Abstract
Background
Palbociclib plus endocrine therapy (ET) demonstrated significant progression-free survival (PFS) benefit in Young Pearl, a randomized phase ll trial comparing palbociclib + ET versus capecitabine in premenopausal women with hormone receptor positive, HER2 negative metastatic breast cancer (MBC). This exploratory analysis investigated potential biomarkers of palbociclib plus ET on PFS.
Patients and methods
Of 178 patients randomized (92 palbociclib plus ET; 86 capecitabine), we performed targeted sequencing (141 patients) and whole transcriptome sequencing (165 patients) using baseline tumor samples to examine genomic alteration in relation to drug response on PFS. Hazard ratios (HRs) were estimated using unstratified Cox proportional hazards models.
Results
PIK3CA (41%) and TP53 (33%) mutations and CCND1 copy number variation (29%) were found most frequently in targeted sequencing of 141 patients. ESR1 mutations were found only in 3.5% of patients of this population. Luminal type showed better prognosis in palbociclib + ET arm but no impact on PFS difference in capecitabine arm. High TMB, TP53 mutation, PTEN loss of function mutation and RB1 pathway alteration showed worse prognosis in palbociclib plus ET arm. Patients with BRCA2 pathogenic mutations showed worse prognosis regardless of PAM50 subtypes. AURKA mutation/amplification, BRIP1/MYC/RAD51C amplification were significantly associated to the patients with short PFS <6 month.
Conclusion
Of palbociclib plus ET, luminal type showed better prognosis and BRCA2 pathogenic mutation showed worse prognosis regardless luminal/non-luminal type. Further exploration of molecular variables is warranted to determine and validate biomarkers of efficacy and resistance.
Keywords: Palbociclib, Next-generation sequencing, Progression-free survival, Biomarker
Highlights
-
•
Luminal type is the most important prognostic marker in palbociclib plus ET treated premenopausal patients.
-
•
High TMB, TP53 mutation, PTEN loss of function mutation and RB1 pathway alteration showed worse prognosis in palbociclib plus ET treated patients.
-
•
Patients with BRCA2 pathogenic mutations showed worse prognosis regardless of PAM50 subtypes.
-
•
AURKA mutation/amplification, BRIP1/MYC/RAD51C amplification were significantly associated to the patients with short PFS <6 month.
-
•
Immunescore had a more significant effect in capecitabine treated patients, and patients with higher Immunescore showed worse prognosis.
1. Introduction
Hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) metastatic breast cancer (MBC) consists of a clinically heterogenous group of tumors with different prognoses and responses to endocrine therapy (ET) and chemotherapy. Recently the addition of cyclin dependent kinase 4 and 6 (CDK4/6) inhibitors to ET has been considered the standard therapy for most patients with HR+, HER2- MBC regardless of menopausal status because of clinically meaningful increases in progression-free survival (PFS) [1,2] and overall survival (OS) [[3], [4], [5]]. Young-PEARL was a study that showed superior PFS of palbociclib plus ET compared to the capecitabine in premenopausal women with HR+, HER2- MBC [6]. The addition of CDK4/6 inhibitors to ET maintained health-related quality of life and improved some symptomatic scores without compromising treatment efficacy [[7], [8], [9], [10], [11], [12]].
However, many patients exhibit primary resistance to CDK4/6 inhibition and do not derive any benefit from these agents, often switching to chemotherapy within 6 months. Some patients initially benefit from treatment, but later develop secondary resistance. It is important to Identify subgroups of patients and intrinsic molecular subtypes of breast cancer that are sensitive to or resistant to CDK4/6 inhibitors plus ET in optimizing patients’ therapy selection. Preclinical data suggested numerous genes which involved in the cyclin D-CDK4/6-RB pathway, cell-cycle regulatory proteins governing G1-S phase transition [13]. Despite a broad biomarker research, palbociclib plus letrozole demonstrated consistent PFS gains versus placebo plus letrozole, with no single biomarker or a set of markers associated with lack of benefit from combination treatment [14]. Addition of palbociclib to fulvestrant demonstrated efficacy in all biomarker groups, although high CCNE1 mRNA expression was associated with relative resistance to palbociclib [15]. Prat et al. confirmed that the prognostic value of intrinsic subtype was maintained in the context of ET plus ribociclib phase lll trial and showed that ribociclib could restore endocrine sensitivity even in HER2 enriched subtype [16].
To investigate further information on patients who received benefit from the addition of a CDK4/6 inhibitor in HR+, HER2- MBC, Young Pearl study required baseline tumor tissues obligatory. Using these tissues, we performed a comprehensive preplanned assessment evaluating DNA, mRNA and protein biomarkers and correlated with clinical outcomes such as PFS in this analysis.
2. Materials and methods
2.1. Patients and study design
The details of the Young Pearl study (NCT02592746) have been previously published [6]. Briefly, it was an open-labeled, randomized phase ll study to receive palbociclib 125 mg daily (3 weeks of treatment, then 1 week off) plus exemestane 25 mg daily with leuprolide 3.75 mg subcutaneously every 4 weeks (arm A, N = 92) or capecitabine 1250mg/m2 twice a day (arm B, N = 86) in premenopausal women who had not received prior aromatase inhibitor for HR+, HER2- MBC. The median follow-up was 17 months (IQR 9–22). This study showed median PFS of 20.1 months in the palbociclib plus ET versus 14.4 months in the capecitabine (hazard ratio 0.659 [95% CI 0.437–0.994], log-rank p = 0.0235). This multicenter study was approved by an Institutional Review Board at each study site. The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki. All patients provided written informed consent before enrollment.
