Abstract
Background
House dust mites (HDM) are the major causative allergen for allergic rhinitis. The sole disease-modifying therapy for allergic rhinitis is allergen immunotherapy (AIT). Rush immunotherapy is the accelerated build-up schedules to reach the target maintenance dose.
Objective
To evaluate the kinetic changes of peripheral blood CD4+CD25+FOXP3+ regulatory T cells (Treg) and serum cytokines in children undergoing 2-day modified rush HDM AIT.
Methods
Children aged 5–15 years with allergic rhinitis were enrolled for a 2-day modified rush HDM AIT. Peripheral blood CD4+CD25+FOXP3+ Treg, serum interleukin (IL)-4, IL-13, interferon-γ, and IL-10 were measured at baseline, finishing rush, achieving maintenance dose, 6 months, and 12 months after reaching maintenance dose. Specific IgE (sIgE) to HDM was evaluated at baseline and 12 months after getting the maintenance dose. Rhinitis symptoms were assessed daily using a daily card.
Results
A total of 12 children with a mean age of 13 years were enrolled. Rhinitis symptom-free days per month increased significantly after reaching the maintenance dose compared to baseline (from 9.5 days to 19.5 days, p = 0.002), and the maximum improvement was seen at 1 year. The levels of Treg were significantly increased at 6 months after maintenance dose compared to baseline level (6.27%±1.63% vs. 3.83%±1.80%, p < 0.001). After treatment, there were significantly decreased serum IL-13 at 1 year after maintenance but no significant changes in sIgE to HDM. The systemic reaction during AIT occurred 7 episodes from 119 shots (5.9%).
Conclusion
Two-day modified rush HDM AIT provides acceptable systemic reactions and increases the number of CD4+CD25+FOXP3+ Treg in children.
Keywords: Subcutaneous immunotherapy, Dust mite, Rhinitis, Regulatory T cells, Cytokines
INTRODUCTION
Allergen immunotherapy (AIT) is the only etiological treatment available for allergic diseases. AIT can be administered subcutaneously or sublingually (SLIT) leads to decreased clinical symptoms, less usage of drugs, and improved quality of life in patients with allergic rhinitis [1]. The mechanism of immune tolerance from AIT was proposed to involve an increase in the number of regulatory T cells [2,3], increases of interferon (IFN)-γ and interleukin (IL)-10 [4], and decreases of IL-4 and IL-5 [5]. The dose of AIT needs to achieve the recommended target or maintenance (MN) dose to get the immune tolerance. Conventional AIT requires 3–6 months to reach the MN dose, but accelerated protocols such as rush AIT have shortened the build-up phase into several weeks to months. Rush AIT for inhalant allergen also provides quicker effectiveness with more acceptable reactions than conventional AIT [1]. House dust mite (HDM) is the most common causative allergen for allergic rhinitis pateints in Southeast Asian countries, especially Thailand [6]. Patients with HDM allergy can receive HDM subcutaneous or sublingual immunotherapy. However, HDM SLIT is approved only in adolescents. Besides, there are some differences in HDM rush AIT protocols, especially in children. There is also scarce evidence on the dynamic change of regulatory T cells and cytokines after rush HDM AIT in children with allergic rhinitis. The current study was aimed to evaluate the changes of CD4+CD25+FOXP3+ regulatory T cells in allergic rhinitis children undergoing 2-day modified rush HDM AIT and monitoing for 12 months after reaching MN dose. In addition, serum cytokines including serum IL-4, IL-13, IFN-γ, and IL-10 were evaluated.
MATERIALS AND METHODS
Patients
This prospective study included children aged 6–15 years with allergic rhinitis from February 2017 to July 2019 at the Department of Pediatrics, Faculty of Medicine Ramathibodi Hospital. All participants were hospitalized for 2-day modified rush HDM AIT using the standardized mite (Dermatophagoides farinae and Dermatophagoides pteronyssinus) (ALK-Abello Pharm. Inc., Round Rock, TX, USA). Premedication with oral antihistamine and oral prednisolone (1 mg/kg/dose) were administered in the morning at least 30 minutes before the first dose of AIT. The detail of the modified 2-day modified rush AIT administration is shown in Table 1. After 2-day of modified rush AIT, the patients received the injection twice a week until reaching the MN dose (D. farina 1,000 AU and D. pteronyssinus 300 AU). Premedication with oral antihistamine 30 minutes was given before each injection. Systemic reactions from AIT were graded according to The World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System [7]. Specific IgE to Der p using ImmunoCap (Phadia, Biomed diagnostics, Bangkok, Thailand) was evaluated at baseline before starting AIT and after 12 months of MN dose. Peripheral blood mononuclear cells (PBMCs) and serum were collected at baseline before AIT, after rush phase, achieving MN dose, 6 months after MN dose (MN 6 Mo), and 12 months after MN dose (MN 12 Mo). All participating subjects and parents were informed of the contents and the study's aims, and they gave their written consent. The study was reviewed and approved by the human rights and Ethics Committee of Faculty of Medicine Ramathibodi Hospital, Mahidol University (ID 01-60-08).
