Abstract
A cDNA expression library prepared from Babesia gibsoni merozoite mRNA was screened with B. gibsoni-infected dog serum. cDNA encoding a 50-kDa protein was cloned and designated the P50 gene. The complete nucleotide sequence of the P50 gene was 1,922 bp. Computer analysis suggested that the sequence of the P50 gene contained an open reading frame of 1,401 bp with a coding capacity of approximately 50 kDa. The complete genomic nucleotide sequence of the P50 gene has been analyzed and shown to contain a single intron of 37 bp. Southern blotting analysis indicated that the P50 gene was present at a single copy in the B. gibsoni genome. The native P50 protein of B. gibsoni with a molecular mass of 50 kDa was identified by Western blotting with anti-recombinant P50 mouse serum. Confocal laser microscopic analysis showed that the P50 protein was located on the surface of B. gibsoni merozoites. The recombinant P50 protein expressed by baculovirus in insect cells was used as the antigen in an enzyme-linked immunosorbent assay (ELISA). The ELISA was able to differentiate between B. gibsoni-infected dog serum and B. canis-infected dog serum or noninfected dog serum. Furthermore, the antibody response against the recombinant P50 protein was maintained until the chronic stage of infection in dogs experimentally infected with B. gibsoni was developed. These results demonstrate that the recombinant P50 protein might be a useful diagnostic reagent for detection of antibodies to B. gibsoni in dogs.
Babesia gibsoni is a tick-borne hemoprotozoan parasite which causes a piroplasmosis in dogs. The disease is characterized by remittent fever, progressive anemia, hemoglobinuria, and marked splenomegaly and hepatomegaly and sometimes causes death. B. gibsoni infection is endemic in many regions of Asia, Africa, Europe, and the Americas (7, 21, 25). Recently, this disease has been found to occur frequently in companion animals and has become a big problem clinically (4, 10). In chronically infected dogs, the disease recurs and causes advanced anemia after an operation or while a dog is on immunosuppressive therapy. Therefore, the diagnosis and detection of dogs that are carriers of this disease or that have a chronic form of this disease are very important. Generally, the diagnosis of acute babesiosis is carried out by detection of intraerythrocytic Babesia organisms by microscopy of a Giemsa-stained thin blood smear film. However, detection of intraerythrocytic Babesia organisms is very difficult in dogs with inapparent or chronic infection because of low levels of parasitemia.
Recently, it has become possible to detect Babesia infection in an animal by PCR (6, 16) or indirectly by measurement of antibody levels by serological tests (20, 26). PCR offers the advantages of high degrees of sensitivity and specificity, but the disadvantage of the test is the requirement for specialized laboratory equipment and facilities and well-trained laboratory personnel. On the other hand, the indirect fluorescent-antibody test (IFAT) and enzyme-linked immunosorbent assay (ELISA) with whole parasite as the antigen have been used for serological diagnosis of B. gibsoni infection (5, 6, 26). These tests are particularly useful for identification of chronically infected dogs with significantly low levels of parasitemia. In general, IFAT and ELISA for babesial parasites are highly sensitive but only moderately specific because of antigenic cross-reactions with other closely related Babesia species (26). In addition, when whole parasites are used as antigens, their quantities can vary from batch to batch. Also, the production of antigen for these tests requires experimentally infected dogs, making production time-consuming and expensive. Moreover, the serum from B. gibsoni-infected dogs sometimes cross-reacts with erythrocytes from healthy dogs or B. canis (1, 2, 3, 26). Therefore, the development of a high-quality system is required for the diagnosis of B. gibsoni infection.
In the present study, in order to isolate a large amount of antigen that is significantly recognized by B. gibsoni-infected dog serum, we have screened a cDNA library prepared from B. gibsoni merozoite mRNA with sera derived from dogs experimentally infected with B. gibsoni and identified a major surface antigen designated P50. Our data indicate that the recombinant P50 protein expressed in insect cells by baculovirus is a useful diagnostic reagent for the detection of antibodies to B. gibsoni.
MATERIALS AND METHODS
Parasite.
