Abstract
Man is increasingly being faced with many health conditions, including viral infection, some of which increases the risk to cancer. These infectious agents contribute to the large number of persons with cancer and the worrisome number that die from the diseases. A good range of drugs are currently in place for treating patients infected with viruses, however, some of the drugs' effectiveness are limited by the emergence of drug-resistant strains of the viruses, as well as adverse effects of the drugs. Similarly, the inability of many anticancer drugs to selectively kill cancer cells while sparing hosts’ normal cells limit their use. This warrants more research for newer drugs, especially from chemicals naturally encrypted in plants with anticancer and antiviral activities. In response to infection with cancer-inducing viruses, plants such as Salvia species synthesize and store secondary metabolites to protect themselves and kill these viruses as well as inhibit their ability to induce carcinogenesis. Hence, this review presented a discussion on the potential application of Salvia species in the prevention and management of cancer and viral infection. The study also discusses the cellular mechanisms of action of these herbal products against cancer cells and viruses, where available and provided suggestions on future research directions. The study is believed to spur more research on how to exploit Salvia phytochemicals as candidates for the development of nutraceuticals and drugs for managing cancers and viral infection.
Keywords: Salvia species, Anticancer, Antiviral, Phytochemicals, Nutraceuticals
Salvia species; Anticancer; Antiviral; Phytochemicals; Nutraceuticals.
1. Introduction
The human cells divide into daughter cells that develop into functional cells and when aged or damaged beyond repair, cells are signaled to undergo apoptosis - programmed cell death. However, there are situation where mutations in some genes of cells render them resistant to normal death programming, making them cancerous. Several factors such as exposure to large doses of radiation, some viruses and other carcinogens induce the loss of native cellular programming, leading to the loss of contact inhibition, and acquisition of cellular immortality, ability to metastasize and undergo angiogenesis, among others [1].
Cancer generally represents a group of dangerous diseases that are leading cause of death globally. In 2020, cancer contributed to about ten million deaths [2], while an estimate of 19 million new cancer cases were diagnosed [3]. The incidence of cancer is increasing globally, both in developed and developing regions of the world due to factors like a rise in the aging population, adoption of sedentary lifestyle, poor diet and others. At present, cancers are managed using radiation and chemical therapies, and surgeries, or a combination of them. Although some of these approaches, especially radiotherapy and surgeries are still clinically useful, they cause serious discomfort to cancer patients. Similarly, many anticancer drugs have serious side effects while some cancer cells have developed resistance to some anticancer drugs [4, 5]. Hence, there is a dire need for clinically-effective drugs that are selectively toxic to cancer cells while sparing normal cells.
Natural products derived from plants, animal protein-originated peptides and marine organisms are gaining scientific attention as accumulating literature are positioning them as potential sources of new anticancer agents [6]. In folkloric practices, plants such as Salvia spp., Zanthoxylum spp., Allium spp., and Zingiber spp. among others are applied in treating many kinds of tumors and cancers [6, 7, 8, 9, 10, 11, 12, 13]. Among these plants, species belonging to the genus Salvia appear to stand out due to their reservoir of many chemicals with cytotoxic properties against cancer cells, antimicrobial, anti-inflammatory and antioxidant capabilities [14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25]. The genus Salvia belong to Lamiaceae - a family of mint flowering plant that is composed of over 230 genera and 7000 plant species. Salvia spp. is made up of more than 900 herbaceous and woody plant species found commonly in Eurasia, Central America and in the Mediterranean region. Extracts of Salvia spp. or their phytochemicals have been demonstrated to possess cytotoxic activities against cancer cells while causing minimal damages to normal cells [24, 26, 27].
Viruses such as Epstein–Barr virus [28, 29], human T-cell leukemia virus (HTLV-1), hepatitis C virus (HCV), human papillomavirus (HPV), hepatitis B virus (HBV), kaposi's sarcoma-associated herpesvirus (KSHV)/human herpes virus 8 (HHV8) [30, 31, 32, 33, 34, 35] and SARS-COV-2 virus [36] among others have been estimated to cause about 20% of cancers, including cervical, bladder, head, neck and hepatic among other carcinomas. These viruses alter the stability of host genomes, increase the frequency of carcinogenic mutations while inhibiting the action of tumor suppressor genes and their gene products, as well as increasing tumor survival [37, 38, 39]. Considering the link between viral infection and cancer initiation and progression, this review discusses the potential application of plants in genus Salvia and their phytochemicals in the prevention and treatment of cancer and viral infection. This study adopted literature search strategy of our previous study [13], to retrieve studies published in peer reviewed journals and deposited in PubMed, ScienceDirect and Google Scholar, on antiviral and anticancer activities of plants in genus Salvia. Keywords such as Salvia, Sage, Lamiaceae, phytochemistry, isolated compounds, anticancer salvia species, antitumor salvia species, antiviral salvia species and others were used alone or in combinations for the searches. At first, abstracts were scanned and studies that reported anticancer and antiviral activities of Salvia spp in English language were retrieved, while studies reported in non-English languages as well as those reporting other pharmacological properties different from anticancer and antiviral activities were excluded. The cellular mechanisms of action of the plant extracts and their phytochemicals in relation to cancer and viral infection were comprehensively discussed in this paper. It is our hope that this review will inspire more research and project multifunctional and broad-spectrum phytochemicals derived from Salvia species as good nutraceutical and drug candidates for the prevention and treatment of cancer and viral infections.
2. Application of Salvia species in cancer prevention and management
Extracts of some Salvia spp. or their phytochemicals have demonstrated potentials to inhibit carcinogenesis, proliferation and metastasis of cancer cells, while causing minimal damages to normal cells [24, 27, 40]. This section will discuss the inhibitory roles of Salvia spp. on carcinogenesis, and in proliferation of cancer cells, as well as inhibition of cellular invasion and metastasis of cancer cells, based on findings from cell culture and animal studies.
2.1. Suppression of mutagenesis
Carcinogenesis occurs when a cell acquires malignant traits as a result of DNA damage, and mammals become at risk when exposed to carcinogens. These carcinogens are either ingested/inhaled from the environment or produced endogenously from precursors in consumer products. For example, apart from being ingested/inhaled through meat, water, tobacco smoke, nitrosamines are produced endogenously through the reaction of secondary/tertiary amino compounds with nitrite/nitrate under acidic or iron-rich condition in the mouth or stomach [41, 42, 43, 44]. Metabolic activation of these carcinogens by cytochrome P450 enzymes produce DNA reactive species, which can impair DNA replication, leading to genetic instability and malignancy [45]. Also, free radicals such as reactive oxygen species (ROS), reactive nitrogen species (RNS), and reactive sulfur species (RSS) that are endogenously produced during cellular metabolism, have potentials to cause oxidative damages to cells [46]. Without their detoxification, these reactive species can oxidize membrane lipids to form lipid peroxides, which can break down into malondialdehyde among other products that react with DNA and low density lipoproteins to form adducts (e.g. malondialdehyde-deoxyguanosine and MDA-LDL) which have been implicated in carcinogenesis and cancer progression [47, 48]. Thus, entities that can decrease carcinogens' formation/bioactivation, increase carcinogens’ excretion, prevent DNA damage (via alkylation or oxidation) and/or suppress cell replication (needed for maintenance of the mutated DNA sequences) are expected to prevent or delay carcinogenesis [49].
Some plants in the genus Salvia have been documented to contain some phytochemicals such as vitamin C [50, 51], rosmarinic acid [49, 52], manool [40], chrysin [20, 53], and tanshinones [54, 55] that suppress carcinogenesis. Vitamin C (ascorbic acid), in combination with polyphenols, helps in the prevention/reduction of oxidative damages by acting as an antioxidant and by regenerating other antioxidant molecules (such as β-carotene, α-tocopherol, urate and glutathione) [48]. Interestingly, the intake of vitamin C has been correlated with reduced risks of cancer [56]. Vitamin C has also been credited with the suppression of nitrosamines formation in vitro [42, 57], and increasing the content of its isomer, erythorbic acid, from 250 to 1000 mg/kg sausages, also suppressed the formation of nitrosamines (specifically, nitrosohydroxyproline formation was suppressed by over 70%) in nitrite-preserved cooked sausages [58]. In male Wistar rat models administered rosmarinic acid (RA) [extracted from dried leaves of Salvia rosmarinus (Syn. Rosmarinus officinalis)] for seven days before a single dose injection of a carcinogen, 1,2-dimethylhydrazine (DMH) (40 mg/kg bw/day), there were reduced extents of DNA damages by 55.2, 55.4 and 49%, respectively for 4, 8, or 16 mg/kg bw/day of RA when compared with those injected with DMH alone. Also, the injection of RA at the same doses respectively reduced the formation of aberrant crypt foci (ACF) in the colon by 62.7, 65.4 and 59.5% after DMH challenge [59]. The reduction in ACF, an indicator of early stage of colon cancer indicates that RA should be further explored as candidate nutraceutical for preventing and treating colon cancer. In another study involving male albino rats injected DMH (40 mg/kg bw), treatment with 250 mg/kg chrysin for 8 weeks significantly reduced the expression of CYP2E1 by 45% and prevented the development of histopathological disorders suggestive of tumorigenesis [60]. CYP2E1 is one of the cytochromes P450 enzymes needed for metabolic activation of DMH [61]. Significant reduction in the levels of malondialdehyde (MDA) by 32.7 and 52.4%, respectively and nitric oxide (NO) by 22.8 and 31.4%, respectively, and concomitant increase in level of reduced glutathione (GSH) by 67.3 and 188.5%, respectively was observed in the rats fed chrysin (125 and 250 mg/kg bw) compared with rats injected DMH without treatment. The reference drug in the study, daidzein, was more effective than chrysin; at 5 and 10 mg/kg bw, daidzein reduced CYP2E1 level by 20.5 and 52.8%, respectively, NO level by 33.4 and 44%, respectively and MDA level by 26.1 and 29%, respectively, but increased GSH level by 135 and 182.7%, respectively.
In a recent investigation by Nocolella et al. [25], manool, a diterpene from S. officinalis protected V79 cells from DNA damages. Manool significantly inhibited micronuclei formation in V79 cells exposed doxorubicin (DXR) and hydrogen peroxide, and NO synthesis in LPS exposed macrophages (Table 1). In DMH-injected rats (40 mg/kg bw, i.p), manool (0.3125–5.0 mg/kg bw) reduced ACF formation by 59.7–84.7%, compared to those treated with only DMH. The diterpene also reduced DXR (10 mg/kg bw, i.p)-induced chromosomal damage by 79.5% at 1.25 mg/kg b.w., p.o, suggesting that its anti-mutagenic potential could be associated with inhibition of inflammation and oxidative stress, the two major mechanisms through which DXR induces chromosomal damage [62, 63]. Additionally, consumption of the crude Salvia spp has also been credited with DNA-protective effects. In an in vivo study, a prophylactic administration of water extract (herbal tea) of S. officinalis (13.3 g/L, p.o for 2 weeks) prevented azoxymethane (AOM)-induced DNA damages on colonocytes and on lymphocytes by 20% and 65% respectively; reduced H2O2-induced DNA damages on colonocytes only by 28%, and inhibited by 30.5% the AOM-induced proliferation (needed for maintenance of the mutated DNA sequences) of mucosal cells [49]. Although these results interestingly suggest that more research efforts are needed to clarify the plant's phytoconstituent(s) responsible for the cancer-protective properties which are potential candidates for cancer prophylaxis, the study fails to compare the effects of the plant tea with known anticancer agent(s). On the other hand, the use of the leaves as an herbal tea in the experiment may be to mimic the traditional application of the whole leaves, either as part of dishes or boiled for nutritional and medicinal purposes. This will allow synergistic and combinatorial interaction of the phytochemicals in the herbal tea compared to when the phytochemicals are taken alone.
Table 1.