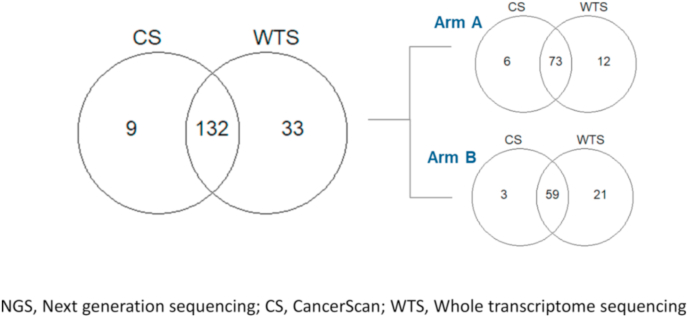
Collection of formalin-fixed paraffin-embedded (FFPE) tumor samples (a tumor block or slides) was mandatory from Young Pearl study, and a metastatic sample was preferable. The timing of collection was at screening but archival histological tissues collected prior to progression of tamoxifen or previous chemotherapy were allowed. We performed targeted sequencing (CancerSCAN™) containing 375 cancer-related genes (141 patients) and whole transcriptome sequencing (165 patients) using baseline tumor samples to examine genomic alteration in relation to PFS (Fig. 1).
Fig. 1.
NGS study design. NGS, Next generation sequencing; CS, CancerScan; WTS, Whole transcriptome sequencing.
2.2. Methods
2.2.1. DNA sequencing
For mutation detection, CancerSCAN™ was performed for 141 patients with more than 300X (660X in average), which is tumor-only 375-gene targeted panel sequencing using HiSeq 2500 (Illumina). We made variant calls using Mutect for SNV, Pindel for INDEL, Contra for CNV, and Juli for Fusion with default parameters, after germline filtering using public and in-house Korean whole exome sequencing databases. Sixty-two patients performed germline BRCA testing. Tumor mutational burden (TMB) was divided into two groups: high TMB (≥10mutations/mb) and low TMB (<10mutations/mb).
2.2.2. RNA-sequencing
Sequencing libraries were prepared with TruSeq RNA Access Library Prep Kit (Illumina, Inc.) to use FFPE tissues following manufacturer's protocols. Paired-end sequencing of the RNA libraries was performed on HiSeq 2500 Sequencing Platform (Illumina, Inc.). After trimming poor quality bases from the FASTQ files, we aligned the reads to the human reference genome (hg19) with STAR (v2.5.2b) and estimated gene expression in terms of Transcripts Per Million (TPM) using RSEM (v1.3). Gene set enrichment analysis was performed based on the GSVA score using GSVA R package (v1.34.0). Research-based PAM50 classification was performed using genefu R package (v2.18.1) to determine molecular intrinsic subtype. Expression greater than 70% quantile was defined as high expression. Immune score was evaluated by ESTIMATE R package (v1.0.13) and relative proportion of immune cell subpopulation was deconvoluted by CIBERSORT. Immune score greater than 70% quantile was defined as high.
2.3. Statistical analysis
Survival end-points were assessed using Kaplan-Meier estimates, and the intrinsic subtype and the influence of gene mutations on PFS was analyzed using Cox proportional hazards model and log-rank tests. Multivariable analyses of baseline patient characteristics (age, prior chemotherapy, Eastern Cooperative Oncology Group performance status, presence of visceral disease such as liver or lung metastases, presence of bone-only metastases, number of metastatic sites, prior ET, and presence of de novo metastatic disease) was conducted to confirm findings and adjust for population bias. All statistical analyses were performed using R version 3.6.1 and p < 0.05 was considered as statistically significant.
3. Results
Between June 15, 2016, and December 10, 2018, 184 patients were randomized, of whom 178 received at least one dose of study treatment. The median age was 44-years old and progesterone receptor status was generally balanced between treatment arms and response rate was 20% and 17% on both arms, respectively (Table 1). Archival tumor samples were consisted of primary tumor (125, 72%) and metastatic tumor (49, 28%).
Table 1.
Study population and clinical outcomes in Young Pearl.