A B. gibsoni strain isolated from a hunting dog in the Hyougo Prefecture of Japan, designated strain NRCPD (14), was used to experimentally infect splenectomized beagles or SCID mice whose red blood cells were replaced by canine red blood cells and was maintained in these animals as described previously (12). The B. gibsoni-infected dog erythrocytes were collected from the experimentally infected dog at peak parasitemia (14%) and stored at −80°C.
Dogs.
One-year-old beagle dogs were used. The dogs were confirmed to be free of natural B. gibsoni infection by detection of specific antibody prior to use in the experiments.
Construction and immunoscreening of cDNA expression library.
Total RNA was prepared from B. gibsoni-infected dog erythrocytes (erythrocyte volume, 10 ml; parasitemia, 14%) by acid guanidinium thiocyanate-phenol-chloroform extraction methods (8), and then polyadenylated RNA was purified with Oligotex-dT 30 (Takara, Tokyo, Japan). The cDNA was synthesized by using a Zap-cDNA synthesis kit, ligated to a λ Zap II phage expression vector, and packaged by using a Gigapack III packaging system (Stratagene, San Diego, Calif.). The cDNA library (105 PFU) was screened with serum from a B. gibsoni-infected dog. Immunoscreening of the cDNA expression library was performed as described previously (13, 19).
cDNA sequencing.
Restriction enzyme-generated fragments for sequencing were subcloned into pBluescript SK(+) vectors. Nucleotide sequencing of both strands was performed with double-stranded plasmid templates by the Taq polymerase cycle sequencing method with Taq polymerase supplied by Applied Biosystems (Foster City, Calif.), and then analyzed with a model 377A ABI sequencer (Applied Biosystems). Sequence data were analyzed with a computer program (MacVector, version 6.5.3; Oxford Molecular, Hunt Valley, Calif.).
Isolation of the P50 genomic clone.
As shown in Table 1, two sets of oligonucleotide primers derived from P50 cDNA were used. The nucleotide sequences of each primer, including an EcoRI restriction enzyme site and their corresponding positions on cDNA, are indicated in Table 1. The amplified products were inserted into the EcoRI site of pBluescript SK(+) and sequenced with M13 reverse and universal primers as described above.
TABLE 1.
Primers used for gene sequencing
| Primer group and primer | Sequence (5′-3′)a | Corresponding position on cDNA |
|---|---|---|
| Group I | ||
| F1 | ACGAATTC TAATATGAATGTCGTT | 24–39 |
| R1 | ACGAATTC TGGAGCTTCTGCACGT | 1323–1338 |
| Group II | ||
| F2 | TCGAATTC TAAGCTTGAGGTAGCAGT | 939–956 |
| R2 | TCGAATTC AGCTTAAAAGACAGCGAT | 1414–1431 |
P50 sequences representing restriction enzyme sites are shown in italics.
Northern and Southern blotting.
Northern blotting and Southern blotting were performed as described previously (11, 13, 18).
Expression of the P50 gene in Escherichia coli
The P50 gene inserted into pBluescript SK(+) vectors was subcloned into plasmid pGEMEX-2 (Promega, Madison, Wis.) of the bacterial expression vector after digestion with EcoRI and XhoI. The resulting plasmid, pGEMEX-2/P50, was checked for accurate insertion by restriction enzyme analysis. The recombinant protein was expressed as a fusion protein of the bacteriophage T7 gene 10 protein in E. coli JM109 (DE3) according to the instructions of the manufacturer (Promega) and designated the gene 10-P50 protein.
Production of anti-gene 10-P50 serum.
Antiserum against the gene 10-P50 protein was produced in mice. One hundred micrograms of the recombinant fusion protein in Freund's complete adjuvant (Difco Laboratories, Detroit, Mich.) was intraperitoneally injected into mice (BALB/c mice; age, 8 weeks). The same antigen in Freund's incomplete adjuvant (Difco) was intraperitoneally injected into the mice on day 14 and again on day 28. Sera were collected from immunized mice 14 days after the last immunization.
Expression of P50 gene in insect cells.
The entire P50 gene in pBluescript SK(+) vectors was recovered after digestion with EcoRI and XhoI, blunt ended with a Klenow fragment of DNA polymerase, and then ligated into the SmaI site of baculovirus transfer vector pBacPAK8 (Clontech, Palo Alto, Calif.). The structure of recombinant plasmid pBP50 was checked by restriction enzyme analysis. Construction of a recombinant baculovirus carrying the P50 gene (AcP50) was performed as described previously (22, 23, 24).