Summary of anticancer effects of Salvia spp extracts and phytochemicals.
| Salvia species | Concentration tested/dose administered | Study model (cell lines or animal models) | Pharmacological effects | References |
|---|---|---|---|---|
|
Crude extract and solvent fraction | ||||
| Water extract of S. officinalis | 13.3 g/l | Female Fischer 344 rats | Reduced AOM-induced DNA damages on colonocytes and on lymphocytes by 20% and 65% respectively. Reduced H2O2-induced DNA damages on colonocytes by 28%. Reduced AOM-induced proliferation by 30.5%. Reduced AOM-induced ACF formation by 53%. | [49] |
| Hydro-ethanol extract of S. chorassanica roots | 6.25–1000 μg/ml | AGS and MKN-45 | Suppressed proliferation of AGS (IC50 = 9.262 μg/ml) and MKN-45 (IC50 = 271.73 μg/ml) cells. | [22] |
| PDE sub-fractions of methanol extracts of S. chloroleuca roots | 25–200 μg/ml | MCF-7 and PC-3 | Reduced viability of MCF-7 and PC-3 cell lines, with IC50 values ranging from 24.19 to 47.15 μg/ml. PDE sub-fractions induced apoptosis in PC-3 cells with DCM sub-fraction increasing the Bax/Bcl-2 ratio by 650% compared to negative control, and cleaving PARP | [64] |
| Petroleum ether sub-fractions of methanol extracts S. atropatana roots | 2.5–200 μg/ml | PC-3, MCF-7 and MDA-MB-231 | Reduced proliferation of PC-3 (IC50 = 8.73 μg/ml), MCF-7 (IC50 = 32.40 μg/ml) and MDA-MB-231 (IC50 = 77.65 μg/ml) by cleaving PARP. | [69] |
| n-hexane sub-fraction of methanol extracts of aerial parts of S. macrosiphon | N.D | A549, MCF-7, MDA-MB-231 and normal HDF | Cytotoxic against A549, MCF-7, MDA-MB-231 and HDF with IC50 values of 20.89, 10.24, 20.98 and 26.90 μg/ml, respectively. | [24] |
| Dichloromethane:methanol extract of S. rosmarinus leaves | 5–150 μg/ml | PC-3 | Cytotoxic against the cancer cell and inhibited its migrationPC-3 cells (IC50 = 19.72 μg/ml) by increasing level of PARP cleavage by 75% (compared to control). The extract suppressed the migration of PC-3 cells by 48% via reduction in total AKT phosphorylated AKT by 27% and 70%), while total mTOR and phosphorylated mTOR reduced by 40% and 65%, respectively. | [27] |
| DMSO extract of S. rosmarinus leaves | 2–10 μg/ml | 184-B5/HER | Inhibited the proliferation of 184-B5/HER cells (IC50 = 4.6 μg/ml) by inducing cell cycle arrests at G1 and G2/M phases and inducing apoptosis (increased Bax/Bcl-2 ratio by 225%). | [70] |
| Methanol extract of S. Rosmarinus |
200 mg/kg b.w for in vivo study |
HGUE-C-1, SW480, and HT-29 cells as well as athymic mice |
Induced necrosis in HGUE-C-1, HT-29 and SW480 cells. At 40 μg/ml, RE increased LDH leakage in HGUE-C-1, SW480 and HT-29 cells by 32.6%, 20.0% and 14.3%, respectively, increased ROS generation by 280, 300 and 250%, respectively; and decreased MMP by 35, 40 and 30%, respectively. At 30 and 40 μg/ml, RE inhibited the migration of HGUE-C-1 cells by 28.8 and 66.1%, respectively, HT-29 cells by 22.1 and 92.5%, respectively and SW480 cells by 5.6 and 76.7%, respectively. At 200 mg/kg.b.w RE reduced HT-29 tumor volume by 34.1% (if RE was administered 2 weeks before and for another 5 weeks after HT-29 cells injection) and by 27.5% (if RE was administered for 5 weeks only after HT-29 cells injection). |
[71] |
|
Isolated compounds | ||||
| A mixture of saprorthoquinone and aethiopinone | 2.5–100 μg/ml | PC-3, MCF-7 and MDA-MB-231 | Cytotoxic against PC-3 (IC50 = 8.83 μg/ml), MCF-7 (IC50 = 26.15 μg/ml) and MDA-MB-231 (IC50 = 66.23 μg/ml) by increasing PARP cleavage (increased PARP cleavage by 220% at 12.5 μg/ml); increasing caspase-3 level (increased caspase-3 level by 1700% at 6.25 μg/ml); and increasing Bax/Bcl-2 ratio (increased Bax/Bcl-2 ratio by 200% at 6.25 μg/ml). | [69] |
| 13-Epimanoyl oxide | N.D | A549, MCF-7, MDA-MB-231 and normal HDF | Cytotoxic against A549, MCF-7 and MDA-MB-231 with IC50 values of 19.37, 15.79 and 22.24 μg/ml, respectively. | [24] |
| Aegyptinone A and tebesinone B | N.D | MCF-7, B16F10, PC-3, and C26 | Aegyptinone A inhibited viability of MCF-7, B16F10, PC-3 and C26 cells (with IC50 values of 4.82, 4.82, 1.24 and 4.09 μg/ml, respectively while the IC50 values of tebesinone B against the cancer cells were 4.45, 6.09, 3.22 and 3.23 μg/ml, respectively by. | [19] |
| Taxodione and sahandinone | N.D | MOLT-4, HT-29) and MCF-7 | Cytotoxic against MOLT-4, HT-29 and MCF-7 (with IC50 values of 0.54, 2.76 and 3.87 μg/ml, respectively by taxodione and IC50 values of 0.41, 1.78 and 3.20 μg/ml, respectively by sahandinone. | [72] |
| Taxodione | 0 - 30 mΜ | BCR-ABL-positive K562 cells | Inhibited proliferation (IC100 = 17.66 μg/ml) and induced apoptosis by 120% upregulation of ROS generation. | [73] |
| 15-deoxyfuerstione, horminon, microstegiol and 14-deoxycoleon U | N.D | K562 and MCF-7 | The phytochemicals were toxic to both cells via inhibition of topoisomerase I. The IC50 values of 15-deoxyfuerstione, horminon, microstegiol and 14-deoxycoleon U were 4.70, 9.60, 3.30 and 2.63 μg/ml, respectively against K562 and 5.13, 11.8, 4.67 and 2.70 μg/ml, respectively against MCF-7. | [76] |
| Ferruginol (FRG) | 6–48 μM | MCF-7 | Induced apoptosis via 340% increase in caspase-3, 225% increase in caspase-9, 400% increase in TBARS, and 271.4% increase in ROS generation. It also reduced the levels of NF-κB, MMP, SOD, CAT and GSH by 65%, 62.5%, 72, 68.4 and 72% respectively. | [74] |
| 10–320 μM | SK-Mel-28 | Cytotoxic against SK-Mel-28 (IC50 = 50.03 μg/ml) by elevating caspase-3-mediated apoptosis. | [75] | |
| Chrysin | 5–100 μM | ES2 and OV90 | Induced apoptosis through the loss of MMP (by 350% and 50%, respectively), and increase in cytoplasmic Ca2+ level (by 550% and 275%, respectively) and ROS generation (by 450% and 120%, respectively). | [77] |
| 10–160 μM and 20 mg/kg b.w.for in vivo study | MKN-45 cells nude mice | Cytotoxic to MKN-45 cells at ≥ 80 μM by increasing Bax/Bcl-2 ratio and TET1 enzymes (by 167%). At 40 μM chrysin reduced MKN-45 cells' migration and invasion by about 44 and 72%, respectively via elevation in TET1 expression by 61.54%. Oral administration of chrysin at 20 mg/kg.bw inhibited tumor growth by reducing 20% of tumor volume and upregulating the expression of TET1 enzymes by 50%. | [80] | |
| 10–200 μg/ml and 8 and 10 mg/kg b.w. | CT-26 and mice | Induced apoptosis in CT26 cells (IC50 = 80 μg/ml) through elevation in caspase-3 and caspase-9 activities by 97.9 and 126.7% respectively. 2-week oral administration of chrysin at 8 and 10 mg/kg.bw to mice resulted in about 21 and 37% reduction in CT-26 tumor volume respectively by decreasing sall4 level and increasing Bax level. | [79] | |
| 125 and 250 mg/kg.bw for in vivo study | SW620 cells for cell culture study and male albino rat for in vivo study | Inhibited proliferation of SW620 cells (IC50 value = 70 μM) via reduction in p-ERK/ERK and p-AKT/AKT ratios by 51 and 24.8% respectively. Treatment of rats with 125 and 250 mg/kg.bw of chrysin for 8–16 weeks inhibited DMH-induced tumorigenesis by reducing CYP2E1 level (by 7.7 and 45%, respectively) MDA level (by 32.7 and 35.3%, respectively), NO level (by 22.8 and 31.4%, respectively), CXCL1 level (by 27.7 and 55.6%, respectively), AREG level (by 21 and 27.4%, respectively), and MMP-9 level (by 21.33 and 47%, respectively), as well as increasing GSH level (by 67.3 and 188.5 %, respectively). | [60] | |
| DHTS | 1.25–5 μM and 10 mg/kg b.w. | EOMA cells and mice | At 1.25 and 2.5 μM, DHTS inhibited the ability of EOMA cells to align and form tubes through reduction in expression of VEGFR2 and MMP-9 by 28.2% and 77%, respectively. At 10 mg/kg bw, DHTS also depressed the expression of VEGFR2 and MMP-9 in mice. | [84] |
| Rosmarinic acid | 4–16 mg/kg b.w | Male Wistar rat models | For 4, 8 or 16 mg/kg bw. treatments, there were respective reductions in DNA damages by 55.2, 55.4 and 49%, and reductions in ACF formation in the distal colon by 62.7, 65.4 and 59.5%. | [59] |
| Manool | 0.5–2.0 μg/ml (for in vitro genotoxicity test), 0.5–4 μg/ml for ex vivo NO synthesis test, 1.25–20.0 mg/kg bw for in vivo genotoxicity assay, and 0.3125–5.0 mg/kg bw for ACF assay | V79 cells (for in vitro genotoxicity test), macrophages (for LPS-induced NO synthesis), rats (for ACF assay) and mice (for in vivo genotoxicity assay) | At 0.5 μg/ml, manool depressed DXR-generated micronuclei formation in V79 cells by 64.54%; at 0.5 and 1.0 μg/ml, manool inhibited hydrogen peroxide-induced micronuclei formation in V79 cells by 37 and 40% respectively and also suppressed LPS-induced NO synthesis in macrophages by 15.0% and 21.6% respectively. At 0.3125–5.0 mg/kg b.w. dose rage, manool reduced ACF formation in rats by 59.7–84.7% and reduced DXR-induced chromosomal damage in mice by 79.5% at 1.25 mg/kg b.w. | [25] |
Abbreviations: N.D = not defined; DHTS = 15,16-Dihydrotanshinone I; DCM = dichloromethane; PDE = petroleum ether, dichloromethane and ethyl acetate subfraction; NO = nitric oxide; LPS = lipopolysaccharides; MMP = mitochondrial membrane potential; TBARS = thiobarbituric acid reactive substance; SOD = superoxide dismutase; GSH = reduced glutathione; ROS = reactive oxygen species; AOM = azoxymethane; ERK = extracellular signal-regulated protein kinase; AKT = protein kinase B; VEGFR2 = vascular endothelial cell growth factor receptor 2; LDH = lactate dehydrogenase; CAT = catalase; ACF = aberrant crypt foci; DNA = deoxyribonucleic acid; DXR = doxorubicin; NF-kB = nuclear factor kappa B; mTOR = mammalian target of rapamycin.
2.2. Suppression of proliferation via induction of apoptosis or necrosis
Apart from being able to limit DNA damages that cause malignancy, extracts of Salvia spp and their phytochemicals have been credited with selective suppression of proliferation of malignant cells in in vitro and in vivo studies, by inducing apoptosis, necrosis and cell cycle arrest as discussed below.
