Abstract
Purpose
An adequate minimal surgical margin for partial nephrectomy (PN) has not yet been conclusively established. Therefore, we aimed to compare PN recurrence rates according to surgical margin status and to establish an adequate minimal surgical margin.
Materials and Methods
We retrospectively studied patients with clinically localized renal cell carcinoma who underwent PN between 2005 and 2014. Surgical margin width (SMW) was assessed for all surgical tissues and divided into three groups: SMW <1 mm, SMW ≥1 mm, and positive surgical margin (PSM). The data were analyzed using the Kaplan-Meier method with log-rank tests and multivariate Cox regression models.
Results
Of 748 patients (median age, 55 years; interquartile range, 46–64 years; 220 female), 704 (94.2%) and 44 (5.8%) patients had negative and PSMs, respectively. Recurrence-free survival was significantly lower in patients with PSMs (p<0.001) and was not significantly different between SMW ≥1 mm and <1 mm groups (p=0.604). PSM was a significant predictor of recurrence (hazard ratio: 8.03, 95% confidence interval: 2.74–23.56, p<0.001), in contrast to SMW <1 mm (p=0.680).
Conclusion
A PSM after PN significantly increases the risk of recurrence. We discovered that even a submillimeter safety surgical margin may be enough to prevent recurrence. To maximize normal renal parenchyma preservation and to avoid cancer recurrence in renal parenchymal tumor patients, PN may be a safe treatment, except for those with a PSM in the final pathology.
Keywords: Neoplasm recurrence, partial nephrectomy, surgical margin, kidney cancer, retrospective study
INTRODUCTION
Owing to developments in imaging techniques, renal parenchymal tumors are usually detected incidentally as small lesions, and surgical resection is the primary treatment. However, the choice of surgical procedure employed can differ depending on the size and stage of the tumor. Because of advances in surgical equipment and techniques, the prognosis of cancer control with partial nephrectomy (PN) is similar to that with radical nephrectomy (RN), with improved functional preservation. Therefore, all recent guidelines suggest that the nephron-saving technique be used as the treatment of choice for small, clinical stage T1 renal parenchymal tumors.1,2,3,4
A positive surgical margin (PSM) can lead to a poor prognosis in various tumor types. In contrast to RN, PN is associated with a risk of PSM because of the nature of this surgical technique.5 Nonetheless, for a renal parenchymal tumor with PSM, the prognosis and follow-up strategy remain controversial, and some researchers argue that PSM is not associated with cancer-free survival. Furthermore, an adequate minimal surgical margin has not yet been conclusively established.6 Traditionally, to prevent recurrence, a surgical margin of least 1 cm is recommended after PN. However, later studies have reported that a surgical margin of 5 mm or even less is sufficient for cancer control.7 Moreover, the PSM rate of tumor enucleation surgery has been reported to be similar to that of PN.8
The safety of a surgical margin width (SMW) of 5 mm has been evaluated previously. Meanwhile, as tumor enucleation techniques usually obtain a SMW within a submillimeter, accounting for one-fourth of negative surgical margin (NSM) cases, we set out to evaluate the safety of a submillimeter SMW. To do so, we aimed to investigate associations between PSM and cancer recurrence rates in patients with clinically localized renal cell carcinoma (RCC) treated with PN. Additionally, we compared recurrence rates between patients with a SMW <1 mm and ≥1 mm to address the uncertainty of surgical margin recommendations for PN and tumor enucleation.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board of Severance Hospital in Seoul, Korea (IRB No: 4-2019-0215) and was performed in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The requirement to obtain written informed consent was waived owing to the retrospective study design. We enrolled 855 patients with clinically localized renal parenchymal tumors who had been treated with PN at our institution between 2005 and 2014. After excluding patients with a benign pathology and those with incomplete pathologic or follow-up data, 748 patients were included in the final analysis. PN was performed by open, laparoscopic, and robotic methods. The patients were divided into the following three groups for comparison according to the surgical margin: 1) SMW <1 mm, 2) SMW ≥1 mm, and 3) PSM. PSM was defined when the tumor extended to the inked specimen edge.
