Abstract
Background:
Atrial fibrillation (AF) and heart failure often occur concomitantly, representing a clinical phenotype at high-risk for poor outcomes. Differences in the characteristics, management, and in-hospital outcomes of AF among those with heart failure with preserved ejection fraction (HFpEF) and those with heart failure with reduced ejection fraction (HFrEF) are not well characterized.
Methods and Results:
Using the National Inpatient Sample, we identified hospitalizations in 2008–2012 for HFpEF and for HFrEF, with and without AF based on ICD-9-CM codes. We examined patient characteristics, procedural rates, and in-hospital outcomes. AF was common among both HFpEF and HFrEF, and increased in prevalence over the study period. A very low proportion of the cohort underwent either direct-current cardioversion or catheter-ablation. Compared to those without AF, those with AF experienced higher in-hospital mortality regardless of heart failure subtype. In multivariable regression analysis, AF was associated with in-hospital mortality in HFpEF (OR 1.10, CI [1.08–1.11]), but not in HFrEF (OR 0.93 [0.92–0.94], p-for-interaction<0.001).
Conclusions:
Our study revealed that the prevalence and adverse impact of AF on those with HFpEF is substantial, providing a rationale to rigorously investigate strategies, such as rhythm-control, to improve outcomes for this particularly vulnerable subpopulation.
Keywords: heart failure, mortality, atrial fibrillation, epidemiology
1.1. INTRODUCTION
Heart failure1 and atrial fibrillation (AF)2 each affect over 5 million people across the United States, and represent conditions independently associated with poor outcomes.3,4 Frequently occurring together, heart failure can beget AF, and AF can beget heart failure.5,6 When they concurrently occur, heart failure and AF synergistically confer a poor prognosis compared to those without these conditions or with either condition alone.7
Characterizing this vulnerable patient population and identifying factors associated with poor outcomes is critical to improving the outcomes of those with concurrent heart failure and AF. Although there are a myriad of studies that have characterized patients with heart failure with reduced ejection fraction (HFrEF) and AF, much less is known about patients with HFpEF and AF. A recent meta-analysis performed on about 54,000 patients comparing outcomes among HFpEF with concurrent AF (HFpEF-AF) and HFrEF with concurrent AF (HFrEF-AF) revealed some insight,8 but incorporated studies comprised of a very select subgroup of patients, limiting inferences that could be drawn. Moreover, this study specifically focused on long-term outcomes, and did not describe the independent association of AF on mortality. Consequently, despite recognition that HFpEF-AF is a potentially important phenotype of HFpEF,9 the relative impact of AF on in-hospital mortality within HFpEF remains poorly characterized.
Our study sought to use a nationally-representative cohort to examine HFpEF with AF in relation to HFrEF with AF, and quantify the relative impact of AF on mortality.
METHODS
2.1. Data source and study population:
We used data from the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project—National Inpatient Sample (NIS) files from 2008–2012.10 The National Inpatient Sample is a 20% stratified sample of all nonfederal US hospitals. Hospitalizations are weighted based on a sampling scheme to permit inferences for a nationally representative population. Accordingly, we weighted hospitalizations based on the NIS sampling scheme, and performed all analyses on weighted data in order to provide nationally-representative estimates, as studies using the NIS have previously done.11,12 Each record in the NIS represents a patient hospitalization, and includes all procedure and diagnosis International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) codes recorded for each discharge.
We included all hospitalizations with a diagnosis of acute heart failure among adults aged ≥18 years in 2008–2012 for analysis. We identified acute heart failure hospitalizations based on ICD-9-CM codes—acute heart failure with preserved ejection fraction: 428.31 and 428.33; acute heart failure with reduced ejection fraction: 428.21 and 428.23. The approach of identifying heart failure hospitalizations according to ICD-9-CM codes has extensively been used in several prior studies examining both Medicare13–15 and the NIS.16,17 Although codes specifically for HFrEF and HFpEF have not been formally validated, they have been used in prior work and have identified cohorts of HFpEF and HFrEF whose characteristics correlate well with those observed in clinical trials and community-based studies.18 To avoid systematic bias of including an emerging phenotype of heart failure known as “heart failure with mid-range ejection fraction,”19 those with combined acute systolic and diastolic heart failure were excluded from analysis. The presence of AF was based on ICD-9-CM code 427.31, which has previously demonstrated good diagnostic performance.20
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee. Given the retrospective nature of this study, the Weill Cornell Institutional Review Board did not require informed consent for this study.
2.2. Baseline Characteristic and Outcome Variables
We collected all baseline characteristic and outcome variables from the NIS. We collected patient-level variables including socio-demographics and comorbid conditions based on previously validated Elixhauser methods. We also collected hospital characteristics derived from the American Hospital Association Annual Survey Database, and all-cause mortality. Based on patient characteristics, we calculated CHADS2 and CHA2DS2-VASc21 scores for each hospitalization. We also calculated an Elixhauser score, a summary index score based on a validated weighted-calculation of the Elixhauser comorbidities,22 for each hospitalization. Finally, we identified the performance of direct-current cardioversion (DCCV) and catheter-ablations based on the presence of ICD-9-CM procedure codes 99.61 (atrial cardioversion) and 37.34 (ablation of heart tissue via a peripherally inserted catheter) respectively. To ensure DCCV was performed for AF, we did not count cases of DCCV accompanied by diagnostic codes for other atrial arrhythmias such as atrial flutter, atrioventricular nodal tachycardia, and paroxysmal supraventricular tachycardia (Supplemental table 1). To ensure catheter-ablation was performed for AF, we did not count cases of ablation accompanied by diagnostic codes for other arrhythmias, or those who likely underwent ablation of the atrioventricular junction, similar to prior studies23,24 (Supplemental Table 2). The primary outcome measure was in-hospital all-cause mortality.
2.3. Statistical Analysis:
To compare baseline characteristics, we used Student’s t tests for continuous variables, and Pearson chi-squared tests for categorical variables. To compare in-hospital mortality, we used the Pearson chi-squared tests. To assess temporal trends for AF prevalence and use of procedures over the 5-year study period, we used the autoregressive integrated moving average model for time series.
We performed multivariable regression analyses to identify correlates of the use of DCCV and catheter-ablation. We also used multivariable regression analysis to determine the association of AF with in-hospital mortality. In these models, we included socio-demographic factors (age, sex, race, payer status), hospital characteristics (hospital size, geographic region, urban setting, and academic status), and comorbidity burden via the Elixhauser score. We used the Taylor linearization method “with replacement” design to compute the standard errors of the regression coefficients for all regression analyses; the Taylor linearization approach is based on a first-order Taylor series linear approximation of the derivative of the log weighted likelihood function.25 All statistical tests were 2-sided, and a p-value of < 0.01 was set to be statistically significant given the size of the cohort. All statistical analyses were conducted using SAS, version 9.2 (SAS Institute, Cary, NC) or SPSS, version 20 (IBM Corporation, Armonk, NY).
