Abstract
Objective: To describe real-world outcomes for youth using the Tandem t:slim X2 insulin pump with Control-IQ technology (“Control-IQ”) for 6 months at a large pediatric clinic.
Methods: Youth with type 1 diabetes, who started Control-IQ for routine care, were prospectively followed. Data on system use and glycemic control were collected before Control-IQ start, and at 1, 3, and 6 months after start. Mixed models assessed change across time; interactions with baseline hemoglobin A1c (HbA1c) and age were tested.
Results: In 191 youth (median age 14, 47% female, and median HbA1c 7.6%), percent time with glucose levels 70–180 mg/dL (time-in-range [TIR]) improved from 57% at baseline to 66% at 6 months (P < 0.001). The proportion of participants reaching TIR target (>70%) doubled from 23.5% at baseline to 47.8% at 3 months, sustaining at 46.7% at 6 months (P < 0.001). Glucose management indicator (approximation of HbA1c) improved from 7.5% at baseline to 7.1% at 3 months and 7.2% at 6 months (P < 0.001). Those with higher baseline HbA1c experienced the most substantial improvements in glycemic control. Percent time using the Control-IQ feature was 86.4% at 6 months, and <4% of cohort discontinued use.
Conclusion: The Control-IQ system clinically and significantly improved glycemic control in a large sample of youth. System use was high at 6 months, with only a small proportion discontinuing use, indicating potential for sustaining results long term.
Keywords: Automated insulin delivery, Hybrid closed loop, Type 1 diabetes, Pediatrics
Introduction
Type 1 diabetes (T1D) is the most common form of diabetes in youth,1 affecting over 1.25 million people in the United States, and incidence is increasing yearly.1–5 To reduce the risk of morbidity and mortality from T1D, the American Diabetes Association (ADA) now recommends children and adults with T1D target a hemoglobin A1c (HbA1c) of <7%,6,7 lower than the previous recommendation of 7.5%. However, only 21% of adults and less than 17% of children meet this target.8,9 In addition to HbA1c metrics, both the ADA and international consensus guidelines have recommended that children and adults with T1D aim for >70% time spent in glucose range 70–180 mg/dL, known as time-in-range (TIR), which correlates with an HbA1c of 7%.10 To help achieve these goals, the ADA Standards of Medical Care In Diabetes recommends that children with T1D use an intensive insulin regimen (such as multiple daily injections or insulin pump) and frequent glucose monitoring (by fingerstick or use of a continuous glucose monitor [CGM]), and further suggests automated insulin delivery to improve glycemic control.6 Automated insulin delivery currently includes hybrid closed-loop systems, which consist of an insulin pump, an integrated CGM, and an algorithm to dynamically increase or decrease programmed insulin delivery to keep glucose levels in a target range. Current systems still require the user to deliver boluses of insulin for carbohydrate consumption and hyperglycemia.
The most recently available hybrid closed-loop system in the United States is the Tandem t:slim X2 insulin pump with Control-IQ technology (Tandem Diabetes Care, San Diego, CA; referred to in this study as “Control-IQ”), which has been approved by the Food and Drug Administration for ages 6 years and older.11 The Control-IQ system consists of an X2 insulin pump, an integrated Dexcom G6 CGM, and an embedded “Control-IQ” algorithm. The algorithm predicts glucose levels 30 min into the future and then increases or decreases programmed basal insulin rates to maximize glucose levels between 112.5 and 160 mg/dL. The algorithm considers current CGM glucose readings, glucose trend, and amount of insulin already administered by the pump (insulin on board). In addition, the system delivers automatic correction boluses of insulin when glucose levels are predicted to rise above 180 mg/dL.
Control-IQ users additionally have the option to program a “Sleep Activity” setting overnight, which narrows the algorithm target range to 112.5–120 mg/dL, and an “Exercise Activity,” which raises the algorithm target to 140–160 mg/dL to reduce risk of hypoglycemia during exercise. The Control-IQ algorithm remains active as long as the pump is receiving CGM sensor data or until the user chooses to turn off the feature. When not active, the user receives insulin based on the manually programmed pump settings.
In the pivotal randomized control trial of the Control-IQ system, adolescents and adults using the system increased their mean TIR (70–180 mg/dL) from 61% at baseline to 71% in 6 months,12 and youth 6–14 years improved from 53% at baseline to 67% over a 16-week period.13 Commercially, Control-IQ became available during the first quarter of 2020, so real-world evidence is now emerging about glycemic outcomes and system use in the real world. The purpose of this study is to describe these real-world outcomes in youth and young adults with T1D using the Control-IQ system, characterizing glycemic control and system use over a 6-month period.
