Abstract
Background:
In PIONEER-HF, the in-hospital initiation of sacubitril/valsartan in patients hospitalized for acute decompensated heart failure (ADHF) was well-tolerated and led to improved outcomes. We aim to determine the representativeness of the PIONEER-HF trial among patients hospitalized for ADHF using real-world data.
Methods:
The study population was derived from all patients discharged alive for ADHF in the Get With The Guidelines-HF (GWTG-HF) registry from 2006 to 2018 with HF with reduced ejection fraction (HFrEF) (“all HFrEF with ADHF”). We then determined the proportion of patients meeting PIONEER-HF eligibility criteria (“PIONEER-HF eligible”) and those meeting a set of limited eligibility criteria (“actionable” cohort). Rates of HF readmissions and all-cause mortality were then compared between the “all HFrEF with ADHF”, “PIONEER-HF eligible”, and “actionable” cohorts using linked Medicare claims data.
Results:
A total of 99,767 patients with HFrEF in GWTG-HF were hospitalized for ADHF. PIONEER-HF inclusion criteria were met by 71,633 (71.8%) patients, and both inclusion and exclusion criteria were met by 20,704 (20.8%) patients. 68,739 (68.9%) patients met the criteria for the “actionable” cohort. Among the CMS-linked patients, the HF rehospitalization rate at 1 year was 35.1% (95% CI 34.5, 35.8) for “all HFrEF with ADHF” patients, 32.6% (95% CI 31.3, 33.9) for the “PIONEER-HF eligible” cohort, and 33.1% (95% CI 32.3, 33.9) for the “actionable” cohort. The 1-year all-cause mortality was 36.7% (95% CI 36.1, 37.4) for “all HFrEF with ADHF” patients, 31.6% (95% CI 30.3, 32.9) for the “PIONEER-HF eligible” cohort, and 32.2% (95% CI 31.4, 33.0) for the “actionable” cohort.
Conclusions:
Patient characteristics and clinical outcomes for patients eligible for PIONEER-HF only modestly differ when compared with those encountered in routine practice, suggesting that the in-hospital initiation of sacubitril/valsartan should be routinely considered for patients with HFrEF hospitalized for ADHF.
Keywords: Sacubitril valsartan, heart failure with reduced ejection fraction, clinical trial, registry
Nearly 1 million people are hospitalized annually for acute decompensated heart failure (ADHF) in the United States alone 1, Despite national efforts to improve transitional and post-discharge care, unplanned 30-day readmission and 30-day mortality rates after a hospitalization for ADHF remain high at 21% and 10%, respectively 2, 3. In addition, the costs associated with unplanned readmissions for heart failure (HF) are >$900 million annually for Medicare patients alone 4. Improving post-discharge outcomes after a hospitalization for ADHF is an important goal for multiple stakeholders, including patients, clinicians and payers.
The PIONEER-HF trial (Comparison of Sacubitril/Valsartan Versus Enalapril on Effect on NT-pro BNP in Patients Stabilized From an Acute Heart Failure Episode) was conducted to determine the safety and efficacy of in-hospital initiation of sacubitril/valsartan vs enalapril in patients with heart failure with a reduced ejection fraction (HFrEF) who were hospitalized for ADHF 5. The in-hospital initiation of sacubitril/valsartan, compared with enalapril, in trial participants hospitalized for ADHF was safe and well-tolerated, achieved an approximately 30% reduction in NT-proBNP levels, and reduced CV death or rehospitalization for HF within 8 weeks of discharge 5–7.
Given this, it is important to determine if the results of the PIONEER-HF trial apply to patients encountered in routine care. In this study, we aimed to: (1) determine the eligibility for the PIONEER-HF trial among patients in a contemporary, United States-based registry cohort with acute HFrEF (“all HFrEF with ADHF”), (2) determine the eligibility for sacubitril/valsartan using criteria most relevant to daily clinical practice (“actionable” cohort), and (3) compare long-term outcomes between the “all HFrEF with ADHF”, “PIONEER-HF eligible”, and “actionable” cohorts using linked Medicare claims data.
Methods
Data sources
The registry population utilized in this analysis encompassed patients enrolled in Get with the Guidelines – Heart Failure (GWTG–HF). GWTG–HF is a contemporary registry established by the American Heart Association (AHA), and includes a diverse cohort of patients hospitalized for HF or who developed significant HF symptoms during hospitalization in the United States 8. Baseline characteristics and subsequent data is collected via case report forms and includes demographics, medical history, laboratory and biochemical data, in –hospital treatment and subsequent outcomes 8.
In this study, we included patients discharged alive in GWTG-HF between January 2006 – June 2018 across 317 sites in the United States. Only patients with non – missing information on age, quantitative or qualitative left ventricular ejection fraction (LVEF), and NT – proBNP or brain natriuretic peptide (BNP) were included in the PIONEER-HF cohort comparison. The baseline characteristics of the PIONEER-HF trial participants that were used for comparison have been previously published and described 9.
