Abstract
Background:
Acute hepatitis B virus (HBV) infections in the United States occur predominantly among persons aged 30–59 years. The Centers for Disease Control and Prevention (CDC) recommends vaccination of adults at increased risk for HBV infection. Completing the hepatitis B (HepB) vaccine dose-series is critical for optimal immune response.
Objectives:
CDC funded 14 health departments (awardees) from 2012 to 2015 to implement a pilot HepB vaccination program for high-risk adults. We evaluated the pilot program to assess vaccine utilization; vaccine dose-series completion, including by vaccination location type; and implementation challenges.
Methods:
Awardees collaborated with sites providing health care to persons at increased risk for HBV infection. Awardees collected information on doses administered, vaccine dose-series completion, and challenges completing and tracking vaccinations, including use of immunization information systems (IIS). Data were reported by each awardee in aggregate to CDC.
Results:
Six of 14 awardees administered 47,911 doses and were able to report patient-level dose-series completion. Among persons who received dose 1, 40.4% received dose 2, and 22.3% received dose 3. Local health department clinics had the highest 3–dose-series completion, 60.6% (531/876), followed by federally qualified health centers at 38.0% (923/2432). While sexually transmitted diseases (STD) clinics administered the most doses in total (17,173 [35.8% of 47,911 doses]), 3–dose-series completion was low (17.1%). The 14 awardees reported challenges regarding completing and tracking dose-series, including reaching high-risk adults for follow-up and inconsistencies in use of IIS or other tracking systems across sites.
Conclusions:
Dose-series completion was low in all settings, but lowest where patients may be less likely to return for follow-up (e.g., STD clinics). Routinely assessing HepB vaccination needs of high-risk adults, including through use of IIS where available, may facilitate HepB vaccine dose-series completion.
Keywords: Hepatitis B, Hepatitis B vaccine, Immunization information systems, Vaccine registry, Adults, Vaccine implementation, Vaccination rates, Dose-series completion
1. Introduction
1.1. Background
Hepatitis B (HepB) is an acute or chronic infectious disease resulting from infection with the hepatitis B virus (HBV). HBV is spread through contact with blood or other bodily fluids. Infection can occur through unprotected sexual contact, sharing of needles, occupational exposure (e.g., among health care personnel), and perinatal transmission [1,2]. HBV is highly infectious; exposures may occur in the absence of visible blood, and HBV may remain viable 7 days or more on hard surfaces [3,4]. Acute HBV infection can result in severe illness and death from liver failure, or lead to chronic HBV infection and development of cirrhosis, liver cancer, liver failure, and death. About 50% of adult HBV infections are asymptomatic [1,5–9].
Universal HepB vaccination of children was recommended in the United States beginning in 1991, resulting in a >90% decline in pediatric cases of HepB [1,2,6]. U.S. cases now occur predominately among adults 30–59 years [6]. The most commonly reported risk factors for acute HBV infection include using injection drugs; having sexual contact with a suspected or confirmed HBV-infected person; being a man who has sex with men (MSM); having ≥2 sex partners concurrently; and having household contact with a person suspected or confirmed to be infected with HBV [6]. However, most acute HepB cases reported to CDC do not include information on risk factors, or the risk factor is unknown [6].
CDC’s Advisory Committee on Immunization Practices (ACIP) recommends vaccination of adults at risk for HBV infection, such as adults at risk from percutaneous exposure to blood (e.g., by injection drug use) and sexual contact with a HBV-infected person. ACIP also recommends vaccination of adults requesting HepB vaccination without reporting a specific risk factor [2,10]. Until April 2018, only alum-adjuvanted single antigen recombinant HepB vaccines (i.e., Energix-B® or Recombivax®) or a combined hepatitis A and hepatitis B vaccine (i.e., Twinrix®), given as 3-dose series at 0, 1, and 6 months, were recommended for HepB prevention. In April 2018, ACIP recommended a newly licensed HepB vaccine (Heplisav-B®) with a novel antigen that is given as a 2-dose series (0 and 1 month) for adults ≥18 years [10].
When vaccinated with only one or two doses in a 3-dose series of alum-adjuvanted HepB vaccine—the type of HepB vaccine used in this pilot—serologic protection from HBV infection is diminished. A protective antibody response among healthy adults <40 years of age is estimated to occur in 30%–55% after one dose, 75% after two doses, and >90% after three doses of alum-adjuvanted HepB vaccine. The third dose is also important for long-term duration of protection after vaccination [1,2,10–14].
As a strategy to reach high-risk adults, ACIP recommends HepB vaccination without assessment of risk factors in certain settings where a high proportion of individuals have risk factors for HBV infection (i.e. universal settings). Examples of such settings include HIV testing or treatment facilities, sexually transmitted disease (STD) treatment facilities, facilities providing drug-abuse treatment and prevention services, health care settings targeting services to injection drug users (IDU), correctional facilities, and health care settings targeting services to men who have sex with men (MSM) [2]. ACIP also recommends HepB vaccination in other settings where patients with risk factors for HepB can be identified and vaccinated (i.e., non-universal settings). Although ACIP has recommended HepB vaccination of adults with risk factors for HBV infection since before 1991 [1,2], vaccination coverage among high-risk adults remains low [15].
In 2012, U.S. immunization programs were able to submit proposals for a CDC-funded HepB vaccination pilot project. The purpose of the pilot was to reduce the incidence of acute HBV infection through targeted HepB vaccination of adults who presented for medical care in universal settings (i.e., those settings where all patients are likely to be at high risk) and adults at increased risk of HBV infection seen in other (i.e., non-universal) settings (Table 1) [2]. We analyzed data from the reports of 14 awardees regarding implementation of the HepB vaccination pilot and data from six of the 14 awardees that were able to track patient-level HepB vaccination dose-series completion. We evaluated the pilot program to assess vaccine utilization; vaccine dose-series completion, including by vaccination location type; use of strategies to improve vaccine uptake; and implementation challenges encountered.
Table 1.
Groups of persons at increased risk of hepatitis B virus (HBV) infection targeted by hepatitis B vaccination pilot program.1
|
The pilot project did not include targeting hepatitis B vaccination of healthcare personnel, persons with end-stage renal disease, or international travelers to regions with high or intermediate levels of endemic HBV infection. Some awardees reported using some vaccine for non-pilot program targeted settings and patients such as at college events, persons with diabetes seen for care in FQHCs, and community outreach events targeting homless, minority, and immigant populations.
