Abstract
Objective or Purpose:
To compare rates of short-term retinal detachment of infants treated for type 1 retinopathy of prematurity (ROP) with intravitreal anti-vascular endothelial growth factor (anti-VEGF) to infants treated with laser. The choice between these two treatments remains controversial. Comparative data are limited and describe retreatment rates rather than retinal structural outcomes predictive of long-term vision. Anti-VEGF acts faster than laser, which may be beneficial for more aggressive ROP.
Design:
Non-randomized, comparative cohort study
Subjects, Participants, and Controls:
The study included 1,167 eyes of 640 infants treated for type 1 ROP. Among these, 164 eyes received anti-VEGF and 1,003 eyes received laser.
Methods, Intervention, and Testing:
Pre- and post-treatment examinations, and treatments, were completed by ophthalmologists with expertise in ROP. The study was a secondary analysis of data from the retrospective G-ROP-1 study (2006–2012) and prospective G-ROP-2 study (2015–2017).
Main Outcome Measure:
Rate of retinal detachment (ROP stages 4A, 4B, or 5) within 8 weeks of initial treatment, an endpoint predictive of poor long-term vision. The results were stratified by PMA at treatment as occurring before versus at or after 36 0/7 weeks, because earlier disease may be considered more aggressive.
Results:
Among 458 eyes treated before PMA 36 0/7 weeks, short-term RD rate was higher after laser (29/368 eyes, 7.9%) than after anti-VEGF (0/90 eyes, 0%) (p < 0.001). Of 709 eyes treated at or after PMA 36 0/7 weeks, short-term RD risk did not differ between groups (laser 20/635 eyes, 3.1%; anti-VEGF 1/74 eyes, 1.4%; p = 0.27).
Conclusion:
Anti-VEGF results in better short-term structural outcomes than laser when type 1 ROP is treated prior to 36 weeks PMA. After this age, both treatments have very low rates of short-term retinal detachments. The faster action of anti-VEGF is likely responsible for these findings.
Keywords: Retinopathy of Prematurity, Retinal Detachment, Anti-vascular endothelial growth factor, Laser photocoagulation
Retinopathy of prematurity (ROP) is a potentially blinding condition. Careful screening is required to identify infants who require treatment to minimize the risk of blindness.1 The Early Treatment of ROP Study (ET-ROP) established pan-retinal photocoagulation laser eye surgery as an effective method of reducing blindness in infants with type 1 pre-threshold ROP. Despite the efficacy of laser photocoagulation, 9.1% of 331 eyes with type 1 ROP treated with laser suffered a poor structural outcome.2
Intravitreal injection of anti-vascular endothelial growth factor (anti-VEGF) agents for treatment of type 1 ROP has been reported and shows promising results.3 The Bevacizumab Eliminates the Angiogenic Threat of ROP (BEAT-ROP) Study demonstrated a higher need for re-treatment in eyes with type 1 ROP in zone 1 or posterior zone 2 treated with laser versus anti-VEGF: 26% vs 4%, respectively.4 Barry et al. reported fewer short-term retinal detachments in infants treated for type 1 ROP with anti-VEGF compared to laser specifically prior to post-menstrual age (PMA) 36 0/7 weeks.5 Earlier PMA was considered by the authors to be a surrogate measure for more aggressively acting disease that was preferable to zone of disease as a marker of disease severity because zone depends upon the subjective judgment of the examiner while PMA is typically a known value. The authors hypothesized that the faster acting effect of anti-VEGF injection versus laser demonstrated a greater relative benefit during earlier PMA because earlier disease is generally more aggressive. However, the study was a single center study with a limited number of eyes treated with anti-VEGF.
We sought to further evaluate the hypothesis that infants treated with anti-VEGF for type 1 ROP prior to PMA 36 0/7 weeks will demonstrate fewer short-term retinal detachments than infants treated with laser using data from the Postnatal Growth and ROP (G-ROP) studies, two large, North American multi-center studies.6–8
METHODS
We conducted a secondary analysis of data from the G-ROP-1 study and the G-ROP-2 study.6–8 These studies were approved by the Institutional Review Boards of the Children’s Hospital of Philadelphia (the study headquarters) and all participating hospitals, and adhered to the tenants of the Declaration of Helsinki. Clinical data were collected at each hospital by trained data abstractors covering a period from 2006 to 2012 retrospectively at 29 hospitals in G-ROP-1 and from 2015 to 2017 prospectively at 41 hospitals in G-ROP-2.6–8 During the study periods, ophthalmologists with expertise in ROP practicing at each hospital determined the presence and severity of ROP using International Classification of ROP terminology during serial diagnostic examinations and made decisions about treatment modality using their clinical judgement. The results of these diagnostic examinations and treatments, including stage, zone, presence of plus disease, timing and type of ROP treatment, as well as the results of post-treatment ROP examinations were collected. In G-ROP-1, post-treatment outcomes were collected through age 15 months, and in G-ROP-2, post-treatment examination results were collected through PMA 50 weeks. Extensive medical and demographic information were also collected for these studies.
For the current analysis, we included infants treated with laser or anti-VEGF for type 1 ROP in one or both eyes during G-ROP-1 or G-ROP-2. Exclusion criteria included initial treatment with pars plana vitrectomy, use of the other treatment modality (e.g., laser after anti-VEGF, or vice versa) within 7 days of the initial treatment, treatment for ROP not meeting type 1 criteria, and insufficient outcome data at 8 weeks, including death within 8 weeks of initial ROP treatment. Both G-ROP-1 and G-ROP-2 were observational studies, and choice of treatment modality and anti-VEGF dosage were at the discretion of the treating ophthalmologist.
The primary outcome for the current analysis was the development of retinal detachment (ROP stages 4A, 4B, or 5) within 8 weeks following treatment for type 1 ROP. This outcome was chosen as a representation of short-term treatment failure. The primary outcome was compared between eyes treated with laser and eyes treated with anti-VEGF. Treated eyes were stratified a priori by their PMA at treatment, which was categorized as treatment before 36 0/7 weeks PMA or treatment at or after 36 0/7 weeks PMA. The choice of time point was based upon the aforementioned single center study conducted at Albany Medical Center, which suggested a difference between groups before 36 0/7 weeks PMA but not after.5 The rationale for this distinction was that ROP reaching criteria for type 1 at an earlier PMA is generally more aggressive with faster progression and might show a preferential benefit for a faster-acting treatment modality. Of note, we did not use a time-to-event analysis, because time to retinal detachment over the short period of eight weeks post treatment would not add meaningful information in the context of whether or not there was simple failure to halt the acute progression of ROP. Treated children are typically followed closely during this time period and progression is likely to be identified in a timely fashion.
