Abstract
Gut microbiome composition is associated with mood-relating behaviours, including those reflecting depression-like phenotypes. Repetitive transcranial magnetic stimulation (rTMS), a non-invasive neuromodulation technique, is an effective treatment for depression, but its effects on the gut microbiome remain largely unknown. This study assessed microbial changes from rat faecal samples longitudinally following chronic restraint stress (CRS) and 10 Hz low-intensity rTMS treatment. CRS increased abundance within the Proteobacteria (Deltaproteobacteria, Desulfovibrionales) and Firmicutes (Anaerostipes, Frinsingococcus), with decreases in Firmicutes family (Acidaminococcaceae) and genera (Roseburia, Phascolarctobacterium and Fusicatenibacter) persisting for up to 4 weeks post CRS. The decrease in Firmicutes was not observed in the handling control and LI-rTMS groups, suggesting that handling alone may have sustained changes in gut microbiome associated with CRS. Nonetheless, LI-rTMS was specifically associated with an increase in Roseburia genus that developed 2 weeks after treatment, and the abundance of both Roseburia and Fusicatenibacter genera was significantly correlated with rTMS behavioural and MRI outcomes. In addition, LI-rTMS treated rats had a reduction in apoptosis pathways and several indicators of reduced inflammatory processes. These findings provide evidence that the brain can influence the gut microbiome in a “top-down” manner, presumably via stimulation of descending pathways, and/or indirectly via behavioural modification.
Keywords: Microbiome, rTMS, Chronic restraint stress, Depression, Animal model
1. Introduction
Major depressive disorder (MDD) is a debilitating neuropsychiatric syndrome with significant morbidity and mortality due to the risk of suicide. Whilst the exact cause of depression is still largely unknown, current research suggests that mental illnesses such as MDD are caused by a complex array of genetic, neurological, hormonal, immunological, environmental, and psychological factors. These factors may be integrated via the gut-brain axis, the bidirectional communication network between the central and enteric nervous systems consisting of neural and immune pathways as well as the hypothalamic-pituitary-adrenal axis (Capuco et al., 2020; Cruz-Pereira et al., 2020). There is increasing evidence that gut-brain signalling is altered in MDD, with with an altered gut microbiome reported as well as a link between MDD and gut symptoms (e.g. IBS) (Kochar et al., 2018; Barandouzi et al., 2020). However the causal relationships between the “bottom” (gut, microbiome), and the “top” (mood and brain function) of the gut-brain axis remain unclear.
Currently, most studies have investigated the bottom-up effects of the gut microbiome on brain chemistry and relevant behaviour in depression. The gut, including the composition of the microbiome, influences mood, cognition and other neurological functions (Clapp et al., 2017; Makris et al., 2021). For example, a faecal microbiota transplant from stressed animals or patients with depression to healthy animals has been shown to increase anxiety and depression-like behaviours in recipients, whereas the transplant of healthy microbiota to patients with depression conversely decreases depression symptoms (Chinna Meyyappan et al., 2020). Similarly, probiotic treatment has been shown to reduce anxiety- and depression-related behaviours (Bravo et al., 2011) and restore brain changes related to depression and emotion processing (Bercik et al., 2010; Tillisch et al., 2013). However, the evidence for a top-down influence of the brain on the microbiome is less clear. While the brain regulates gastrointestinal motility, secretory activity and immune responses (Mayer, 2011), and psychological stress can change microbiome composition (Galley et al., 2014), a direct influence of brain signalling on the microbiome has not been definitively demonstrated. Additionally, many antidepressant medications targeting brain function and mood are delivered orally and have antimicrobial properties (Valles-Colomer et al., 2019; Ait Chait et al., 2020), making it difficult to differentiate between potential top-down effects of neurological change on the gut, and the direct effect of oral medication on microbiome composition (Lukić et al., 2019; McGovern et al., 2019).
The use of brain-specific treatment methods can help to dissect the top-down effects of depression treatment on the gut microbiome. Non-invasive brain stimulation has exhibited potential positive effects on the gut microbiome in patients with weight disorders through subsequent improvements in weight (Artifon et al., 2020; Ferrulli et al., 2021). Here we use repetitive transcranial magnetic stimulation (rTMS), a non-invasive FDA-approved treatment for depression (O'Reardon et al., 2007) that uses electromagnetic induction to alter the excitability of the brain (George et al., 2000). Given the evidence for perturbed gut-brain signalling in depression, we hypothesised that this brain-specific treatment for depression would allow us to isolate any potential top-down effects of the brain on the gut microbiome. Because of the variability of human subjects not only in terms of the gut microbiome but also in their response to rTMS, here we use a well characterised chronic restraint intervention in rats to: 1) validate the effects of a chronic restraint stress model of depression on gut microbial composition and diversity in male Sprague-Dawley rats and 2) identify the effects of low-intensity rTMS treatment on the gut microbiome in this depression model.
2. Methods
All experimental procedures were approved by the University of Western Australia (UWA) Animal Ethics Committee (RA/3/100/1640) and conducted in accordance with the National Health and Medical Research Council Australian code for the care and use of animals for scientific purposes. All investigators were trained in animal handling by the UWA Programme in Animal Welfare, Ethics, and Science (PAWES) and had valid Permission to Use Animals (PUA) licenses.
2.1. Animals
The microbiome samples analysed here were collected from several cohorts of rats over a 2-year period. In addition to CRS and rTMS described in detail below, animals underwent behavioural testing and MRI imaging procedures to measure brain anatomy, resting-state functional MRI (rs-fMRI) and neurotransmitter levels (Magnetic Resonance Spectroscopy) (Fig. 1). The outcomes of MRI (including rs-fMRI, hippocampal volume and neurotransmitter levels) and behavioural tests are used in the present study for correlation analysis, and have been submitted for publication in full elsewhere. Summary of behavioural data are available on the Figshare repository (https://figshare.com/), with https://doi.org/10.6084/m9.figshare.16866790. Full raw behavioural and MRI datasets are available on request to the corresponding author.
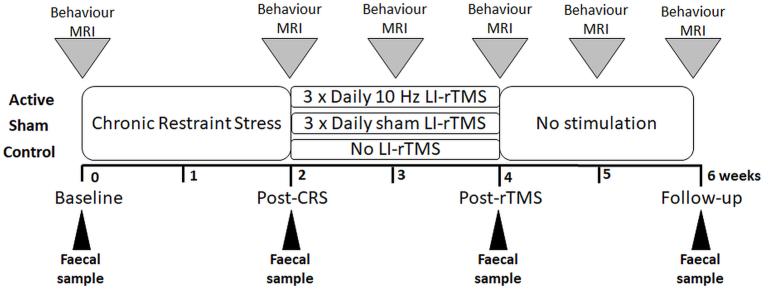
Fig. 1.
Experimental timeline. The research design consisted of an initial one-week period of habituation upon arrival of the animal. Rats then underwent chronic restraint stress for 2.5 h daily for 13 consecutive days. Animals were randomly allocated to 3 groups: active animals received 10 min of 10 Hz LI-rTMS three times daily (1 h apart, five days a week for two weeks), sham animals received a sham version of the stimulation protocol and depression control animals received no stimulation or extra handling. Faecal samples were collected at baseline (before the start of CRS), after the end of the 13 days of CRS (Post-CRS), after the end of the treatment period (Post-rTMS) and two weeks after the end of the treatment period (Follow-up).
Young adult male Sprague-Dawley rats (5–6 weeks old, 150–200g) from the Animal Resources Centre (Perth, Western Australia) were maintained in a temperature-controlled animal care facility on a 12-h light-dark cycle with food and water ad libitum (except during restraint). Animals were allowed to acclimatise to the facility for one week following their arrival. Cages contained two acidified water bottles (acidified as per safety regulations) and 500g of food pellets. To ensure the influence of diet on the gut microbiome was minimal, the diet for all animals was kept consistent and the weights of the animals were recorded weekly. Animals were randomly assigned to one of the following groups: 1) active group consisting of animals (n = 12) which underwent CRS and received accelerated 10 Hz LI-rTMS (10 min three times daily, 1 h apart, five days/week for two weeks) to the left hemisphere; 2) sham group consisting of (n = 12) animals which underwent CRS and sham LI-rTMS; and 3) control group consisting of animals (n = 7) which underwent CRS but did not receive any stimulation or extra handling during the treatment period (Fig. 1). Animals were housed in pairs, such that those within the same group were co-housed to prevent transmission of faecal dysbiosis (Sun et al., 2013). At the end of the study, animals were euthanised by intraperitoneal overdose of pentobarbitone (Lethabarb) anaesthesia (160 mg/kg i.p.).
The CRS procedure was carried out for 2.5 h/day for 13 consecutive days as described previously (Seewoo et al., 2020). Animals receiving LI-rTMS were habituated to handling and the coil as described previously (Rodger et al., 2012; Makowiecki et al., 2014). LI-rTMS was delivered at a frequency of 10 Hz using a custom-built round coil at an intensity of approximately 13 mT at the surface of the cortex, which is below the rodent's motor threshold (Seewoo et al., 2018). Stimulation was delivered following an accelerated protocol: 3 times daily at 1-h intervals for 2 weeks (Seewoo et al., 2019, 2021). During the sham procedure, the coil was disconnected. Faecal pellets were collected from rats in all groups prior to CRS (baseline), after the last CRS procedure (post-CRS), at the end of the treatment period (post-rTMS) and two weeks after end of the treatment period (follow-up) (Fig. 1).
