Abstract
Due to global concerns over coronavirus disease 2019 (COVID-19) vaccine-associated allergic reactions; the Hong Kong Institute of Allergy (HKIA) formulated an initial set of consensus statements (CS) on COVID-19 Vaccine Allergy Safety (VAS) in early 2021. Following accumulation of both local and international experience on and COVID-19 VAS, the HKIA task force reformed to update the Hong Kong consensus on COVID-19 VAS. A nominated task force of experts managing patients with drug and vaccine allergies in Hong Kong formulated the updated CS by unanimous decision. A total of 9 new statements were established. Individuals with history of food allergies and anaphylaxis unrelated to the components of COVID-19 vaccines do not require allergist review prior to vaccination. Individuals with history suspicious of an excipient allergy may now be vaccinated with a non-PEG containing vaccine without prior allergist assessment. Individuals with suspected mild allergic reactions following prior COVID-19 vaccination can proceed with the next dose. Only individuals who present with immediate-type allergic reaction with systemic symptoms or more severe nonimmediate type reactions should defer their next dose until allergist review. The remaining statements regarding adequate safety during vaccination and advocation for legislative changes regarding excipient disclosure in Hong Kong remained unchanged from the prior CS. The updated CS are updated in accordance with local and international experience thus far and serve as guidance for local frontline healthcare providers to further promote safe COVID-19 vaccine uptake in Hong Kong.
Keywords: Vaccine allergy, COVID-19, Allergy, Vaccine
INTRODUCTION
In February 2021, Hong Kong launched its vaccination campaign against coronavirus disease 2019 (COVID-19). Two vaccines were authorized for emergency use in Hong Kong: the Fosun Pharma/ BioNTech Comirnaty mRNA vaccine (BNT), and the Sinovac CoronaVac inactivated virus vaccine (SV). Due to global concerns over vaccine-associated allergic reactions, the Hong Kong Institute of Allergy formed a task force of experts to formulate a set of consensus statements (CS) on COVID-19 Vaccine Allergy Safety (VAS) in early 2021 [1]. The CS were intended to guide local frontline healthcare providers to maintain vaccine safety and promote vaccine uptake. The recommendations were taken up by the Department of Health and served as population-wide guidance on VAS in Hong Kong.
After months of vaccinations and billions of doses administered globally, the safety profiles of both Comirnaty and CoronaVac have proven to be reliable. Although many countries released interim guidelines early on regarding COVID-19 VAS, these strict precautions have since been relaxed by worldwide health authorities to allow more individuals to receive vaccinations. Hong Kong also published its initial experience in VAS patients attending VAS services provided by both the public and private sector over a period of 4 months [2]. In the survey, 98% of patients who were referred for prevaccination assessment were recommended for subsequent vaccination, and only a quarter underwent any allergy testing, reinforcing that COVID-19 vaccines are very safe.
Hong Kong has appreciated a low rate of COVID-19 vaccine-associated anaphylaxis (only 0.5 per million doses administered), largely due to guidance from the original CS that prioritized vaccine safety and prevention of vaccine-related allergic reactions. This low anaphylaxis rate was essential to boost vaccine confidence and to encourage uptake in the initial stages of the vaccination campaign. However, the uptake rate of vaccines remained suboptimal, with less than half of the population vaccinated by July [3], hindering achievement of herd immunity. Barriers to vaccination include a high proportion of inappropriate referrals to specialists, incorrect diagnoses of anaphylaxis, and inability to diagnose excipient allergies. In view of this, the HKIA task force reformed to update the CS on COVID-19 VAS in accordance with emerging evidence from both international and local data.
TASK FORCE OF EXPERTS
The same task force of experts managing patients with drug and vaccine allergies in Hong Kong that were nominated to formulate the first set of CS updated the statements. All members of this task force were nominated representatives of the HKIA, consisting of 2 academic representatives (PHL and ASYL), 2 public sector representatives working in the VAS Clinic of the Hospital Authority Hong Kong West Cluster (VC and EYLA), 2 private sector representatives (THL and AYYW), and 2 professional body representatives (MHKH and GWKW).