Secondary outcomes for the current analysis included a comparison of short-term retinal detachment rates between eyes receiving laser versus anti-VEGF (1) with stratification by most posterior zone of ROP at time of treatment instead of PMA at treatment and (2) with no stratification at all; as well as the short-term rate of re-treatment (re-treatment during the first 8 weeks post initial treatment).
Cluster bootstrap analysis was used to account for inter-eye correlation when determining statistical significance, because some infants had treatment of type 1 ROP in both eyes, and the number of retinal detachments in the anti-VEGF treatment group was too low for statistical modelling.9 The 95% confidence intervals for the RD rates were calculated based on the 2.5% percentile and 97.5% percentile of 2000 bootstrap replications. Comparisons of the RD rates following laser and anti-VEGF were based on normal approximations of 2000 bootstrap replications. A generalized estimating equation was used for comparison of retreatment rates and number of retreatments between laser and anti-VEGF. For these comparisons, adjustment for birth weight (BW) and gestational age (GA) could not be made due to the small number of outcome events.
RESULTS
A total of 818 (5.5%) of 14,966 eyes in the G-ROP-1 study and 378 (4.7%) of the 7,960 eyes in the G-ROP-2 study were treated for type 1 ROP. Among these treated eyes, 7 eyes from the G-ROP-1 study and 22 eyes from the G-ROP-2 study were excluded for the current analysis, including 13 eyes that received a second treatment modality within 7 days of the initial treatment, 1 eye that was initially treated with pars plana vitrectomy, and 15 eyes of infants who died within 8 weeks of initial treatment. Therefore, a total of 1,167 eyes of 640 infants (811 eyes from the G-ROP-1 study and 356 eyes from the G-ROP-2 study) were included in this study (Figure 1). One hundred sixty-four eyes were treated initially with anti-VEGF and 1,003 eyes were treated initially with laser. One hundred forty-seven of 164 eyes (89.6%) treated with anti-VEGF received bevacizumab, while 17 of 164 eyes (10.4%) received ranibizumab. Infants treated with anti-VEGF had lower mean BW (658 vs 709 g, p = 0.01) and lower mean PMA at treatment (35.8 vs 36.7 weeks, p = 0.001) than infants treated with laser, respectively (Table 1). Among 1,167 included eyes, 458 (39.2%) eyes were treated before a PMA of 36 0/7 weeks, and 709 (60.8%) eyes were treated at or after PMA 36 0/7 weeks. Infants with eyes treated before PMA 36 0/7 weeks had a lower mean BW (663 vs 726 g, p < 0.001) and mean GA (24.2 vs. 25.3 weeks, p < 0.001) than infants with eyes treated at or after PMA 36 0/7 weeks, respectively. Within these subgroups based upon PMA at treatment, infants treated with anti-VEGF before 36 weeks PMA had a lower mean BW (621 vs 674 g, p = 0.02) than infants treated with laser before 36 weeks PMA. There were no significant differences in GA or PMA at treatment between anti-VEGF treated and laser treated eyes within treatment subgroups before and after PMA 36 weeks. Of the 8 infants who were excluded due to death within 8 weeks of initial treatment, 4 were treated with only laser, 2 with only anti-VEGF, and 2 with both laser and anti-VEGF.
Figure 1.
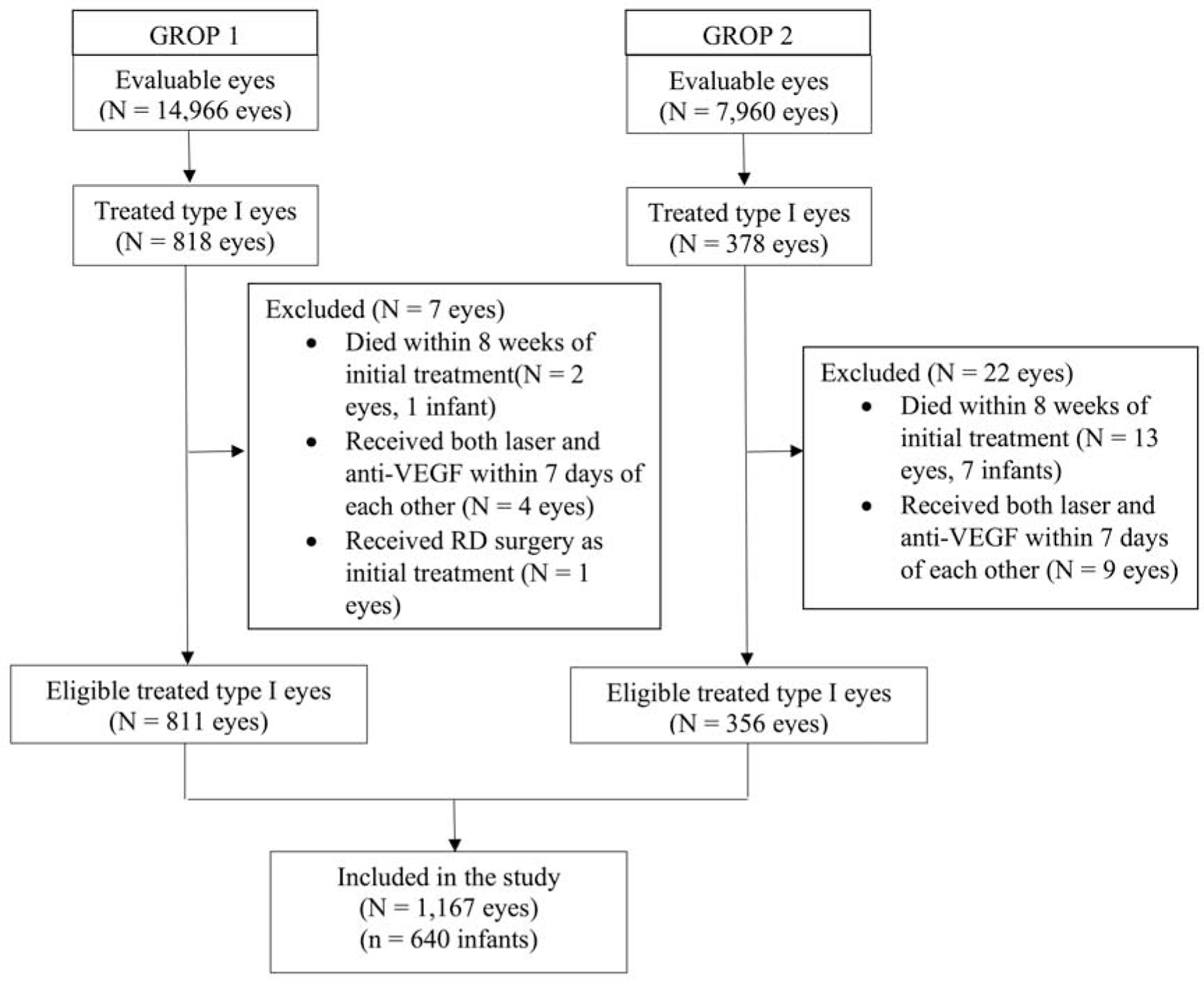
Flowchart of eligible eyes included and excluded in the study.