Methods
Study design and participants
This was a prospective observational study of youth using the Control-IQ system for routine diabetes management at a large pediatric diabetes clinic. Young adults and youth receiving care in our clinic, and their caregivers, were invited to participate if they were prescribed the Control-IQ system between January 2020 and November 2020; they were able to complete online surveys written in English. Training on the system was provided as per clinical protocols (not part of the study) by the device manufacturer, either by online training modules or with a certified trainer. Additional clinical follow-up occurred at our center by diabetes educators during the first few weeks of use, including a structured phone contact with initial data review and assessment.14
Study participation involved allowing research staff to record glycemic and system use information from device downloads for research purposes, and completing online surveys at baseline, 3 months, and 6 months (survey data not yet published). The Colorado Multiple Institutional Review Board approved this study; young adults and caregivers provided informed consent, and youth provided assent.
Measures
Participants' age, sex, race/ethnicity, insurance status, and previous insulin delivery method were obtained from the electronic health record at study enrollment. Glycemic and system use measures were collected from commercial Control-IQ download software (https://tconnecthcp.tandemdiabetes.com) or Tidepool (www.Tidepool.org) for 2-week periods at baseline and 1, 3, and 6 months when available from clinic downloads. Device use measures included percent time using the Control-IQ hybrid closed-loop automation, percent time wearing CGM, total daily dose of insulin, number of meal boluses given per day, average number of hours per night using Sleep Activity, and average number of uses of Exercise Activity. Blood glucose meter information was not obtained, as users could use CGM glucose values for insulin dosing and diabetes management decisions.15
For the 6-month timepoint, the number of high and low sensor glucose alerts was tabulated. Glycemic data included percent time CGM sensor glucose values <54 mg/dL (“Level 2” hypoglycemia), <70 mg/dL (“Level 1” hypoglycemia), 70–180 (TIR), >180 (“Level 1” hyperglycemia), and >250 mg/dL (“Level 2” hyperglycemia), mean sensor glucose, and standard deviation as per international consensus guidelines.10 We also calculated a Composite Glucose Index (COGI) for each timepoint, an index that characterizes glucose profiles from 0 to 100 based on TIR, percent time <70 mg/dL, and standard deviation.16
Due to the coronavirus disease 2019 (COVID-19) pandemic that occurred during the 2020 calendar year, in-person clinic visits were severely restricted, making HbA1c data difficult to obtain. For baseline HbA1c stratification, HbA1c levels were included if they were obtained up to 90 days before or up to 7 days after starting Control-IQ. Glucose management indicator (GMI), an approximation of HbA1c, was calculated for each timepoint based on mean sensor glucose value for the past 2 weeks of data (https://www.jaeb.org/gmi/) and was used as a proxy measurement for overall glucose control comparison across timepoints.17
Statistical analysis
Descriptive statistics reported include counts and percentages, means and standard deviations, median and interquartile range, and percentiles. Mixed-effects models were used to examine changes in the outcomes over time. Least-squares means and standard errors were used to report the results of mixed-effects models. For variables such as CGM measures that were recorded at baseline, the baseline visit was used as the comparison to months 3 and 6. For Control-IQ variables, month 1 was used as the comparison to months 3 and 6. Pairwise comparisons of means at each visit were adjusted for multiple testing using a Tukey adjustment. In addition, models with an interaction between time and baseline age (≤13, 14–17, and ≥18 years) or HbA1c (≤7%, 7%–9%, and ≥9%) were used to determine if the changes in outcomes over time differed by group. R (R Core Team, Vienna) software was used for all analyses.
Results
A total of 201 youth started the Control-IQ system and enrolled in the study. Five participants were lost to follow-up after 1 month, and five additional participants discontinued using the Control-IQ system by 3 months. The remaining 191 participants were included in the analysis [median age 14 years,10,16 HbA1c 7.6% (6.9 and 8.4), 47.4% female, 87.8% non-Hispanic white, and 95.8% previous insulin pump users] (Table 1). There were two episodes of diabetic ketoacidosis during the study duration, one due to viral gastroenteritis and one due to a likely infusion set failure. There was no severe hypoglycemic event reported in the medical record.
Table 1.