Table 1. Protocol for modified rush house dust mite allergen immunotherapy.
| Rush (1-hour interval between injections) | Build-up | ||||
|---|---|---|---|---|---|
| Day 1 | Day 2 | ||||
| Concentration | Volume (mL) | Concentration | Volume (mL) | Concentration | Volume (mL) |
| 1:1000 vol/vol (green label) |
0.1 | 1:10 vol/vol (yellow label) |
0.1 | 1:10 vol/vol (yellow label) |
0.25 |
| 0.2 | 0.2 | 0.30 | |||
| 0.4 | 0.35 | ||||
| 0.40 | |||||
| 0.45 | |||||
| 0.50 | |||||
| 1:100 vol/vol (blue label) |
0.1 | 1:1 vol/vol (red label) |
0.10 | ||
| 0.3 | 0.15 | ||||
| 0.5 | 0.20 | ||||
| 0.25 | |||||
| 0.30 | |||||
| 0.35 | |||||
| 0.40 | |||||
| 0.45 | |||||
| 0.50 | |||||
Materials and reagents
FOXP3+ Treg identification
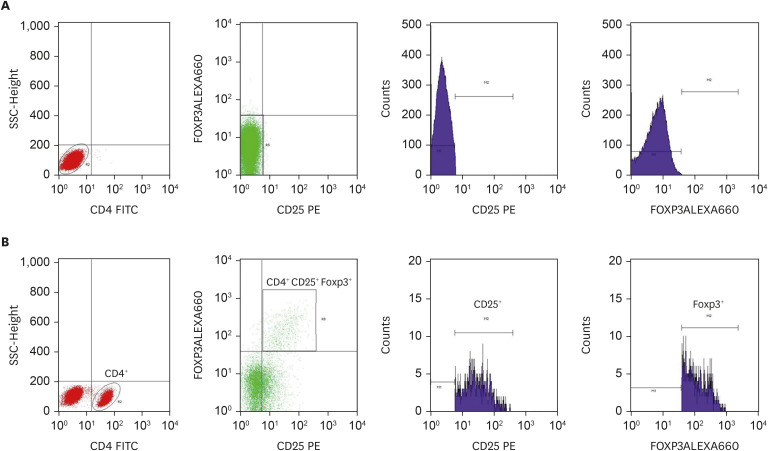
In brief, fresh heparinized whole blood samples (6 mL) were collected from patients at each time point. PBMCs were isolated using SepMate-15 tubes (STEMCELL Technologies, Vancouver, Canada) and Lymphoprep (Alere Technologies AS, Oslo, Norway), centrifuged with 1,200 g for 20 minutes in room temperature. PBMC layer at the interface was collected and twice washed at 3,500 rpm by phosphate buffer saline for 5 minutes. PBMCs were resuspended with RPMI medium-1640 (Glico technology, New Youk, NY, USA), which was used to study the number of FoxP3 Treg by flow cytometry. 1×106 cells of PBMCs were incubated with mouse anti-human CD4 FITC and anti-human CD25 FITC (BD Bioscience, San Jose, CA, USA) for extracellular staining and anti-human Foxp3 (eFluor 660, eBioscience, San Diego, CA, USA) for intracellular staining. Moreover, Isotype control was used anti-FOXP3 isotype (Rat IhG2 K, eFluor 660, eBioscience). To determine CD4+CD25+FOXP3+ Treg cells were analyzed by flow cytometry using BD FACSCalibur, BDBioscience (Fig. 1).