The task force formulated the updated CS by unanimous decision after multiple rounds of discussion. All members were invited to express opinions and suggest refinements until all statements reached unanimous agreement.
No ethics approval was required as no human or animal subjects were involved.
CONSENSUS STATEMENTS
A total of 9 new statements were established to replace the prior 11 published CS (Table 1). Three statements were on prevaccination recommendations, 2 statements on postvaccination guidance, and the remaining 4 statements on vaccination and legislation. These CS are for those live in Hong Kong. Individuals from localities where multiple vaccines containing various excipients are available should follow advice from respective local authorities.
Table 1. Updated consensus statements (CS) on COVID-19 Vaccine Allergy Safety in Hong Kong*.
| CS #1: People with a history of immediate-type allergic reaction with systemic symptoms to prior COVID-19 vaccination should not receive further COVID-19 vaccination until allergist evaluation. |
| CS #2: People with a history of nonimmediate type allergic reaction to prior COVID-19 vaccination which required medical attention should seek allergist advice prior to further COVID-19 vaccination. |
| CS #3: People with a history of severe immediate-type allergy to multiple classes of drugs may have an undiagnosed excipient (such as polyethylene glycol [PEG]) allergy and they may be vaccinated with a non-PEG-containing vaccine†. |
| CS #4: Allergy testing with PEG or PEG-containing surrogates appear to be poorly predictive and should not be routinely performed. In cases where these tests are used, results should be interpreted in the context of a detailed clinical history by an allergist. |
| CS #5: Patients with allergic rhinitis, asthma, atopic dermatitis, chronic urticaria, drug and food allergies, and anaphylaxis unrelated to COVID-19 vaccines (without other precautions) do not need to see an allergist for evaluation of COVID-19 vaccine allergy risk. |
| CS #6: Healthcare providers should be sufficiently prepared to recognize and treat allergic reactions properly, with adrenaline and antihistamines readily available. |
| CS #7: When an immediate-type allergic reaction following COVID-19 vaccination is suspected, blood for serum tryptase should be saved from 30 minutes to 4 hours (preferably within 2 hours) of symptom onset. |
| CS #8: People should be routinely observed for at least 15 minutes after COVID-19 vaccination. Those at higher risk of COVID-19 vaccine-associated allergic reactions should be observed for at least 30 minutes after vaccination. |
| CS #9: Full excipient lists should be mandated and made available in all product inserts of registered drugs. |
COVID-19, coronavirus disease 2019.
*Individuals in other countries where multiple vaccines containing various excipients are available should follow advice from respective local authorities. †Individuals with strong preference to receive PEG-containing vaccines may consider referral to an allergist.
CS #1: People with a history of immediate-type allergic reaction with systemic symptoms to prior COVID-19 vaccination should not receive further COVID-19 vaccination until allergist evaluation.
CS #2: People with a history of nonimmediate type allergic reaction to prior COVID-19 vaccination which required medical attention should seek allergist advice prior to further COVID-19 vaccination.
CS #3: People with a history of severe immediate-type allergy to multiple classes of drugs may have an undiagnosed excipient (such as polyethylene glycol [PEG]) allergy and they may be vaccinated with a non-PEG-containing vaccine (Individuals with strong preference to receive PEG-containing vaccines may consider referral to an allergist).
CS #4: Allergy testing with PEG or PEG-containing surrogates appear to be poorly predictive and should not be routinely performed. In cases where these tests are used, results should be interpreted in the context of a detailed clinical history by an allergist.
CS #5: Patients with allergic rhinitis, asthma, atopic dermatitis, chronic urticaria, drug and food allergies, and anaphylaxis unrelated to COVID-19 vaccines (without other precautions) do not need to see an allergist for evaluation of COVID-19 vaccine allergy risk.
CS #6: Healthcare providers should be sufficiently prepared to recognize and treat allergic reactions properly, with adrenaline and antihistamines available.
CS #7: When an immediate-type allergic reaction following COVID-19 vaccination is suspected, blood for serum tryptase should be saved from 30 minutes to 4 hours (preferably within 2 hours) of symptom onset.
CS #8: People should be routinely observed for at least 15 minutes after COVID-19 vaccination. Those at higher risk of COVID-19 vaccine-associated allergic reactions should be observed for at least 30 minutes after vaccination.
