Abstract
Either PCR-mediated single strand conformation polymorphism (SSCP) analysis or DNA sequencing of rpoB DNA (157 bp) can be used as a rapid screening method for the detection of mutations related to the rifampin resistance of Mycobacterium tuberculosis. However, due to the nonspecific amplification of rpoB DNA from nontuberculous mycobacteria these methods cannot be directly applied to clinical specimens such as sputa. We developed a nested PCR method that can specifically amplify the rpoB DNA of M. tuberculosis on the basis of rpoB DNA sequences of 44 mycobacteria. Nested PCR-linked SSCP analysis and the DNA sequencing method were applied directly in order to detect M. tuberculosis and determine its rifampin susceptibility in 56 sputa. The results obtained by nested PCR-SSCP and DNA sequencing were concordant with those of conventional drug susceptibility testing and DNA sequencing performed with culture isolates.
Mycobacterium tuberculosis is still regarded as a causative agent of high morbidity and mortality throughout the world (5). Due to the spread of human immunodeficiency virus infection, the decline in the incidence of tuberculosis which had been brought about by advanced antituberculosis chemotherapy and improved living conditions was reversed in the mid-1980s. Human immunodeficiency virus-related tuberculosis led to a rise in the frequency of multidrug-resistant M. tuberculosis (1, 7). The rapid detection of resistance to first-line drugs such as rifampin and isoniazid is essential for the efficient control of multidrug-resistant strains (3, 9, 16). Although the period required for culturing is shortened by the BACTEC system, drug susceptibility testing in a liquid medium still requires 1 to 2 weeks for final determination and report to the clinicians (19), calling for further reduction of the detection period.
Recently, molecular methods exploiting the genetic mechanism of drug resistance have markedly improved the diagnosis of drug-resistant tuberculosis (17, 20). Rifampin resistance of M. tuberculosis is largely associated with point mutations in a region of rpoB (20), mutations which cause rifampin resistance to a high level in Escherichia coli (10, 14). Various molecular methods have been applied to detect these unique mutations, including PCR-mediated single-strand conformation polymorphism (SSCP) analysis (12, 15, 21), single-tube heminested PCR-SSCP (23), the dideoxy fingerprinting method (6), line probe assay (4), and DNA sequence analysis (11, 20). For the rapid detection or determination of rifampin resistance, it is desirable to apply these methods directly to primary specimens, such as sputa, as well as cultures. Among these methods, PCR-SSCP may be the most cost-effective method for detecting point mutations within the 69-nucleotide region. However, the direct detection of rifampin-resistant M. tuberculosis in sputa by conventional PCR-SSCP has a drawback in that it lacks sensitivity. Nonspecific amplification due to the highly conserved sequences of rpoB DNA among GC-rich bacteria that may reside in the respiratory tract has led to difficulty in interpreting PCR-SSCP results (21). For this reason, earlier studies using this technique required the use of pure cultures of M. tuberculosis. To resolve these problems, a heminested PCR method based on M. tuberculosis signature nucleotides was introduced (23). Although heminested PCR was effective in amplifying M. tuberculosis-specific rpoB DNA directly from sputum samples, applying this method to SSCP analysis could not resolve mutations at codon 526 (in E. coli numbering). Furthermore, when compared with the conventional PCR-SSCP analysis of 157-bp DNA, this method required a longer electrophoresis time for the clear differentiation of bands in several resistant strains (6).
In this study, we developed a nested PCR that can specifically amplify the rpoB DNA of M. tuberculosis. Based on the 44 rpoB sequences of mycobacteria (11), we were able to design M. tuberculosis-specific primers for nested PCR. Nested PCR-linked SSCP analysis and a direct DNA sequencing method were applied to detect mutations of M. tuberculosis in sputa. This enabled us to detect mutations related to rifampin resistance occurring within the 69-nucleotide region of rpoB DNA derived from 56 sputa. The results were compared with those obtained by conventional PCR-SSCP, drug susceptibility testing, and the DNA sequence analysis of cultured M. tuberculosis from the same sputum samples.
MATERIALS AND METHODS
Mycobacteria and DNA preparation.
Twenty-eight reference strains (19 strains of mycobacteria and 9 strains of nonmycobacteria) and 48 clinical isolates of mycobacteria that had been isolated from patients with mycobacterial infections were used to determine the specificity of a first-round PCR (Table 1). Fifty-six sputum specimens from patients with suspected M. tuberculosis infections and M. tuberculosis isolates from culture of the same specimens were provided by the Korean Institute of Tuberculosis for the nested PCR-linked SSCP and direct sequencing. Amplification of IS6110 DNAs (536 bp) and positive cultures also supported the presence of M. tuberculosis in all of the sputa. Clinical isolates were identified by conventional biochemical tests and partial 16S rDNA sequencing.
TABLE 1.