Fig. 1. The gating strategy of the percentage of CD4+ CD25+ Foxp3+ regulatory T (Treg) cells population Isotype (A), and test (B) were gated to allow determination of population densities. Data from the children patients (n = 12; with house dust mites subcutaneous immunotherapy) were acquired using The BD FACSCalibur. CD4+ population gated by SSC and CD4 FITC, then Treg gated from CD4+ by CD25+ Foxp3+ population. Histograms of CD25+ and Foxp3+ showed purity of CD4+ CD25+ Foxp3+ (Treg) cells population gating.
Cytokines determination
The levels of serum IL-4, IL-13, IFN-γ, and IL-10 in the serum were detected using the BioLegend LEGENDplex commercial kit (Cat. According to the manufacturers' instructions, no. 740723, Human cytokine panel (13-plex) with the filter plate. Briefly, the stored serum was thawed and diluted by 2-fold dilution with assay buffer before assay. These experiments are done in triplicate. First, the plasma was incubated with beads coat antibody detection in a rotator for 2 hours at room temperature. After that, each sample wells was added SA-PE reagent and incubated for 30 minutes. Then, washed twice and kept in the dark until determined by flow cytometry.
Statistical analysis
Statistical analysis was performed with the SigmaPlot V14 software. Data from flow cytometry and serum cytokine levels were analyzed using the repeated measure analysis of variance. The data are presented as median and interquartile range for nonparametric data and mean±standard deviation (SD) for parametric data.
RESULTS
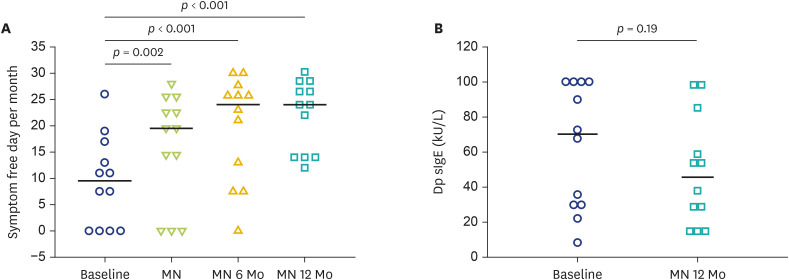
A total of 12 children (7 boys and 5 girls) aged 6–15 years with a mean age of 13 years completed the prospective study. The mean (SD) of sIgE to Der p was 63.01 (10.27) kU/L. During 2-day modified rush HDM AIT, the systemic reactions occurred in 7 episodes from 119 shots (5.9%) (Table 2) and 8 out of 225 shots (3.6%) during the build-up phase. All children responded well to the treatment and had no other systemic reactions from HDM AIT during MN. All enrolled children reached the MN dose. After treatment with HDM AIT, a significant increase in rhinitis symptom-free days per month was observed when reaching the MN dose, and this improvement was maximumly shown at MN 6 Mo and MN 12 Mo (Fig. 2A). However, there were no significant changes in sIgE to Der p after MN 12 Mo (Fig. 2B).
Table 2. Characteristic of patients who had systemic reactions during rush allergen immunotherapy.
| Age (yr) | Sex | Dose with reaction | Symptoms | Systemic reaction grading* | Treatments |
|---|---|---|---|---|---|
| 13 | Female | 1:10 v/v 0.2 mL | Acute urticaria | Grade 3 | IV antihistamine |
| Wheezing | Epinephrine IM | ||||
| Decreased PEFR | |||||
| 8 | Male | 1:10 v/v 0.2 mL | Acute urticaria | Grade 3 | IV antihistamine |
| Wheezing | Epinephrine IM | ||||
| Decreased PEFR | |||||
| 13 | Female | 1:10 v/v 0.1 mL | Flushing | Grade 2 | IV antihistamine |
| Decreased PEFR | Salbutamol nebulization | ||||
| 13 | Male | 1:10 v/v 0.1 mL | Acute urticaria | Grade 1 | Oral antihistamine |
| 7 | Female | 1:10 v/v 0.2 mL | Acute urticaria | Grade 1 | Oral antihistamine |
| 10 | Male | 1:10 v/v 0.1 mL | Acute urticaria | Grade 1 | Oral antihistamine |
| 5 | Male | 1:10 v/v 0.2 mL | Acute urticaria | Grade 1 | Oral antihistamine |
PEFR, peak expiratory flow rate; IM, intramuscular injection; IV, intraveouns injection.