Patient characteristics
We reviewed the medical records at our institution to obtain the patients’ clinical and pathologic characteristics, including age, sex, tumor size, Fuhrman grade, tumor histology, tumor stage, and surgical margin status of the PN specimen. Tumor stage was determined according to the 7th edition of the American Joint Committee on Cancer Staging Manual.9
Pathologic analysis
All specimens were sent to dedicated urological pathologists at our institution for analysis. Tumor size was determined by measuring the maximal diameter of the tumor at the time of the pathologic examination. For malignant tumors only, the histology was modeled as a categorical variable as follows: clear cell, papillary (no type difference), chromophobe, and unclassified renal cancer.
Follow-up assessments
The patients were assessed every 3 months for the first year after surgery and then semi-annually or annually. Follow-up assessments involved history taking, physical examinations, routine laboratory blood tests, chest radiography, and abdominal imaging (computed tomography or magnetic resonance imaging). The follow-up evaluations of PSM cases were performed at the same intervals. Recurrence was defined as a radiologically verified, newly observed, abnormal enhanced lesion during the study period. A newly observed lesion adjacent to the operative site was confirmed as local recurrence, and that in a distant organ was verified as distant recurrence.
Statistical analysis
Among the patients’ baseline characteristics and pathologic outcomes, we compared categorical data, such as sex, Fuhrman grade, tumor histology, tumor stage, and surgical margin status, using the chi-square test, whereas the continuous variables of age and tumor size were analyzed using the Mann-Whitney U test. We used the Kaplan-Meier method with log-rank tests to estimate and compare the probabilities of recurrence among groups. Cox proportional hazards models were used to investigate associations between variables and the risk of recurrence. Significant variables from the univariate analysis were included in the multivariate model. All statistical analyses were conducted using SPSS software, version 23.0 (IBM Corp., Armonk, NY, USA). Comparisons were conducted using the two-tailed test, and p-values <0.05 were considered statistically significant.
RESULTS
Of the 748 patients enrolled in this study, 44 (5.8%) had PSM. Among the patients without PSM, 532 (75.6%) and 172 (24.4%) patients were classified as having an SMW ≥1 mm and <1 mm, respectively. The median follow-up period after PN was 58 months (interquartile range: 40–78 months).
Table 1 shows the differences in clinical and pathologic characteristics according to surgical margin status and width. Compared to SMW ≥1 mm and <1 mm, patients with PSM were less likely to have a clear cell type and pathologic T1a stage (p=0.002 and <0.001, respectively). No statistically significant differences were identified between the SMW ≥1 mm and <1 mm groups in terms of patient age, sex, tumor size, Fuhrman grade, tumor histology, or tumor stage.
Table 1. Comparison of Clinical and Pathologic Characteristics according to Surgical Margin Status and Width.