2.2. Sample preparation and sequencing
Between 2 and 6 faecal pellets per animal were collected four timepoints, each separated by two weeks: baseline, post CRS, post LI-rTMS and follow up (Fig. 1). Pellets were collected during behavioural testing sessions when they were spontaneously and abundantly expelled in a clean and controlled environment with no additional interventions. Pellets were frozen within 10 min of production at each collection timepoint and stored separately at −80 °C until the completion of the animal cohort. Using aseptic techniques to avoid cross contamination, rat faecal pellets were subsequently weighed into microcentrifuge tubes, and a 10 g mid-section of each pellet was sent to Australian Genome Research Facility (AGRF, Australia) for DNA extraction and sequencing of the 16S rRNA gene V3–V4 region on an Illumina MiSeq platform using the 2 × 300 bp V3 chemistry. The V3V4 primers used by AGRF were a modified 341F (5′-CCTAYGGGRBGCASCAG-3′) and a modified 806R (5′-GGACTACNNGGGTAT CTAAT-3′) (Yu et al., 2005). The 16S raw sequencing data is publicly available at Figshare repository (https://figshare.com/), with https://doi.org/10.6084/m9.figshare.16866790.
2.3. 16S data processing
Raw sequencing reads were subjected to quality and adapter trimming using the bbduk.sh command available in the BBTools suite (version 38.87) (https://jgi.doe.gov/data-and-tools/bbtools/), with the following input parameters: qtrim = r trimq = 20 ktrim = r k = 23 mink = 11 minlen = 200 hdist = 1 tpe tbo. Next, the MeFiT pipeline (version 1.0) (Parikh et al., 2016) was run with default parameters to merge trimmed overlapping paired-end reads. The summary statistics of the trimmed and merged sequence reads are available in Table S1. For filtering and clustering of merged reads into operational taxonomic units (OTUs), the MICCA software (version 1.7.2) was used (Albanese et al., 2015). In detail, merged sequences of less than 420 bp were filtered using the micca filter command. Then, the micca otu command was run to perform de novo greedy clustering of the sequences into OTUs using a sequence identity threshold of 97%, along with the removal of chimeric sequences and singleton OTUs. For each representative OTU sequence, taxonomic information was assigned using the Bayesian LCA-based taxonomic classification method (version 2.3-alpha) with a minimum e-value of 1e-10 and 100 bootstrap replications, against the NCBI 16S ribosomal RNA database (Gao et al., 2017). A minimum confidence score of 80 was used to accept a taxonomic assignment at each level. Multiple sequence alignment of the OTU representative sequences was performed using PASTA (Mirarab et al., 2014), where the output alignment file was used for phylogenetic tree construction using FastTree (Price et al., 2010) under the GTR model with CAT approximation. The OTU table, as well as the taxonomic information, are available in Table S2.
Prior to analysing both alpha and beta diversities using the phyloseq (McMurdie and Holmes, 2013) and amplicon (https://rdrr.io/github/microbiota/amplicon) R packages, rarefaction of the OTU table was performed at 8462 sequences per sample. For alpha diversity analysis, four indices were evaluated, including the number of observed OTUs, abundance-based coverage estimator (ACE), Shannon entropy and inverse Simpson. Comparison of alpha diversity between groups and different timepoints was performed using ANOVA with post-hoc Tukey test. For beta diversity analysis, principal coordinates analysis (PCoA) was performed at the OTU level using both weighted and unweighted UniFrac distance metrics, followed by the analysis of similarity test (ANOSIM) to test for differences in microbial communities between groups and different timepoints. Volatility analysis was performed for each group of rat subjects by comparing the Aitchison distances for OTUs between successive samples collected from the same animal over time (Bastiaanssen et al., 2021). Prediction of the microbial communities for KEGG functional pathways was performed using PICRUSt2 (version 2.3.0-b) (Douglas et al., 2020). The generated KEGG pathway abundances are available in Table S3.
2.4. Statistical analysis
To identify significant bacterial taxa and KEGG pathways between groups and different timepoints, the analysis of composition of microbiomes (ANCOM) procedure (Lin and Peddada, 2020) was performed on unfiltered absolute abundance data, adjusted for cage and study batch effects. The p-values were adjusted using the Benjamini-Hochberg procedure at the significance level of 0.05. Significant bacterial taxa and KEGG pathways were declared by using ANCOM's W-statistic with a threshold of 0.7. All ANCOM test results are available in Table S4. For each differentially abundant taxa and KEGG pathways identified by ANCOM, further statistical testing was performed on the centered log-ratio (CLR)-transformed raw taxa and KEGG abundance values as described below.
2.4.1. Effect of CRS
Bacterial diversity and composition were similar between groups at baseline. Due to the small sample size, data from the baseline and post-CRS timepoints were pooled from the three groups receiving restraint to determine the effects of CRS on the gut microbiome. The difference in bacterial composition between baseline and post-CRS timepoints was examined by the Wilcoxon signed rank test (n = 29/timepoint).
2.4.2. Effect of LI-rTMS
To determine the effects of LI-rTMS on the gut microbiome, the Quade test was used to determine if there was a significant effect of timepoint (post-CRS, post-rTMS and follow-up) in each group, followed by Post hoc Wilcoxon signed rank test to determine paired differences between timepoints. The Benjamini-Hochberg false discovery rate (FDR) method was used to correct for multiple comparisons at the significance level of 0.05.
2.4.3. Correlation analysis
Magnetic resonance imaging (MRI) and behavioural data were acquired at the four timepoints using previously published methods (Seewoo et al., 2020). Spearman correlations were performed between the microbiome data and the following MRI and behavioural measures from unpublished results: GABA and glutamate concentration (as a ratio to total creatine concentration) obtained from proton magnetic resonance spectroscopy (1H-MRS) within the sensorimotor cortex; hippocampal volume obtained from T2-weighted anatomical MRI data; cingulate cortex functional connectivity obtained from seed-based analysis of resting-state functional MRI (rs-fMRI) data; functional connectivity within the interoceptive network, salience network and default mode network obtained from independent component analysis of rs-fMRI data; number of exits from the open arms of the elevated plus maze (EPM); time spent immobile during the forced swim test (FST); time spent swimming during FST; and latency, which is the time taken to exhibit the first immobility behaviour during FST.
3. Results
3.1. Effect of CRS on the gut microbiome
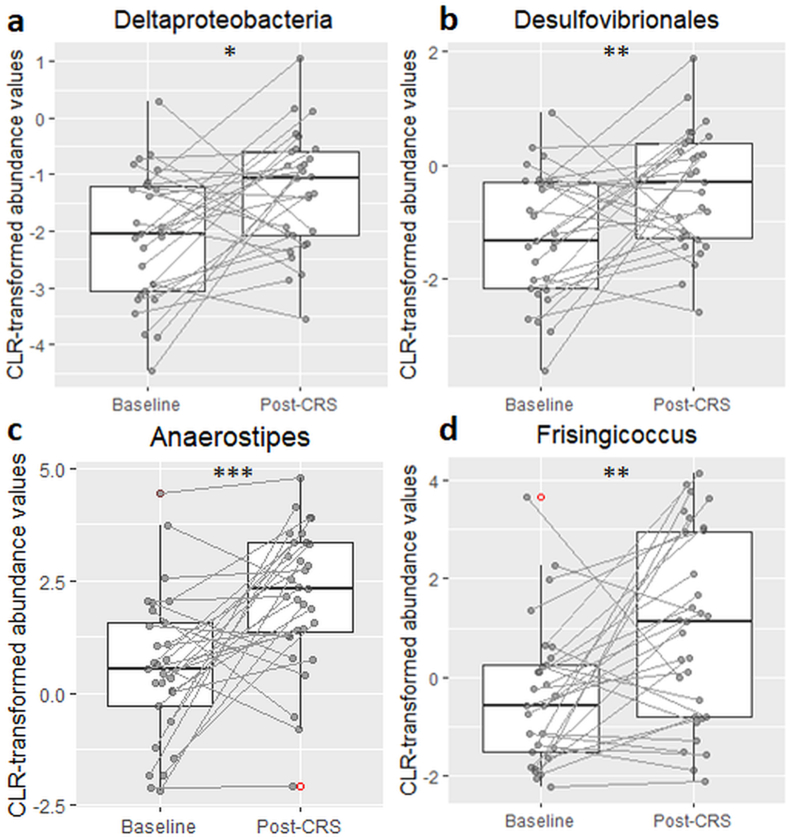
CRS did not induce any changes in alpha diversity or beta diversity (Supplementary Figs. S1–S10). However, class-level analyses revealed a significant increase in Deltaproteobacteria post-CRS (V = 102, p = 0.011) which was accompanied by an increase in the corresponding Desulfovibrionales order (V = 98, p = 0.009; Fig. 2). Genus-level analysis showed marked increases in both Anaerostipes and Frisingicoccus of the Clostridia class (V = 68, p < 0.001; V = 86, p = 0.004 respectively; Fig. 2). There were no significant changes in any other taxa or KEGG pathways.