2. Methods
2.1. Selection of awardees
All 64 local and state health department immunization programs in the United States were eligible to apply for the HepB vaccination pilot if their jurisdiction had an incidence of acute HepB ≥ 1.2 cases per 100,000 total population, based on 2010 data from the National Notifiable Disease Surveillance System [6]. The initial project period was 2 years (September 2012–2014); 12 of 14 awardees requested a one-year extension to September 2015. Applicants were required to indicate they could begin vaccinating in fall 2012, and to demonstrate their ability to target vaccination efforts in settings that served adults at increased risk for acute HepB (Table 1). Programs with existing relationships with settings where HepB vaccination for all adults is recommended were considered most likely to be able to initiate vaccinations before fall 2012. Programs were also asked to provide summary information on doses administered that were recorded in their jurisdiction’s Immunization Information Systems (IIS).
The pilot program targetted vaccines for certain high-risk persons, as indicated in Table 1. However, the pilot program did not include targeting HepB vaccination for health care personnel, persons with end-stage renal disease, or international travelers to regions with high or intermediate levels of endemic HBV infection [1,2,10]. Some awardees reported using some vaccine for non-pilot program targeted settings and patients such as at college events, persons with diabetics seen for care in federally qualified health centers (FQHCs), and community outreach events targeting homeless, minority and immigrant populations. However, the proportion of vaccine administered to persons in non-targeted pilot program groups during the program period is likely small given that the vaccine was administered mostly in universal settings and only 5% of vaccine was administered in “other” settings, such as homeless shelters, mental health facilities, tuberculosis clinics, nursing homes associated with HBV outbreaks, community centers, and community outreach events.
Awardees were recommended to follow ACIP recommendations on HepB vaccination implementation and implement strategies for vaccination as recommended by the US Preventative Services Task Force [1,2,16]. Testing for markers of prior or existing HBV infection, prior HepB vaccination, or immune response to vaccination was conducted at medical providers’ discretion; funding for testing was not included.
The pilot programs, including the evaluation, were reviewed by CDC for human subjects research determination. None of the projects were determined to be research and therefore IRB review was not needed. Only aggregated data was sent to CDC to allow for pilot program evaluation.
Operational costs for HepB pilot awardees were included in this pilot, because the lack of operational funds was reported as a significant barrier to implementing a previous Adult Hepatitis B Vaccination Initiative in 2007–2009 [17]. Operational funds were designated for identifying specific populations and settings and implementing evidence-based strategies to increase HepB vaccination, per ACIP HepB vaccination recommendations [1,2] and the Community Guide to Preventive Services [16,18,19].
Applications included estimates of the number of HepB vaccine doses that the awardees and their partners projected administering. Single-antigen alum-adjuvanted HepB vaccines (Energix-B® and Recombivax®) were made available to awardees through direct assistance from CDC. Applicants’ proposed operational budgets could include <50% of the total vaccine cost.
2.2. Data collection and summary reports
CDC provided templates to awardees to standardize the information collected and reported to CDC (Appendix Table A1). Awardees submitted monthly narrative reports, quarterly narrative and quantitative reports, and a final report. Monthly reports included the number of vaccine doses ordered, number of doses administered, and challenges encountered. Quarterly reports included the number of doses ordered and administered by facility type and by demographics, and the number of doses administered by vaccine dose-series order (i.e., first, second, or third dose). Non-universal vaccination sites (e.g., FQHCs serving the general population) were asked to collect HepB risk factors of vaccination recipients. In the final report, the awardees summarized the elements collected in the monthly and quarterly reports, as well as reported on pilot implementation practices used, challenges, and lessons learned.
Reporting on the number of unused, wasted, and invalid doses during the one-year extension period of the project (year 3) was added to the reporting template for awardees that had HepB vaccine pilot doses remaining at the end of the 2-year pilot.
2.3. Data analysis
Aggregated awardee data from quarterly reports on HepB vaccine doses ordered, administered, and wasted; summary demographic information regarding pilot HepB vaccine dose recipients; dose-series completion; reported risk factors; and vaccination settings were analyzed using SAS 9.3 (SAS Institute Inc., Cary, NC).
3. Results
3.1. Overview of awardees and activities
Fourteen awardees were selected: Alabama, Chicago, Florida, Kentucky, Louisiana, Maryland, Michigan, Nevada, New York City, Oregon, San Antonio, Tennessee, Virginia, and West Virginia. All except San Antonio had participated in the Adult Hepatitis B Vaccination Initiative during 2007–2009 [17].
Program operations costs of $200,963–$400,000 were provided to each awardee (totaling $4,211,936 for all 14 awardees). CDC allotted 176,990 HepB vaccine doses for the pilot project based on awardees’ and their partners’ initial projections of the number of doses that they would administer.
A total of 459 settings participated in the pilot project (Table 2). Health departments (n = 159, 34.6%), correctional facilities (n = 81, 17.6%), and STD clinics (n = 77, 16.8%) accounted for 69.1% (317/459) of the settings.
Table 2.
Hepatitis B vaccination pilot facility types by awardees, September 2012–September 2015.
| Number of Facilities by Type of Facility by Awardee | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Facility Type | AL | Chicago | FL | KY | LA | MD | MI | NV | New York City | OR | San Antonio | TN | VA | WV | Total |
| Department of Corrections | 20 | 4 | 0 | 7 | 0 | 3 | 0 | 0 | 11 | 14 | 0 | 17 | 0 | 5 | 81 |
| Drug treatment facility | 0 | 13 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 6 | 0 | 0 | 5 | 27 |
| Federally qualified health center | 0 | 15 | 0 | 6 | 1 | 6 | 0 | 2 | 2 | 0 | 0 | 0 | 12 | 0 | 44 |
| Health care facility targeting IDU | 0 | 4 | 0 | 0 | 0 | 1 | 11 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 17 |
| Health care setting targeting MSM | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 3 |
| HIV clinic | 0 | 1 | 0 | 0 | 1 | 4 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 3 | 12 |
| Local health department clinic | 67 | 0 | 7 | 67 | 4 | 10 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 159 |
| STD clinic | 0 | 0 | 0 | 0 | 3 | 7 | 9 | 1 | 1 | 0 | 1 | 38 | 0 | 17 | 77 |
| Other1 | 0 | 4 | 0 | 3 | 0 | 11 | 1 | 2 | 2 | 0 | 16 | 0 | 0 | 0 | 39 |
| Totals | 87 | 42 | 7 | 83 | 9 | 44 | 23 | 10 | 17 | 14 | 26 | 55 | 12 | 30 | 459 |
Abbreviations: AL = Alabama; FL = Florida; IDU = injection drug user; KY = Kentucky; LA = Louisiana; MD = Maryland; MI = Michigan; MSM = men who have sex with men; NV = Nevada; OR = Oregon; STD = sexually transmitted disease; TN = Tennessee; VA = Virginia; WV = West Virginia.
