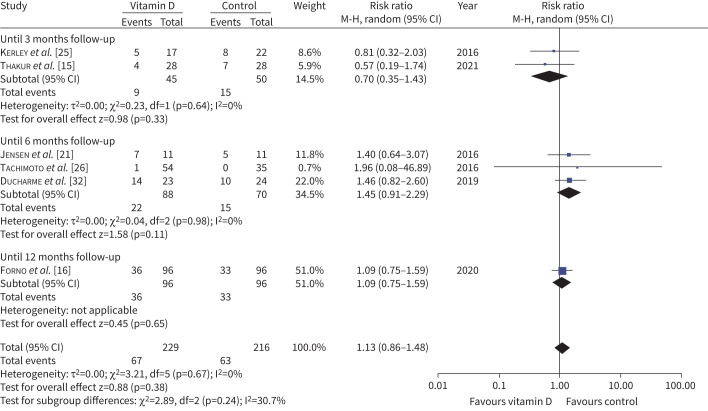
The primary outcome data are presented in figure 2. Nine trials reported data on corticosteroid use [9, 15, 16, 21, 25, 26, 31, 32, 36]. Seven trials (654 participants) compared the requirement of rescue systemic steroids in an asthma attack [14–16, 21, 25, 26, 32]. However, only six (445 participants) provided data for pooled analysis. Overall, 29.3% participants in vitamin D group and 29.2% in placebo/control group required rescue systemic steroids for asthma control (RR 1.13, 95% CI 0.86–1.48; I2=0%, p=0.7) (moderate-certainty evidence) (table 3). As the duration of supplementation and follow-up varied across trials and can affect the primary outcome, we also assessed the impact of the duration of follow-up (figure 2). None of the trials showed any benefit with vitamin D supplementation and there were no significant subgroup differences (based on follow-up period, which closely mimics supplementation duration). Jat et al. [14] did not observe any difference in the median number of courses of oral corticosteroids during the study period.
FIGURE 2.
Forest plot showing the proportion of children with asthma exacerbations requiring rescue systemic steroids. M-H: Mantel-Haenszel; df: degrees of freedom.
TABLE 3.
Summary of findings (primary and secondary outcomes)
| Outcomes | Number of participants (studies) | Relative effect, RR (95% CI) | Anticipated absolute effects (95% CI) | GRADE certainty of the evidence | |
| Risk with placebo | Risk with vitamin D | ||||
| Number of children requiring systemic corticosteroids for asthma exacerbations | 445 (6 RCTs) | 1.13 (0.86–1.48) | 292 per 1000 | 330 per 1000 (251–432) | +++− Moderate# |
| Number of children with one or more asthma exacerbations | 1132 (11 RCTs) | 0.84 (0.65–1.09) | 452 per 1000 | 380 per 1000 (294–493) | +−−− Very low#,¶,+ |
| Number of children requiring emergency/unscheduled visits | 361 (3 RCTs) | 0.97 (0.89–1.07) | 669 per 1000 | 649 per 1000 (595–715) | ++−− low#,¶ |
| Number of children requiring hospitalisations for asthma exacerbation | 275 (2 RCTs) | 1.38 (0.52–3.66) | 70 per 1000 | 18 per 1000 (22–124) | ++−− Low¶,§ |
| Number of children with well-controlled asthma | 442 (4 RCTs) | 1.00 (0.97–1.04) | 941 per 1000 | 941 per 1000 (913–979) | ++−− Low#,¶ |
| FEV1 | 314 (4 RCTs) | MD −2.64 (−7.04– +1.77) | ++−− Low#,+ |
||
| F ENO | 94 (2 RCTs) | MD −2.87 (−24.66– +18.91) | +−−− Very low#,+,§ |
||
| Vitamin D levels post-intervention | 857 (8 RCTs) | MD +10.68 (+6.3– +15.05) | ++−− Lowƒ |
||
| Number of children with serious adverse events | 525 (3 RCTs) | 1.30 (0.55–3.07) | 31 per 1000 | 41 per 1000 (17–97) | ++−− Low#,§ |
RR: risk ratio; GRADE: Grading of Recommendations Assessment, Development and Evaluation; FEV1: forced expiratory volume in 1 s; FENO: exhaled nitric oxide fraction; RCT: randomised controlled trial; MD: mean difference. #: 95% confidence interval crosses the null line; +: I2>50%; ¶: includes high risk of bias trials; §: extremely wide 95% confidence interval; ƒ: I2>75%.
