Abstract
Ciliary neurotrophic factor (CNTF) is produced by astrocytes which have been implicated in regulating stress responses. We found that CNTF in the medial amygdala (MeA) promotes despair or passive coping, i.e., immobility in an acute forced swim stress, in female mice, while having no effect in males. Neutralizing CNTF antibody injected into the MeA of wildtype females reduced activation of downstream STAT3 (Y705) 24 and 48 h later. In concert, the antibody reduced immobility in the swim test in females and only after MeA injection, but not when injected in the central or basolateral amygdala. Antibody injected into the male MeA did not affect immobility. These data reveal a unique role of CNTF in female MeA in promoting despair or passive coping behavior. Moreover, 4 weeks of chronic unpredictable stress (CUS) increased immobility in the swim test and reduced sucrose preference in wildtype CNTF+/+, but not CNTF−/− littermate, females. Following CUS, 10 min of restraint stress increased plasma corticosterone levels only in CNTF+/+ females. In males, the CUS effects were present in both genotypes. Further, CUS increased CNTF expression in the MeA of female, but not male, mice. CUS did not alter CNTF in the female hippocampus, hypothalamus and bed nucleus of stria terminalis. This suggests that MeA CNTF has a female-specific role in promoting CUS-induced despair or passive coping, behavioral anhedonia and neuroendocrine responses. Compared to CNTF+/+ mice, CNTF−/− mice did not show differences in CUS-induced anxiety-like behavior and sensorimotor gating function as measured by elevated T-Maze, open field and pre-pulse inhibition of the acoustic startle response. Together, this study reveals a novel CNTF-mediated female-specific mechanism in stress responses and points to opportunities for developing treatments for stress-related disorders in women.
Keywords: Chronic unpredictable stress, Passive stress-coping, Anhedonia, Neuroendocrine response, Neutralizing antibody, Stereotaxic injection
Abbreviations: BLA, basolateral amygdala; BNST, bed nucleus of stria terminalis; CeA, central amygdala; CNTF, ciliary neurotrophic factor; CRF, corticotropin releasing factor; CUS, chronic unpredictable stress; HPA, hypothalamic-pituitary-adrenal; Hyp, hypothalamus; IL-6, interleukin-6; LIF, leukemia inhibitory factor; MeA, medial amygdala; mPFC, medial prefrontal cortex; PAG, periaqueductal grey; PTSD, post-traumatic stress disorder; PVN, paraventricular nucleus; TNF, tumor necrosis factor
Graphical abstract
Highlights
-
•
CNTF in the MeA promotes despair or passive coping behavior in female mice only.
-
•
Chronic stress upregulates CNTF in female but not male MeA.
-
•
CNTF contributes to chronic stress-induced despair or passive coping, anhedonia and neuroendocrine responses in females only.
-
•
CNTF does not affect anxiety-like behavior and sensorimotor gating function.
-
•
These data reveal a novel CNTF-mediated female-specific mechanism in stress responses.
1. Introduction
Chronic stress is a potent triggering factor for numerous mental disorders, including depression and post-traumatic stress disorder (PTSD) (Chrousos, 2009; McEwen, 2007). The dysfunction of stress responses is linked to these disorders (Deussing and Chen, 2018; Godoy et al., 2018). Women are much more susceptible to these disorders than men, possibly due to more robust responses to stress (Chrousos, 2009; McEwen, 2007; Rincon-Cortes et al., 2019) but the underlying mechanisms are not understood well.
Chronic stress enhances immobility in the forced swim test (Dunn and Swiergiel, 2008; Lam et al., 2018). The immobility response during the forced swim has been labeled as depression-like behavior or despair. Recent findings also recommend that acquired immobility is passive coping or adaptive behavior to an inescapable stressor, including the forced swim (Commons et al., 2017; de Kloet and Molendijk, 2016; Molendijk and de Kloet, 2015). Chronic stress also increases anhedonia, i.e., a reduced capacity to experience pleasure (Stanton et al., 2019), and hypothalamus-pituitary-adrenal (HPA) response (Deussing and Chen, 2018). These stress responses are positively associated with stress-related mental disorders, such as depression and PTSD (Deussing and Chen, 2018; Godoy et al., 2018). Importantly, sex differences have been found in these stress responses and females have more robust responses than males (Kokras and Dalla, 2014; Xing et al., 2013). Identifying the sex-specific mechanisms underlying these responses will provide novel therapeutic targets for developing treatments for stress-related mental disorders specifically for women or men.
Ciliary neurotrophic factor (CNTF) is a member of the interleukin-6 (IL-6) cytokine family and expressed almost exclusively in the nervous system (Stockli et al., 1989). In the central nervous system, CNTF is produced by astrocytes and increased following injury (Kang et al., 2013; Yang et al., 2008). CNTF increases adult neurogenesis (Emsley and Hagg, 2003; Jia et al., 2018; Yang et al., 2008) and promotes neuronal survival (Hagg et al., 1992; Hagg and Varon, 1993; Kang et al., 2013). CNTF acts through the CNTF-specific receptor, CNTFRα, expressed by neurons (Ip et al., 1993). Astrocytes are essential in regulating stress responses by downregulating gap-junction coupling and impairing glutamate uptake and transmission (Murphy-Royal et al., 2019). Stress responses help to maintain homeostasis by regulating multiple neural circuits and effector molecules in the brain (Deussing and Chen, 2018). A recent study shows that chronic intermittent cold stress reduces CNTF in the rat orbitofrontal cortex, which leads to reversal learning deficit (Girotti et al., 2019), suggesting a contribution of CNTF in stress response. We previously identified a striking sex-specific effect of CNTF on immobility in the forced swim in mice. CNTF promotes immobility in female mice while reducing it in males (Jia et al., 2019). The switch from active (swim) to passive (immobility) coping is controlled by a top-down pathway in the brain, i.e., the medial prefrontal cortex (mPFC), via the bed nucleus of stria terminalis (BNST), connects to the periaqueductal grey (PAG) (Molendijk and de Kloet, 2019; Molendijk and de Kloet, 2021). Other brain areas, including the amygdala, modulate stress-coping response by projections into these nuclei (Molendijk and de Kloet, 2019; Molendijk and de Kloet, 2021). Female mice have higher levels of CNTF in the amygdala, but not the cortex, hippocampus or hypothalamic PVN, than males (Jia et al., 2019), suggesting that the CNTF effect on immobility may be through mechanism(s) in the amygdala.
Here, we first defined the region-specific effect of CNTF within the amygdala on the immobility responses to inescapable swim stress, using CNTF antibody injections into the subnuclei of the amygdala. Secondly, we determined the role of CNTF in chronic stress responses in females vs. males, using wildtype and knockout mice.
2. Materials and methods
2.1. Animals
A total of 455 mice were used. The CNTF+/+ and CNTF−/− mice (Valenzuela et al., 2003) are on a C57 background and were backcrossed to JAX C57BL/6 mice ten times (=99.9% C57BL/6). Genotyping protocol was provided by Regeneron Pharmaceuticals who provided the original breeders. We bred CNTF heterozygous mice to produce sex-matched littermates and experiments started when they were 6–8 weeks old. All mice were housed with food and water available ad libitum, and maintained on a 12 h light:12 h dark cycle. All animal procedures were approved by the East Tennessee State University Committee, which is consistent with the NIH Guide on Care and Use of Animals.
2.2. Chronic unpredictable stress (CUS)
CUS included environmental and social stress without food and water deprivation and nociceptive events. There were two groups: CUS and control handling group. For mice in the CUS group, CUS was implemented for 4 weeks using a previously published protocol with minor modifications (Hu et al., 2012; Papadopoulou et al., 2015; Willner, 2005). Briefly, mice were subjected to seven pairs of stressors with one pair each day. Day 1: 1 h on an orbital shaker (100 rpm) followed by 12 h damp bedding. Day 2: 1 h immobilization in 50 ml Falcon tube followed by 12 h in a tilted cage (45°). Day 3: 1 h exposure to overcrowding by placing four to five mice in a plastic box (10 × 10 × 5cm) with ventilation holes followed by 1 h cage shaking. Day 4: 1 h immobilization followed by 12 h tilted cage. Day 5: 1 h exposure to overcrowding followed by 12 h damp bedding. Day 6: 1 h cage shaking followed by 12 h tilted cage. Day 7: 1 h immobilization followed by 24 h light on (no dark period). The same cycle was repeated for 4 weeks. Control mice were handled in the morning of every day while the CUS was performed.