RESULTS
There were 4,641,890 hospitalizations with acute heart failure that met inclusion criteria. Among them, 45.3% were for HFpEF and 54.7% were for HFrEF.
3.1. Baseline Characteristics
Among those with HFpEF, 41.9% had concurrent atrial fibrillation (HFpEF-AF). This prevalence increased from 37.4% in 2008 to 44.7% in 2012 (Figure 1). Those with HFpEF-AF were older, more commonly white, and more commonly recipients of Medicare compared to those with HFpEF without AF (Table 1). With regard to comorbidity, those with HFpEF-AF were more likely to have hypothyroidism and valvular heart disease compared to those with HFpEF without AF; and less likely to have anemia, chronic renal failure, diabetes, and obesity compared to those with HFpEF without AF (Table 1). The mean Elixhauser score was slightly lower for those with AF compared to those without. The CHADS2 and CHA2DS2-VASc scores were slightly higher for those with AF compared to those without.
Figure 1. Prevalence of Concurrent AF with HF.
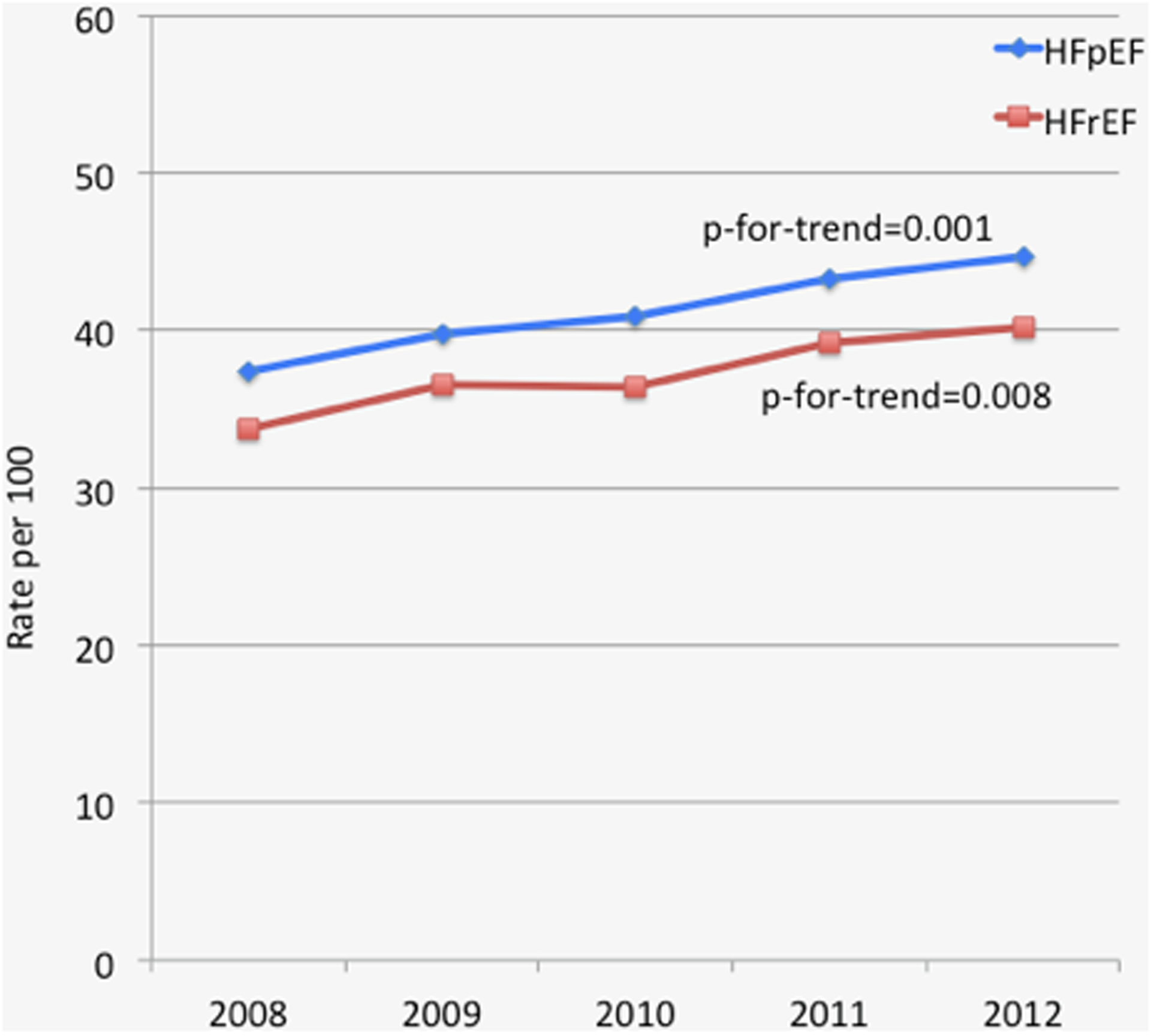
Line graph demonstrating prevalence of atrial fibrillation with heart failure
Table 1.