Mycobacteria and nonmycobacteria used to determine the specificity of the first-round PCR
| Species | Type strainsa | No. of clinical isolatesb |
|---|---|---|
| Mycobacteria | ||
| M. avium | ATCC 25291 | 6 |
| M. fortuitum | ATCC 6841 | 4 |
| M. gordonae | ATCC 14470 | 2 |
| M. kansasii | ATCC 12478 | 5 |
| M. nonchromogenicum | ATCC 19530 | 0 |
| M. scrofulaceum | ATCC 19981 | 0 |
| M. smegmatis | ATCC 19420 | 0 |
| M. terrae | ATCC 15755 | 0 |
| M. triviale | ATCC 23292 | 0 |
| M. vaccae | ATCC 15483 | 0 |
| M. chelonae | ATCC 35749 | 3 |
| M. gastri | ATCC 15754 | 0 |
| M. intracellulare | ATCC 13950 | 8 |
| M. malmoense | ATCC 29571 | 0 |
| M. phlei | ATCC 11758 | 0 |
| M. simiae | ATCC 25275 | 0 |
| M. szulgai | ATCC 35799 | 0 |
| M. tuberculosis | ATCC 27294 | 20 |
| M. ulcerans | ATCC 19423 | 0 |
| Nonmycobacteria | ||
| Rhodococcus equi | IMSNU20114 | 0 |
| Rhodococcus erythropolis | IMSNU20115 | 0 |
| Rhodococcus rhodochrous | IMSNU20349 | 0 |
| Nocardia otitidiscaviarum | IMSNU21221 | 0 |
| Nocardia nova | IMSNU21197 | 0 |
| Corynebacterium diphtheriae | MSNU | 0 |
| Corynebacterium glutamicum | IMSNU21196 | 0 |
| Neisseria meningitidis | MSNU | 0 |
| Haemophilus influenzae | MSNU | 0 |
MSNU, Department of Microbiology, Seoul National University College of Medicine; IMSNU, Institute of Microbiology, Seoul National University.
Clinical isolates were identified by biochemical tests and partial 16S rDNA sequencing.
DNAs were purified by a previously described method (2, 11). A loopful culture of each strain was suspended with 200 μl of TEN buffer (10 mM Tris-HCl, 1 mM EDTA, 100 mM NaCl [pH 8.0]) and placed in a 2.0-ml screw-cap microcentrifuge tube filled with 100 μl (packed volume) of glass beads (diameter, 0.1 mm; Biospec Products, Bartlesville, Okla.), and then 200 μl of phenol-chloroform-isoamylalcohol (50:49:1) was added to this mixture. The screw-cap tube filled with the mixture was oscillated on a Mini-Bead beater (Biospec Products) for 1 min to disrupt the bacteria. The tube was centrifuged (12,000 × g, 5 min), and the aqueous phase was transferred into another clean tube, to which 10 μl of 3 M sodium acetate and 130 μl of isopropyl alcohol were added. The DNA pellet was washed with 70% ethanol and solubilized with 60 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0). Two microliters of purified DNA was used as the template in the PCRs. The sputa were processed by 1% NaOH for liquefaction-decontamination and sedimentation (12,000 × g, 15 min) (18). The sediments were resuspended in 1.5 ml of phosphate buffer (pH 6.8) and 0.5 ml was inoculated onto Löwenstein-Jensen media. DNAs were isolated from the residual sediments (1.0 ml) of smear-positive sputa as above and dissolved in 60 μl of TE buffer (pH 8.0). Two microliters of prepared DNA in TE buffer was used as the template in both the conventional PCR and the nested PCR.
Identification and rifampin susceptibility testing of M. tuberculosis.
Clinical isolates of M. tuberculosis were tested at the Korean Institute of Tuberculosis for drug susceptibility by agar dilution (1% proportion method) (13). Fifty-six sputa and the above-mentioned clinical isolates underwent nested PCR-SSCP and sequencing without prior information as to their rifampin susceptibility. Separate PCRs (22) were performed on these specimens to detect and identify M. tuberculosis in both sputa and cultures using an IS6110 kit (catalog no. N5811; Bioneer, Chungbuk, Korea) according to the manufacturer's instruction.
Conventional PCR-linked SSCP and DNA sequencing.
Conventional PCR-SSCP was performed as previously described, using TR9 and TR8 primers (12, 21). Separately, 56 culture isolates of M. tuberculosis derived from the same specimens were analyzed by conventional PCR-DNA sequencing. The amplified products were purified by QIAEX II gel elution system (QIAGEN, Hilden, Germany). Nucleotide sequences were directly determined from the purified PCR product (157 bp) with forward and reverse primers using an Applied Biosystems 373A automatic sequencer and BigDye Terminator Cycle Sequencing kit (PE Applied Biosystems, Warrington, United Kingdom). For the sequencing reaction, 60 ng of PCR-amplified DNA, 3.2 pmol of either the forward or the reverse primer and 8 μl of BigDye Terminator RR mix (part no. 4303153; PE Applied Biosystems) were mixed and adjusted to a final volume of 20 μl by adding distilled water. The reaction was run using 5% (vol/vol) dimethylsulfoxide for 30 cycles of 15 s at 95°C, 10 s at 50°C, and 4 min at 60°C. Both strands were sequenced for cross check.
Nested PCR-linked SSCP and DNA sequencing.