Study cohorts
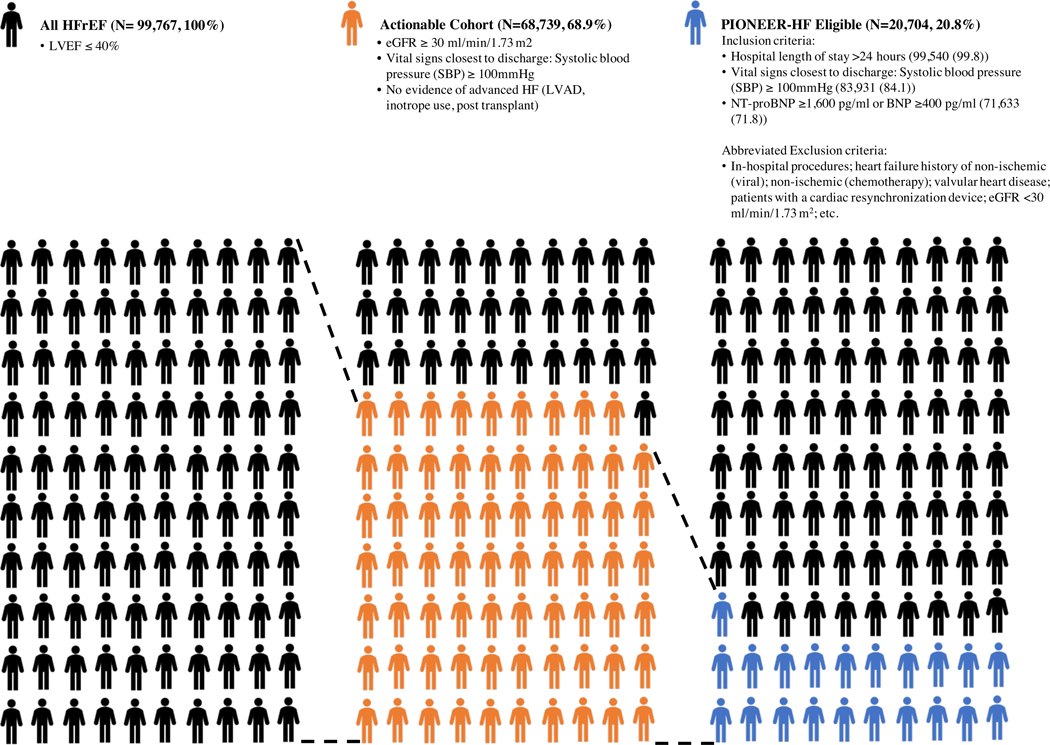
The initial study cohort (“all HFrEF with ADHF”) was derived by selecting for all patients discharged alive in GWTG-HF between January 2006 – June 2018 with LVEF < 40% on quantitative assessment. Next, we derived two separate groups by applying: (1) PIONEER-HF trial inclusion and exclusion criteria (“PIONEER-HF eligible”) and (2) a minimal set of clinically relevant criteria (“actionable”) to the overall HFrEF population in GWTG-HF. This “actionable” cohort was constructed to allow an understanding of the number of HFrEF patients encountered in clinical practice for whom clinicians might consider sacubitril/valsartan therapy, and comprised of three criteria: (i) estimated glomerular filtration rate (eGFR) ≥ 30 ml/min/1.73 m2, (ii) systolic blood pressure (SBP) ≥ 100 mmHg, and (iii) no evidence of advance HF as determined by the use of a left –ventricular assist device, inotropes, or post –cardiac transplant.
Data on all-cause mortality, and HF readmission rate at 1 year were obtained by linking the GWTG-HF registry with the Center for Medicare & Medicaid (CMS) Part A inpatient fee-for-service claims. Medicare Part A is national health insurance program in the United States that covers inpatient treatment for individuals aged over 65. By linking the Medicare Part A claims of individuals over age 65 with a diagnosis of HFrEF in GWTG-HF between the year 2006 and 2015 (Supplemental Table 1), we were able to determine post-discharge outcomes in HFrEF patients and infer how each cohort faired following index HF discharge.
Statistical analysis
All analyses were carried out in SAS version 9.4 (SAS Institute, Cary, North Carolina). The Institutional Review Board of Duke Clinical Research Institute approved the study and granted a waiver of analyzing de-identified patient data for research purposes.
Results
A total of 206,207 patients were discharged alive in the GWTG-HF registry between January 2006 – June 2018. Of this cohort, a total of 99,767 (N) patients in GWTG-HF were hospitalized for acute HF in the setting of HFrEF. PIONEER-HF inclusion criteria were met by 71,633 (71.8%) patients, and both inclusion and exclusion criteria were met by 20,704 (20.8%) patients. 68,739 (68.9%) patients met the criteria for the “actionable” cohort (Table 1, Figure 1, Supplementary Figure 1). The most common reasons GWTG–HfrEF patients were excluded from the PIONEER-HF study were the following: patients with contraindications to ARB therapy (17.6% of N), patients with contraindications to sacubitril/valsartan therapy (7% of N), and eGFR <30 ml/min/1.73 m2 as calculated by the Modification in Diet in Renal Disease (5.9% of N). The most common reason the GWTG–HfrEF patients were excluded from the “actionable” cohort was not meeting the criteria for eGFR ≥ 30 ml/min/1.73 m2 (23.6% of N excluded).