Sensitivity analysis
All six trials reporting primary outcomes were at low risk of bias, thereby precluding the need for sensitivity analysis. In addition, there was no statistical heterogeneity among them; the results largely remained unchanged with fixed-effect analysis (RR 1.09, 95% CI 0.83–1.43). Only one trial exclusively enrolled VDD (10–30 ng·mL−1) children and they did not find any difference in severe asthma (requiring systemic rescue steroids) [16]. When we excluded this trial in sensitivity analysis, the results remained unchanged (five trials with 253 participants; RR 1.17, 95% CI 0.80–1.72).
Regression analysis
A significant overlap and variability in the disease severity, dosage and route of vitamin D supplementation across the studies precluded the subgroup analysis on these variables (table 2). To investigate the effect of these variables, we did a random-effects metaregression analysis. We aimed to perform metaregression for sample size, dosage, duration, use of vitamin D (maintenance dose) in the control group, baseline vitamin D levels, disease severity and other cointerventions. Due to significant heterogeneity in intervention dose (500–300 000 IU), duration (weeks to a year), dosing schedule (daily, weekly or combined), use of bolus (different intervals and doses), it was not possible to analyse individual covariates. Therefore, we decided to use cumulative dose as a covariate to include both dose and duration. In addition, vitamin D is a fat-soluble vitamin with a more extended washout period, so the cumulative dose is important. Due to the limited number of studies reporting baseline vitamin D, it was eliminates from the covariates. Therefore, the final metaregression included sample size, cumulative dose, active intervention in the control group and cotreatment (steroids, subcutaneous immunotherapy, etc.) as covariates (e-table 3). We did not find any significant relationship of either of the covariates with the use of rescue systemic corticosteroids.
Secondary outcomes
Pooled meta-analysis of 11 trials (1132 participants) did not find a significant effect of vitamin D supplementation on the proportion of children with at least one asthma attack (RR 0.84, 95% CI 0.65–1.09; I2=58%, p=0.007) (very low-certainty evidence) (e-figure 2). As the trials have different supplementation and follow-up durations, and substantial heterogeneity (I2=58%, p<0.001), we explored the relationship of asthma exacerbation with the follow-up period (e-figure 3). As for the primary outcome, we did not see any significant difference in the proportion of participants with acute attacks at various follow-up time points. On metaregression analysis (e-table 3), we did not find any significant relationship between the covariates and the asthma attacks (any severity).
Eight trials reported data on unscheduled/emergency healthcare visits for asthma attacks (table 2). However, only three provided data for quantitative synthesis [17, 26, 32]. The pooled data (three trials with 361 participants) suggest that vitamin D does not reduce the need for unscheduled hospital visits (RR 0.97, 95% CI 0.89–1.07; I2=0%, p=0.4) (low-certainty evidence). In the remaining four, vitamin D did not significantly affect emergency visits [14–16, 21]. Two trials reporting the need for hospitalisation did not find a significant difference (RR 1.38, 95% CI 0.52–3.66; I2=0%, p=0.8) (low-certainty evidence) [16, 26]. The proportion of participants with well-controlled asthma was similar in vitamin D (95%) and placebo (94.1%) groups (four trials with 442 participants; RR 1.00, 95% CI 0.97–1.04; I2=0%, p=0.9) (low-certainty evidence). Only one trial (206 participants) reported data on β2-agonists and they did not find any difference in rescue β2-agonist use (RR 1.15, 95% CI 0.71–1.85) [14].
Different scores (GINA, ACT, C-ACT and ATAQ) were used to assess asthma control. Except for two trials [9, 26], none reported a significant difference (detailed in table 2). Two trials (276 participants) provided C-ACT scores for quantitative synthesis. There was no significant difference in post-intervention C-ACT scores (MD 0.22, 95% CI −0.51– +0.94; I2=0%) (e-figure 4). 12 trials assessed pulmonary function tests (table 2). 10 trials reported the effect of vitamin D on forced expiratory volume in the first second, of which nine did not show any significant impact of vitamin D. Meta-analysis of four trials (314 participants) did not observe any significant benefit with vitamin D supplementation (MD −2.64, 95% CI −7.04–1.77; I2=62%, p=0.05). The other pulmonary function tests (exhaled nitric oxide fraction and peak expiratory flow rate) were similar in the two groups (table 3 and e-figure 5).