Baseline Characteristics of Control-IQ Users
| Strata | Result | |
|---|---|---|
| n | 191 | |
| Age, years (median [IQR]) | 14.0 [10.0, 16.0] | |
| Age category [n, (%)] | ≤13 years | 92 (48.4) |
| 14–17 years | 67 (35.5) | |
| 18+ years | 31 (16.3) | |
| HbA1c, % (median [IQR])a | 7.6 [6.9, 8.4] | |
| HbA1c category [n, (%)]a | ≤7% | 38 (29.5) |
| >7% to <9% | 71 (55.0) | |
| ≥9% | 20 (15.5) | |
| Sex [n, (%)] | Female | 90 (47.4) |
| Male | 100 (52.6) | |
| Ethnicity [n, (%)] | Hispanic or Latino | 6 (3.2) |
| Not Hispanic or Latino | 166 (87.8) | |
| Unknown/not reported | 17 (9.0) | |
| Race [n, (%)] | White | 166 (87.8) |
| Black or African American | 2 (1.1) | |
| More than one race | 6 (3.2) | |
| Unknown/not reported | 15 (7.9) | |
| Insurance status (%) | Public | 19 (10.1) |
| Private | 151 (80.3) | |
| Other | 18 (9.6) | |
| Uninsured | 0 (0.0) | |
| Prior insulin | Multiple daily injections | 8 (4.2) |
| Tandem pump | 177 (92.7) | |
| Other insulin pump | 6 (3.1) |
Thirty-two percent were missing due to lack of HbA1c data available due to COVID-19 pandemic.
COVID-19, coronavirus disease 2019; HbA1c, hemoglobin A1c; IQR, interquartile range.
System-use outcomes
CGM sensor wear improved from 85.0% at baseline to 90.3% at 3 months (post hoc P = 0.003) and sustained at 90.0% at 6 months (P = 0.97) (Table 2). Sleep Activity feature usage increased throughout the first 6 months, meaning Sleep Activity was used for more hours per night, on average, across time (P < 0.001). Sleep Activity was used more than 15 h a day in 3.3% of users at 3 months and 4.8% of users at 6 months. The use of the Exercise Activity decreased over time (P = 0.001). The percent of time youth used the Control-IQ automation remained high (≥86%) across all time points (P = 0.16). Furthermore, the number of meal boluses remained unchanged (P = 0.07), although there was a trend for a decrease between baseline and 6 months (post hoc P = 0.05). At 6 months, participants experienced an average of 3.0 high glucose alerts and 1.2 low glucose alerts each day.
Table 2.
Use of the Control-IQ System and Glycemic Outcomes Over a 6-Month Period
| Strata | Baseline | 3 months | 6 months | P a | |
|---|---|---|---|---|---|
| % Time using Control-IQ automation [%, (SE)] | 88.3 (1.2)b | 86.0 (1.2) | 86.4 (1.3) | 0.16 | |
| % Time continuous glucose monitor use [%, (SE)] | 85.0 (1.4) | 90.3 (1.4) | 90.0 (1.4) | <0.002 | |
| Total daily dose [units, (SE)] | 47.0 (2.0) | 49.9 (1.9) | 50.4 (2.0) | <0.001 | |
| Number of meal boluses/day [#, (SE)] | 4.4 (0.2) | 4.2 (0.2) | 4.0 (0.2) | 0.07 | |
| Sleep activity use [# hours/night, (SE)] | 6.6 (0.3)b | 7.8 (0.3) | 8.4 (0.3) | <0.001 | |
| Exercise activity use [# uses/week (SE)] | 1.0 (0.1)b | 0.8 (0.1) | 0.6 (0.1) | 0.001 | |
| % TIR 70–180 mg/dL [%, (SE)] | 56.8 (1.2) | 68.0 (1.2) | 66.2 (1.2) | <0.001 | |
| Baseline HbA1c ≤7% | 73.1 (2.1) | 75.7 (2.1) | 76.1 (2.2) | <0.003 | |
| Baseline HbA1c >7% to <9% | 51.2 (1.6) | 66.1 (1.5) | 62.2 (1.6) | <0.001 | |
| Baseline HbA1c ≥9% | 38.8 (3.2) | 52.6 (2.8) | 51.6 (3.2) | <0.001 | |
| GMI [%, (SE)] | 7.5 (0.1) | 7.2 (0.1) | 7.2 (0.1) | <0.001 | |
| Baseline HbA1c ≤7% | 6.8 (0.1) | 6.8 (0.1) | 6.7 (0.10) | 1.0 | |
| Baseline HbA1c >7% to <9% | 7.7 (0.1) | 7.3 (0.1) | 7.4 (0.1) | <0.001 | |
| Baseline HbA1c ≥9% | 8.5 (0.1) | 7.9 (0.1) | 7.9 (0.1) | <0.001 | |
| Average glucose [mg/dL, (SE)] | 175.0 (2.2) | 160.5 (2.2) | 162.5 (2.3) | <0.001 | |
| COGI [COGI, (SE)] | 65.4 (0.73) | 73.2 (0.71) | 72.1 (0.75) | <0.001 | |
| Baseline HbA1c ≤7% | 73.4 (1.4) | 77.6 (1.4) | 76.3 (1.5) | 0.37 | |