2.3. Behavioral analyses
Behavioral tests were conducted 1–3 days after the termination of CUS. All behavioral tests were conducted from 10 a.m. to 12 p.m. during the light phase. To attempt to minimize the order or carryover effect of multiple behavior tests, we used three different cohorts of mice (Supplemental Fig. 1). Each cohort was given one or three tests that would unlikely produce a carryover or order effect, although we concede this may not have been completely eliminated. One cohort of mice was tested on sucrose preference, forced swim and open field. The second cohort of mice was tested on the elevated T-maze. The third cohort of mice was tested on pre-pulse inhibition of the acoustic startle response. The wire hanging test was performed in non-stressed naïve mice.
2.3.1 Forced swim test, as we used previously (Jia et al., 2019), was performed in a circular pool of water (23–25 °C). All mice were tested in a single 6 min trial with the last 4 min used for data analysis. The duration of immobility in seconds were recorded using AnyMaze behavioral scanning software (Stoelting Co., Wood Dale, IL). Immobility was defined as the cessation of all movements except those necessary to stay floating.
2.3.2 Sucrose preference test. Behavioral anhedonia was measured by sucrose preference (Higuchi et al., 2016). Briefly, mice were subjected to water deprivation for 16 h, and then two pre-weighed bottles with one containing tap water and the other one containing 1% sucrose solution were presented for 90 min. The positions of water and sucrose bottles (left and right) were switched every 30 min. The bottles were weighed during each position switch and at the end of test. The weight differences during the last 60 min were used to calculate the volume intake from each bottle. The sucrose preference was expressed as a percentage of sucrose intake relative to the total liquid (sucrose + water) intake.
2.3.3 Open field test measures locomotor function that served as a control for possible motor deficits that might confound performance on other behavioral tests and performed as we described previously (Jia et al., 2019). The test was conducted in a square white Plexiglas arena and locomotor activity was recorded using a digital camera mounted above the arena. The session was 10 min and a digital grid was superimposed on the box floor and activity was monitored by AnyMaze software. The distance traveled for each mouse was recorded in meter (m). Further, times spent in the center vs. peripheral area were also used to assess anxiety-like behavior.
2.3.4 Wire hanging test measures global muscle function and coordination (Olivan et al., 2015). Briefly, each mouse was placed on a wire cage top over the home cage. Then the cage top was inverted and suspended above the home cage. The latency for the mouse to fall into the home cage was recorded. Each mouse had three trials (a maximum of 5 min per trial) per day and was tested for 3 days. The average latency to fall was calculated.
2.3.5 Elevated T-Maze test was conducted as we did previously (Jia et al., 2019) to measure anxiety-like behavior. There were a total of four trials. The first three trials were placing the mouse into the closed arm and recording the latency to leave the walled area to enter the open arm. The first trial is considered habituation, and trial 2 and 3 were scored as acquisition trials. The last trial was an escape trial by placing the mouse on either end of the open arms and recording the latency to enter the closed arm.
2.3.6 Pre-pulse inhibition of the acoustic startle response measures sensorimotor gating function (Shelton et al., 2021). There were three PPI chambers (Kinder Scientific, Poway, CA) and each mouse was tested in the same chamber on each day. Each daily session began with a 5-min habituation period with only the background noise (70 dB) present. After this habituation was complete, animals were subjected to three different, randomly assigned trial types, which included pulse, prepulse, and no stimulus trials. The pulse trial was a 120 decibel (dB) startle pulse administered by itself. The prepulse trial was an auditory stimulus that was either 3, 6, or 12 dB above the 70-dB background noise. The no stimulus trial was when a stimulus was not provided. A total of 5 pulse, 5 no stimulus, and 15 prepulse trials (5 trials of each 73, 76, and 82 dB) were presented in each session. The animal response was recorded and measured in Newtons within a 250-ms window immediately following stimulus presentation through a computer interface. Animals were tested for three consecutive days. The intertrial interval given on each trial averaged 15 s. PPI was calculated using the following equation:100−[(mean prepulse response/mean pulse Response) × 100].
2.4. Stereotaxic intra-amygdala injections
Intracerebral stereotaxic injections were performed similarly to past procedures in our laboratory (Jia et al., 2018, 2020). Briefly, following anesthesia with an i.p. injection of Avertin (i.p., 0.4 g/kg), the mouse was placed into Kopf stereotaxic apparatus using ear bars. A total of 0.3 μl of purified goat IgG (1 μg/μl, PP40, EMD Millipore, RRID: AB_97837) or goat anti-mouse/rat CNTF neutralizing IgG antibody (1 μg/μl, AB-557-NA, R & D Systems, RRID: AB_354368, (Yang et al., 2008)) was bilaterally injected using a 26 gauge Hamilton syringe. Volumes of 0.35–1 μl have been used for intra-MeA injections and do not result in cell loss or tissue damage (Shemesh et al., 2016). The injection was made over 3 minutes, with 2 minutes waiting periods before and after to reduce backflow. The stereotaxic coordinates from Bregma were AP = −1.7 mm, ML = ±2.25 mm and DV = −5.3 mm for the medial amygdala, MeA (Shemesh et al., 2016); AP = −1.2 mm, ML = ±2.5 mm and DV = −4.6 mm for the central amygdala, CeA (Beckerman and Glass, 2012), and AP = −1.4 mm, ML = ±3.3 mm and DV = −5.0 mm for the basolateral amygdala, BLA (Heldt and Ressler, 2010).
2.5. Tissue collection
Mice were briefly anesthetized with 4% isoflurane for 0.5 min. After rapid decapitation by guillotine, trunk blood was collected using EDTA-coated microcapillary blood collection tubes (cat# 07–6011, RAM Scientific) and centrifuged at 3000 g for 20 minute at 4 °C. The plasma was stored at −80 °C. Blood collection was in the afternoon between 1 and 3 pm, which precludes any time-dependent dynamics of corticosterone during the day (Chen et al., 2006; Deussing et al., 2010). A 10 min of restraint stress was applied to some of the mice prior to blood collection by placing the mouse into a 50 ml Falcon tube. The brain was dissected and flash frozen in wet dry ice with isopentane and stored at −80 °C. The MeA, hypothalamic PVN and hippocampus were punched out from 700 μm thick coronal brain cryostat sections from Bregma −1.2 to −1.9 (Jia et al., 2019). The BNST was punched out from a 500 μm thick coronal brain cryostat sections from Bregma 0.0 to −0.5. All samples were stored at −80 °C for mRNA and protein analysis.
2.6. RT-qPCR, Western blotting, immunostaining and ELISA
RT-qPCR and Western blotting were performed, as we did previously (Jia et al., 2019). Primers from ThermoFisher Scientific included Mouse CNTF (Mm00446373-ml), LIF (Mm00434762_g1), IL-6 (Mm0044619_ml), TNF (Mm00443258_ml) and GAPDH (Mm99999915-gl). Data analysis was performed with ΔΔCt method and GAPDH was used as an endogenous loading control. The antibodies used in Western blots included CNTF antibody (MAB338, EMD Millipore, RRID: AB_2083064), phospho-STAT3-Tyr705 (pSTAT3705, #9131, Cell signaling, RRID: AB_331586), STAT3 (#9132, Cell Signaling, RRID: AB_823645), and β-actin (Cat# 4967, Cell Signaling, RRID: AB_330288). For fluorescence western blot, donkey anti-rabbit IRDye 800CW (926–32213, LI-COR) and anti-mouse IRDye 680RD (926–68072, LI-COR) secondary antibodies were used. For chemiluminescence western blot, HRP-conjugated secondary antibodies were used. Images were taken by Odyssey XF Imaging System (LI-COR) and quantified using Image Studio Ver 5.2 (LI-COR). Donkey anti-goat IgG-conjugated to Alex Fluor-488 was used for immunostaining. The levels of plasma corticosterone were measured using ELISA kit (ab108821, Abcam).
2.7. Statistical analyses
Statistical significance was determined by p < 0.05 (GraphPad Prism 7.0). Two-tailed student t tests were performed when two groups were compared. A one-way or two-way ANOVA was applied when there were three or more groups to test one factor or two factors, such as genotypes and treatments. The Newman-Keuls test was used for post hoc multiple comparisons as appropriated. Data are presented as mean + SEM.