Adverse events
Vitamin D supplementation was safe (e-table 4). There was no statistically significant difference between the two groups regarding the minor (headache, nausea, vomiting, rash, abdominal pain or rash) or serious adverse effects (RR 1.30, 95% CI 0.55–3.07; I2=0%, p=0.9) (table 3).
Effect in VDD children
None of the trials enrolled children exclusively in the deficient range (<12 ng·mL−1); therefore, we included RCTs with participants having 25(OH)D levels <20 ng·mL−1 before enrolment collectively under the deficient/insufficient category for subgroup analysis. Three trials enrolled children with 25(OH)D levels <20 ng·mL−1 [14, 29, 35]. However, only one study provided data on asthma exacerbation [14]. That study did not observe any significant effect of vitamin D supplementation on any reported outcomes.
As a part of sensitivity analysis, we pooled the data from low risk of bias studies (e-table 5). There was no significant change in any of the outcomes. Similarly, we also performed sensitivity analysis for outcomes with heterogeneity <50% using the fixed-effect model [18]. Again, none of the results differed between the two groups (e-table 6).
Publication bias
As the primary outcome had only six studies, we could not assess publication bias for it, but we further explored this aspect for another important and generalised outcome (children with one or more asthma exacerbation) reported in 11 studies. One high-risk study [9] falls outside the pseudo 95% confidence limits (e-figure 6) but the rest are symmetrically distributed around the log RR. There was no relationship between the study size and effect size; therefore, significant publication bias is unlikely. Considering the limitations of the funnel plot, we performed a more robust Egger's linear regression test. Egger's test did not show any significant small study effect (coefficient 0.081, 95% CI −0.11–0.27; p=0.2).
Discussion
This systematic review and meta-analysis did not find any protective effect of adjuvant vitamin D supplementation on reducing asthma attacks requiring rescue systemic corticosteroids in children. In addition, vitamin D did not decrease asthma exacerbations, need for emergency/unscheduled emergency visits or hospitalisation for asthma attacks. Very low-certainty evidence suggests that adjuvant vitamin D does not improve pulmonary function either. Extremely few (0.8%) participants had severe adverse events (apart from hospitalisation due to asthma attack) and none was attributed to vitamin D supplementation.
Considering the heterogeneity and high risk of bias in observational studies, we limited our analysis to RCTs. Except for four studies, all were of moderate to good quality. Even after limiting to high-quality trials, we did not observe any positive effect of vitamin D supplementation, reinforcing the robustness of the conclusions (moderate-certainty evidence). An IPD-MA observed a protective effect of vitamin D supplementation in VDD adults but not among those with sufficient vitamin D levels [7]. Only three trials enrolled VDD/vitamin D-insufficient children in our meta-analysis and only one reported the effect on asthma attacks. Therefore, these results should not be extrapolated to VDD children.
Initial systematic review and meta-analysis showed that vitamin D might protect against moderate to severe asthma attacks (requiring rescue systemic steroids). However, the effect size was small and level of certainty was low [5, 7, 12, 13, 39]. Contrary to previous reviews, we did not observe any protective efficacy of vitamin D supplementation on any of the clinical or spirometry parameters. The main reason for the contrary results is the inclusion of recent, larger sample size RCTs published in the past 5 years, which were not part of previous systematic reviews. The earlier systematic review included five to eight small studies (including adult studies) with an aggregate sample size of 149–573 [12, 13, 39, 40]. Our review consists of 17 trials (1572 participants) exclusively performed in children and is much larger than the previous reviews. Thus, even if we restrict to low risk of bias studies, moderate-certainty evidence suggests that vitamin D supplementation does not reduce asthma attacks or the need for rescue systemic steroids.