| Variables | NSM (n=704) | PSM (n=44) | p value* | SMW ≥1 mm (n=532) | SMW <1 mm (n=172) | p value* | |
|---|---|---|---|---|---|---|---|
| Age, yr | 55 (46–64) | 57 (47–66) | 0.510 | 55 (46–64) | 55 (45–64) | 0.946 | |
| Sex, male | 500 (71.0) | 28 (63.6) | 379 (71.2) | 121 (70.3) | 0.847 | ||
| Tumor size, cm | 2.5 (1.8–3.4) | 2.8 (1.6–4.7) | 0.179 | 2.5 (1.8–3.4) | 2.5 (1.9–3.4) | 0.406 | |
| Fuhrman grade | 0.338 | 0.154 | |||||
| 1 | 63 (8.9) | 5 (11.4) | 52 (9.8) | 11 (6.4) | |||
| 2 | 390 (55.4) | 21 (47.7) | 303 (57.0) | 87 (50.6) | |||
| 3 | 219 (31.1) | 14 (31.8) | 150 (28.2) | 69 (40.1) | |||
| 4 | 7 (1.0) | 0 (0.0) | 7 (1.3) | 0 (0.0) | |||
| Missing | 25 (3.6) | 4 (9.1) | 20 (3.7) | 5 (2.9) | |||
| Tumor histology | 0.002† | 0.935 | |||||
| Clear cell | 615 (87.4) | 33 (75.0) | 466 (87.5) | 149 (86.6) | |||
| Papillary | 52 (7.4) | 3 (6.8) | 37 (7.0) | 15 (8.7) | |||
| Chromophobe | 36 (5.1) | 7 (15.9) | 28 (5.3) | 8 (4.7) | |||
| Other | 1 (0.1) | 1 (2.3) | 1 (0.2) | 0 (0.0) | |||
| Tumor stage | <0.001† | 0.670 | |||||
| T1a | 629 (89.3) | 29 (65.9) | 477 (89.7) | 152 (88.4) | |||
| ≥T1b | 75 (10.7) | 15 (34.1) | 55 (10.3) | 20 (11.6) | |||
NSM, negative surgical margin; PSM, positive surgical margin; SMW, surgical margin width; IQR, interquartile range.
Data are presented as median (IQR) or n (%).
*The chi-square test was used for categorical data, whereas the Mann-Whitney U test was used for continuous data, †p<0.05.
In the entire cohort, 17 patients (2.3%) experienced recurrence during the follow-up period after PN: 6 (0.8%) had local recurrence, and 11 (1.5%) had distant recurrence. Of these, 7 (15.9%) were from the PSM group, 2 (1.2%) were from the SMW <1 mm group, and 8 (1.5%) were from the SMW ≥1 mm group (Table 2). The Kaplan-Meier curves in Fig. 1 show that recurrence-free survival was significantly lower in patients with PSM (log-rank test, p<0.001). When the cases were stratified by SMW ≥1 mm and <1 mm, no significant difference in recurrence-free survival was observed between the two groups (log-rank test, p=0.604). Five-year recurrence free survival rates were 13.3%, 1.1% and 1.3% for the PSM, SMW <1 mm and SMW >1 mm groups, respectively.
Table 2. Recurrence Sites after Partial Nephrectomy.
| Local (n=6) | Distant | |||||
|---|---|---|---|---|---|---|
| Bone (n=1) | Liver (n=1) | Lung (n=8) | Lymph node (n=1) | |||
| Margin status | ||||||
| SMW ≥1 mm (n=532) | 1 (0.2)* | 1 (0.2) | 5 (0.9) | 1 (0.2) | ||
| SMW <1 mm (n=172) | 1 (0.6) | 1 (0.6) | ||||
| PSM (n=44) | 5 (11.3) | 2 (4.5) | ||||
| Tumor stage | ||||||
| T1a (n=658) | 2 (0.3) | 4 (0.6) | 1(0.2) | |||
| ≥T1b (n=90) | 4 (4.4) | 1 (1.1) | 1 (1.1) | 4 (4.4) | ||
| Tumor histology | ||||||
| Clear cell (n=648) | 6 (0.9) | 1 (0.2) | 1 (0.2) | 6 (0.9) | 1 (0.2) | |
| Papillary (n=55) | 2 (3.6) | |||||
| Fuhrman grade | ||||||
| 1 (n=68) | ||||||
| 2 (n=411) | 4 (1) | 1 (0.2) | 2 (0.5) | 1 (0.2) | ||
| 3 (n=233) | 2 (0.9) | 1 (0.4) | 4 (1.7) | |||
| 4 (n=7) | 1 (14) | |||||
| Missing (n=29) | 1 (3.4) | |||||
SMW, surgical margin width; PSM, positive surgical margin.
Data are presented as n (%).
*Clear cell, Fuhrman grade 2, 3.0×2.5 cm, has been recurred to ipsilateral perinephric fat.