Characteristics of HFpEF and HFrEF, stratified by presence of AF
| HFpEF | HFrEF | |||||
|---|---|---|---|---|---|---|
| Variable | AF (n=794,013) | No AF (n=1,094,287) | p | AF (n=858,435) | No AF (n=1,413,994) | p |
| Age (Mean ± SD, years) | 79.6 ± 10.3 | 73.1 ± 13.8 | <0.001 | 75.8 ± 12.0 | 69.2 ± 15.1 | <0.001 |
| Women | 64.3 | 64.0 | <0.001 | 40.7 | 42.7 | <0.001 |
| Race (%) | ||||||
| White | 84.0 | 68.8 | 80.0 | 65.1 | ||
| Black | 7.8 | 19.5 | <0.001 | 11.2 | 22.6 | <0.001 |
| Other | 8.2 | 11.7 | 8.8 | 12.2 | ||
| Primary Payer (%) | ||||||
| Medicare | 87.8 | 77.2 | 81.7 | 68.8 | ||
| Medicaid | 2.5 | 7.1 | <0.001 | 4.3 | 9.7 | <0.001 |
| Private including HMO | 7.9 | 11.6 | 10.7 | 14.6 | ||
| Self pay | 0.8 | 2.4 | 1.9 | 4.5 | ||
| Hospital Region (%) | ||||||
| Northeast | 25.3 | 23.2 | 23.4 | 22.1 | ||
| Midwest | 26.6 | 24.4 | <0.001 | 25.7 | 23.2 | <0.001 |
| South | 32.9 | 38.0 | 36.4 | 40.9 | ||
| West | 15.2 | 14.4 | 14.5 | 13.7 | ||
| Hospital Setting (%) | ||||||
| Academic | 44.8 | 45.3 | <0.001 | 46.1 | 47.4 | <0.001 |
| Urban | 89.2 | 88.0 | <0.001 | 88.4 | 88.1 | <0.001 |
| Hospital Size (%) | ||||||
| Small | 12.9 | 12.4 | 11.7 | 11.0 | ||
| Medium | 26.1 | 25.6 | <0.001 | 23.7 | 23.5 | <0.001 |
| Large | 61.0 | 62.0 | 64.6 | 65.5 | ||
| Comorbidity | ||||||
| Anemia | 32.4 | 35.8 | <0.001 | 26.8 | 26.8 | 0.82 |
| Coronary artery disease | 42.1 | 41.1 | <0.001 | 59.9 | 58.6 | <0.001 |
| Chronic pulmonary disease | 38.2 | 37.6 | <0.001 | 33.0 | 31.8 | <0.001 |
| Chronic renal failure | 34.9 | 39.1 | <0.001 | 37.2 | 35.7 | <0.001 |
| Diabetes | 36.8 | 46.6 | <0.001 | 37.4 | 42.8 | <0.001 |
| Hypertension | 68.9 | 67.9 | <0.001 | 65.1 | 64.3 | <0.001 |
| Hypothyroidism | 21.0 | 16.3 | <0.001 | 17.2 | 12.3 | <0.001 |
| Liver disease | 2.0 | 2.7 | <0.001 | 2.3 | 2.6 | <0.001 |
| Obesity | 16 | 21.4 | <0.001 | 12 | 13.2 | <0.001 |
| Peripheral vascular disorders | 12 | 12.1 | 0.06 | 12.6 | 12.6 | 0.31 |
| Pulmonary circulation disorders | 7.1 | 6.2 | <0.001 | 3.8 | 3.0 | <0.001 |
| Valvular Disease | 9.4 | 6.7 | <0.001 | 7.1 | 4.9 | <0.001 |
| Elixhauser Comorbidity Summary Score (Mean ± SD) | 7.8 ± 7.1 | 8.2 ± 7.2 | <0.001 | 7.2 ± 6.9 | 6.9 ± 7.0 | <0.001 |
| CHADS2 Score (Mean ± SD) | 2.9 ± 0.9 | 2.7 ± 0.9 | <0.001 | 2.7 ± 0.9 | 2.5 ± 0.9 | <0.001 |
| CHA2DS2-VASc Score (Mean ± SD) | 4.5 ± 1.2 | 4.2 ± 1.3 | <0.001 | 4.1 ± 1.3 | 3.7 ± 1.4 | <0.001 |
| In-hospital Mortality (%) | 4.7 | 4.0 | <0.001 | 5.3 | 5.1 | <0.001 |
Abbreviations: HFpEF=heart failure with preserved ejection fraction; HFrEF=heart failure with reduced ejection fraction; AF=atrial fibrillation.
Among those with HFrEF, 37.6% had concurrent atrial fibrillation (HFrEF-AF). This prevalence increased from 33.7% in 2008 to 40.2% in 2012 (Figure 1). Those with HFrEF-AF were older, more commonly white, and more commonly recipients of Medicare compared to those with HFrEF without AF (Table 1). With regard to comorbidity, those with HFrEF-AF were more likely to have hypothyroidism and valvular heart disease compared to those with HFrEF without AF; and less likely to have diabetes compared to those with HFrEF without AF (Table 1). The mean Elixhauser, CHADS2, and CHA2DS2-VASc scores were slightly higher for those with AF compared to those without.
3.2. Use of Direct-current Cardioversion and Catheter-ablation
Rates of DCCV and catheter-ablation were low for both HFpEF-AF and HFrEF-AF. Among HFpEF-AF, DCCV was performed in 10 out of every 1000 hospitalizations and catheter-ablation was performed in just 1 out of every 1000 hospitalizations. Among HFrEF-AF, DCCV was performed in 10 out of every 1000 hospitalizations and catheter-ablation was performed in 2 out of every 1000 hospitalizations. The rates of procedure use were stable across the study period (HFpEF: DCCV p-for-trend=1.00, catheter-ablation p-for-trend=0.99; HFpEF: DCCV p-for-trend=0.99, catheter-ablation p-for-trend=1.00).
Factors associated with the use of DCCV among HFpEF-AF and HFrEF-AF included younger age (less than 75 years) and White race (Figure 2A). Elixhauser score was inversely associated with DCCV in both groups, where each Elixhauser point reduced the odds of undergoing a DCCV by 7% among HFpEF-AF and HFrEF-AF. Notably, women were more likely to undergo a DCCV among HFpEF-AF and men were more likely to undergo DCCV among HFrEF-AF.
Figure 2. Factors Associated with DCCV, Catheter-ablation, and In-hospital Mortality.