Footnotes: Chicago “Other” included 3 Asian community based-agencies and 1 homeless shelter; Kentucky “Other” included 3 rural health clinics and community clinics combined; Maryland “Other” included 9 community-based organizations, 1 rescue mission, and 1 addictions clinic; Michigan “Other” included 1 Asian Liver Center; Nevada “Other” included 1 university setting and 1 pan-Asian community clinic (none or only a few vaccines administered at this site); New York City “Other” included 2 community-based organizations: African Services Committee and Korean Community Services; San Antonio “Other” included 1 homeless shelter, 4 mental health facilities, 6 college events, 2 HIV/AIDS resource fairs, 1 community health fair, 1 community back-to-school block party, and 1 outreach with a celebrity rapper; and West Virginia reported 17 county health department/STD clinics, which are included in the STD category, and 5 substance abuse treatment/IDU facilities, which are included in the drug treatment facility category for this table.
Evidence-based strategies that awardees reported were implemented by vaccination sites included standing orders, use of IIS, client reminder/recall systems, provider assessment and feedback, provider reminder systems, and reducing patient out-of-pocket costs [16]. All but one awardee reported utilization of their IIS to some extent. Client or family incentive rewards were used in one awardee’s site, and five awardees used combinations of community-based interventions (e.g., community education and health fairs) with other interventions. (Appendix Table A2) However, the pilot was not designed to quantify the consistency with which strategies were used or the impact of individual strategies.
3.2. Awardees’ hepatitis B vaccine use
During September 2012–September 2015, 161,171 HepB vaccine doses were ordered and distributed, representing 91% of the initial number of needed doses estimated by awardees (176,990 doses). (Table A3). Of the 161,171 doses ordered, awardees reported 139,110 (86.3%) were administered; 17,045 (10.6%) were unused; and 7251 (4.5%) were wasted (e.g., due to expiration of doses or interruption in the cold chain). Barriers such as delays in staff hiring or staff turnover, fewer vaccinators available at locations and lower vaccine acceptance than anticipated, and challenges with follow-up for next vaccine doses were cited for lower-than-projected vaccine use. Reporting the use of pilot HepB vaccine doses after September 2015 was not initially required as part of the project. However, eight awardees with vaccine remaining at the end of the third year of the project reported that an approximate 8000 additional doses were administered after September 2015. Thus, at least 91% (147,110 of 161,171) of distributed pilot doses were administered.
3.3. Description of vaccinated persons
The number of vaccine doses administered by age group, race/ethnicity, and sex are illustrated in Fig. 1. Most doses were administered to non-Hispanic white men 25–44 years old. Among vaccinated persons for whom risk factor information was reported (n = 44,355), 47% did not want to report a risk factor and 24% were evaluated for an STD (Fig. 2).
Fig. 1.
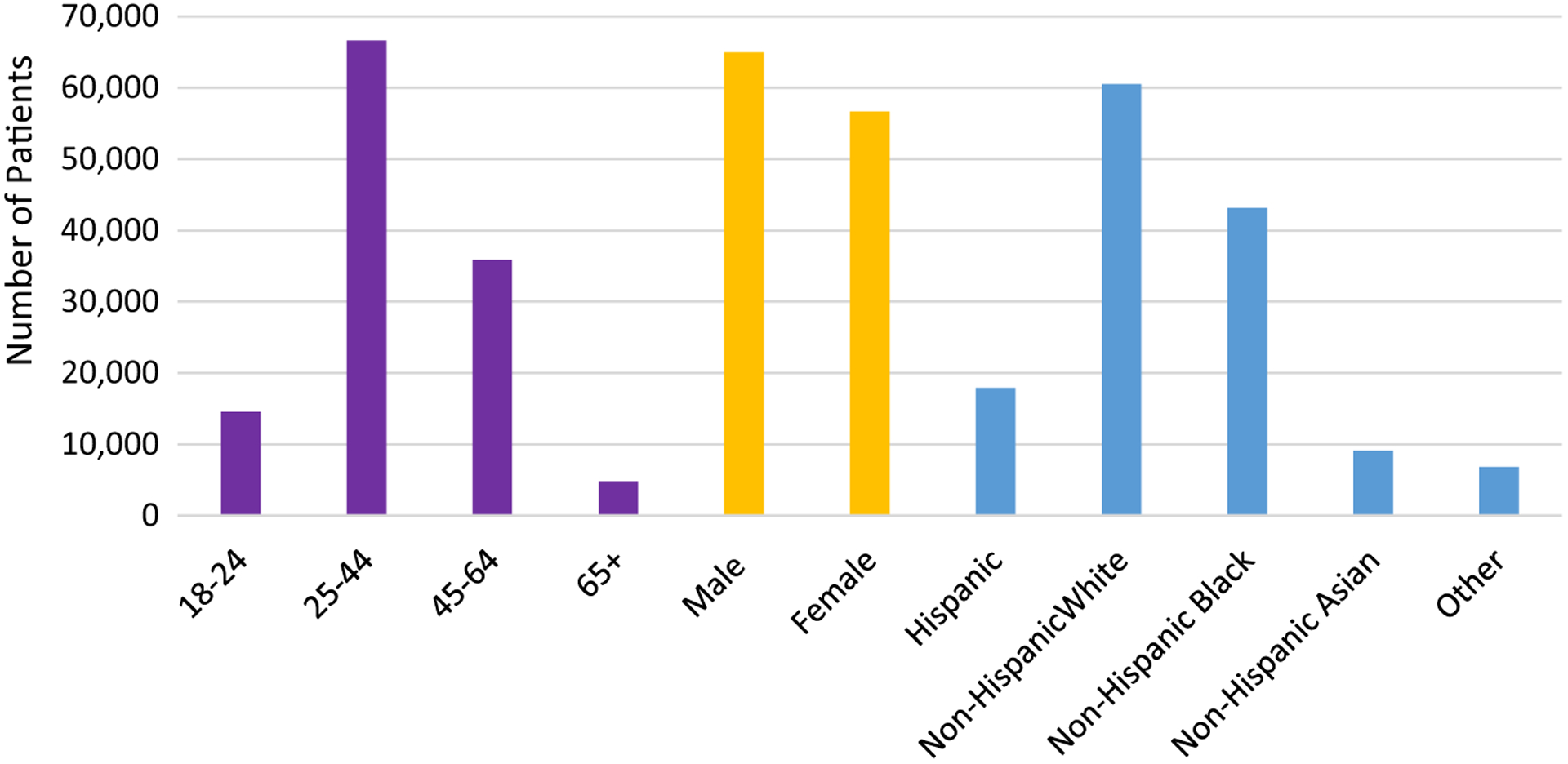
Reported demographic characteristics of patients that received hepatitis B vaccine doses adminstered through the pilot project, 2012–2015.1–3.
