Abstract
5‐Aminolevulinic acid is a new‐generation photosensitizer with high tumor specificity. It has been used successfully in the diagnosis, treatment, and screening of urological cancers including bladder cancer; specifically, it has been used in photodynamic diagnosis to detect tumors by illuminating the lesion with a specific wavelength of light to produce fluorescence in the lesion after administration of 5‐aminolevulinic acid, in photodynamic therapy, which induces tumor cell death via production of cytotoxic reactive oxygen species, and in photodynamic screening, in which porphyrin excretion in the blood and urine is used as a tumor biomarker after administration of 5‐aminolevulinic acid. In addition to these applications in urological cancers, 5‐aminolevulinic acid–based photodynamic technology is expected to be used as a novel strategy for a large number of cancer types because it is based on a property of cancer cells known as the Warburg effect, which is a basic biological property that is common across all cancers.
The mechanism of 5‐aminolevulinic acid‐based photodynamic therapy.
1. INTRODUCTION
In a recent study, Malik and Lugaci 1 described the research and development of 5‐aminolevulinic acid (5‐ALA) as a new‐generation photosensitizer with high tumor specificity. The use of 5‐ALA–based photodynamic technology has since been examined in a number of studies worldwide, such as in photodynamic diagnosis (PDD), in which it is used to detect tumors by illuminating the lesion with a specific wavelength of light to produce fluorescence after administration of 5‐ALA; photodynamic therapy (PDT), in which it is used to induce cell death; and in photodynamic screening (PDS), in which porphyrin excretion in the blood and urine is used as a tumor biomarker. Thus, a number of applications using 5‐ALA are expected to emerge in the clinical setting.
1.1. 5‐Aminolevulinic acid (5‐ALA) as a photosensitizer
5‐ALA is a natural amino acid produced in plants and animals, and it is a common precursor of hemoglobin and chlorophyll (Figures 1, 2). Endogenous 5‐ALA is generated from glycine and succinyl CoA in the mitochondria by an enzymatic reaction induced by the 5‐ALA synthetic enzyme, whereas exogenous 5‐ALA may also be introduced into cells by its administration. In normal cells, both endogenous and exogenous 5‐ALA produce a precursor through the same biosynthetic and metabolic pathways in the cytoplasm, and the precursor is transported to the mitochondria via the ATP‐binding cassette (ABC) subfamily B member 6 (ABCB6) to produce protoporphyrin IX (PpIX). Subsequently, ferrochelatase catalyzes the insertion of ferrous iron into PpIX to form heme and bilirubin (Figure 1). Heme is an important protein that is incorporated into the electron transport chain, which regulates the production of adenosine triphosphate (ATP) in aerobic metabolism.
FIGURE 1.
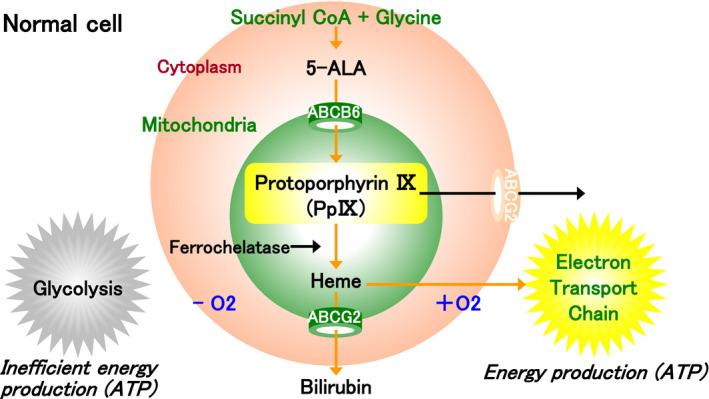
The porphyrin biosynthesis pathway in the normal cell. In normal cells, endogenous 5‐aminolevulinic acid (5‐ALA) is generated from glycine and succinyl CoA in the mitochondria. It is converted into several precursors in the cytoplasm, and protoporphyrin IX (PpIX) is synthesized in the mitochondria. Subsequently, ferrochelatase catalyzes the insertion of ferrous iron into PpIX to form heme and bilirubin. Heme is an important protein that is incorporated into the electron transport chain, which regulates the production of adenosine triphosphate (ATP) in aerobic metabolism
FIGURE 2.