| Treated arm | Patient Number | Age (yr) (median, range) | IHC |
Best response |
||||
|---|---|---|---|---|---|---|---|---|
| PR+ | PR- | CR | PR | SD | PD | |||
| Arm A | 91 (52%) | 44 (31–58) | 69 (40%) | 22 (13%) | 1 (1%) | 33 (19%) | 54 (31%) | 3 (2%) |
| Arm B | 83 (48%) | 44 (28–53) | 62 (36%) | 21 (12%) | 3 (2%) | 26 (15%) | 48 (28%) | 5 (3%) |
3.1. Mutational landscape
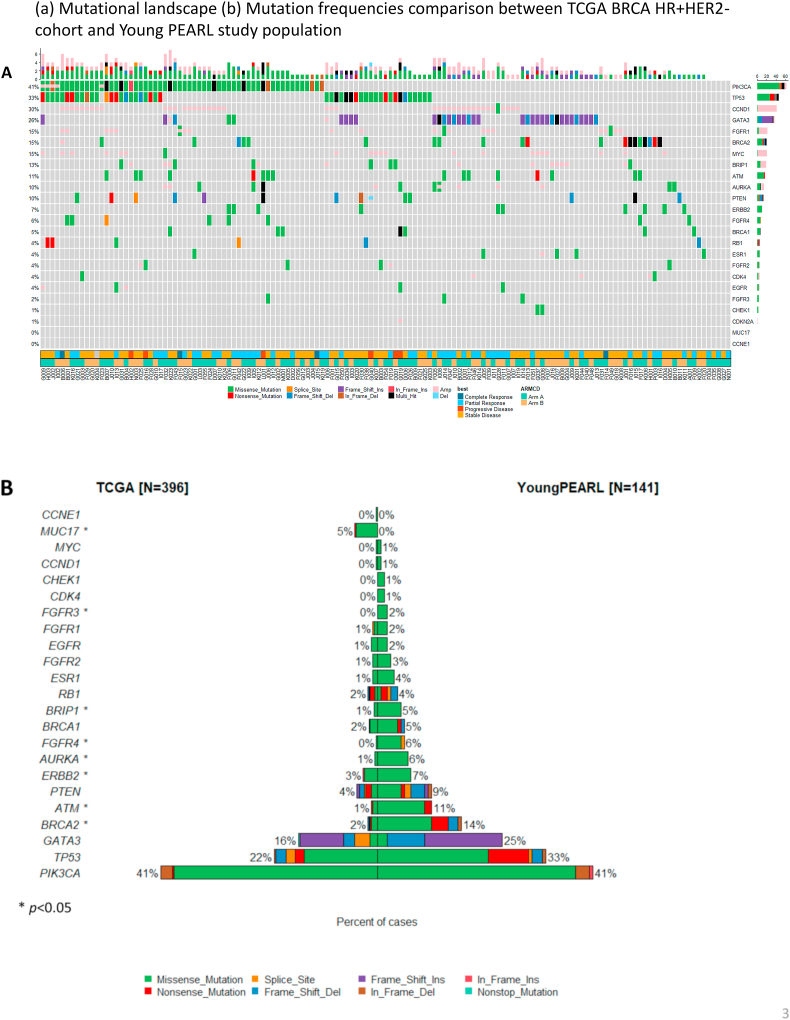
Of 141 targeted sequenced all patients, PIK3CA (41%) were most frequently mutated and TP53 (33%), CCND1 copy number variation (29%), GATA3 (25%), BRCA2 (14%) mutation were frequently detected in this population (Fig. 2A). ESR1 mutations were found in only 3.5% of patients. We compared mutation frequencies between The Cancer Genome Atlas (TCGA) BRCA HR + HER2-cohort and Young PEARL study population. In contrast to the TCGA data that overwhelmingly included postmenopausal women (307/396 = 77.5%), the Young Pearl premenopausal study showed that genetic mutations such as BRCA2, ERBB2, AURKA, FGFR4, and BRIP1 mutations more frequently reported with statistically significant (Fig. 2B).
Fig. 2.
Molecular profiling of Young Pearl study. (A) Mutational landscape. (B) Mutation frequencies comparison between TCGA BRCA HR + HER2-cohort and Young PEARL study population.
3.2. Prognosis in each treatment arm based on intrinsic subtype
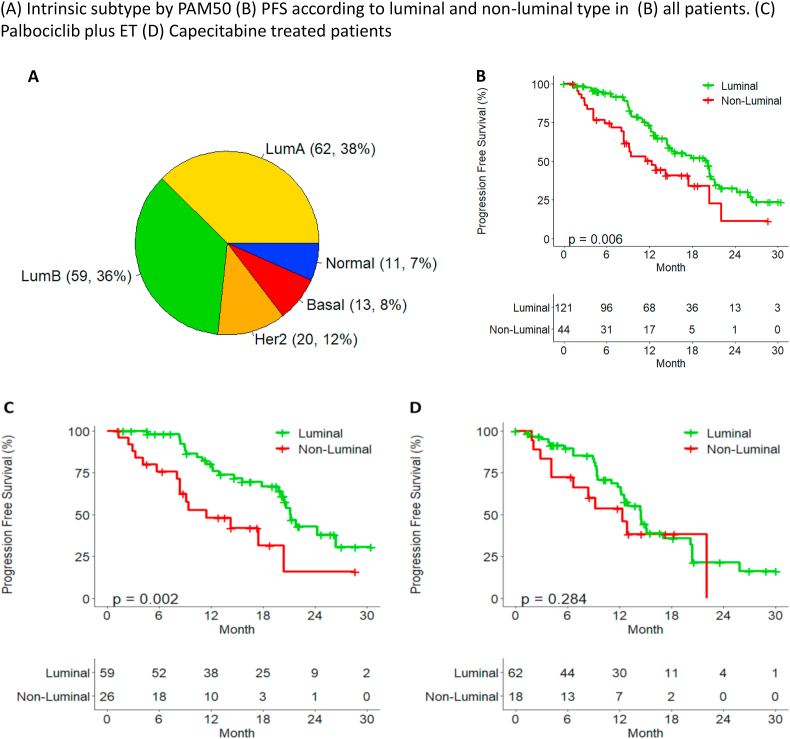
We classified the study population by PAM50 (Fig. 3A) and explored whether the benefit of palbociclib plus ET or capecitabine may differ by intrinsic breast cancer subtype. In total, 73% of patients was classified as Luminal type (including luminal A and B), and showed better prognosis than non-luminal type of patients in all patients (Fig. 3B). There was a difference in PFS between the luminal and non-luminal type in arm A (p < 0.05), but not in arm B (p = 0.284) (Fig. 3C and D). When analyzing the difference in PFS according to subtypes, arm A showed better prognosis than arm B in luminal A (p = 0.004) and no statistically significant difference in PFS in other subtypes (Fig. S1).