*The World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System.
Fig. 2. Comparison of rhinitis symptom-free day changes after house dust mites (HDM) allergen immunotherapy (A) and serum specific IgE to HDM at baseline and 12 months after maintenance (MN) dose (MN 12 Mo) (B). MN 6 Mo, 6 months after MN dose.
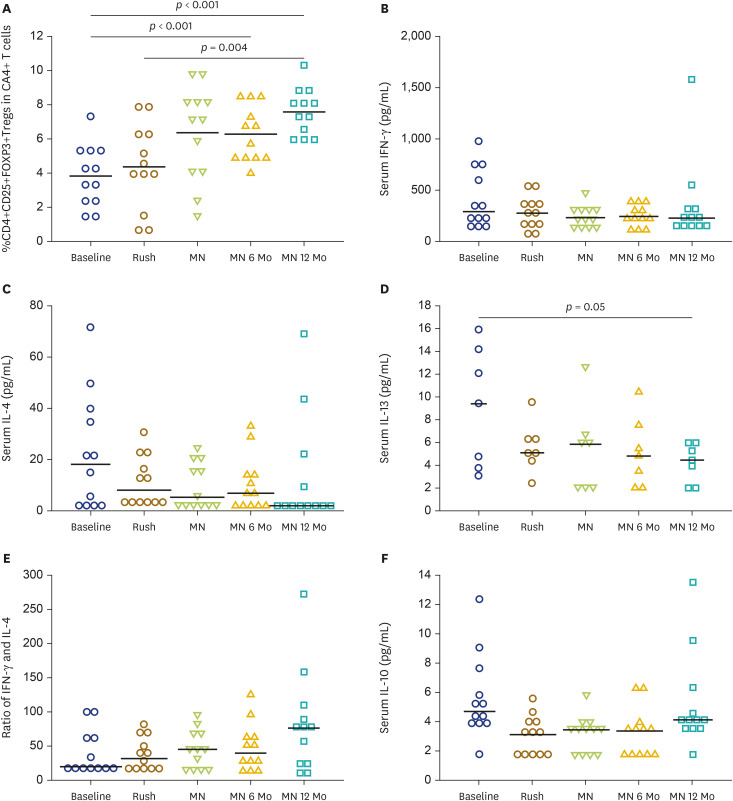
Dynamic changes of CD4+CD25+FOXP3+ regulatory T (Treg) cells and serum cytokines
There was a trend towards increasing Treg when achieving MN dose, but this was failed to reach statistically significant. However, the percentage of Treg in CD4+ cells was significantly increased at MN 6 Mo and MN 12 Mo compared to those of the baseline and MN time points (Fig. 3A). No correlations between sIgE and the percentage of regulatory T cell at baseline and after MN 12 Mo were demonstrated. There were no significant changes of the IFN-γ and IL-4 in the serum after HDM AIT at MN dose, MN 6 Mo, and MN 12 Mo compared to the baseline levels (Fig. 3B, C). There was a modest decrease in serum IL-13 after HDM AIT treatment at MN 12 Mo and a trend toward increases in IFN-γ /IL-4 ratio in the serum at MN 12 Mo compared to the baseline levels (Fig. 3D, E). There were no significant changes in serum IL-10 after HDM AIT treatment (Fig. 3F).
Fig. 3. Dynamic changes of the percentage of CD4+CD25+FOXP3+ (Treg) in CD4+ cells (A) at baseline, at maintenance (MN) dose, 6 months after MN dose (MN 6 Mo), and 12 months after MN dose (MN 12 Mo), serum IFN-γ (B), IL-4 (C), serum IL-13 (D), ratio of IFN-γ and IL-4 (E), and serum IL-10 (F).
DISCUSSION
In the present study, we have demonstrated that our 2-day modified rush HDM AIT protocol in children with allergic rhinitis has a similar systemic reaction compared to the previous report [8]. In addition, all children significantly increased rhinitis symptoms free-day/month after treatment. We have also shown the trend toward increasing the number of CD4+CD25+FOXP3+Treg after reaching the MN dose. CD4+CD25+FOXP3+Treg was significantly increased at MN 6 Mo, and it was sustained increased at 12 months. Additionally, serum IL-13 decreased significantly after receiving the MN dose of AIT for 1 year.