Production of anti-SfP50 serum.
Spodoptera frugiperda (Sf9) cells infected with AcP50 (SfP50) were cultured for 4 days and washed three times with phosphate-buffered saline (PBS) by centrifugation. The resulting pellets were frozen and thawed three times. The cell lysate antigens were used for immunization of mice as described above.
IFAT and confocal laser microscopic observation.
A thin blood smear film of a B. gibsoni-infected blood sample collected from a B. gibsoni-infected SCID mouse whose red blood cells were replaced by canine red blood cells was fixed with methanol for 20 min and incubated with anti-SfP50 serum at 37°C for 1 h. The slide was washed with PBS for 10 min and incubated with fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G antibody (Bethyl Laboratories) at 37°C for 1 h. Then, the slide was washed with PBS for 10 min and incubated with propidium iodide (Molecular Probes, Eugene, Oreg.) and RNase A (50 μg/ml) for 10 min and mounted in 50% glycerol for confocal laser microscopic observation.
Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting were carried out as described previously (15, 23).
ELISA.
Sf9 cells infected with AcP50 were cultured in TC-100 medium containing 10% fetal calf serum for 4 days. The cells were centrifuged at 10,000 × g for 10 min after they were washed two times with PBS and lysed with 1% Triton X-100. The supernatant was dialyzed against antigen coating buffer (0.05 M carbonate-bicarbonate buffer [pH 9.6]) and then used as the antigen for ELISA. The ELISA was performed as described previously (24).
Sera.
Serum samples from six dogs experimentally infected with B. gibsoni (NRCPD strain) or a dog experimentally infected with B. canis and negative serum samples from healthy dogs were used. Eight serum samples from field dogs infected with B. gibsoni were also used.
Nucleotide sequence accession number.
The sequence of the P50 gene of B. gibsoni has been submitted to the DDBJ database under accession no. AB051834.
RESULTS
Cloning and sequencing of P50 cDNA clones.
A total of 22 positive clones were obtained by immunoscreening of the cDNA expression library prepared from B. gibsoni (105 PFU) with dog antisera raised against B. gibsoni. The insert sizes for these clones ranged from 1,500 to 2,500 bp. Phagemids were excised from the clones and partially sequenced with M13 and universal primers. The clones were categorized into eight groups, and two clones, clones 1 and 20, from one group were chosen for further analysis. The two clones with insert DNAs that were digested with HindIII and subcloned into the HindIII site of pBluescript SK(+) vectors were completely sequenced with M13 and universal primers. The cDNA sequence of clone 20 is shown in Fig. 1. Starting with methionine at position 28, a single open reading frame of 1,401 nucleotides was present. The open reading frame encodes a polypeptide of 466 amino acid residues, with a size of 50 kDa, as calculated with a computer, and the gene was designated P50. A computer-aided search of the GenBank databases by BLAST analysis did not reveal any known homologous genes from other species including protozoa.
FIG. 1.
Nucleotide sequence of the coding region of the P50 cDNA and its flanking sequences. The predicted amino acid is indicated below each codon.
Characterization of P50 gene.
A probe from a P50 cDNA clone was hybridized to the total RNA isolated from B. gibsoni merozoites by Northern blotting. The mRNA of the P50 gene is about 2.3 kb (data not shown).
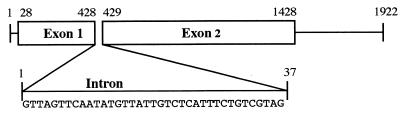
A probe derived from the P50 cDNA clone was hybridized to B. gibsoni DNA fragments by Southern blotting. Genomic DNA was digested with the restriction enzymes EcoRI, BamHI, BglII, and BclI. The cDNA sequence, which did not contain any EcoRI or BamHI sites, contained only a single BglII site and a single BclI site. Only one band was obtained in EcoRI and BamHI digestions, and two bands were obtained in BglII and BclI digestions (data not shown). These results suggested that the P50 gene occurs as a single copy in the genome of B. gibsoni. B. gibsoni genomic DNA was amplified by PCR with two sets of primers, namely, primers of groups I and II (Table 1). The resulting DNA fragments were ca. 1,300 and 500 bp. These amplified DNA fragments were molecularly cloned into each plasmid vector. The plasmids containing genes from each representative group were isolated and subjected to DNA sequencing analysis. Comparisons of the sequences of the plasmids with the P50 cDNA revealed interruption of the coding region by a 37-bp intron, starting at position 428 and ending at position 429 of the P50 cDNA sequence (Fig. 2). The sequence of the splice junctions of this intron was similar to those found in other species of protozoan parasites (9, 17).