AMC = Albany Medical Center, GROP 1 = Postnatal Growth and Retinopathy of Prematurity Study 1, GROP 2 = Postnatal Growth and Retinopathy of Prematurity Study 2, RD = Retinal detachment.
Table 1.
Baseline characteristics of 1,167 eyes of 640 infants treated for type 1 retinopathy of prematurity, stratified by modality of treatment and postmenstrual age at treatment.
Anti-VEGF, anti-vascular endothelial growth factor; PMA, post-menstrual age; ROP, retinopathy of prematurity.
| PMA < 36 weeks | PMA >= 36 weeks | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
Laser
(N = 368 eyes) |
Anti-VEGF
(N = 90 eyes) |
p value |
Laser
(N = 635 eyes) |
Anti-VEGF
(N = 74 eyes) |
p value |
Laser
(N = 1003 eyes) |
Anti-VEGF
(N = 164 eyes) |
p value | |
| Birth weight (g) | 0.02 | 0.44 | 0.01 | ||||||
| Mean (SD) | 673.6 (138.0) | 620.9 (131.3) | 729.2 (207.4) | 702.9 (201.1) | 708.8 (186.8) | 657.9 (170.8) | |||
| Median | 650.0 | 610.0 | 682.0 | 655.0 | 670.0 | 628.0 | |||
| Range | (380.0–1235.0) | (390.0–875.0) | (380.0–1692.0) | (370.0–1273.0) | (380.0–1692.0) | (370.0–1273.0) | |||
| Gestational Age (weeks) | 0.40 | 0.99 | 0.12 | ||||||
| Mean (SD) | 24.2 (1.0) | 24.0 (1.4) | 25.3 (1.6) | 25.3 (1.7) | 24.9 (1.5) | 24.6 (1.7) | |||
| Median | 24.0 | 24.0 | 25.0 | 25.0 | 25.0 | 24.0 | |||
| Range | (22.0–28.0) | (22.0–27.0) | (22.0–31.0) | (22.0–32.0) | (22.0–31.0) | (22.0–32.0) | |||
| PMA at first type 1 treatment | 0.98 | 0.25 | 0.001 | ||||||
| Mean (SD) | 34.1 (1.0) | 34.1 (0.9) | 38.2 (2.0) | 37.8 (1.7) | 36.7 (2.6) | 35.8 (2.3) | |||
| Median | 34.0 | 34.0 | 38.0 | 37.0 | 36.0 | 35.0 | |||
| Range | (30.0–35.0) | (32.0–35.0) | (36.0–46.0) | (36.0–42.0) | (30.0–46.0) | (32.0–42.0) | |||
| Gender | 0.96 | 0.09 | 0.25 | ||||||
| Female | 158 (42.9%) | 39 (43.3%) | 293 (46.1%) | 24 (32.4%) | 451 (45.0%) | 63 (38.4%) | |||
| Male | 210 (57.1%) | 51 (56.7%) | 342 (53.9%) | 50 (67.6%) | 552 (55.0%) | 101 (61.6%) | |||
| Ethnicity | 0.73 | 0.07 | 0.52 | ||||||
| Hispanic or Latino | 35 (9.5%) | 9 (10.0%) | 59 (9.3%) | 2 (2.7%) | 94 (9.4%) | 11 (6.7%) | |||
| Not Hispanic or Latino | 175 (47.6%) | 48 (53.3%) | 397 (62.5%) | 42 (56.8%) | 572 (57.0%) | 90 (54.9%) | |||
| Unknown | 158 (42.9%) | 33 (36.7%) | 179 (28.2%) | 30 (40.5%) | 337 (33.6%) | 63 (38.4%) | |||
| Race | 0.27 | 0.21 | 0.04 | ||||||
| White/Caucasian | 194 (52.7%) | 50 (55.6%) | 348 (54.8%) | 33 (44.6%) | 542 (54.0%) | 83 (50.6%) | |||
| Asian/Asian American | 10 (2.7%) | 4 (4.4%) | 18 (2.8%) | 2 (2.7%) | 28 (2.8%) | 6 (3.7%) | |||
| Black/African American | 75 (20.4%) | 9 (10.0%) | 139 (21.9%) | 9 (12.2%) | 214 (21.3%) | 18 (11.0%) | |||
| American Indian/Alaskan Native | 6 (1.6%) | 0 (0.0%) | 1 (0.2%) | 0 (0.0%) | 7 (0.7%) | 0 (0.0%) | |||
| Native Hawaiian/Other Pacific Islander | 2 (0.5%) | 0 (0.0%) | 4 (0.6%) | 0 (0.0%) | 6 (0.6%) | 0 (0.0%) | |||
| Other | 46 (12.5%) | 11 (12.2%) | 40 (6.3%) | 6 (8.1%) | 86 (8.6%) | 17 (10.4%) | |||
| Unknown | 35 (9.5%) | 16 (17.8%) | 82 (12.9%) | 21 (28.4%) | 117 (11.7%) | 37 (22.6%) | |||
| Greater than 1 race checked | 0 (0.0%) | 0 (0.0%) | 3 (0.5%) | 3 (4.1%) | 3 (0.3%) | 3 (1.8%) | |||
| Birth Location | 0.07 | 0.80 | 0.28 | ||||||