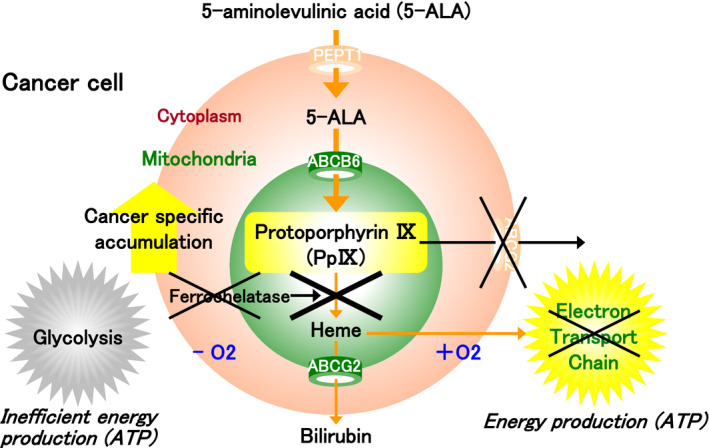
The porphyrin biosynthesis pathway in the cancer cell. In tumor cells, ferrochelatase is inactive, and heme production from protoporphyrin IX (PpIX) is suppressed. Furthermore, the activity of ABCG2 is downregulated, and the excretion of PpIX is suppressed. On the other hand, PpIX production is promoted in tumor cells due to the activation of the porphyrin synthetic enzyme and peptide transporter 1 (PEPT1). As a result, PpIX accumulates in excess in the mitochondria of tumor cells. The basic biological property of tumor cells to rely on anaerobic metabolism is common across all cancer types and is known as the Warburg effect
Rather than using the process of oxidative phosphorylation in the mitochondria via the tricarboxylic acid (TCA) cycle and the electron transport chain to produce ATP, tumor cells rely on a more inefficient glucose metabolism via oxygen‐independent glycolysis to produce ATP irrespective of whether the environment is anaerobic or aerobic (Figure 2). This phenomenon, known as the Warburg effect, is a basic biological property of all cancers and was first described by Otto Warburg, who subsequently was awarded the Nobel Prize in Physiology or Medicine in 1931. 2
In various cancer types including bladder cancer, ferrochelatase is inactive, as electrons needed to reduce ferric ion to ferrous ion are lacking due to downregulation of the TCA cycle. This leads to a lack of heme production and downregulation of PpIX catabolism. Furthermore, ABC superfamily G member 2 (ABCG2), used to excrete PpIX, is also inactivated in tumor cells, resulting in the downregulation of PpIX excretion from the cells. 3 , 4 On the other hand, PpIX production is promoted in tumor cells due to the activation of the 5‐ALA synthetic enzyme and 5‐ALA influx transporter known as peptide transporter 1 (PEPT1). As a result, PpIX accumulates in excess in the mitochondria of tumor cells. 3 , 4 In urothelial cancer (UC), excessive PpIX accumulation in tumor cells was shown to be approximately 17 times higher than in the normal epithelium. 5 , 6 , 7 , 8 PpIX, which is synthesized from 5‐ALA, is a photoactive compound and can be excited at a particular wavelength to emit light. Thus, it can be used in the diagnosis, treatment, and screening of cancer, and several applications are expected to emerge in clinical settings. 9
1.2. Photodynamic diagnosis (PDD)
PpIX, which is synthesized from 5‐ALA, is photoactive (Figure 3). When excited by visible blue light in the range of 375‐445 nm, PpIX that has specifically accumulated in excess in the mitochondria of tumor cells emits red fluorescent light in the range of 600‐740 nm. By using 5‐ALA as a photosensitizer to produce red fluorescence in tumor cells, tumor cells can be detected by fluorescence navigation in a diagnostic procedure known as ALA‐PDD (Figure 3).
FIGURE 3.
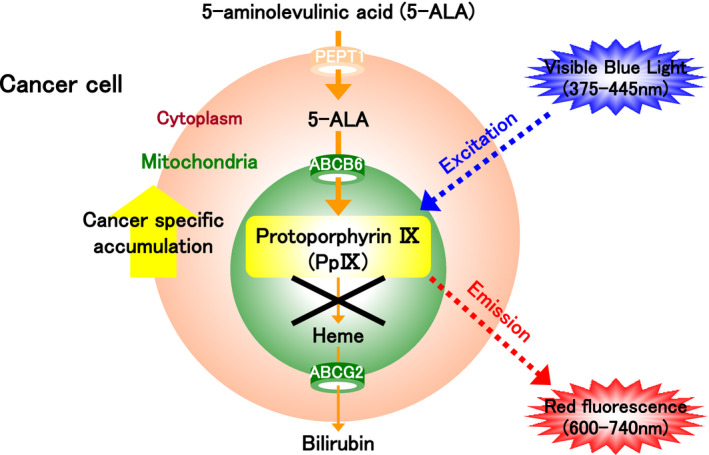
The mechanism of photodynamic diagnosis (PDD). Protoporphyrin IX (PpIX) is synthesized from 5‐aminolevulinic acid (5‐ALA) and specifically accumulates in excess in the mitochondria of tumor cells. It can be excited by blue visible light (375‐445 nm) to emit red fluorescence (600‐740 nm). 5‐ALA can therefore be used as a photosensitizer to produce red fluorescence in tumor cells. The diagnostic procedure based on fluorescence navigation using 5‐ALA is called ALA‐PDD
ALA‐PDD for bladder cancer is superior to conventional endoscopic diagnosis performed under white light for the detection of flat lesions such as microcarcinoma and carcinoma in situ (CIS), as well as flat lesions that connect to raised lesions. Kriegmair et al 6 first reported the clinical evidence for PDD in 1994, whereby 5‐ALA was administered in the bladder. Subsequently, many clinical studies were conducted in Europe and the United States that demonstrated the safety and utility of the procedure. 10 , 11 , 12 As 5‐ALA is a soluble compound, a fat‐soluble ester compound hexaminolevulinate hydrochloride (HAL) was developed to further improve the accumulation of PpIX in tumor cells. HAL was approved in Europe as Hexvix® (PHOTOCURE ASA) in 2005, 13 and in the United States as Cysview® (PHOTOCURE ASA) in 2010, 14 and it has been used in clinical settings. Notably, HAL has limited use because it is highly cytotoxic and cannot be administered systemically. 15 Japan was the first to approve a 5‐ALA diagnostic agent for oral administration. The agent, called Alaglio®, was approved in 2017 for intraoperative visualization of non–muscle‐invasive bladder cancer (NMIBC) during transurethral resection of bladder tumors (TURBT). 