Fig. 2.
Effect of chronic restraint stress on abundance of Deltaproteobacteria class (a), and its corresponding Desulfovibrionales order (b), and Anaerostipes (c), and Frisingicocus (d) genera of the Clostridia class . The figure shows boxplots of centered log-ratio transformed taxa abundances for taxa identified as significant in the ANCOM's W-statistic (threshold of 0.7). Between-timepoint comparisons were made by Wilcoxon signed rank test. *p < 0.05; **p < 0.01; ***p < 0.001.
3.2. Effect of LI-rTMS on the gut microbiome
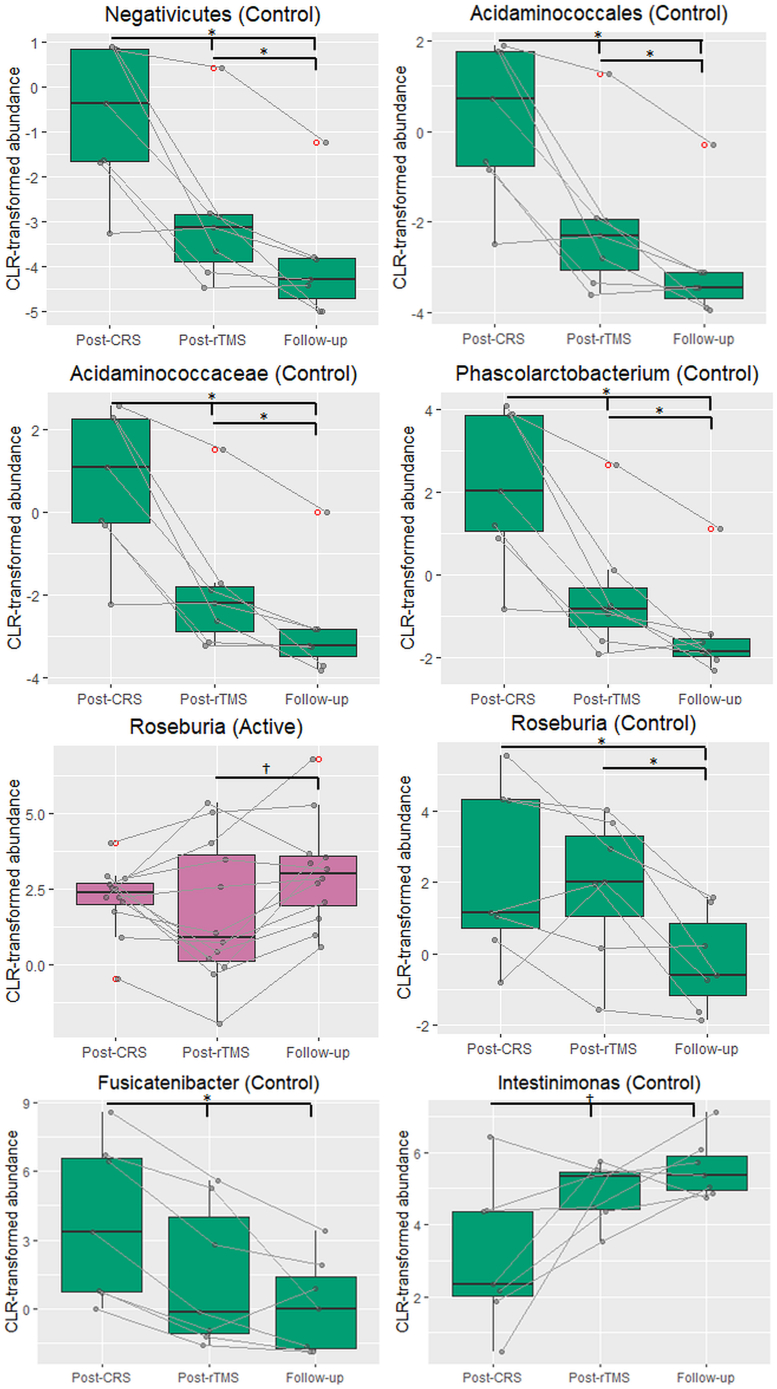
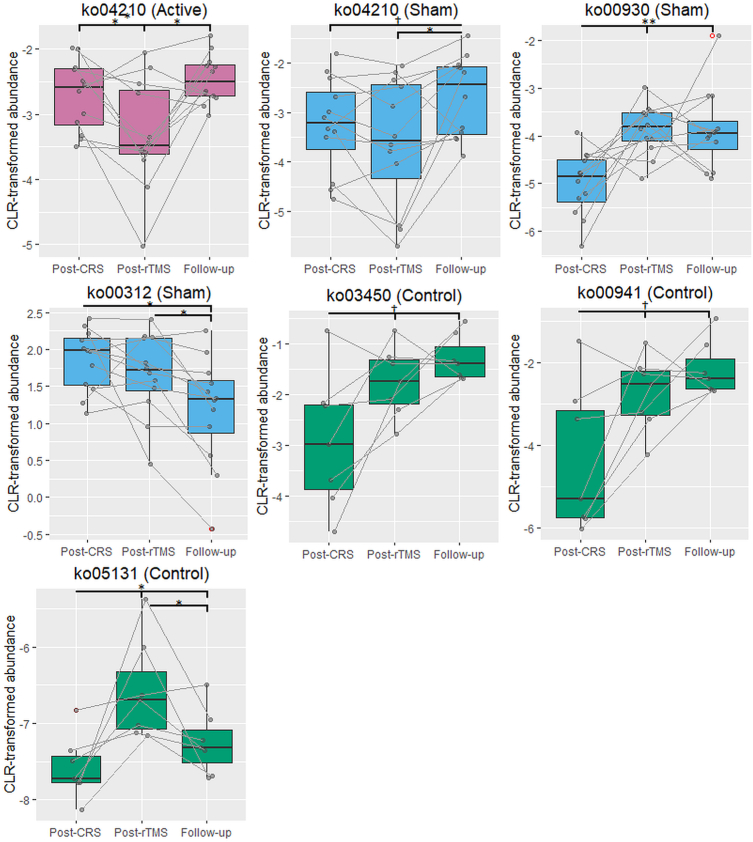
LI-rTMS did not induce any changes in alpha diversity or beta diversity (Supplementary Figs. S1–S10). Class-level analyses revealed a significant decrease in the Negativicutes class over time in the depression control group (F(2,12) = 12.20, p = 0.001; pFDR = 0.031 for all; Fig. 3). This decrease was accompanied by a significant decrease in the corresponding Acidaminococcales order (F(2,12) = 12.20, p = 0.001; pFDR = 0.047 for all). Within the Negativicutes class, Acidaminococcaceae family also significantly decreased over time in the depression control group (F(2,12) = 20.37, p < 0.001; pFDR = 0.031 for Post-CRS vs Post-rTMS, pFDR = 0.023 for Post-CRS vs Follow-up and Post-rTMS vs Follow-up), as did the corresponding Phascolarctobacterium genus (F(2,12) = 14.14, p < 0.001; pFDR = 0.023 for Post-CRS vs Post-rTMS and Follow-up, pFDR = 0.031 for Post-rTMS vs Follow-up). Additionally, there was a significant effect of timepoint for the Roseburia genus from the Clostridia class in the active (F(2,22) = 4.569, p = 0.022) and depression control groups (F(2,12) = 5.243, p = 0.023). There was a trend for an increase in Roseburia genus between the post-rTMS and follow-up timepoints (pFDR = 0.081) in the active group while Roseburia genus decreased significantly at the follow-up timepoint compared to both post-CRS and post-rTMS timepoints in the depression control group (pFDR = 0.047 for both). Furthermore, within the Clostridia class in the control group, there was a significant decrease in Fusicatenibacter genus from the post-CRS timepoint to the post-rTMS and follow-up timepoints (F(2,12) = 15.23, p < 0.001; pFDR = 0.047 for both) and a trend for an increase in Intestinimonas genus from the post-CRS timepoint to the post-rTMS and follow-up timepoints (F(2,12) = 7.868, p = 0.007; pFDR = 0.07 for both). There were several significant changes in KEGG functional pathways (Table 1, Fig. 4).
Fig. 3.
Changes in abundance of taxa between timepoints within the active, sham and depression control groups. The figure shows boxplots of centered log-ratio transformed taxa abundances for taxa identified as significant in the ANCOM's W-statistic (threshold of 0.7). Quade test was used to determine if there was a significant effect of timepoint (post-CRS, post-rTMS and follow-up) in each group. Between-timepoint comparisons were made by Post hoc Wilcoxon signed rank test with Benjamini-Hochberg FDR correction. †p < 0.1; *p < 0.05.
Table 1.