| Inborn | 158 (42.9%) | 52 (57.8%) | 373 (58.7%) | 45 (60.8%) | 531 (52.9%) | 97 (59.1%) | |||
| Outborn | 210 (57.1%) | 38 (42.2%) | 262 (41.3%) | 29 (39.2%) | 472 (47.1%) | 67 (40.9%) | |||
| Stage, Zone, Plus at type 1 ROP treatment | 0.08 | 0.14 | <0.001 | ||||||
| Stage 1,Zone I,Plus | 6 (1.6%) | 7 (7.8%) | 0 (0.0%) | 3 (4.1%) | 6 (0.6%) | 10 (6.1%) | |||
| Stage 2,Zone I,Plus | 11 (3.0%) | 5 (5.6%) | 3 (0.5%) | 1 (1.4%) | 14 (1.4%) | 6 (3.7%) | |||
| Stage 2,Zone II,Plus | 35 (9.5%) | 5 (5.6%) | 63 (9.9%) | 5 (6.8%) | 98 (9.8%) | 10 (6.1%) | |||
| Stage 3,Zone I,No plus | 15 (4.1%) | 6 (6.7%) | 6 (0.9%) | 2 (2.7%) | 21 (2.1%) | 8 (4.9%) | |||
| Stage 3,Zone I,Plus | 67 (18.2%) | 28 (31.1%) | 13 (2.0%) | 8 (10.8%) | 80 (8.0%) | 36 (22.0%) | |||
| Stage 3,Zone I,Pre-plus | 20 (5.4%) | 9 (10.0%) | 14 (2.2%) | 10 (13.5%) | 34 (3.4%) | 19 (11.6%) | |||
| Stage 3,Zone II,Plus | 205 (55.7%) | 30 (33.3%) | 494 (77.8%) | 45 (60.8%) | 699 (69.7%) | 75 (45.7%) | |||
| Type I ROP, Not specified, Not specified | 0 (0.0%) | 0 (0.0%) | 4 (0.6%) | 0 (0.0%) | 4 (0.4%) | 0 (0.0%) | |||
| Type I ROP,Not specified, Plus | 0 (0.0%) | 0 (0.0%) | 1 (0.2%) | 0 (0.0%) | 1 (0.1%) | 0 (0.0%) | |||
| Type I ROP,Zone II, Plus | 9 (2.4%) | 0 (0.0%) | 35 (5.5%) | 0 (0.0%) | 44 (4.4%) | 0 (0.0%) | |||
| Type I ROP,Zone II, Pre-plus | 0 (0.0%) | 0 (0.0%) | 2 (0.3%) | 0 (0.0%) | 2 (0.2%) | 0 (0.0%) | |||
| Anti-VEGF agent | |||||||||
| Bevacizumab | 85 (94.4%) | 62 (83.8%) | 147 (89.6%) | ||||||
| Ranibizumab | 5 (5.6%) | 12 (16.2%) | 17 (10.4%) | ||||||
P values were from logistic regression with generalized estimating equation to account for the correlation between eyes within the same infant.
When treatment for type 1 ROP occurred prior to PMA 36 0/7 weeks, eyes treated with anti-VEGF were less likely to develop a retinal detachment within 8 weeks after treatment (0/90 eyes with RD, 0%) than eyes treated with laser (29/368 eyes with RD, 7.9%; p < 0.001) (Table 2) (Figure 2). In contrast, when treatment occurred at or after PMA 36 0/7 weeks, there was no significant difference in retinal detachments within 8 weeks after treatment between eyes treated with anti-VEGF (1/74 eyes with RD, 1.4%) and eyes treated with laser (20/635 eyes with RD, 3.1%; p = 0.27).
Table 2.
Retinal detachment rates within 8 weeks after treatment of type 1 retinopathy of prematurity with laser and intravitreal anti-VEGF, stratified by post-menstrual age at treatment, and zone of disease.
Anti-VEGF, anti-vascular endothelial growth factor; CI, confidence interval; PMA, postmenstrual age
| RD Rate | Laser, % 95% CI A |
Anti-VEGF, % 95% CI A |
P value A |
|---|---|---|---|
|
PMA < 36 weeks
(N=458 eyes) |
29/368 (7.9%) 4.7%, 11.3% |
0/90 (0.0%) NA |
<0.001 |
|
PMA ≥ 36 weeks
(N=709 eyes) |
20/635 (3.1%) 1.6%, 4.9% |
1/74 (1.4%) 0.0%, 4.5% |
0.27 |
|
Zone I B
(N=234 eyes) |
12/155 (7.7%) 3.1%, 13.2% |
1/79 (1.3%) 0.0%, 4.3% |
0.02 |
|
Zone II B
(N=928 eyes) |
37/843 (4.4%) 2.8%, 6.1% |
0/85 (0.0%) NA |
<0.001 |
| Total | 49/1003 (4.9%) 3.4%, 6.5% |
1/164 (0.6%) 0.0%, 2.0% |
<0.001 |
Based on Bootstrap method
5 eyes with unknown zones were excluded
NA: 95% CI could not be calculated due to zero retinal detachments.
Figure 2.