Footnotes:
1Data tracking issues (e.g., coding errors in distinguishing doses funded by the pilot and doses funded by other sources) resulted in some- over and under-counting of doses among awardees.
2Florida’s data were not included in figure, as their age groups and race/ethnicity were collected using different categories from other awardees. Florida reported vaccination of persons in the following age groups: 16–18 years (n = 14), 19 years (n = 42), 20–49 years (n = 7082), and 50–64 years (n = 3468). Florida reported vaccination among the following racial and ethnic groups: white (n = 8410), black (n = 3770), American Indian/Alaskan (n = 68), and Asian/Pacific Islander (n = 185), and Hispanic (n = 3,407), Non-Hispanic (n = 8701), Haitian (n = 307), and no answer (n = 18)].
3Individuals could report more than one race/ethnicity.
Fig. 2.
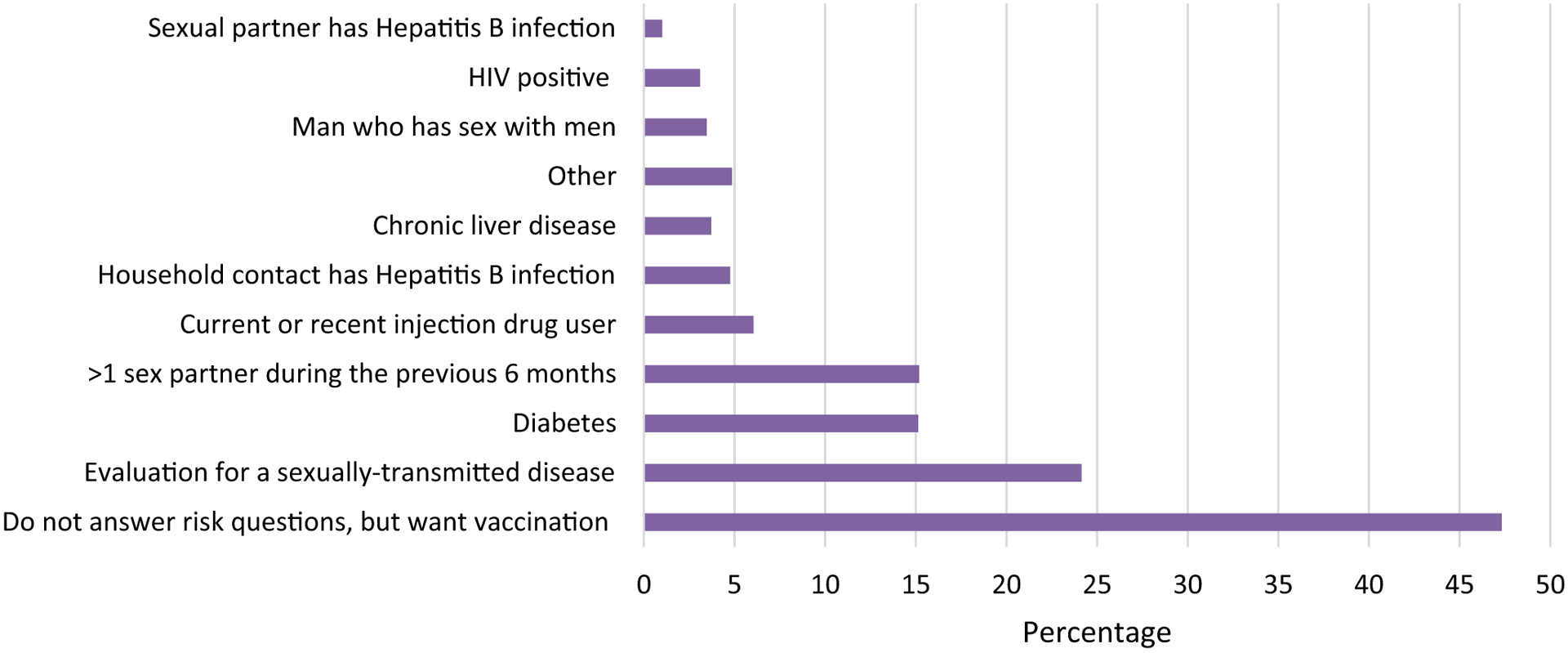
Percentages of hepatitis B vaccinees reporting one or more risk factors (N = 44,355), 2012–2015.1–5.
Footnotes:
1Louisiana, Michigan, New York City, Oregon, and West Virginia did not report on risk factors.
2Chicago reported an aggregate count for each risk factor across facility types which included correctional facilities, drug treatment facilities, federally qualified health centers, HIV clinics, health care facilities targeting injection drug users, local health department clinics, health care settings targeting men who have sex with men, sexually transmitted diseases clinic, and other (e.g., community-based organizations, health fairs, homeless shelters).
3Patients could report more than one risk factor. Data tracking issues (e.g., coding errors in distinguishing doses funded by the pilot and doses funded by other sources) resulted in some over- and under-counting of doses among awardees.
4“Other” is not specified.
5Did not answer questions regarding risk factors for hepatitis B virus exposure, but wanted hepatitis B vaccination.
3.4. Vaccine dose tracking and use of immunization information systems
Among doses administered through September 2015, awardees reported the number of doses administered by dose-series (dose 1, 2, or 3) by setting (Fig. 3). Among 138,665 doses with reported information on series dose, 52.7% of doses were dose 1 in the 3-dose series, 23.3% were dose-series dose 2, and 18.0% were dose 3.
Fig. 3.