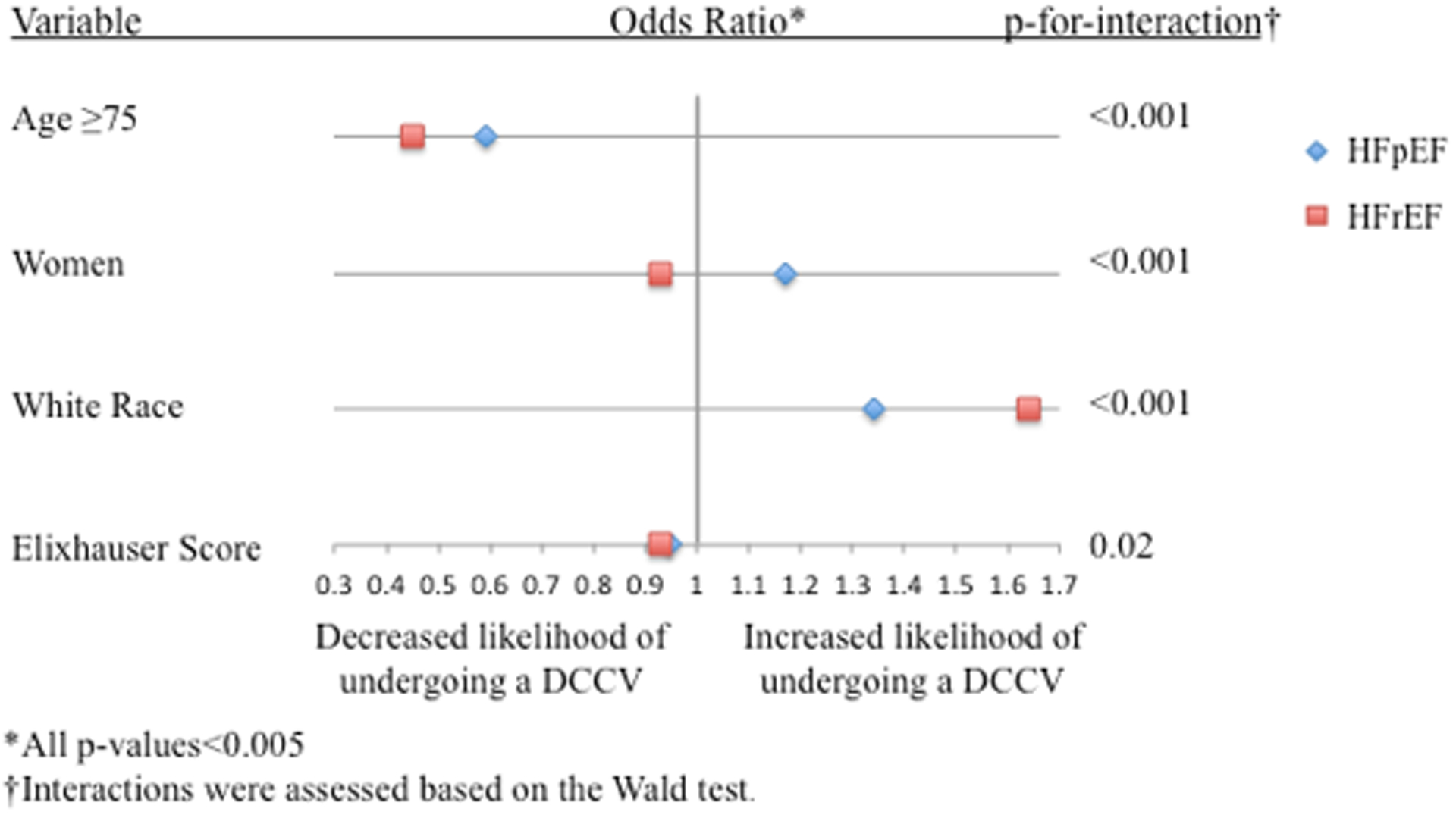
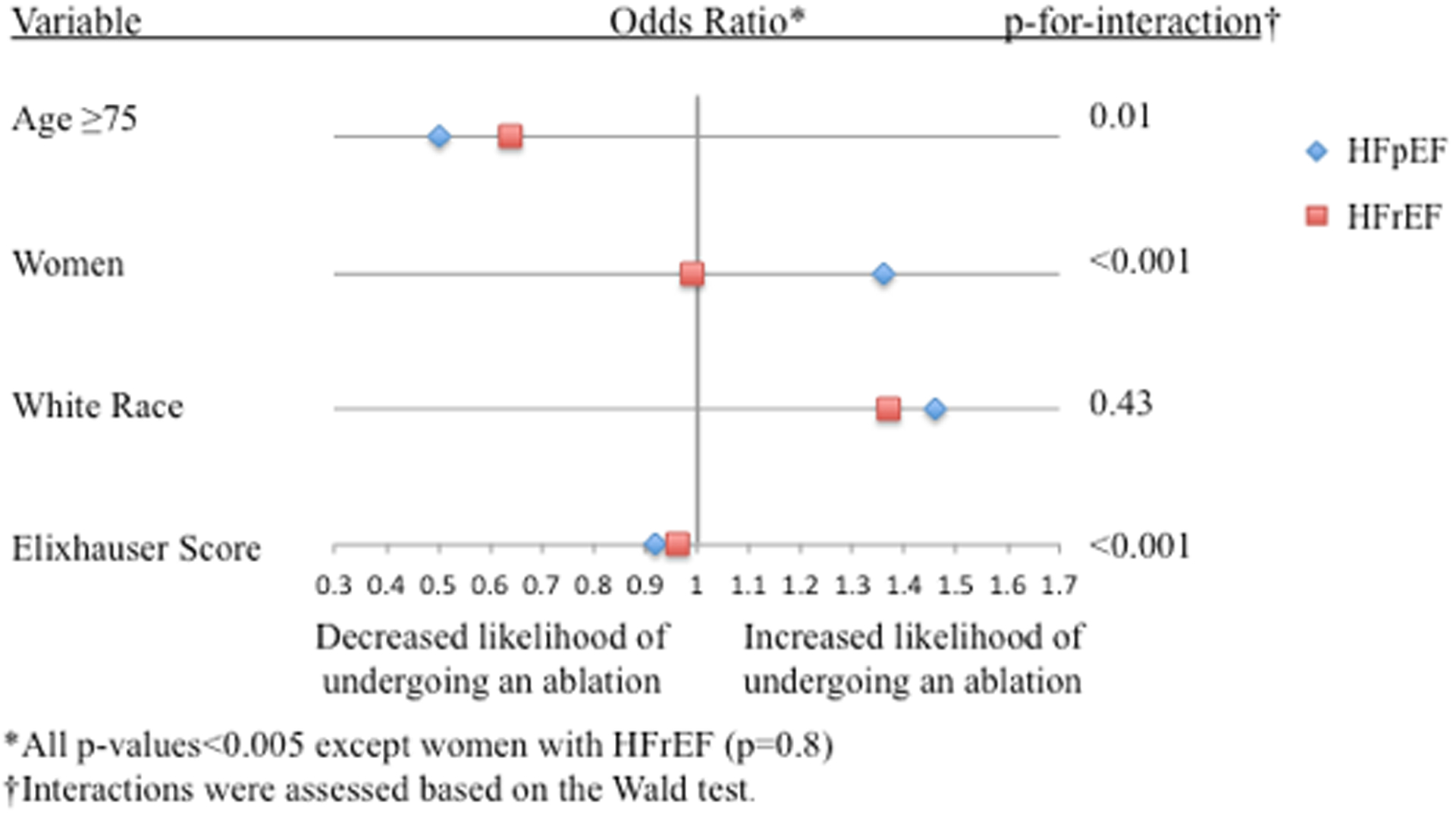
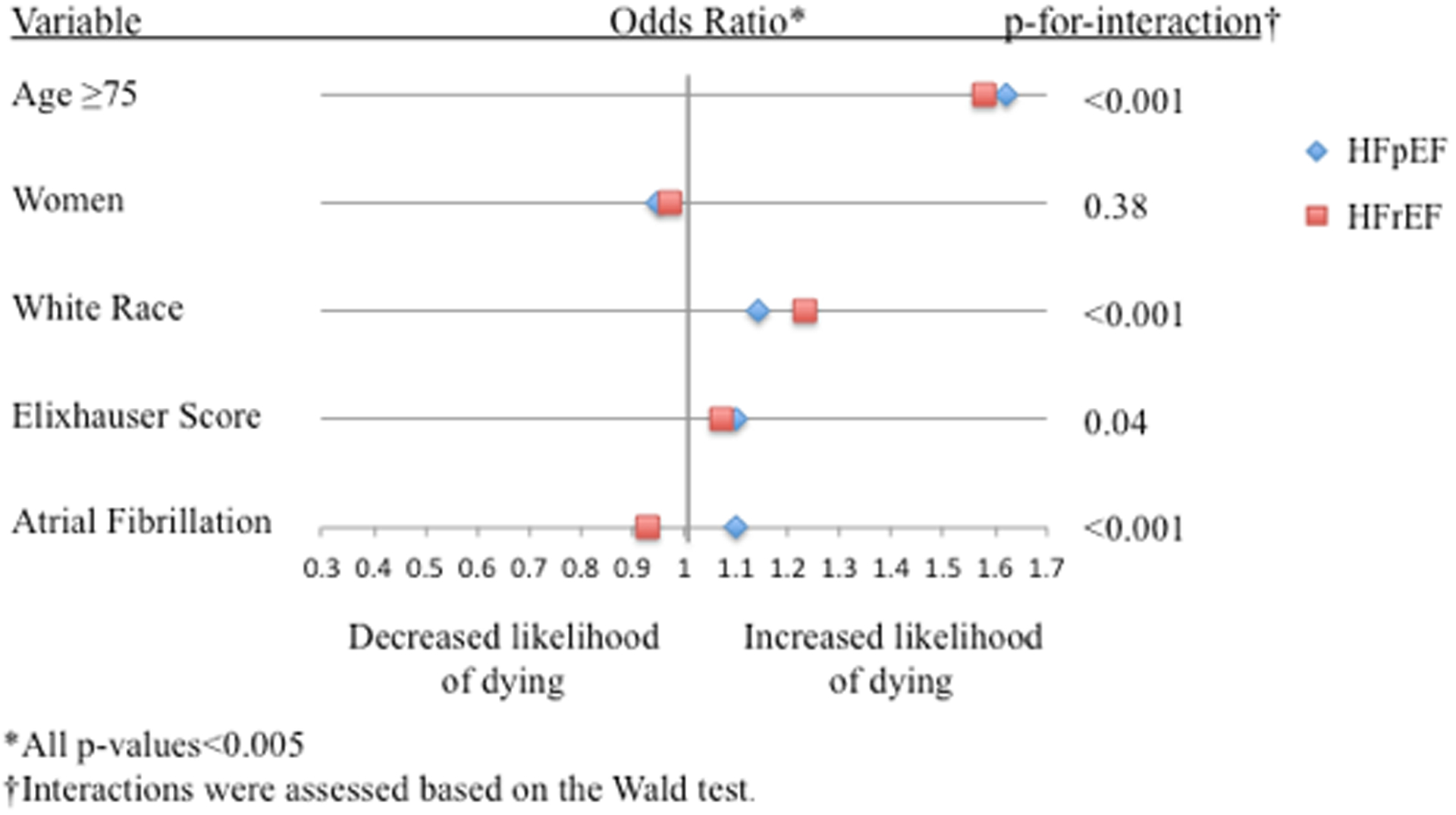
Forest plot showing factors associated with A.) DCCV use, B.) Catheter-ablation use, and C.) In-hospital mortality
Factors associated with the use of catheter-ablation among HFpEF-AF and HFrEF-AF also included younger age (less than 75 years) and White race (Figure 2B). Elixhauser score was inversely associated with catheter-ablation in both groups, where each Elixhauser point reduced the odds of undergoing a catheter-ablation among HFpEF-AF by 8% and among HFrEF-AF by 4%. Notably, women were more likely to undergo a catheter-ablation among HFpEF-AF, and sex did not influence the use of catheter-ablation among HFrEF-AF.
3.3. In-hospital mortality
Among HFpEF, those with concurrent AF experienced a higher unadjusted in-hospital mortality rate compared to those without AF (Table 1). On the other hand, among HFrEF, those with concurrent AF and those without AF experienced similar unadjusted in-hospital mortality rates (Table 1).
In multivariable regression analysis (Figure 2C), correlates of in-hospital mortality included age ≥75 years, men, white race, and Elixhauser score for both HFpEF-AF and HFrEF-AF. Interestingly, the association of AF with in-hospital mortality diverged in relation to heart failure subtype. Among those with HFpEF, AF was associated with in-hospital mortality, whereas AF was inversely associated among those with HFrEF.
4.1. DISCUSSION
This is the largest epidemiologic study of hospitalized HFpEF patients with AF to date, revealing several important findings. First, HFpEF with concurrent AF was common and became increasingly prevalent over the study period. Second, rates of DCCV and catheter-ablation were very low in both HFpEF and HFrEF, and were less likely to be performed in those who were non-white and those with higher comorbidity burden. Third, AF was associated with in-hospital mortality among those with HFpEF, but not those with HFrEF.
Consistent with other cohorts of hospitalized patients,26–29 the prevalence of AF in our study was about 40%, with comparable rates among those with HFpEF and HFrEF. Prevalent AF was associated with advanced age and white race, observations that parallel the general population.30 The prevalence was noted to increase for both HFpEF and HFrEF, an observation that may be related to increasing awareness and recognition of this important phenotype.