16 , 17 , 18 Currently, 3 years after its approval, Alaglio® has been used in approximately 13 000 cases across approximately 370 institutions. The ALA‐PDD procedure for bladder cancer was included in the Clinical Practice Guidelines for Bladder Cancer in Japan, which was revised in 2019. 19 In response to clinical question 1 (CQ1): “Is tumor visualization technology (PDD, narrow‐band imaging [NBI]) recommended for diagnosing bladder cancer?,” the guidelines indicate that the “use of tumor visualization technology in the diagnosis of bladder cancer is recommended because of improved cancer detection sensitivity (PDD: strength of recommendation 1, certainty of evidence A; NBI: strength of recommendation 1, certainty of evidence B).” Furthermore, in response to CQ4: “Is PDD or NBI recommended when treating NMIBC?,” the guidelines indicate that the use of PDD is recommended because it lowers the recurrence rate of bladder cancer (strength of recommendation 1, certainty of evidence A). In comparison, whereas NBI improves the cancer detection rate, it is unclear whether it lowers the recurrence rate of bladder cancer (strength of recommendation 2, certainty of evidence B). Guidelines in Europe and the United States strongly recommend the use of ALA‐PDD, stating that it markedly improves the diagnostic accuracy, especially the detection rate of bladder CIS, and that PDD‐TURBT may also improve the relapse‐free survival rate. 20 , 21
There are a large number of reports of meta‐analyses and systematic reviews of ALA‐PDD for bladder cancer. These reports generally demonstrated that, whereas ALA‐PDD has superior detection sensitivity of over 90%, it has relatively poor specificity of 57%‐79%, resulting in a high rate of false‐positive diagnoses. 22 , 23 ALA‐PDD also increased the rate of detection, with additional CIS detection rates of 25%‐40.8% per lesion and 19% per patient, showing its effectiveness. 24 , 25 , 26 , 27 In terms of the prognosis, ALA‐PDD–guided endoscopic resection was shown to be superior to conventional endoscopic resection under white light in reducing residual tumors after the procedure 22 , 24 , 25 , 28 and in improving the relapse‐free survival rate. 22 , 24 , 25 , 26 , 27 , 29 , 30 , 31 However, there is no consensus as to whether ALA‐PDD improved the progression‐free survival rate. 22 , 25 , 29 , 31 , 32
In addition to the indications related to the bladder, ALA‐PDD has also been shown to be effective in the diagnosis of ureteropelvic UC in recent studies. An investigator‐initiated clinical study conducted in Japan demonstrated that ALA‐PDD was as effective as in the bladder, 33 and other studies are being conducted to examine the use of ALA‐PDD in other types of cancers.
1.3. Photodynamic therapy (PDT)
PpIX that has specifically accumulated in excess in the mitochondria of tumor cells can be excited at low excitation wavelengths in the red (600‐740 nm) and green (450‐580 nm) visible ranges (Figure 4). Once the light is absorbed, PpIX returns from the excited state to the ground state while releasing energy. This results in the production of cytotoxic reactive oxygen species (ROS), such as hydroxyl radicals, singlet oxygen, hydrogen peroxide, and superoxide, in tumor cells. ROS subsequently cause damage to the mitochondria and induce apoptosis of tumor cells, leading to cell death. This mechanism underlies the effect of ALA‐PDT (Figure 4). 9 Cells undergoing apoptosis are characterized by condensation and blebbing of cytoplasm, as well as nuclear fragmentation. Apoptotic cells are phagocytosed rapidly by macrophages and neutrophils while retaining their contents. Thus, unlike in necrosis, where an inflammatory response is induced due to leakage of cell contents, apoptosis causes little harm to surrounding cells and tissues. In other words, as ALA‐PDT primarily elicits its antitumor effect through apoptosis, there are likely few side effects when used in the clinical setting. Furthermore, ALA‐PDT is a painless procedure because the antitumor effect is induced by low‐energy excitation. Thus, patients do not need to be anesthetized, and the procedure can be performed repeatedly, unlike radiation therapy. Furthermore, like ALA‐PDD, ALA‐PDT is cancer specific because it targets PpIX that has specifically accumulated in excess in tumor cells. 9 As such, it has already been approved and is in clinical use in Europe for the treatment of actinic keratosis and skin cancer. 34 In vitro and in vivo preclinical studies have also demonstrated that ALA‐PDT is highly effective against bladder and prostate cancer. 35 , 36
FIGURE 4.