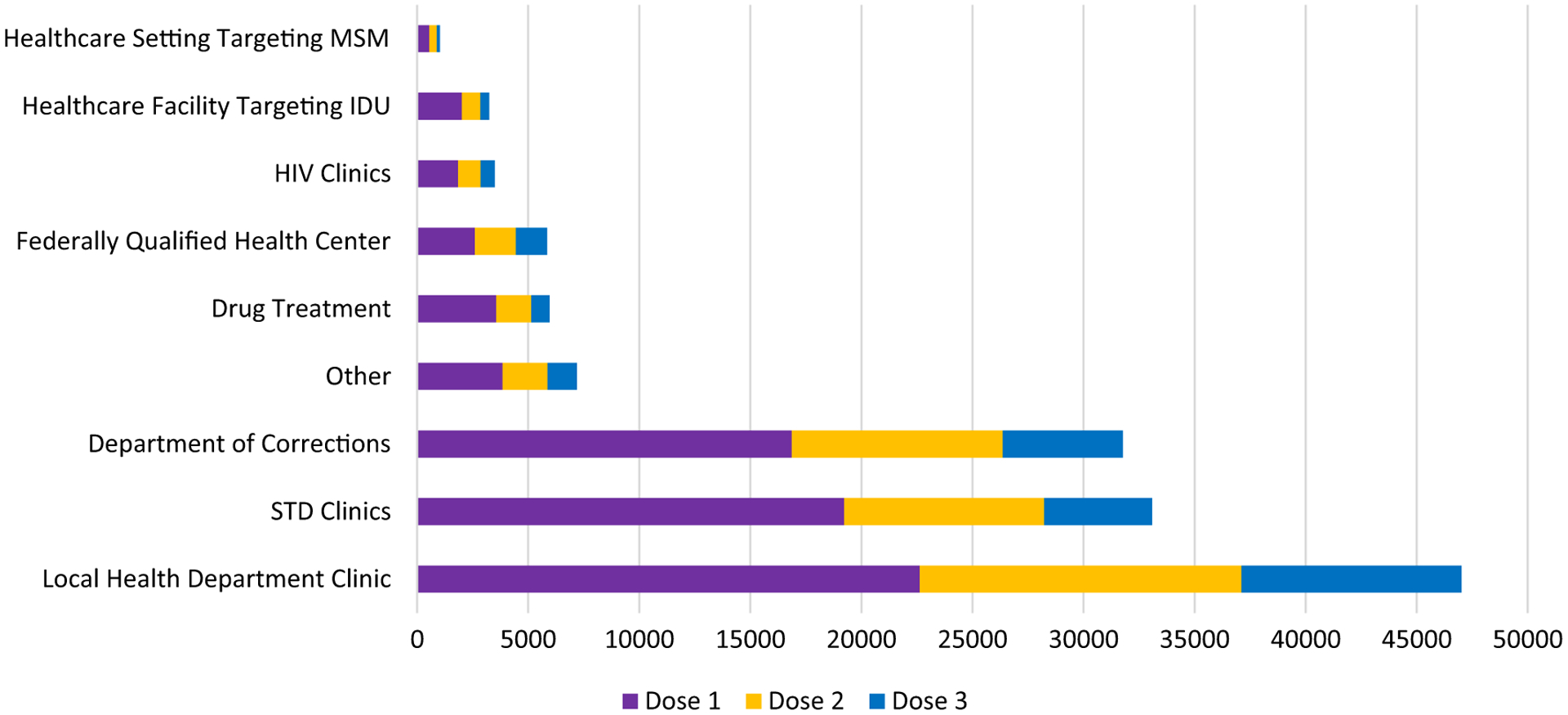
Hepatitis B vaccination doses administered through the pilot by setting type and dose in vaccine series, September 2012-September 2015.1–3
Footnotes: Awardees reported doses administered by setting and dose-number of the 3-dose series. This figure includes awardees who did and did not track individual patient-level dose-series completion.
1Abbreviations: STD = Sexually transmitted disease; MSM = men who have sex with men; IDU = injection drug user.
2Data regarding doses administered that did not include which dose in the series was given were excluded from this figure. Data tracking issues (e.g., coding errors in distinguishing doses funded by the pilot and doses funded by other sources) resulted in some over- and under-counting of doses among awardees.
3“Other” includes community-based organizations (e.g., those that serve Africans, Asian/Pacific Islanders), rural health clinics, community health clinics, mental health clinics, family planning clinics, HIV/AIDS resource fairs, health fairs, homeless shelters, college events, college health fairs, a celebrity rapper, and community outreach events (e.g., community health fair, block parties).
Only six awardees (Chicago, Michigan, Maryland, New York City, Oregon, and San Antonio) reported that they were able to capture patient-level pilot vaccine dose-series completion (Table 3). All 14 awardees had an IIS; however, use of the IIS during the pilot project and data elements captured varied among the awardees and among vaccination locations. One awardee did not have a functioning IIS during the pilot period. Another awardee was not able to merge IIS data with data collected using paper forms from vaccination sites. Other awardees reported challenges using their IIS due to difficulties and costs of modifying their IIS to incorporate data elements requested for the pilot evaluation, such as risk factors for hepatitis B infection. In order to collect the pilot information, they created new data collection instruments to be completed for each vaccine dose administered and had challenges linking the pilot-collected data to specific patients in the IIS. In addition, in many cases the IIS did not include data collection elements that could be used to distinguish HepB vaccinations administered as part of the pilot versus non-pilot doses. Awardees reported that vaccination sites used a combination of IIS with and without other methods to collect information on demographics, risk factors, doses administered, and dose-series completion.
Table 3.
Hepatitis B vaccination dose-series completion among persons who received a first dose and for whom patient-level vaccination information was available among 6 awardee sites able to track patient-level dose-series completions, September 2012–September 2015.1
| Setting Type | Number of persons who received dose 1 | Number (%) of dose 1 recipients who received dose 2 | Number (%) of dose 1 recipients who received dose 3 | Total number of doses 1–3 administered by setting type |
|---|---|---|---|---|
| STD clinic | 11,245 | 4000 (35.6) | 1928 (17.1) | 17,173 |
| Department of Corrections | 5150 | 2058 (40.0) | 908 (17.6) | 8116 |
| Other2 | 3447 | 1552 (45.0) | 1079 (31.3) | 6078 |
| Federally qualified health center | 2432 | 1359 (55.9) | 923 (38.0) | 4714 |
| Drug treatment facility | 2564 | 791 (30.9) | 349 (13.6) | 3704 |
| Health care facility targeting IDU2 | 2008 | 674 (33.6) | 325 (16.2) | 3007 |
| HIV clinic2 | 1278 | 551 (43.1) | 379 (29.7) | 2208 |
| Local health department clinic | 876 | 585 (66.8) | 531 (60.6) | 1992 |
| Health care setting targeting MSM2 | 457 | 327 (71.6) | 135 (29.5) | 919 |
| Total | 29,457 | 11,897 (40.4) | 6557 (22.3) | 47,911 |
Abbreviations: STD = sexually transmitted disease; MSM = men who have sex with men; IDU = injection drug user.
Data included only from 6 of the 14 hepatitis B pilot project awardees that were able to track dose series (Chicago, Michigan, Maryland, New York City, Oregon, San Antonio);
“Other” includes 3 Asian community based-agencies and 1 homeless shelter in Chicgo; 9 community-based organizations, 1 rescue mission, and 1 addiction clinic in Maryland; 1 Asian Liver Center in Michigan; 2 community-based organizations (African Services Committee and Korean Community Services) in New York City; and 1 homeless shelter, 4 mental health facilities, 6 college events, 2 HIV/AIDS resource fairs, 1 community health fair, 1 community back-to-school block party, and 1 outreach with a celebrity rapper in San Antonio.