Table 1.
Baseline characteristics
| Variable | PIONEER-HF trial participants (9) | All HFrEF patients in GWTG | PIONEER-HF eligible patients in GWTG | Actionable patients in GWTG | % Std. Diff. vs PIONEER-HF patients | ||
|---|---|---|---|---|---|---|---|
| (N=440) | N=99,767 | N=20,704 | N=68,739 | HFrEF | Eligible | Actionable | |
| Demographics | |||||||
| Age | 61 (51 – 71) | 70 (58 – 81) | 71 (59 – 82) | 69 (57 – 80) | |||
| Female | 113 (25.7) | 36,490 (36.6) | 7,905 (38.2) | 24,182 (35.2) | 23.7 | 27.1 | 20.8 |
| Race | |||||||
| Black | 158 (35.9) | 25,488 (25.5) | 5,204 (25.1) | 18,671 (27.2) | 22.6 | 23.6 | 18.9 |
| White | 261 (59.3) | 61,925 (62.1) | 12,878 (62.2) | 41,826 (60.8) | 5.6 | 5.9 | 3.1 |
| Medical History | |||||||
| Atrial fibrillation/Atrial flutter | 147 (33.4) | 33,111 (33.3) | 5,794 (28.1) | 22,581 (32.9) | 0.3 | 11.6 | 1.0 |
| CVA/TIA | 44 (10.0) | 15,435 (15.5) | 2,918 (14.1) | 10,369 (15.1) | 16.6 | 12.7 | 15.5 |
| Previous myocardial infarction | 27 (6.1) | 26,996 (27.1) | 5,079 (24.6) | 17,763 (25.9) | 58.7 | 53.0 | 55.9 |
| Hyperlipidemia | 159 (36.1) | 51,580 (51.8) | 9,824 (47.6) | 34,909 (50.9) | 32.0 | 23.4 | 30.1 |
| Hypertension | 384 (87.3) | 79,392 (79.7) | 16,478 (79.9) | 55,447 (80.8) | 20.4 | 20.1 | 17.7 |
| Diabetes | 79 (18.0) | 44,137 (44.3) | 8,690 (42.1) | 29,596 (43.1) | 59.4 | 54.6 | 56.8 |
| Renal Insufficiency (SCr>2) | 130 (29.5) | 20,067 (20.2) | 1,827 (8.9) | 7,916 (11.5) | 21.9 | 54.4 | 45.7 |
| Prior percutaneous coronary intervention | 2 (0.5) | 18,630 (18.7) | 3,286 (15.9) | 12,558 (18.3) | 65.2 | 58.8 | 64.3 |
| Prior coronary artery bypass graft | 18 (4.1) | 20,280 (20.4) | 3,471 (16.8) | 13,345 (19.5) | 51.3 | 42.5 | 49.1 |
| Implantable cardioverter defibrillator only | 80 (18.2) | 18,067 (18.1) | 3,552 (17.2) | 11,625 (16.9) | 0.1 | 2.5 | 3.3 |
| Cardiac resynchronization therapy | 43 (9.8) | 9,343 (9.4) | 171 (0.8) | 5,913 (8.6) | 1.3 | 40.7 | 4.0 |
| Medical History panel missing | – | 201 (0.2) | 68 (0.3) | 136 (0.2) | – | – | – |
| Discharge Measures | |||||||
| Systolic Blood Pressure, mmHg | 118 (110 – 133) | 116 (104 – 131) | 120 (110 – 134) | 120 (110 – 133) | |||
| Heart rate, bpm | 81 (72 – 92) | 76 (68 – 86) | 76 (68 – 86) | 76 (68 – 86) | |||
| Body mass index | 30.5 (25.9 – 37.1) | 27.2 (23.2 – 32.7) | 27.1 (23.2 – 32.3) | 27.7 (23.5 – 33.3) | |||
| NT-BNP, pg/mL | 4,821 (3,109 – 8,767)* | 5,196 (2,424 – 10,618) | 4,810 (2,448 – 9,245) | 4,576 (2,083 – 9,287) | |||
| Ejection Fraction Quantitative, % | 24 (18 – 30) | 25 (20 – 33) | 26 (20 – 35) | 25 (20 – 34) | |||
| Potassium, mEq | 4.2 (4.0 – 5.0) | 4.0 (3.7 – 4.4) | 4.0 (3.7 – 4.3) | 4.0 (3.7 – 4.3) | |||
| Prior Medications | |||||||
| Sacubitril/valsartan | – | 838 (0.9) | – | 580 (0.9) | – | – | – |
| ACEi/ARB | 208 (47.3) | 49,755 (52.0) | 11,890 (61.1) | 36,284 (55.1) | 9.4 | 27.9 | 15.6 |
| Beta-Blocker | 262 (59.5) | 62,213 (65.0) | 11,421 (58.7) | 42,099 (63.9) | 11.2 | 1.8 | 8.9 |
| Aldosterone antagonist | 48 (10.9) | 16,271 (17.0) | 2,655 (13.6) | 11,177 (17.0) | 17.6 | 8.3 | 17.5 |
| Loop diuretics | 262 (59.5) | 60,307 (63.0) | 11,166 (57.3) | 40,504 (61.5) | 7.1 | 4.5 | 3.9 |
| Hydralazine | 30 (6.8) | 9,118 (9.5) | 1,127 (5.8) | 5,225 (7.9) | 9.9 | 4.2 | 4.3 |