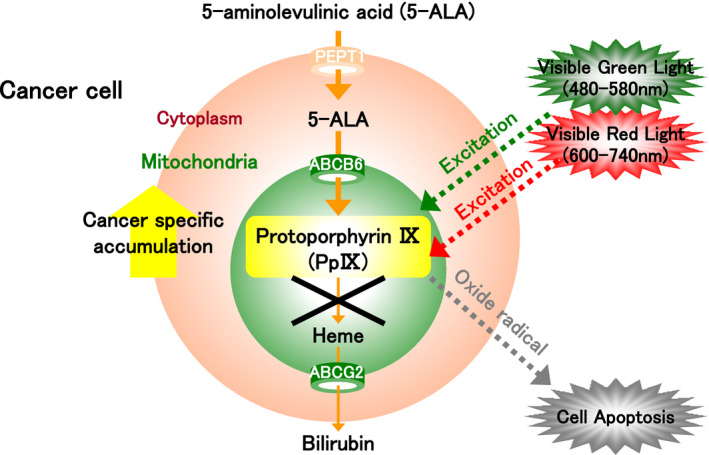
The mechanism of photodynamic therapy (PDT). Protoporphyrin IX (PpIX) is synthesized from 5‐aminolevulinic acid (5‐ALA) and specifically accumulates in excess in the mitochondria of tumor cells. It can be excited at low excitation wavelengths in the red (600‐740 nm) and green (450‐580 nm) visible ranges. Once the light is absorbed, PpIX returns from the excited state to the ground state while releasing energy. This results in the production of cytotoxic reactive oxygen species (ROS), such as hydroxyl radicals, singlet oxygen, hydrogen peroxide, and superoxide, in tumor cells. ROS subsequently cause damage to the mitochondria and induce apoptosis of tumor cells, leading to cell death. This mechanism is the basis of ALA‐PDT
A clinical study of PDT for bladder cancer was first reported in 1976 by Kelly et al, who used a hematoporphyrin derivative and a mercury arc lamp. 37 In Japan, Hisazumi et al reported the use of PDT with a hematoporphyrin derivative and argon dye laser in 1983 to treat 46 tumors in nine NMIBC patients. 38 However, PDT with a hematoporphyrin derivative did not become prevalent due to the high incidence of adverse events. In 1996, Kriegmair et al 39 first reported the use of 5‐ALA in PDT for the treatment of bladder cancer, leading to subsequent clinical studies. 40 , 41 , 42 , 43 , 44 , 45 These trials were performed primarily in patients with treatment‐resistant bladder cancer and bladder CIS, and excitation wavelengths in the green (514 nm), red (630‐635 nm), and white (380‐700 nm) ranges were used because they were delivered at a low heat density of 15‐100 J/cm2. The studies demonstrated that ALA‐PDT was effective in achieving early response rates of 60%‐100% within 3‐4 months of treatment, and response rates of 30%‐50% within 1.5‐3 years of treatment. 40 , 41 , 42 , 43 , 44 , 45 Furthermore, a phase I clinical study was conducted in 2013 in which the 5‐ALA derivative HAL was used as a photosensitizer to perform PDT with white light (380‐700 nm) as postoperative adjuvant therapy for treatment‐resistant NMIBC after TURBT. 46 In that trial, 17 patients received PDT three times with 1.5 months between treatment cycles, and the treatment was successful in eliminating lesions in nine patients (52.9%) at 6 months, four patients (23.5%) at 9 months, and two patients (11.8%) at 21 months, showing good outcomes, as well as the safety of the procedure. Although ALA‐PDT is yet to be approved for use as a treatment for bladder cancer, it is a highly accurate and minimally invasive procedure that is widely applicable and may replace Bacillus Calmette‐Guerin (BCG) intravesical therapy for bladder CIS.
In addition to 5‐ALA, HAL, and a hematoporphyrin derivative Photofrin® (porfimer sodium), various photosensitizers have been examined for use in PDT for bladder cancer. They include Fotolon® (chlorin e6), which is nonaromatic, unlike porphyrin, as well as a chlorine derivative, Radachlorin®. 47
1.4. Photodynamic screening (PDS)
Conventionally, urine cytology is frequently performed to screen for bladder cancer (Figure 5). However, it is limited in terms of diagnostic accuracy, particularly its low sensitivity. 48 More recently, a multitarget fluorescence in situ hybridization assay called the UroVysion assay was shown to be superior to urine cytology in terms of its sensitivity and to be effective in predicting recurrence of bladder cancer after TURBT. As such, the assay was approved for insurance coverage in 2019 as a diagnostic aid for possible recurrence of bladder cancer and has been used in clinical practice. 49 , 50 However, the assay alone is insufficient in the detection of bladder cancer and as a follow‐up procedure, and cystoscopy remains necessary. 51
FIGURE 5.