A previous systematic review concluded that high-dose vitamin D might be useful [39]; however, we did not observe any effect of cumulative dose or duration of treatment on asthma attacks on meta-regression. Jolliffe et al. [7] performed an IPD-MA of paediatric and adult populations, and observed significant effects of vitamin D supplementation. They observed benefits in VDD (<25 nmol·L−1) individuals (three trials with 92 participants) but not in those with normal vitamin D levels. As 91 out of 92 VDD individuals included in that IPD-MA were adults, the findings are not applicable to children. In our meta-analysis, minimal evidence did not support vitamin D supplementation in this subpopulation; however, we are uncertain about this outcome. As many of these trials enrolled children with vitamin D levels in the deficiency range, an IPD-MA limited to VDD children would be helpful.
Our review has several limitations. There was wide variability in the population characteristics (race, ethnicity, disease severity and vitamin D levels), intervention (dose, duration and follow-up) and outcome (definition of attack, therapy and asthma control scores). Although we tried to address these variabilities by performing appropriate analyses, we are unsure of the impact on our study outcomes. One may argue that the dosage of vitamin D supplementation was relatively low in some trials and many might not have achieved so-called normal vitamin D levels, which might have affected the outcomes. However, it is unlikely to be accurate as trials using very high doses (up to 500 000 IU) also did not find a beneficial effect.
This review includes four high risk of bias studies and many small studies with wide confidence intervals. However, sensitivity analysis of the low risk of bias studies showing similar results with a better level of certainty is reassuring. In addition, there was no significant difference in the effect size between the small and relatively large trials. Moreover, we downgraded the level of evidence for heterogeneity, wide confidence intervals and risk of bias. Since we do not have robust data on VDD children, these results might not apply to them.
In conclusion, this systematic review and meta-analysis did not find any protective effect of adjuvant vitamin D supplementation in preventing moderate to severe asthma exacerbations requiring rescue systemic corticosteroids in children. However, for the rest of the outcomes level of certainty is low to very low. Further, more extensive trials are needed to assess its efficacy in VDD children to improve the confidence of the evidence.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00662-2021.SUPPLEMENT (317.2KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
This protocol is registered at https://www.crd.york.ac.uk/prospero/ with identifier number CRD42021229450. The original data are in the public domain. Sorted information is available from the corresponding author and can be provided on written request.
Author contributions: J. Kumar, J. Meena, P. Kumar and J.P. Goyal performed the literature search. C. Thakur and P. Choudhary collected the data. J. Charan, A. Gupta and K. Singh supervised data collection. J. Kumar, P. Kumar and J.P. Goyal drafted the manuscript. J. Charan, A. Gupta and K. Singh critically revised the manuscript. All authors designed the study, analysed and interpreted the data, and performed quality assessment. All authors have seen the final manuscript and approved it for submission.
This article has supplementary material available from openres.ersjournals.com
Conflict of interest: J. Kumar has nothing to disclose.
Conflict of interest: P. Kumar has nothing to disclose.
Conflict of interest: J.P. Goyal has nothing to disclose.
Conflict of interest: C. Thakur has nothing to disclose.
Conflict of interest: P. Choudhary has nothing to disclose.
Conflict of interest: J. Meena has nothing to disclose.
Conflict of interest: J. Charan has nothing to disclose.
Conflict of interest: K. Singh has nothing to disclose.
Conflict of interest: A. Gupta has nothing to disclose.
References
- 1.Fu L-S, Tsai M-C. Asthma exacerbation in children: a practical review. Pediatr Neonatol 2014; 55: 83–91. doi: 10.1016/j.pedneo.2013.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Lung Association. Asthma and Children Fact Sheet . https://www.lung.org/lung-health-diseases/lung-disease-lookup/asthma/learn-about-asthma/asthma-children-facts-sheet. Date last accessed: 21 April 2021.
- 3.World Health Organization . Asthma. https://www.who.int/news-room/fact-sheets/detail/asthma. Date last accessed: 21 April 2021.