3. Results
3.1. CNTF in the MeA promotes despair or passive coping behavior in female but not male mice
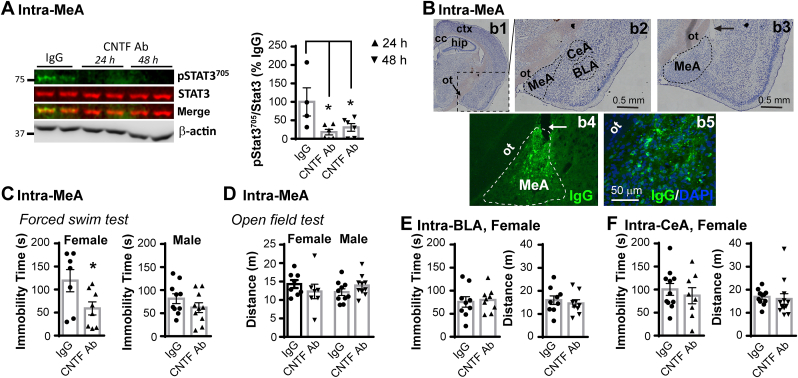
To determine region-specific CNTF effects within the amygdala, IgG or CNTF neutralizing antibody was stereotaxically injected into the MeA, BLA or CeA of female and male C57BL/6 mice. At 24 and 48 h after intra-MeA CNTF antibody in females, pSTAT3705, which is downstream of CNTF, was reduced in extracts of the MeA by 82% and 69%, respectively (Fig. 1A, F(2, 13) = 5.093, p = 0.023, one-way ANOVA), confirming the efficacy of the antibody. Injected IgG in the MeA, BLA or CeA was validated by immunostaining for IgG in purified IgG-injected mice (Fig. 1B, Supplemental Fig. 2). After antibody injection, the females and males were tested in the forced swim test at 24 h and in the open field test at 48 h. Neutralizing CNTF in the MeA reduced the immobility time in the forced swim test by 50% in females (Fig. 1C, t(13) = 2.226, p = 0.044), while not producing any effect in males, suggesting a female-specific effect of MeA CNTF on promoting despair or passive coping behavior. This sex-specific CNTF effect did not attribute to changes in motor function since there was no effect of CNTF antibody on spontaneous locomotor activity tested in the open field in either male or female mice (Fig. 1D). Injection of CNTF antibody into the BLA (Fig. 1E) or CeA (Fig. 1F) of female mice did not affect immobility time in the forced swim test and locomotor activity in the open field, suggesting that CNTF in these areas are not involved in the behavioral response to an acute stressor. Collectively, these data indicate that CNTF in the MeA has a female-specific effect on promoting despair or passive coping behavior to inescapable acute stress.
Fig. 1.
CNTF in the MeA promotes immobility during acute inescapable swim stress in female but not male mice. A) Injection of CNTF Ab into the MeA of C57BL/6 mice reduced the levels of pStat3705 24 and 48 h later in female mice as shown by double fluorescence Western blot and quantification. Quantification of pStat3705 was performed using the same areas in the blots as the total Stat3 bands, which was especially necessary for pStat3705 at 24 and 48 h when signals were very weak. N = 4,6,6 female mice, *p < 0.05 (one-way ANOVA followed by Bonferroni test. B) Confirmation of intra-MeA injection. In b1-b2, crystal violet-stained coronal brain sections indicate the location of the MeA, BLA and CeA. ctx-cortex, cc-corpus callosum, hip-hippocampus, ot-optic tract. In b3, the arrow indicates an intra-MeA injection tract. The most ventral position of the needle was purposefully kept just dorsal to the nucleus to prevent its injury. In b4, immunostaining for IgG in an IgG-injected mouse validates the spread of injected IgG within the MeA. A high magnification image of the MeA in b4 is shown in b5. C) Intra-MeA injection of CNTF Ab reduced immobility time in the forced swim tested at 24 h post-injection in females but not males. D) The CNTF Ab did not affect locomotor activity tested in an open field at 48 h post-injection in either sex. CNTF Ab injection into the E) BLA or F) CeA did not affect the immobility time or locomotor activity tested at 24 and 48 h post-injection, respectively. N = 7,8 females and 10,10 males in C-D, N = 9,9 females in E and 11,8 females in F, *p < 0.05 (Two-tailed t-test). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. Knockout of CNTF in female but not male mice blocks chronic stress-induced despair or passive coping, behavioral anhedonia and neuroendocrine response
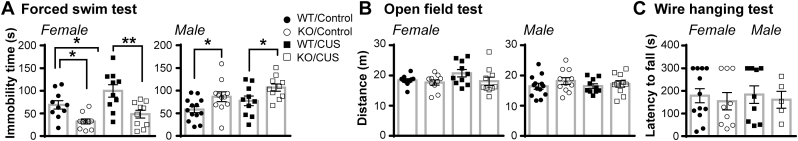
After 4 weeks CUS both female and male CNTF+/+ and CNTF−/− mice weighed less than control mice handled daily, without genotype differences (Supplemental Fig. 3), confirming the CUS effect. The forced swim test was performed at 24 h after termination of control handling or CUS to measure immobility (Fig. 2A). In female mice, a two-way ANOVA showed significant main effects of CUS (F(1, 36) = 5.818, p = 0.021) and genotype (F(1, 36) = 20.57, p < 0.0001). Post hoc comparisons revealed that CUS increased the immobility time in CNTF+/+ mice by 45%, but did not affect CNTF−/− females. Further, CNTF+/+ females had longer immobility time than CNTF−/− littermates in control-handled mice, which is consistent with our previous study (Jia et al., 2019), and in the CUS-treated group. These data indicate that a lack of CNTF in female mice blocks chronic stress-induced despair or passive coping to an inescapable acute stressor. In male mice, a two-way ANOVA also demonstrated significant main effects of CUS (F(1, 40) = 4.724, p = 0.036) and genotype (F(1, 40) = 10.06, p = 0.003). Post hoc comparisons demonstrated that knockout of CNTF increased immobility time in both control- and CUS-treated mice. These data suggest that CNTF knockout increases despair or passive coping in males, which is consistent with previous data (Jia et al., 2019), and CUS has an overall promoting effect on it. Neither CUS nor CNTF knockout altered locomotor function tested in an open field at 48 h after termination of control handling or CUS (Fig. 2B), suggesting that the effects on immobility were not due to motor deficits. Further, CNTF+/+ and CNTF−/− mice had similar muscle strength and coordination measured in a wire hanging test in both sexes at 10–14 weeks of age, when other behaviors were tested (Fig. 2C), supporting CNTF knockout effect does not attribute to motor deficits.
Fig. 2.
CNTF−/− female, but not male, mice have reduced chronic stress-induced immobility. Mice were subjected to 4 weeks of control handling or CUS. A) 24 h afterwards, CUS increased immobility time in the forced swim test in female wildtype CNTF+/+ (99.9% C57BL/6 background) but not CNTF−/− females. In males, immobility times were higher in both control- and CUS-treated CNTF−/− mice compared to their respective CNTF+/+ controls. B) 48 h after CUS, locomotor activity in an open field was not affected in either sexes or genotypes. N = 10 mice/group in females, N = 13,11,10,10 males, *p < 0.05, **p < 0.01 (Two-way ANOVA followed by Newman-Keuls multiple comparisons). C) Knockout of CNTF did not affect muscle strength and coordination tested by wire hanging in naïve 10–14 week old female and male mice. N = 12,9 females and 9,5 males.
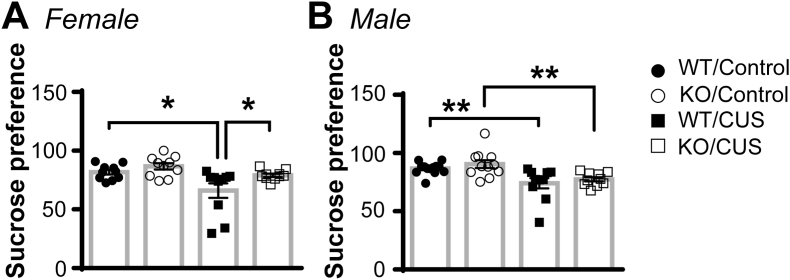
Sucrose preference was measured 4 h after the termination of 4 weeks of control handling or CUS. In female mice (Fig. 3A), a two-way ANOVA showed significant main effects of CUS (F(1, 36) = 10.56, p = 0.003) and genotype (F(1, 36) = 6.247, p = 0.017). CUS reduced sucrose preference in CNTF+/+ (by 20%), but not CNTF−/−, females. In CUS-treated groups, CNTF−/− females had higher levels of sucrose preference than CNTF+/+ females. Thus, knockout of CNTF in female mice attenuates chronic stress-induced behavioral anhedonia in females. In male mice (Fig. 3B), there was a significant main effect of CUS (F(1, 40) = 19.64, p < 0.0001). CUS reduced sucrose preference in both CNTF+/+ and CNTF−/− mice without genotype difference, suggesting CNTF does not affect chronic stress-induced anhedonia in males.
Fig. 3.