Fig. 3.
Intrinsic subtype and PFS. (A) Intrinsic subtype by PAM50. PFS according to luminal and non-luminal type in (B) all patients. (C) Palbociclib plus ET. (D) Capecitabine treated patients.
3.3. Potential prognostic biomarkers to predict worse prognosis of palbociclib plus ET by targeted sequencing
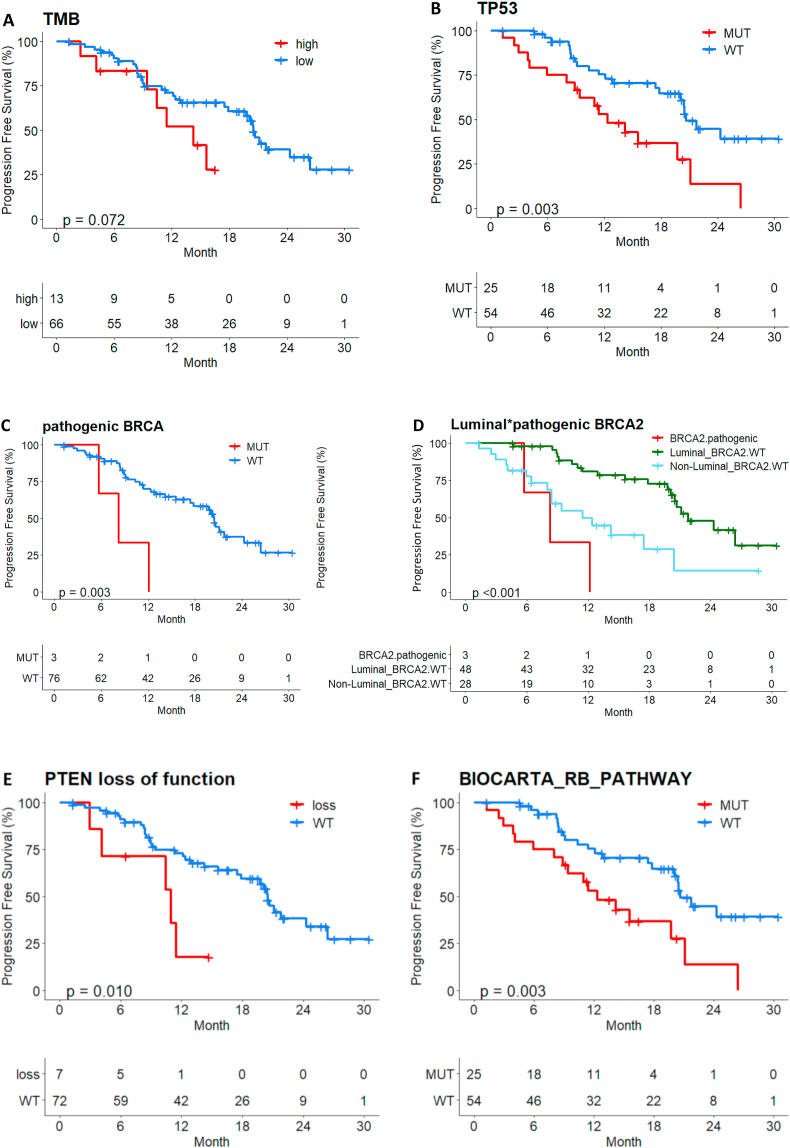
In palbociclib plus ET treated patients, High TMB (N = 13, 17%), TP53 mutation (N = 25, 32%) showed worse prognosis (p = 0.072, p = 0.003 respectively). Even though the number of patients with BRCA2 pathogenic mutation was small (N = 3, 4%), these patients showed worse prognosis regardless PAM50 subtypes (p = 0.003). On the contrary, luminal patients without BRCA2 pathogenic mutations showed longer PFS compared to non-luminal patients (p < 0.001). PTEN loss of function mutation (N = 7, 8.9%) and RB pathway alteration, defined as a mutation (SNV/INDEL) was detected in any of the genes belonging to the 26 BIOCARTA_RB_PATHWAY genes (TP53, ATM, RB1, CDK4, CHEK1 genes included in CancerSCAN™ panel) (N = 25, 31.6%) were also associated with poor prognosis (Fig. 4A–F).
Fig. 4.
PFS by several molecular alterations in palbociclib plus ET treated patients (a) Tumor mutation burden (b) TP53 mutation (c) pathogenic BRCA2 mutation (d) Luminal and pathogenic BRCA2 mutation (e) PTEN loss of function (f) RB pathway alteration.