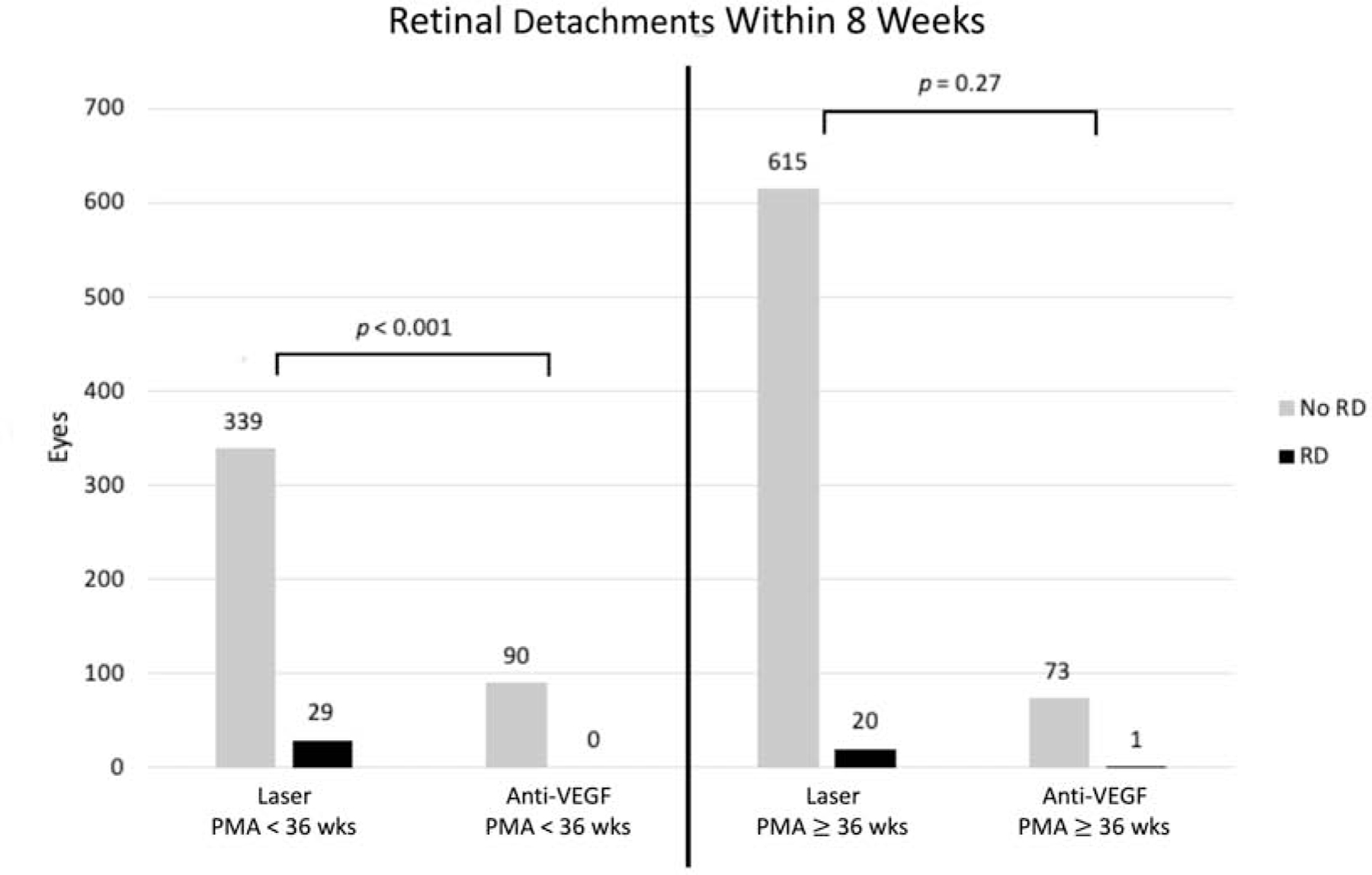
Retinal detachments within 8 weeks following treatment for type 1 retinopathy of prematurity with intravitreal anti-vascular endothelial growth factor versus laser photocoagulation, stratified by post-menstrual age before and after 36 weeks at time of treatment.
Anti-VEGF = Anti-vascular endothelial growth factor, PMA = post-menstrual age, RD = Retinal detachment
When all included eyes were considered without stratification by PMA at treatment, fewer short-term retinal detachments were observed in eyes treated with anti-VEGF (1/164 eyes with RD, 0.6%) than in eyes treated with laser (49/1,003 eyes with RD, 4.9%; p < 0.001). When stratified by zone of ROP, fewer short-term retinal detachments were observed among eyes treated for type 1 ROP in zone 1 with anti-VEGF (1/79 eyes with RD, 1.3%) compared to eyes treated with lasers (12/155 eyes with RD, 7.7%; p = 0.02). Eyes with type 1 ROP in zone 2 were also less likely to develop retinal detachment within 8 weeks when treated with anti-VEGF (0/85 eyes with RD, 0%) compared to eyes treated with laser (37/843 eyes with RD, 4.4%; p < 0.001) (Table 2).
Among eyes treated with laser, more retinal detachments were noted in eyes treated before PMA 36 0/7 weeks (29/368 eyes with RD, 7.9%) than at or after 36 0/7 weeks (20/635 eyes with RD, 3.1%; p = 0.01). There was no difference in the rate of short-term retinal detachment after laser if ROP at treatment was in zone 1 (12/155 eyes with RD, 7.7%) or zone 2 (37/843 eyes with RD, 4.4%; p = 0.22). With regard to re-treatment, 27 (16.5%) of 164 eyes initially treated with anti-VEGF and 73 (7.3%) of 1,003 eyes initially treated with laser required retreatment within 8 weeks of initial treatment (p = 0.03). Among infants treated before PMA 36 0/7 weeks, retreatments occurred in 14 (15.6%) of 90 eyes initially treated with anti-VEGF and 41 (11.1%) of 368 eyes initially treated with laser (p = 0.45). Among infants treated at or after PMA 36 0/7 weeks, retreatment was performed in 13 (17.6%) of 74 eyes treated with anti-VEGF and 32 (5.0%) of 635 eyes initially treated with laser (p = 0.053) (Table 3).
Table 3.
Characteristics of retreatment within 8 weeks following initial treatment with laser or intravitreal anti-VEGF, stratified by postmenstrual age at initial treatment.
PMA, post-menstrual age; anti-VEGF, anti-vascular endothelial growth factor
| PMA < 36 weeks | PMA >= 36 weeks | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
Laser
(N = 368 eyes) |
Anti-VEGF
(N = 90 eyes) |
p value |
Laser
(N = 635 eyes) |
Anti-VEGF
(N = 74 eyes) |
p value |
Laser
(N = 1003 eyes) |
Anti-VEGF
(N = 164 eyes) |
p value | |
| # of retreatments | 0.46 | 0.07 | 0.10 | ||||||
| 0 | 327 (88.9%) | 76 (84.4%) | 603 (95.0%) | 61 (82.4%) | 930 (92.7%) | 137 (83.5%) | |||
| 1 | 40 (10.9%) | 12 (13.3%) | 29 (4.6%) | 13 (17.6%) | 69 (6.9%) | 25 (15.2%) | |||
| 2 | 1 (0.3%) | 2 (2.2%) | 3 (0.5%) | 0 (0.0%) | 4 (0.4%) | 2 (1.2%) | |||
| Retreatment rate | 41 (11.1%) | 14 (15.6%) | 0.45 | 32 (5.0%) | 13 (17.6%) | 0.053 | 73 (7.3%) | 27 (16.5%) | 0.03 |
| First retreatment type | 0.06 | 0.22 | 0.31 | ||||||
| Laser | 37 (90.2%) | 7 (50.0%) | 24 (75.0%) | 12 (92.3%) | 61 (83.6%) | 19 (70.4%) | |||
| Anti-VEGF | 4 (9.8%) | 7 (50.0%) | 8 (25.0%) | 1 (7.7%) | 12 (16.4%) | 8 (29.6%) | |||
DISCUSSION
We found a short-term structural benefit of treating type 1 ROP with intraocular anti-VEGF injection compared to laser when treatment was required prior to 36 0/7 weeks PMA. While there appeared to be fewer short-term retinal detachments overall in eyes treated with anti-VEGF than in eyes treated with laser, the overall benefit of anti-VEGF over laser was driven by the subgroup of eyes that were treated before 36 weeks PMA, who presumably had more aggressive ROP and among whom the rates of short-term detachments were 7.9% following laser and 0% following anti-VEGF. In contrast, there was no significant difference in short-term detachments between treatment groups after 36 0/7 weeks PMA. The concept of using “PMA less than 36 0/7 weeks at time of treatment of type 1 ROP” as a relative marker of disease aggression instead of zone of ROP was first introduced by Barry et al., who reported short-term structural superiority of treatment with anti-VEGF compared to laser in this subgroup in a single-center cohort.5 Our larger, multi-center study validates those earlier findings.