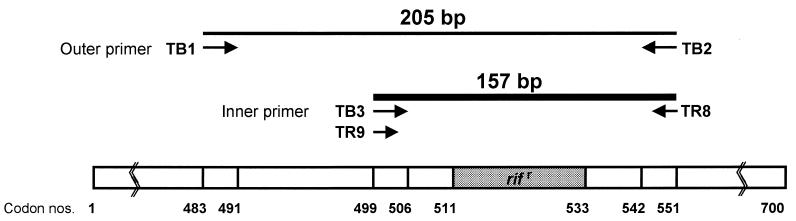
M. tuberculosis-specific nucleotides have been found in the rpoB sequences (8, 11). The outer primers TB1 (5′-ACGTGGAGGCGATCACACCGCAGACGT-3′) and TB2 (5′-TGCACGTCGCGGACCTCCAGCCCGGCA-3′) were modified from Rpo105 (23) and TR8, respectively (21) (modifications are in bold). The inner primers for the second-round PCR were TB3 (5′-TCGCCGCGATCAAGGAGTTCTTC-3′), which was modified from TR9, and TR8 (21) (Fig. 1). The first-round PCR was performed in a 20-μl PCR mixture tube (AccuPower PCR PreMix; Bioneer) containing 2 U of Taq polymerase, 10 mM Tris-HCl (pH 8.3), and 1.5 mM MgCl2, and 20 pmol of each primer was added. The volume was adjusted to 20 μl. The reaction mixture was subjected to 30 cycles of amplification (30 s at 95°C, 60 s at 78°C) followed by a 5-min extension at 78°C in a Perkin-Elmer Cetus Model 9600 thermalcycler. The first-round PCR product (205 bp) was diluted (100-fold) and used as a template for the second-round PCR (157 bp), which was performed in 30 cycles (30 s at 95°C, 1 min at 72°C) followed by a 5-min extension at 72°C. SSCP analysis was performed as described above, except that 0.1 μl (0.1 μCi) of [α-32P]dCTP (Amersham International) was added to the second reaction mixture. DNA sequencing was directly performed with nested PCR products as above.
FIG. 1.
Primers used for PCR and nested PCR. Conventional PCR was performed with the TR9-TR8 primer set as previously described. The first-round PCR was performed with the TB1-TB2 primer set amplifying 205-bp rpoB DNA. The second-round PCR was performed with the TB3-TR8 primer set to amplify 157-bp DNA from the first-round PCR product. Numbers indicate rpoB codons of E. coli.
Southern blotting.
First-round PCR products were electrophoresed on a 1.5% (wt/vol) agarose gel (Sigma, Steinheim, Germany). The DNA on agarose gel was denatured with a solution of 1.5 M NaCl and 0.5 N NaOH and neutralized with a solution of 1 M Tris (pH 7.4) and 1.5 M NaCl. Subsequently, the denatured DNA was transferred to nylon membranes (Nytran 77593; Schleicher & Schuell, Inc., Keene, N.H.) by the capillary transfer method. The membranes were hybridized with 32P-labeled 157-bp rpoB fragment probes, which were amplified by PCR using the TR9-TR8 primer set from Mycobacterium avium (ATCC 25291). The random primer labeling kit (Amersham, Arlington Heights, Ill.) was used for radiolabeling. Hybridization was performed at 68°C with a solution containing 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) 0.5% sodium dodecyl sulfate, 100 μg of salmon sperm DNA per ml, and 5× Denhardt solution. The membrane was washed twice at 65°C with a solution of 2× SSC and 0.1% sodium dodecyl sulfate for 30 min.
RESULTS
Amplification of rpoB DNA by nested PCR.
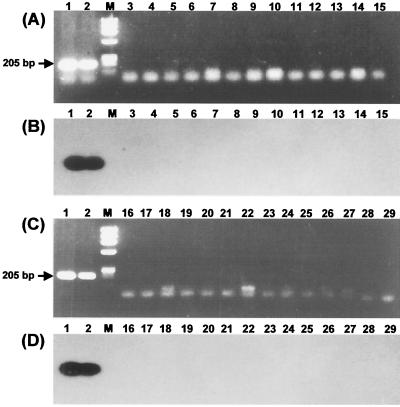
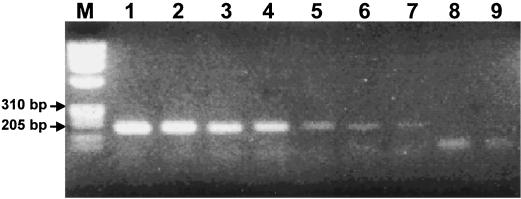
The PCR product amplified by the first-round PCR (TB1-TB2 primer set) was 205-bp rpoB DNA comprising a 157-bp fragment which was previously used for SSCP analysis (12, 15, 21). The specificity of the first-round PCR was tested with the DNAs from the reference strains of 19 mycobacteria and 9 nonmycobacteria and 48 clinical isolates of mycobacteria. The products were amplified only from the type strain and 20 clinical isolates of M. tuberculosis. The specificity of the first-round PCR was verified by Southern blotting (Fig. 2). Nothing was amplified when the template DNAs were diluted to less than 10 fg. Thus, the sensitivity of nested PCR assay in terms of DNA amount in a reaction could be determined as 10 fg of DNA in ethidium bromide-stained gels (Fig. 3).
FIG. 2.
Specific amplification of M. tuberculosis rpoB DNA by first-round PCR (TB1-TB2 primer set). The Southern blot shows that the PCR product (205 bp) was amplified from only M. tuberculosis by the first-round PCR. (A and B) Lanes: 1, M. tuberculosis H37Rv; 2, clinical isolate of M. tuberculosis; M, φX174/RF DNA/HaeIII digest; 3, M. avium; 4, M. fortuitum; 5, M. gastri; 6, M. gordonae; 7, M. intracellulare; 8, M. kansasii; 9; M. malmoense; 10, M. nonchromogenicum; 11, M. phlei; 12, M. scrofulaceum; 13, M. simiae; 14, M. smegmatis; 15, M. terrae. (C and D) Lanes 16, M. triviale; 17, M. vaccae; 18, M. chelonae; 19, M. szulgai; 20, M. ulcerans; 21, Rhodococcus equi; 22, Rhodococcus erythropolis; 23, Rhodococcus rhodochrous; 24, Nocardia otitidiscaviarum; 25, Nocardia nova; 26, Corynebacterium glutamicum; 27, Corynebacterium diphtheriae; 28, Neisseria meningitidis; 29, Haemophilus influenzae.
FIG. 3.