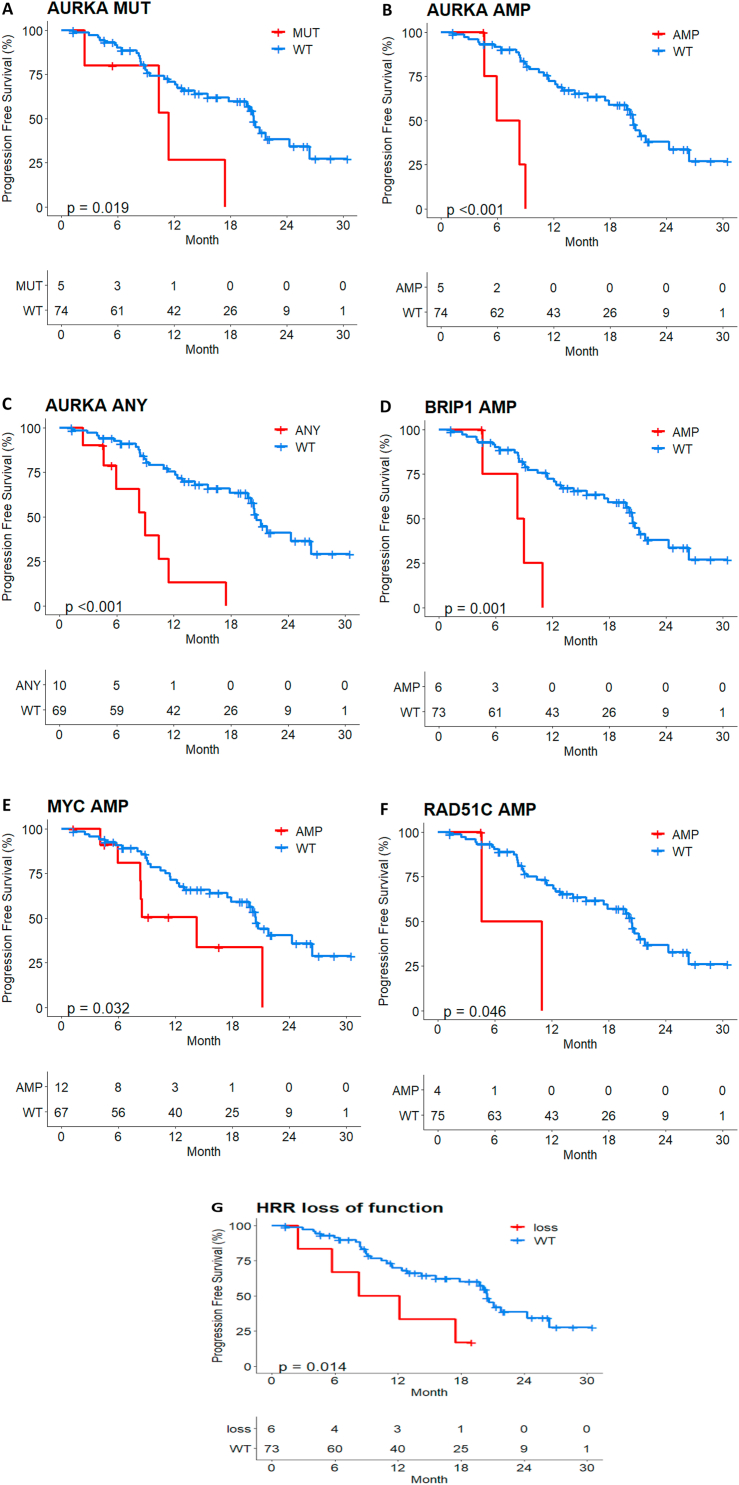
Although the sample size was not enough, AURKA mutation/amplification, BRIP1/MYC/RAD51C amplification and homologous related repair (HRR) loss of function mutation were significantly associated with poor prognosis (Fig. 5A–G). HRR loss of function (splicing, stop-gain, frameshift, insertion/deletion) was detected in any of the 15 genes from FoundationOne® CDx panel. It is noteworthy that AURKA, BRIP1 and RAD51C amplification were enriched in the patients with short PFS <6 months (Fig. S2).
Fig. 5.
PFS by AURKA molecular alteration. (A) mutation. (B) amplification. (C) any mutation or amplification and (D) BRIP1 amplification. (E) MYC amplification. (F) RAD51C amplification. (G) HRR loss of function in palbociclib plus ET treated patients.
3.4. Gene expression analysis using RNA-sequencing data
Analyzing gene expression showed that the baseline gene expression levels (higher or lower based on dichotomization by 70%) of RB1, ESR1/2 didn't affect PFS by the addition of palbociclib to ET in palbociclib plus ET treated patients (Fig. S3). We compared whether genes reported as amplification in DNA sequencing are reported as expression in RNA sequencing. AURKA and BRIP amplification were reported to be highly correlated with RNA expression, whereas MYC or RB loss of function was not reported to correlate with RNA expression level (Fig. S4).
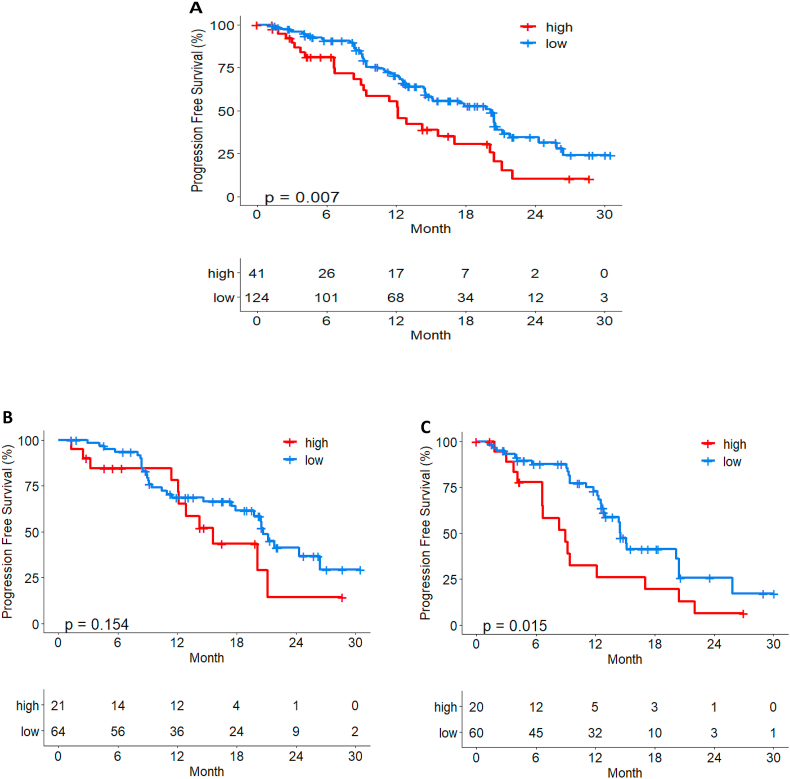
On immunescore analysis, patients with high immunescore showed a poor prognosis (median PFS in high 12.1 months (9.2–20.1) vs low 20.2 months (14.7–21.8) with statistically significance (p = 0.007) in all Young Pearl cohort (Fig. 6A). Immunescore had a more significant effect in capecitabine treated patients, and patients with higher Immunescore showed worse prognosis (p < 0.05) (Fig. 6B and C).