- 4.Zahran HS, Bailey CM, Damon SA, et al. Vital Signs: asthma in children — United States, 2001–2016. MMWR Morb Mortal Wkly Rep 2018; 67: 149–155. doi: 10.15585/mmwr.mm6705e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jat KR, Khairwa A. Vitamin D and asthma in children: a systematic review and meta-analysis of observational studies. Lung India 2017; 34: 355–363. doi: 10.4103/0970-2113.209227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bush A. Pathophysiological mechanisms of asthma. Front Pediatr 2019; 7: 68. doi: 10.3389/fped.2019.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med 2017; 5: 881–890. doi: 10.1016/S2213-2600(17)30306-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urashima M, Segawa T, Okazaki M, et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr 2010; 91: 1255–1260. doi: 10.3945/ajcn.2009.29094 [DOI] [PubMed] [Google Scholar]
- 9.Yadav M, Mittal K. Effect of vitamin D supplementation on moderate to severe bronchial asthma. Indian J Pediatr 2014; 81: 650–654. doi: 10.1007/s12098-013-1268-4 [DOI] [PubMed] [Google Scholar]
- 10.Darabi B, Moin M, Purpbak Z. The effect of vitamin D supplementation over asthma outcome. Iran J Allergy Asthma Immunol 2013; 12: S87. [Google Scholar]
- 11.Majak P, Olszowiec-Chlebna M, Smejda K, et al. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol 2011; 127: 1294–1296. doi: 10.1016/j.jaci.2010.12.016 [DOI] [PubMed] [Google Scholar]
- 12.Riverin BD, Maguire JL, Li P. Vitamin D supplementation for childhood asthma: a systematic review and meta-analysis. PLoS ONE 2015; 10: e0136841. doi: 10.1371/journal.pone.0136841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martineau AR, Cates CJ, Urashima M, et al. Vitamin D for the management of asthma. Cochrane Database Syst Rev 2016; 9: CD011511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jat KR, Goel N, Gupta N, et al. Efficacy of vitamin D supplementation in asthmatic children with vitamin D deficiency: a randomized controlled trial (ESDAC trial). Pediatr Allergy Immunol 2021; 32: 479–488. doi: 10.1111/pai.13415 [DOI] [PubMed] [Google Scholar]
- 15.Thakur C, Kumar J, Kumar P, et al. Vitamin-D supplementation as an adjunct to standard treatment of asthma in children: a randomized controlled trial (ViDASTA Trial). Pediatr Pulmonol 2021; 56: 1427–1433. doi: 10.1002/ppul.25287 [DOI] [PubMed] [Google Scholar]
- 16.Forno E, Bacharier LB, Phipatanakul W, et al. Effect of vitamin D3 supplementation on severe asthma exacerbations in children with asthma and low vitamin D levels: the VDKA randomized clinical trial. JAMA 2020; 324: 752–760. doi: 10.1001/jama.2020.12384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alansari K, Davidson BL, Yousef KI, et al. Rapid vs maintenance vitamin D supplementation in deficient children with asthma to prevent exacerbations. Chest 2017; 152: 527–536. doi: 10.1016/j.chest.2017.06.021 [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.2. Cochrane, 2021. [Google Scholar]
- 19.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khadilkar A, Khadilkar V, Chinnappa J, et al. Prevention and treatment of vitamin D and calcium deficiency in children and adolescents: Indian Academy of Pediatrics (IAP) guidelines. Indian Pediatr 2017; 54: 567–573. doi: 10.1007/s13312-017-1070-x [DOI] [PubMed] [Google Scholar]
- 21.Jensen ME, Mailhot G, Alos N, et al. Vitamin D intervention in preschoolers with viral-induced asthma (DIVA): a pilot randomised controlled trial. Trials 2016; 17: 353. doi: 10.1186/s13063-016-1483-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr 2020; 74: 1498–1513. doi: 10.1038/s41430-020-0558-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satia MC, Mukim AG, Tibrewala KD, et al. A randomized two way cross over study for comparison of absorption of vitamin D3 buccal spray and soft gelatin capsule formulation in healthy subjects and in patients with intestinal malabsorption. Nutr J 2015; 14: 114. doi: 10.1186/s12937-015-0105-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Virchow JC, Backer V, de Blay F, et al. Defining moderate asthma exacerbations in clinical trials based on ATS/ERS joint statement. Respir Med 2015; 109: 547–556. doi: 10.1016/j.rmed.2015.01.012 [DOI] [PubMed] [Google Scholar]