Awardees reported that staff at vaccination sites with IIS-use capabilities received training on how to use their IIS, and were encouraged to use IIS to assess patient vaccine history before any vaccinations were given and to record administered HepB vaccine doses. However, awardees reported that IIS were inconsistently utilized across vaccination locations. IIS-related challenges that awardees and their partners encountered included limited internet connectivity at some sites, time constraints resulting from high patient volume, and lack of staff time dedicated to vaccination and vaccination needs screening. Some correctional facility sites did not allow vaccine providers to bring electronics within the facility site, preventing access to IIS during the patient encounter. Another significant challenge for all awardees was that many adults were not yet included in the IIS, increasing the time burden on sites to enter new patients’ information into the IIS system. The variability in use of IIS, use of non-IIS databases for tracking and collection of demographic information, and challenges distinguishing pilot from non-pilot doses resulted in both under- and over-counting of pilot doses administered. However, the exact impact of tracking and reporting errors could not be estimated.
3.5. Hepatitis B dose-series completion
Among patients vaccinated through one of the six awardees (Chicago, Michigan, Maryland, New York City, Oregon, and San Antonio) that reported patient-level dose-series completion, an average of 40.4% of persons who received dose 1 received a second dose, and an average of 22.3% of those who received a first dose received a third dose during the project period. Series completion varied by vaccination setting (Table 3). Local health department clinics had the highest hepatitis B 3–dose-series completion at 60.6%, followed by FQHCs at 38.0%. The setting with the lowest 3-dose series completion rate was drug treatment facilities (13.6%). STD clinics administered the most doses of any setting (35.8% of the 47,911 total doses), but 3-dose vaccine series completion was 17.1%. Correctional facilities administered 8116 vaccinations, the second most of any setting, and had a 3–dose-series completion rate of 17.6% (Table 3).
A summary of challenges and lessons learned reported by the 14 awardees and their partners is listed in Table 4. Challenges not already noted above included delays in pilot start-up and onboarding of vaccination locations, hiring delays, and staff turnover, which impeded implementation and delayed use of ordered vaccine. In addition, awardees and their partners overestimated the number of doses that could be utilized at vaccination locations, which contributed to dose wasting due to vaccine expiration. Challenges tracking pilot doses versus non-pilot HepB vaccine doses may have resulted in under- or over-counting of vaccines administered and inaccuracies in dose-series completion estimates.
Table 4.
Summary of challenges to hepatitis B vaccination pilot program implementation and facilitators of implementation reported by awardees.
| Challenges | Facilitators |
|---|---|
| Project startup and staffing | |
| Delays in project start up and staff hiring | Confirming partners’ capacity to provide vaccine services and the need in the communities they serve |
| Turnover among project staff and clinical vaccination site staff | Identifying clinic site vaccination champions |
| Incorporating vaccination services in settings without dedicated staff time for vaccination | Having buy-in from all front-line staff at vaccination sites |
| Limited experience and staff time for use of immunization information system (IIS) data lookup and data entry | Providing vaccination sites with infrastructure funds available for supplies, vaccine storage units, hiring staff, and project data collection and reporting costs |
| Other work demands competing for staff time | Hiring temporary nursing services if existing staff are unable to add vaccination services |
| Data and dose tracking | |
| Inconsistent/incomplete use of IIS across vaccination sites | Providing IIS initial training and retraining as staff changed |
| Inconsistent/incomplete use of non-IIS tracking systems across vaccination sites | Developing and utilizing non-IIS tracking systems |
| IIS not able to be modified to collect risk factor and other demographic variables | |
| Difficulty distinguishing pilot doses from non-pilot doses | |
| Tracking of patient-level dose-series completion | |
| Vaccine use and dose-series completion | |
| Over-estimating doses that would be administered by partner organizations | Using reminder/recall systems such as postcards and IIS reminder-recall capabilities |
| Frequent changes in high-risk patients’ contact information; limited ability to conduct reminder/recalls for follow-up doses | Utilizing IIS and/or clinic or health care system electronic medical records prompts |
| Less vaccination acceptance by patients and fewer second and third doses administered than anticipated | Use of standing orders |
| Use of alternative vaccination clinic times, including community events |
Activities reported by awardees that facilitated vaccination use included implementation of evidence-based practices, such as use of patient and provider reminders and standing orders. In addition, ensuring vaccination clinic locations and their staff were able to incorporate vaccination assessment and administration into their clinic services, and had staff support for the project, including having a clinic vaccination champion, helped facilitate vaccination implementation (Table 4).
4. Discussion
Through the HepB vaccine pilot, more than 147,000 HepB vaccine doses were administered to adults at high risk for HBV infection. The pilot also elucidated significant challenges to completing the HepB vaccine dose series among high-risk adults, especially adults seen for care in high-risk settings such as STD clinics and correctional facilities. Although a large number of doses were administered in these settings, indicating capabilities to provide vaccination services, dose-series completion was low. While high-risk settings may be ideal places to initiate vaccinations like HepB, challenges with follow-up indicate the necessity of instituting strategies that generate reminders for patients and remind providers that additional doses are due [16,19,20]. Utilization of IIS can serve both of these functions, and electronic records or other systems changes also can be implemented to prompt review of vaccinations and identify needed vaccines. Many awardees reported difficulties with dose-series completion tracking. Expanding the use of IIS in clinics that provide vaccinations to adults could facilitate reminder and recall of patients for needed doses. However, use of IIS would not eliminate challenges, given that many persons at high risk for HBV infection will remain difficult to reach with reminder/recall notifications and that many adults are not included in IIS.
Few studies have looked at HepB vaccine dose-series completion. However, the dose-series completion among the 6 pilot awardees is substantially lower than that found in the literature that we identified. Among adults enrolled in managed care organizations, 53–71% of adults completed the 3-dose series [19]. Among MSM who initiated HepB vaccination at a single STD clinic in San Diego during 1998–2003, 64% received 2 doses and 43% received all doses in the 3-dose series [20]. This clinic routinely assessed patients for HepB vaccination needs, tracked dose-series completion using a clinic-specific database, conducted reminders/recalls for subsequent doses, and provided feedback to staff regarding the clinic’s HepB vaccination performance.
Our lower overall estimates of series completion likely reflect the variety of locations used, inclusion of mobile populations difficult to reach for follow-up, limited timeframe for the pilot, limited capacity to enter doses given in IIS, and limited capacity to send reminders. Our estimated series completion may differ from actual series completion given (1) the challenges with vaccine dose tracking and incomplete reporting to IIS of adult vaccinations in general; (2) challenges specific to the settings and populations where most vaccines were given (i.e., STD clinics, drug treatment facilities, and correctional institutions) where return visits for continuing care may be less likely compared to other settings; and (3) the possibility that some vaccinations may have occurred using non-pilot vaccine doses and were not reported as part of the pilot dose-series completion.