Procedures like DCCV and catheter-ablation were infrequently performed in our cohort. Although this cohort had a high burden of comorbidity, it did not appear that comorbidity severity (as quantified by Elixhauser score) had a major influence on the performance of either procedure. Thus, we speculate that our findings stem from the paucity of data demonstrating the benefit of a rhythm-control strategy in heart failure. The largest study to date examining a rhythm control strategy among heart failure patients was the AF-CHF study,31 which revealed that a rhythm control strategy primarily using anti-arrhythmic drugs did not improve outcomes compared to a rate-control strategy. Notably, this study evaluated the impact of pharmacotherapy, which is not the only form of rhythm control. DCCV and catheter-ablation offer alternative strategies to maintain rhythm control, and have been shown to be safe.32,33 However, DCCV has not been prospectively studied without the use of anti-arrhythmic drugs; and although there is promising data on the use of catheter-ablation in heart failure patients,34–36 large prospective studies are still lacking. With ongoing randomized-controlled trials like RAFT-AF (NCT01420393) and CASTLE-AF (NCT00643188), which aim to determine the benefit of catheter-ablation, more will soon be known about the role of catheter ablation, at least in the HFrEF subset of heart failure patients
The observation that racial minorities were less likely to undergo either DCCV or catheter-ablation among those with HFpEF and HFrEF warrants additional investigation. One possible explanation for this finding is that racial minorities may be less symptomatic from their AF, and therefore less likely to have a guideline-based indication3 for a rhythm-control strategy. In a study of 432 individuals confirmed to have AF on electrocardiogram from the REGARDS cohort, Meschia found that blacks were much less likely to be aware that they had AF, even after controlling for payer status and access to health care.37 The authors suggested that this observation may be attributable to an increased likelihood of asymptomatic (silent) AF among blacks. Since our multivariable regression analysis controlled for hospital characteristics including size and location, access to electrophysiology services was unlikely to have made a significant contribution to this finding. However, it is not known whether other aspects of health-care related disparities, which have influenced patterns of other electrophysiological procedure use,38,39 contributed to this finding. Our data, therefore, reinforces the need to further examine racial differences in presentation and management of those with concurrent heart failure and AF.