Fig. 1. Kaplan-Meier curve for recurrence-free survival for all groups. NSM, negative surgical margin; PSM, positive surgical margin; SMW, surgical margin width.
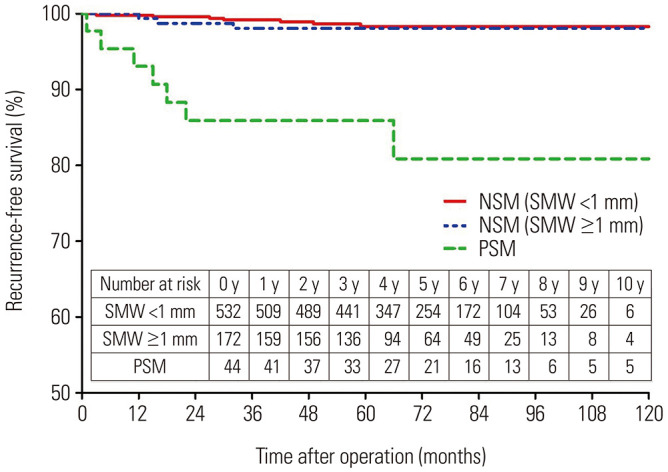
Table 3 shows the results of the univariate and multivariate Cox regression analyses for predicting recurrence after PN. In the multivariate regression analysis, age [hazard ratio (HR)10: 1.06, 95% confidence interval (CI): 1.01–1.11, p=0.009], tumor stage ≥T1b (HR: 5.60, 95% CI: 2.10–14.91, p<0.001), and a PSM (HR: 8.03, 95% CI: 2.74–23.56, p<0.001) were identified as significant predictors of recurrence, whereas an SMW <1 mm was not (p=0.680).
Table 3. Univariate and Multivariate Analyses of Factors Associated with Recurrence.
| Variables | Univariate | Multivariate* | |||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| Age | 1.07 (1.02–1.12) | 0.002† | 1.06 (1.01–1.11) | 0.009† | |
| Sex | |||||
| Female | 1 (Ref) | 0.305 | |||
| Male | 1.92 (0.55–6.69) | ||||
| Fuhrman grade | 0.373 | ||||
| Low (1 and 2) | 1 (Ref) | ||||
| High (3 and 4) | 1.57 (0.58–4.21) | ||||
| Tumor histology | |||||
| Clear cell | 1 (Ref) | ||||
| Papillary | 1.51 (0.35–6.61) | 0.584 | |||
| Chromophobe | Not applicable | ||||
| Other | Not applicable | ||||
| Tumor stage | |||||
| T1a | 1 (Ref) | 1 (Ref) | |||
| ≥T1b | 6.56 (1.39–30.92) | 0.017† | 5.60 (2.10–14.91) | <0.001† | |
| Margin status | |||||
| NSM (SMW ≥1 mm) | 1 (Ref) | 1 (Ref) | |||
| NSM (SMW <1 mm) | 1.41 (0.36–5.46) | 0.619 | 1.33 (0.34–5.15) | 0.680 | |
| PSM | 12.94 (4.54–36.90) | <0.001† | 8.03 (2.74–23.56) | <0.001† | |
CI, confidence interval; HR, hazard ratio; NSM, negative surgical margin; PSM, positive surgical margin; SMW, surgical margin width; Ref, reference.
*Significant variables in the univariate analysis were included in the multivariate model, †p<0.05.
DISCUSSION
In this large-sample retrospective study, we examined the effects of the surgical margin on the prognosis of patients undergoing PN. Our study confirmed once again that PSM is an important predictor of recurrence. Additionally, it was confirmed that maintaining a submillimeter SMW in accordance with recent trends aimed at maximizing normal renal parenchyma protection is safe without jeopardizing cancer control.