Summary of changes in abundance for KEGG functional pathways following chronic restraint stress (CRS) and between timepoints within the active, sham and depression control groups. Active animals received 10 min of 10 Hz LI-rTMS three times daily (1 h apart, five days a week for two weeks), sham animals received a sham version of the stimulation protocol with the coil turned off and control animals received no stimulation or extra handling.
| KEGG | Functional pathway | Group | Quade test | Timepoint comparison | Mean ± SD |
|---|---|---|---|---|---|
| ko04210 | Apoptosis | Active | F(2,22) = 8.272, p = 0.002 | Post-CRS vs Post-rTMS pFDR = 0.031 Post-rTMS vs Follow-up pFDR = 0.028 |
Post-CRS = −2.71 ± 0.53 Post-rTMS = −3.32 ± 0.83 Follow-up = −2.46 ± 0.37 |
| Sham | F(2,22) = 7.286, p = 0.003 | Post-CRS vs Follow-up pFDR = 0.078 Post-rTMS vs Follow-up pFDR = 0.037 |
Post-CRS = −3.25 ± 0.96 Post-rTMS = −3.60 ± 1.29 Follow-up = −2.67 ± 0.82 |
||
| ko00930 | Caprolactam degradation | Sham | F(2,22) = 5.016, p = 0.016 | Post-CRS vs Post-rTMS pFDR = 0.007 Post-CRS vs Follow-up pFDR = 0.007 |
Post-CRS = −5.00 ± 0.67 Post-rTMS = −3.87 ± 0.53 Follow-up = −3.88 ± 0.84 |
| ko00312 | beta-Lactam resistance | Sham | F(2,22) = 6.790, p = 0.005 | Post-CRS vs Follow-up pFDR = 0.031 Post-rTMS vs Follow-up pFDR = 0.010 |
Post-CRS = 1.85 ± 0.42 Post-rTMS = 1.66 ± 0.56 Follow-up = 1.17 ± 0.74 |
| ko03450 | Non-homologous end-joining | Control | F(2,12) = 7.455, p = 0.008 | Post-CRS vs Post-rTMS pFDR = 0.070 Post-CRS vs Follow-up pFDR = 0.070 |
Post-CRS = −2.94 ± 1.35 Post-rTMS = −1.75 ± 0.69 Follow-up = −1.28 ± 0.45 |
| ko00941 | Flavonoid biosynthesis | Control | F(2,12) = 5.243, p = 0.023 | Post-CRS vs Post-rTMS pFDR = 0.070 Post-CRS vs Follow-up pFDR = 0.070 |
Post-CRS = −4.37 ± 1.77 Post-rTMS = −2.74 ± 0.91 Follow-up = −2.15 ± 0.67 |
| ko05131 | Shigellosis | Control | F(2,12) = 17.71, p < 0.001 | Post-CRS vs Post-rTMS pFDR = 0.023 Post-CRS vs Follow-up pFDR = 0.023 Post-rTMS vs Follow-up pFDR = 0.031 |
Post-CRS = −7.58 ± 0.41 Post-rTMS = −6.57 ± 0.66 Follow-up = −7.25 ± 0.42 |
Fig. 4.
Changes in KEGG pathways between timepoints within the active, sham and depression control groups. The figure shows boxplots of centered log-ratio transformed KEGG pathway abundances for pathways identified as significant in the ANCOM's W-statistic (threshold of 0.7). Quade test was used to determine if there was a significant effect of timepoint (post-CRS, post-rTMS and follow-up) in each group. Between-timepoint comparisons were made by Post hoc Wilcoxon signed rank test with Benjamini-Hochberg FDR correction. †p < 0.1; *p < 0.05; **p < 0.01.
3.3. Correlation between the gut microbiome and brain and behavioural measures
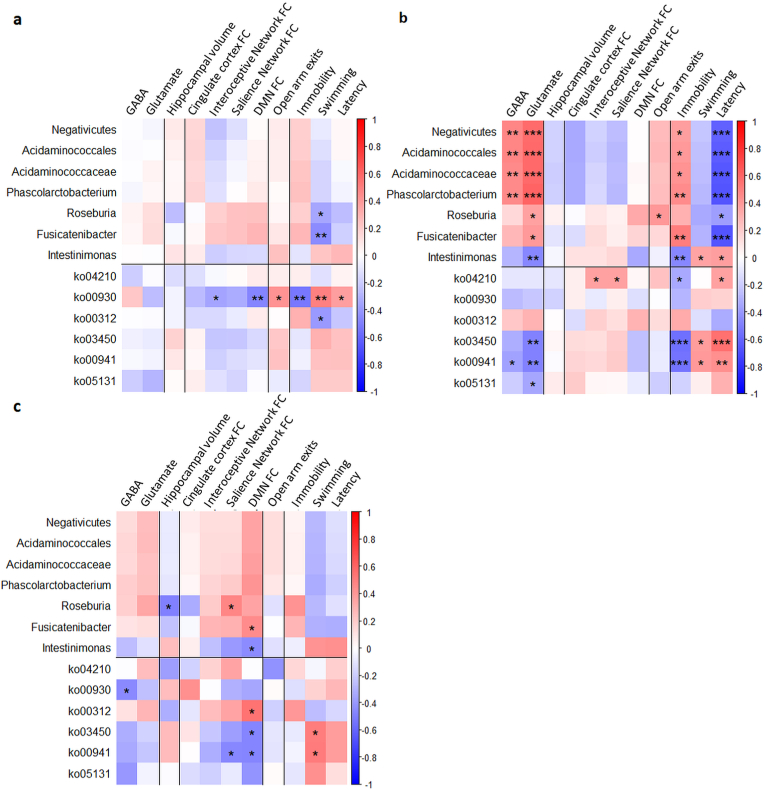
Spearman's rank correlation test using data from all groups and timepoints revealed significant correlations of microbiome composition and KEGG pathways with several MRI and behavioural measures (Fig. 5). However, none of these correlations survived multiple comparison correction in the active and depression control groups. In the sham group, GABA was positively correlated to Negativicutes class (r = 0.47, p = 0.005, pFDR = 0.037), Acidaminococcales order (r = 0.48, p = 0.005, pFDR = 0.037), Acidaminococcaceae family (r = 0.45, p = 0.007, pFDR = 0.047) and Phascolarctobacterium genus (r = 0.46, p = 0.006, pFDR = 0.045). Glutamate was positively correlated to Negativicutes class (r = 0.58, p < 0.001, pFDR = 0.005), Acidaminococcales order (r = 0.58, p < 0.001, pFDR = 0.004), Acidaminococcaceae family (r = 0.59, p < 0.001, pFDR = 0.004) and Phascolarctobacterium genus (r = 0.60, p < 0.001, pFDR = 0.003), but negatively correlated to the Intestinimonas genus (r = –0.46, p = 0.005, pFDR = 0.037) and ko03450 (r = –0.48, p = 0.003, pFDR = 0.033) and ko00941 (r = –0.49, p = 0.003, pFDR = 0.029) pathways. For the forced swim test, time spent immobile was positively correlated to the Phascolarctobacterium (r = 0.44, p = 0.007, pFDR = 0.048) and Fusicatenibacter genera (r = 0.51, p = 0.002, pFDR = 0.020), but negatively correlated to the Intestinimonas genus (r = –0.47, p = 0.004, pFDR = 0.037) and ko03450 (r = –0.56, p < 0.001, pFDR = 0.007) and ko00941 (r = –0.54, p < 0.001, pFDR = 0.010) pathways. Latency to first immobility behaviour was positively correlated to the ko03450 pathway (r = 0.56, p < 0.001, pFDR = 0.007), but negatively correlated to the Negativicutes class (r = –0.62, p < 0.001, pFDR = 0.002), Acidaminococcales order (r = –0.63, p < 0.001, pFDR = 0.002), Acidaminococcaceae family (r = –0.65, p < 0.001, pFDR = 0.001) and both Phascolarctobacterium and Fusicatenibacter genera (r = –0.66, p < 0.001, pFDR = 0.001 for both).
Fig. 5.
Correlations between microbiome data and MRI and behavioural measures. Centered log-ratio transformed abundances of taxa and KEGG pathways for taxa and pathways identified as significant in the ANCOM's W-statistic (threshold of 0.7) were correlated to the following parameters by Spearman correlations: GABA/tCr and Glutamate/tCr ratios from 1H-MRS data; hippocampal volume; connectivity (average z-scores) of the cingulate cortex, interoceptive network, salience network and default mode network from the rs-fMRI data; number of exits from the open arms from elevated plus maze data; and time spent immobile (Immobility) and swimming, and latency time to exhibit first immobility behaviour from forced swim test data. *p < 0.05, **p < 0.01, ***p < 0.001 (no multiple comparison correction).
4. Discussion
There is increasing evidence that the gut-brain axis plays a key role in the aetiology and treatment of depression. Our study confirmed previously reported changes in the gut resident bacteria composition following CRS in rats and adds that handling the rats after CRS caused a significant effect on the abundance of Firmicutes phylum. Our main finding was that LI-rTMS increased the abundance of the anti-inflammatory Roseburia genus, which was correlated with behavioural improvement in the forced swim test. Taken together with the decrease in apoptosis pathways following LI-rTMS, as well as the correlation of caprolactam pathway with functional outcomes only in rTMS treated animals, our results suggest that LI-rTMS may have anti-inflammatory and protective effects on the gut microbiome that are associated with its therapeutic effects.