In vitro cytotoxicity of crude extracts of Salvia species and their solvent fractions: The hydro-ethanol extract of S. chorassanica roots, stem and leaves reduced the proliferation of AGS and MKN-45 cancer cells, with highest cytotoxicity observed when AGS cells were treated with the roots extract (IC50 = 9.262 μg/ml) [22]. The petroleum ether, dichloromethane and ethyl acetate (PDE) sub-fractions of methanol extracts of S. chloroleuca roots showed cytotoxicity against human breast adenocarcinoma (MCF-7) and prostate (PC-3) cell lines, with IC50 values ranging from 24.19 to 47.15 μg/ml, which were however, less active than the reference anticancer agent, DRX (IC50 = 2.78 and 2.49 μg/ml for MCF-7 and PC-3, respectively) [64]. Notably, the PDE sub-fractions induced apoptosis in PC-3 cells, as indicated by increase in sub-G1 peaks in flow cytometry histograms; with dichloromethane (DCM) sub-fraction achieving this by increasing the Bax/Bcl-2 ratio by 650% compared to negative control, and cleaving poly (ADP-ribose) polymerase (PARP). Overexpression of PARP has been shown to contribute to the promotion of carcinogenesis and survival of cancer cells, hence, its inhibition is a major current target of managing ovarian [65], prostate, breast [66], renal [67], pancreatic [68], and other cancers. Hence, phytochemicals responsible for the above bioactivity by PDE sub-fractions should be isolated via activity-guided fractionation and should be further explored as broad-spectrum anticancer entities.
In a related study, petroleum ether sub-fraction of methanol extract of S. atropatana root and a mixture of two O-naphthoquinone-containing isomers (saprorthoquinone and aethiopinone) isolated from it were cytotoxic against PC-3 cells with IC50 values of 8.83 and 8.73 μg/ml, respectively through induction of apoptosis [69], suggesting that the isomers accounted for majority of the cytotoxic activity of the petroleum ether sub-fraction. Although DXR was more effective (IC50 = 4.03 μg/ml) than the herbal materials, the safety concerns of DXR makes the herbal products credible for further development as potential alternatives.
In a recent study by Balaei-Kahnamoei et al. [24], the n-hexane sub-fraction of methanol extracts of aerial parts of S. macrosiphon was cytotoxic against A549, MCF-7, and MDA-MB-231 cancer cells with IC50 values of 20.89, 10.24 and 20.98 μg/ml, respectively. The reported activity of the sub-fraction was similar to the reference anticancer drug, etoposide against A549 and MDA-MB-231 with IC50 values of 16.58 and 20.30 μg/ml, respectively, and higher than etoposide against MCF-7 (with IC50 value of 22.08 μg/ml). Despite this interesting cytotoxicity, the sub-fraction has low therapeutic value as it was also toxic to normal human dermal fibroblast (HDF) with low IC50 value of 26.90 μg/ml. However, 13-epimanoyl oxide (EMO), among other compounds isolated from this sub-fraction, displayed cytotoxicity against A549, MCF-7, and MDA-MB-231 cells (IC50 = 11.40, 9.29 and 13.09 μg/ml, respectively), while having minimal effects on normal human dermal fibroblast (HDF) cell lines with an IC50 value of 337.58 μg/ml. Interestingly, the cytotoxic effects of EMO against the above cancer (A549, MCF-7, and MDA-MB-231) cells were stronger than that of etoposide (IC50 = 16.58, 22.08 and 20.30 μg/ml, respectively). Based on its higher therapeutic index (lower toxicity to HDF) compared with etoposide (IC50 = 92.70 μg/ml against HDF), EMO should be further explored as an important candidate for nutraceutical intervention for cancer management.
A study showed that exposure of PC-3 cells to dichloromethane:methanol extract of S. rosmarinus leaves reduced the proliferation (IC50 = 19.72 μg/ml) and induced apoptosis (increased the level of PARP cleavage by 75% compared to control), without substantial effect on the viability of normal PNT1A cells even at 150 μg/ml, emphasizing the selectivity of the extract against cancerous cells [27]. At 50 μg/ml, the extract proved more effective than 10 nM of docetaxel and paclitaxel which served as reference cytotoxic agents, further buttressing the nutraceutical usefulness of the plant for cancer chemoprevention and therapy. Similarly, DMSO extract of S. rosmarinus leaves inhibited the proliferation of 184-B5/HER cells (IC50 = 4.6 μg/ml) by inducing apoptosis (increased Bax/Bcl-2 ratio by 225% compared to negative control) and cell cycle arrest at G1 and G2/M phases [70]. On the other hand, Pérez-Sánchez et al. (2019) reported that cell death in HGUE-C-1, SW480, and HT-29 cells exposed to S. rosmarinus leaves extract was due to necrosis resulting from elevation of ROS generation, loss of mitochondrial membrane potential (MMP) and loss of plasma membrane integrity. Thus, cell death induced in cancer cells by Salvia spp extracts and their phytochemicals involves different mechanisms, including necrosis and apoptosis.
In vitro cytotoxicity of phytochemicals isolated from Salvia species: Eghbaliferiz et al. (2018) isolated four quinones from dichloromethane sub-fraction of methanol extracts of S. tebesana roots, among which aegyptinone A and tebesinone B were cytotoxic against MCF-7, melanoma (B16F10), PC-3, and colon (C26) cancer cells by unknown mechanisms, with PC-3 being most susceptible with IC50 values of 1.24 and 3.22 μg/ml for aegyptinone A and tebesinone B, respectively. The anticancer activities of these phytochemicals were lower than DRX which was cytotoxic against MCF-7, B16F10, PC-3 and C26 with IC50 values of 1.01, 0.40, 1.08 and 0.15 μg/ml respectively). Phytochemicals such as ferruginol (FRG), taxodione (TXD), sahandinone, 4-dehydrosalvilimbinol and labda-7,14-dien-13-ol were isolated and characterized from dichloromethane roots extracts of S. lachnocalyx, another important Salvia spp used for making dishes in Asia [72]. Interestingly, all the compounds, by unknown mechanisms, displayed cytotoxicity against acute lymphoblastic leukemia (MOLT-4), colorectal adenocarcinoma (HT-29) and human breast cancer (MCF-7) cells, with taxodione and sahandinone being most active (IC50 values ranging from 0.41 to 3.87 μg/ml), and also slightly more effective than the reference anticancer agent, cisplatin, except against MCF-7 (cisplatin IC50 values against MOLT-4, HT-29, MCF-7 cells were 0.75, 3.41 and 1.7 μg/ml, respectively). Understanding the molecular mechanisms via which the extract and its phytochemicals inhibit the proliferation of cancer cells will increase the likelihood of their exploitation for nutraceutical application in cancer prevention and management; hence, future study should clarify the mechanisms via which the herbal drugs act.
In another study, TXD (30 μM) totally inhibited the proliferation of BCR-ABL-positive K562 cells by inducing apoptosis via intracellular ROS generation (at 10 μM, TXD increased ROS generation by 120% compared to negative control) [73]. Notably, TXD was more effective than the reference anticancer agent, imatinib. Comparatively, at 20 μM, imatinib reduced viability by 40% which was lower than over 80% reduction in viability recorded when BCR-ABL-positive K562 cells were exposed to same concentration of TXD [73]. FRG inhibited the proliferation of MCF-7 cells (IC50 = 7.06 μg/ml) through induction of apoptosis by increasing the expression of caspases-3 by 340% and caspase-9 by 225% and increasing intracellular ROS generation by 271.4% in the cancerous cells. It reduced the expression of NF-κB (a signaling protein that is involved in the proliferation, survival, angiogenesis, and metastasis of cancerous cells) by 65%, and levels of MMP by 62.5% and antioxidant proteins (SOD, CAT and GSH were reduced by 72, 68.4 and 72%, respectively), causing the oxidation of membrane-bound lipids, as shown by the elevation of thiobarbituric acid reactive substance (TBARS) level by 400% [74]. In SK-Mel-28 cancer cells, FRG induced cytotoxicity (IC50 = 50.03 μg/ml) through the induction of caspase-3 (at IC75, FRG raised caspase-3 level by 700%)-mediated apoptosis via phosphorylation of p38 protein and translocation of NF-κB into the nucleus [75]. However, these studies failed to compare the activity of FRG and known anticancer agent(s), limiting the clinical interpretation of the findings. A subsequent investigation showed that diterpenoids such as 15-deoxyfuerstione, horminon, microstegiol, and 14-deoxycoleon U isolated from S. lachnocalyx inhibited K562 and MCF-7 cells (IC50 values ranging from 2.63–11.83 μg/ml) by inhibiting topoisomerase I activity comparable with cisplatin [76]. Interestingly, 14-deoxycoleon U gave better inhibitory activity against K562 and MCF-7 cells (IC50 values = 2.63 and 2.7 μg/ml, respectively) than cisplatin (IC50 = 2.91 and 12.49 μg/ml, respectively).
Another potential anticancer candidate from Salvia spp is chrysin. The phytochemical was reported to be cytotoxic against ES2 (IC55 = 5.89 μg/ml) and OV90 (IC75 = 29.43 μg/ml) cancer cells by inducing apoptosis through the reduction of MMP level by 350 and 50%, respectively, while increasing cytoplasmic Ca2+ level by 550 and 275%, respectively, and ROS generation by 450 and 120%, respectively [77]. The induction of apoptosis through intracellular ROS generation by chrysin was also observed by Lima [78] against RT4, 5637 and T24 cells (IC50 = 107.6, 82.8 and 104.9 μM, respectively). In several other studies, chrysin inhibited the proliferation of CT26 cells (IC50 = 80 μg/ml via elevation in caspases-3 and 9 activities by 97.9 and 126.7%, respectively) [79], SW620 cells [IC50 value = 70 μM via reduction in levels of phosphorylated extracellular signal-regulated protein kinase (ERK) and protein kinase B (AKT) by 51 and 24.8%, respectively] [60], MKN-45 cells (IC50 = 80 μM via increased Bax/Bcl-2 ratio and expression of ten-eleven translocation 1 (TET1) enzymes by 167%) [80] and also synergized with 5-fluorouracil (5-FU) (40 μM/20 μM or 50 μM/25 μM of chrysin/5-FU respectively) to induce cell cycle arrest at the G2/M phase and apoptosis (via decrease in AKT phosphorylation and upregulation of p21 expression) in 5-FU-resistant AGS/FR cells [81].
In vivo anticancer activities of Salvia species via inhibition of tumor growth: Apart from inhibition of growth and killing of cancerous cells in vitro, extracts and phytochemicals of Salvia spp have also been demonstrated to suppress tumor growth in animal models. The oral administration of 200 mg/kg bw. of S. rosmarinus extract (RE) to athymic mice infected with HT-29 cells reduced tumor volume by 34.1% (if RE was administered 2 weeks before and for another 5 weeks after HT-29 cells injection) and by 27.5% (if RE was administered for 5 weeks only after HT-29 cells injection) [71]. Similarly, a 2-week gavage administration of chrysin at 20 mg/kg bw inhibited tumor growth (decreased tumor volume by about 20%) in nude mice infected with MKN-45 cells by inducing about 50% upregulation of expression of TET1 enzymes [80]. Furthermore, 2-week oral administration of chrysin at 8 and 10 mg/kg bw to mouse models infected with CT-26 cells resulted in about 21 and 37% reduction in tumor volume respectively by decreasing sall4 level and increasing Bax level [79]. It is possible that the doses of chrysin administered are sub-effective doses (lower than what is needed to totally inhibit tumor growth); hence, further study should clarify the clinical effectiveness of using higher but safe doses of chrysin to prevent tumor growth. The lower in vivo anti-tumor activity of chrysin, compared to its in vitro activity, suggest that the phytochemical may have poor bioavailability and biostability. The poor bioavailability agrees with the findings that chrysin is easily metabolized by detoxification via glucuronidation and sulfonation to its metabolites that are effluxed by mainly anion transporters [82]. Hence, further study should explore measures to improve the bioavailability of chrysin by incorporating the flavonoid into carrier molecules that will increase the transepithelial transport and by extension, its biological activities.