Fig. 6.
The impact of ImmuneScore on PFS in (A) all Young Pearl cohort (B) Palbociclib plus ET (C) Capecitabine treated patients.
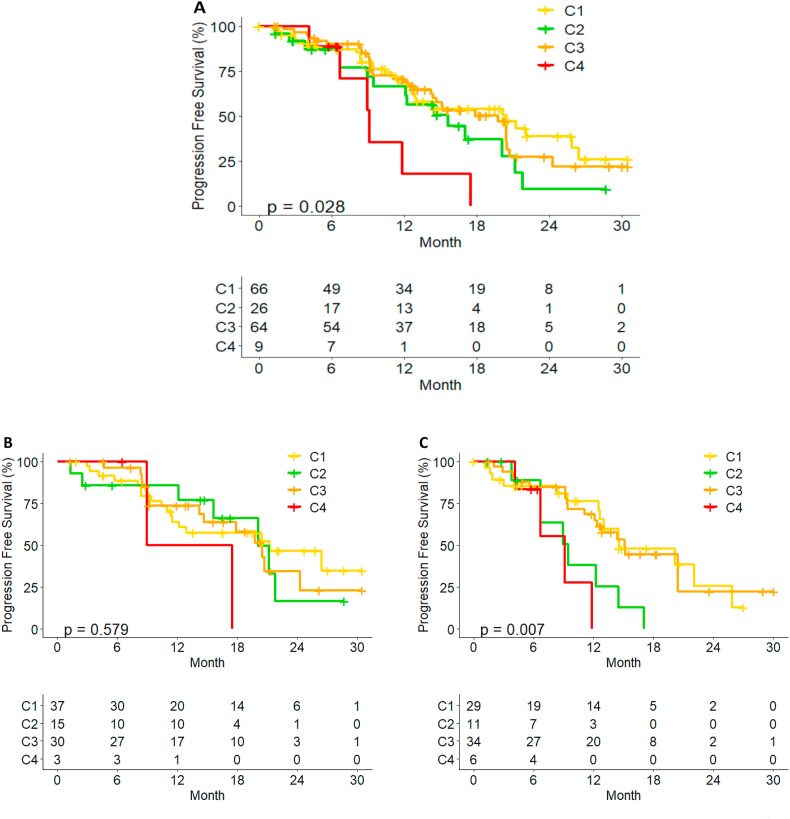
We performed immune gene expression profiling and conducted unsupervised consensus clustering of 165 tumors using expression of 130 immune-related genes. The best separation was achieved by dividing the patients into four subtypes (Fig. S5). C4 group showed low interferon and high regulatory T cell expression showed and reported worse prognosis not only analyzing by arm, but also within the entire study group (Fig. 7A–C).
Fig. 7.
PFS by Immune-related gene cluster. (A) all Young Pearl cohort. (B) Palbociclib plus ET. (C) Capecitabine treated patients.
4. Discussion
CDK4/6 inhibitors have emerged as a significant advance for the treatment of patients with HR+, HER2- MBC. While many CDK4/6 inhibitor clinical studies, including the Young Pearl study focusing on premenopausal patients, have demonstrated to improve clinical outcomes such as PFS and OS, there is no suggestion to direct therapy other than the presence of estrogen receptor (ER) expression.
We attempted an analysis using the NGS techniques to find biomarkers predicting efficacy and resistance of treatment in palbociclib plus ET or capecitabine. In the palbociclib plus ET treated arm, patients with luminal type showed better prognosis and BRCA2 pathogenic mutation showed worse prognosis regardless luminal/non-luminal subtype. ESR1 mutation was found in low frequency (3.4%) which is related to the absence of aromatase inhibitor treatment of participants in this study. Some molecular alteration such as high TMB, TP53 mutation, PTEN loss of function and RB pathway alteration may be associated with resistance of palbociclib plus ET treated patients as consistently reported in other studies [17]. Even though the sample size was not enough AURKA alteration (mutation, amplification and expression), BRIP1/MYC/RAD51C amplification and HRR loss of function mutation may predict the poor prognosis among palbocliclib plus ET treated patients. It is noteworthy that AURKA, BRIP alteration were associated with shorter PFS <6 month which need to be further explored.