Every surgery has its own goals, and for PN, there are three main goals, the so-called “trifecta.” Hung, et al.11 first described the concept of the trifecta in PN as consisting of the following factors: a NSM, no renal function decline, and no urologic complications. Because controlling the cancer may be the most vital goal of oncologic surgery, achieving NSM is of utmost importance. Furthermore, because of a decrease in renal function after PN, the resection volume of healthy tissue is also a critical factor for surgical success. Therefore, the width of the surgical margin may be a conflicting factor between successful cancer control and the preservation of renal function.
The development of surgical techniques has reduced the recommended margin width from the traditional threshold of 1 cm to a few millimeters for tumor enucleation. A pilot study by Piper, et al.12 with 67 patients questioned the textbook concept that the SMW has to be >1 cm in PN for effective cancer control. The authors concluded that an SMW >1 mm around the tumor may be adequate to prevent its recurrence. Furthermore, evidence from many studies supports the concept that tumor enucleation is also a reliable treatment option with favorable oncological outcomes and functional recovery comparable with that achieved using PN.8,13 However, research has also indicated that renal cell cancer with a diameter of 4–7 cm (T1b stage) may have extra pseudo-capsular cancer located within 3 mm of the primary tumor.14 Despite the current trend towards narrower surgical margins, there is still no consensus regarding their safety range. To date, the recommended SMW of >1 mm for preventing recurrence has remained the narrowest margin investigated.
Although disputed in literature, PSM should be avoided in PN. In a large study population of 2256 patients, Wood, et al.15 established that recurrence after PN is associated with PSM and higher pathologic stage. However, some studies have reported a different relationship between PSM after PN and tumor recurrence. For example, Bensalah, et al.16 reported that although PSM after PN showed a trend towards being associated with local recurrence, the association was not statistically significant, and PSM did not influence cancer-specific survival in their study. Nonetheless, several authors have asserted that even if PSM after PN does not influence recurrence, vigilant follow up is mandatory.17,18 This follow up requires additional and repeated examinations; the burden of the additional medical costs is borne by the patient, and these procedures can be stressful for both the surgeon and the patient. Thus, all possible efforts should be made to avoid PSM.
In our study, the incidence of PSM was 5.8%, which was not significantly different from that reported in previous studies.19,20 Patients with PSM had a significantly higher T stage disease and presented with the chromophobe type of RCC more frequently than those with an NSM. This significant intergroup difference in the frequency of this RCC type may stem from innate characteristic of being large in size at the initial diagnosis.21 The recurrence rate was higher in patients with PSM than in those with SMW ≥1 mm and <1 mm. In our multivariate analysis, both tumor stage ≥T1b and PSM were strong prognosticators of cancer recurrence. Individuals with an SMW <1 mm also tended to exhibit a higher HR for recurrence than those with an SMW ≥1 mm. However, the observed difference was not statistically significant, and the HR value in the group with an SMW <1 mm was not comparable with that determined for the PSM group (HR: 1.33 vs. 8.03). Although, there were no statistical difference in clinical and pathological characteristics between the SMW ≥1 mm and SMW <1 mm groups, it can be assumed that factors that were not include in this study, such as tumor location or method of surgery, may have affected the difference in HRs. Long-term follow-up studies should be conducted in the future to examine the effects of an SMW <1 mm or different surgical methods on recurrence or survival.
Our study has several strengths worth noting. First, the mean follow-up period was 58 months, which is longer than that reported in previous studies, and thus, provides a better understanding of the effects of PSM on recurrence. Second, although there are many studies on PSM, to the best of our knowledge, none have suggested that an SMW <1 mm may be safe. Third, our study confirms that if NSM can be achieved, tumor enucleation, which usually results in a narrow surgical margin, is a safe operation method. Fourth, our single-institutional cohort ensures data uniformity.