FIG. 2.
Structure and nucleotide sequence of the genomic P50 gene. The nucleotide sequence of the intron is shown.
Expression of P50 in E. coli by pGEMEX-2 vector.
The P50 gene was ligated into the bacterial expression vector pGEMEX-2, and then P50 was expressed as a fusion protein with the bacteriophage T7 gene 10 protein in E. coli. The molecular masses of the gene 10 and the gene 10-P50 fusion proteins were estimated to be 35 and 85 kDa, respectively, as expected (data not shown). Mice immunized with the gene 10-P50 fusion protein induced specific antibodies against B. gibsoni by IFAT (data not shown).
Identification of native P50 protein.
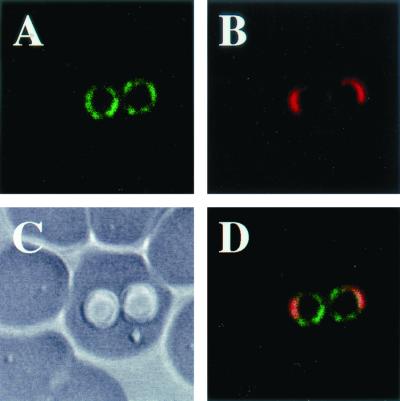
The lysates of B. gibsoni-infected dog erythrocytes were analyzed by Western blotting with mouse antisera against the gene 10-P50 protein. As shown in Fig. 3, a specific band of 50 kDa was detected in B. gibsoni-infected erythrocytes. In addition, the 50-kDa band was detected as a major antigen in Western blotting with B. gibsoni-infected dog serum (data not shown). To determine the localization of the P50 protein in parasites, B. gibsoni merozoites were examined by IFAT with anti-SfP50 serum (see below) with a confocal laser microscope. As shown in Fig. 4, the specific fluorescence was localized on the parasite cell surface.
FIG. 3.
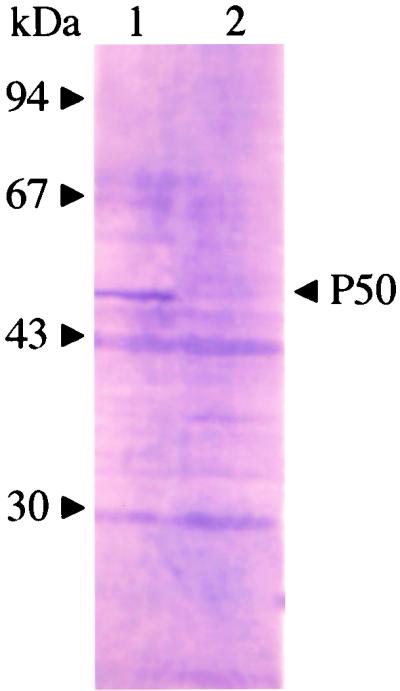
Western blots of native P50 protein obtained with mouse anti-gene 10-P50 serum. Lane 1, B. gibsoni-infected erythrocytes; lane 2, erythrocytes from healthy dogs. The positions of the molecular mass standards are indicated on the left.
FIG. 4.
Localization of antigens recognized by anti-SfP50 mouse serum in confocal laser micrographs. (A) Immunofluorescent staining of B. gibsoni merozoites with anti-SfP50 mouse serum. (B) Propidium iodide staining of B. gibsoni merozoite nuclei. (C) The phase-contrast images of B. gibsoni merozoites. (D) Panels A and B overlaid on panel C. The images were derived from a single section.
Expression of P50 in insect cells by recombinant baculovirus.