4.1. Diversity and volatility
Diversity of the gut microbiome is often used as an index for health, with an increase in overall diversity and species richness being a good indicator of health and a reduction or lack of diversity often signalling disease (Shreiner et al., 2015). However, there is no consensus in the literature regarding whether or how the gut microbiota richness and diversity changes in human depression and preclinical models (Barandouzi et al., 2020; Simpson et al., 2021). In the present study, neither CRS nor LI-rTMS induced changes in alpha or beta diversity parameters, or in volatility; in the literature, there is evidence for no change (Naseribafrouei et al., 2014; Zheng et al., 2016), a decrease (Kelly et al., 2016; Huang et al., 2018) and an increase in diversity (Jiang et al., 2015). This disparity is likely due to the high individual variability in gut microbiome composition together with underpowered studies and the presence of confounders such as effect of antidepressants or other oral prescription medications. The ability to control for potential effects of diet, lifestyle and environment on the gut microbiome is an important advantage of performing animal studies, and can potentially increase the sensitivity of detecting microbiome changes that can then be validated in humans (Bear et al., 2020).
However, the timing of our study may have contributed to the lack of changes in diversity and volatility after CRS: in our studies the animals received CRS for only 2 weeks, which may not be sufficient for differences to appear. Other studies in mice performed CRS daily for 5 weeks and found more extensive changes compared to those reported here (Yang et al., 2021). rTMS treatment was also short because we applied an accelerated treatment (3x daily for 2 weeks) instead of the conventional single daily treatment for 4–6 weeks. The trend towards a reduction in volatility over time in our data suggests that more time may be required for the microbiome to change in response to environmental stressors and treatment, and our data may reflect overlapping changes due to CRS and treatment.
Despite the lack of change in overall diversity, our results identify changes in the sub-levels of two of the major phyla (Proteobacteria and Firmicutes) following CRS, similar to changes described in human patients with depression. We observed an increase in Deltaproteobacteria class of the Proteobacteria phylum, principally due to an increase in the Desulfovibrionales order. Proteobacteria have consistently been shown to be upregulated in a chronic unpredictable mild stress mouse model (Tian et al., 2019) and in patients with depression (Barandouzi et al., 2020; Cheng et al., 2020; Simpson et al., 2021), matching our findings.
4.2. CRS and LI-rTMS increase abundance of Firmicutes
The most significant changes in our study were in the Firmicutes which is one of the most abundant phyla in the microbiome of rats and humans and have been linked to a wide range of human health conditions including obesity and mental illness (Barandouzi et al., 2020). Several studies have shown that patients with depression have altered abundance of Firmicutes, but results remain inconsistent: both increases and decreases in the overall abundance of Firmicutes have been reported in patients with depression (Barandouzi et al., 2020; Capuco et al., 2020). Other analysis approaches such as the Firmicutes/Bacteroidetes ratio have been related to several neurological diseases and inflammatory conditions (Zhang et al., 2021), including obesity (Crovesy et al., 2020), autism spectrum disorders (Coretti et al., 2018) and inflammatory bowel diseases (Stojanov et al., 2020), with a reduced ratio associated with improvement. However more recent studies have highlighted the difficulty in associate the Firmicutes/Bacteroidetes ratio with health status, not only because of the challenges of controlling for confounders (Magne et al., 2020), but also because of the variation of this ratio across the human lifespan (Mariat et al., 2009).
Despite the lack of change in overall diversity, we observed differences between groups in abundance of specific sub-taxa. Members of the Lachnospiraceae family (Clostridia class: Anaerostipes and Frisingicoccus genera) significantly increased following CRS, supporting previous findings that link increased Firmicutes with pro-inflammatory processes also implicated in depression (Huang et al., 2018; Barandouzi et al., 2020). However, the changes in Firmicutes that occurred in the weeks following CRS are more difficult to interpret. The most consistent changes were detected in the control group, with downregulation of the Lachnospiraceae family, suggesting a spontaneous decrease in the effects of CRS over time once the restraint intervention was stopped. However, the decreases were not observed in the active and sham groups, suggesting that handling the rats during LI-rTMS procedures had a significant impact on their microbiome, potentially sustaining the effects of CRS. While we cannot rule out that bacteria were directly transmitted from the researchers to the rats, this is unlikely because of the strict physical containment and personal protection equipment protocols in place in the animal care facility (PC2). Rather, handling has been shown to have significant effects on rodent behaviour and wellbeing, most often reducing anxiety (Schmitt and Hiemke, 1998). However, some studies have shown that handling may have different outcomes depending on the animal's experiences and environment (Pritchard et al., 2013). It is therefore possible that the handling associated with delivering active and sham LI-rTMS sustained the stress induced by CRS.
While the data suggest that the handling component of rTMS is primarily responsible for maintaining high abundance of Firmicutes genera in the weeks following CRS, an increase in Roseburia genus only in the rTMS group identifies a specific effect of rTMS. In contrast to other Firmicutes genera, Roseburia is considered a beneficial bacterium that has a positive impact on the immune system: the flagellar system of Roseburia intestinalis reduces intestinal inflammation by suppressing IL-17 in the host (Zhu et al., 2018), and its abundance has been reported to decrease under various disease conditions (Patterson et al., 2017; Tamanai-Shacoori et al., 2017). However, recently, a higher abundance of Roseburia has also previously been linked to changes in gut microbiome in mice and to cerebral hypometabolism (Sanguinetti et al., 2018). Interestingly, previous studies of the gut microbiome following brain stimulation (using different protocols in patients with weight disorders) have shown an increase in Clostridia, the class to which Roseburia belongs, and these were interpreted as being anti-inflammatory and therefore beneficial (Artifon et al., 2020; Ferrulli et al., 2021), although different taxonomic sub-levels were involved compared to our study. Our study highlights that it is difficult to interpret changes in bacteria at the phyla level because taxa within the Firmicutes phylum have different functions.
4.3. Functional and pathway implications of microbiome changes
Although we found no changes in KEGG pathways following CRS, the characteristics of the different bacterial sub-taxa can suggest mechanisms whereby microbial changes might contribute to depression after chronic stress. Notably, genera in the Desulfovibrionales order are sulphate reducing and have been implicated in gastrointestinal inflammation through hydrogen sulphide accumulation in the gut (Dordević et al., 2021). In addition, the expression of lipopolysaccharides on the outer cell membrane of this order are thought to stimulate a host immune response through pro-inflammatory receptor stimulation and cytokine release (Hakansson and Molin, 2011). An extensive body of literature supports the association between depression and a chronic low-grade inflammatory response (Berk et al., 2013) and therefore, a higher abundance of these pro-inflammatory bacteria may play a role in development and/or maintenance of depression (Barandouzi et al., 2020; Kunugi, 2021).
Only one KEGG pathway was significantly altered following active LI-rTMS: apoptosis was decreased following treatment but returned to post-CRS levels at the 2 week follow up. Interestingly, anti-apoptotic effects of LI-rTMS have been reported in the brain: LI-rTMS has been shown to decrease genes related to apoptosis and inflammation (Grehl et al., 2015; Clarke et al., 2021) and when delivered at higher intensities promotes survival signalling pathways after a stroke (Caglayan et al., 2019). rTMS also increases the pro-survival factors such as BDNF, not only in the brain but also peripherally in serum (Makowiecki et al., 2014; Niimi et al., 2016; Heath et al., 2018; Feng et al., 2019). Taken together, our data showing a decrease in the apoptotic pathway and an increase in the anti-inflammatory Roseburia genus, specifically following active LI-rTMS, suggest that this brain-specific treatment may reduce inflammation and dysbiosis in the gut. It will be important to determine in future studies whether the impact of TMS on the microbiome is due to vagal nerve activation, or if circulating factors such as cytokines and/or BDNF are involved.
4.4. Correlations of microbiome with behaviour and MRI
Using data collected from the same animals and published in previous studies (Seewoo et al., 2019, 2021), we correlated behavioural and MRI outcomes with the sub-taxa of interest identified in our current analysis. A key finding was that in the active LI-rTMS group, Fusicatenibacter and Roseburia were negatively correlated with swimming behaviour in the FST. These genera are both members of the Lachnospiraceae family which has been consistently negatively correlated with symptoms of depression in humans (Vacca et al., 2020). Correlation of KEGG pathways with behavioural and MRI outcomes identified only the caprolactam pathway in active LI-rTMS (Rampelli et al., 2020). The caprolactam pathway is involved in the degradation of carbon compounds found in man made products such as nylon, synthetic fibres and plasticisers. Increases in the caprolactam pathway are interesting because they have been associated with longevity in humans, and high levels of caprolactam metabolising bacteria may provide a protection against toxins in the environment. Environmental toxins such as plastics and synthetic organic compounds have an impact on mental health (Daniel et al., 2020; Minatoya and Kishi, 2021; Schirmer et al., 2021) and our results raise the possibility that LI-rTMS may facilitate the growth of micro-organisms that can breakdown these compounds, potentially provide a protective effect. It will be important in future studies to determine whether the bacterial changes identified in this study actively contribute to the changes in behaviour, or are merely a consequence of the different interventions.