Amplification of rpoB DNA by nested PCR using serially diluted M. tuberculosis DNA as templates. Amplified products are observed only in the lanes that used more than 10 fg of template DNA. Lanes: M, φX174/RF DNA/HaeIII digest; 1, 10 ng; 2, 1 ng; 3, 100 pg; 4, 10 pg; 5, 1 pg; 6, 100 fg; 7, 10 fg; 8, 1 fg; 9, negative control without DNA.
PCR- and nested PCR-SSCP analysis for sputa.
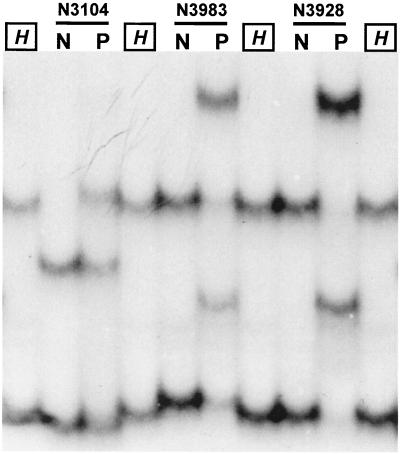
When conventional PCR-SSCP using the TR9-TR8 primer set was directly applied to sputa, rifampin susceptibility of 11 of 56 samples could not be determined (Table 2). The targeted rpoB DNAs were rarely amplified from eight sputum specimens (N2895, N2994, N3440, N3448, N3903, N3912, N3990, and N4064). Three sputum specimens (N3104, N3928, and N3983) showed ambiguous SSCP patterns displaying more than three bands, which was possibly caused by simultaneous amplification of the rpoB DNA from both M. tuberculosis and other bacteria. Furthermore, one (N3928) of these three sputum specimens showed a false-positive PCR-SSCP result. Because the SSCP pattern differed from that of the rifampin-susceptible reference strain, it was identified as a rifampin-resistant strain (Fig. 4). In contrast, the nested PCR successfully produced a 157-bp DNA directly from all of the specimens. Therefore, the presence of mutations in the amplified rpoB DNA from all specimens could be determined easily by SSCP analysis (Tables 2 and 3). Typical SSCP patterns of M. tuberculosis rpoB DNA were observed (Fig. 4). The three sputum specimens that had shown ambiguous results by conventional PCR-SSCP analysis were clearly determined to be two rifampin-resistant strains (N3104 and N3983) and one susceptible strain (N3928) of M. tuberculosis by nested PCR-SSCP analysis (Fig. 4), susceptibility testing, and PCR direct sequencing of rpoB DNA from the culture (Table 3). The results of the nested PCR-SSCP method performed on sputum specimens were entirely concordant with those of cultures analyzed by drug susceptibility testing and conventional PCR-SSCP. Based on the result of susceptibility testing performed with cultures, conventional PCR-SSCP analysis for sputa showed only 75.8% sensitivity and 87% specificity (Table 2).
TABLE 2.
Rifampin susceptibility of M. tuberculosis in sputa determined by conventional PCR-SSCP and nested PCR-SSCP analyses
| Method | Result | No. of sputum samples found by rifampin susceptibility testinga to be:
|
|
|---|---|---|---|
| Susceptible (n = 33) | Resistant (n = 23) | ||
| PCR-SSCP | Susceptible | 25 | 0 |
| Resistant | 0 | 20 | |
| Not determinedb | 8 | 3 | |
| Nested PCR-SSCP and DNA sequencing | Susceptible | 33 | 0 |
| Resistant | 0 | 23 | |
| Not determined | 0 | 0 | |
Agar dilution method performed with cultures from the same sputa used for SSCP analysis.
SSCP patterns could not be interpreted due to no amplification or more than three bands.
FIG. 4.
Comparison of PCR-SSCP and nested PCR-SSCP performed with three sputum specimens, N3104 (His526 [CAC]→Arg [CGC]), N3928 (wild type), and N3983 (Ser531 [TTG]→Leu [TCG]). Patterns of conventional PCR-SSCP using the TR9-TR8 primer set showed more than two bands (lanes P). They were unusual in PCR-SSCP analysis performed with culture. However, nested PCR-SSCP showed only two bands by which the rifampin resistance of M. tuberculosis could be determined as other reports (lanes N). H, M. tuberculosis H37Rv.
TABLE 3.