| Baseline HbA1c >7% to <9% | 62.0 (1.0) | 72.0 (1.0) | 70.0 (1.1) | <0.001 | |
| Baseline HbA1c ≥9% | 57.5 (2.2) | 65.9 (1.9) | 64.2 (2.2) | <0.001 | |
| Glucose <70 mg/dL [%, (SE)] | 2.2 (0.16) | 1.8 (0.2) | 1.8 (0.2) | 0.01 | |
| Glucose <54 mg/dL [%, (SE)] | 0.4 (0.1) | 0.4 (0.1) | 0.4 (0.1) | 0.90 | |
| Glucose >180 mg/dL [%, (SE)] | 41.8 (1.2) | 31.7 (1.2) | 34.0 (1.3) | <0.001 | |
| Glucose >250 mg/dL [%, (SE)] | 17.3 (1.0) | 12.6 (0.9) | 13.0 (1.0) | <0.001 | |
| Glucose standard deviation [mean, (SE)] | 65.3 (1.2) | 57.9 (1.2) | 58.7 (1.2) | <0.001 |
P value is for change over time. In post hoc analysis, all significant changes were between baseline and 3 months. There were no significant changes between 3 and 6 months.
For Control-IQ system specific features, month 1 was considered baseline data.
COGI, Composite Glucose Index; GMI, glucose management indicator; SE, standard error; TIR, time-in-range.
A total of seven participants (3.5%) discontinued using the Control-IQ automation by 6 months (5 by 3 months and 2 by 6 months): 1 stopped using an insulin pump altogether, but continued using the CGM, 1 returned to using a different insulin pump, and 5 continued-use of the Tandem insulin pump but discontinued using the CGM (which disables the Control-IQ automation). User-cited reasons for discontinuing Control-IQ were difficulty using the CGM (including skin irritation, connectivity issues between CGM and pump, and general discomfort/dissatisfaction) and dissatisfaction with glycemic control when using the Control-IQ automation.
Three participants had intermittent use of Control-IQ automation, meaning they were not using it at the 3-month timepoint, but were using it at 6 months. Reasons for intermittent use of the Control-IQ automation were inconsistent CGM use (not using CGM at 3 months but using CGM at 6 months) and confusion on how to use Control-IQ (e.g., using CGM, but CGM not connected to the insulin pump, so the Control-IQ automation could not engage). Finally, Control-IQ automation use was unknown for 12 participants at 6 months due to available pump reports not displaying the percent Control-IQ use.
Glycemic outcomes
TIR (70–180 mg/dL) significantly increased from 57.0% at baseline to 68.1% at 3 months (an improvement of 2 h 40 min per day) and sustained at 66.2% at 6 months (P < 0.001) (Table 2). For every 1% use of the Control-IQ automation, TIR increased by 0.18% (P < 0.001). The number of meal boluses positively associated with TIR (P < 0.001). The number of low glucose alerts positively correlated to TIR, while the number of high glucose alerts negatively correlated to TIR (r = 0.51 and r = −0.63, respectively; P < 0.001 for both). The proportion of participants reaching goal TIR of >70% doubled from 23.5% at baseline to 47.8% at 3 months, sustaining at 46.7% at 6 months (P < 0.001). GMI also improved from 7.5% at baseline to 7.1% at 3months and sustained at 7.2% at 6 months (P < 0.001). Similarly, the proportion of participants reaching GMI goal of <7% increased from 26.5% (baseline) to 43.9% and 41.1% at 3 and 6 months. respectively (P < 0.001). COGI increased over time (P < 0.001).
Time spent with glucose levels <70 mg/dL (hypoglycemia) decreased from baseline to 3 and 6 months (P = 0.01), although was at or below the target goal of <4% hypoglycemia7,10 across all timepoints. There was no change in time <54 mg/dL (serious hypoglycemia), which was less than 1% for all timepoints, and at target goal. These trends in percent TIR, percent <70 mg/dL, and GMI were similar across all ages and were not different by age strata (P = 0.37).