A faster mechanism of action of anti-VEGF compared to laser may explain our study findings. Tractional retinal detachment is the primary source of blindness in eyes with type 1 ROP.10,11 Laser photocoagulation ablates hypoxic avascular retina, the primary source of excessive VEGF and subsequent fibrovascular proliferation in ROP. By destroying this source of VEGF, laser can be effective in preventing progression to retinal detachment from type 1 ROP. Response to laser typically takes a week or more to be visible on clinical examination, presumably because VEGF present in the vitreous at the time of laser takes time to clear. In contrast, visible regression of ROP is faster if treated with anti-VEGF, as intravitreal anti-VEGF agents rapidly sequester VEGF in the vitreous at the time of treatment.12,13 This difference in rapidity of effect would be expected to have a more pronounced effect with ROP that is progressing more quickly. Shah et. al. demonstrated fewer retinal detachments in eyes with aggressive posterior ROP treated with anti-VEGF compared to laser.14 These findings also support the hypothesis that anti-VEGF demonstrates greater efficacy than laser for rapidly progressing ROP.
While zone is a traditional marker of disease severity, PMA and zone of ROP are closely related, and there are advantages to using PMA as a marker of ROP aggression. Natural history data from the Cryotherapy for ROP Study demonstrated that ROP follows a typical course tied to developmental age (PMA) and that developmental age is a more reliable indicator of ROP risk than chronologic age.15 More posterior ROP occurs earlier in development and the more posterior the location of ROP, generally the more aggressive the ROP state. Presumably, type 1 ROP in zone 1 involves greater area of avascular retina, higher VEGF production, and more aggressive ROP when compared to type 1 ROP in zone 2. Many studies have used zone 1 as a marker of aggression of type 1 ROP.4,16–18 While zone of ROP is clearly defined in the International Classification of ROP,19 clinical distinction of zone 1 from zone 2 is subjective and carries significant inter-observer variability, even among experienced clinicians.20 Perhaps such variability explains why we observed no difference in rate of retinal detachment between laser-treated eyes in zone 1 compared to zone 2. In contrast to zone, PMA at diagnosis is a known objective measure and therefore is easier to reproduce across physicians and institutions. Our data suggest that diagnosis of type 1 ROP prior to PMA 36 0/7 weeks may be a more practical clinical marker of retinal detachment risk, and therefore disease aggression, than zone of disease.
We chose a short-term outcome for this study, development of retinal detachment within 8 weeks of treatment, because this is a more direct sign of treatment failure, as opposed to disease reactivation. In addition, many retinal detachments after laser occur within this time period,5,21 and the half-lives of most anti-VEGF agents suggest their effects will primarily occur in the first 8 weeks after treatment.22–24 While long-term visual acuity would be an ideal clinical outcome, data from the ET-ROP study suggest retinal detachment is closely associated with poor long-term visual outcomes and is a good proxy for such long-term outcomes.11 Finally, short-term risk of retinal detachment is more directly relevant to long-term visual outcome than “disease recurrence requiring treatment,” which has been the focus of prior studies comparing anti-VEGF and laser; the goal of treatment for ROP is to prevent imminent progression to retinal detachment. If acute progression is not halted, prognosis is poor. Nevertheless, it is important to recognize the need for long-term monitoring of eyes treated with anti-VEGF for late reactivation that may benefit from additional treatment.
The large number of treated eyes in our study enabled a comparison of laser and anti-VEGF stratified by PMA at treatment. The geographically and racially diverse sample across many hospitals and many different treating physicians improves the generalizability of the findings. However, there are potential limitations to consider. Despite the large overall number of treated eyes in this study, the number of eyes treated with anti-VEGF in some sub-groups, such as treatment at or after PMA 36 weeks, may have limited the power to detect differences between groups. Infants were not randomized to treatment modality. If there was a tendency to use anti-VEGF for what was perceived to be more aggressive ROP, this would bias the results towards worse outcomes for anti-VEGF eyes, which would not change the conclusions for the groups treated prior to PMA 36 0/7 weeks but might change the conclusions for the group treated at or after PMA 36 0/7 weeks, in which a statistical difference was not found. With regard to outcome, we considered only retinal detachment and not other adverse structural outcomes, such as macular folds, data for which were available for G-ROP-1 but not G-ROP 2. Macular fold was considered a poor structural outcome in ET-ROP and is associated with poor long-term visual acuity.10 In G-ROP-1, 11 lasered eyes resulted in macular fold without retinal detachment.21 We did not consider longer-term outcomes that might influence clinician treatment choice. Eyes treated with anti-VEGF may have persistent avascular retina, placing them at risk for late reactivation and retinal detachment, even years after their initial treatment.25–29 Additional treatment for eyes treated with anti-VEGF may need to be considered, including after the 8-week endpoint reported in this study. Reported rates of retreatment after initial monotherapy with anti-VEGF have varied considerably.3,5,30
Our study also does not address safety concerns about the use of anti-VEGF agents for ROP.31,32 Systemic VEGF levels are depressed for up to 12 weeks after intraocular bevacizumab for ROP with uncertain effects on developing brain, lung, and kidneys.33–36 Systemic VEGF levels recover more rapidly following ranibizumab injection, but are still suppressed initially.37–41 Studies comparing neurodevelopmental outcomes between infants treated with laser versus anti-VEGF have yielded inconclusive results. Some show no adverse effect from anti-VEGF,3,42,43 and others suggest worse motor outcomes and higher mortality among infants treated with bevacizumab compared to laser.44,45 These studies should be interpreted with caution, as treatment modalities were generally not randomized and sicker infants tended to be treated with anti-VEGF agents instead of laser.46 Finally, ideal dosing of bevacizumab for ROP has yet to be established.47–49 Wallace, et al. recently demonstrated good results with 0.004 mg, considerably less than the 0.625 mg used in BEAT-ROP.50
The decision of whether to treat type 1 ROP with laser or intravitreal anti-VEGF injections remains a complicated, multifaceted one. Our data confirm a clear short-term structural benefit of anti-VEGF over laser prior to PMA 36 0/7 weeks and suggest that the more objective measure of PMA at type 1 diagnosis may be preferable to the subjective judgement of zone of disease. However, this benefit must be considered along with other risks and benefits, including long-term structural outcomes, long-term visual acuity outcomes, and short-term and long-term safety data of patients treated with anti-VEGF.