Comparison of results determined by three different methods to determine rifampin susceptibility of M. tuberculosis in sputa and their culture isolates
| Specimen no. | Clinical specimen designation | Resistance toa:
|
Result from:
|
|||||
|---|---|---|---|---|---|---|---|---|
| RIF | INH | STR | EMB | Conventional PCR-SSCP or direct sequencingb | Nested PCR-SSCP or direct sequencingb | DNA sequencing | ||
| 1 | N2838 | S | S | S | S | Wild type | Wild type | No mutation |
| 2 | N2872 | S | S | S | S | Wild type | Wild type | No mutation |
| 3 | N2877 | R | R | S | R | Abnormal | Abnormal | Mutation |
| 4 | N2893 | R | R | S | R | Abnormal | Abnormal | Mutation |
| 5 | N2895 | R | R | S | S | Not determinedc | Abnormal | Mutation |
| 6 | N2902 | R | R | S | R | Abnormal | Abnormal | Mutation |
| 7 | N2903 | R | R | S | R | Abnormal | Abnormal | Mutation |
| 8 | N2904 | S | S | S | S | Wild type | Wild type | No mutation |
| 9 | N2910 | R | R | R | R | Abnormal | Abnormal | Mutation |
| 10 | N2984 | R | R | S | S | Abnormal | Abnormal | Mutation |
| 11 | N2994 | S | S | S | S | Not determined | Wild type | No mutation |
| 12 | N2999 | S | S | S | S | Wild type | Wild type | No mutation |
| 13 | N3000 | S | S | S | S | Wild type | Wild type | No mutation |
| 14 | N3012 | S | S | S | S | Wild type | Wild type | No mutation |
| 15 | N3085 | S | S | S | S | Wild type | Wild type | No mutation |
| 16 | N3096 | S | S | S | S | Wild type | Wild type | No mutation |
| 17 | N3104 | R | R | R | S | Ambiguousd | Abnormal | Mutation |
| 18 | N3105 | S | S | S | S | Wild type | Wild type | No mutation |
| 19 | N3106 | S | S | S | S | Wild type | Wild type | No mutation |
| 20 | N3426 | S | S | S | S | Wild type | Wild type | No mutation |
| 21 | N3428 | S | S | S | S | Wild type | Wild type | No mutation |
| 22 | N3440 | R | R | S | R | Not determined | Abnormal | Mutation |
| 23 | N3448 | S | S | S | S | Not determined | Wild type | No mutation |
| 24 | N3471 | R | R | S | S | Abnormal | Abnormal | Mutation |
| 25 | N3474 | R | R | S | S | Abnormal | Abnormal | Mutation |
| 26 | N3496 | R | R | R | R | Abnormal | Abnormal | Mutation |
| 27 | N3500 | S | S | S | S | Wild type | Wild type | No mutation |
| 28 | N3501 | R | R | S | S | Abnormal | Abnormal | Mutation |
| 29 | N3507 | S | S | S | S | Wild type | Wild type | No mutation |
| 30 | N3522 | R | R | S | R | Abnormal | Abnormal | Mutation |
| 31 | N3523 | R | R | S | S | Abnormal | Abnormal | Mutation |
| 32 | N3524 | R | R | S | R | Abnormal | Abnormal | Mutation |
| 33 | N3536 | R | R | S | S | Abnormal | Abnormal | Mutation |
| 34 | N3606 | S | S | S | S | Wild type | Wild type | No mutation |
| 35 | N3874 | R | R | S | S | Abnormal | Abnormal | Mutation |
| 36 | N3878 | S | S | S | S | Wild type | Wild type | No mutation |
| 37 | N3903 | S | S | S | S | Not determined | Wild type | No mutation |
| 38 | N3912 | S | S | S | S | Not determined | Wild type | No mutation |
| 39 | N3914 | S | S | S | S | Wild type | Wild type | No mutation |
| 40 | N3924 | S | S | S | S | Wild type | Wild type | No mutation |
| 41 | N3928 | S | S | S | S | Ambiguous | Wild type | No mutation |
| 42 | N3983 | R | R | S | S | Ambiguous | Abnormal | Mutation |
| 43 | N3986 | R | R | S | R | Abnormal | Abnormal | Mutation |
| 44 | N3990 | S | S | S | S | Not determined | Wild type | No mutation |
| 45 | N3992 | S | S | S | S | Wild type | Wild type | No mutation |
| 46 | N3993 | S | S | S | S | Wild type | Wild type | No mutation |
| 47 | N4025 | R | R | S | R | Abnormal | Abnormal | Mutation |
| 48 | N4030 | R | R | R | R | Abnormal | Abnormal | Mutation |
| 49 | N4062 | S | S | S | S | Wild type | Wild type | No mutation |
| 50 | N4064 | S | S | S | S | Not determined | Wild type | No mutation |
| 51 | N4069 | S | R | S | R | Wild type | Wild type | No mutation |
| 52 | N4115 | R | R | S | S | Abnormal | Abnormal | Mutation |
| 53 | N4116 | S | S | S | S | Wild type | Wild type | No mutation |
| 54 | N4123 | S | S | S | S | Wild type | Wild type | No mutation |
| 55 | N4159 | S | S | S | S | Wild type | Wild type | No mutation |
| 56 | N4165 | S | S | S | S | Wild type | Wild type | No mutation |
RIF, rifampin; INH, isoniazid; STR, streptomycin; EMB, ethambutol; S, susceptible; R, resistant.
Wild type or Abnormal, identical SSCP patterns and sequences or different from that of reference strain, respectively.
Products were poorly amplified for the SSCP analysis.
The extra bands originating from other bacteria which may coexist with M. tuberculosis interfered with SSCP analysis.
Nested PCR-linked DNA sequencing for sputa.
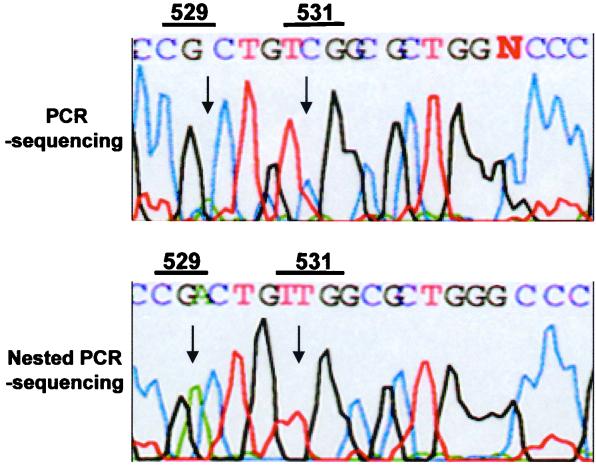
We also applied both the conventional and the nested PCR sequencing methods directly to sputa. The advantage of nested PCR-linked sequencing was revealed when rpoB DNA from the three specimens (N3104, N3928, and N3983) which had shown ambiguous results in conventional PCR-SSCP was selectively amplified by nested PCR. Thus, mutations related to rifampin resistance were definitely determined by direct sequencing. It was interesting to note that the results of conventional PCR sequencing did not coincide with those of nested PCR sequencing. The peaks on the electropherogram of conventional PCR sequencing were confusing in one sample (N3983) (Fig. 5, upper panel). The third nucleotide (CGA) at codon 529, which is a specific nucleotide of M. tuberculosis (8, 11), was not clearly determined by conventional PCR direct sequencing. Furthermore, PCR sequencing misidentified codon 531 as TCG, which reflects the rifampin-susceptible strain. However, the second nucleotide at codon 531, which is the most frequent site of mutation related to rifampin resistance, was determined as TTG by nested PCR sequencing (Fig. 5, lower panel). The sequence analysis of rpoB DNA performed on a culture isolate from the same sputum (N3983) showed a mutation at Ser531 (TCG→TTG).