Several limitations of our study should also be mentioned. First, as this was a retrospective study, there is a possibility of selection bias and information inaccuracy. Second, although recurrence was analyzed, cancer-specific survival and overall survival were not. An analysis of survival might have provided more in-depth results on the influence PSM has on recurrence. Third, we did not consider the tumor location, identity of the surgeon, and method of surgery, all of which may have affected the findings. Fourth, according to the literature, there is no difference in cancer control between RN and PN. However, because we did not compare RN and tumor enucleation, further evaluation is needed regarding their effects on recurrence.
In conclusion, our study shows that PSM resulting from PN increases the risk of recurrence in patients with RCC. Moreover, we demonstrate that a surgical margin in the sub-millimeter range may be adequate for preventing recurrence. To maximize normal renal parenchyma preservation and to avoid cancer recurrence in renal parenchymal tumor patients, PN may be a safe treatment, except in instances of PSM in the final pathology. To substantiate our findings, additional large-scale long-term studies are required.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Won Sik Jang, Won Sik Ham, Woong Kyu Han, Koon Ho Rha, and Young Deuk Choi.
- Data curation: Jongsoo Lee, Jinu Kim, and Jong Chan Kim.
- Formal analysis: Jongsoo Lee, Won Sik Jang, Jinu Kim, and Jong Chan Kim.
- Investigation: Jongsoo Lee and Won Sik Jang.
- Methodology: Jongsoo Lee and Won Sik Jang.
- Project administration: Won Sik Jang, Koon Ho Rha, and Young Deuk Choi.
- Supervision: Won Sik Jang, Won Sik Ham, Woong Kyu Han, Koon Ho Rha, and Young Deuk Choi.
- Validation: Jongsoo Lee and Won Sik Jang.
- Visualization: Jongsoo Lee, Jinu Kim, and Jong Chan Kim.
- Writing—original draft: Jongsoo Lee, Won Sik Jang, Jinu Kim, and Jong Chan Kim.
- Writing—review & editing: Won Sik Jang, Jongsoo Lee, Won Sik Ham, and Woong Kyu Han.
- Approval of final manuscript: all authors.
References
- 1.Dash A, Vickers AJ, Schachter LR, Bach AM, Snyder ME, Russo P. Comparison of outcomes in elective partial vs radical nephrectomy for clear cell renal cell carcinoma of 4-7 cm. BJU Int. 2006;97:939–945. doi: 10.1111/j.1464-410X.2006.06060.x. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Jonasch E, Agarwal N, Bhayani S, Bro WP, Chang SS, et al. Kidney cancer, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:804–834. doi: 10.6004/jnccn.2017.0100. [DOI] [PubMed] [Google Scholar]
- 3.Campbell S, Uzzo RG, Allaf ME, Bass EB, Cadeddu JA, Chang A, et al. Renal mass and localized renal cancer: AUA guideline. J Urol. 2017;198:520–529. doi: 10.1016/j.juro.2017.04.100. [DOI] [PubMed] [Google Scholar]
- 4.Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913–924. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Leibovich BC, Blute M, Cheville JC, Lohse CM, Weaver AL, Zincke H. Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. J Urol. 2004;171:1066–1070. doi: 10.1097/01.ju.0000113274.40885.db. [DOI] [PubMed] [Google Scholar]
- 6.Zini L, Perrotte P, Capitanio U, Jeldres C, Shariat SF, Antebi E, et al. Radical versus partial nephrectomy: effect on overall and noncancer mortality. Cancer. 2009;115:1465–1471. doi: 10.1002/cncr.24035. [DOI] [PubMed] [Google Scholar]