In vitro and in vivo anticancer properties of Salvia species via inhibition of angiogenesis, invasion and metastasis: Tumors have been described as benign- if they remain localized at their origins, or as cancerous/malignant- if they metastasize to other sites in the body; localized tumors are generally easier to treat via surgery and radiotherapy than tumors that spread, which require systemic treatments [83]. Solvent extracts of Salvia spp and their chemical constituents have been credited with anti-metastatic effects. They inhibited metastasis by, among other mechanisms, suppressing the activities of enzymes involved in degradation of extracellular matrix, suppressing the migration of cells and/or by depressing the expression of growth factors and receptors needed for formation of new vessels [60, 71, 74, 80, 84]. In an in vitro study involving endothelioma (EOMA) cell line, treatment with 1.25 and 2.5 μM of 15,16-dihydrotanshinone I (DHTS), a tanshinone isolated from S. miltiorrhiza roots inhibited the ability of EOMA cells to align and form tubes by 30.77 and 75.4%, respectively [84]. In an in vivo study by the same researcher, DHTS (10 mg/kg bw, i.p.) depressed the expression of vascular endothelial cell growth factor receptor 2 (VEGFR2) and matrix metalloproteinase 9 (MMP-9) by ten folds higher than propranolol (a commercial drug used in treating infantile hemangiomas) [84]. Salvia rosmarinus extract (RE) significantly suppressed the migration of cultured HGUE-C-1, HT-29 and SW480 cells [71]; it inhibited migration of HGUE-C-1 cells by 28.8 and 66.1%, respectively, HT-29 cells by 22.1 and 92.5%, respectively and SW480 cells by 5.6 and 76.7%, respectively at concentrations of 30 and 40 μg/ml. In PC-3 cells, RE was shown to inhibit cellular migration by 48% through the reduction in mRNA expression of AKT and mTOR by 27% and 40%, respectively, and their phosphorylation by 70% and 65%, respectively [27], suggesting that RE inhibit the migration of the cancer cell by modulating PI3K/AKT/mTOR signaling pathway [85]. This is because PI3K/AKT/mTOR signaling pathway has been implicated in development and progression of many cancer cells [86, 87, 88, 89], making it a good target for new anticancer agents in pipeline such as coptisin [90], zingerone [91], mangiferin [92], erianin [93], melatonin [94, 95] and rapamycin [96]. A similar result, although not compared with any reference anticancer agent, was reported in another in vitro study, where chrysin, at 40 μM (less than the toxic dose of 80 μM) elevated TET1 expression in MKN-45 cells by 61.54%, thereby reducing the cells’ migration and invasion by about 44 and 72%, respectively [80].
In experimentally-induced colorectal cancer (induced by subcutaneous injection with 40 mg/kg bw of DMH for 16 weeks) in rats, concomitant subcutaneous injection of chrysin for an average of 8 weeks (week 8–16) reduced the level of metastasis markers, including chemokine (C-X-C motif) ligand 1 (CXCL1), MMP-9 and the epidermal growth factor receptor, amphiregulin (AREG) [60]. At 125 and 250 mg/kg.bw, chrysin reduced the levels of CXCL1 by 27.7% and 55.6%, respectively, AREG by 21% and 27.4%, respectively, and MMP-9 by 21.33% and 47%, respectively, compared to those injected with DMH alone. Chrysin was however, less effective than daidzein; 5 and 10 mg/kg.bw daidzein reduced the levels of CXCL1 by 39% and 55.6%, respectively, AREG by 26% and 32.4%, respectively, and MMP-9 by 45.4% and 64.4%, respectively in DMH-injected-daidzein-treated rats compared to those injected with DMH alone.
Generally, the available literature indicates that Salvia spp are reservoirs of chemicals that are good candidates for nutraceutical and drug development for cancer treatment; however, it is noteworthy to mention that the clinical interpretation of some of the studies reviewed such as Balaei-Kahnamoei et al [24], Eghbaliferiz et al. [19]; and Mirzaei et al. [72] among others are limited due to deficiency of mechanisms behind the reported biological activities. Additionally, some had poor study designs such as lack of positive controls (reference anticancer drugs) to enable proper comparison of the anticancer activities of the herbal substance with that of known anticancer agent(s). Table 1 summarizes the anticancer activities of extracts of Salvia spp and their phytochemicals while Figure 1 shows the mechanisms through which Salvia spp and their phytochemicals inhibit the growth of and/or kill cancer cells.
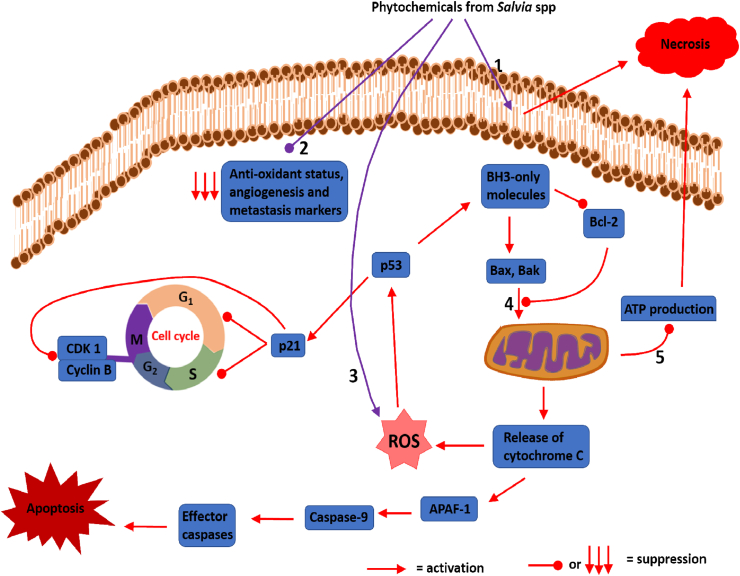
Figure 1.
Proposed mechanisms of anti-cancer activities of phytochemicals from Salvia spp. 1) Induction of plasma membrane permeabilization in cancer cells; 2) Reduction of anti-oxidant status of cancer cells and suppression of angiogenesis and metastasis markers; 3) Elevation of ROS in cancer cells; 4) Induction of MOMP; 5) Suppression of ATP production. Phytochemicals from Salvia spp induce plasma membrane permeabilization in cancer cells, leading to death by necrosis. They also elevate ROS production, leading to activation of p53, which induce cell cycle arrest and senescence via p21. P53 also induce the intrinsic apoptotic pathway via upregulation in expression of BH3-only molecules (e.g. PUMA and Noxa). BH3-only molecules elevate the levels of pro-apoptotic molecules (Bax and Bak) and depress the levels of anti-apoptotic molecules (Bcl-2). Induction of MOMP by Bax and/or Bak lead to release of cytochrome C, leading to apoptosis. Induction of MOMPS also leads to ATP depletion (suppression of ATP production), which can lead to death by necrosis. Salvia spp phytochemicals also suppress the levels of anti-oxidant molecules in cancer cells, allowing membrane lipids' peroxidation (which can also activate p53 to bring about cell death). Salvia spp phytochemicals also suppress angiogenesis and metastasis markers. Abbreviations: ROS = Reactive oxygen species; MOMP = Mitochondrial outer membrane permeabilization; ATP = Adenosine triphosphate; CDK 1 = Cyclin-dependent kinase-1; APAF-1 = Apoptosis protein activating factor-1; Bax = Bcl-2-associated X protein; Bak = Bcl-2 homologous antagonist/killer; BH-3 = Bcl-2 homology 3; PUMA = p53 upregulated modulator of apoptosis.
2.3. Application of Salvia species in viral infection
Virus particles are very recalcitrant and their resultant health challenges and fatality rates have caused several global panics expressed as epidemics, endemics, and pandemics. Several approaches towards combating viral infections with their different levels of efficacies have been reviewed [97]. Moreover, the use of natural products such as plant phytochemicals have been reported to be very attractive because of their minimal cytotoxicity to the host cells, while effectively combating viral infection [98]. Out of the brimming bioactivities that have been identified in several plant species in genus Salvia, their interesting antiviral properties reported in a few studies are yet to be fully harnessed [99]. This session presents an up-to-date review of studies on Salvia spp that have successfully been identified to be potent against a number of viral particles. Suggestions were made on the potential application of Salvia spp in combating global threats of viral pandemics such as the current SARS-CoV-2 pandemic.
An earlier study on S. fructicosa reported the antiviral potency of its essential oils on Herpes simplex virus 1 (HSV-1) with a 0.2% of the oil fraction concentration inhibiting 80% plaque forming unit (PFU) of the virus [100]. The study identified and characterized thujone as the interesting antiviral phytochemical present in S. fructicosa, and on analysis for anti-HSV-1 activities, 0.1% of thujone concentration successfully inhibited 95% PFU of HSV-1 [100]. Other studies have shown chloroform extract of S. cyanescens and S. microstegia leaves [101], methanolic extract of S. limbata leaves [102], and 20% ethanol extract of S. officinalis [103] to effectively inactivate HSV-1, at doses which are not cytotoxic to normal cells. A more recent study characterized the essential oil fraction of Salvia desoleana Atzei and discovered linalyl acetate, alpha terpinyl acetate, and germacrene D as its component with potent antiviral properties against acyclovir sensitive (AS) and acyclovir resistant (AR) strains of herpes simplex virus-2 (HSV-2) [104]. Among the isolated compounds from the essential oil, only β-caryophyllene was shown to exhibit substantial anti-HSV-1 activity (EC50 = 5.02 μg/ml) [104].
Endemic viral strains of influenza virus such as the avian strain (H7N7), the human influenza stain (H3N2), and the H1N1 strain, which have caused several epidemics in the world, with challenging level of genetic drifts have been reported to be managed in vitro with extracts of a number of Salvia spp [23, 105, 106]. Specifically, methanol extracts of S. sclarea and S. cedronel reduced H7N7/H3N2 influenza virus strains-induced cytopathy on Madin-Darby canine kidney (MDCK) cells [102, 107]. Similarly, recent studies reported that the methanol extract of S. plebeian and the essential oil from S. dentate inhibit the H1N1 strain of influenza virus (reducing plaque formation by 93% at 11.18 mM) [16, 106]. Moreover, characterization of essential oils fraction of Salvia sclarea flower identified β–myrcene, linalool, α–terpineol, cinnamaldehyde, linalyl acetate and geranyl acetate as the major component which was responsible for the 52% higher H1N1-CPE inhibition compared to the standard drug, oseltamivir [108]. Other strains such as the avian influenza H5N1 virus were reported to have been inactivated by camphor and α-thujone component of the essential oil fraction of Salvia officinalis L. [23]. Lastly, polysaccharide fractions of S. plebeia demonstrated potent inhibitory activities against cultured respiratory syncytial virus (RSV) with therapeutic index of 123 relative to 141 by ribavirin that served as reference anti-RSV. In experimentally-induced RSV infection in mice, intragastric administration of polysaccharide fraction of S. plebeia (17.6 mg/20 g/d) for one week gave 60% lung index inhibition ratio, and suppressed viral load and prevented RSV-induced lesions in the lung tissue by inhibiting the mRNA expression of toll-like receptors (TLR-3 and TLR-4) lungs [17]. The polysaccharide fraction also up-regulated anti-inflammatory signal mediators, IFN-γ and IL-2 expression in mouse serum while the expression of pro-inflammatory signal molecule, TNF-α was down-regulated suggesting that the herbal material does not only inhibit the viral replication but suppress immune system's overactivity in response to the viral infection leading to the damages seen in lungs and other tissues of RSV-infected persons.
Due to the valuable antiviral activities of some Salvia spp, several patents have been filed in the past to protect intellectual properties for discoveries of novel compounds and extracts of Salvia spp with interesting inhibitory activities against a number of viral diseases. Han and Lee [109] successfully filed two patents for salvianolic acid and its conjugated compounds from both S. officinalis and S. vunnanensis with remarkable anti-retroviral properties (US6043276A and WO1998024460A1). The novel β-(3,4-dihydroxyphenyl) lactic acid and/or caffeic acid isolated from S. officinalis was demonstrated to inhibit the integrase activities of HIV-1 when conjugated under alkaline condition, even at a concentration of less than 1 μg/ml [110]. Several conjugations and polymerization of β-(3,4-dihydroxyphenyl) lactic acid and/or caffeic acid which resulted in active formulation were present in the patent document (US6043276A). Moreover, the aqueous extract of S. vunnanensis with anti-integrase activities against many retroviruses was also patented recently [111].
Salvia spp-derived phenolic compounds such as chrysin and Cappariloside A have been demonstrated to elicit antiviral activities against a handful of viruses. For example, chrysin has been demonstrated, using in vitro experiments, to suppress influenza viruses H1N1 and H3N2, PIV3, ADV, influenza A/Puerto Rico/8 H1N1, enterovirus 71 (EV71) and coxsackievirus B3 (CVB3) replication through the inhibition of viral 3C protease activity [112, 113, 114, 115]. Other phenolic acids from the aerial parts of Salvia plebeia R. Br including 6-hydroxyluteolin 7-O-β-d-glucoside, nepitrin, homoplantaginin, hispidulin, nepetin, rosmarinic acid methyl ester and luteolin were demonstrated to exhibit anti-influenza A virus effect by inhibiting the activity of H1N1 neuraminidase, the viral enzyme that hydrolysis sialic acid in host's respiratory tract [116, 117]. For more information on other phytochemicals isolated from Salvia miltiorrhiza, Salvia cedronella, and Salvia plebeia R. Br with antiviral properties, see the recent reviews [17, 118, 119].