CS #9: Full excipient lists should be mandated and made available in all product inserts of registered drugs.
DISCUSSION
Given the novel nature of COVID-19 vaccines and associated allergies that emerged during the initial doses of vaccinations worldwide, expert working groups set forth to provide interim recommendations to support the global vaccination effort [4,5,6]. Most of the recommendations erred on the side of caution to minimize allergy-related events, and prioritized vaccine safety to facilitate COVID-19 vaccination uptake. Individual allergy history such as a history of anaphylaxis, severe drug, food, or venom allergies, were all assumed as clinical predictors associated with higher risk of COVID-19 vaccine-related allergy [7]. However, they were primarily based on collective expert opinion, and were expected to evolve along with clinical practices as more evidence became available.
Months into the vaccination program, COVID-19 vaccines, regardless of their formulations, have proven to be very safe. Countries have published data that support the safety profiles of the vaccines, with rates of anaphylaxis and allergy-related events comparable to those of other vaccines [8]. In Hong Kong, the anaphylaxis rate to COVID-19 vaccines was at 0.5 per 1 million doses administered, and other vaccine-associated allergic events were kept similarly low. Hong Kong was also able to characterize its experience of the initial months of COVID-19 VAS in Hong Kong [2]. This comprehensive data set allowed us to assess risk factors for vaccine-associated allergy and vaccination outcomes, and supported the updates of our CS.
Updates to risk stratify reactions following COVID-19 vaccinations were necessary given the prevalence of such reactions, likely attributed to the increased immunogenicity of the novel mRNA vaccines compared to traditional vaccines. There is an increase in reactogenic symptoms, including injection-site reactions, myalgia, headache, fever, fatigue, and in some cases, a transient rash. As seen in local postvaccine patients, mucocutaneous manifestations were largely mild and self-limiting, and did not correlate with the reproducibility nor severity of symptoms following next dose of vaccination [9,10]. In fact, proceeding with a second dose has been shown to be safe even in more severe reactions and cases of anaphylaxis [11]. However, given the paucity of these cases, we decided to set the threshold for deferring next dose at a similar level to widely adapted guidelines, such as those provided by the United Kingdom’s Green Book, COVID-19 chapter 14a [12].
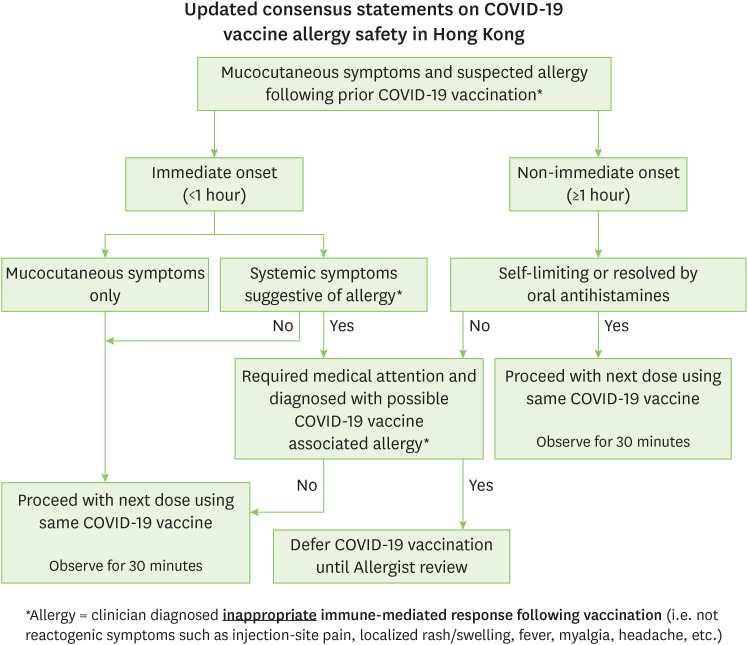
Individuals with suspected mild allergic reactions following prior COVID-19 vaccination can proceed with the next dose. Only individuals who present with immediate-type allergic reaction with systemic symptoms or more severe nonimmediate type reactions should defer their next dose until allergist review. We supplemented these 2 conditions with an algorithm that can be easily accessible by first-line doctors who assess these reactions, adapted from the Green Book guidance for mucocutaneous reactions following vaccinations [12] (Fig. 1). It is safe for patients with mild reactions to their first dose to receive their next dose of vaccines, and that the benefits of completion of vaccination outweigh the risks of mild allergic reactions.