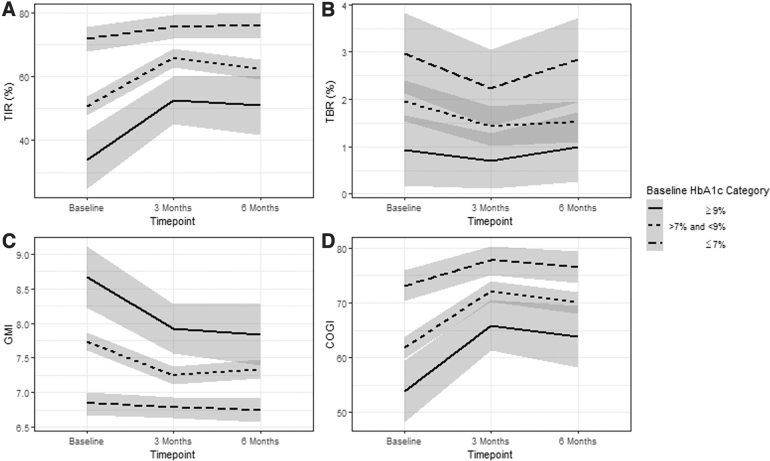
The percent TIR was significantly different by baseline HbA1c strata (P < 0.001), although all strata demonstrated improvement in TIR (Fig. 1). Those with baseline HbA1c levels ≥9% had the largest improvement in TIR over 6 months at 13.8% increase (absolute) or 35.6% relative increase. The GMI was significantly different by baseline HbA1c strata (P < 0.001) with the high and middle HbA1c groups improving GMI by 6 months, but the <7% HbA1c group remaining the same (P = 1.0). Similarly, COGI was significantly different by baseline HbA1c strata (P = 0.007), with the high and middle HbA1c groups improving COGI by 6 months, but the <7% HbA1c group not significantly improving (P = 0.37). There was no difference in change across time for time spent in hypoglycemia by baseline HbA1c.
FIG. 1.
Change in TIR 70–180 mg/dL (A), TBR (B), GMI (C), and COGI (D) across time stratified by baseline HbA1c (95% confidence interval shaded). COGI, Composite Glucose Index; GMI, glucose management indicator; HbA1c, hemoglobin A1c; TBR, time below range; TIR, time-in-range.
Discussion
This is the first study to report on improvement in glycemic TIR for youth and young adults using the Control-IQ hybrid closed-loop system in the real world for management of T1D. System use remained very high throughout the first 6 months, indicating the potential for sustainability of therapy and sustained glycemic control.
It is noteworthy that the glycemic outcomes in this observational study are comparable to glycemic outcomes reported in rigorously controlled clinical trials of hybrid closed-loop systems. In this study, youth achieved a mean TIR of 66% at 6 months, which is similar to the clinical trials of Control-IQ in youth ages 6–13 years (TIR = 67%),13 the MiniMed 670G in adolescents ages 14 to 21 years (TIR = 67%),18 and the 670G in ages 7–13 years (TIR = 65%).19 Typically, real-world results are more attenuated than in controlled trials, whereas in this case, use of the Control-IQ system yielded similar outcomes in a less controlled environment and broader aged population of youth. These are encouraging early data to support the idea that the system may perform as well in clinical practice as it did in clinical trials.
TIR improved in this cohort by 9%, which is similar to the 10% TIR increase reported in the adolescent/adult pivotal trial for Control-IQ,12 although less than the 14% TIR improvement in the youth trial (ages 6–13 years old).13 An improvement in TIR of 10% is often considered clinically significant, associated with an HbA1c drop of 0.5%–0.8%.10 A TIR improvement of 5% could also be considered a substantial improvement, equating to an additional 1 h per day spent in target range. The improvement in TIR for this study was primarily due to reduced exposure to hyperglycemia, which is important for reducing risk of long-term macrovascular and microvascular complications.
Time spent <70 mg/dL was reduced significantly, although time spent <54 mg/dL was unchanged (though meeting clinical targets). It is possible that the Control-IQ system's basal attenuation was able to gently reverse mild hypoglycemia, while unable to compensate for steeper declines in glucose levels that led to lower nadir glucose, possibly from insulin delivered by bolus. The GMI and COGI composite metric also improved from baseline to 3 months, except for those with baseline HbA1c ≤7%, indicating a favorable weighting of TIR, hypoglycemia, and standard deviation with use of Control-IQ. Those who were already meeting HbA1c targets at baseline did not demonstrate worsening GMI or COGI.