Knockout of CNTF blocks chronic stress-induced reduction of sucrose preference only in female mice. Sucrose preference was performed at 4 h after control handling or CUS to evaluate behavioral anhedonia. A) After 4 weeks of CUS, CNTF+/+, but not CNTF−/−, females had reduced sucrose preference compared to their own genotype controls. N = 10 mice/group. B) In male mice, CUS reduced sucrose preference in CNTF+/+ and CNTF−/− mice without genotype difference. N = 13,11,10,10 mice, *, p < 0.05, **p < 0.01 (Two-way ANOVA followed by Newman-keuls multiple comparisons).
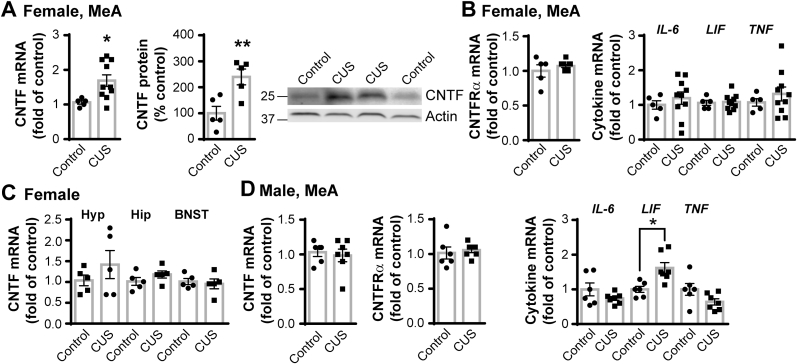
To exclude the potential compensatory effects on receptor or cytokines related to CNTF, we measured the mRNA levels of CNTF receptor, CNTFRα, leukemia inhibitory factor (LIF) and interleukin-6 (IL-6) in female mouse brains. CNTF−/− females has the same expression levels as their CNTF+/+ littermates in the MeA (Supplemental Figs. 4A and B). CNTF knockout also had no effect on LIF and IL-6 in the hypothalamic PVN, hippocampus and BNST (Supplemental Figs. 4C–E).
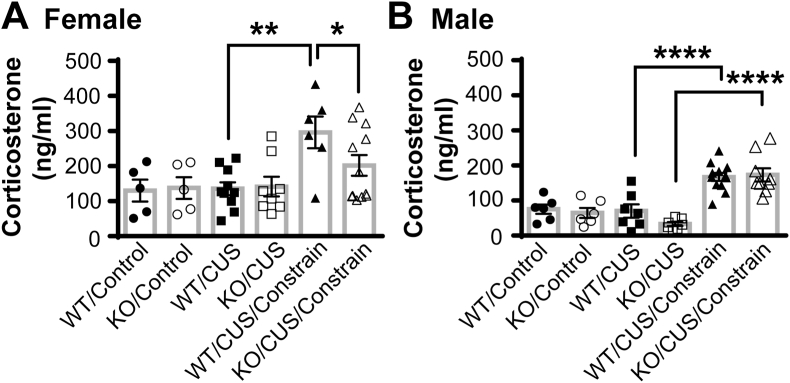
Plasma corticosterone levels were measured at 24 h after the termination of control handling or CUS, and in CUS mice challenged with 10 min of immobilization. Two-way ANOVA analyses showed a main effect of treatment (control vs. CUS vs. CUS + immobilization challenge) in both female (F(2, 40) = 8.757, p = 0.0007, Fig. 4A) and male (F(2, 41) = 40.94, p < 0.0001, Fig. 4B) mice. There were no genotype differences in the basal levels of corticosterone in either control- and CUS-treated mice of both sexes. An acute challenge of 10 min of restraint stress increased corticosterone (more than 2-fold) in CNTF+/+, but not CNTF−/−, females (Fig. 4A). In males, restraint stress increased corticosterone levels in CNTF+/+ and CNTF−/− mice without genotype difference (Fig. 5B). Together, these data indicate that CNTF knockout reduces the neuroendocrine response following chronic stress only in females.
Fig. 4.
CNTF−/− female, but not male, mice have a reduced restraint-induced neuroendocrine response following CUS. Plasma levels of corticosterone were measured at 24 h after control handling or CUS, with or without a subsequent 10 min of restraint challenge. A) In female mice, basal levels of corticosterone were not affected by CUS or genotype. Restraint challenge increased plasma corticosterone in CNTF+/+, but not CNTF−/−, females. B) In males, restraint challenge increased plasma corticosterone in both CNTF+/+ and CNTF−/− mice without genotype difference. N = 5,5,10,8,6,12 female mice and 6,6,7,7,12,9 male mice, *p < 0.05, **p < 0.01, ****p < 0.0001 (Two-way ANOVA followed by Newman-Keuls multiple comparisons).
Fig. 5.
CNTF knockout does not affect chronic stress-induced anxiety-like behavior in both sexes. Elevated T-maze and open field tests were performed at 24 and 48 h after 4 weeks of control handling or CUS. A) In the elevated T maze test, CUS increased latencies to enter the open arm in both sexes and genotypes, without genotype difference. B) CUS reduced latency to escape the open arm in female CNTF+/+ mice. N = 11, 11, 7, 11 females and 11, 9, 9, 8 males, *p < 0.05, **p < 0.01 (Two-way ANOVA followed by Newman-Keuls multiple comparisons). In the open field test, CUS increased time spent in the center area (C) of the open arena and reduced time spent in the peripheral area (D). N = 10, 10, 10, 10 females and 13, 11, 10, 10 males, *p < 0.05, **p < 0.01, ***p < 0.001 (Two-way ANOVA followed by Newman-Keuls multiple comparisons).
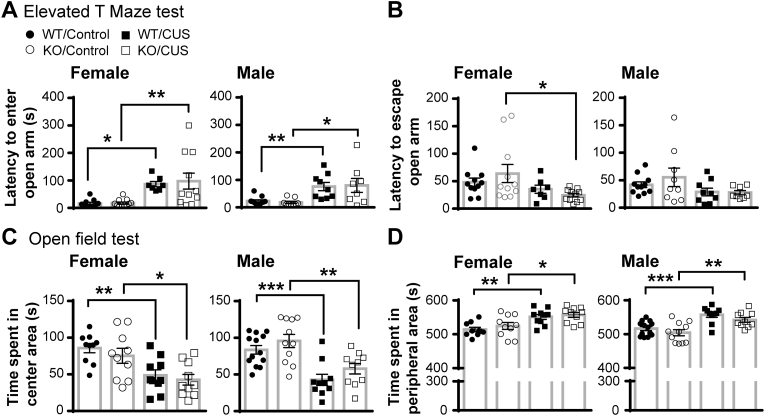
3.3. Knockout of CNTF does not affect anxiety-like behavior and sensorimotor gating function in both sexes
We measured anxiety-like behavior at 24 and 48 h after control handling or CUS in CNTF+/+ and CNTF−/− mice using elevated T maze and open field tests, respectively. The elevated T-maze test measures the conflict anxiety and panic-like escape behavior using latency to enter and escape open arm (Donner and Lowry, 2013). CUS increased latency to enter the open arm (Fig. 5A) in both females and males 3–5 fold without genotype differences. Two-way ANOVA analyses revealed main effects of CUS (F(1, 36) = 19.19, p < 0.0001 in females and F(1, 33) = 17.69, p = 0.0002 in males). CUS also had an overall effect on reducing latency to escape open arm (Fig. 5B) in both sexes (F(1, 36) = 6.024, p = 0.019 in females and F(1, 33) = 4.584, p = 0.040 in males). We also assessed anxiety-like behavior by measuring times spent in the center vs. peripheral area in the open field test. Both CUS-treated females and males spent less time in the center area of the open arena (Fig. 5C) and consequently longer time in the peripheral area (Fig. 5D) than their respective control-handled mice and no genotype differences were found. Two-way ANOVA analyses demonstrated main effects of CUS at F(1, 36) = 19.53, p < 0.0001 in females and F(1, 33) = 17.69, p = 0.0002 in males. Together, these data indicate that chronic stress promotes anxiety-like behavior in both sexes but that CNTF has no effect under physiological conditions, consistent with our previous study (Jia et al., 2019), or following chronic stress.