Fig. 1. Algorithm for suspected allergic reactions to prior coronavirus disease 2019 (COVID-19) vaccination.
Precautions for vaccine-naïve individuals have been reviewed and updated by multiple healthcare authorities around the world, as emerging evidence shows a lack of association between allergic conditions with risk of developing vaccine-associated allergic reactions [10]. Our local study similarly found that a history of anaphylaxis, multiple food allergies, and drug allergies were not associated with higher risk of vaccine-associated allergies. In view of this, prior precautions including history of severe, immediate-type allergy to multiple food groups and history of anaphylaxis unrelated to COVID-19 vaccines and their relevant components were removed from our updated recommendations. Additionally, we reassure healthcare providers that patients with atopic conditions, chronic urticaria, and history of allergies can all be safely vaccinated and do not require any prevaccination evaluation by an allergist.
Immediate-type reactions to COVID-19 vaccines are postulated to be caused by relevant vaccine excipients [13,14,15]. While a history of severe, immediate-type allergy to multiple classes of drugs is unlikely to be associated with COVID-19 vaccine allergy, we recommend these patients to receive a non-PEG containing vaccine instead. This is due to Hong Kong’s problematic drug legislation, which does not require full disclosure of excipients in listed drugs, making it extremely difficult to diagnose excipient allergies [16]. In our local study, although only 2% of prevaccination patients were recommended to defer vaccinations, over 80% of these patients had multiple, suspicious drug allergy histories which could point towards potentially undiagnosed excipient allergies. Healthcare authorities of other countries such as the Medicines and Healthcare products Regulatory Agency (UK), Australian Society of Clinical Immunology and Allergy, the National Advisory Committee on Immunization (Canada), and the Centers for Disease Control and Prevention (US), have listed known excipient allergies as contraindications to their respective specific excipient-containing vaccines [17,18,19,20,21]. Since the excipient in question cannot be discerned without full excipient lists, this statement serves as a surrogate for possible PEG allergy, and these patients should avoid PEG-containing vaccines. This highlights a major flaw in Hong Kong’s pharmaceutical legislation, and we continue to urge that full excipient lists be made available for all drugs.
In line with international experience, assessment of possible COVID-19 vaccine allergy with traditional skin testing (including skin prick tests and intradermal skin tests) has shown to be inconclusive. Testing with PEG, PEG-containing surrogates, and even COVID-19 vaccines appear to be poorly predictive [5,22,23]. In addition, adverse cutaneous reactions following vaccine skin testing are known and well-reported, ranging in severity that does not correlate clinically [24]. We therefore caution the use of such procedures, which should not be routinely performed. In cases where these tests are used, results should be interpreted in the context of a detailed clinical history by an allergist.
Statements pertaining to the vaccination process remain unchanged from our previous CS. They include monitoring requirements and management of potential allergic reactions which are in keeping with current practice for anaphylaxis management [25]. Adrenaline remains the first-line treatment for all patients at risk of anaphylaxis. An acute serum tryptase level should be saved from 30 minutes to 4 hours following an acute reaction, to help support or exclude a diagnosis of anaphylaxis. Postvaccine observation for at least 15 minutes and extended to 30 minutes for those at higher risk as per standard care continues to balance safety and practicality.
CONCLUSION
The original CS have proved to be successful in maximizing vaccination safety in order to bolster vaccine confidence and uptake. However, those recommendations were based largely on expert opinion and preliminary data, and were expected to be superseded by more robust and specific evidence-based guidance. The updated CS are updated in accordance with local and international experience thus far and serve as guidance for local frontline healthcare providers to further promote safe COVID-19 vaccine uptake in Hong Kong, bringing us closer to our goal of herd immunity.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Valerie Chiang, Philip H. Li.
- Formal analysis: Valerie Chiang, Philip H. Li.