4.5. Study limitations and future directions
The current study has several limitations. Firstly, we did not include a control group of rats that had no intervention (ie. Non-CRS and non-rTMS group). While this group would have been useful to track the spontaneous changes in gut microbiome over the 6 weeks of the study, our aims and statistical approach were to compare animals longitudinally over time to explore how the microbiome changes over time in individual animals in response to CRS and rTMS. This longitudinal approach is useful for understanding individual changes that are likely to occur in humans. Secondly, only male rats were utilised in this study, as the CRS model is most reliable in male Sprague Dawley rats. Given that there are strong sex differences in depression aetiology and treatment and in the gut-brain axis, future studies should expand the applicability of present results by investigating microbiome changes in female rats (Eid et al., 2019; Holingue et al., 2020). Thirdly, the rats in this study were young adults. Age has been shown to have a significant effect on the composition of the gut microbiome (Agans et al., 2011; Radjabzadeh et al., 2020). Six months or older rats that have fully completed cerebral development (Mengler et al., 2014) could be used in future studies to exclude potentially confounding effects of brain-maturation with age. Lastly, avoiding the effects of co-housing animals is difficult. While animals in the same treatment group were co-housed in the present study to avoid microbial transfer between study groups, the fact that co-housed animals tend to have more similar microbiome could mask or exaggerate the results.
We also acknowledge the widely recognised challenges and limitations of modelling and measuring depression-like behaviour in rats: depression is diagnosed in humans based on subjective measures that are difficult to evaluate in rats. In choosing our behavioural tests, we selected tests that are considered the gold standard in the literature (EPM and FST) and test for anxiety and learned helplessness/stress responses respectively (Seewoo et al., 2020). Although performing additional tests could potentially provide more information about the models and interventions, we believe that our design represents an acceptable trade-off that optimises the quality of the data without redundancy (Cryan and Mombereau, 2004; Shoji et al., 2016) or “over testing” that could increase stress levels and compromise outcomes.
4.6. Conclusion
In conclusion, this study is the first of its kind to investigate the effects of CRS and LI-rTMS treatment on the gut microbiome longitudinally in male Sprague Dawley rats. Specifically, we found that chronic stress induced gut microbial dysbiosis, which was associated with high levels of anxiety and depression as well as brain changes. Our results after CRS highlight that although handling had a major impact on microbiome composition, there was evidence supporting a top-down effect of rTMS on the gut microbiome, with the main outcome being an increase in abundance of the Roseburia genus. The change in the microbiome was observed only in the LI-rTMS group, and not in the sham or no-handling control groups, suggesting that it was a consequence of altering brain activity with LI-rTMS. However, it remains unclear whether the increase in Roseburia is a direct effect via stimulation of neural pathways that connect the brain to the gut, or whether it is an indirect effect, resulting from the behavioural (mood) improvements. Given that Fusicatenibacter abundance was also significantly correlated with behaviour, but was not significantly altered by rTMS at the group level, and that handling alone had a significant impact on the microbiome, it is likely that there is complex feedback between microbiome, behaviour (including stress levels) and brain function: future studies should investigate faecal transplants and vagal nerve transection interventions to further explore the interplay of the gut brain axis components with behaviour and mood.
CRediT authorship contribution statement
Bhedita J. Seewoo: Conceptualization, Data curation, Formal analysis, Investigation, Supervision, Visualization, Writing – original draft, Writing – review & editing. Eng Guan Chua: Data curation, Formal analysis, Methodology, Visualization, Writing – review & editing. Yasmin Arena-Foster: Data curation, Investigation, Writing – original draft, Writing – review & editing. Lauren A. Hennessy: Investigation, Methodology, Project administration, Writing – review & editing. Anastazja M. Gorecki: Data curation, Investigation, Methodology, Writing – review & editing. Ryan Anderton: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing – review & editing. Jennifer Rodger: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Declaration of competing interest
The author(s) declare no competing financial and/or non-financial interests in relation to the work described.
Acknowledgements
The authors thank Dr Kirk Feindel, Dr Sarah Etherington, Ms Marissa Penrose-Menz, Ms Kerry Leggett, Ms Elizabeth Jaeschke-Angi, Ms Leah Mackie, Ms Kaylene Schutz, Mr Rex Edwards, Mr Samuel Bolland, Ms Abbey Figliomeni, Ms Elena Faessler, Mr Erik Stefan, Ms Tess Wheeler, Ms Samantha Musgrave, Mr David Gerard, Ms Yashvi Bhatt and Mr Parth Patel for their assistance with the experiments. The authors also thank the team at UWA M Block Animal Care Services for their assistance with animal care and transport. This research was funded by The University of Western Australia. BJS is supported by a Forrest Research Foundation Scholarship, an International Postgraduate Research Scholarship, and a University Postgraduate Award. LAH is supported by a University Postgraduate Award at the University of Western Australia, and the Commonwealth Government's ‘ Australian Government Research Training Program Fees Offset’. JR was supported by a Fellowship from MSWA and the Perron Institute for Neurological and Translational Science, and by a grant from the Perpetual Philanthropy: the Helen Leech Endowment.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2022.100430.
Contributor Information
Bhedita J. Seewoo, Email: bseewoo@gmail.com.
Eng Guan Chua, Email: eng.chua@uwa.edu.au.
Yasmin Arena-Foster, Email: 21989068@student.uwa.edu.au.
Lauren A. Hennessy, Email: lauren.hennessy@research.uwa.edu.au.
Anastazja M. Gorecki, Email: anastazja.gorecki@research.uwa.edu.au.
Ryan Anderton, Email: ryan.anderton@nd.edu.au.
Jennifer Rodger, Email: jennifer.rodger@uwa.edu.au.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Agans R., Rigsbee L., Kenche H., Michail S., Khamis H.J., Paliy O. Distal gut microbiota of adolescent children is different from that of adults. FEMS Microbiol. Ecol. 2011;77:404–412. doi: 10.1111/j.1574-6941.2011.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait Chait Y., Mottawea W., Tompkins T.A., Hammami R. Unravelling the antimicrobial action of antidepressants on gut commensal microbes. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-74934-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanese D., Fontana P., De Filippo C., Cavalieri D., Donati C. MICCA: a complete and accurate software for taxonomic profiling of metagenomic data. Sci. Rep. 2015;5:9743. doi: 10.1038/srep09743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artifon M., Schestatsky P., Griebler N., Tossi G.M., Beraldo L.M., Pietta-Dias C. Effects of transcranial direct current stimulation on the gut microbiome: a case report. Brain Stimul.: Basic, Transl. Clin. Res. Neuromodulat. 2020;13:1451–1452. doi: 10.1016/j.brs.2020.07.019. [DOI] [PubMed] [Google Scholar]
- Barandouzi Z.A., Starkweather A.R., Henderson W.A., Gyamfi A., Cong X.S. Altered composition of gut microbiota in depression: a systematic review. Front. Psychiatr. 2020;11 doi: 10.3389/fpsyt.2020.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaanssen T.F.S., Gururajan A., van de Wouw M., Moloney G.M., Ritz N.L., Long-Smith C.M., Wiley N.C., Murphy A.B., Lyte J.M., Fouhy F., Stanton C., Claesson M.J., Dinan T.G., Cryan J.F. Volatility as a concept to understand the impact of stress on the microbiome. Psychoneuroendocrinology. 2021;124 doi: 10.1016/j.psyneuen.2020.105047. [DOI] [PubMed] [Google Scholar]
- Bear T.L.K., Dalziel J.E., Coad J., Roy N.C., Butts C.A., Gopal P.K. The role of the gut microbiota in dietary interventions for depression and anxiety. Adv. Nutr. 2020;11:890–907. doi: 10.1093/advances/nmaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P., Verdu E.F., Foster J.A., Macri J., Potter M., Huang X., Malinowski P., Jackson W., Blennerhassett P., Neufeld K.A., Lu J., Khan W.I., Corthesy–Theulaz I., Cherbut C., Bergonzelli G.E., Collins S.M. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112. doi: 10.1053/j.gastro.2010.06.063. [DOI] [PubMed] [Google Scholar]