There were also important differences with regard to men and women undergoing procedures. Though differences were small among those with HFrEF, women were more likely than men to undergo DCCV or catheter-ablation among those with HFpEF. The likelihood of having symptomatic AF may again play a pivotal role in patterns observed in our study. Among the general population, women with AF are more symptomatic, more likely to have recurrence, and experience higher mortality rates compared to men.31,40,41 This likely stems, at least in part, from important differences in left ventricular response to stressors between men and women, where women have a higher predilection for developing concentric left ventricular hypertrophy and greater diastolic dysfunction.42–44 Consequently, women with HFpEF may be more intolerant of AF compared to men, a notion supported by the observation in I-PRESERVE that AF conferred an increased mortality risk in women compared to men.45 For this reason, coupled with the fact that women are less likely to respond to pharmacologic therapy,46–49 providers may more likely pursue DCCV or catheter-ablation as a rhythm control strategy for women with HFpEF.
While the impact of AF on long-term outcomes among patients with heart failure is well-described, its impact on short-term hospital outcomes is poorly characterized. Our study showed that those with HFpEF experienced lower unadjusted in-hospital mortality compared to those with HFrEF, revealing in-hospital outcomes to parallel long-term outcomes.8. We further aimed to compare the relative contributions of AF to in-hospital mortality between HFpEF and HFrEF. Studies to date have shown that concurrent AF is associated with worse outcomes, but such studies have lacked sufficient power and/or national-representation to permit inferences with regard to the influence of AF on in-hospital outcomes. Our study of almost 5 million hospitalizations facilitated a robust multivariable regression analysis revealing that AF was a correlate of in-hospital mortality among those with HFpEF but not those with HFrEF, again paralleling patterns observed for long-term outcomes.50,51 This underscores the importance of left atrial morphology and function on outcomes in HFpEF. In the setting of reduced ventricular compliance and impaired diastolic filling in HFpEF, the contribution of atrial contraction may be particularly relevant to cardiac output and normalization of filling pressures. Indeed, the presence of AF in patients with HFpEF has been linked to declining left atrial function, rising left atrial wall tension, and elevated filling pressures.52,53 While similar findings can be seen in HFrEF, the association between left atrial abnormalities and outcome are more strongly linked in HFpEF compared to HFrEF,52 and likely underlies our finding that AF is an independent predictor of in-hospital mortality in HFpEF, but not HFrEF. Whether restoration of sinus rhythm resulting in improved hemodynamics among patients with HFpEF can improve outcomes remains unknown and likely warrants additional investigation. The degree to which the HFpEF-AF phenotype overlaps with other important phenotypes, like HFpEF associated with obesity or HFpEF associated with pulmonary hypertension and right ventricular dysfunction, is not well-characterized and represents an additional area for future research.
Strengths of this study included the large number of hospitalizations and nationally-representative nature of the cohort including all payers. The NIS sampling design has been validated11,54 and is commonly used to examine patterns in national healthcare among a range of subpopulations including HFpEF.18 There were also some important limitations to our study. Echocardiographic and electrocardiographic data were not available to confirm the diagnoses of heart failure or AF. Laboratory values like b-type natriuretic peptide were also unavailable. This could have led to misclassification errors, a source of information bias. However, the use of ICD-9-CM codes to identify heart failure13,55 and to identify AF20,56 have been extensively used with reasonable diagnostic performance. Further, the prevalence of AF in our cohort matched that of other cohorts of hospitalized heart failure patients,26–29 suggesting that the effect of misclassification may have been limited, strengthening the internal validity of our findings. While we were able to subtype heart failure into HFpEF and HFrEF using methodology previously described,18 we were unable to subtype AF into paroxysmal or permanent AF, or differentiate between incident and prevalent AF. The presence of AF or sinus rhythm at discharge was also unavailable. These factors may have had an impact on the prevalence of AF, performance rates of rhythm-control procedures, and outcomes reported in this study. Because cases represented de-identified hospitalizations, overrepresentation of certain factors in the cases of repeat hospitalizations could not be excluded. Finally, we did not have information on unmeasured variables including medications like anti-arrhythmic drugs and anticoagulation, which may have had an impact on outcomes. Despite lacking this granularity, large administrative databases have been cited as a useful resource for hypothesis-generation,57 which was the primary intention of this study.
In conclusion, our study demonstrates the prevalence and adverse impact of AF on those with HFpEF, providing a rationale to rigorously investigate strategies, such as rhythm-control, to improve outcomes for this particularly vulnerable subpopulation.
Supplementary Material
Funding Sources:
This work was supported by the Michael Wolk Heart Foundation and the New York Cardiac Center, Inc. The Michael Wolk Heart Foundation and the New York Cardiac Center, Inc. had no role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript. Dr. Goyal was supported by the 2016-2017 Glorney-Raisbeck Fellowship Award in Cardiovascular Disease from the New York Academy of Medicine during a portion of this work. Dr. Goyal is currently supported by National Institute on Aging grant R03AG056446. Dr. Kamel is supported by National Institute of Neurological Disorders and Stroke (NINDS) grants K23NS082367, R01NS097443, and the Michael Goldberg Stroke Research Fund.