The expansion of circulating CD4+CD25+FOXP3+Treg T cells after HDM AIT is in line with the previous study in adult patients receiving conventional dust mite AIT [9,10]. An increase in the number of CD4+CD25+FOXP3+Treg cells after AIT was suggested to play a pivotal role in developing allergen-specific immune tolerance [11]. After rush venom immunotherapy in children, we have also demonstrated a rise in CD4+CD25+FOXP3+Treg cells [12].
We found a significantly decreased serum IL-13 at 1 year after MN of AIT, but there were no changes in serum IFN-γ or IL-4. However, there was a trend toward increasing the IFN-γ/IL-4 ratio. There is an increase in intracellular IFN-γ/IL-5 ratio of the CD3+ and CD4+ cells in children receiving rush dust mite AIT [13]. A recent study demonstrated the decreased the production of IL-5 and IL-13 from PBMCs of asthmatic adult recieving HDM AIT [14]. The unsignificant changes in the serum IFN-γ and IL-4 after rush HDM AIT in the current study may be explained by the difference in the method of detecting the cytokine. The changes in these cytokines after AIT may be modestly detected in the serum but can be detected better intracellular levels. The current study's increase in Treg percentage is consistent with earlier research in adults receiving HDM AIT [10]. These results may be implied that HDM AIT is equally effective in adults and children.
We also found the significant clinical improvement of rhinitis symptom as evaluated by the symptom-free day per month when the children recieving MN dose and the clinical improvement continuing increased up to MN 12 Mo. This finding is in line with the findings of a recent study in Korean children with asthma and allergic rhinitis, which found that HDM subcuateous immunotherapy improved symptom scores more than the drug-only group [15].
The systemic reaction in our 2-day modified rush HDM AIT throughout the rush phase and build-up phase was found to be comparable to a recent report for inhalant AIT [8]. Of 12 enrolled children, 2 (16.7%) developed grade III systemic reactions requiring intramuscular epinephrine injection during rush AIT. The incidence of systemic reactions in our rush AIT protocol is in line with other rush AIT protocols, ranging from 14.7%–38% [8].
In summary, the present study demonstrates the feasibility of 2-day modified rush HDM AIT and evidence of immunological tolerance in children with allergic rhinitis. Therefore, this 2-day modified rush HDM AIT could be a treatment option for allergic rhinitis children candidates for HDM AIT.
ACKNOWLEDGEMENTS
This study was financially supported by research funds from the Faculty of Medicine Ramathibodi Hospital, Mahidol University, Thailand. We would like to thank Ms Cherapat Sasisakulporn for helping in enrolling the participating subjects.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Tipyapa Rattanamanee, Putthapoom Lumjiaktase, Wiparat Manuyakorn.
- Formal analysis: Tipyapa Rattanamanee, Wiparat Manuyakorn.
- Investigation: Tipyapa Rattanamanee, Putthapoom Lumjiaktase, Nanthisa Kemawichanura, Potjanee Kiewnga, Wanlapa Jotikasthira, Wiparat Manuyakorn.
- Methodology: Tipyapa Rattanamanee, Putthapoom Lumjiaktase, Nanthisa Kemawichanura, Potjanee Kiewnga, Wanlapa Jotikasthira, Wiparat Manuyakorn.
- Project administration: Tipyapa Rattanamanee, Wiparat Manuyakorn.
- Writing - original draft: Tipyapa Rattanamanee.
- Writing - review & editing: Wiparat Manuyakorn, Putthapoom Lumjiaktase.