Generally, the antiviral potential of phytochemicals from Salvia spp has been underexploited. The results of the studies reviewed, taken together showed that the plant species have great promise in the prevention and management of respiratory viral infections such as the diverse strains of influenza virus, parainfluenza virus and respiratory syncytial virus (RSV) [17, 101, 108]. The ongoing COVID-19 pandemic, caused by SARS-CoV-2, have distorted virtually a lot of global activities [120], demanding concerted solutions. Several studies and reviews have compared the etiology and symptoms of SARS-CoV-2 with other known respiratory viral diseased conditions. Since there is a wealth of evidence about the therapeutic potentials of phytoconstituents of Salvia spp, against several strains of respiratory viruses who share close relationship with SARS-CoV-2 [121], there are possibilities that the world solutions to the prevent or manage COVID-19 pandemics might be encrypted in Salvia plants. A few studies have shown that some phytochemicals from different Salvia spp are potent anti-COVID druggable molecules [21]. For example, three salvianolic acids, salvianolic acid A, salvianolic acid B, and salvianolic acid C, isolated from S. miltiorrhiza was reported to inhibits SARS-CoV-2 entry by binding in the interface between the RBD of the SARS-CoV-2 and the ACE2 receptors, hence, inhibiting entry to the viral entry [122]. Other studies have reported that S. officinalis plays a potent inhibitory role against SARS-CoV-2 and have potential applicability in the manufacture of Unani hand sanitizers [21, 123]. Recently, phytochemicals of Salvia spp-origin such as phenolic compound, rutin and a benzoylated monoterpene glycoside, plebeioside B have been shown inhibit SARS-CoV-2 protease, indicating potential application in managing COVID-19 replication [124]. There is a need for more studies to unravel the unexploited antiviral nuggets encrypted in different species of genus Salvia. Table 2 summarizes the antiviral activities of extracts of Salvia spp and some of their phytochemicals while Figure 2 presents the structures of some compounds from Salvia spp with anticancer and antiviral properties.
Table 2.
Antiviral activities of extracts of Salvia species and their phytochemicals.
| Salvia species | Virus | Cell culture, Study model | Assay | Duration of treatment/Incubation time | Activities (IC50/EC50) | References |
|---|---|---|---|---|---|---|
| Salvia cyanescens Boiss. & Balansa | HSV-1 PI- 3 |
MDBK and Vero cell lines | cytopathogenic effect (CPE) concentrations | 48 h | 16–1.0 mg/ml against both HSV-1 and PI-3 | [101] |
| Salvia dentata | H1N1 | MDCK cells | plaque reduction assay (PRA) | 108 h | 93% | [16] |
| Salvia microstegia Boiss. & Balansa | HSV-1 PI- 3 |
MDBK and Vero cell lines | cytopathogenic effect (CPE) concentrations | 48 h | 16–1.0 mg/ml against both HSV-1 and PI-3 | [101] |
| Salvia cedronella Boiss | H7N7, H3N2,HSV-1 & HSV-2 | MDCK and MDBK cells | Cytopathogenic effect (CPE) reduction assay | 24 h | 0.30 mg/ml (H7N7), 0.60 mg/ml (H3N2), 0.60 mg/ml (HSV-1) & 0.50 mg/ml (HSV-2) | [107] |
| Salvia desoleana Atzei | Acyclovir sensitive (AS) and Acyclovir resistant (AR) strains HSV-2 |
Vero, (ATCC CCL-81), the epithelial cell lines Hep-2 (ATCC CCL-23) and A549 (ATCC CCL-185) | HSV inhibition assay Tim of Addition Assay Virus inactivation assay |
2 h | 23.72 μg/ml (AS) 28.57 μg/ml (AR) | [104] |
| Salvia fructicosa Mill. | HSV-1 | Vero cells | plaque assay | 48 h | 0.2% oil fraction inhibits 80 % PFU 0.1% thujone inhibits 95% PFU |
[100] |
| Salvia limbata C.A.Mey. | HSV-1 and HSV-2 | MDCK and MDBK | Cytopathic effect (CPE) reduction assay. The 50% end point titration technique (EPTT) |
48–72 h | 0.12 (mg/ml) (HSV-1) & 0.05 (mg/ml) (HSV-2) | [102] |
|
Salvia miltiorrhiza Bunge |
EV71 | Vero, MRC-5 & rhabdomyosarcoma cells | Plaque Assay, Cytopathic Effect Test & MTT Assay | 3 h | 0.742 mg/ml (SA1) 0.585 mg/ml (SA2) |
[125] |
|
Salvia miltiorrhiza Bunge |
EV71 | COS-1 cell line | Cytotoxicity & Anti-EV71 infection assays | 48 h | MLB IC50 value -0.09 mM - TI value: 10.52 RA IC50 value: 0.50 mM - TI value: 2.97 |
[126] |
| Salvia officinalis L. | HSV-1 | WISH human amnion epithelial cells | reduction of CPE | 5 h | 199.0 μg/ml | [127] |
| Salvia officinalis L. | HSV-1 | Vero cells | inhibition of plaque formation | 48 h | 1.41–1.88 μg/ml | [128] |
| Salvia officinalis L. | HSV-1 and HSV-2 | RC-37 cells | Reduction in plague formation assays | 72 h | 0.18 μg/ml (HSV-1) & 0.04 μg/ml (HSV-2) | [103] |
| Salvia officinalis L. | H5N1 | MDCK cells | Plaque reduction assay | 72 h | (IC50) 0.41 μg/ml | [23] |
| Salvia plebeian R.Br. | H1N1 | MDCK cells | Cytopathic Effect (CPE) Inhibition Assay | 24 h | 6.58–11.67 μg/ml | [106] |
| Salvia plebeian R.Br. | RSV | RPMI 1640 culture solution | MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetr-azolium bromide] assay Time-of-addition assay |
48 h | 60 % lung index inhibition ratio with high dose High-dose (17.6 mg/20 g/d) of treatment | [17] |
| Salvia sclarea L. | H1N1 | MDCK cells | Cytopathic effect (CPE) reduction method. | 48 h | CPE inhibition of 52% greater than the standard drug oseltamivir, with no cytotoxicity at a concentration of 100 μg/ml | [108] |
| Salvia sclarea L. | (H7N7) (H3N2) |
MDCK and MDBK cells | Cytopathic effect (CPE) reduction assay. The 50% end point titration technique (EPTT) |
48–72 h | 0.13 (mg/ml) (H7N7) 0.13 (mg/ml) (H3N2) |
[102] |
Abbreviations: Herpes simplex (type-1) (HSV-1); parainfluenza (type-3); chicken influenza virus (H7N); human influenza virus (H3N2); Enterovirus 71 (EV71); avian influenza virus H5N1; respiratory syncytial virus (RSV); Madin-Darby bovine kidney (MDBK) cell lines; Madin-Darby canine kidney (MDCK); African green monkey fibroblastoid kidney cells (Vero cell).
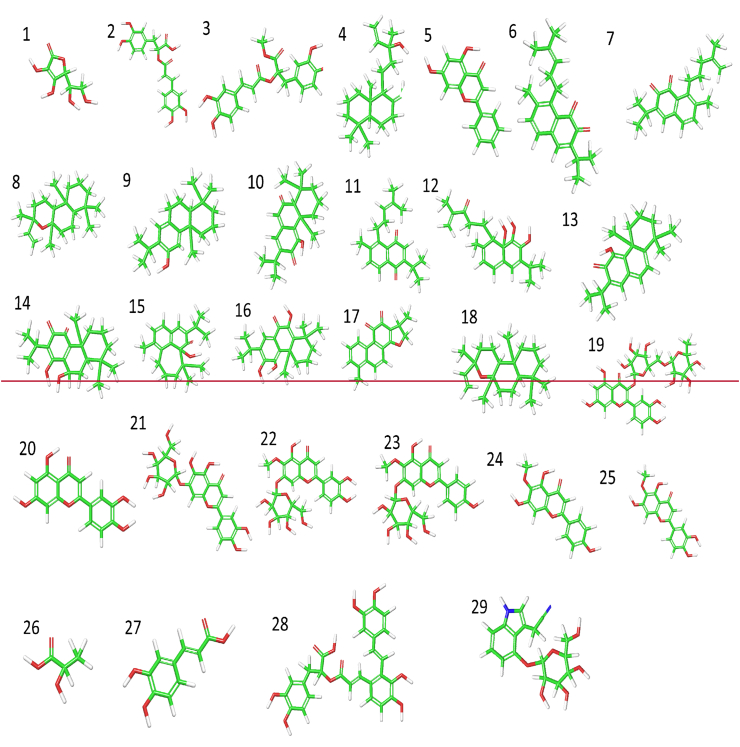
Figure 2.
Structures of some compounds from Salvia spp with anticancer and antiviral properties. Key: Green colour represents carbon chain, red colour represents oxygen atom, blue colour represents nitrogen atom while white colour represents hydrogen atom. Vitamin C (1), Rosmarinic acid (2), Rosmarinic acid methyl ester (3), Manool (4), Chrysin (5), Saprorthoquinone (6), Aethiopinone (7), Epimanoyl oxide (8), Ferruginol (9), Taxodione (10), Sahandinone (11), 4-Dehydrosalvilimbinol (12), 15-Deoxyfuerstione (13), Horminon (14), Microstegiol (15), 14-Deoxycoleon (16), 15,16-Dihydrotanshinone I (17), 13-Epimanoyl oxide (18), Rutin (19), Luteolin (20), 6-Hydroxyluteolin 7-O-β-d-glucoside (21), Nepitrin (22), Homoplantaginin (23), Hispidulin (24), Nepetin (25), Lactic acid (26), Caffeic acid (27), Salvianolic acid (28) & Cappariloside A (29).