FIG. 5.
Electropherograms of automatic DNA sequencing after the amplification of rpoB DNA by PCR and nested PCR which were directly performed with the DNA from sputum sample N3983. The PCR DNA sequencing (upper panel) showed ambiguous results due to nonspecific amplification of rpoB DNA. However, nested PCR DNA sequencing (lower panel) revealed a signature nucleotide at codon 529 (CGA) and a mutation at codon 531 (TTG) in the rpoB DNA of M. tuberculosis. N3983 was confirmed by susceptibility testing and sequence analysis of culture isolate as a rifampin-resistant strain harboring a mutation at Ser531 (TCG→TTG).
Rifampin susceptibility of culture isolates.
Culture isolates of M. tuberculosis from sputa were analyzed in order to determine their rifampin susceptibility using the agar dilution method and PCR direct sequencing. Results from the phenotypic and genotypic analyses were identical. All the sequences determined by PCR sequencing of culture isolates were concordant with those of sputa determined by nested PCR sequencing but not with those determined by conventional PCR sequencing.
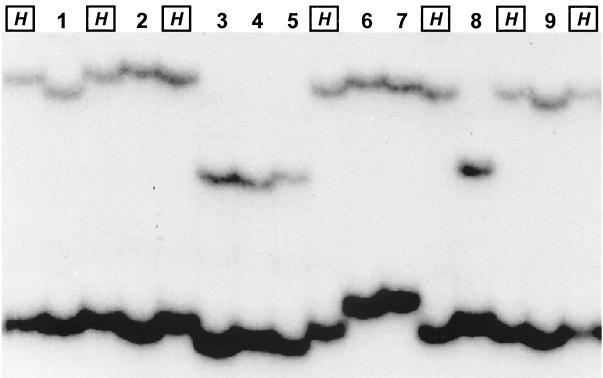
Twenty-three strains displayed point mutations that are correlated with rifampin resistance as determined by the agar dilution method. Mutations in each codon of eight amino acids (Gln513, Met515, Asp516, Gla517, Asn518, His526, Ser531, and Leu533) were found. The highest frequency (61%) was observed at Ser531. Resistant strains were also easily differentiated in a sequence-specific manner with the PCR-SSCP analysis (Fig. 6). Interestingly, novel genotypes were observed in two specimens. One strain (N3471) harbored 5 mutations (ATG GAC CAG AAC→ATC GCC AAC TAC) at four consecutive codons, which led to changes of four amino acids (Met515Asp516Gla517Asn518→Ile515Ala516Asn517Tyr518). The other (N3522) harbored a double mutation (TCG→ATG) at Ser531. These two strains also showed novel SSCP patterns (Fig. 6).
FIG. 6.
Nested PCR-SSCP patterns of rifampin-resistant strains (lanes 1 to 9) identified in this study. Lanes: H, M. tuberculosis H37Rv; 1, N3523 (Gln513→Pro); 2, N2893 (Asp516→Val); 3, N2910 (His526→Tyr); 4, N3501 (His526→Asp); 5, N2902 (His526→Arg); 6, N2895 (Ser531→Leu); 7, N3522 (Ser531→Met); 8, N2877 (Leu533→Pro); 9, N3471 (Met515, Asp516, Gla517, Asn518→Ile, Ala, Asn, Tyr).
DISCUSSION
Missense mutations, usually confined to the 69-nucleotide region of the rpoB gene, are related to the rifampin resistance of M. tuberculosis. Using molecular techniques to detect these mutations is more rapid than rifampin susceptibility testing which depends on culture and therefore requires an additional 4 to 6 weeks after the primary isolation to obtain the results. Although problems due to silent mutations were recently described (12), rifampin resistance in M. tuberculosis was successfully determined by PCR-SSCP (12, 15, 21) and PCR direct sequencing (11, 20) when the DNA was prepared from cultures. However, it has not been easy to apply these methods directly to sputa because of the poor sensitivity and specificity of PCR (8, 20). If PCR could be performed with sputa as well as culture isolates, the entire identification process for M. tuberculosis would be shortened to several days.
For this purpose, a line probe assay (4) and single-tube heminested PCR protocol (23) have been developed. Although there are several advantages to the line probe assay, it is more expensive than other methods and requires several probes for reverse hybridization to determine the mutation. Heminested PCR uses signature nucleotides of M. tuberculosis to avoid amplification of DNA from other GC-rich bacteria. It is a powerful method to detect rpoB DNA of M. tuberculosis directly from sputa. However, when this method was applied to detect M. tuberculosis rifampin resistance, further analysis such as sequencing or dideoxy fingerprinting after the amplification of 193-bp rpoB DNA by heminested PCR was required. This method can be linked to SSCP analysis performed directly on a DNA sample without a postamplification step but, compared to SSCP analysis which targets the 157-bp rpoB DNA, this method has problems in detecting several specific mutations. In order to detect the most frequent mutation at codon 531 (Ser [TCG]→Leu [TTG]), it required longer electrophoresis. Furthermore, the C-to-T transition mutation in codon 526 (His [CAC]→Tyr [TAC]) was not differentiated (6).