Breast cancer that develops in premenopausal women may have biologic differences from that which develops in postmenopausal women [18]. According to recently updated OS data with ribociclib, we can compare OS of 41 months [3] in premenopausal women to 51 months [19] in postmenopausal patients treated with ET alone (control arm) in the MBC frontline setting. It can be expected that the premenopausal breast cancer biology would be more aggressive than postmenopausal one. According to multi-omics profiling, younger Korean breast cancers appeared to harbor significant molecular differences from western breast cancers. These younger cohort expressed ER at lower levels along with weaker expression signature ER signaling, suggesting that these tumors were less dependent on estrogen signaling than older BCs. Weaker tumor addiction to ER signaling coupled with co-occurring oncogenic drivers could in part explain that HR + young breast cancer responded more poorly to ET than their older counterparts [20]. The very similar design of PEARL study, the postmenopausal version of Young Pearl trial, showed the limited efficacy of palbociclib plus exemestane (8 months PFS) compared to capecitabine (10.6 months PFS) even in wild-type ESR1 population [21]. Distinctive molecular features of young breast cancer would be the reason that premenopausal women might greater benefit from the addition of CDK4/6 inhibitor and overcome treatment resistance to ET alone.
In this study, high immune score subtype did not show a survival advantage among premenopausal patients with HR+, HER2- MBC and negatively correlated with PFS in capecitabine treated patients with statistically significance. Young BC patients appeared to harbor more inflammatory immune microenvironments than elderly BC patients, as indicated by higher expression levels of cytotoxic T-cell markers and checkpoint mediators such as PD-L1 [20]. This finding can be helpful to develop future treatment options for premenopausal patients with HR+, HER2- MBC.
We mainly performed targeted DNA sequencing and compared with RNA sequencing data to find out if there are strong biomarkers that can predict the prognosis. Unfortunately, there was little correlation between the DNA mutation/amplification and the RNA expression level in this study. We didn't analyze the entire genes of the cell-cycle signaling pathway related to the mechanism of action of CDK4/6 inhibitors due to limited panel of targeted DNA sequencing. As an ad hoc exploratory analysis of Young-PEARL study with small number of sample size, it was not possible to clarify the meaning of specific molecular genetic variations under the lack of statistical power.
Additional limitation was that we were unable to address whether the type of tumor tissue affects prognosis or treatment benefit. Our tissue sample was obtained primary archival breast tissue (72%) and metastatic tumor tissue (28%), and it is possible that this heterogeneity might influence the results. Analysis of half of all patients was performed for searching biomarkers with palbociclib plus ET at this moment and further analysis of capecitabine treated patients will be performed and comprehensively compare the entire results. Finally, it is necessary to perform additional analysis to see if there are useful biomarkers to explain OS in addition to PFS.
In conclusion, we investigated multiple genomic alterations which were associated with clinical outcome in Young-Pearl study. Several potential genes and immune gene cluster that could predict the prognosis of palbociclib plus ET treatment were analyzed. We found some molecular variables to be warranted as a biomarker of early progression and resistance. CDK4/6 inhibitors plus ET shows good treatment effect in most of HR + breast cancer patients, but some patients show early rapid progression and primary resistance during treatment course. We hope that this study will serve as an exploratory basis for finding such resistant signals.
Funding
This work was supported by a grant from the South Korean Ministry of Health and Welfare (HA17C0055) and by the South Korean National R&D Program for Cancer Control, Ministry of Health and Welfare (1720150).
Author contributions
Soohyeon Lee: Conceptualization, Writing – original draft preparation, rewriting and editing kyunghee Park: Methodology, Data curation, Formal analysis, Software, Validation Gun Min Kim, Kyung Hae Jung, Seok Yun Kang, In Hae Park, Jee Hyun Kim, Hee Kyung Ahn: Investigation, Data collection, Resources, Writing - Review Woong-Yang Park: Methodology, Software, Writing - Review Seock-Ah Im: Methodology, Investigation, Writing – review & editing, Supervision, Yeon Hee Park: Conceptualization, Funding acquisition, Methodology, Investigation, Writing – review & editing, Supervision.
Declaration of competing interest
SA Im reports grants from Pfizer, drug supply from Pfizer, Shinpoong, Daewoong, and Takeda (during the conduct of the study); grants from AstraZeneca and Pfizer (outside the submitted work); and advisory role for Hanmi, GSK, Pfizer, Eisai, Amgen, Lilly, MSD, and Roche (outside the submitted work). KHJ reports grants from Ono Pharma Korea (outside the submitted work). HKA reports personal fees and non-financial support from AstraZeneca, Roche, Boehringer Ingelheim, Menarini, Pfizer, Bristol-Myers Squibb, Ono, and Boryung, and grants from Samyang (outside the submitted work). KHJ reports personal fees from AstraZeneca Korea and Roche Korea (outside the submitted work). YHP reports grants and non-financial support from Pfizer, AstraZeneca, Novartis, Merck, and Roche (during the conduct of this study), and grants from Eisai (outside the submitted work). All other authors declare no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.01.014.
Contributor Information
Seock-Ah Im, Email: moisa@snu.ac.kr.