Footnotes
This author takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation
Conflicts of Interest: None.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics C, Stroke Statistics S. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):e38–360. [DOI] [PubMed] [Google Scholar]
- 2.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112(8):1142–1147. [DOI] [PubMed] [Google Scholar]
- 3.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr., Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW, American College of Cardiology/American Heart Association Task Force on Practice G. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–76. [DOI] [PubMed] [Google Scholar]
- 4.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810–1852. [DOI] [PubMed] [Google Scholar]
- 5.Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, Wang TJ, Levy D, Benjamin EJ, Ho JE. Atrial Fibrillation Begets Heart Failure and Vice Versa: Temporal Associations and Differences in Preserved Versus Reduced Ejection Fraction. Circulation. 2016;133(5):484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M. Heart Failure With Preserved Ejection Fraction and Atrial Fibrillation: Vicious Twins. J Am Coll Cardiol. 2016;68(20):2217–2228. [DOI] [PubMed] [Google Scholar]
- 7.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D’Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107(23):2920–2925. [DOI] [PubMed] [Google Scholar]
- 8.Kotecha D, Chudasama R, Lane DA, Kirchhof P, Lip GY. Atrial fibrillation and heart failure due to reduced versus preserved ejection fraction: A systematic review and meta-analysis of death and adverse outcomes. Int J Cardiol. 2016;203:660–666. [DOI] [PubMed] [Google Scholar]
- 9.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation. 2016;134(1):73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2007–2009. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/nisoverview.jsp [Google Scholar]
- 11.Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, Pavri BB, Kurtz SM. Trends in permanent pacemaker implantation in the United States from 1993 to 2009: increasing complexity of patients and procedures. J Am Coll Cardiol. 2012;60(16):1540–1545. [DOI] [PubMed] [Google Scholar]
- 12.Papolos A, Narula J, Bavishi C, Chaudhry FA, Sengupta PP. U.S. Hospital Use of Echocardiography: Insights From the Nationwide Inpatient Sample. J Am Coll Cardiol. 2016;67(5):502–511. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Dharmarajan K, Wang Y, Krumholz HM. National trends in heart failure hospital stay rates, 2001 to 2009. J Am Coll Cardiol. 2013;61(10):1078–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krumholz HM, Lin Z, Keenan PS, Chen J, Ross JS, Drye EE, Bernheim SM, Wang Y, Bradley EH, Han LF, Normand SL. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto-Filho JA, Kim N, Bernheim SM, Suter LG, Drye EE, Krumholz HM. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure-associated hospitalizations in the United States. J Am Coll Cardiol. 2013;61(12):1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Win TT, Davis HT, Laskey WK. Mortality Among Patients Hospitalized With Heart Failure and Diabetes Mellitus: Results From the National Inpatient Sample 2000 to 2010. Circ Heart Fail. 2016;9(5):e003023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goyal P, Almarzooq ZI, Horn EM, Karas MG, Sobol I, Swaminathan RV, Feldman DN, Minutello RM, Singh HS, Bergman GW, Wong SC, Kim LK. Characteristics of Hospitalizations for Heart Failure with Preserved Ejection Fraction. Am J Med. 2016;129(6):635 e615–626. [DOI] [PubMed] [Google Scholar]
- 19.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M, Document R. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891–975. [DOI] [PubMed] [Google Scholar]
- 20.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21 Suppl 1:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goyal P, Rich MW. Electrophysiology and heart rhythm disorders in older adults. J Geriatr Cardiol. 2016;13(8):645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626–633. [DOI] [PubMed] [Google Scholar]
- 23.Piccini JP, Sinner MF, Greiner MA, Hammill BG, Fontes JD, Daubert JP, Ellinor PT, Hernandez AF, Walkey AJ, Heckbert SR, Benjamin EJ, Curtis LH. Outcomes of Medicare beneficiaries undergoing catheter ablation for atrial fibrillation. Circulation. 2012;126(18):2200–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deshmukh A, Patel NJ, Pant S, Shah N, Chothani A, Mehta K, Grover P, Singh V, Vallurupalli S, Savani GT, Badheka A, Tuliani T, Dabhadkar K, Dibu G, Reddy YM, Sewani A, Kowalski M, Mitrani R, Paydak H, Viles-Gonzalez JF. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93 801 procedures. Circulation. 2013;128(19):2104–2112. [DOI] [PubMed] [Google Scholar]
- 25.Wolter KM (2007) Introduction to Variance Estimation. 2nd ed. New York: Springer. [Google Scholar]
- 26.Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, Hochadel M, Komajda M, Lassus J, Lopez-Sendon JL, Ponikowski P, Tavazzi L, EuroHeart Survey I, Heart Failure Association ESoC. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27(22):2725–2736. [DOI] [PubMed] [Google Scholar]
- 27.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC, Committee ASA, Investigators. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47(1):76–84. [DOI] [PubMed] [Google Scholar]
- 28.O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF Jr., Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365(1):32–43. [DOI] [PubMed] [Google Scholar]
- 29.Khazanie P, Liang L, Qualls LG, Curtis LH, Fonarow GC, Hammill BG, Hammill SC, Heidenreich PA, Masoudi FA, Hernandez AF, Piccini JP. Outcomes of medicare beneficiaries with heart failure and atrial fibrillation. JACC Heart Fail. 2014;2(1):41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. [DOI] [PubMed] [Google Scholar]
- 31.Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JM, Buxton AE, Camm AJ, Connolly SJ, Dubuc M, Ducharme A, Guerra PG, Hohnloser SH, Lambert J, Le Heuzey JY, O’Hara G, Pedersen OD, Rouleau JL, Singh BN, Stevenson LW, Stevenson WG, Thibault B, Waldo AL, Atrial F, Congestive Heart Failure I. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358(25):2667–2677. [DOI] [PubMed] [Google Scholar]
- 32.Botkin SB, Dhanekula LS, Olshansky B. Outpatient cardioversion of atrial arrhythmias: efficacy, safety, and costs. Am Heart J. 2003;145(2):233–238. [DOI] [PubMed] [Google Scholar]
- 33.Wilton SB, Fundytus A, Ghali WA, Veenhuyzen GD, Quinn FR, Mitchell LB, Hill MD, Faris P, Exner DV. Meta-analysis of the effectiveness and safety of catheter ablation of atrial fibrillation in patients with versus without left ventricular systolic dysfunction. Am J Cardiol. 2010;106(9):1284–1291. [DOI] [PubMed] [Google Scholar]