Acknowledgments
Financial Support: This study was supported by grant R01EY021137 from the National Institutes of Health and by the Richard Shafritz Endowed Chair in Ophthalmology Research at the Children’s Hospital of Philadelphia.
Abbreviations/Acronyms:
- Anti-VEGF
anti-vascular endothelial growth factor
- BEAT-ROP
Bevacizumab Eliminates the Angiogenic Threat of Retinopathy of Prematurity Study
- BW
birth weight
- ET-ROP
Early Treatment of Retinopathy of Prematurity Study
- GA
gestation age
- GROP
Postnatal Growth and Retinopathy of Prematurity Study
- PMA
post-menstrual age
- RD
retinal detachment
- ROP
retinopathy of prematurity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: None
Conflict of Interest: No conflicting relationship exists for any author.
REFERENCES:
- 1.Fierson WM. Screening Examination of Premature Infants for Retinopathy of Prematurity. Pediatrics 2018;142(6). [DOI] [PubMed] [Google Scholar]
- 2.Group ETFROPC. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 2003;121(12):1684–1694. [DOI] [PubMed] [Google Scholar]
- 3.Zhang MH, Blair MP, Ham SA, Rodriguez SH. Two-Year Outcomes Comparing Anti-VEGF Injections to Laser for ROP Using a Commercial Claims Database. Ophthalmic Surg Lasers Imaging Retina 2020;51(9):486–493. [DOI] [PubMed] [Google Scholar]
- 4.Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 2011;364(7):603–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barry GP, Tauber KA, Fisher M, Greenberg S, Zobal-Ratner J, Binenbaum G. Short-term retinal detachment risk after treatment of type 1 retinopathy of prematurity with laser photocoagulation versus intravitreal bevacizumab. J aapos 2019;23(5):260.e261–260.e264. [DOI] [PubMed] [Google Scholar]
- 6.Binenbaum G, Tomlinson LA, de Alba Campomanes AG, et al. Validation of the Postnatal Growth and Retinopathy of Prematurity Screening Criteria. JAMA Ophthalmol 2019;138(1):31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binenbaum G, Bell EF, Donohue P, et al. Development of Modified Screening Criteria for Retinopathy of Prematurity. JAMA Ophthalmology 2018;136(9):1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binenbaum G, Tomlinson LA. Postnatal Growth and Retinopathy of Prematurity Study: Rationale, Design, and Subject Characteristics. Ophthalmic Epidemiol 2017;24(1):36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Efron B GG. A Leisurely Look at the Bootstrap, the Jackknife, and Cross-Validation. The American Statistician 1983;37 (1):36–48. [Google Scholar]
- 10.Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 2003;121(12):1684–1694. [DOI] [PubMed] [Google Scholar]
- 11.Repka MX. Outcome of Eyes Developing Retinal Detachment During the Early Treatment for Retinopathy of Prematurity Study. Archives of Ophthalmology 2011;129(9):1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueller B, Salchow DJ, Waffenschmidt E, et al. Treatment of type I ROP with intravitreal bevacizumab or laser photocoagulation according to retinal zone. British Journal of Ophthalmology 2017;101(3):365–370. [DOI] [PubMed] [Google Scholar]
- 13.Cabrera MT, Chia T, Wallace DK, et al. Short-term computer-assisted quantification of plus disease after treatment of type 1 retinopathy of with intravitreal bevacizumab or retinal laser photocoagulation. Retin Cases Brief Rep 2018. [DOI] [PubMed] [Google Scholar]
- 14.Shah PK, Subramanian P, Venkatapathy N, Chan RVP, Chiang MF, Campbell JP. Aggressive posterior retinopathy of prematurity in two cohorts of patients in South India: implications for primary, secondary, and tertiary prevention. J aapos 2019;23(5):264.e261–264.e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer EA, Flynn JT, Hardy RJ, et al. Incidence and early course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology 1991;98(11):1628–1640. [DOI] [PubMed] [Google Scholar]
- 16.Roohipoor R, Torabi H, Karkhaneh R, Riazi-Eafahani M. Comparison of intravitreal bevacizumab injection and laser photocoagulation for type 1 zone II retinopathy of prematurity. J Curr Ophthalmol 2019;31(1):61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karkhaneh R, Torabi H, Khodabande A, Roohipoor R, Riazi-Esfahani M. Efficacy of Intravitreal Bevacizumab for the Treatment of Zone I Type 1 Retinopathy of Prematurity. J Ophthalmic Vis Res 2018;13(1):29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon JM, Shin DH, Kim SJ, et al. Outcomes after laser versus combined laser and bevacizumab treatment for type 1 retinopathy of prematurity in zone I. Retina 2017;37(1):88–96. [DOI] [PubMed] [Google Scholar]
- 19.The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005;123(7):991–999. [DOI] [PubMed] [Google Scholar]
- 20.Campbell JP, Ryan MC, Lore E, et al. Diagnostic Discrepancies in Retinopathy of Prematurity Classification 2016;123(8):1795–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison D, Shaffer J, Ying GS, Binenbaum G. Ocular complications following treatment in the Postnatal Growth and Retinopathy of Prematurity (G-ROP) Study. J aapos 2018;22(2):128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel S, Klufas M. Evidence to date: ranibizumab and its potential in the treatment of retinopathy of prematurity. Eye and Brain 2019;Volume 11:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinapis Sinapis CI, Sinapis DI, et al. Pharmacokinetics of intravitreal bevacizumab (Avastin®) in rabbits. Clinical Ophthalmology 2011:697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakri SJ SM, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology 2007;114(5):855–859. [DOI] [PubMed] [Google Scholar]
- 25.Hajrasouliha AR, Garcia-Gonzales JM, Shapiro MJ, Yoon H, Blair MP. Reactivation of Retinopathy of Prematurity Three Years After Treatment With Bevacizumab. Ophthalmic Surg Lasers Imaging Retina 2017;48(3):255–259. [DOI] [PubMed] [Google Scholar]