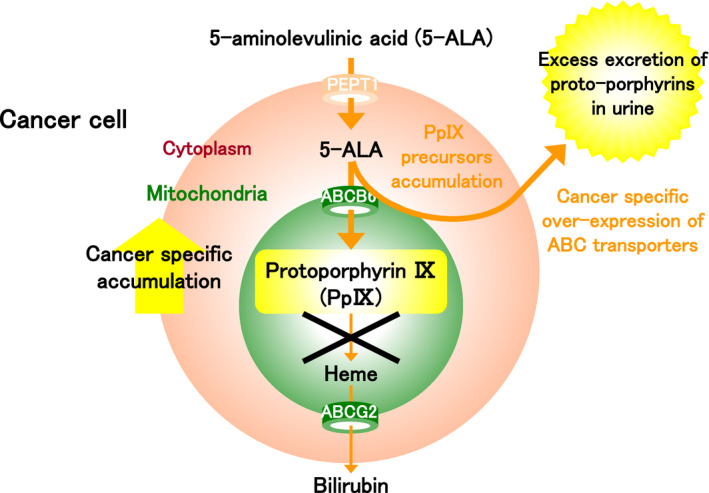
The mechanism of photodynamic screening (PDS). Protoporphyrin IX (PpIX) is synthesized from 5‐aminolevulinic acid (5‐ALA) and specifically accumulates in excess in the mitochondria of tumor cells. The accumulation of PpIX leads to saturation of porphyrin precursors including uroporphyrin (UP) and coproporphyrin (CP). Furthermore, due to tumor‐specific upregulation of transporters used to excrete these precursors of PpIX, it is hypothesized that the amounts of porphyrin precursors of PpIX excreted in excess in the urine and blood are increased in cancer patients. ALA‐PDS is a screening method based on the quantification of porphyrin excretion following oral administration of 5‐ALA
In this context, 5‐ALA–based cancer screening methods are being developed. Specifically, such methods aim to measure the amount of porphyrin excreted in urine and blood after oral administration of 5‐ALA. As previously highlighted, in various cancer types including bladder cancer, exogenous 5‐ALA that has been administered is metabolized and specifically accumulates in excess as PpIX in the mitochondria of tumor cells due to the abnormal activities of various transporters and enzymes. 1 Excessive accumulation of PpIX leads to saturation of porphyrin precursors of PpIX including uroporphyrin (UP) and coproporphyrin (CP). Furthermore, due to tumor‐specific upregulation of transporters used to excrete these precursors of PpIX, it is hypothesized that the precursors of PpIX are excreted excessively in the urine and blood. In ALA‐PDS, cancer screening is performed based on the quantification of porphyrin excretion following oral administration of 5‐ALA (Figure 5). 52
Studies performed in animal models of hepatocellular carcinoma used high‐performance liquid chromatography to demonstrate that the administration of 5‐ALA markedly increased the amount of PpIX, UP, and CP in blood 53 and urine. 54 Thus, the amount of porphyrin in blood and urine after the oral administration of 5‐ALA may be a highly sensitive and stable biomarker of cancer, irrespective of the type and nature of cancer. In fact, the procedure has been shown to be effective for bladder, colorectal, and pancreatic cancers in clinical studies. 55 , 56 , 57 In particular, in a study in which 1.0 g of 5‐ALA was given orally to 66 patients with bladder cancer and 20 healthy volunteers, the concentration of porphyrin in urine was found to be significantly higher in bladder cancer patients than in healthy volunteers. 55 The study also demonstrated that urine UPI and CPI had high sensitivity (UPI: 100%, CPI: 100%) and specificity (UPI: 96.4%, CPI: 91.4%) for bladder cancer 8 hours after the administration of 5‐ALA. Thus, it may be widely applicable as a novel tumor marker in clinical settings.
2. CONCLUSION
ALA‐PDD, ALA‐PDT, and ALA‐PDS are photodynamic procedures that use 5‐ALA. These techniques are based on the property known as the Warburg effect, which is a basic biological property that is common across all cancers. Thus, in addition to urological cancers such as bladder cancer, the techniques are expected to be used as a novel therapeutic strategy applicable to a large number of cancer types and be beneficial for patients with various cancers.
DISCLOSURE
All authors have no conflict of interest.