- 34.Khan MN, Jais P, Cummings J, Di Biase L, Sanders P, Martin DO, Kautzner J, Hao S, Themistoclakis S, Fanelli R, Potenza D, Massaro R, Wazni O, Schweikert R, Saliba W, Wang P, Al-Ahmad A, Beheiry S, Santarelli P, Starling RC, Dello Russo A, Pelargonio G, Brachmann J, Schibgilla V, Bonso A, Casella M, Raviele A, Haissaguerre M, Natale A, Investigators P-C. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 2008;359(17):1778–1785. [DOI] [PubMed] [Google Scholar]
- 35.Machino-Ohtsuka T, Seo Y, Ishizu T, Sugano A, Atsumi A, Yamamoto M, Kawamura R, Machino T, Kuroki K, Yamasaki H, Igarashi M, Sekiguchi Y, Aonuma K. Efficacy, safety, and outcomes of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. J Am Coll Cardiol. 2013;62(20):1857–1865. [DOI] [PubMed] [Google Scholar]
- 36.Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, Reddy M, Jais P, Themistoclakis S, Dello Russo A, Casella M, Pelargonio G, Narducci ML, Schweikert R, Neuzil P, Sanchez J, Horton R, Beheiry S, Hongo R, Hao S, Rossillo A, Forleo G, Tondo C, Burkhardt JD, Haissaguerre M, Natale A. Ablation Versus Amiodarone for Treatment of Persistent Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted Device: Results From the AATAC Multicenter Randomized Trial. Circulation. 2016;133(17):1637–1644. [DOI] [PubMed] [Google Scholar]
- 37.Meschia JF, Merrill P, Soliman EZ, Howard VJ, Barrett KM, Zakai NA, Kleindorfer D, Safford M, Howard G. Racial disparities in awareness and treatment of atrial fibrillation: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2010;41(4):581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez AF, Fonarow GC, Liang L, Al-Khatib SM, Curtis LH, LaBresh KA, Yancy CW, Albert NM, Peterson ED. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA. 2007;298(13):1525–1532. [DOI] [PubMed] [Google Scholar]
- 39.Farmer SA, Kirkpatrick JN, Heidenreich PA, Curtis JP, Wang Y, Groeneveld PW. Ethnic and racial disparities in cardiac resynchronization therapy. Heart Rhythm. 2009;6(3):325–331. [DOI] [PubMed] [Google Scholar]
- 40.Paquette M, Roy D, Talajic M, Newman D, Couturier A, Yang C, Dorian P. Role of gender and personality on quality-of-life impairment in intermittent atrial fibrillation. Am J Cardiol. 2000;86(7):764–768. [DOI] [PubMed] [Google Scholar]
- 41.Friberg J, Scharling H, Gadsboll N, Truelsen T, Jensen GB, Copenhagen City Heart S. Comparison of the impact of atrial fibrillation on the risk of stroke and cardiovascular death in women versus men (The Copenhagen City Heart Study). Am J Cardiol. 2004;94(7):889–894. [DOI] [PubMed] [Google Scholar]
- 42.Krumholz HM, Larson M, Levy D. Sex differences in cardiac adaptation to isolated systolic hypertension. Am J Cardiol. 1993;72(3):310–313. [DOI] [PubMed] [Google Scholar]
- 43.Piro M, Della Bona R, Abbate A, Biasucci LM, Crea F. Sex-related differences in myocardial remodeling. J Am Coll Cardiol. 2010;55(11):1057–1065. [DOI] [PubMed] [Google Scholar]
- 44.Scantlebury DC, Borlaug BA. Why are women more likely than men to develop heart failure with preserved ejection fraction? Curr Opin Cardiol. 2011;26(6):562–568. [DOI] [PubMed] [Google Scholar]
- 45.Lam CS, Carson PE, Anand IS, Rector TS, Kuskowski M, Komajda M, McKelvie RS, McMurray JJ, Zile MR, Massie BM, Kitzman DW. Sex differences in clinical characteristics and outcomes in elderly patients with heart failure and preserved ejection fraction: the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail. 2012;5(5):571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suttorp MJ, Kingma JH, Koomen EM, van ‘t Hof A, Tijssen JG, Lie KI. Recurrence of paroxysmal atrial fibrillation or flutter after successful cardioversion in patients with normal left ventricular function. Am J Cardiol. 1993;71(8):710–713. [DOI] [PubMed] [Google Scholar]
- 47.Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJ, Tijssen JG, Crijns HJ, Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study G. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347(23):1834–1840. [DOI] [PubMed] [Google Scholar]
- 48.Rienstra M, Van Veldhuisen DJ, Hagens VE, Ranchor AV, Veeger NJ, Crijns HJ, Van Gelder IC, Investigators R. Gender-related differences in rhythm control treatment in persistent atrial fibrillation: data of the Rate Control Versus Electrical Cardioversion (RACE) study. J Am Coll Cardiol. 2005;46(7):1298–1306. [DOI] [PubMed] [Google Scholar]
- 49.Kerr CR, Humphries K. Gender-related differences in atrial fibrillation. J Am Coll Cardiol. 2005;46(7):1307–1308. [DOI] [PubMed] [Google Scholar]
- 50.Olsson LG, Swedberg K, Ducharme A, Granger CB, Michelson EL, McMurray JJ, Puu M, Yusuf S, Pfeffer MA, Investigators C. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J Am Coll Cardiol. 2006;47(10):1997–2004. [DOI] [PubMed] [Google Scholar]
- 51.Linssen GC, Rienstra M, Jaarsma T, Voors AA, van Gelder IC, Hillege HL, van Veldhuisen DJ. Clinical and prognostic effects of atrial fibrillation in heart failure patients with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail. 2011;13(10):1111–1120. [DOI] [PubMed] [Google Scholar]
- 52.Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8(2):295–303. [DOI] [PubMed] [Google Scholar]
- 53.Lam CS, Rienstra M, Tay WT, Liu LC, Hummel YM, van der Meer P, de Boer RA, Van Gelder IC, van Veldhuisen DJ, Voors AA, Hoendermis ES. Atrial Fibrillation in Heart Failure With Preserved Ejection Fraction: Association With Exercise Capacity, Left Ventricular Filling Pressures, Natriuretic Peptides, and Left Atrial Volume. JACC Heart Fail. 2017;5(2):92–98. [DOI] [PubMed] [Google Scholar]
- 54.Pant S, Patel NJ, Deshmukh A, Golwala H, Patel N, Badheka A, Hirsch GA, Mehta JL. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol. 2015;65(19):2070–2076. [DOI] [PubMed] [Google Scholar]
- 55.Li Q, Glynn RJ, Dreyer NA, Liu J, Mogun H, Setoguchi S. Validity of claims-based definitions of left ventricular systolic dysfunction in Medicare patients. Pharmacoepidemiol Drug Saf. 2011;20(7):700–708. [DOI] [PubMed] [Google Scholar]
- 56.Patel NJ, Deshmukh A, Pant S, Singh V, Patel N, Arora S, Shah N, Chothani A, Savani GT, Mehta K, Parikh V, Rathod A, Badheka AO, Lafferty J, Kowalski M, Mehta JL, Mitrani RD, Viles-Gonzalez JF, Paydak H. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation. 2014;129(23):2371–2379. [DOI] [PubMed] [Google Scholar]
- 57.Mayer-Schonberger V Big Data for cardiology: novel discovery? Eur Heart J. 2016;37(12):996–1001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