Yeon Hee Park, Email: yhparkhmo@skku.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cristofanilli M., Turner N.C., Bondarenko I., Ro J., Im S.A., Masuda N., et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 2.Finn R.S., Martin M., Rugo H.S., Jones S., Im S.A., Gelmon K., et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 3.Im S.A., Lu Y.S., Bardia A., Harbeck N., Colleoni M., Franke F., et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381:307–316. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 4.Hortobagyi G.N., Stemmer S.M., Burris H.A., Yap Y.S., Sonke G.S., Paluch-Shimon S., et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2019;30:1842. doi: 10.1093/annonc/mdz215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sledge G.W., Jr., Toi M., Neven P., Sohn J., Inoue K., Pivot X., et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6:116–124. doi: 10.1001/jamaoncol.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park Y.H., Kim T.Y., Kim G.M., Kang S.Y., Park I.H., Kim J.H., et al. Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer (KCSG-BR15-10): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2019;20:1750–1759. doi: 10.1016/S1470-2045(19)30565-0. [DOI] [PubMed] [Google Scholar]
- 7.Rugo H.S., Dieras V., Gelmon K.A., Finn R.S., Slamon D.J., Martin M., et al. Impact of palbociclib plus letrozole on patient-reported health-related quality of life: results from the PALOMA-2 trial. Ann Oncol. 2018;29:888–894. doi: 10.1093/annonc/mdy012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verma S., O'Shaughnessy J., Burris H.A., Campone M., Alba E., Chandiwana D., et al. Health-related quality of life of postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer treated with ribociclib + letrozole: results from MONALEESA-2. Breast Cancer Res Treat. 2018;170:535–545. doi: 10.1007/s10549-018-4769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman P.A., Toi M., Neven P., Sohn J., Grischke E.M., Andre V., et al. Health-related quality of life in MONARCH 2: abemaciclib plus fulvestrant in hormone receptor-positive, HER2-negative advanced breast cancer after endocrine therapy. Oncol. 2020;25:e243–e251. doi: 10.1634/theoncologist.2019-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goetz M.P., Martin M., Tokunaga E., Park I.H., Huober J., Toi M., et al. Health-related quality of life in MONARCH 3: abemaciclib plus an aromatase inhibitor as initial therapy in HR+, HER2- advanced breast cancer. Oncol. 2020;25:e1346–e1354. doi: 10.1634/theoncologist.2020-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harbeck N., Iyer S., Turner N., Cristofanilli M., Ro J., Andre F., et al. Quality of life with palbociclib plus fulvestrant in previously treated hormone receptor-positive, HER2-negative metastatic breast cancer: patient-reported outcomes from the PALOMA-3 trial. Ann Oncol. 2016;27:1047–1054. doi: 10.1093/annonc/mdw139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S., Im S.A., Kim G.M., Jung K.H., Kang S.Y., Park I.H., et al. Patient-reported outcomes of palbociclib plus exemestane with GnRH agonist versus capecitabine in premenopausal women with hormone receptor-positive metastatic breast cancer: a prospective, open-label, randomized phase ll trial (KCSG-BR 15-10) Cancers. 2020;12 doi: 10.3390/cancers12113265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoninger S.F., Blain S.W. The ongoing search for biomarkers of CDK4/6 inhibitor responsiveness in breast cancer. Mol Cancer Therapeut. 2020;19:3–12. doi: 10.1158/1535-7163.MCT-19-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finn R.S., Liu Y., Zhu Z., Martin M., Rugo H.S., Dieras V., et al. Biomarker analyses of response to cyclin-dependent kinase 4/6 inhibition and endocrine therapy in women with treatment-naive metastatic breast cancer. Clin Cancer Res. 2020;26:110–121. doi: 10.1158/1078-0432.CCR-19-0751. [DOI] [PubMed] [Google Scholar]
- 15.Turner N.C., Liu Y., Zhu Z., Loi S., Colleoni M., Loibl S., et al. Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2019;37:1169–1178. doi: 10.1200/JCO.18.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prat A., Chaudhury A., Solovieff N., Pare L., Martinez D., Chic N., et al. Correlative biomarker analysis of intrinsic subtypes and efficacy across the MONALEESA phase III studies. J Clin Oncol. 2021;39:1458–1467. doi: 10.1200/JCO.20.02977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bardia A., Su F., Solovieff N., Im S.A., Sohn J., Lee K.S., et al. Genomic profiling of premenopausal HR+ and HER2- metastatic breast cancer by circulating tumor DNA and association of genetic alterations with therapeutic response to endocrine therapy and ribociclib. JCO Precis Oncol. 2021;5 doi: 10.1200/PO.20.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chollet-Hinton L., Anders C.K., Tse C.K., Bell M.B., Yang Y.C., Carey L.A., et al. Breast cancer biologic and etiologic heterogeneity by young age and menopausal status in the Carolina Breast Cancer Study: a case-control study. Breast Cancer Res. 2016;18:79. doi: 10.1186/s13058-016-0736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hortobagyi G.N., Stemmer S.M., Burris H.A., Yap Y.S., Sonke G.S., Hart L., et al. Overall survival (OS) results from the phase III MONALEESA-2 (ML-2) trial of postmenopausal patients (pts) with hormone receptor positive/human epidermal growth factor receptor 2 negative (HR+/HER2L) advanced breast cancer (ABC) treated with endocrine therapy (ET) +/- ribociclib (RIB) Ann Oncol. 2021;32:S1290–S1291. doi: 10.1016/j.annonc.2021.08.2090. [DOI] [Google Scholar]
- 20.Kan Z., Ding Y., Kim J., Jung H.H., Chung W., Lal S., et al. Multi-omics profiling of younger Asian breast cancers reveals distinctive molecular signatures. Nat Commun. 2018;9:1725. doi: 10.1038/s41467-018-04129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin M., Zielinski C., Ruiz-Borrego M., Carrasco E., Turner N., Ciruelos E.M., et al. Palbociclib in combination with endocrine therapy versus capecitabine in hormonal receptor-positive, human epidermal growth factor 2-negative, aromatase inhibitor-resistant metastatic breast cancer: a phase III randomised controlled trial-PEARL. Ann Oncol. 2021;32:488–499. doi: 10.1016/j.annonc.2020.12.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.