In this study, we used a nested PCR strategy targeting the 157-bp rpoB DNA, which has been most widely used for PCR-SSCP analysis and sequencing in order to detect mutations related to rifampin resistance. This approach confers a higher sensitivity and specificity than conventional PCR on SSCP and sequence analysis. Unlike heminested PCR, which is based on limited numbers of mycobacterial species (8, 23), the M. tuberculosis-specific primers for nested PCR can be selected from the signature nucleotides on the basis of rpoB DNA sequences of 44 mycobacteria (11). Therefore, the possibility of nonspecific amplification of rpoB DNA from NTM or nonmycobacteria should be greatly reduced. The signature nucleotides for M. tuberculosis were located at the 3′ hydroxyl termini of each primer as previously described (23). Thus, amplification of rpoB DNA from NTM or nonmycobacteria could be avoided by first-round PCR performed at a high annealing temperature and direct detection and determination of the rifampin susceptibility of M. tuberculosis in sputum were possible. Misidentification of rpoB sequences (codons 529 and 531 of N3983) by PCR sequencing may have resulted from simultaneous amplification of the rpoB DNA from M. tuberculosis and other bacteria, but this problem was solved by the nested PCR.
Conventional PCR-SSCP did not detect the rpoB sequences in 11 of 56 sputa (19.6%). Although the rpoB DNAs were not amplified (or detected) by conventional PCR in eight specimens, IS6110 PCR and cultures identified M. tuberculosis in all cases. Moreover, nested PCR amplified rpoB DNA from these specimens, and thus permitted rifampin susceptibilities to be determined by SSCP and sequencing. Another example of the high specificity of nested PCR-SSCP was found in the results for 3 of the 11 specimens which showed ambiguous results by conventional PCR-SSCP. Each had three bands that may have been caused by the simultaneous amplification of the rpoB DNA from both M. tuberculosis and other bacteria. Judging from SSCP patterns that were clearly different from that of the rifampin-susceptible reference strain (M. tuberculosis H37Rv), they might be regarded as rifampin resistant. Considering that NTM and nonmycobacteria have sequence variations in the corresponding region (157 bp) of amplified rpoB DNA, these bands may also represent false-positive results. Nested PCR-SSCP and DNA sequencing definitively identified these strains to be either rifampin-resistant or rifampin-susceptible M. tuberculosis. In contrast, specimen N3928 was quite interesting in that it was identified as a rifampin-resistant strain by PCR-SSCP but was proven to be rifampin susceptible by nested PCR-SSCP, susceptibility testing, and PCR-direct sequencing of rpoB DNA from pure culture. These results suggest that rpoB amplification from bacteria other than M. tuberculosis, causing a false-positive result, may be excluded by the nested PCR protocol used in this study.
The results of the nested PCR-SSCP method applied to sputa were entirely concordant with those of culture and conventional drug susceptibility testing as well as those obtained by conventional PCR-SSCP analysis. Furthermore, nested PCR-SSCP analysis enabled the direct detection of rifampin resistance from primary clinical specimens, such as sputa. Although the nested PCR-SSCP has a great advantage in reducing the time required for the primary culture and specific identification of M. tuberculosis in sputa, it still is limited by the requirements of radiolabeled PCR products, extensive labor, and a level of technical expertise not found in most clinical laboratories. It does, however, provide a significant advance in the rapid detection of multidrug-resistant M. tuberculosis directly from a clinical specimen.
ACKNOWLEDGMENTS
This work was supported by grant 98-N1-02-01-A-08 from the National Project for Medical Research, funded by the Korean Ministry of Science and Technology (MOST), and supported in part by project BK21 for Medicine, Dentistry, and Pharmacy.