Inoue K, Fukuhara H, Yamamoto S, et al. Current status of photodynamic technology for urothelial cancer. Cancer Sci.2022;113:392–398. 10.1111/cas.15193
REFERENCES
- 1. Malik Z, Lugaci H. Destruction of erythroleukaemic cells by photoactivation of endogenous porphyrins. Br J Cancer. 1987;56(5):589‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koppenol WH, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11(5):325‐337. [DOI] [PubMed] [Google Scholar]
- 3. Hagiya Y, Endo Y, Yonemura Y, et al. Pivotal roles of peptide transporter PEPT1 and ATP‐binding cassette (ABC) transporter ABCG2 in 5‐aminolevulinic acid (ALA)‐based photocytotoxicity of gastric cancer cells in vitro. Photodiagnosis Photodyn Ther. 2012;9(3):204‐214. [DOI] [PubMed] [Google Scholar]
- 4. Hagiya Y, Fukuhara H, Matsumoto K, et al. Expression levels of PEPT1 and ABCG2 play key roles in 5‐aminolevulinic acid (ALA)‐induced tumor‐specific protoporphyrin IX (PpIX) accumulation in bladder cancer. Photodiagnosis Photodyn Ther. 2013;10(3):288‐295. [DOI] [PubMed] [Google Scholar]
- 5. Inoue K, Takashi K, Kamada M, et al. Regulation of 5‐aminolevulinic acid‐mediated protoporphyrin IX‐accumulation in human urothelial carcinomas. Pathobiology. 2009;76(6):303‐314. [DOI] [PubMed] [Google Scholar]
- 6. Kriegmair M, Waidelich R, Baumgartner R, Lumper W, Ehsan A, Hofstetter A. Photodynamic therapy of superficial bladder cancer. An alternative to radical cystectomy? Urologe A. 1994;33(4):276‐280. [PubMed] [Google Scholar]
- 7. Steinbach P, Weingandt H, Baumgartner R, Kriegmair M, Hofstadter F, Knuchel R. Cellular fluorescence of the endogenous photosensitizer protoporphyrin IX following exposure to 5‐aminolevulinic acid. Photochem Photobiol. 1995;62(5):887‐895. [DOI] [PubMed] [Google Scholar]
- 8. Kriegmair M, Ehsan A, Baumgartner R, et al. Fluorescence photodetection of neoplastic urothelial lesions following intravesical instillation of 5‐aminolevulinic acid. Urology. 1994;44(6):836‐841. [DOI] [PubMed] [Google Scholar]
- 9. Ishizuka M, Abe F, Sano Y, et al. Novel development of 5‐aminolevurinic acid (ALA) in cancer diagnoses and therapy. Int Immunopharmacol. 2011;11(3):358‐365. [DOI] [PubMed] [Google Scholar]
- 10. Kriegmair M, Baumgartner R, Knuechel R, et al. Fluorescence photodetection of neoplastic urothelial lesions following intravesical instillation of 5‐aminolevulinic acid. Urology. 1994;44(6):836‐841. [DOI] [PubMed] [Google Scholar]
- 11. Inoue K, Fukuhara H, Shimamoto T, et al. Comparison between intravesical and oral administration of 5‐aminolevulinic acid in the clinical benefit of photodynamic diagnosis for non‐muscle invasive bladder cancer. Cancer. 2012;118(4):1062‐1074. [DOI] [PubMed] [Google Scholar]
- 12. Hungerhuber E, Stepp H, Kriegmair M, et al. Seven years' experience with 5‐aminolevulinic acid in detection of transitional cell carcinoma of the bladder. Urology. 2007;69(2):260‐264. [DOI] [PubMed] [Google Scholar]
- 13. Witjes JA, Redorta JP, Jacqmin D, et al. Hexaminolevulinate‐guided fluorescence cystoscopy in the diagnosis and follow‐up of patients with non‐muscle‐invasive bladder cancer: review of the evidence and recommendations. Eur Urol. 2010;57(4):607‐614. [DOI] [PubMed] [Google Scholar]
- 14. Lotan Y, Bivalacqua TJ, Downs T, et al. Blue light flexible cystoscopy with hexaminolevulinate in non‐muscle‐invasive bladder cancer: review of the clinical evidence and consensus statement on optimal use in the USA – update 2018. Nat Rev Urol. 2019;16(6):377‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bourré L, Giuntini F, Eggleston IM, Wilson M, MacRobert AJ. 5‐Aminolaevulinic acid peptide prodrugs enhance photosensitization for photodynamic therapy. Mol Cancer Ther. 2008;7(6):1720‐1729. [DOI] [PubMed] [Google Scholar]
- 16. Inoue K, Anai S, Fujimoto K, et al. Oral 5‐aminolevulinic acid mediated photodynamic diagnosis using fluorescence cystoscopy for nonmuscle‐invasive bladder cancer: a randomized, double‐blind, multicentre phase II/III study. Photodiagnosis Photodyn Ther. 2015;12(2):193‐200. [DOI] [PubMed] [Google Scholar]
- 17. Inoue K, Matsuyama H, Fujimoto K, et al. The clinical trial on the safety and effectiveness of the photodynamic diagnosis of non‐muscle‐invasive bladder cancer using fluorescent light–guided cystoscopy after oral administration of 5‐aminolevulinic acid (5‐ALA). Photodiagnosis Photodyn Ther. 2016;13:91‐96. [DOI] [PubMed] [Google Scholar]
- 18. Nakai Y, Inoue K, Tsuzuki T, et al. Oral 5‐aminolevulinic acid‐mediated photodynamic diagnosis using fluorescence cystoscopy for non‐muscle‐invasive bladder cancer: a multicenter phase III study. Int J Urol. 2018;25(8):723‐729. [DOI] [PubMed] [Google Scholar]