3. Conclusions
Many plants in genus Salvia are traditionally used as vegetable for making dishes by Asians due to the believed health-promoting potentials. Solvent extracts of some of the plants and their phytochemicals have been documented to offer some health benefits, including anticancer and antiviral properties, among others. Specifically, chrysin, taxodione and ferruginol are the most outstanding chemicals from the species with broad-spectrum activities against initiation, progression and survival of many cancer cells, making them good candidates for nutraceutical and drug development for cancer prevention and treatment. On the other hand, the antiviral activities of the species and their phytochemicals such as rosmarinic acid, thujone and linalool are highly underexploited, demanding further studies to elucidate the chemicals responsible for the broad-spectrum antiviral activities of the extracts of the species. It is also important to state that majority of the studies were carried out in vitro and ex vivo, creating a question on how biostable and bioavailable are the extracts or their phytochemicals when orally ingested? Hence, future studies should focus on animal and human studies to explore the clinical applicability of the findings. In addition, the molecular mechanisms through which the extracts and their phytochemicals elicit the health benefits were not investigate in many of the studies reviewed, limiting their practical usefulness. Hence, future studies should investigate the molecular mechanisms of action of these extracts and their phytochemicals, and compare their biological activities with known reference drugs. Finally, some of the studies failed to use the taxonomically accepted names of the plants; hence, future researchers should sort the help of experts in taxonomy for proper identification and authentication of the plants, and compare the information with features in standard plant databases.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.World Health Organization (WHO) 2021. Cancer.https://www.who.int/news-room/fact-sheets/detail/cancer Available from: [Google Scholar]
- 2.Ferlay J., Colombet M., Soerjomataram I., Parkin D., Piñeros M., Znaor A., Bray F. Cancer statistics for the year 2020: an overview. Int. J. Cancer. 2021;149:778–789. doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 3.Sung H., Ferlay J., Siegel R., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Yoon S.M., Chu F.-I., Ruan D., Steinberg M.L., Raldow A., Lee P. Assessment of toxic effects associated with dose-fractionated radiotherapy among patients with cancer and comorbid collagen vascular disease. JAMA Netw. Open. 2021;4:e2034074. doi: 10.1001/jamanetworkopen.2020.34074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang K., Tepper J. Radiation therapy-associated toxicity: etiology, management, and prevention. CA: Cancer J. Clin. 2021;71:437–454. doi: 10.3322/caac.21689. [DOI] [PubMed] [Google Scholar]
- 6.Lichota A., Gwozdzinski K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19113533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adewole K.E. Nigerian antimalarial plants and their anticancer potential: a review. J. Integr. Med. 2020;18:92–113. doi: 10.1016/j.joim.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Akhtar M.S., Swamy M.K. Anticancer plants: properties and application. Anticancer Plants: Prop. Appl. 2018;1:1–582. [Google Scholar]
- 9.Marcotullio M.C., Curini M., Becerra J.X. An ethnopharmacological, phytochemical and pharmacological review on lignans from Mexican Bursera spp. Molecules. 2018;23 doi: 10.3390/molecules23081976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zengin G., Llorent-Martínez E.J., de Córdova M.L.F., Bahadori M.B., Mocan A., Locatelli M., Aktumsek A. Chemical composition and biological activities of extracts from three Salvia species: S. blepharochlaena, S. euphratica var. leiocalycina, and S. verticillata subsp. amasiaca. Ind. Crop. Prod. 2018;111:11–21. [Google Scholar]
- 11.Mocan A., Babotă M., Pop A., Fizeșan I., Diuzheva A., Locatelli M., Carradori S., Campestre C., Menghini L., Sisea C.R., Sokovic M., Zengin G., Păltinean R., Bădărău S., Vodnar D.C., Crișan G. Chemical constituents and biologic activities of sage species: a comparison between salvia officinalis l., s. glutinosa l. and s. transsylvanica (schur ex griseb. & schenk) schur. Antioxidants. 2020;9 doi: 10.3390/antiox9060480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mollica A., Zengin G., Locatelli M., Picot-Allain C.M.N., Mahomoodally M.F. Multidirectional investigations on different parts of Allium scorodoprasum L. subsp. rotundum (L.) Stearn: phenolic components, in vitro biological, and in silico propensities. Food Res. Int. 2018;108:641–649. doi: 10.1016/j.foodres.2018.03.064. [DOI] [PubMed] [Google Scholar]
- 13.Okagu I.U., Ndefo J.C., Aham E.C., Udenigwe C.C. Zanthoxylum species: a review of traditional uses, phytochemistry and pharmacology in relation to cancer, infectious diseases and sickle cell anemia. Front. Pharmacol. 2021:2475. doi: 10.3389/fphar.2021.713090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alavi M.S., Fanoudi S., Rahbardar M.G., Mehri S., Hosseinzadeh H. An updated review of protective effects of rosemary and its active constituents against natural and chemical toxicities. Phytother Res. 2020;35:1313–1328. doi: 10.1002/ptr.6894. [DOI] [PubMed] [Google Scholar]
- 15.Mahendran G., Rahman L.-U. Ethnomedicinal, phytochemical and pharmacological updates on Peppermint (Mentha × piperita L.)—a review. Phytother Res. 2020;34:2088–2139. doi: 10.1002/ptr.6664. [DOI] [PubMed] [Google Scholar]
- 16.Najar B., Mecacci G., Nardi V., Cervelli C., Nardoni S., Mancianti F., Ebani V.V., Giannecchini S., Pistelli L. Volatiles and antifungal-antibacterial-antiviral activity of South African salvia spp. essential oils cultivated in uniform conditions. Molecules. 2021;26:2826. doi: 10.3390/molecules26092826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.yu Liang Y., wei Li K., jv Niu F., Li Y., cheng Wei H., lei Dai Y., yu Wang Y., zheng Zhou C., huan Wan X. Salvia plebeia R. Br. polysaccharides (SPP) against RSV (respiratory syncytial virus) infection: antiviral effect and mechanisms of action. Biomed. Pharmacother. 2021;141:111843. doi: 10.1016/j.biopha.2021.111843. [DOI] [PubMed] [Google Scholar]
- 18.Yu M., Gouvinhas I., Rocha J., Barros A.I.R.N.A. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep. 2021;11:1–14. doi: 10.1038/s41598-021-89437-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eghbaliferiz S., Emami S.A., Tayarani-Najaran Z., Iranshahi M., Shakeri A., Hohmann J., Asili J. Cytotoxic diterpene quinones from Salvia tebesana Bunge. Fitoterapia. 2018;128:97–101. doi: 10.1016/j.fitote.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Jasicka-Misiak I., Poliwoda A., Petecka M., Buslovych O., Shlyapnikov V.A., Wieczorek P.P. Antioxidant phenolic compounds in salvia officinalis L. And salvia sclarea L. Ecol. Chem. Eng. S. 2018;25:133–142. [Google Scholar]
- 21.Pamukoff-Michelson R. Salvia officinalis: antimicrobial activity against coronaviruses and other pathogens. Application in respiratory diseases. Gen. Med. 2020;22:80–83. [Google Scholar]
- 22.Karami F., Yazdi A.D., Salahshourifar I., Beigi M.M. Investigating the effects of salvia chorassanica bunge and shoot extracts on gastric cancer cells: evidence of different behavior on various tumor grades. Pharmaceut. Sci. 2021;27:378–384. [Google Scholar]
- 23.Abou-Baker D.H., Amarowicz R., Kandeil A., Ali M.A., Ibrahim E.A. Antiviral activity of Lavandula angustifolia L. and Salvia officinalis L. essential oils against avian influenza H5N1 virus. J. Agric. Food Res. 2021;4:100135. doi: 10.1016/j.jafr.2021.100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balaei-Kahnamoei M., Eftekhari M., Ardekani M.R.S., Akbarzadeh T., Saeedi M., Jamalifar H., Safavi M., Sam S., Zhalehjoo N., Khanavi M. Phytochemical constituents and biological activities of Salvia macrosiphon Boiss. BMC Chem. 2021;15:1–7. doi: 10.1186/s13065-020-00728-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicolella H.D., Fernandes G., Ozelin S.D., Rinaldi-Neto F., Ribeiro A.B., Furtado R.A., Senedese J.M., Esperandim T.R., Veneziani R.C.S., Tavares D.C. Manool, a diterpene from Salvia officinalis, exerts preventive effects on chromosomal damage and preneoplastic lesions. Mutagenesis. 2021;36:177–185. doi: 10.1093/mutage/geab001. [DOI] [PubMed] [Google Scholar]
- 26.Garcia C.S.C., Menti C., Paula A., Lambert F., Barcellos T., Moura S., Calloni C., Branco C.S., Salvador M., Roesch-Ely M., Henriques J.A.P. Pharmacological perspectives from Brazilian Salvia officinalis (Lamiaceae): antioxidant, and antitumor in mammalian cells. An. Acad. Bras. Cienc. 2016;88:281–292. doi: 10.1590/0001-3765201520150344. [DOI] [PubMed] [Google Scholar]
- 27.Jaglanian A., Termini D., Tsiani E. Rosemary (Rosmarinus officinalis L.) extract inhibits prostate cancer cell proliferation and survival by targeting Akt and mTOR. Biomed. Pharmacother. 2020;131:110717. doi: 10.1016/j.biopha.2020.110717. [DOI] [PubMed] [Google Scholar]
- 28.Munz C. Latency and lytic replication in Epstein-Barr virus-associated oncogenesis. Nat. Rev. Microbiol. 2019;17:691–700. doi: 10.1038/s41579-019-0249-7. [DOI] [PubMed] [Google Scholar]
- 29.Cao Y., Xie L., Shi F., Tang M., Li Y., Hu J., Zhao L., Zhao L., Yu X., Luo X., Liao W., Bode A.M. Targeting the signaling in Epstein–Barr virus-associated diseases: mechanism, regulation, and clinical study. Signal Transduc. Target. Ther. 2021;6:1–33. doi: 10.1038/s41392-020-00376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLaughlin-Drubin M.E., Munger K. Viruses associated with human cancer. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2008;1782:127–150. doi: 10.1016/j.bbadis.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith A.J., Smith L.A. Viral carcinogenesis. Progr. Mol. Biol. Transl. Sci. 2016;144:121–168. doi: 10.1016/bs.pmbts.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Araldi R., Sant’Ana T., Módolo D., de Melo T., Spadacci-Morena D., de Cassia Stocco R., Cerutti J., de Souza E. The human papillomavirus (HPV)-related cancer biology: an overview. Biomed. Pharmacother. Biomed. Pharmacother. 2018;106:1537–1556. doi: 10.1016/j.biopha.2018.06.149. [DOI] [PubMed] [Google Scholar]
- 33.Zapatka M., Borozan I., Brewer D.S., Iskar M., Grundhoff A., Alawi M., Desai N., Sültmann H., Moch H., Cooper C.S., Eils R., Ferretti V., Lichter P. The landscape of viral associations in human cancers. Nat. Genet. 2020;52:320–330. doi: 10.1038/s41588-019-0558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai H., Feng Y., Fan P., Guo Y., Kuerban G., Chang C., Yao X., Peng Y., Wang R. HPV16 E6-specific T cell response and HLA-A alleles are related to the prognosis of patients with cervical cancer. Infect. Agents Cancer. 2021:1–11. doi: 10.1186/s13027-021-00395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zella D., Gallo R. Viruses and bacteria associated with cancer: an overview. Viruses. 2021;13 doi: 10.3390/v13061039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stingi A., Cirillo L. SARS-CoV-2 infection and cancer. Bioessays. 2021;43:2000289. doi: 10.1002/bies.202000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kennedy G., Komano J., Sugden B. Epstein-Barr virus provides a survival factor to Burkitt’s lymphomas. Proc. Natl. Acad. Sci. Unit. States Am. 2003;100:14269–14274. doi: 10.1073/pnas.2336099100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gruhne B., Sompallae R., Marescotti D., Kamranvar S.A., Gastaldello S., Masucci M.G. The Epstein–Barr virus nuclear antigen-1 promotes genomic instability via induction of reactive oxygen species. Proc. Natl. Acad. Sci. Unit. States Am. 2009;106:2313–2318. doi: 10.1073/pnas.0810619106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mesri E.A., Feitelson M., Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host Microbe. 2014;15:266. doi: 10.1016/j.chom.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Oliveira P.F., Munari C.C., Nicolella H.D., Veneziani R.C.S., Tavares D.C. Manool, a Salvia officinalis diterpene, induces selective cytotoxicity in cancer cells. Cytotechnology. 2015;68:2139–2143. doi: 10.1007/s10616-015-9927-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farré M.J., Insa S., Lamb A., Cojocariu C., Gernjak W. Occurrence of N-nitrosamines and their precursors in Spanish drinking water treatment plants and distribution systems. Environ. Sci.: Water Res. Technol. 2019;6:210–220. [Google Scholar]