REFERENCES
- 1.Barnes P, Bloch A B, Davidson P T, Snider D E., Jr Tuberculosis in patients with immunodeficiency virus infection. N Engl J Med. 1991;324:1644–1650. doi: 10.1056/NEJM199106063242307. [DOI] [PubMed] [Google Scholar]
- 2.Boom R, Sol C J A, Salimans M M M, Jansen C L, Wertheim-van Dillen P M E, Van Der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.British Thoracic Association. A controlled trial of six months chemotherapy in pulmonary tuberculosis. Am Rev Respir Dis. 1982;126:460–462. doi: 10.1164/arrd.1982.126.3.460. [DOI] [PubMed] [Google Scholar]
- 4.De Beenhouwer H, Lhiang Z, Jannes G, Mijs W, Machtelinckx L, Rossau R, Traore H, Portalels F. Rapid detection of rifampin resistance in sputum and biopsy specimens from tuberculosis patients by PCR and line probe assay. Tuber Lung Dis. 1995;76:425–430. doi: 10.1016/0962-8479(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 5.Des Prez R M, Heim C R. Mycobacterium tuberculosis. In: Mandell G L, Douglas R G, Bennett J E, editors. Principles and practice of infectious diseases. 2nd ed. New York, N.Y: Churchill Livingstone; 1990. pp. 1877–1906. [Google Scholar]
- 6.Felmlee T A, Liu Q, Whelen A C, Williams D, Sommer S S, Persing D H. Genotypic detection of Mycobacterium tuberculosis rifampin resistance: comparison of single-strand conformation polymorphism and dideoxy fingerprinting. J Clin Microbiol. 1995;33:1617–1623. doi: 10.1128/jcm.33.6.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frieden T R, Sterling T, Pablos-Mendez A, Kilburn J O, Cauthen J O, Dooley S W. The emergence of drug-resistant tuberculosis in New York City. N Engl J Med. 1993;328:521–526. doi: 10.1056/NEJM199302253280801. [DOI] [PubMed] [Google Scholar]
- 8.Hunt J M, Roberts G D, Stockmann L, Felmlee T A, Persing D D. Detection of a genetic locus encoding resistance to rifampin in mycobacterial cultures and in clinical specimens. Diagn Microbiol Infect Dis. 1994;18:219–227. doi: 10.1016/0732-8893(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 9.Iseman M. Treatment of multidrug-resistant tuberculosis. N Engl J Med. 1993;329:784–791. doi: 10.1056/NEJM199309093291108. [DOI] [PubMed] [Google Scholar]
- 10.Jin D, Gross C A. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J Mol Biol. 1988;202:45–58. doi: 10.1016/0022-2836(88)90517-7. [DOI] [PubMed] [Google Scholar]
- 11.Kim B J, Lee S H, Lyu M A, Kim S J, Bai G H, Kim S J, Chae G T, Kim E C, Cha C Y, Kook Y H. Identification of mycobacterial species by comparative sequence analysis of the RNA polymerase gene (rpoB) J Clin Microbiol. 1999;37:1714–1720. doi: 10.1128/jcm.37.6.1714-1720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim B J, Kim S Y, Park B H, Lyu M A, Park I K, Bai G H, Kim S J, Cha C Y, Kook Y H. Mutations in the rpoB gene of Mycobacterium tuberculosis that interfere with PCR-single-strand-conformation polymorphism analysis for rifampin susceptibility testing. J Clin Microbiol. 1997;35:492–494. doi: 10.1128/jcm.35.2.492-494.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S J, Hong Y P. Drug resistance of Mycobacterium tuberculosis in Korea. Tuber Lung Dis. 1992;73:219–224. doi: 10.1016/0962-8479(92)90090-7. [DOI] [PubMed] [Google Scholar]
- 14.Lisitsyn N A, Sverdlov E D, Moiseyeva E P, Danilevskaya O N, Nikiforov V G. Mutation to rifampin resistance at the beginning of the RNA polymerase beta subunit gene in Escherichia coli. Mol Gen Genet. 1984;196:173–174. doi: 10.1007/BF00334112. [DOI] [PubMed] [Google Scholar]
- 15.Matsiota-Bernard P, Vrioni G, Marinis E. Characterization of rpoB mutations in rifampin-resistant clinical Mycobacterium tuberculosis isolates from Greece. J Clin Microbiol. 1998;36:20–23. doi: 10.1128/jcm.36.1.20-23.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchison D A, Nunn A J. Influence of initial drug resistance on the response to short-course chemotherapy of pulmonary tuberculosis. Am Rev Respir Dis. 1986;133:423–430. doi: 10.1164/arrd.1986.133.3.423. [DOI] [PubMed] [Google Scholar]
- 17.Morris S, Bai G H, Suffys P, Portillo-Gomez L, Fairchok M, Rouse D. Molecular mechanisms of multiple drug resistance in clinical isolates of Mycobacterium tuberculosis. J Infect Dis. 1995;171:954–960. doi: 10.1093/infdis/171.4.954. [DOI] [PubMed] [Google Scholar]
- 18.Nolte F S, Metchock B. Mycobacterium. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. Washington, D.C.: American Society for Microbiology; 1995. pp. 400–437. [Google Scholar]
- 19.Roberts G D, Goodman N L, Heifets L, Larsh H W, Lindner T H, McClatchy J K, McGinnis M R, Siddiqi S H, Wright P. Evaluation of the BACTEC radiometric method for recovery of mycobacteria and drug susceptibility testing of Mycobacterium tuberculosis from acid-fast smear-positive specimens. J Clin Microbiol. 1983;18:689–696. doi: 10.1128/jcm.18.3.689-696.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Telenti A, Imboden P, Marchesi F, Lowrie D, Cole S, Colston M J, Matter L, Schopfer K, Bodmer T. Detection of rifampin-resistance mutations in Mycobacterium tuberculosis. Lancet. 1993;341:647–650. doi: 10.1016/0140-6736(93)90417-f. [DOI] [PubMed] [Google Scholar]
- 21.Telenti A, Imboden P, Marchesi F, Schmidheini T, Bodmer T. Direct, automated detection of rifampin-resistant Mycobacterium tuberculosis by polymerase chain reaction and single-strand conformation polymorphism analysis. Antimicrob Agents Chemother. 1993;37:2054–2058. doi: 10.1128/aac.37.10.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thierry D, Cave M D, Gsenach K D, Crawford J T, Bates J H, Gloquel B, Guesdon J L. IS6110, an IS-like element of Mycobacterium tuberculosis complex. Nucleic Acids Res. 1990;18:188. doi: 10.1093/nar/18.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whelen A C, Felmlee T A, Hunt J M, Wiliams D L, Roberts G D, Stockman L, Persing D H. Direct genotypic detection of Mycobacterium tuberculosis rifampin resistance in clinical specimens by using single-tube heminested PCR. J Clin Microbiol. 1995;33:556–561. doi: 10.1128/jcm.33.3.556-561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]