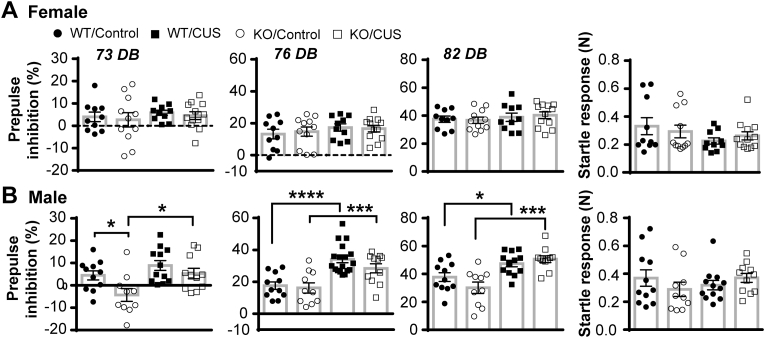
Sensorimotor gating function was assessed by pre-pulse inhibition for three consecutive days after control handling or CUS. In female mice (Fig. 6A), CUS did not alter pre-pulse inhibition nor the acoustic startle response in both genotypes. In male mice (Fig. 6B), CUS increased pre-pulse inhibition without genotype differences. CUS did not alter the startle response in males, either. Two-way ANOVA analyses revealed main effects of CUS at 73, 76 and 83 DB (F(1, 40) = 8.822, 53.67 and 25.20, p = 0.005, p < 0.0001 and p < 0.0001). These data suggest that chronic stress improves sensorimotor gating function in males, but not through CNTF.
Fig. 6.
Knockout of CNTF does not affect sensorimotor gating function.Pre-pulse inhibition was performed at 24, 48 and 72 h after 4 weeks of control handling or CUS. A) In female mice, CUS did not affect pre-pulse inhibition and startle response in both genotypes. B) In males, CUS increased pre-pulse inhibition without altering the startle response in both genotypes. N = 10,11,10,12 females and 11,10,12,11 males, *p < 0.05, ***p < 0.001, ****p < 0.0001 (Two-way ANOVA followed by Newman-Keuls comparison tests).
3.4. Chronic stress upregulates CNTF expression in female but not male MeA
Next, we determined the effect of chronic stress on CNTF expression in the MeA using the CNTF+/+ mice shown in Fig. 4 that did not undergo restraint stress. In females, CUS increased CNTF mRNA in the MeA by 70% (t(13) = 2.552, p = 0.024) and protein by 2.4 fold (t(8) = 3.459, p = 0.008, Fig. 7A). CUS did not affect mRNA levels of CNTFRα, or CNTF-related cytokines IL-6 and LIF, or pro-inflammatory tumor necrosis factor (TNF)(Fig. 7B). CUS did not alter CNTF mRNA levels in the hypothalamic PVN, hippocampus and BNST of the same females (Fig. 7C). In males, CUS did not change CNTF, CNTFRα, IL-6 and TNF, while increasing LIF mRNA in the MeA (Fig. 7D, t(9) = 2.876, p = 0.018). Together, these data suggest that chronic stress has an effect in females, but not in males, on CNTF expression in the MeA, which may contribute to chronic stress-induced passive coping behavior in females.
Fig. 7.
Chronic stress upregulates CNTF expression in female, but not male, MeA. Female and male wildtype CNTF+/+ mice treated with 4 weeks of control handling or CUS, without subsequent restraint, in Fig. 4 were used to measure gene expression. A) In the female MeA, CUS increased CNTF mRNA measured by RT-qPCR and protein by densitometry of Western blots. B) CUS did not alter CNTFRα, or IL-6, LIF and TNF mRNA in the female MeA. N = 5,10 mice for mRNA analysis and N = 5 mice/group for protein analysis. *p < 0.05, **p < 0.01 (Two-tailed t-test). C) CUS did not affect CNTF mRNA expression in the hypothalamic PVN (Hyp), hippocampus (Hip) and BNST of the same females. N = 5,5 mice. D) In the male MeA, CUS had no effect on CNTF, CNTFRα, IL-6 or TNF, but increased LIF mRNA expression. N = 6,7 mice. *p < 0.05, **p < 0.01 (Two-tailed t-test).
4. Discussion
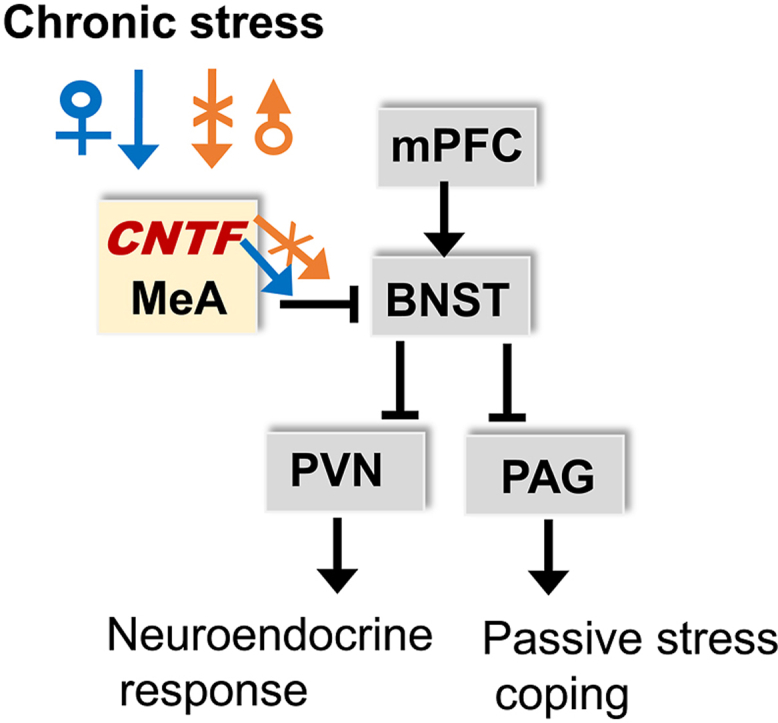
This study, for the first time, reveals a female-specific role of CNTF specifically in the MeA to increase despair or passive coping behavior in response to inescapable acute stress. Importantly, our data suggest that, in females, CNTF also increases chronic stress-induced despair or passive coping, anhedonia, and corticosterone response, which could be mediated in part by the MeA. Increased stress responses are associated with stress-related mental disorders, including depression and PTSD (Deussing and Chen, 2018; Godoy et al., 2018). The female-specific CNTF mechanism provides a novel therapeutic target for developing treatments for women with these disorders, which affect them more severely than men.
4.1. Female-specific role of CNTF in the MeA in despair or passive coping to acute inescapable stress
Sex differences have been reported in rodent forced swim tests (Kokras et al., 2015; Kokras and Dalla, 2017). Several studies found that females displayed higher levels of immobility than male controls (Dalla et al., 2008; Drossopoulou et al., 2004; Hong et al., 2012; Kokras et al., 2012; Leussis and Andersen, 2008; Li et al., 2015; Pitychoutis et al., 2009; Tonelli et al., 2008), whereas others reported opposite results or no sex differences (Andrade et al., 2007; Brotto et al., 2000; Brummelte et al., 2006; Fonken et al., 2016; Martinez-Mota et al., 2011). These could be due to different species (rat vs. mouse) and different mouse strains (Dalla et al., 2010; Voikar et al., 2001). In CNTF mice who have a C57 background, we found that female mice display higher levels of immobility than males and, importantly, that CNTF−/− females have reduced immobility compared to wildtype CNTF+/+ littermates (Jia et al., 2019). This suggests that CNTF plays an important role in despair or passive coping behavior and this role is sex-specific. Stress-coping behavior is controlled by the mPFC-BNST-PAG pathway, in which mPFC inhibits PAG via GABAergic projections from BNST to PAG (Molendijk and de Kloet, 2019; Molendijk and de Kloet, 2021). Inescapable stress reduces BNST activation from mPFC, thus disinhibiting PAG, which switches active to passive coping (Molendijk and de Kloet, 2019; Molendijk and de Kloet, 2021). The BNST is a critical node for modulating this pathway by receiving projections from various brain regions (Radley and Sawchenko, 2011). The amygdala sends strong and direct projections to the BNST, providing an anatomical basis for amygdala-mediated modulation of stress-coping. Our previous (Jia et al., 2019) and current data suggest that CNTF produced by astrocytes in the MeA of female mice may modulate its input on the BNST to promote passive coping behavior.