- Investigation: Valerie Chiang, Agnes S.Y. Leung, Elaine Y.L. Au, Marco H.K. Ho, Tak Hong Lee, Adrian Y.Y. Wu, Gary W.K. Wong, Philip H. Li.
- Methodology: Valerie Chiang, Agnes S.Y. Leung, Elaine Y.L. Au, Marco H.K. Ho, Tak Hong Lee, Adrian Y.Y. Wu, Gary W.K. Wong, Philip H. Li.
- Project administration: Philip H. Li.
- Writing - original draft: Valerie Chiang.
- Writing - review & editing: Valerie Chiang, Agnes S.Y. Leung, Elaine Y.L. Au, Marco H.K. Ho, Tak Hong Lee, Adrian Y.Y. Wu, Gary W.K. Wong, Philip H. Li.
References
- 1.Chiang V, Leung ASY, Au EYL, Ho MHK, Lee TH, Wu AYY, Wong GWK, Li PH. Consensus statements on the approach to COVID-19 Vaccine Allergy Safety in Hong Kong. Front Allergy. 2021 doi: 10.3389/falgy.2021.690837. July 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiang V, Mok SWS, Chan JKC, Leung WY, Ho CTK, Au EYL, Lau CS, Lee TH, Li PH. Experience of the first 1127 COVID-19 Vaccine Allergy Safety patients in Hong Kong - clinical outcomes, barriers to vaccination, and urgency for reform. World Allergy Organ J. 2022;15:100622. doi: 10.1016/j.waojou.2021.100622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong Kong Vaccination Dashboard [Internet] Hong Kong: The government of the Hong Kong Special Administrative Region; 2021. [cited 2021 Jul 21]. Available from: https://www.covidvaccine.gov.hk/en/ [Google Scholar]
- 4.CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine - United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenhawt M, Shaker M, Golden DBK. PEG/polysorbate skin testing has no utility in the assessment of suspected allergic reactions to SARS-CoV-2 vaccines. J Allergy Clin Immunol Pract. 2021;9:3321–3322. doi: 10.1016/j.jaip.2021.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner PJ, Ansotegui IJ, Campbell DE, Cardona V, Ebisawa M, El-Gamal Y, Fineman S, Geller M, Gonzalez-Estrada A, Greenberger PA, Leung ASY, Levin ME, Muraro A, Sánchez Borges M, Senna G, Tanno LK, Yu-Hor Thong B, Worm M WAO Anaphylaxis Committee. COVID-19 vaccine-associated anaphylaxis: a statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ J. 2021;14:100517. doi: 10.1016/j.waojou.2021.100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US-December 14, 2020-January 18, 2021. JAMA. 2021;325:1101–1102. doi: 10.1001/jama.2021.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hourihane JOB, Byrne AM, Blümchen K, Turner PJ, Greenhawt M. Ascertainment bias in anaphylaxis safety data of COVID-19 vaccines. J Allergy Clin Immunol Pract. 2021;9:2562–2566. doi: 10.1016/j.jaip.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krantz MS, Kwah JH, Stone CA, Jr, Phillips EJ, Ortega G, Banerji A, Blumenthal KG. Safety evaluation of the second dose of messenger RNA COVID-19 vaccines in patients with immediate reactions to the first dose. JAMA Intern Med. 2021;181:1530–1533. doi: 10.1001/jamainternmed.2021.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shavit R, Maoz-Segal R, Iancovici-Kidon M, Offengenden I, Haj Yahia S, Machnes Maayan D, Lifshitz-Tunitsky Y, Niznik S, Frizinsky S, Deutch M, Elbaz E, Genaim H, Rahav G, Levy I, Belkin A, Regev-Yochay G, Afek A, Agmon-Levin N. Prevalence of allergic reactions after Pfizer-BioNTech COVID-19 vaccination among adults with high allergy risk. JAMA Netw Open. 2021;4:e2122255. doi: 10.1001/jamanetworkopen.2021.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krantz MS, Bruusgaard-Mouritsen MA, Koo G, Phillips EJ, Stone CA, Jr, Garvey LH. Anaphylaxis to the first dose of mRNA SARS-CoV-2 vaccines: don’t give up on the second dose! Allergy. 2021;76:2916–2920. doi: 10.1111/all.14958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.UK Health Security Agency. Coronavirus (COVID-19) vaccination information for public health professionals. London: UK Health Security Agency; 2020. [Google Scholar]