| Nitrates | 43 (9.8) | 17,780 (18.6) | 3,094 (15.9) | 11,058 (16.8) | 25.4 | 18.4 | 20.8 |
| Digoxin | 41 (9.3) | 14,574 (15.2) | 2,776 (14.3) | 10,074 (15.3) | 18.1 | 15.4 | 18.2 |
| Prior Medications panel missing | – | 4,030 (4.0) | 1,231 (5.9) | 2,842 (4.1) | – | – | – |
Abbreviations: CVA = cerebrovascular accident; TIA = transient ischemic attack; SCr = serum creatinine; NT-proBNP = N terminal pro brain natriuretic peptide; ACEi = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker. *NT-proBNP at screening. Data presented as median (IQR) and N (%)
Figure 1.
Abbreviated flow diagram for the derivation of the “all HFrEF with ADHF”, “PIONEER-HF eligible” and “actionable” cohort.
Population characteristics
Table 1/Supplementary Table 2 compares the baseline characteristics between the PIONEER-HF trial participants and the three GWTG-HF cohorts. There were notable differences between PIONEER-HF trial participants and GWTG-HF cohorts. A notable, difference was a lower median age of PIONEER-HF trial participants when compared to any of the three GWTG cohorts [PIONEER-HF 61 (51–71) years vs. “all-HfrEF with ADHF” – 70 (58–81) years vs. “actionable” 69 (57–80) years vs. “PIONEER-HF eligible” 71 (59–82) years]. The proportion of women was lower in the PIONEER-HF trial when compared to the GWTG cohorts (“PIONEER-HF eligible” 25.7% vs. “all-HfrEF with ADHF” – 36.6% vs. “actionable” 35.2% vs. “PIONEER-HF eligible” 38.2%). Further, the proportion of patients in PIONEER-HF who self-identified as black were higher in the PIONEER-HF trial (35.9%) compared to 25.5% of the “all HfrEF with ADHF”, 27.2% of the “actionable” cohort, and 25.1% of PIONEER-HF eligible.
Within the GWTG cohorts, “PIONEER-HF eligible” patients generally had lower rates of comorbid disease when compared to the “all-HfrEF with ADHF” and “actionable” patient cohorts (atrial fibrillation: 28.1% vs. 33.3% vs. 32.9 %; coronary artery disease: 50.4% vs. 52.9% vs. 51.1%; hypertension: 79.9% vs. 79.7% vs 80.8%; diabetes: 41.5% vs. 43.8% vs. 42.5% and renal insufficiency: 8.9% vs 20.2% vs 11.5%).
Outcomes
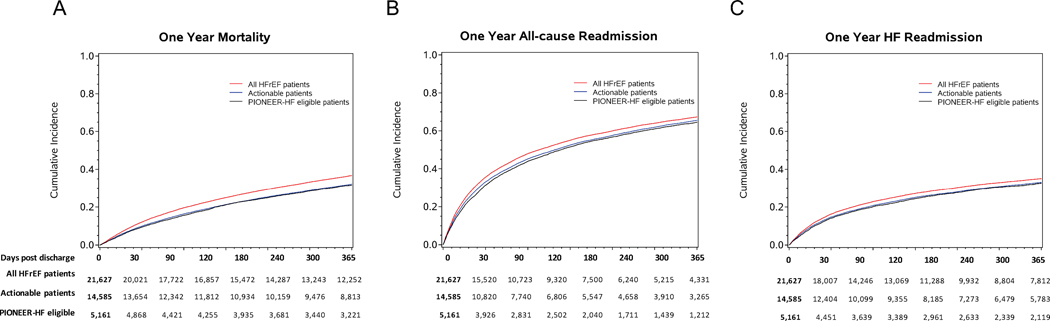
A total of 21,627 patients were linked within CMS to determine post discharge outcomes (47.4% of total) (Supplementary Table 3). Among the CMS-linked patients, the 1-year all-cause mortality and its 95% CI in the “all HfrEF with ADHF” cohort was higher (36.7%, 95% CI 36.1, 37.4) than then the upper limits of the “actionable” (32.2%, 95% CI 31.4, 33.0) and the “PIONEER-HF eligible” cohorts (31.6%, 95% CI 30.3, 32.9). This indicates significantly higher all-cause mortality in “all HfrEF with ADHF” than in the “actionable” or “PIONEER-HF eligible” cohorts (Table 2, Figure 2). The all-cause readmission rate at 1 year for “PIONEER-HF eligible” patients was the lowest with 64.5% (95% CI 63.2, 65.8), 65.5% (95% CI 64.7, 66.3) for the “actionable” cohort and 67.3% (95% CI 66.7, 68.0) for the “all HfrEF with ADHF” cohort. The HF rehospitalization rate at 1 year was 35.1% (95% CI 34.5, 35.8) for “all HfrEF with ADHF” patients, 33.1% (95% CI 32.3, 33.9) for the “actionable” cohort and 32.6% (95% CI 31.3, 33.9) for the “PIONEER-HF eligible” cohort.