- Berk M., Williams L.J., Jacka F.N., O'Neil A., Pasco J.A., Moylan S., Allen N.B., Stuart A.L., Hayley A.C., Byrne M.L., Maes M. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. doi: 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U.S.A. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caglayan A.B., Beker M.C., Caglayan B., Yalcin E., Caglayan A., Yulug B., Hanoglu L., Kutlu S., Doeppner T.R., Hermann D.M., Kilic E. Acute and post-acute neuromodulation induces stroke recovery by promoting survival signaling, neurogenesis, and pyramidal tract plasticity. Front. Cell. Neurosci. 2019;13 doi: 10.3389/fncel.2019.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuco A., Urits I., Hasoon J., Chun R., Gerald B., Wang J.K., Kassem H., Ngo A.L., Abd-Elsayed A., Simopoulos T., Kaye A.D., Viswanath O. Current perspectives on gut microbiome dysbiosis and depression. Adv. Ther. 2020;37:1328–1346. doi: 10.1007/s12325-020-01272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S., Han B., Ding M., Wen Y., Ma M., Zhang L., Qi X., Cheng B., Li P., Kafle O.P., Liang X., Liu L., Du Y., Zhao Y., Zhang F. Identifying psychiatric disorder-associated gut microbiota using microbiota-related gene set enrichment analysis. Briefings Bioinf. 2020;21:1016–1022. doi: 10.1093/bib/bbz034. [DOI] [PubMed] [Google Scholar]
- Chinna Meyyappan A., Forth E., Wallace C.J.K., Milev R. Effect of fecal microbiota transplant on symptoms of psychiatric disorders: a systematic review. BMC Psychiatr. 2020;20:299. doi: 10.1186/s12888-020-02654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp M., Aurora N., Herrera L., Bhatia M., Wilen E., Wakefield S. Gut microbiota's effect on mental health: the gut-brain axis. Clin. Pract. 2017;7 doi: 10.4081/cp.2017.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke D., Beros J., Bates K.A., Harvey A.R., Tang A.D., Rodger J. Low intensity repetitive magnetic stimulation reduces expression of genes related to inflammation and calcium signalling in cultured mouse cortical astrocytes. Brain Stimul. 2021;14:183–191. doi: 10.1016/j.brs.2020.12.007. [DOI] [PubMed] [Google Scholar]
- Coretti L., Paparo L., Riccio M.P., Amato F., Cuomo M., Natale A., Borrelli L., Corrado G., Comegna M., Buommino E., Castaldo G., Bravaccio C., Chiariotti L., Berni Canani R., Lembo F. Gut microbiota features in young children with autism spectrum disorders. Front. Microbiol. 2018;9:3146. doi: 10.3389/fmicb.2018.03146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crovesy L., Masterson D., Rosado E.L. Profile of the gut microbiota of adults with obesity: a systematic review. Eur. J. Clin. Nutr. 2020;74:1251–1262. doi: 10.1038/s41430-020-0607-6. [DOI] [PubMed] [Google Scholar]
- Cruz-Pereira J.S., Rea K., Nolan Y.M., O'Leary O.F., Dinan T.G., Cryan J.F. Depression's unholy trinity: dysregulated stress, immunity, and the microbiome. Annu. Rev. Psychol. 2020;71:49–78. doi: 10.1146/annurev-psych-122216-011613. [DOI] [PubMed] [Google Scholar]
- Cryan J.F., Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol. Psychiatr. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- Daniel S., Balalian A.A., Whyatt R.M., Liu X., Rauh V., Herbstman J., Factor-Litvak P. Perinatal phthalates exposure decreases fine-motor functions in 11-year-old girls: results from weighted Quantile sum regression. Environ. Int. 2020;136 doi: 10.1016/j.envint.2019.105424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dordević D., Jančíková S., Vítězová M., Kushkevych I. Hydrogen sulfide toxicity in the gut environment: meta-analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes. J. Adv. Res. 2021;27:55–69. doi: 10.1016/j.jare.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas G.M., Maffei V.J., Zaneveld J.R., Yurgel S.N., Brown J.R., Taylor C.M., Huttenhower C., Langille M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020;38:685–688. doi: 10.1038/s41587-020-0548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid R.S., Gobinath A.R., Galea L.A.M. Sex differences in depression: insights from clinical and preclinical studies. Prog. Neurobiol. 2019;176:86–102. doi: 10.1016/j.pneurobio.2019.01.006. [DOI] [PubMed] [Google Scholar]
- Feng J., Zhang Q., Zhang C., Wen Z., Zhou X. The Effect of sequential bilateral low-frequency rTMS over dorsolateral prefrontal cortex on serum level of BDNF and GABA in patients with primary insomnia. Brain Behav. 2019;9 doi: 10.1002/brb3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrulli A., Drago L., Gandini S., Massarini S., Bellerba F., Senesi P., Terruzzi I., Luzi L. Deep transcranial magnetic stimulation affects gut microbiota composition in obesity: results of randomized clinical trial. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22094692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley J.D., Nelson M.C., Yu Z., Dowd S.E., Walter J., Kumar P.S., Lyte M., Bailey M.T. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014;14:189. doi: 10.1186/1471-2180-14-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Lin H., Revanna K., Dong Q. A Bayesian taxonomic classification method for 16S rRNA gene sequences with improved species-level accuracy. BMC Bioinf. 2017;18:247. doi: 10.1186/s12859-017-1670-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George M.S., Nahas Z., Molloy M., Speer A.M., Oliver N.C., Li X.B., Arana G.W., Risch S.C., Ballenger J.C. A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol. Psychiatr. 2000;48:962–970. doi: 10.1016/s0006-3223(00)01048-9. [DOI] [PubMed] [Google Scholar]
- Grehl S., Viola H.M., Fuller-Carter P.I., Carter K.W., Dunlop S.A., Hool L.C., Sherrard R.M., Rodger J. Cellular and molecular changes to cortical neurons following low intensity repetitive magnetic stimulation at different frequencies. Brain Stimul. 2015;8:114–123. doi: 10.1016/j.brs.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Hakansson A., Molin G. Gut microbiota and inflammation. Nutrients. 2011;3 doi: 10.3390/nu3060637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath A., Lindberg D.R., Makowiecki K., Gray A., Asp A.J., Rodger J., Choi D.-S., Croarkin P.E. Medium- and high-intensity rTMS reduces psychomotor agitation with distinct neurobiologic mechanisms. Transl. Psychiatry. 2018;8:126. doi: 10.1038/s41398-018-0129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holingue C., Budavari A.C., Rodriguez K.M., Zisman C.R., Windheim G., Fallin M.D. Sex differences in the gut-brain axis: implications for mental health. Curr. Psychiatr. Rep. 2020;22:83. doi: 10.1007/s11920-020-01202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Shi X., Li Z., Shen Y., Shi X., Wang L., Li G., Yuan Y., Wang J., Zhang Y., Zhao L., Zhang M., Kang Y., Liang Y. Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatric Dis. Treat. 2018;14:3329–3337. doi: 10.2147/NDT.S188340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Ling Z., Zhang Y., Mao H., Ma Z., Yin Y., Wang W., Tang W., Tan Z., Shi J., Li L., Ruan B. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Kelly J.R., Borre Y., O' Brien C., Patterson E., El Aidy S., Deane J., Kennedy P.J., Beers S., Scott K., Moloney G., Hoban A.E., Scott L., Fitzgerald P., Ross P., Stanton C., Clarke G., Cryan J.F., Dinan T.G. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Kochar B., Barnes E.L., Long M.D., Cushing K.C., Galanko J., Martin C.F., Raffals L.E., Sandler R.S. Depression is associated with more aggressive inflammatory bowel disease. Am. J. Gastroenterol. 2018;113:80–85. doi: 10.1038/ajg.2017.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunugi H. Gut microbiota and pathophysiology of depressive disorder. Ann. Nutr. Metab. 2021;77(Suppl. 2):11–20. doi: 10.1159/000518274. [DOI] [PubMed] [Google Scholar]
- Lin H., Peddada S.D. Analysis of compositions of microbiomes with bias correction. Nat. Commun. 2020;11:3514. doi: 10.1038/s41467-020-17041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukić I., Getselter D., Ziv O., Oron O., Reuveni E., Koren O., Elliott E. Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl. Psychiatry. 2019;9:133. doi: 10.1038/s41398-019-0466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magne F., Gotteland M., Gauthier L., Zazueta A., Pesoa S., Navarrete P., Balamurugan R. The Firmicutes/Bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. 2020;12 doi: 10.3390/nu12051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowiecki K., Harvey A.R., Sherrard R.M., Rodger J. Low-intensity repetitive transcranial magnetic stimulation improves abnormal visual cortical circuit topography and upregulates BDNF in mice. J. Neurosci. 2014;34:10780–10792. doi: 10.1523/JNEUROSCI.0723-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris A.P., Karianaki M., Tsamis K.I., Paschou S.A. The role of the gut-brain axis in depression: endocrine, neural, and immune pathways. Hormones (Basel) 2021;20:1–12. doi: 10.1007/s42000-020-00236-4. [DOI] [PubMed] [Google Scholar]
- Mariat D., Firmesse O., Levenez F., Guimarăes V.D., Sokol H., Doré J., Corthier G., Furet J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer E.A. Gut feelings: the emerging biology of gut–brain communication. Nat. Rev. Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern A.S., Hamlin A.S., Winter G. A review of the antimicrobial side of antidepressants and its putative implications on the gut microbiome. Aust. N. Z. J. Psychiatr. 2019;53:1151–1166. doi: 10.1177/0004867419877954. [DOI] [PubMed] [Google Scholar]