CNTF binding of CNTFRα on neurons (Ip et al., 1993) maintains their excitability in the locus coeruleus and acute stress-induced CNTF release into the 3rd ventricle affects neuronal excitation in the prefrontal cortex (Alpar et al., 2018), suggesting that CNTF can sustain neural activity. Our data show that neutralization of CNTF in the MeA of females reduced passive coping already one day later, suggesting that CNTF constantly regulates the mPFC-BNST-PAG pathway. Surprisingly, neutralization of CNTF in the other amygdala nuclei, BLA or CeA, did not affect passive coping behavior. It would suggest that there are regional differences in CNTF expression or that these nuclei differ in their innervation of the BNST. The BLA primarily sends excitatory and the CeA primarily sends inhibitory projections to the BNST to modulate fear, anxiety and addiction-related behaviors (Lebow and Chen, 2016; Phelps and LeDoux, 2005; Stamatakis et al., 2014). The MeA projects both excitatory and inhibitory inputs to the BNST to mediate sociosexual behavior (Lebow and Chen, 2016; Miller et al., 2019; Nordman et al., 2020). Enhancement of inhibitory input from the MeA would decrease BNST GABAergic inhibition of the PAG, favoring passive stress-coping. It is possible that neutralizing CNTF in the CeA was ineffective due to the counteracting effect of additional direct inhibitory projections from the CeA to the PAG (Hopkins and Holstege, 1978). It remains to be determined whether and which projections from the MeA to the BNST are involved.
Another potential mechanism underlying the effect of CNTF on MeA-mediated passive coping could be via urocortin-3 expressing MeA neurons projecting to the BNST (Deussing et al., 2010). Urocortins are neuropeptides belonging to corticotropin releasing factor (CRF) family, present in the MeA and mediate stress responses (Deussing and Chen, 2018). The majority of BNST neurons that express urocortin-3 receptors (CRF-R2) are GABAergic (Henckens et al., 2017; Shemesh et al., 2016). CNTF can regulate urocortin-1 expression in hypothalamic neurons in vitro (Purser et al., 2013). Whether CNTF regulates urocortin-3 in the MeA remains to be determined.
In contrast to female mice, neutralization of CNTF in the MeA did not affect immobility in males and is consistent with our finding that male CNTF knockout does not have reduced immobility in the forced swim test. Both estrogen and progesterone mitigate passive stress-coping behavior in rodent models by modulating neurotransmission and increasing hippocampal BDNF (Douma et al., 2005; Frye, 2011). We have shown that progesterone but not estrogen inhibits CNTF expression in astroglioma C6 cells, a cell model of astrocyte (Jia et al., 2019). Further, progesterone alleviated ovariectomy-induced passive stress-coping behavior in mice through inhibiting CNTF in the amygdala. Progesterone receptors are enriched in the MeA (Brinton et al., 2008). Thus, progesterone may play a role in the female-specific effect of MeA CNTF on passive stress-coping.
4.2. Female-specific detrimental role of CNTF in chronic stress responses
Chronic stress increases despair or passive coping to acute inescapable stressors (Dunn and Swiergiel, 2008; Lam et al., 2018), which we also found in CNTF+/+ mice. Chronic uncontrollable stress attenuates excitatory output from the mPFC (McKlveen et al., 2016), increasing passive coping through reduced BNST inhibition of the PAG. CUS could act through a similar mechanism. CUS did not increase immobility in female CNTF−/− mice but did so in males, suggesting that CNTF increases chronic stress-induced passive coping only in females. The CNTF effects were not due to motor deficits that have been reported in 28 week old CNTF−/− mice (Masu et al., 1993), probably because we used 10–14 week old mice. Chronic stress also causes anhedonia, a reduced capacity to experience pleasure (Stanton et al., 2019). Our data are consistent with others (Kokras and Dalla, 2014; Xing et al., 2013) that the decrease in sucrose intake was greater in females than males. Knockout of CNTF increases sucrose preference (Jia et al., 2019) and blocked the CUS-induced reduction of sucrose preference in female but not male mice, indicating a sex-specific role of CNTF in behavioral anhedonia.
The hypothalamus-pituitary-adrenal (HPA) axis is widely used to measure stress response (Kajantie and Phillips, 2006; Kudielka and Kirschbaum, 2005) and has marked sex differences. Men have higher adrenocorticotropic hormone (ACTH) levels than women, but cortisol levels are comparable (Roelfsema et al., 1993), suggesting a high sensitivity of the adrenal cortex in women. Female rodents have higher levels of basal and stress-induced ACTH and corticosterone than males (Eliot and Richardson, 2016; Verma et al., 2011). Our CUS-treated wildtype CNTF+/+ female mice also had higher corticosterone levels than males (135.5 ± 18.48 vs. 70.26 ± 18.88, p = 0.03, two-tailed t-test). Moreover, restraint stress-induced corticosterone following CUS was absent in female but not male CNTF−/− mice, suggesting that CNTF promotes the neuroendocrine response in females only. A 5 min isoflurane anesthesia increases plasma corticosterone in female rats only (Bekhbat et al., 2016) even though it does not affect immediate early and stress-associated genes in rat brains of both sexes (Bekhbat et al., 2016; Hamaya et al., 2000; Wu et al., 2015). This suggests that females have greater HPA activity (Eliot and Richardson, 2016; Verma et al., 2011). Our isoflurane anesthesia was for only half a min making it less to contribute to the CNTF effect in the female corticosterone response. This is also supported by finding no differences in corticosterone levels between CNTF+/+ and CNTF−/− mice without restraint stress.
Together, our antibody and CNTF knockout data suggest that MeA CNTF is a key female-specific regulator of chronic stress-induced despair or passive coping. Our knockout data also suggest an involvement in chronic stress-induced anhedonia and neuroendocrine responses in females but we cannot rule out its role in other brain areas. CUS did not alter CNTF in stress-related brain areas, including the PVN, hippocampus and BNST. The PVN directly controls neuroendocrine responses suggesting the involvement of MeA-PVN and/or MeA-BNST-PVN circuits (Radley and Sawchenko, 2011). Activation of MeA neurons triggers dopamine release in the nucleus accumbens, suggesting that CNTF in the MeA regulates the function of reward circuitry that is closely linked to anhedonia. Others have found that chronic intermittent cold stress reduces CNTF in rat orbitofrontal cortex of both sexes, which leads to reversal learning deficit (Girotti et al., 2019). The apparent discrepancy with our finding that stress increases CNTF in females could be due to the paradigm of stress, brain areas, and/or endpoint measurements. Indeed, CNTF has no effects on anxiety-like behavior, measured by two commonly used assays, elevated T maze and open field tests, or on sensorimotor gating function, measured by pre-pulse inhibition. A single study using only female mice showed that CNTF−/− mice displayed increased startle response and pre-pulse inhibition and had motor deficits at 8–15 weeks of age tested in the dark phase (Peruga et al., 2012). These discrepancies with our findings could be due to either a carryover effect of the battery of behavioral tests they performed (McIlwain et al., 2001) and/or circadian rhythms (Benstaali et al., 2001; Fodor et al., 2016). Whether CNTF affects diurnal rhythms is unknown. Fonken et al. reported that microRNA-155 deletion reduced passive coping and anxiety-like behavior as well as increased sucrose preference in both sexes, in concert with increased CNTF in the hippocampus of female mice only (Fonken et al., 2016). This would suggest that CNTF in the hippocampus is not directly involved in the sex-specific effect, which is in line with our previous study showing that there is no sex-specific difference in hippocampal CNTF expression and that ovariectomy increases despair or passive coping without changing hippocampal CNTF (Jia et al., 2019) and our current data that chronic stress did not alter CNTF expression in the hippocampus of female mice. Chronic stress prolongs the estrous cycle in rats by exhibiting an extended diestrus (Fu et al., 2018). Thus, the low level of progesterone in the diestrus phase (Jenkins et al., 2001) may also contribute to CNTF expression in the MeA, which remains to be investigated.
5. Conclusion
Together, this study reveals a novel CNTF-mediated female-specific mechanism in stress responses and points to opportunities for female-specific treatments for stress-related disorders, e.g., inhibitors of CNTF expression or CNTFRα antagonists.
Disclosures
The authors declare no competing financial interests or potential conflicts of interest.
CRediT authorship contribution statement
Cuihong Jia: Conceptualization, Methodology, Data curation, Writing – original draft, preparation, Investigation, Supervision, Funding acquisition. W. Drew Gill: Methodology, Investigation. Chiharu Lovins: Methodology, Investigation. Russell W. Brown: Data curation, Writing – review & editing. Theo Hagg: Conceptualization, Funding acquisition, Writing – review & editing.
Declaration of competing interest
The authors declare no competing financial interests or potential conflicts of interest.