Table 2.
All-cause mortality, all-cause and HF-related readmission at 1-year post discharge compared between: All HFrEF, Actionable, and PIONEER-HF eligible.
| At 30 Days | At 90 Days | At 1 Year | ||||
|---|---|---|---|---|---|---|
| Outcome | n | Cumulative Incidence (95% CI) | n | Cumulative Incidence (95% CI) | n | Cumulative Incidence (95% CI) |
| All-cause Mortality | ||||||
| All HFrEF patients | 1,410 | 6.6 (6.2, 6.9) | 3,411 | 16.0 (15.5, 16.4) | 7,645 | 36.7 (36.1, 37.4) |
| Actionable patients | 799 | 5.5 (5.1, 5.9) | 1,894 | 13.1 (12.6, 13.7) | 4,502 | 32.2 (31.4, 33.0) |
| PIONEER-HF eligible patients | 257 | 5.0 (4.4, 5.6) | 645 | 12.6 (11.7, 13.5) | 1,581 | 31.6 (30.3, 32.9) |
| All-cause Readmission | ||||||
| All HFrEF patients | 5,119 | 23.8 (23.2, 24.4) | 9,016 | 42.3 (41.6, 42.9) | 13,975 | 67.3 (66.7, 68.0) |
| Actionable patients | 3,174 | 21.9 (21.2, 22.6) | 5,679 | 39.5 (38.7, 40.3) | 9,143 | 65.5 (64.7, 66.3) |
| PIONEER-HF eligible patients | 1,039 | 20.2 (19.1, 21.3) | 1,945 | 38.2 (36.8, 39.5) | 3,207 | 64.5 (63.2, 65.8) |
| HF Readmission | ||||||
| All HFrEF patients | 2,195 | 10.2 (9.8, 10.6) | 4,228 | 19.8 (19.3, 20.4) | 7,249 | 35.1 (34.5, 35.8) |
| Actionable patients | 1,338 | 9.2 (8.8, 9.7) | 2,580 | 18.0 (17.3, 18.6) | 4,596 | 33.1 (32.3, 33.9) |
| PIONEER-HF eligible patients | 433 | 8.4 (7.7, 9.2) | 886 | 17.4 (16.4, 18.5) | 1,611 | 32.6 (31.3, 33.9) |
Figure 2.
Cumulative incidence plots for the three cohorts: “all HFrEF with ADHF”, “PIONEER-HF eligible” and “actionable”. (A) One year mortality; (B) One year all-cause readmission; (C) One year HF readmission
Discussion
In a contemporary HF registry of patients hospitalized for ADHF in the United States, one out of every five individuals (20.8%) met the PIONEER-HF inclusion and exclusion criteria. Relaxing the criteria to an “actionable” cohort increased the eligibility to 68.9% of all patients with HfrEF. Baseline characteristics and clinical outcomes at 1 year within the GWTG-HF cohorts indicated an all-over comparable but somewhat lower risk profile of patients eligible for PIONEER-HF as compared with patients with HfrEF encountered in routine practice.
With nearly one in three patients experiencing mortality within 1 year of hospitalization for ADHF in clinical practice, there is a substantial opportunity to improve the care and outcomes of these patients. PIONEER-HF provided data on the safety and efficacy of sacubitril/valsartan in patients recently treated for ADHF. The PIONEER-HF data suggests that in-hospital initiation of sacubitril/valsartan can both reduce NT-proBNP levels and readmissions through 8 weeks 7, 10. Our analysis found that PIONEER-HF trial participants were comparable to patients with HfrEF hospitalized for ADHF in routine clinical practice in the United States. Yet, the PIONEER-HF trial participants and the selected GWTG-HF cohorts had some notable differences in the distribution of age, sex, and race as well as comorbid burden, suggesting that GWTG-HF registry cohort was all-over sicker. While the clinical outcomes seem mostly comparable between the “PIONEER-HF eligible” and the “actionable” cohort, patients who did not meet either the PIONEER-HF trial inclusion and exclusion criteria as well as the reduced “actionable” criteria were associated with a worse clinical outcome. Thus, the impact of PIONEER-HF on the in-hospital initiation of sacubitril/valsartan in patients with ADHF is poised to be significant, especially when extended to capture a broader population (“actionable” cohort). The importance of starting GDMT during hospitalization, in order to leverage this critical timepoint to optimize a patient’s HF treatment plan appears to be safe, is likely to improve adherence to medication and clinical outcomes 11–14.