Control-IQ is one of two hybrid closed-loop systems available in the United States for youth and adults with T1D. The MiniMed 670G was the first system, commercially available in 2016. Our group previously reported that our real-world youth cohort achieved 56.9% TIR after 6 months of 670G use,20 which is less than the 66% achieved in this real-world study of Control-IQ. The reason for the difference is likely not the efficacy of the hybrid closed-loop algorithms in each device, which have demonstrated similar glycemic results in clinical trials.13,19 It is more likely due to adherence to using the hybrid closed-loop automation in each system.
Real-world studies of the MiniMed 670G report relatively low time (44%–76% at 6 months) using “Auto Mode,” the hybrid closed-loop automation feature.20–23 Our study found a higher average use of the Control-IQ automation at 86%. Furthermore, higher discontinuation rates have been reported for the 670G system at 6 months (30% in youth24 and 34% in a mixed youth and adult sample,21 whereas the discontinuation of Control-IQ in this cohort was only 3.5%).
One likely explanation for these adherence differences is the relative task burden needed to maintain hybrid closed loop—the 670G system requires the user to perform fingerstick blood glucose checks 2–6 times/day for sensor calibration and for hybrid closed-loop use and can exit the user from “Auto Mode” if these requests are ignored and for other reasons. The Control-IQ system does not require fingersticks in routine use and does not exit the user from Control-IQ automation unless the user chooses to turn the feature off or the CGM is discontinued. The amount of work and troubleshooting that a device requires is a known barrier to device use in both adolescents25 and adults,26 and plays an important role in continued use.
In clinical care, use of diabetes technologies is associated with better glycemic outcomes. In a recent T1D Exchange Registry report, youth using CGM achieved mean HbA1c of 7.9%–8.3%, and insulin pump users achieved mean HbA1c of 8.0%–8.8% (depending on age).9 Although HbA1cs were not collected in this study due to the COVID-19 pandemic, the equivalent median GMI was 7.1%, substantially lower (better) than the Registry data, and closer to the ADA goal of <7%. The TIR achieved in this study at 6 months was 66%, which approaches the clinical goal for TIR of >70%.7,10 These encouraging glycemic achievements may indicate that hybrid closed-loop technologies enable youth to achieve better outcomes than those who use an insulin pump or CGM alone, although larger registry analyses would be needed to confirm this.
In addition to hybrid closed-loop use being very high, the mean use of the Sleep Activity feature increased over time. The interpretation is difficult, however, because it is unclear if the mean number of hours of use was due to the Sleep Activity being set for longer durations, or if it was turned on for more nights of use. Our clinicians have observed that some Control-IQ users accidentally set the Sleep Activity for one night of the week, when intending to set it for all nights. Careful education around how to set this feature may be important. Furthermore, <5% of the cohort used the feature >15 h a day. Anecdotally, all-day use of Sleep Activity is used as a strategy to improve TIR by lowering the target throughout the day but was not utilized in a large percentage of our participants.
Regardless of device choice, self-management behaviors remain important considerations for glycemic improvement. The number of user-initiated meal insulin boluses in this study slightly decreased from 4.4 to 4 average boluses/day from baseline to 6 months. This did not reach statistical significance in the overall model; however, there was a trend for decrease between baseline and 6 months (post hoc P = 0.05). This is important to evaluate in longer studies, as the number of boluses is strongly associated with glycemic control,27,28 and a decrease in bolusing could indicate hybrid closed-loop users may get complacent about bolusing over time.
It was not possible to evaluate the mean number of hyperglycemia correction boluses (insulin administered specifically for a high blood glucose) in this analysis, because commercially available reports for Control-IQ do not distinguish between the automated hyperglycemia correction boluses and user-initiated correction boluses on composite reports, and only show the information on the daily view, which was not analyzed. This would be an important addition to future iterations of hybrid closed-loop software, as user behavior around hyperglycemia is important for understanding overall diabetes self-management practices. Furthermore, the number of total boluses was not analyzed, as auto-corrections are a part of the modular Control-IQ hyperglycemia mitigation strategy and do not contribute further understanding of user behavior nor complete algorithm response to hyperglycemia.