- McMurdie P.J., Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengler L., Khmelinskii A., Diedenhofen M., Po C., Staring M., Lelieveldt B.P.F., Hoehn M. Brain maturation of the adolescent rat cortex and striatum: changes in volume and myelination. Neuroimage. 2014;84:35–44. doi: 10.1016/j.neuroimage.2013.08.034. [DOI] [PubMed] [Google Scholar]
- Minatoya M., Kishi R. A review of recent studies on Bisphenol A and phthalate exposures and child neurodevelopment. Int. J. Environ. Res. Publ. Health. 2021;18:3585. doi: 10.3390/ijerph18073585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirarab S., Nguyen N., Guo S., Wang L.-S., Kim J., Warnow T. PASTA: ultra-large multiple sequence alignment for nucleotide and amino-acid sequences. J. Comput. Biol. 2014;22:377–386. doi: 10.1089/cmb.2014.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseribafrouei A., Hestad K., Avershina E., Sekelja M., Linløkken A., Wilson R., Rudi K. Correlation between the human fecal microbiota and depression. Neuro Gastroenterol. Motil. 2014;26:1155–1162. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- Niimi M., Hashimoto K., Kakuda W., Miyano S., Momosaki R., Ishima T., Abo M. Role of brain-derived neurotrophic factor in beneficial effects of repetitive transcranial magnetic stimulation for upper limb hemiparesis after stroke. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reardon J.P., Solvason H.B., Janicak P.G., Sampson S., Isenberg K.E., Nahas Z., McDonald W.M., Avery D., Fitzgerald P.B., Loo C., Demitrack M.A., George M.S., Sackeim H.A. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol. Psychiatr. 2007;62:1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Parikh H.I., Koparde V.N., Bradley S.P., Buck G.A., Sheth N.U. MeFiT: merging and filtering tool for illumina paired-end reads for 16S rRNA amplicon sequencing. BMC Bioinf. 2016;17:491. doi: 10.1186/s12859-016-1358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M.N., Dehal P.S., Arkin A.P. FastTree 2 – approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard L.M., Van Kempen T.A., Zimmerberg B. Behavioral effects of repeated handling differ in rats reared in social isolation and environmental enrichment. Neurosci. Lett. 2013;536:47–51. doi: 10.1016/j.neulet.2012.12.048. [DOI] [PubMed] [Google Scholar]
- Radjabzadeh D., Boer C.G., Beth S.A., van der Wal P., Kiefte-De Jong J.C., Jansen M.A.E., Konstantinov S.R., Peppelenbosch M.P., Hays J.P., Jaddoe V.W.V., Ikram M.A., Rivadeneira F., van Meurs J.B.J., Uitterlinden A.G., Medina-Gomez C., Moll H.A., Kraaij R. Diversity, compositional and functional differences between gut microbiota of children and adults. Sci. Rep. 2020;10:1040. doi: 10.1038/s41598-020-57734-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampelli S., Soverini M., D'Amico F., Barone M., Tavella T., Monti D., Capri M., Astolfi A., Brigidi P., Biagi E., Franceschi C., Turroni S., Candela M., David Lawrence A. Shotgun metagenomics of gut microbiota in humans with up to extreme longevity and the increasing role of xenobiotic degradation. mSystems. 2020;5 doi: 10.1128/mSystems.00124-20. e00124-00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger J., Mo C., Wilks T., Dunlop S.A., Sherrard R.M. Transcranial pulsed magnetic field stimulation facilitates reorganization of abnormal neural circuits and corrects behavioral deficits without disrupting normal connectivity. Faseb. J. 2012;26:1593–1606. doi: 10.1096/fj.11-194878. [DOI] [PubMed] [Google Scholar]
- Schirmer E., Schuster S., Machnik P. Bisphenols exert detrimental effects on neuronal signaling in mature vertebrate brains. Commun. Biol. 2021;4:465. doi: 10.1038/s42003-021-01966-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt U., Hiemke C. Strain differences in open-field and elevated plus-maze behavior of rats without and with pretest handling. Pharmacol. Biochem. Behav. 1998;59:807–811. doi: 10.1016/s0091-3057(97)00502-9. [DOI] [PubMed] [Google Scholar]
- Seewoo B., Feindel K., Etherington S., Hennessy L., Croarkin P., Rodger J. M85. Validation of the chronic restraint stress model of depression in rats and investigation of standard vs accelerated rTMS treatment. Neuropsychopharmacology. 2019;44:122–123. [Google Scholar]
- Seewoo B.J., Feindel K.W., Etherington S.J., Rodger J. Resting-state fMRI study of brain activation using low-intensity repetitive transcranial magnetic stimulation in rats. Sci. Rep. 2018;8:6706. doi: 10.1038/s41598-018-24951-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seewoo B.J., Feindel K.W., Won Y., Joos A.C., Figliomeni A., Hennessy L.A., Rodger J. White matter changes following chronic restraint stress and neuromodulation: a diffusion magnetic resonance imaging study in young male rats. Biol. Psychiatr.: Glob. Open Sci. 2021 doi: 10.1016/j.bpsgos.2021.08.006. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seewoo B.J., Hennessy L.A., Feindel K.W., Etherington S.J., Croarkin P.E., Rodger J. Validation of chronic restraint stress model in young adult rats for the study of depression using longitudinal multimodal MR imaging. eneuro. 2020;7 doi: 10.1523/ENEURO.0113-20.2020. ENEURO.0113-0120.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji H., Takao K., Hattori S., Miyakawa T. Age-related changes in behavior in C57BL/6J mice from young adulthood to middle age. Mol. Brain. 2016;9:11. doi: 10.1186/s13041-016-0191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreiner A.B., Kao J.Y., Young V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015;31:69–75. doi: 10.1097/MOG.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson C.A., Diaz-Arteche C., Eliby D., Schwartz O.S., Simmons J.G., Cowan C.S.M. The gut microbiota in anxiety and depression – a systematic review. Clin. Psychol. Rev. 2021;83 doi: 10.1016/j.cpr.2020.101943. [DOI] [PubMed] [Google Scholar]
- Stojanov S., Berlec A., Štrukelj B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020;8:1715. doi: 10.3390/microorganisms8111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Zhang M., Chen C.-C., Gillilland M., 3rd, Sun X., El-Zaatari M., Huffnagle G.B., Young V.B., Zhang J., Hong S.-C., Chang Y.-M., Gumucio D.L., Owyang C., Kao J.Y. Stress-induced corticotropin-releasing hormone-mediated NLRP6 inflammasome inhibition and transmissible enteritis in mice. Gastroenterology. 2013;144:1478–1487. doi: 10.1053/j.gastro.2013.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian P., Wang G., Zhao J., Zhang H., Chen W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutr. Biochem. 2019;66:43–51. doi: 10.1016/j.jnutbio.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Tillisch K., Labus J., Kilpatrick L., Jiang Z., Stains J., Ebrat B., Guyonnet D., Legrain–Raspaud S., Trotin B., Naliboff B., Mayer E.A. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394–1401. doi: 10.1053/j.gastro.2013.02.043. e1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca M., Celano G., Calabrese F.M., Portincasa P., Gobbetti M., De Angelis M. The controversial role of human gut Lachnospiraceae. Microorganisms. 2020;8:573. doi: 10.3390/microorganisms8040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles-Colomer M., Falony G., Darzi Y., Tigchelaar E.F., Wang J., Tito R.Y., Schiweck C., Kurilshikov A., Joossens M., Wijmenga C., Claes S., Van Oudenhove L., Zhernakova A., Vieira-Silva S., Raes J. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019;4:623–632. doi: 10.1038/s41564-018-0337-x. [DOI] [PubMed] [Google Scholar]
- Yang H.-l., Li M.-M., Zhou M.-F., Xu H.-S., Huan F., Liu N., Gao R., Wang J., Zhang N., Jiang L. Links between gut dysbiosis and neurotransmitter disturbance in chronic restraint stress-induced depressive behaviours: the role of inflammation. Inflammation. 2021 doi: 10.1007/s10753-021-01514-y. [DOI] [PubMed] [Google Scholar]
- Yu Y., Lee C., Kim J., Hwang S. Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol. Bioeng. 2005;89:670–679. doi: 10.1002/bit.20347. [DOI] [PubMed] [Google Scholar]
- Zhang W., Qu W., Wang H., Yan H. Antidepressants fluoxetine and amitriptyline induce alterations in intestinal microbiota and gut microbiome function in rats exposed to chronic unpredictable mild stress. Transl. Psychiatry. 2021;11:131. doi: 10.1038/s41398-021-01254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P., Zeng B., Zhou C., Liu M., Fang Z., Xu X., Zeng L., Chen J., Fan S., Du X., Zhang X., Yang D., Yang Y., Meng H., Li W., Melgiri N.D., Licinio J., Wei H., Xie P. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol. Psychiatr. 2016;21:786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- Zhu C., Song K., Shen Z., Quan Y., Tan B., Luo W., Wu S., Tang K., Yang Z., Wang X. Roseburia intestinalis inhibits interleukin-17 excretion and promotes regulatory T cells differentiation in colitis. Mol. Med. Rep. 2018;17:7567–7574. doi: 10.3892/mmr.2018.8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.