- 25.Kerley CP, Hutchinson K, Cormican L, et al. Vitamin D3 for uncontrolled childhood asthma: a pilot study. Pediatr Allergy Immunol 2016; 27: 404–412. doi: 10.1111/pai.12547 [DOI] [PubMed] [Google Scholar]
- 26.Tachimoto H, Mezawa H, Segawa T, et al. Improved control of childhood asthma with low-dose, short-term vitamin D supplementation: a randomized, double-blind, placebo-controlled trial. Allergy 2016; 71: 1001–1009. doi: 10.1111/all.12856 [DOI] [PubMed] [Google Scholar]
- 27.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 28.Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group, 2013. [Google Scholar]
- 29.Najmuddin F, Lahiri K. Vitamin D in pediatric asthma and allergic rhinitis: benefits beyond skeletal health. Insights Allergy Asthma Bronchitis 2017; 3: 1. doi: 10.21767/2471-304X.100019 [DOI] [Google Scholar]
- 30.Han Y-Y, Forno E, Rosser FJ, et al. Effect of vitamin D supplementation on lung function and asthma-related outcomes in children with asthma and low vitamin D levels. Am J Respir Crit Care Med 2021; 203: A3117. doi: 10.1164/ajrccm-conference.2021.203.1_MeetingAbstracts.A3117 [DOI] [Google Scholar]
- 31.Baris S, Kiykim A, Ozen A, et al. Vitamin D as an adjunct to subcutaneous allergen immunotherapy in asthmatic children sensitized to house dust mite. Allergy 2014; 69: 246–253. doi: 10.1111/all.12278 [DOI] [PubMed] [Google Scholar]
- 32.Ducharme FM, Jensen M, Mailhot G, et al. Impact of two oral doses of 100,000 IU of vitamin D3 in preschoolers with viral-induced asthma: a pilot randomised controlled trial. Trials 2019; 20: 138. doi: 10.1186/s13063-019-3184-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis E, Fernandez C, Nella A, et al. Relationship of 25-hydroxyvitamin D and asthma control in children. Ann Allergy Asthma Immunol 2012; 108: 281–282. doi: 10.1016/j.anai.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 34.Bar Yoseph R, Livnat G, Schnapp Z, et al. The effect of vitamin D on airway reactivity and inflammation in asthmatic children: a double-blind placebo-controlled trial. Pediatr Pulmonol 2015; 50: 747–753. doi: 10.1002/ppul.23076 [DOI] [PubMed] [Google Scholar]
- 35.Swangtrakul N, Manuyakorn W, Mahachoklertwattana P, et al. Effect of vitamin D on lung function assessed by forced oscillation technique in asthmatic children with vitamin D deficiency: a randomized double-blind placebo-controlled trial. Asian Pac J Allergy Immunol 2019; [ 10.12932/AP-010519-0553]. [DOI] [PubMed] [Google Scholar]
- 36.Majak P, Rychlik B, Stelmach I. The effect of oral steroids with and without vitamin D3 on early efficacy of immunotherapy in asthmatic children. Clin Exp Allergy 2009; 39: 1830–1841. doi: 10.1111/j.1365-2222.2009.03357.x [DOI] [PubMed] [Google Scholar]
- 37.Schou AJ, Heuck C, Wolthers OD. Does vitamin D administered to children with asthma treated with inhaled glucocorticoids affect short-term growth or bone turnover? Pediatr Pulmonol 2003; 36: 399–404. doi: 10.1002/ppul.10379 [DOI] [PubMed] [Google Scholar]
- 38.Randev S, Kumar P, Guglani V. Vitamin D supplementation in childhood – a review of guidelines. Indian J Pediatr 2018; 85: 194–201. doi: 10.1007/s12098-017-2476-0 [DOI] [PubMed] [Google Scholar]
- 39.Pojsupap S, Iliriani K, Sampaio TZAL, et al. Efficacy of high-dose vitamin D in pediatric asthma: a systematic review and meta-analysis. J Asthma 2015; 52: 382–390. doi: 10.3109/02770903.2014.980509 [DOI] [PubMed] [Google Scholar]
- 40.Fares MM, Alkhaled LH, Mroueh SM, et al. Vitamin D supplementation in children with asthma: a systematic review and meta-analysis. BMC Res Notes 2015; 8: 23. doi: 10.1186/s13104-014-0961-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00662-2021.SUPPLEMENT (317.2KB, pdf)