References
- 1.Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, Nelson M, Weber R, Bernstein DI, Blessing-Moore J, Khan DA, Lang DM, Nicklas RA, Oppenheimer J, Portnoy JM, Randolph C, Schuller DE, Spector SL, Tilles S, Wallace D. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(1 Suppl):S1–55. doi: 10.1016/j.jaci.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 2.Pereira-Santos MC, Baptista AP, Melo A, Alves RR, Soares RS, Pedro E, Pereira-Barbosa M, Victorino RM, Sousa AE. Expansion of circulating Foxp3+)D25bright CD4+ T cells during specific venom immunotherapy. Clin Exp Allergy. 2008;38:291–297. doi: 10.1111/j.1365-2222.2007.02887.x. [DOI] [PubMed] [Google Scholar]
- 3.Boonpiyathad T, Sözener ZC, Akdis M, Akdis CA. The role of Treg cell subsets in allergic disease. Asian Pac J Allergy Immunol. 2020;38:139–149. doi: 10.12932/AP-030220-0754. [DOI] [PubMed] [Google Scholar]
- 4.Bellinghausen I, Metz G, Enk AH, Christmann S, Knop J, Saloga J. Insect venom immunotherapy induces interleukin-10 production and a Th2-to-Th1 shift, and changes surface marker expression in venom-allergic subjects. Eur J Immunol. 1997;27:1131–1139. doi: 10.1002/eji.1830270513. [DOI] [PubMed] [Google Scholar]
- 5.Jutel M, Pichler WJ, Skrbic D, Urwyler A, Dahinden C, Müller UR. Bee venom immunotherapy results in decrease of IL-4 and IL-5 and increase of IFN-gamma secretion in specific allergen-stimulated T cell cultures. J Immunol. 1995;154:4187–4194. [PubMed] [Google Scholar]
- 6.Traiyan S, Manuyakorn W, Kanchongkittiphon W, Sasisakulporn C, Jotikasthira W, Kiewngam P, Kamchaisatian W, Benjaponpitak S. Skin prick test versus phadiatop as a tool for diagnosis of allergic rhinitis in children. Am J Rhinol Allergy. 2021;35:98–106. doi: 10.1177/1945892420938300. [DOI] [PubMed] [Google Scholar]
- 7.Cox L, Larenas-Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: The World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J Allergy Clin Immunol. 2010;125:569–574. 574.e1–574.e7. doi: 10.1016/j.jaci.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 8.Cox L. Advantages and disadvantages of accelerated immunotherapy schedules. J Allergy Clin Immunol. 2008;122:432–434. doi: 10.1016/j.jaci.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Xian M, Feng M, Dong Y, Wei N, Su Q, Li J. Changes in CD4+CD25+FoxP3+ regulatory T cells and serum cytokines in sublingual and subcutaneous immunotherapy in allergic rhinitis with or without asthma. Int Arch Allergy Immunol. 2020;181:71–80. doi: 10.1159/000503143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boonpiyathad T, van de Veen W, Wirz O, Sokolowska M, Rückert B, Tan G, Sangasapaviliya A, Pradubpongsa P, Fuengthong R, Thantiworasit P, Sirivichayakul S, Ruxrungtham K, Akdis CA, Akdis M. Role of Der p 1-specific B cells in immune tolerance during 2 years of house dust mite-specific immunotherapy. J Allergy Clin Immunol. 2019;143:1077–86.e10. doi: 10.1016/j.jaci.2018.10.061. [DOI] [PubMed] [Google Scholar]
- 11.Sahiner UM, Durham SR. Hymenoptera venom allergy: how does venom immunotherapy prevent anaphylaxis from bee and wasp stings? Front Immunol. 2019;10:1959. doi: 10.3389/fimmu.2019.01959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rattanamanee T, Lumjiaktase P, Kemawichanura N, Kiewgnam P, Jotikasthira W, Manuyakorn W. Regulatory T cell and cytokine changes in children undergoing 3 days rush venom immunotherapy. Asian Pac J Allergy Immunol. 2020 doi: 10.12932/AP-140520-0843. [Epub] [DOI] [PubMed] [Google Scholar]
- 13.Kim HB, Jin HS, Lee SY, Kim JH, Kim BS, Park SJ, Hong SJ. The effect of rush immunotherapy with house dust mite in the production of IL-5 and IFN-gamma from the peripheral blood T cells of asthmatic children. J Korean Med Sci. 2009;24:392–397. doi: 10.3346/jkms.2009.24.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchida T, Nakagome K, Iemura H, Naito E, Miyauchi S, Uchida Y, Soma T, Nagata M. Clinical evaluation of rush immunotherapy using house dust mite allergen in Japanese asthmatics. Asia Pac Allergy. 2021;11:e32. doi: 10.5415/apallergy.2021.11.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim CK, Callaway Z, Park JS, Kwon E. Efficacy of subcutaneous immunotherapy for patients with asthma and allergic rhinitis in Korea: effect on eosinophilic inflammation. Asia Pac Allergy. 2021;11:e43. doi: 10.5415/apallergy.2021.11.e43. [DOI] [PMC free article] [PubMed] [Google Scholar]