Historically, studies also evaluate the number of fingerstick blood glucose measurements as an important self-management behavior. This was not relevant in this study due to the use of the Dexcom G6 CGM, which does not require fingerstick measurements for calibration or routine diabetes management. The percent time using CGM may be considered an alternative measure of adherence, and CGM use was high at every time point, although significantly improved over 6 months in the full model. Since hybrid closed-loop automation relies on CGM use, this adherence metric is especially important for these systems.
Strengths of this study include the large sample of youth and young adults who started Control-IQ, and longitudinal data over a 6-month period. A large number of glycemic and diabetes management variables were assessed to draw meaningful conclusions about real-world use. There are limitations to this study as well. Most participants were non-Hispanic white with private insurance, seen in a tertiary diabetes center, limiting the generalizability of the results, although commensurate with national demographic registry profiles of T1D.9 Furthermore, earlier adoption of technology has been associated with higher socioeconomic status.29 The participants in this study already had well-controlled diabetes relative to the general clinic population (registry data indicating average HbA1c in children being 8.1%–9.3%).9 In addition, the majority of participants were already using insulin pumps and CGMs before starting Control-IQ, so it is unknown if the levels of adherence to the system and glycemic outcomes would be achievable to individuals less familiar with diabetes technology. Finally, in the time of the COVID-19 pandemic, HbA1c levels were not obtainable, and GMI (calculated from mean glucose level for the past 2 weeks) was used as a proxy.
Conclusion
Youth and young adults with T1D using a new hybrid closed-loop system improved glycemic control in the first 3 months compared to baseline, and this improvement was sustained through 6 months. High use of the system and significant glycemic improvements indicate the potential for long-term sustainability, reducing overall morbidity and mortality. Further longitudinal studies are needed to test this hypothesis.
Acknowledgments
We would like to thank the PANTHER (Practical Advanced Therapies for Diabetes) research team, including Estella Escobar, Angela Karami, and Lindsey Towers for recruitment and data collection for this study. We also thank the additional PANTHER investigators for their support and help, including Dr. Robert Slover and Dr. Paul Wadwa. Finally, we thank our amazing research participants at the Barbara Davis Center, for sharing their experience with our clinical and research teams.
Authors' Contributions
Dr. L.H.M. conceptualized and designed the study, interpreted the data, drafted the initial article, and reviewed and revised the article. Ms. C.B. conceptualized and designed the study, verified data acquisition, interpreted the data, and reviewed and revised the article. Mr. T.V. and Dr. L.P. designed the study, performed data analysis and interpretation, and reviewed and revised the article. Dr. E.C. contributed to study design, interpreted the data, and reviewed and revised the article. Dr. K.A.D. and Dr. G.P.F. designed the study, interpreted the data, and reviewed and revised the article. All authors approved the final article as submitted and agree to be accountable for all aspects of the work.
Author Disclosure Statement
L.H.M. has received speaking/consulting honoraria from Tandem Diabetes, Dexcom, and Capillary Biomedical, and is a certified trainer for Medtronic Minimed. G.P.F. has received speaking consulting honoraria from Tandem Diabetes, Dexcom, Medtronic, Insulet, Abbott, Lilly, and Beta Bionics. His institution receives research/project grants from Medtronic, Tandem Diabetes, Insulet, Dexcom, Abbott, Lilly, and Beta Bionics. C.B., L.P., T.V., E.C., and K.D. have nothing to disclose.
Funding Information
JDRF early career patient-oriented diabetes research award (5-ECR-2019-736-A-N) awarded to Dr. G.P.F.