- 7.Lee HJ, Liss MA, Derweesh IH. Outcomes of partial nephrectomy for clinical T1b and T2 renal tumors. Curr Opin Urol. 2014;24:448–452. doi: 10.1097/MOU.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 8.Cao DH, Liu LR, Fang Y, Tang P, Li T, Bai Y, et al. Simple tumor enucleation may not decrease oncologic outcomes for T1 renal cell carcinoma: a systematic review and meta-analysis. Urol Oncol. 2017;35:661.e15–661.e21. doi: 10.1016/j.urolonc.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Byrd DR, Carducci MA, Compton CC, Fritz AG, Greene F. AJCC cancer staging handbook. 7th ed. New York: Springer; 2010. [Google Scholar]
- 10.Timsit MO, Bazin JP, Thiounn N, Fontaine E, Chrétien Y, Dufour B, et al. Prospective study of safety margins in partial nephrectomy: intraoperative assessment and contribution of frozen section analysis. Urology. 2006;67:923–926. doi: 10.1016/j.urology.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 11.Hung AJ, Cai J, Simmons MN, Gill IS. “Trifecta” in partial nephrectomy. J Urol. 2013;189:36–42. doi: 10.1016/j.juro.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 12.Piper NY, Bishoff JT, Magee C, Haffron JM, Flanigan RC, Mintiens A, et al. Is a 1-cm margin necessary during nephron-sparing surgery for renal cell carcinoma? Urology. 2001;58:849–852. doi: 10.1016/s0090-4295(01)01393-0. [DOI] [PubMed] [Google Scholar]
- 13.Blackwell RH, Li B, Kozel Z, Zhang Z, Zhao J, Dong W, et al. Functional implications of renal tumor enucleation relative to standard partial nephrectomy. Urology. 2017;99:162–168. doi: 10.1016/j.urology.2016.07.048. [DOI] [PubMed] [Google Scholar]
- 14.Chen XS, Zhang ZT, Du J, Bi XC, Sun G, Yao X. Optimal surgical margin in nephron-sparing surgery for T1b renal cell carcinoma. Urology. 2012;79:836–839. doi: 10.1016/j.urology.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Wood EL, Adibi M, Qiao W, Brandt J, Zhang M, Tamboli P, et al. Local tumor bed recurrence following partial nephrectomy in patients with small renal masses. J Urol. 2018;199:393–400. doi: 10.1016/j.juro.2017.09.072. [DOI] [PubMed] [Google Scholar]
- 16.Bensalah K, Pantuck AJ, Rioux-Leclercq N, Thuret R, Montorsi F, Karakiewicz PI, et al. Positive surgical margin appears to have negligible impact on survival of renal cell carcinomas treated by nephron-sparing surgery. Eur Urol. 2010;57:466–471. doi: 10.1016/j.eururo.2009.03.048. [DOI] [PubMed] [Google Scholar]
- 17.Yossepowitch O, Thompson RH, Leibovich BC, Eggener SE, Pettus JA, Kwon ED, et al. Positive surgical margins at partial nephrectomy: predictors and oncological outcomes. J Urol. 2008;179:2158–2163. doi: 10.1016/j.juro.2008.01.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marszalek M, Carini M, Chlosta P, Jeschke K, Kirkali Z, Knüchel R, et al. Positive surgical margins after nephron-sparing surgery. Eur Urol. 2012;61:757–763. doi: 10.1016/j.eururo.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 19.Shah PH, Moreira DM, Okhunov Z, Patel VR, Chopra S, Razmaria AA, et al. Positive surgical margins increase risk of recurrence after partial nephrectomy for high risk renal tumors. J Urol. 2016;196:327–334. doi: 10.1016/j.juro.2016.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiavina R, Serni S, Mari A, Antonelli A, Bertolo R, Bianchi G, et al. A prospective, multicenter evaluation of predictive factors for positive surgical margins after nephron-sparing surgery for renal cell carcinoma: the RECORd1 Italian project. Clin Genitourin Cancer. 2015;13:165–170. doi: 10.1016/j.clgc.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Lee WK, Byun SS, Kim HH, Rha KH, Hwang TK, Sung GT, et al. Characteristics and prognosis of chromophobe non-metastatic renal cell carcinoma: a multicenter study. Int J Urol. 2010;17:898–904. doi: 10.1111/j.1442-2042.2010.02630.x. [DOI] [PubMed] [Google Scholar]