Sf9 cells were infected at 10 PFU/cell with AcP50 or with a control recombinant baculovirus carrying the GFP gene (AcGFP). After 4 days of incubation, cells infected with AcP50 were analyzed by Western blotting with B. gibsoni-infected dog serum. As shown in Fig. 5, bands with molecular masses of 27 to 51 kDa were detected in both solubilized and unsolubilized fractions of cells lysed with 1% Triton X-100. The smaller proteins (less than 51 kDa) found in infected cell extracts might be the degradation products of the 51-kDa protein. The molecular mass of the recombinant P50 protein was similar to that of the native P50 protein from B. gibsoni.
FIG. 5.
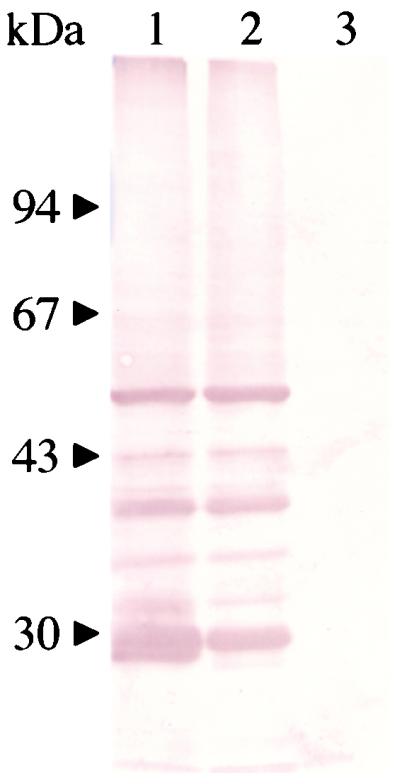
Western blots of the recombinant P50 protein expressed in insect cells with B. gibsoni-infected dog serum. Lane 1, solubilized fraction of AcP50-infected cells; lane 2, unsolubilized fraction of AcP50-infected cells; lane 3, AcGFP-infected cells. The positions of the molecular mass standards are indicated on the left.
Diagnosis of B. gibsoni infection in dogs by ELISA with recombinant P50 as antigen.
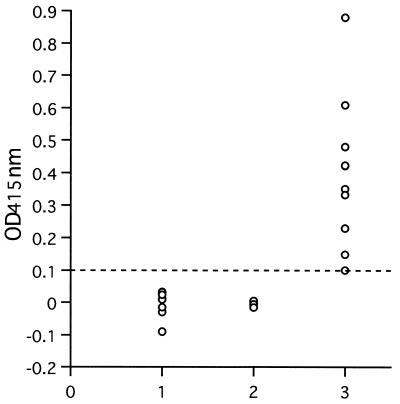
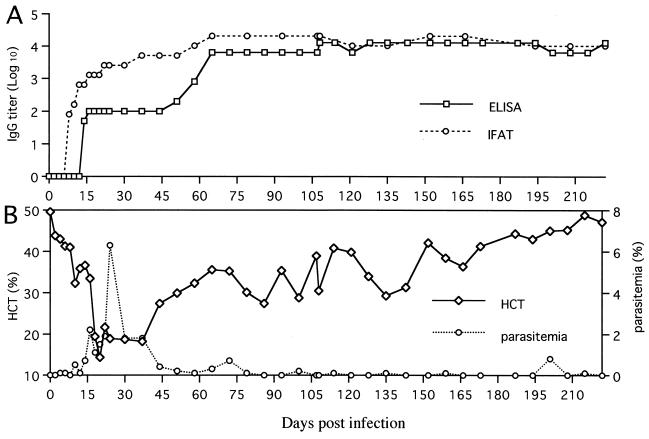
To evaluate whether recombinant P50 expressed by baculovirus can be a suitable antigen for diagnosis of B. gibsoni infection in dogs, the solubilized fraction from AcP50-infected cells was tested by ELISA. As shown in Fig. 6, all serum samples from dogs experimentally infected with B. gibsoni were positive (optical density, >0.1), whereas the serum samples from uninfected dogs and B. canis-infected dogs were negative (optical density, <0.1). A dog experimentally infected with B. gibsoni developed a significant antibody response to P50 antigen by day 14, as determined by the ELISA (Fig. 7A). The antibody response was maintained at high levels until 222 days postinfection, even if it became the chronic stage of infection, which was characterized by a recovering hematocrit rate and a significantly low level of parasitemia (Fig. 7B). By IFAT with B. gibsoni-infected erythrocytes as the antigen, antibodies to B. gibsoni were detected from 8 to 222 days postinfection (Fig. 7A). In addition, all eight serum samples from field dogs infected with B. gibsoni were positive by the ELISA (data not shown).