The PIONEER-HF trial was novel in several ways. It was the first study to suggest benefit of in-hospital initiation of sacubitril/valsartan in a diverse population of patients hospitalized for HF. Over a third of patients enrolled in the trial identified as black, and approximately half were not being treated with an angiotensin converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) at the time of their admission 9. Successful therapies for the treatment of ADHF or evidence to support initiation of chronic treatments instituted in the setting of a HF hospitalization have been limited. In regards to sacubitril/valsartan the early uptake of this drug was slow, amounting to only 2.3% of patients hospitalized for HfrEF in the US in the first 12 months post approval by the Food and Drug Administration 15.
PIONEER-HF demonstrated that initiating patients on sacubitril/valsartan at this critical juncture in their disease course can positively impact both the trajectory of disease-specific biomarkers and patient outcomes 9, 10. Our findings are distinct from results by Parikh et al. who compared the scope of sacubitril/valsartan eligibility in the PARADIGM-HF trial (Prospective Comparison of Angiotensin Receptor-Neprilysin Inhibitor) which enrolled outpatients with a more narrow LVEF inclusion criterion (≤ 35%). Yet, more patients in the GWTG-HF registry met PARADIGM-HF eligibility criteria, given in parts to more relaxed natriuretic peptide [PARADIGM-HF: LVEF ≤ 35%, NT-proBNP ≥ 600 pg/mL or BNP ≥ 150 pg/mL (or, if hospitalized for HF within 6 months, NT-proBNP ≥ 400 pg/mL or BNP ≥ 100 pg/mL) and PIONEER-HF: LVEF < 40%, NT-proBNP ≥ 1600pg/mL or BNP ≥ 400 pg/mL]16.
The use of pharmacological therapies to block neurohormonal pathways has improved outcomes for patients in the stable, chronic phase of HF. Thus, it is important that the majority of patients be considered for these medications following stabilization of acute HF. Despite improving outcomes in patients with HfrEF, several missed opportunities to improve clinical outcomes remain. Recent work emphasized the low rates of guideline directed medical therapy (GDMT) in outpatients with HfrEF. Among eligible patients, 27%, 33%, and 67% were not prescribed ACEI/ARB/ARNI, beta-blocker, and mineralocorticoid receptor antagonist therapy, respectively. Further, when medications were prescribed, few patients were receiving target doses of ACEI/ARB (17%), ARNI (14%), and beta-blocker (28%) 17. Only 1% of the eligible patient population received recommended target doses of all classes of medications. Unfortunately, up-titration of medications does not occur frequently during follow-up thus the majority of patients either do not get started on the appropriate medications or their target doses 18. In many instances a hospitalization for HF presents an opportunity to start or up-titrate patients on their GDMT for HF. Yet, in the setting of a hospitalization, in many instances medications for HF get discontinued or dose reduced due to hemodynamic or cardiorenal considerations 19. Not surprisingly the use of GDMT at discharge is low 20. This represents an extensive gap in how the medical therapy for HF could be further optimized and lends further support to ensuring that patients are on the most efficacious doses of their GDMT prior to discharge 21–25.
As shown in our analysis the number of patients who could potentially benefit from sacubitril/valsartan can be significantly increased when only a reduced number of inclusion and exclusion criteria is applied (“PIONEER-HF eligible” to “actionable”). It seems reasonable to offer sacubitril/valsartan therapy to a wider population of symptomatic HfrEF patients in the absence of absolute contraindications such as persistent hypotension and advanced renal disease 26. Our analysis confirms that patient characteristics are similar amongst patients meeting PIONEER-HF inclusion and exclusion criteria and those in the actionable cohort. It is likely that the expansion to patients outside of the PIONEER-HF trial inclusion and exclusion criteria may derive a comparable benefit as it was seen with other HF medical therapies 27.
Limitations
Notably, not all PIONEER-HF inclusion and exclusion criteria were available in GWTG-HF, thus some criteria were replaced by surrogate criteria. Further, although prior studies have suggested patients enrolled in GWTG-HF have similar characteristics to national cohorts, GWTG-HF may not represent all patients hospitalized for ADHF in the United States. Next, the “actionable” and “PIONEER -HF eligible” cohorts originate from the “All HfrEF” cohort. Thus, the interpretation of the results needs to be considered in this context. Finally, we acknowledge that the definition of the “actionable cohort”, while clinically meaningful is subjective.
Conclusions
Patient characteristics and clinical outcomes in patients eligible for PIONEER-HF only modestly differ when compared with those encountered in routine practice, indicating that result from PIONEER-HF may be broadly generalizable. These results further support clinicians to apply guideline recommendations to optimize GDMT for their patients prior to discharge for their ADHF hospitalization.