Acknowledgement
This work was supported by a grant from the East Tennessee State University Research Development Committee-Major Grants Program (CJ), the National Institutes of Health (AG029493, TH, and C06RR0306551), and funds from Quillen College of Medicine. We express our gratitude to Donald Lovins, Shantaya Andrews and Nausheen Siddiqui for technical support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2022.100435.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Alpar A., et al. Hypothalamic CNTF volume transmission shapes cortical noradrenergic excitability upon acute stress. EMBO J. 2018;37 doi: 10.15252/embj.2018100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade S., et al. Gender differences of acute and chronic administration of dehydroepiandrosterone in rats submitted to the forced swimming test. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2007;31:613–621. doi: 10.1016/j.pnpbp.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Beckerman M.A., Glass M.J. The NMDA-NR1 receptor subunit and the mu-opioid receptor are expressed in somatodendritic compartments of central nucleus of the amygdala neurons projecting to the bed nucleus of the stria terminalis. Exp. Neurol. 2012;234:112–126. doi: 10.1016/j.expneurol.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M., et al. Brief anesthesia by isoflurane alters plasma corticosterone levels distinctly in male and female rats: implications for tissue collection methods. Behav. Brain Res. 2016;305:122–125. doi: 10.1016/j.bbr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benstaali C., et al. Circadian rhythms of body temperature and motor activity in rodents their relationships with the light-dark cycle. Life Sci. 2001;68:2645–2656. doi: 10.1016/s0024-3205(01)01081-5. [DOI] [PubMed] [Google Scholar]
- Brinton R.D., et al. Progesterone receptors: form and function in brain. Front. Neuroendocrinol. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotto L.A., et al. Sex differences in forced-swim and open-field test behaviours after chronic administration of melatonin. Eur. J. Pharmacol. 2000;402:87–93. doi: 10.1016/s0014-2999(00)00491-x. [DOI] [PubMed] [Google Scholar]
- Brummelte S., et al. High post-partum levels of corticosterone given to dams influence postnatal hippocampal cell proliferation and behavior of offspring: a model of post-partum stress and possible depression. Horm. Behav. 2006;50:370–382. doi: 10.1016/j.yhbeh.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Chen A., et al. Urocortin 2-deficient mice exhibit gender-specific alterations in circadian hypothalamus-pituitary-adrenal axis and depressive-like behavior. J. Neurosci. 2006;26:5500–5510. doi: 10.1523/JNEUROSCI.3955-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Commons K.G., et al. The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chem. Neurosci. 2017;8:955–960. doi: 10.1021/acschemneuro.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C., et al. Sex differences in the effects of two stress paradigms on dopaminergic neurotransmission. Physiol. Behav. 2008;93:595–605. doi: 10.1016/j.physbeh.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Dalla C., et al. Sex differences in animal models of depression and antidepressant response. Basic Clin. Pharmacol. Toxicol. 2010;106:226–233. doi: 10.1111/j.1742-7843.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- de Kloet E.R., Molendijk M.L. Coping with the forced swim stressor: towards understanding an adaptive mechanism. Neural Plast. 2016;2016:6503162. doi: 10.1155/2016/6503162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deussing J.M., et al. Urocortin 3 modulates social discrimination abilities via corticotropin-releasing hormone receptor type 2. J. Neurosci. 2010;30:9103–9116. doi: 10.1523/JNEUROSCI.1049-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deussing J.M., Chen A. The corticotropin-releasing factor family: physiology of the stress response. Physiol. Rev. 2018;98:2225–2286. doi: 10.1152/physrev.00042.2017. [DOI] [PubMed] [Google Scholar]
- Donner N.C., Lowry C.A. Sex differences in anxiety and emotional behavior. Pflugers Arch. 2013;465:601–626. doi: 10.1007/s00424-013-1271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douma S.L., et al. Estrogen-related mood disorders: reproductive life cycle factors. ANS Adv. Nurs. Sci. 2005;28:364–375. doi: 10.1097/00012272-200510000-00008. [DOI] [PubMed] [Google Scholar]
- Drossopoulou G., et al. Sex differences in behavioral, neurochemical and neuroendocrine effects induced by the forced swim test in rats. Neuroscience. 2004;126:849–857. doi: 10.1016/j.neuroscience.2004.04.044. [DOI] [PubMed] [Google Scholar]
- Dunn A.J., Swiergiel A.H. Effects of acute and chronic stressors and CRF in rat and mouse tests for depression. Ann. N. Y. Acad. Sci. 2008;1148:118–126. doi: 10.1196/annals.1410.022. [DOI] [PubMed] [Google Scholar]
- Eliot L., Richardson S.S. Sex in context: limitations of animal studies for addressing human sex/gender neurobehavioral Health disparities. J. Neurosci. 2016;36:11823–11830. doi: 10.1523/JNEUROSCI.1391-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley J.G., Hagg T. Endogenous and exogenous ciliary neurotrophic factor enhances forebrain neurogenesis in adult mice. Exp. Neurol. 2003;183:298–310. doi: 10.1016/s0014-4886(03)00129-8. [DOI] [PubMed] [Google Scholar]
- Fodor A., et al. The prepulse inhibition deficit appearance is largely independent on the circadian cycle, body weight, and the gender of vasopressin deficient Brattleboro rat. Endocr. Regul. 2016;50:16–23. doi: 10.1515/enr-2016-0004. [DOI] [PubMed] [Google Scholar]
- Fonken L.K., et al. MicroRNA-155 deletion reduces anxiety- and depressive-like behaviors in mice. Psychoneuroendocrinology. 2016;63:362–369. doi: 10.1016/j.psyneuen.2015.10.019. [DOI] [PubMed] [Google Scholar]
- Frye C.A. Progesterone attenuates depressive behavior of younger and older adult C57/BL6, wildtype, and progesterone receptor knockout mice. Pharmacol. Biochem. Behav. 2011;99:525–531. doi: 10.1016/j.pbb.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X.Y., et al. Effects of chronic unpredictable mild stress on ovarian reserve in female rats: feasibility analysis of a rat model of premature ovarian failure. Mol. Med. Rep. 2018;18:532–540. doi: 10.3892/mmr.2018.8989. [DOI] [PubMed] [Google Scholar]
- Girotti M., et al. Ciliary neurotrophic factor signaling in the rat orbitofrontal cortex ameliorates stress-induced deficits in reversal learning. Neuropharmacology. 2019;160:107791. doi: 10.1016/j.neuropharm.2019.107791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy L.D., et al. A comprehensive overview on stress neurobiology: basic concepts and clinical implications. Front. Behav. Neurosci. 2018;12:127. doi: 10.3389/fnbeh.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagg T., et al. Ciliary neurotrophic factor prevents neuronal degeneration and promotes low affinity NGF receptor expression in the adult rat CNS. Neuron. 1992;8:145–158. doi: 10.1016/0896-6273(92)90116-u. [DOI] [PubMed] [Google Scholar]
- Hagg T., Varon S. Ciliary neurotrophic factor prevents degeneration of adult rat substantia nigra dopaminergic neurons in vivo. Proc. Natl. Acad. Sci. U. S. A. 1993;90:6315–6319. doi: 10.1073/pnas.90.13.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaya Y., et al. The effects of pentobarbital, isoflurane, and propofol on immediate-early gene expression in the vital organs of the rat. Anesth. Analg. 2000;90:1177–1183. doi: 10.1097/00000539-200005000-00034. [DOI] [PubMed] [Google Scholar]
- Heldt S.A., Ressler K.J. Amygdala-specific reduction of alpha1-GABAA receptors disrupts the anticonvulsant, locomotor, and sedative, but not anxiolytic, effects of benzodiazepines in mice. J. Neurosci. 2010;30:7139–7151. doi: 10.1523/JNEUROSCI.0693-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens M., et al. CRF receptor type 2 neurons in the posterior bed nucleus of the stria terminalis critically contribute to stress recovery. Mol. Psychiatr. 2017;22:1691–1700. doi: 10.1038/mp.2016.133. [DOI] [PubMed] [Google Scholar]
- Higuchi F., et al. Hippocampal MicroRNA-124 enhances chronic stress resilience in mice. J. Neurosci. 2016;36:7253–7267. doi: 10.1523/JNEUROSCI.0319-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S., et al. Social isolation in adolescence alters behaviors in the forced swim and sucrose preference tests in female but not in male rats. Physiol. Behav. 2012;105:269–275. doi: 10.1016/j.physbeh.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins D.A., Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp. Brain Res. 1978;32:529–547. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- Hu Y., et al. Hippocampal nitric oxide contributes to sex difference in affective behaviors. Proc. Natl. Acad. Sci. U. S. A. 2012;109:14224–14229. doi: 10.1073/pnas.1207461109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip N.Y., et al. The alpha component of the CNTF receptor is required for signaling and defines potential CNTF targets in the adult and during development. Neuron. 1993;10:89–102. doi: 10.1016/0896-6273(93)90245-m. [DOI] [PubMed] [Google Scholar]