- 42.de La Pomélie D., Santé-Lhoutellier V., Gatellier P. Mechanisms and kinetics of tryptophan N-nitrosation in a gastro-intestinal model. Food Chem. 2017;218:487–495. doi: 10.1016/j.foodchem.2016.08.131. [DOI] [PubMed] [Google Scholar]
- 43.Karwowska M., Kononiuk A. Nitrates/nitrites in food—risk for nitrosative stress and benefits. Antioxidants. 2020;9:241. doi: 10.3390/antiox9030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moradi S., Shariatifar N., Akbari-adergani B., Molaee Aghaee E., Arbameri M. Analysis and health risk assessment of nitrosamines in meat products collected from markets, Iran: with the approach of chemometric. J. Environ. Health Sci. Eng. 2021;2021:1–11. doi: 10.1007/s40201-021-00692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rendic S.P., Guengerich F.P. Human Family 1–4 cytochrome P450 enzymes involved in the metabolic activation of xenobiotic and physiological chemicals: an update. Arch. Toxicol. 2021:395–472. doi: 10.1007/s00204-020-02971-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ali S.S., Ahsan H., Zia M.K., Siddiqui T., Khan F.H. Understanding oxidants and antioxidants: classical team with new players. J. Food Biochem. 2020;44:e13145. doi: 10.1111/jfbc.13145. [DOI] [PubMed] [Google Scholar]
- 47.Kanner J., Selhub J., Shpaizer A., Rabkin B., Shacham I., Tirosh O. Redox homeostasis in stomach medium by foods: the Postprandial Oxidative Stress Index (POSI) for balancing nutrition and human health. Redox Biol. 2017;12:929–936. doi: 10.1016/j.redox.2017.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toh J.W.T., Wilson R.B. Pathways of gastric carcinogenesis, Helicobacter pylori virulence and interactions with antioxidant systems, vitamin C and phytochemicals. Int. J. Mol. Sci. 2020;21:6451. doi: 10.3390/ijms21176451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pedro D.F.N., Ramos A.A., Lima C.F., Baltazar F., Pereira-Wilson C. Colon cancer chemoprevention by sage tea drinking: decreased DNA damage and cell proliferation. Phytother Res. 2016;30:298–305. doi: 10.1002/ptr.5531. [DOI] [PubMed] [Google Scholar]
- 50.Sari A., Kursat M., Civelek S. Vitamin contents of some Salvia L. Taxa growing in Turkey. Chem. Nat. Compd. 2009;45:944–946. [Google Scholar]
- 51.Yaman C. Lemon balm and sage herbal teas: quantity and infusion time on the benefit of the content Chás de sálvia e erva-cidreira: quantidade e do tempo de infusão no benefício do conteúdo. Food Sci. Technol. 2020;44:1–11. [Google Scholar]
- 52.Lima C.F., Andrade P.B., Seabra R.M., Fernandes-Ferreira M., Pereira-Wilson C. The drinking of a Salvia officinalis infusion improves liver antioxidant status in mice and rats. J. Ethnopharmacol. 2005;97:383–389. doi: 10.1016/j.jep.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 53.Kivrak Ş., Göktürk T., Kivrak I., Kaya E., Karababa E. Investigation of phenolic profiles and antioxidant activities of some Salvia species commonly grown in Southwest Anatolia using UPLC-ESI-MS/MS. Food Sci. Technol. 2019;39:423–431. [Google Scholar]
- 54.He X., Yang J., Huang Y., Zhang Y., Wan H., Li C. Green and efficient ultrasonic-assisted extraction of bioactive components from salvia miltiorrhiza by natural deep eutectic solvents. Molecules. 2020;25:140. doi: 10.3390/molecules25010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu L., Gao H., Wen T., Gu T., Zhang S., Yuan Z. Tanshinone IIA attenuates AOM/DSS-induced colorectal tumorigenesis in mice via inhibition of intestinal inflammation. Pharmaceut. Biol. 2021;59:89–96. doi: 10.1080/13880209.2020.1865412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang D., Xu P., Li Y., Wei B., Yang S., Zheng Y., Lyu L., Deng Y., Zhai Z., Li N., Wang N., Lyu J., Dai Z. Association of vitamin C intake with breast cancer risk and mortality: a meta-analysis of observational studies. Aging (Albany NY) 2020;12:18435. doi: 10.18632/aging.103769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keuleyan E., Bonifacie A., Gatellier P., Ferreira C., Blinet S., Promeyrat A., Nassy G., Santé-Lhoutellier V., Théron L. Design of an in vitro model to screen the chemical reactivity induced by polyphenols and vitamins during digestion: an application to processed meat. Foods. 2021;10:2230. doi: 10.3390/foods10092230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herrmann S.S., Granby K., Duedahl-Olesen L. Formation and mitigation of N-nitrosamines in nitrite preserved cooked sausages. Food Chem. 2015;174:516–526. doi: 10.1016/j.foodchem.2014.11.101. [DOI] [PubMed] [Google Scholar]
- 59.Furtado R.A., Oliveira B.R., Silva L.R., Cleto S.S., Munari C.C., Cunha W.R., Tavares D.C. Chemopreventive effects of rosmarinic acid on rat colon carcinogenesis. Eur. J. Cancer Prev. 2015;24:106–112. doi: 10.1097/CEJ.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 60.Salama A.A.A., Allam R.M. Promising targets of chrysin and daidzein in colorectal cancer: amphiregulin, CXCL1, and MMP-9. Eur. J. Pharmacol. 2021;892:173763. doi: 10.1016/j.ejphar.2020.173763. [DOI] [PubMed] [Google Scholar]
- 61.Reed L., Arlt V.M., Phillips D.H. The role of cytochrome P450 enzymes in carcinogen activation and detoxication: an in vivo–in vitro paradox. Carcinogenesis. 2018;39:851–859. doi: 10.1093/carcin/bgy058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taymaz-Nikerel H., Karabekmez M.E., Eraslan S., Kırdar B. Doxorubicin induces an extensive transcriptional and metabolic rewiring in yeast cells. Sci. Rep. 2018;8:1–14. doi: 10.1038/s41598-018-31939-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qiao X., van der Zanden S.Y., Wander D.P.A., Borràs D.M., Song J.-Y., Li X., van Duikeren S., van Gils N., Rutten A., van Herwaarden T., van Tellingen O., Giacomelli E., Bellin M., Orlova V., Tertoolen L.G.J., Gerhardt S., Akkermans J.J., Bakker J.M., Zuur C.L., Pang B., Smits A.M., Mummery C.L., Smit L., Arens R., Li J., Overkleeft H.S., Neefjes J. Uncoupling DNA damage from chromatin damage to detoxify doxorubicin. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117:15182–15192. doi: 10.1073/pnas.1922072117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shakeri A., Delavari S., Ebrahimi S.N., Asili J., Emami S.A., Tayarani-Najaran Z. A new tricyclic abietane diterpenoid from Salvia chloroleuca and evaluation of cytotoxic and apoptotic activities. Revista Brasileira de Farmacognosia. 2019;29:30–35. [Google Scholar]
- 65.Della Corte L., Foreste V., Claudia D.F., Giampaolino P., Bifulco G. 2021. Poly (ADP-Ribose) Polymerase (PARP) as Target for the Treatment of Epithelial Ovarian Cancer: what to Know; pp. 543–554. [DOI] [PubMed] [Google Scholar]
- 66.Taylor A., Chan D., Tio V., Patil S., Traina T., Robson M., Khasraw M. PARP (Poly ADP-Ribose Polymerase) inhibitors for locally advanced or metastatic breast cancer. Cochrane Database Syst. Rev. 2021;4 doi: 10.1002/14651858.CD011395.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pletcher J.P., Bhattacharjee S., Doan J.P., Wynn R., Sindhwani P., Nadiminty N., Petros F.G. The emerging role of poly (ADP-Ribose) polymerase inhibitors as effective therapeutic agents in renal cell carcinoma. Front. Oncol. 2021:2559. doi: 10.3389/fonc.2021.681441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singh H.M., Bailey P., Hübschmann D., Berger A.K., Neoptolemos J.P., Jäger D., Siveke J., Springfeld C. Poly(ADP-ribose) polymerase inhibition in pancreatic cancer. Gene Chromosome Cancer. 2021;60:373–384. doi: 10.1002/gcc.22932. [DOI] [PubMed] [Google Scholar]
- 69.Shakeri A., Farahmand S.S., Tayarani-Najaran Z., Emami S.A., Kúsz N., Hohmann J., Boozari M., Tavallaie F.Z., Asili J. 4,5-Seco-5,10-friedo-abietane-type diterpenoids with anticancer activity from Salvia atropatana Bunge. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020;394:241–248. doi: 10.1007/s00210-020-01967-2. [DOI] [PubMed] [Google Scholar]
- 70.Telang N. Anti-proliferative and pro-apoptotic effects of rosemary and constituent terpenoids in a model for the HER-2-enriched molecular subtype of clinical breast cancer. Oncol. Lett. 2018;16:5489–5497. doi: 10.3892/ol.2018.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pérez-Sánchez A., Barrajón-Catalán E., Ruiz-Torres V., Agulló-Chazarra L., Herranz-López M., Valdés A., Cifuentes A., Micol V. Rosemary (Rosmarinus officinalis) extract causes ROS-induced necrotic cell death and inhibits tumor growth in vivo. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-018-37173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mirzaei H.H., Firuzi O., Schneider B., Baldwin I.T., Jassbi A.R. Cytotoxic diterpenoids from the roots of Salvia lachnocalyx. Revista Brasileira de Farmacognosia. 2017;27:475–479. [Google Scholar]
- 73.Uchihara Y., Tago K., Taguchi H., Narukawa Y., Kiuchi F., Tamura H., Funakoshi-Tago M. Elsevier; 2018. Taxodione Induces Apoptosis in BCR-ABL-Positive Cells through ROS Generation. [DOI] [PubMed] [Google Scholar]
- 74.Rengarajan T., Keerthiga S., Duraikannu S., Periyannan V. Exploring the anticancer and anti-inflammatory activities of ferruginol in MCF-7 breast cancer cells. Cancer. 2020;1:1–12. [Google Scholar]
- 75.Jia Y., Wu C., Zhang B., Zhang Y., Li J. Ferruginol induced apoptosis on SK-Mel-28 human malignant melanoma cells mediated through P-p38 and NF-κB. Hum. Exp. Toxicol. 2018 doi: 10.1177/0960327118792050. [DOI] [PubMed] [Google Scholar]
- 76.Mirzaei H.H., Firuzi O., Jassbi A.R. Diterpenoids from roots of salvia lachnocalyx;In-silico and in-vitro toxicity against human cancer cell lines. Iran. J. Pharm. Res. (IJPR) 2020;19:94. doi: 10.22037/ijpr.2019.15429.13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lim W., Ryu S., Bazer F.W., Kim S.-M., Song G. Chrysin attenuates progression of ovarian cancer cells by regulating signaling cascades and mitochondrial dysfunction. J. Cell. Physiol. 2017;233:3129–3140. doi: 10.1002/jcp.26150. [DOI] [PubMed] [Google Scholar]
- 78.Lima A.P.B., Almeida T.C., Barros T.M.B., Rocha L.C.M., Garcia C.C.M., da Silva G.N. Toxicogenetic and antiproliferative effects of chrysin in urinary bladder cancer cells. Mutagenesis. 2020;35:361–371. doi: 10.1093/mutage/geaa021. [DOI] [PubMed] [Google Scholar]
- 79.Bahadori M., Baharara J., Amini E. Anticancer properties of chrysin on colon cancer cells, in vitro and in vivo with modulation of caspase-3, -9, bax and sall4. Iran. J. Biotechnol. 2016;14:117–124. doi: 10.15171/ijb.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhong X., Liu D., Jiang Z., Li C., Chen L., Xia Y., Liu D., Yao Q., Wang D. Chrysin induced cell apoptosis and inhibited invasion through regulation of TET1 expression in gastric cancer cells. OncoTargets Ther. 2020;13:3287. doi: 10.2147/OTT.S246031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee S., Lee S.K., Jung J. Potentiating activities of chrysin in the therapeutic efficacy of 5-fluorouracil in gastric cancer cells. Oncol. Lett. 2021;21:7. doi: 10.3892/ol.2020.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Walle U., Galijatovic A., Walle T. Transport of the flavonoid chrysin and its conjugated metabolites by the human intestinal cell line Caco-2. Biochem. Pharmacol. 1999;58:431–438. doi: 10.1016/s0006-2952(99)00133-1. [DOI] [PubMed] [Google Scholar]
- 83.Patel A. Benign vs malignant tumors. JAMA Oncol. 2020;6:1488. doi: 10.1001/jamaoncol.2020.2592. [DOI] [PubMed] [Google Scholar]