The pilot was conducted before licensure of a new adjuvanted HepB vaccine, HEPLISAV-B®([HepB-CpG]), in November 2017 [21]. In April 2018, ACIP recommended HepB-CpG for use in persons aged ≥18 years, administered as a 2-dose series (0 and 1 month) as opposed to the 3-dose series (0, 1, and 6 months) for vaccines used in this pilot [10]. HepB-CpG is prepared with a 1018 adjuvant containing small synthetic immunostimulatory cytidine-phosphate-guanosine oligodeoxynucleotide (CpG-ODN) motifs that binds to toll-like receptor 9 to stimulate a directed immune response to hepatitis B surface antigen [10]. HepB-CpG provides greater immunity against HBV infection after 2 doses compared to 3 doses of alum-adjuvanted vaccines [HepB-alum] [21]. The HepB-CpG vaccine may provide an advantage for vaccination of persons who may be less likely to return for the final dose in a 3-dose series, and would provide high-risk patients with peak immunity in a shorter amount of time [21–24]. However, the fact that an average of only 40% of persons who received dose 1 received a second dose in our pilot indicates the continued need to develop and implement strategies to increase completion of both 2- and 3-dose series vaccines [25].
Maintaining accurate and complete documentation of how many pilot HepB vaccine doses were administered in hundreds of settings and dose-series completion using pilot doses, and manually entering the information into IIS were significant burdens, compounded by frequent staff turnover and hiring challenges for awardees and their partners. Some awardees reported delays in the initiation of the project due to limited staffing to manage the pilot, and due to facilities’ hesitance to participate because of vaccine storage issues, data collection requirements, lack of existing staff time to administer vaccine, and the limited time period for funding. Efforts to sustain this initiative during the 2-year project period and 1-year extension also competed with awardees’ and their partners’ other projects and routine operations. These challenges enforce the need to improve routine vaccine needs assessment in all settings where high-risk adults obtain care—including in pharmacies, workplace occupational health clinics, and other settings—to ensure high-risk persons receive recommended vaccines [25].
Challenges with overestimating the number of doses that would be given during the pilot and challenges with initiating vaccination efforts in some sites contributed to vaccine wastage of 4.5% of doses. Awardees were required to estimate total needed doses for the project rather than ordering a smaller number of doses as vaccine was administered. In contrast to our results, a study of private pediatric practices found vaccine dose wastage of 0.1% of total doses given [26]. In these practices, vaccine was ordered every two weeks so that supply was closely matched to demand. Future efforts should be designed to allow for frequent smaller vaccine quantity ordering to reduce the number of doses that expire before they can be used.
Analyses of data from this pilot project had a number of limitations. Most notably, some over- and under-counting of doses administered among the awardees likely resulted from data tracking issues, such as limited access to or use of IIS in many vaccination locations, staff not familiar with the IIS, or lack of staff time to record or review vaccinations in the IIS. Awardees also reported identifying coding errors in tracking administration of pilot doses separately from non-pilot doses of HepB vaccine, which may have resulted in over- or under-counting of vaccine doses administered. Despite these limitations, the results of this pilot clearly illustrate the challenges of implementing vaccination programs for high-risk adults in high-risk settings and the need for sustained efforts to routinely offer vaccinations in high-risk settings.
5. Conclusions
This HepB vaccination pilot project resulted in successful administration of more than 147,000 HepB vaccinations to high-risk adults, but also highlighted many challenges to reaching these populations with vaccination services and ensuring dose-series completion. Although maximal effectiveness and duration of immunity occurs with HepB dose-series completion, initiation of vaccination among high-risk persons remains critical to reducing the risk of HBV infection even when many patients may not return for subsequent doses. Sustained efforts are needed to improve the routine assessment for vaccination needs and vaccination of adults in all settings to improve dose-series completion especially among those who may not specifically seek follow-up vaccinations. The availability of a HepB vaccine requiring only a 2-dose series may help with dose-series completion, but improvements in adult immunization infrastructure, including utilizing IIS to assess adults’ vaccination status and record vaccinations, remain critical for identifying and fully vaccinating high-risk adults against HBV infection and other vaccine-preventable diseases [16,19,25].
Acknowledgements
The authors wish to thank all of the health departments and their partners that contributed to this pilot project, including the Alabama Department of Public Health, Chicago Department of Public Health; Phil Reichert and Florida Department of Health; Thaddeus Pham and the Hawaii Department of Health; the Kentucky Immunization Program; Frank Welsh and the Louisiana Office of Public Health; the Prevention and Health Promotion Administration, Maryland Department of Health; Michigan Department of Health & Human Services; Public Health Division, Oregon Health Authority; San Antonio Metropolitan Health District; Tennessee Department of Health; Bethany McCunn, MPH and the Virginia Department of Health Division of Immunization; New York City Department of Health and Mental Hygiene; and West Virginia Bureau for Public Health. We also thank LaTreace Harris, CDC for her review of the manuscript.
Funding
Funding for this pilot project was provided by the Centers for Disease Control and Prevention.
Table A1
Data collected from awardees by report type.
| Report Type | Report Elements |
|---|---|
| Monthly Report |
|
| Quarterly Narrative Update |
|
| Quarterly Quantitative Update |
|
| Final Report |
|
Information about dose wastage was not originally incorporated into the report template, but was included in later revisions.
E.g., doses that were not used prior to reaching their expiration dates or vaccine that was unable to be used because of temperature storage errors or mishaps.
Although three awardees provided some information on testing, each reported information differently and the completeness of reporting of testing could not be determined. Thus, information regarding testing was not included in the final summary report for the HepB Pilot project.
I.e., practices regarding communication and training with sites, vaccination strategies to identify and offer vaccination to persons at risk for HepB, and IIS use to capture doses administered and dose-series completion. Universal settings include those settings where a high proportion of persons who see care have risk factors for HBV infection, such as sexually transmitted disease (STD) clinics, HIV clinics, correctional facilities, and drug treatment centers. Non-universal settings would include general medical care clinics such as the health department, community health centers, or primary care clinics.
E.g., corrections, STD clinics, other health department clinics, drug treatment centers.
Awardees that did not utilize all of their allotted HepB vaccine by the time the project ended in September 2015 (including the 1-year no-cost extension) were asked to report monthly regarding the number of doses administered, number of doses unused, and number of doses that had expired or been unused due to other reasons such as interruptions in maintaining recommended storage conditions.
Such as age, race/ethnicity, sex, specific risk factor(s) for HBV infection, to assess if awardees were accessing persons at greatest risk of HBV infection in their jurisdictions.