FIG. 6.
Values from ELISA with recombinant P50 protein and experimentally infected dog sera. Lane 1, sera from healthy dogs; lane 2, B. canis-infected dog sera; lane 3, B. gibsoni-infected dog sera. OD415nm, optical density at 415 nm.
FIG. 7.
Detection of antibody to recombinant P50 in a dog experimentally infected with B. gibsoni by ELISA and IFAT (A). (B) Parasitemia and hematocrit (HCT) rates. IgG, immunoglobulin G.
DISCUSSION
In the present report we describe the cloning and molecular characterization of a gene encoding a 50-kDa major surface antigen of B. gibsoni. A cDNA expression library prepared from B. gibsoni merozoite mRNA was screened with serum from dogs experimentally infected with B. gibsoni in order to identify antigens that induce high-level antibody responses. This led to the isolation of an antigen called P50, which exhibited strong immunoreactivity with B. gibsoni-infected dog serum. The cDNA encoded a polypeptide of 466 amino acid residues and of 50 kDa, as calculated with a computer. The P50 gene is present at a single copy and contains an intron of 37 bp. The size of the transcription product of the P50 gene was about 2.3 kb, which was slightly larger than the size of the transcription product of the cDNA clone. This result indicated that the P50 gene consists of a single transcription unit. The native protein encoded by the P50 gene, which has a molecular mass of 50 kDa, was detected by Western blotting with anti-gene 10-recombinant P50 serum. The molecular mass of 50 kDa was identical to the expected size obtained from the amino acid sequence.
In the host-parasite interaction, the surface proteins of parasite cells are the main targets of host immune responses, and the surface antigens of the parasites are therefore logical targets for use as subunit vaccines and diagnostic reagents. In the present study, the P50 protein was identified as the major surface antigen on B. gibsoni merozoite cells. Therefore, the recombinant P50 protein expressed by baculovirus was evaluated for its diagnostic potential in an ELISA. The recombinant P50 protein had an apparent molecular mass of 51 kDa, which was similar to that of the native P50 protein of B. gibsoni. In addition, the recombinant P50 protein reacted strongly with B. gibsoni-infected dog serum by Western blotting. These results indicated that the P50 protein expressed in insect cells is similar to the native P50 protein in terms of its molecular structure and antigenicity. By the ELISA, only B. gibsoni-infected dog serum showed a strong reactivity to the recombinant P50 protein, but B. canis-infected dog serum and serum from healthy dogs did not. The sequential serum samples derived from a dog experimentally infected with B. gibsoni became positive at 14 days, and ELISA antibody titers increased thereafter until 222 days postinfection. Sera obtained from the dog at the late stage of infection had stronger reactivity to the recombinant P50 protein than those from the dog at the early stage. In a comparison of ELISA and IFAT, antibodies that recognized B. gibsoni were detected earlier by IFAT than by the ELISA. It may be because IFAT detects many antibodies to different antigens, whereas the ELISA detects limited antibodies to a single antigen. However, in the chronic stages of infection, the antibody titers were almost the same by ELISA and IFAT. This result suggested that the ELISA with the recombinant P50 protein as the antigen has a good potential to detect antibody in dogs chronically infected with B. gibsoni. Furthermore, eight samples from field dogs infected with B. gibsoni showed strong reactivities to the recombinant P50 by ELISA. These results indicated that the recombinant P50 protein expressed in insect cells is suitable for detection of acute and chronic stages of B. gibsoni infection in dogs. Further studies by ELISA with the recombinant P50 protein and large numbers of serum samples from B. gibsoni-infected dogs in the field are necessary. Recently, it was reported that B. gibsoni strains isolated in North America and Asia belong to different species (27, 28); therefore, we should carry out experiments to confirm the reactivity of the recombinant P50 protein with sera from B. gibsoni-infected dogs from North America.