- Jenkins J.A., et al. The influence of gender and the estrous cycle on learned helplessness in the rat. Biol. Psychol. 2001;58:147–158. doi: 10.1016/s0301-0511(01)00111-9. [DOI] [PubMed] [Google Scholar]
- Jia C., et al. Ciliary neurotrophic factor is a key sex-specific regulator of depressive-like behavior in mice. Psychoneuroendocrinology. 2019;100:96–105. doi: 10.1016/j.psyneuen.2018.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia C., et al. Inhibition of astrocyte FAK-JNK signaling promotes subventricular zone neurogenesis through CNTF. Glia. 2018;66:2456–2469. doi: 10.1002/glia.23498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia C., et al. Vitronectin mitigates stroke-increased neurogenesis only in female mice and through FAK-regulated IL-6. Exp. Neurol. 2020;323:113088. doi: 10.1016/j.expneurol.2019.113088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajantie E., Phillips D.I. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kang S.S., et al. Endogenous CNTF mediates stroke-induced adult CNS neurogenesis in mice. Neurobiol. Dis. 2013;49:68–78. doi: 10.1016/j.nbd.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokras N., et al. Forced swim test: what about females? Neuropharmacology. 2015;99:408–421. doi: 10.1016/j.neuropharm.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Kokras N., Dalla C. Sex differences in animal models of psychiatric disorders. Br. J. Pharmacol. 2014;171:4595–4619. doi: 10.1111/bph.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokras N., Dalla C. Preclinical sex differences in depression and antidepressant response: implications for clinical research. J. Neurosci. Res. 2017;95:731–736. doi: 10.1002/jnr.23861. [DOI] [PubMed] [Google Scholar]
- Kokras N., et al. Behavioral sexual dimorphism in models of anxiety and depression due to changes in HPA axis activity. Neuropharmacology. 2012;62:436–445. doi: 10.1016/j.neuropharm.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Kudielka B.M., Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol. Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Lam V.Y.Y., et al. Chronic stress alters behavior in the forced swim test and underlying neural activity in animals exposed to alcohol prenatally: sex- and time-dependent effects. Front. Behav. Neurosci. 2018;12:42. doi: 10.3389/fnbeh.2018.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebow M.A., Chen A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatr. 2016;21:450–463. doi: 10.1038/mp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leussis M.P., Andersen S.L. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62:22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- Li N., et al. Sex-specific diurnal immobility induced by forced swim test in wild type and clock gene deficient mice. Int. J. Mol. Sci. 2015;16:6831–6841. doi: 10.3390/ijms16046831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Mota L., et al. Sex and age differences in the impact of the forced swimming test on the levels of steroid hormones. Physiol. Behav. 2011;104:900–905. doi: 10.1016/j.physbeh.2011.05.027. [DOI] [PubMed] [Google Scholar]
- Masu Y., et al. Disruption of the CNTF gene results in motor neuron degeneration. Nature. 1993;365:27–32. doi: 10.1038/365027a0. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McIlwain K.L., et al. The use of behavioral test batteries: effects of training history. Physiol. Behav. 2001;73:705–717. doi: 10.1016/s0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- McKlveen J.M., et al. Chronic stress increases prefrontal inhibition: a mechanism for stress-induced prefrontal dysfunction. Biol. Psychiatr. 2016;80:754–764. doi: 10.1016/j.biopsych.2016.03.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.M., et al. Divergent medial amygdala projections regulate approach-avoidance conflict behavior. Nat. Neurosci. 2019;22:565–575. doi: 10.1038/s41593-019-0337-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendijk M.L., de Kloet E.R. Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology. 2015;62:389–391. doi: 10.1016/j.psyneuen.2015.08.028. [DOI] [PubMed] [Google Scholar]
- Molendijk M.L., de Kloet E.R. Coping with the forced swim stressor: current state-of-the-art. Behav. Brain Res. 2019;364:1–10. doi: 10.1016/j.bbr.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Molendijk M.L., de Kloet E.R. Forced swim stressor: trends in usage and mechanistic consideration. Eur. J. Neurosci. 2021;00:1–19. doi: 10.1111/ejn.15139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Royal C., et al. Stress-induced structural and functional modifications of astrocytes-Further implicating glia in the central response to stress. Glia. 2019;67:1806–1820. doi: 10.1002/glia.23610. [DOI] [PubMed] [Google Scholar]
- Nordman J.C., et al. Potentiation of divergent medial amygdala pathways drives experience-dependent aggression escalation. J. Neurosci. 2020;40:4858–4880. doi: 10.1523/JNEUROSCI.0370-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivan S., et al. Comparative study of behavioural tests in the SOD1G93A mouse model of amyotrophic lateral sclerosis. Exp. Anim. 2015;64:147–153. doi: 10.1538/expanim.14-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou A., et al. Acute and chronic stress differentially regulate cyclin-dependent kinase 5 in mouse brain: implications to glucocorticoid actions and major depression. Transl. Psychiatry. 2015;5:e578. doi: 10.1038/tp.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peruga I., et al. Endogenous ciliary neurotrophic factor modulates anxiety and depressive-like behavior. Behav. Brain Res. 2012;229:325–332. doi: 10.1016/j.bbr.2012.01.020. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., LeDoux J.E. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pitychoutis P.M., et al. Neurochemical and behavioral alterations in an inflammatory model of depression: sex differences exposed. Neuroscience. 2009;159:1216–1232. doi: 10.1016/j.neuroscience.2009.01.072. [DOI] [PubMed] [Google Scholar]
- Purser M.J., et al. The cytokine ciliary neurotrophic factor (CNTF) activates hypothalamic urocortin-expressing neurons both in vitro and in vivo. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley J.J., Sawchenko P.E. A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J. Neurosci. 2011;31:9683–9695. doi: 10.1523/JNEUROSCI.6040-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon-Cortes M., et al. Stress: influence of sex, reproductive status and gender. Neurobiol. Stress. 2019;10:100155. doi: 10.1016/j.ynstr.2019.100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema F., et al. Sex-dependent alteration in cortisol response to endogenous adrenocorticotropin. J. Clin. Endocrinol. Metab. 1993;77:234–240. doi: 10.1210/jcem.77.1.8392084. [DOI] [PubMed] [Google Scholar]
- Shelton H.W., et al. The effects of a novel inhibitor of tumor necrosis factor (TNF) alpha on prepulse inhibition and microglial activation in two distinct rodent models of schizophrenia. Behav. Brain Res. 2021;406:113229. doi: 10.1016/j.bbr.2021.113229. [DOI] [PubMed] [Google Scholar]
- Shemesh Y., et al. Ucn3 and CRF-R2 in the medial amygdala regulate complex social dynamics. Nat. Neurosci. 2016;19:1489–1496. doi: 10.1038/nn.4346. [DOI] [PubMed] [Google Scholar]
- Stamatakis A.M., et al. Amygdala and bed nucleus of the stria terminalis circuitry: implications for addiction-related behaviors. Neuropharmacology. 2014;76 Pt B:320–328. doi: 10.1016/j.neuropharm.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton C.H., et al. From stress to anhedonia: molecular processes through functional circuits. Trends Neurosci. 2019;42:23–42. doi: 10.1016/j.tins.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockli K.A., et al. Molecular cloning, expression and regional distribution of rat ciliary neurotrophic factor. Nature. 1989;342:920–923. doi: 10.1038/342920a0. [DOI] [PubMed] [Google Scholar]
- Tonelli L.H., et al. Intranasal immune challenge induces sex-dependent depressive-like behavior and cytokine expression in the brain. Neuropsychopharmacology. 2008;33:1038–1048. doi: 10.1038/sj.npp.1301488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela D.M., et al. High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat. Biotechnol. 2003;21:652–659. doi: 10.1038/nbt822. [DOI] [PubMed] [Google Scholar]
- Verma R., et al. Gender differences in stress response: role of developmental and biological determinants. Ind. Psychiatr. J. 2011;20:4–10. doi: 10.4103/0972-6748.98407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voikar V., et al. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol. Behav. 2001;72:271–281. doi: 10.1016/s0031-9384(00)00405-4. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Wu X.Y., et al. Effect of pentobarbital and isoflurane on acute stress response in rat. Physiol. Behav. 2015;145:118–121. doi: 10.1016/j.physbeh.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Xing Y., et al. Gender differences in CMS and the effects of antidepressant venlafaxine in rats. Neurochem. Int. 2013;63:570–575. doi: 10.1016/j.neuint.2013.09.019. [DOI] [PubMed] [Google Scholar]
- Yang P., et al. Ciliary neurotrophic factor mediates dopamine D2 receptor-induced CNS neurogenesis in adult mice. J. Neurosci. 2008;28:2231–2241. doi: 10.1523/JNEUROSCI.3574-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.