Supplementary Material
What is new?
In a contemporary HF registry of patients hospitalized for ADHF in the United States, one out of every five individuals (20.8%) met the PIONEER-HF inclusion and exclusion criteria.
Relaxing the criteria to an “actionable” cohort increased the eligibility to 68.9% of all patients with HFrEF.
Baseline characteristics and clinical outcomes at 1 year indicated an all-over comparable but somewhat lower risk profile of patients eligible for PIONEER-HF as compared with patients with HFrEF encountered in routine practice.
What are the clinical implications?
Patient characteristics and clinical outcomes in participants enrolled in PIONEER-HF only modestly differ when compared with those encountered in routine practice, indicating that result from PIONEER-HF may be broadly generalizable.
These results further support clinicians to apply guideline recommendations to optimize GDMT for their patients prior to discharge for their ADHF hospitalization.
Acknowledgments
All Sources of Funding:
The Get With The Guidelines®–Heart Failure (GWTG-HF) program is provided by the American Heart Association. GWTG-HF is sponsored, in part, by Amgen Cardiovascular and has been funded in the past through support from Medtronic, GlaxoSmithKline, Ortho-McNeil, and the American Heart Association Pharmaceutical Roundtable. Powered by IQVIA, Cambridge, MA. Duke Clinical Research Institute (DCRI) served as the data analysis center.
Non-standard Abbreviations and Acronyms:
- ADHF
acute decompensated heart failure
- BNP
brain natriuretic peptide
- CMS
Center for Medicare and Medicaid
- GWTG-HF
Get With The Guidelines – Heart Failure
- HFrEF
heart failure with a reduced ejection fraction
- LVEF
left ventricular ejection fraction
Footnotes
Disclosures
MF: Grant; American Heart Association. Consultant; Company Relationship; Axon Therapies, Galvani, GCF: Reports grant support from NIH, consulting for Abbott, Amgen, Bayer, CHF Solutions, Janssen, Medtronic, and Novartis.
EJV: Grant; Novartis. Consultant; Company Relationship; Novartis, Amgen, Philips,
AFH: Company Relationship; AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Luitpold Pharmaceuticals, Merck, Novartis. Honoraria; Company Relationship; Bayer, Boston Scientific, Novartis.
ADD: Reports research funding from the American Heart Association, Amgen, AstraZeneca, Bayer, Intra-Cellular Therapies, Luitpold Pharmaceuticals, Medtronic, the NIH, Novartis and Patient Centered Outcomes Research Institute, and consulting with AstraZeneca, Bayer, LivaNova, Mardil Medical, Novartis and Procyrion.
References:
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 2.Medicare Payment Advisory Commission. June 2018. Report to the Congress: Medicare and the Health Care Delivery System. Mandated report: the effects of the Hospital Readmissions Reduction Program. Available at http://www.medpac.gov/. [Google Scholar]
- 3.Wadhera RK, Joynt Maddox KE, Wasfy JH, Haneuse S, Shen C and Yeh RW. Association of the Hospital Readmissions Reduction Program With Mortality Among Medicare Beneficiaries Hospitalized for Heart Failure, Acute Myocardial Infarction, and Pneumonia. JAMA. 2018;320:2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medicare Payment Advisory Commission. June 2008. Report to the Congress: Reforming the Delivery System. Available at http://medpac.gov/.
- 5.Velazquez EJ, Morrow DA, DeVore AD, Ambrosy AP, Duffy CI, McCague K, Hernandez AF, Rocha RA and Braunwald E. Rationale and design of the comParIson Of sacubitril/valsartaN versus Enalapril on Effect on nt-pRo-bnp in patients stabilized from an acute Heart Failure episode (PIONEER-HF) trial. American heart journal. 2018;198:145–151. [DOI] [PubMed] [Google Scholar]
- 6.Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E, PIONEER-HF Investigators. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med. 2019;380:539–548. [DOI] [PubMed] [Google Scholar]
- 7.Morrow DA, Velazquez EJ, DeVore AD, Desai AS, Duffy CI, Ambrosy AP, Gurmu Y, McCague K, Rocha R and Braunwald E. Clinical Outcomes in Patients With Acute Decompensated Heart Failure Randomly Assigned to Sacubitril/Valsartan or Enalapril in the PIONEER-HF Trial. Circulation. 2019;139:2285–2288. [DOI] [PubMed] [Google Scholar]
- 8.Hong Y and LaBresh KA. Overview of the American Heart Association “Get With the Guidelines” Programs: Coronary Heart Disease, Stroke, and Heart Failure. Critical Pathways in Cardiology. 2006;5:179–186. [DOI] [PubMed] [Google Scholar]
- 9.Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R and Braunwald E. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med. 2019;380:539–548. [DOI] [PubMed] [Google Scholar]