In the present study, mice inoculated with both recombinant P50 expressed in E. coli and insect cells had high titers of antibody against blood merozoites of B. gibsoni. Our next step will be to implement immunization trials with dogs to determine the potency of the recombinant P50 protein produced in E. coli and insect cells as a potential subunit vaccine to control canine B. gibsoni infection.
ACKNOWLEDGMENT
This work was supported by grants from the Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
- 1.Adachi K, Makimura S. Changes in anti-erythrocyte membrane antibody level of dogs experimentally infected with Babesia gibsoni. J Vet Med Sci. 1992;54:1221–1223. doi: 10.1292/jvms.54.1221. [DOI] [PubMed] [Google Scholar]
- 2.Adachi K, Tateishi M, Horii Y, Nagatomo H, Shimizu T, Makimura S. Reactivity of serum anti-erythrocyte membrane antibody in Babesia gibsoni-infected dogs. J Vet Med Sci. 1994;56:997–999. doi: 10.1292/jvms.56.997. [DOI] [PubMed] [Google Scholar]
- 3.Adachi K, Tateishi M, Horii Y, Nagatomo H, Shimizu T, Makimura S. Immunologic characteristics of anti-erythrocyte membrane antibody produced in dogs during Babesia gibsoni infection. J Vet Med Sci. 1995;57:121–123. doi: 10.1292/jvms.57.121. [DOI] [PubMed] [Google Scholar]
- 4.Adachi K, Ueno C, Makimura S. Immunosuppression in dogs naturally infected with Babesia gibsoni. J Vet Med Sci. 1993;55:503–505. doi: 10.1292/jvms.55.503. [DOI] [PubMed] [Google Scholar]
- 5.Anderson J F, Magnarelli L A, Sulzer A J. Canine babesiosis: indirect fluorescent antibody test for a North American isolate of Babesia gibsoni. Am J Vet Res. 1980;41:2102–2105. [PubMed] [Google Scholar]
- 6.Bose R, Jorgensen W K, Dalgliesh R J, Friedhoff K T, de Vos A J. Current state and future trends in the diagnosis of babesiosis. Vet Parasitol. 1995;57:61–74. doi: 10.1016/0304-4017(94)03111-9. [DOI] [PubMed] [Google Scholar]
- 7.Casapulla R, Baldi L, Avallone V, Sannino R, Pazzanese L, Mizzoni V. Canine piroplasmosis due to Babesia gibsoni: clinical and morphological aspects. Vet Rec. 1998;142:168–169. doi: 10.1136/vr.142.7.168. [DOI] [PubMed] [Google Scholar]
- 8.Chomczynski P, Sacci N. Single step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 9.Donald R G, Roos D S. Homologous recombination and gene replacement at the dihydrofolate reductase-thymidylate synthase locus in Toxoplasma gondii. Mol Biochem Parasitol. 1994;63:243–253. doi: 10.1016/0166-6851(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 10.Farwell G E, LeGrand E K, Cobb C C. Clinical observations on Babesia gibsoni and Babesia canis infections in dogs. J Am Vet Med Assoc. 1982;180:507–511. [PubMed] [Google Scholar]
- 11.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 12.Fukumoto S, Xuan X, Igarashi I, Zhang S, Mugisha J, Ogata T, Nagasawa H, Fujisaki K, Suzuki N, Mikami T. Morphological changes of Babesia gibsoni proliferated in canine red blood cell-substituted severe combined immunodeficiency mice. J Parasitol. 2000;86:956–959. doi: 10.1645/0022-3395(2000)086[0956:MCOBGG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Ikadai H, Xuan X, Igarashi I, Tanaka S, Kanemaru T, Nagasawa H, Fujisaki K, Suzuki N, Mikami T. Cloning and expression of a 48-kilodalton Babesia caballi merozoite rhoptry protein and potential use of the recombinant antigen in an enzyme-linked immunosorbent assay. J Clin Microbiol. 1999;37:3475–3480. doi: 10.1128/jcm.37.11.3475-3480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishimine T, Makimura S, Kitazawa S, Tamura S, Suzuki N. Pathophysiological findings on blood of beagles experimentally infected with B. gibsoni. Jpn J Trop Med Hyg. 1978;6:15–26. [Google Scholar]