- 84.Cai Y., Lv F., Kaldybayeva N., Zhamilya A., Wu Z., Wu Y. 15, 16-dihydrotanshinone I inhibits hemangiomas through inducing pro-apoptotic and anti-angiogenic mechanisms in vitro and in vivo. Front. Pharmacol. 2018:25. doi: 10.3389/fphar.2018.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li H., Prever L., Hirsch E., Gulluni F. Targeting PI3K/AKT/mTOR signaling pathway in breast cancer. Cancers. 2021;13:3517. doi: 10.3390/cancers13143517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun Z., Wang Z., Liu X., Wang D. New development of inhibitors targeting the PI3K/AKT/mTOR pathway in personalized treatment of non-small-cell lung cancer. Anti Cancer Drugs. 2015;26:1–14. doi: 10.1097/CAD.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 87.Tan A. Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC) Thoracic Cancer. 2020;11:511–518. doi: 10.1111/1759-7714.13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.None I., Pothongsrisit S., Pongrakhananon V. Targeting the PI3K/AKT/mTOR signaling pathway in lung cancer: an update regarding potential drugs and natural products. Molecules (Basel, Switzerland) 2021:26. doi: 10.3390/molecules26134100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rascio F., Spadaccino F., Rocchetti M., Castellano G., Stallone G., Netti G., R. E The pathogenic role of PI3K/AKT pathway in cancer onset and drug resistance: an updated review. Cancers. 2021;13 doi: 10.3390/cancers13163949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim S., Hwangbo H., Kim M., Ji V., Lee H., Kim G., Kwon C., Leem S., Hong S., Cheong J., Choi Y. Coptisine induces autophagic cell death through down-regulation of PI3K/Akt/mTOR signaling pathway and up-regulation of ROS-mediated mitochondrial dysfunction in hepatocellular carcinoma Hep3B cells. Arch. Biochem. Biophys. 2021:697. doi: 10.1016/j.abb.2020.108688. [DOI] [PubMed] [Google Scholar]
- 91.Qian S., Fang H., Zheng L., Liu M. Zingerone suppresses cell proliferation via inducing cellular apoptosis and inhibition of the PI3K/AKT/mTOR signaling pathway in human prostate cancer PC-3 cells. J. Biochem. Mol. Toxicol. 2021;35 doi: 10.1002/jbt.22611. [DOI] [PubMed] [Google Scholar]
- 92.Shi J., Lv H., Tang C., Li Y., Huang J., Zhang H. Mangiferin inhibits cell migration and angiogenesis via PI3K/AKT/mTOR signaling in high glucose- and hypoxia-induced RRCECs. Mol. Med. Rep. 2021;23 doi: 10.3892/mmr.2021.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang H., Xie X., Li G., Chen J., Li M., Xu X., Xiong Q., Chen G., Yin Y., Peng F., Chen V., Peng C. Erianin inhibits human lung cancer cell growth via PI3K/Akt/mTOR pathway in vitro and in vivo. Phytother Res. : PTR. 2021;35:4511–4525. doi: 10.1002/ptr.7154. [DOI] [PubMed] [Google Scholar]
- 94.Zhang X., Niu Y., Huang Y. Melatonin inhibits cell proliferation in a rat model of breast hyperplasia by mediating the PTEN/AKT pathway. Oncol. Rep. 2021;45:1–9. doi: 10.3892/or.2021.8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen K., Zhu P., Chen W., Luo K., Shi X., Zhai W. Melatonin inhibits proliferation, migration, and invasion by inducing ROS-mediated apoptosis via suppression of the PI3K/Akt/mTOR signaling pathway in gallbladder cancer cells. Aging. 2021;13:22502–22515. doi: 10.18632/aging.203561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu T., Liang X., Sun Y., Yang S. Rapamycin suppresses the PI3K/AKT/mTOR signaling pathway by targeting SIRT1 in esophageal cancer. Exp. Ther. Med. 2021;22 doi: 10.3892/etm.2021.10624. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 97.Meganck R.M., Baric R.S. Developing therapeutic approaches for twenty-first-century emerging infectious viral diseases. Nat. Med. 2021;27(3):401–410. doi: 10.1038/s41591-021-01282-0. [DOI] [PubMed] [Google Scholar]
- 98.Ghildiyal R., Prakash V., Chaudhary V.K., Gupta V., Gabrani R. Phytochemicals as antiviral agents: recent updates. Plant-Derived Bioact. 2020;279 [Google Scholar]
- 99.Wu Y.-B., Ni Z.-Y., Shi Q.-W., Dong M., Kiyota H., Gu Y.-C., Cong B. Constituents from salvia species and their biological activities. Chem. Rev. 2012;112:5967–6026. doi: 10.1021/cr200058f. [DOI] [PubMed] [Google Scholar]
- 100.Sivropoulou A., Nikolaou C., Papanikolaou E., Kokkini S., Lanaras T., Arsenakis M. Antimicrobial, cytotoxic, and antiviral activities of salvia fructicosa essential oil. J. Agric. Food Chem. 1997;45:3197–3201. [Google Scholar]
- 101.Özçeli̇k B., Erdoğan Orhan İ., Kan Y. Determination of antiviral activity and cytotoxicity of selected sage (salvia L.) species. Fabad J. Pharm. Sci. 2011;36:155–160. [Google Scholar]
- 102.Öğütçü H., Sökmen A., Sökmen M., Polissiou M., Serkedjieva J., Daferera D., Şahin F., Bariş Ö., Güllüce M. Bioactivities of the various extracts and essential oils of salvia limbata C.A.Mey. and salvia sclarea L. Turk. J. Biol. 2008;32:181–192. [Google Scholar]
- 103.Schnitzler P., Nolkemper S., Stintzing F., Reichling J. Comparative in vitro study on the anti-herpetic effect of phytochemically characterized aqueous and ethanolic extracts of Salvia officinalis grown at two different locations. Phytomedicine : Int. J. Phytother. Phytopharmacol. 2008;15:62–70. doi: 10.1016/j.phymed.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 104.Cagno V., Sgorbini B., Sanna C., Cagliero C., Ballero M., Civra A., Donalisio M., Bicchi C., Lembo D., Rubiolo P. In vitro anti-herpes simplex virus-2 activity of Salvia desoleana Atzei & V. Picci essential oil. PLoS One. 2017;12 doi: 10.1371/journal.pone.0172322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abdelrahman Z., Li M., Wang X. Comparative review of SARS-CoV-2, SARS-CoV, MERS-CoV, and influenza A respiratory viruses. Front. Immunol. 2020:2309. doi: 10.3389/fimmu.2020.552909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bang S., Quy Ha T., Lee C., Li W., Oh W., Shim S. Antiviral activities of compounds from aerial parts of Salvia plebeia R. Br. J. Ethnopharmacol. 2016;192:398–405. doi: 10.1016/j.jep.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 107.Alim A., Goze I., Goze H.M., Tepe B., Serkedjieva J. In vitro antimicrobial and antiviral activities of the essential oil and various extracts of Salvia cedronella Boiss. J. Med. Plants Res. 2009;3:413–419. [Google Scholar]
- 108.Choi H.-J. Chemical constituents of essential oils possessing anti-influenza A/WS/33 virus activity. Osong Publ. Health Res.Perspect. 2018;9:348–353. doi: 10.24171/j.phrp.2018.9.6.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Han M., Lee P. 1999. US6043276A - Compounds Obtained from Salvia Species Having Antiviral Activity - Google Patents.https://patents.google.com/patent/US6043276 [Google Scholar]
- 110.Zhang Y., Xie Y., Liao X., Jia Q., Chai Y. A Chinese patent medicine Salvia miltiorrhiza depside salts for infusion combined with conventional treatment for patients with angina pectoris: a systematic review and meta-analysis of randomized controlled trials. Phytomedicine. 2017;25:100–117. doi: 10.1016/j.phymed.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 111.yu Liang Y., huan Wan X., jv Niu F., min Xie S., Guo H., ying Yang Y., yan Guo L., zheng Zhou C. Salvia plebeia R. Br. : an overview about its traditional uses, chemical constituents, pharmacology and modern applications. Biomed. Pharmacother. 2020;121:109589. doi: 10.1016/j.biopha.2019.109589. [DOI] [PubMed] [Google Scholar]
- 112.Song J.H., Kwon B.E., Jang H., Kang H., Cho S., Park K., Ko H.J., Kim H. Antiviral activity of chrysin derivatives against coxsackievirus B3 in vitro and in vivo. Biomol. Ther. 2015;23:465–470. doi: 10.4062/biomolther.2015.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang J., Zhang T., Du J., Cui S., Yang F., Jin Q. Anti-enterovirus 71 effects of chrysin and its phosphate ester. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li Z., Zhao J., Zhou H., Li L., Ding Y., Li J., Zhou B., Jiang H., Zhong N., Hu W., Yang Z. Cappariloside A shows antiviral and better anti-inflammatory effects against influenza virus via regulating host IFN signaling, in vitro and vivo. Life Sci. 2018;200:115–125. doi: 10.1016/j.lfs.2018.03.033. [DOI] [PubMed] [Google Scholar]
- 115.Kim S.R., Jeong M.S., Mun S.H., Cho J., Seo M.D., Kim H., Lee J., Song J.H., Ko H.J. Antiviral activity of chrysin against influenza virus replication via inhibition of autophagy. Viruses. 2021;13 doi: 10.3390/v13071350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bang S., Quy Ha T.K., Lee C., Li W., Oh W.K., Shim S.H. Antiviral activities of compounds from aerial parts of Salvia plebeia R. Br. J. Ethnopharmacol. 2016;192:398–405. doi: 10.1016/j.jep.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 117.Bang S., Li W., Ha T.K.Q., Lee C., Oh W.K., Shim S.H. Anti-influenza effect of the major flavonoids from Salvia plebeia R.Br. via inhibition of influenza H1N1 virus neuraminidase. Nat. Prod. Res. 2018;32:1224–1228. doi: 10.1080/14786419.2017.1326042. [DOI] [PubMed] [Google Scholar]
- 118.van de Sand L., Bormann M., Schmitz Y., Heilingloh C.S., Witzke O., Krawczyk A. Antiviral active compounds derived from natural sources against herpes simplex viruses. Viruses. 2021;13 doi: 10.3390/v13071386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Akram M., Tahir I.M., Shah S.M.A., Mahmood Z., Altaf A., Ahmad K., Munir N., Daniyal M., Nasir S., Mehboob H. Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: a systematic review. Phytother Res. : PTR. 2018;32:811–822. doi: 10.1002/ptr.6024. [DOI] [PubMed] [Google Scholar]
- 120.Zhu H., Wei L., Niu P. The novel coronavirus outbreak in Wuhan, China. Glob. Health Res. Pol. 2020;5:1–3. doi: 10.1186/s41256-020-00135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Manzanares-Meza L.D., Medina-Contreras O., Manzanares-Meza L.D., Medina-Contreras O. SARS-CoV-2 and influenza: a comparative overview and treatment implications. Boletín Médico Del Hospital Infantil de México. 2020;77:262–273. doi: 10.24875/BMHIM.20000183. [DOI] [PubMed] [Google Scholar]
- 122.Hu S., Wang J., Zhang Y., Bai H., Wang C., Wang N., He L. Three salvianolic acids inhibit 2019-nCoV spike pseudovirus viropexis by binding to both its RBD and receptor ACE2. J. Med. Virol. 2021;93:3143–3151. doi: 10.1002/jmv.26874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Azad M.M., Al Mahmud A., Islam M.S., Gouhar A.I. Common sage (Salvia officinalis) antiviral role: potentiality of a Unani hand sanitizer in COVID-19 (corona virus) second wave control. Asian J. Med. Biol. Res. 2020;6:611–617. [Google Scholar]
- 124.Zackria A.A., Pattabiraman R., Murthy T.P.K., Kumar S.B., Mathew B.B., Biju V.G. Computational screening of natural compounds from Salvia plebeia R. Br. for inhibition of SARS-CoV-2 main protease. Vegetos (Bareilly, India) 2021:1–15. doi: 10.1007/s42535-021-00304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu B.-W., Pan T.-L., Leu Y.-L., Chang Y.-K., Tai P.-J., Lin K.-H., Horng J.-T. Antiviral effects of salvia miltiorrhiza (Danshen) against enterovirus 71. Am. J. Chin. Med. 2012;35:153–168. doi: 10.1142/S0192415X07004709. [DOI] [PubMed] [Google Scholar]
- 126.Chung Y.C., Hsieh F.C., Lin Y.J., Wu T.Y., Lin C.W., Lin C.T., Tang N.Y., Jinn T.R. Magnesium lithospermate B and rosmarinic acid, two compounds present in Salvia miltiorrhiza, have potent antiviral activity against enterovirus 71 infections. Eur. J. Pharmacol. 2015;755:127–133. doi: 10.1016/j.ejphar.2015.02.046. [DOI] [PubMed] [Google Scholar]
- 127.Šmidling D., Mitić-Ćulafić D., Vuković-Gačćić B., Simić D., Knežević-Vukčević J. Evaluation of antiviral activity of fractionated extracts of sage Salvia officinalis L. (Lamiaceae) Arch. Biol. Sci. 2008;60:421–429. [Google Scholar]
- 128.Santoyo S., Jaime L., García-Risco M.R., Ruiz-Rodríguez A., Reglero G. Antiviral properties of supercritical CO2 extracts from oregano and sage. Int. J. Food Prop. 2014;17:1150–1161. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.