- 13.Cabanillas B, Novak N. Allergy to COVID-19 vaccines: a current update. Allergol Int. 2021;70:313–318. doi: 10.1016/j.alit.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caballero ML, Quirce S. Excipients as potential agents of anaphylaxis in vaccines: analyzing the formulations of currently authorized COVID-19 vaccines. J Investig Allergol Clin Immunol. 2021;31:92–93. doi: 10.18176/jiaci.0667. [DOI] [PubMed] [Google Scholar]
- 15.Sellaturay P, Nasser S, Islam S, Gurugama P, Ewan PW. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin Exp Allergy. 2021;51:861–863. doi: 10.1111/cea.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li PH, Yeung HHF, Lau CS, Au EYL. Excipient allergy and importance of complete allergy histories. J Allergy Clin Immunol Pract. 2020;8:2122–2123. doi: 10.1016/j.jaip.2020.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. COVID-19 vaccines reported adverse events. Atlanta (GA): Centers for Disease Control and Prevention; 2021. [Google Scholar]
- 18.Banerji A, Wolfson AR, Wickner PG, Cogan AS, McMahon AE, Saff R, Robinson LB, Phillips E, Blumenthal KG. COVID-19 vaccination in patients with reported allergic reactions: updated evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9:2135–2138. doi: 10.1016/j.jaip.2021.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.COVID-19 vaccines (Pfizer/BioNTech and COVID-19 Vaccine AstraZeneca): current advice [Internet] London: Medicines and Healthcare products Regulatory Agency; 2021. [2021 Jun 23]. Available from: https://www.gov.uk/drug-safety-update/covid-19-vaccines-pfizer-slash-biontech-and-covid-19-vaccine-astrazeneca-current-advice. [Google Scholar]
- 20.Recommendations on the use of COVID-19 vaccines [Internet] Ottawa: Government of Canada; 2021. [2021 Jun 22]. Available from: https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines.html#b9. [Google Scholar]
- 21.Guide: allergy and COVID-19 vaccination [Internet] Brookvale (NSW): Australasian Society of Clinical Immunology and Allergy; 2021. [2021 Jun 21]. Available from: https://www.allergy.org.au/hp/papers/guide-allergy-and-covid-19-vaccination. [Google Scholar]
- 22.Wolfson AR, Robinson LB, Li L, McMahon AE, Cogan AS, Fu X, Wickner P, Samarakoon U, Saff RR, Blumenthal KG, Banerji A. First-dose mRNA COVID-19 vaccine allergic reactions: limited role for excipient skin testing. J Allergy Clin Immunol Pract. 2021;9:3308–3320.e3. doi: 10.1016/j.jaip.2021.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bianchi L, Biondi F, Hansel K, Murgia N, Tramontana M, Stingeni L. Skin tests in urticaria/angioedema and flushing to Pfizer-BioNTech SARS-CoV-2 vaccine: Limits of intradermal testing. Allergy. 2021;76:2605–2607. doi: 10.1111/all.14839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiang V, Mong PPT, Chan EKK, Au EYL, Li PH. Caution against injudicious vaccine allergy skin tests: Adverse reactions after intradermal COVID-19 vaccine testing. Contact Dermatitis. 2021:cod.13977. doi: 10.1111/cod.13977. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li PH, Chua GT, Leung ASY, Chan YC, Chan KKL, Cheung KH, Chong PCY, Ho PPK, Kwan MYW, Lai JCH, Lam KK, Lam TSK, Leung TF, Li TY, Duque JSR, So JLT, Wan KA, Wong HCY, Wu AYY, Lee TH, Ho MHK, Siu AYC. Hong Kong Anaphylaxis Consortium Consensus Statements on prescription of adrenaline autoinjectors in the acute care setting. Asia Pac Allergy. 2021;11:e1. doi: 10.5415/apallergy.2021.11.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]