- 15.Martin W J, Finerty J, Rosenthal A. Isolation of Plasmodium berghei (malaria) parasites by ammonium chloride lysis of infected erythrocytes. Nat New Biol. 1971;233:260–261. doi: 10.1038/newbio233260a0. [DOI] [PubMed] [Google Scholar]
- 16.Persing D H, Mathiesen D, Marshall W F, Telford S R, Spielman A, Thomford J W, Conrad P A. Detection of Babesia microti by PCR. J Clin Microbiol. 1992;30:2097–2103. doi: 10.1128/jcm.30.8.2097-2103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prasartkaew S, Zijlstra N M, Wilairat P, Overdulve J P, de Vries E. Molecular cloning of a Plasmodium falciparum gene interrupted by 15 introns encoding a functional primase 53 kDa subunit as demonstrated by expression in a baculovirus system. Nucleic Acids Res. 1996;24:3934–3941. doi: 10.1093/nar/24.20.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 19.Short J M, Sorge J A, Huse W D. λZAP: a bacteriophage with in vivo excision properties. Nucleic Acids Res. 1988;16:7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waltisbuhl D J, Goodger B V, Wright I G, Commins M A, Mahoney D F. An enzyme linked immunosorbent assay to diagnose Babesia bovis infection in cattle. Parasitol Res. 1987;73:126–131. doi: 10.1007/BF00536468. [DOI] [PubMed] [Google Scholar]
- 21.Wozniak E J, Barr B C, Thomford J W, Yamane I, McDonough S P, Moore P F, Naydan D, Robinson T W, Conrad P A. Clinical, anatomic, and immunopathologic characterization of Babesia gibsoni infection in the domestic dog (Canis familiaris) J Parasitol. 1997;83:692–699. [PubMed] [Google Scholar]
- 22.Xuan X, Nakamura T, Ihara T, Sato I, Tuchiya K, Nosetto E, Ishihama A, Ueda S. Chracterization of pseudorabies virus glycoprotein gII expressed by recombinant baculovirus. Virus Res. 1995;36:151–161. doi: 10.1016/0168-1702(94)00112-p. [DOI] [PubMed] [Google Scholar]
- 23.Xuan X, Maeda K, Mikami T, Otsuka H. Characterization of canine herpesvirus glycoprotein C expressed in insect cells. Virus Res. 1996;46:57–64. doi: 10.1016/s0168-1702(96)01374-3. [DOI] [PubMed] [Google Scholar]
- 24.Xuan X, Larsen A, Ikadai H, Tanaka T, Igarashi I, Nagasawa H, Fujisaki K, Toyoda Y, Suzuki N, Mikami T. Expression of Babesia equi merozoite antigen 1 in insect cells by a recombinant baculovirus and evaluation of its diagnostic potential in an enzyme-linked immunosorbent assay. J Clin Microbiol. 2001;39:705–709. doi: 10.1128/JCM.39.2.705-709.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamane I, Conrad P A, Gardner I. Babesia gibsoni infections in dogs. J Protozool Res. 1993;3:111–125. [PubMed] [Google Scholar]
- 26.Yamane I, Thomford J W, Gardner I A, Dubey J P, Levy M, Conrad P A. Evaluation of the indirect fluorescent antibody test for diagnosis of Babesia gibsoni infections in dogs. Am J Vet Res. 1993;54:1579–1584. [PubMed] [Google Scholar]
- 27.Zahler M, Rinder H, Schein E, Gothe R. Detection of a new pathogenic Babesia microti-like species in dogs. Vet Parasitol. 2000;89:241–248. doi: 10.1016/s0304-4017(00)00202-8. [DOI] [PubMed] [Google Scholar]
- 28.Zahler M, Rinder H, Zweygarth E, Fukata T, Maede Y, Schein E, Gothe R. ‘Babesia gibsoni ’ of dogs from North America and Asia belong to different species. Parasitology. 2000;120:365–369. doi: 10.1017/s0031182099005557. [DOI] [PubMed] [Google Scholar]