- 19. Matsumoto H, Shiraishi K, Azuma H, et al. Clinical practice guidelines for bladder cancer 2019 update by the Japanese Urological Association: summary of the revision. Int J Urol. 2020;27(9):702‐709. [DOI] [PubMed] [Google Scholar]
- 20. Babjuk M, Burger M, Zigeuner R, et al. European Association of Urology. EAU guidelines on non‐muscle‐invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64(4):639‐653. [DOI] [PubMed] [Google Scholar]
- 21. Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007;178(6):2314‐2330. [DOI] [PubMed] [Google Scholar]
- 22. Mowatt G, N'Dow J, Vale L, et al. Photodynamic diagnosis of bladder cancer compared with white light cystoscopy: systematic review and meta‐analysis. Int J Technol Assess Health Care. 2011;27(1):3‐10. [DOI] [PubMed] [Google Scholar]
- 23. Chen C, Huang H, Zhao Y, et al. Diagnostic performance of image technique based transurethral resection for non‐muscle invasive bladder cancer: systematic review and diagnostic meta‐analysis. BMJ Open. 2019;9(10):e028173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kausch I, Sommerauer M, Montorsi F, et al. Photodynamic diagnosis in non‐muscle‐invasive bladder cancer: a systematic review and cumulative analysis of prospective studies. Eur Urol. 2010;57(4):595‐606. [DOI] [PubMed] [Google Scholar]
- 25. Rink M, Babjuk M, Catto JW, et al. Hexyl aminolevulinate‐guided fluorescence cystoscopy in the diagnosis and follow‐up of patients with non‐muscle‐invasive bladder cancer: a critical review of the current literature. Eur Urol. 2013;64(4):624‐638. [DOI] [PubMed] [Google Scholar]
- 26. Burger M, Grossman HB, Droller M, et al. Photodynamic diagnosis of non‐muscle‐invasive bladder cancer with hexaminolevulinate cystoscopy: a meta‐analysis of detection and recurrence based on raw data. Eur Urol. 2013;64(5):846‐854. [DOI] [PubMed] [Google Scholar]
- 27. Di Stasi SM, De Carlo F, Pagliarulo V, et al. Hexaminolevulinate hydrochloride in the detection of nonmuscle invasive cancer of the bladder. Ther Adv Urol. 2015;7(6):339‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shen P, Yang J, Wei W, et al. Effects of fluorescent light‐guided transurethral resection on non‐muscle‐invasive bladder cancer: a systematic review and meta‐analysis. BJU Int. 2012;110(6b):E209‐E215. [DOI] [PubMed] [Google Scholar]
- 29. Yuan H, Qiu J, Liu L, et al. Therapeutic outcome of fluorescence cystoscopy guided transurethral resection in patients with non‐muscle invasive bladder cancer: a meta‐analysis of randomized controlled trials. PLoS One. 2013;8(9):e74142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rolevich AI, Evmenenko AA. A systematic review and meta‐analysis to assess the recurrence‐free survival in non‐muscle invasive bladder cancer after transurethral resection guided by 5‐aminolevulinic acid‐induced photodynamic diagnosis compared with white‐light transurethral resect. Urologiia. 2016;4:137‐146. [PubMed] [Google Scholar]
- 31. Chou R, Selph S, Buckley DI, et al. Comparative effectiveness of fluorescent versus white light cystoscopy for initial diagnosis or surveillance of bladder cancer on clinical outcomes: systematic review and meta‐analysis. J Urol. 2017;197(3 Pt 1):548‐558. [DOI] [PubMed] [Google Scholar]
- 32. Gakis G, Fahmyb O. Systematic review and meta‐analysis on the impact of hexaminolevulinate‐ versus white‐light guided transurethral bladder tumor resection on progression in non‐muscle invasive bladder cancer. Bladder Cancer. 2016;2(3):293‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fukuhara H, Kurabayashi A, Furihata M, et al. 5‐aminolevulinic acid‐mediated photodynamic diagnosis using fluorescence ureterorenoscopy for urinary upper tract urothelial carcinoma ∼Preliminary prospective single centre trial∼. Photodiagnosis Photodyn Ther. 2020;29:101617. [DOI] [PubMed] [Google Scholar]
- 34. Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Cosmet Investig Dermatol. 2014;7:145‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Inoue K, Fukuhara H, Kurabayashi A, et al. Photodynamic therapy involves anti‐angiogenic mechanism and is enhanced by ferrochelatase inhibitor in urothelial carcinoma. Cancer Sci. 2013;104(6):765‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fukuhara H, Inoue K, Kurabayashi A, et al. The inhibition of ferrochelatase enhances 5‐aminolevulinic acid‐based photodynamic action for prostate cancer. Photodiagnosis Photodyn Ther. 2013;10(4):399‐409. [DOI] [PubMed] [Google Scholar]
- 37. Kelly JF, Snell ME. Hematoporphyrin derivative: a possible aid in the diagnosis and therapy of carcinoma of the bladder. J Urol. 1976;115(2):150‐151. [DOI] [PubMed] [Google Scholar]