- 10.Morrow DA, Velazquez EJ, DeVore AD, Prescott MF, Duffy CI, Gurmu Y, McCague K, Rocha R and Braunwald E. Cardiovascular biomarkers in patients with acute decompensated heart failure randomized to sacubitril-valsartan or enalapril in the PIONEER-HF trial. European heart journal. 2019;40:3345–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhagat AA, Greene SJ, Vaduganathan M, Fonarow GC and Butler J. Initiation, Continuation, Switching, and Withdrawal of Heart Failure Medical Therapies During Hospitalization. JACC Heart failure. 2019;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soucier RJ, Miller PE, Ingrassia JJ, Riello R, Desai NR and Ahmad T. Essential Elements of Early Post Discharge Care of Patients with Heart Failure. Current heart failure reports. 2018;15:181–190. [DOI] [PubMed] [Google Scholar]
- 13.Blizzard S, Verbosky N, Stein B, Hale G, Patel N, Chau Y and Cave B. Evaluation of Pharmacist Impact Within an Interdisciplinary Inpatient Heart Failure Consult Service. The Annals of pharmacotherapy. 2019:1060028019842656. [DOI] [PubMed] [Google Scholar]
- 14.Srivastava PK and Fonarow GC. In-Hospital Initiation of Angiotensin Receptor-Neprilysin Inhibitors-The Time Is Now. JAMA Cardiol. 2019;4:195–196. [DOI] [PubMed] [Google Scholar]
- 15.Luo N, Fonarow GC, Lippmann SJ, Mi X, Heidenreich PA, Yancy CW, Greiner MA, Hammill BG, Hardy NC, Turner SJ, et al. Early Adoption of Sacubitril/Valsartan for Patients With Heart Failure With Reduced Ejection Fraction: Insights From Get With the Guidelines-Heart Failure (GWTG-HF). JACC Heart Fail. 2017;5:305–309. [DOI] [PubMed] [Google Scholar]
- 16.Parikh KS, Lippmann SJ, Greiner M, Heidenreich PA, Yancy CW, Fonarow GC and Hernandez AF. Scope of Sacubitril/Valsartan Eligibility After Heart Failure Hospitalization: Findings From the GWTG-HF Registry (Get With The Guidelines-Heart Failure). Circulation. 2017;135:2077–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction: The CHAMP-HF Registry. J Am Coll Cardiol. 2018;72:351–366. [DOI] [PubMed] [Google Scholar]
- 18.Greene SJ, Fonarow GC, DeVore AD, Sharma PP, Vaduganathan M, Albert NM, Duffy CI, Hill CL, McCague K, Patterson JH, et al. Titration of Medical Therapy for Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol. 2019;73:2365–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breu AC, Allen-Dicker J, Mueller S, Palamara K, Hinami K and Herzig SJ. Hospitalist and primary care physician perspectives on medication management of chronic conditions for hospitalized patients. J Hosp Med. 2014;9:303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diamant MJ, Virani SA, MacKenzie WJ, Ignaszewski A, Toma M and Hawkins NM. Medical therapy doses at hospital discharge in patients with existing and de novo heart failure. ESC Heart Failure. 2019;6:774–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 22.Tran RH, Aldemerdash A, Chang P, Sueta CA, Kaufman B, Asafu-Adjei J, Vardeny O, Daubert E, Alburikan KA, Kucharska-Newton AM, et al. Guideline-Directed Medical Therapy and Survival Following Hospitalization in Patients with Heart Failure. Pharmacotherapy. 2018;38:406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaguchi T, Kitai T, Miyamoto T, Kagiyama N, Okumura T, Kida K, Oishi S, Akiyama E, Suzuki S, Yamamoto M, et al. Effect of Optimizing Guideline-Directed Medical Therapy Before Discharge on Mortality and Heart Failure Readmission in Patients Hospitalized With Heart Failure With Reduced Ejection Fraction. The American journal of cardiology. 2018;121:969–974. [DOI] [PubMed] [Google Scholar]
- 24.Hollenberg SM, Warner Stevenson L, Ahmad T, Amin VJ, Bozkurt B, Butler J, Davis LL, Drazner MH, Kirkpatrick JN, Peterson PN, et al. 2019 ACC Expert Consensus Decision Pathway on Risk Assessment, Management, and Clinical Trajectory of Patients Hospitalized With Heart Failure: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2019;74:1966–2011. [DOI] [PubMed] [Google Scholar]
- 25.Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JGF, de Boer RA, Drexel H, Ben Gal T, Hill L, et al. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. European journal of heart failure. 2019;21:1169–1186. [DOI] [PubMed] [Google Scholar]
- 26.DeVore AD, Hill CL, Thomas L, Sharma PP, Albert NM, Butler J, Patterson JH, Spertus JA, Williams FB, Duffy CI, et al. Patient, Provider, and Practice Characteristics Associated With Sacubitril/Valsartan Use in the United States. Circ Heart Fail. 2018;11:e005400. [DOI] [PubMed] [Google Scholar]
- 27.Ambrosy AP, Fudim M and Chioncel O. Should providers prescribe sacubitril/valsartan based on trial eligibility, approval indication, or guideline recommendations? European journal of heart failure. 2019; 21(11):1398–1401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.