References
- 1. Mayer-Davis EJ, Dabelea D, Lawrence JM: Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med 2017;377:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patterson CC, Gyürüs E, Rosenbauer J, et al. : Trends in childhood type 1 diabetes incidence in Europe during 1989–2008: evidence of non-uniformity over time in rates of increase. Diabetologia 2012;55:2142–2147. [DOI] [PubMed] [Google Scholar]
- 3. Patterson C, Guariguata L, Dahlquist G, et al. : Diabetes in the young—a global view and worldwide estimates of numbers of children with type 1 diabetes. Diabetes Res Clin Pract 2014;103:161–175. [DOI] [PubMed] [Google Scholar]
- 4. Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ: Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am 2010;39:481–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DIAMOND Project Group: Incidence and trends of childhood type 1 diabetes worldwide 1990–1999. Diabet Med 2006;23:857–866. [DOI] [PubMed] [Google Scholar]
- 6. American Diabetes Association: Children and adolescents: standards of medical care in diabetes 2021. Diabetes Care 2021;44(Suppl 1):S180–S199. [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association: Glycemic targets: standards of medical care in diabetes 2021. Diabetes Care 2021;44(Suppl 1):S73–S84. [DOI] [PubMed] [Google Scholar]
- 8. Miller KM, Foster NC, Beck RW, et al. : Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care 2015;38:971–978. [DOI] [PubMed] [Google Scholar]
- 9. Foster NC, Beck RW, Miller KM, et al. : State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther 2019;21:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Battelino T, Danne T, Bergenstal RM, et al. : Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019;42:1593–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. U.S. Food and Drug Administration: FDA authorizes first interoperable, automated insulin dosing controller designed to allow more choices for patients looking to customize their individual diabetes management device system 2019. https://www.fda.gov/news-events/press-announcements/fda-authorizes-first-interoperable-automated-insulin-dosing-controller-designed-allow-more-choices#:~:text=The%20U.S.%20Food%20and%20Drug,insulin%20pump%20(ACE%20pump)%20and. Accessed March 11, 2021.
- 12. Brown SA, Kovatchev BP, Raghinaru D, et al. : Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Breton MD, Kanapka LG, Beck RW, et al. : A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med 2020;383:836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Messer LH, Berget C, Ernst A, et al. : Initiating hybrid closed loop: a program evaluation of an educator-led control-IQ follow-up at a large pediatric clinic. Pediatr Diabetes 2021;22:586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. U.S. Food and Drug Administration: FDA expands indication for continuous glucose monitoring system, first to replace fingerstick testing for diabetes treatment decisions 2016. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm534056.htm. Accessed March 11, 2021.
- 16. Leelarathna L, Thabit H, Wilinska ME, et al. : Evaluating glucose control with a novel composite continuous glucose monitoring index. J Diabetes Sci Technol 2020;14:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bergenstal RM, Beck RW, Close KL, et al. : Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care 2018;41:2275–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garg SK, Weinzimer SA, Tamborlane WV, et al. : Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2017;19:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Forlenza GP, Pinhas-Hamiel O, Liljenquist DR, et al. : Safety evaluation of the MiniMed 670G system in children 7–13 years of age with type 1 diabetes. Diabetes Technol Ther 2019;21:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berget C, Messer LH, Vigers T, et al. : Six months of hybrid closed loop in the real-world: an evaluation of children and young adults using the 670G system. Pediatr Diabetes 2020;21:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lal RA, Basina M, Maahs DM, et al. : One year clinical experience of the first commercial hybrid closed-loop. Diabetes Care 2019;42:2190–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Akturk HK, Giordano D, Champakanath A, et al. : Long-term real-life glycaemic outcomes with a hybrid closed-loop system compared with sensor-augmented pump therapy in patients with type 1 diabetes. Diabetes Obes Metab 2020;22:583–589. [DOI] [PubMed] [Google Scholar]
- 23. Duffus SH, Ta'ani ZA, Slaughter JC, et al. : Increased proportion of time in hybrid closed-loop “Auto Mode” is associated with improved glycaemic control for adolescent and young patients with adult type 1 diabetes using the MiniMed 670G insulin pump. Diabetes Obes Metab 2020;22:688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Messer LH, Berget C, Vigers T, et al. : Real world hybrid closed-loop discontinuation: predictors and perceptions of youth discontinuing the 670G system in the first 6months. Pediatr Diabetes 2020;21:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Messer LH, Tanenbaum ML, Cook PF, et al. : Cost, hassle, and on-body experience: barriers to diabetes device use in adolescents and potential intervention targets. Diabetes Technol Ther 2020;22:760–767. [DOI] [PubMed] [Google Scholar]
- 26. Tanenbaum ML, Hanes SJ, Miller KM, et al. : Diabetes device use in adults with type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes Care 2017;40:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patton SR, Driscoll KA, Clements MA: Adherence to insulin pump behaviors in young children with type 1 diabetes mellitus. J Diabetes Sci Technol 2017;11:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clements MA, DeLurgio SA, Williams DD, et al. : Association of HbA1c to BOLUS scores among youths with type 1 diabetes. Diabetes Technol Ther 2016;18:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weiss D, Sund ER, Freese J, Krokstad S: The diffusion of innovative diabetes technologies as a fundamental cause of social inequalities in health. The Nord-Trøndelag Health Study, Norway. Sociol Health Illn 2020;42:1548–1565. [DOI] [PubMed] [Google Scholar]