- 38. Hisazumi H, Misaki T, Miyoshi N. Photoradiation therapy of bladder tumors. J Urol. 1983;130(4):685‐687. [DOI] [PubMed] [Google Scholar]
- 39. Kriegmair M, Baumgartner R, Lumper W, Waidelich R, Hofstetter A. Early clinical experience with 5‐aminolevulinic acid for the photodynamic therapy of superficial bladder cancer. Br J Urol. 1996;77(5):667‐671. [DOI] [PubMed] [Google Scholar]
- 40. Waidelich R, Stepp H, Baumgartner R, Weninger E, Hofstetter A, Kriegmair M. Clinical experience with 5‐aminolevulinic acid and photodynamic therapy for refractory superficial bladder cancer. J Urol. 2001;165(6 Pt 1):1904‐1907. [DOI] [PubMed] [Google Scholar]
- 41. Shackley DC, Briggs C, Gilhooley A, et al. Photodynamic therapy for superficial bladder cancer under local anaesthetic. BJU Int. 2002;89(7):665‐670. [DOI] [PubMed] [Google Scholar]
- 42. Berger AP, Steiner H, Stenzl A, Akkad T, Bartsch G, Holtl L. Photodynamic therapy with intravesical instillation of 5‐aminolevulinic acid for patients with recurrent superficial bladder cancer, a single‐center study. Urology. 2003;61:338‐341. [DOI] [PubMed] [Google Scholar]
- 43. Waidelich R, Beyer W, Knüchel R, et al. Whole bladder photodynamic therapy with 5‐aminolevulinic acid using a white light source. Urology. 2003;61(2):338‐341. [DOI] [PubMed] [Google Scholar]
- 44. Skyrme RJ, French AJ, Datta SN, Allman R, Mason MD, Matthews PN. A phase‐1 study of sequential mitomycin C and 5‐aminolaevulinic acid‐mediated photodynamic therapy in recurrent superficial bladder carcinoma. BJU Int. 2005;95(9):1206‐1210. [DOI] [PubMed] [Google Scholar]
- 45. Inoue K. 5‐Aminolevulinic acid‐mediated photodynamic therapy for bladder cancer. Int J Urol. 2017;24(2):97‐101. [DOI] [PubMed] [Google Scholar]
- 46. Bader MJ, Stepp H, Beyer W, et al. Photodynamic therapy of bladder cancerda phase I study using hexaminolevulinate (HAL). Urol Oncol. 2013;31(7):1178‐1183. [DOI] [PubMed] [Google Scholar]
- 47. Lee JY, Diaz RR, Cho KS, et al. Efficacy and safety of photodynamic therapy for recurrent, high grade nonmuscle invasive bladder cancer refractory or intolerant to bacille Calmette‐Guérin immunotherapy. J Urol. 2013;190(4):1192‐1199. [DOI] [PubMed] [Google Scholar]
- 48. van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol. 2005;47(6):736‐748. [DOI] [PubMed] [Google Scholar]
- 49. Kojima T, Nishiyama H, Ozono S, et al. Clinical evaluation of two consecutive UroVysion fluorescence in situ hybridization tests to detect intravesical recurrence of bladder cancer: a prospective blinded comparative study in Japan. Int J Clin Oncol. 2018;23(6):1140‐1147. [DOI] [PubMed] [Google Scholar]
- 50. Ikeda A, Kojima T, Kawai K, et al. Risk for intravesical recurrence of bladder cancer stratified by the results on two consecutive UroVysion fluorescence in situ hybridization tests: a prospective follow‐up study in Japan. Int J Clin Oncol. 2020;25(6):1163‐1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nagai T, Okamura T, Yanase T, et al. Examination of diagnostic accuracy of UroVysion fluorescence in situ hybridization for bladder cancer in a single community of Japanese hospital patients. Asian Pac J Cancer Prev. 2019;20(4):1271‐1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ogura S. Photodynamic screening of tumor using aminolevulinic acid. In: Okura I, Tanaka T, eds. Part IV Application of Aminolevulinic Acid for Tumor Diagnosis and Therapy. 15. Photodynamic Screening of Tumors Using Aminolevulinic Acid. Aminolevulinic Acid –Science, Technology and Application. SBI ALApromo; 2011:169‐176. [Google Scholar]
- 53. Ogura S, Ishizuka M, Mizokami Y, et al. Analysis of porphyrins in mouse plasma after administration of 5‐aminolevulinic acid as a potential tumor marker. Porphyrins. 2009;18(2–3):25‐30. [Google Scholar]
- 54. Ishizuka M, Hagiya Y, Mizokami Y, et al. Porphyrins in urine after administration of 5‐aminolevulinic acid as a potential tumor marker. Photodiagnosis Photodyn Ther. 2011;8(4):328‐331. [DOI] [PubMed] [Google Scholar]
- 55. Inoue K, Ota U, Ishizuka M, et al. Porphyrins as urinary biomarkers for bladder cancer after 5‐aminolevulinic acid administration: the potential of photodynamic screening for tumors. Photodiagnosis Photodyn Ther. 2013;10(4):484‐489. [DOI] [PubMed] [Google Scholar]
- 56. Kamada Y, Murayama Y, Ota U, et al. Urinary 5‐aminolevulinic acid concentrations as a potential tumor marker for colorectal cancer screening and recurrence. Anticancer Res. 2016;36(5):2445‐2450. [PubMed] [Google Scholar]
- 57. Ikeura T, Hori Y, Mitsuyama T, et al. Effectiveness of photodynamic screening using 5‐aminolevulinic acid for the diagnosis of pancreatic cancer. Anticancer Res. 2020;40(6):3571‐3577. [DOI] [PubMed] [Google Scholar]