- 26.Snyder LL, Garcia-Gonzalez JM, Shapiro MJ, Blair MP. Very Late Reactivation of Retinopathy of Prematurity After Monotherapy With Intravitreal Bevacizumab 2016;47(3):280–283. [DOI] [PubMed] [Google Scholar]
- 27.Hu J, Blair MP, Shapiro MJ, Lichtenstein SJ, Galasso JM, Kapur R. Reactivation of Retinopathy of Prematurity After Bevacizumab Injection 2012;130(8):1000. [DOI] [PubMed] [Google Scholar]
- 28.Ittiara S, Blair MP, Shapiro MJ, Lichtenstein SJ. Exudative retinopathy and detachment: a late reactivation of retinopathy of prematurity after intravitreal bevacizumab. J aapos 2013;17(3):323–325. [DOI] [PubMed] [Google Scholar]
- 29.Mansukhani SA, Hutchinson AK, Neustein R, Schertzer J, Allen JC, Hubbard GB. Fluorescein Angiography in Retinopathy of Prematurity: Comparison of Infants Treated with Bevacizumab to Those with Spontaneous Regression. Ophthalmol Retina 2019;3(5):436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of Intravitreal Bevacizumab for Stage 3+ Retinopathy of Prematurity. New England Journal of Medicine 2011;364(7):603–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quinn GE, Darlow BA. Concerns for Development After Bevacizumab Treatment of ROP. PEDIATRICS 2016;137(4):e20160057–e20162016. [DOI] [PubMed] [Google Scholar]
- 32.Avery RL. Bevacizumab (Avastin) for retinopathy of prematurity: wrong dose, wrong drug, or both? J aapos 2012;16(1):2–4. [DOI] [PubMed] [Google Scholar]
- 33.Huang CY LR, Wang NK, Chao AN, Chen KJ, Chen TL, Hwang YS, Lai CC, Wu WC. Changes in systemic vascular endothelial growth factor levels after intravitreal injection of aflibercept in infants with retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 2018;256(3):479–487. [DOI] [PubMed] [Google Scholar]
- 34.Hong YR, Kim YH, Kim SY, Nam GY, Cheon HJ, Lee SJ. Plasma concentrations of vascular endothelial growth factor in retinopathy of prematurity after intravitreal bevacizumab injection. Retina 2015;35(9):1772–1777. [DOI] [PubMed] [Google Scholar]
- 35.Wu W-C, Lien R, Liao P-J, et al. Serum Levels of Vascular Endothelial Growth Factor and Related Factors After Intravitreous Bevacizumab Injection for Retinopathy of Prematurity. JAMA Ophthalmology 2015;133(4):391. [DOI] [PubMed] [Google Scholar]
- 36.Sato T, Wada K, Arahori H, et al. Serum Concentrations of Bevacizumab (Avastin) and Vascular Endothelial Growth Factor in Infants With Retinopathy of Prematurity. American Journal of Ophthalmology 2012;153(2):327–333.e321. [DOI] [PubMed] [Google Scholar]
- 37.Stahl A, Lepore D, Fielder A, et al. Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): an open-label randomised controlled trial. Lancet 2019;394(10208):1551–1559. [DOI] [PubMed] [Google Scholar]
- 38.Hoerster R, Muether P, Dahlke C, et al. Serum concentrations of vascular endothelial growth factor in an infant treated with ranibizumab for retinopathy of prematurity. Acta Ophthalmologica 2013;91(1):e74–e75. [DOI] [PubMed] [Google Scholar]
- 39.Wu WC, Shih CP, Lien R, et al. Serum Vascular Endothelial Growth Factor After Bevacizumaorer Ranibizumab Treatment For Retinopathy of Prematurity. Retina 2017;37(4):694–701. [DOI] [PubMed] [Google Scholar]
- 40.Avery RL, Castellarin AA, Steinle NC, et al. SYSTEMIC PHARMACOKINETICS AND PHARMACODYNAMICS OF INTRAVITREAL AFLIBERCEPT, BEVACIZUMAB, AND RANIBIZUMAB. Retina 2017;37(10):1847–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen X, Zhou L, Zhang Q, Xu Y, Zhao P, Xia H. Serum Vascular Endothelial Growth Factor Levels before and after Intravitreous Ranibizumab Injection for Retinopathy of Prematurity. Journal of Ophthalmology 2019;2019:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez SH, Peyton C, Lewis K, et al. Neurodevelopmental Outcomes Comparing Bevacizumab to Laser for Type 1 ROP. Ophthalmic Surg Lasers Imaging Retina 2019;50(6):337–343. [DOI] [PubMed] [Google Scholar]
- 43.Kennedy KA, Mintz-Hittner HA. Medical and developmental outcomes of bevacizumab versus laser for retinopathy of prematurity. J aapos 2018;22(1):61–65.e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morin J, Luu TM, Superstein R, et al. Neurodevelopmental Outcomes Following Bevacizumab Injections for Retinopathy of Prematurity. PEDIATRICS 2016;137(4):e20153218–e20152015. [DOI] [PubMed] [Google Scholar]
- 45.Natarajan G, Shankaran S, Nolen TL, et al. Neurodevelopmental Outcomes of Preterm Infants With Retinopathy of Prematurity by Treatment. Pediatrics 2019;144(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blair MP, Shapiro MJ, Berrocal AM, et al. Re: Good: Bevacizumab for retinopathy of prematurity: treatment when pathology is embedded in a normally developing vascular system ( Ophthalmology . 2016;123:1843–1844). Ophthalmology 2017;124(10):e74–e75. [DOI] [PubMed] [Google Scholar]
- 47.Avery RL. Bevacizumab (Avastin) for retinopathy of prematurity: Wrong dose, wrong drug, or both? Journal of American Association for Pediatric Ophthalmology and Strabismus 2012;16(1):2–4. [DOI] [PubMed] [Google Scholar]
- 48.Wallace DK, Kraker RT, Freedman SF, et al. Assessment of Lower Doses of Intravitreous Bevacizumab for Retinopathy of Prematurity: A Phase 1 Dosing Study. JAMA ophthalmology 2017;135(6):654–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallace DK, Dean TW, Hartnett ME, et al. A Dosing Study of Bevacizumab for Retinopathy of Prematurity: Late Recurrences and Additional Treatments. Ophthalmology 2018;125(12):1961–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wallace DK, Kraker RT, Freedman SF, et al. Short-term Outcomes After Very Low-Dose Intravitreous Bevacizumab for Retinopathy of Prematurity. JAMA Ophthalmology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