Table A2
Strategies implemented in universal and non-universal settings during the hepatitis B pilot, September 2012–September 2015.
| Strategy | Awardees implementing strategy |
|---|---|
| Immunization information systems | All awardees except Kentucky |
| Client reminder/recall systems | Alabama, Chicago, Florida, Maryland, Michigan, Nevada, New York City, San Antonio, Tennessee, Virginia, and West Virginia |
| Client or family incentive rewards | Chicago1 |
| Provider assessment and feedback | Chicago, Louisiana, New York City, and Virginia2 |
| Home visits | No awardees |
| Provider reminder systems | Florida, Kentucky, Maryland, Nevada, New York City, Oregon, San Antonio, Tennessee, and Virginia |
| Community-based interventions in combination | Florida, Oregon, Virginia, San Antonio and West Virginia |
| Reducing client out-of-pocket costs | Chicago, Florida, Kentucky, Louisiana, Michigan, New York City, Oregon, San Antonio, Tennessee, Virginia, and West Virginia |
| Standing orders | All awardees |
Chicago’s (CHI) BlueCross BlueShield CareVan provided incentives for first, second, and third doses of hepatitis B vaccinations such as nylon draw string bags, water bottles, and stress balls.
Chicago, Louisiana, New York City, and Virginia provided this information via monthly reports, site visits, phone calls, and emails.
Table A3
Hepatitis B vaccine doses ordered, administered, and wasted as of September 2015.1
| Awardee2 | Vaccinating Sites | Patient Visits to Sites4 | Patients Offered HepB Vaccine4 | Doses Ordered | Doses Given | Doses Unused5 | Doses Wasted6 |
|---|---|---|---|---|---|---|---|
| Alabama3 | 87 | 30,502 | 19,571 | 20,000 | 15,669 | – | – |
| Chicago | 42 | 138,057 | 24,282 | 15,800 | 15,581 | 219 | 0 |
| Florida | 7 | 12,433 | 9102 | 11,760 | 10,921 | 839 | 186 |
| Kentucky | 83 | 239,450 | 61,487 | 10,250 | 9260 | 1002 | 778 |
| Louisiana | 9 | 47,733 | 9343 | 6000 | 3256 | 694 | 2454 |
| Maryland | 44 | 58,989 | 12,461 | 14,500 | 10,014 | 4486 | 1379 |
| Michigan | 23 | 118,040 | 91,639 | 17,000 | 17,023 | 0 | 0 |
| Nevada | 10 | 43,698 | 38,755 | 9450 | 8085 | 1365 | 1099 |
| New York City | 17 | 184,525 | 45,757 | 12,400 | 11,539 | 861 | 38 |
| Oregon | 14 | 307,800 | 3744 | 4500 | 4491 | 9 | 214 |
| San Antonio | 26 | 2979 | 2205 | 3000 | 2061 | 939 | 36 |
| Tennessee | 55 | 754,581 | 108,295 | 17,073 | 15,392 | 1521 | 33 |
| Virginia | 12 | 477,034 | 10,751 | 7954 | 7187 | 2257 | 326 |
| West Virginia | 30 | 7091 | 6525 | 11,484 | 8631 | 2853 | 708 |
| Totals | 459 | 2,422,912 | 443,917 | 161,171 | 139,110 | 17,045 | 7,251 |
Project end date 9/29/2015 (range between 12/29/2014 and 9/29/2015).
Data tracking issues (e.g., coding errors in distinguishing doses funded by the pilot and doses funded by other sources) resulted in some over- and under-counting of doses among awardees.
Due to de-duplication errors with the local database for this project, at least 3000 records were lost. Additionally, at least one large county did not submitt its hepatitis B enrollment forms. The number of doses unused and wasted is unknown.
Some awardees estimated the number of patient visits to sites and the number of patients who were offered hepatitis B vaccine or did not track the number of patient visits or vaccinations offered.
Doses unused as reported in the awardees’ final reports. Awardees with unused doses continued to vaccinate after September 2015 until all doses were administered or had expired.
Doses wasted were reported in the awardees’ final reports. Reported reasons why doses were wasted: not used before expiration date, improper storage and handling, dropped/broken vial, client decided not to be vaccinated, syringe malfunction, lost vaccine, and unknown reasons.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names and commercial source is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services, or the U.S. Centers for Disease Control and Prevention.
Declaration of Competing Interest
All authors report that they have no financial conflicts of interest.
References
- [1].CDC. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). Part II: immunization of adults. MMWR Recomm Rep 2006;55(RR-16):1–33. [PubMed] [Google Scholar]
- [2].Schillie S, Vellozzi C, Reingold A, Harris A, Haber P, Ward JW, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2018;67(RR-1):1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Krugman S, Overby LR, Mushahwar IK, Ling CM, Frosner GG, Deinhardt F. Viral hepatitis, type B—studies on natural history and prevention reexamined. N Engl J Med 1979;300:101–6. [DOI] [PubMed] [Google Scholar]
- [4].Hoofnagle JH, DiBisceglie AM. Serologic diagnosis of acute and chronic viral hepatitis. Semin Liver Dis 1991;11(2):73–83. [DOI] [PubMed] [Google Scholar]
- [5].Roberts H, KruszonMoran D, Ly KN, Hughes E, Iqbal K, Jiles RB, et al. Prevalence of chronic hepatitis B virus (HBV) infection in US households: National Health and Nutrition Examination Survey (NHANES), 1988–2012. Hepatology 2016;63(2):388–97. [DOI] [PubMed] [Google Scholar]
- [6].CDC. Division of Viral Hepatitis. Viral hepatitis surveillance: United States, 201 https://www.cdc.gov/hepatitis/statistics/2016surveillance/pdfs/2016HepSurveillanceRpt.pdf. Revised April 16, 2018 [accessed July 2, 2018]. [Google Scholar]
- [7].Beasley RP. Hepatitis, B virus. The major etiology of hepatocellular carcinoma. Cancer 1988;61(10):1942–56. [DOI] [PubMed] [Google Scholar]
- [8].McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology 2009;49(5 Suppl):S45–55. [DOI] [PubMed] [Google Scholar]
- [9].Mitchell T, Armstrong GL, Hu DJ, Wasley A, Painter JA. The increasing burden of imported chronic Hepatitis B—United States, 1974–2008. PLoS ONE 2013;8(3).10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schillie S, Harris A, Link-Gelles R, Romero J, Ward J, Nelson N. Recommendations of the Advisory Committee on Immunization Practices for use of a hepatitis B vaccine with a novel adjuvant. MMWR Morb Mortal Wkly Rep 2018;67:455–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zajac BA, West DJ, McAleer WJ, Scolnick EM. Overview of clinical studies with hepatitis B vaccine made by recombinant DNA. J Infect 1986;13(SupplA):39–45. [DOI] [PubMed] [Google Scholar]
- [12].Andre FE. Summary of safety and efficacy data on a yeast-derived hepatitis B vaccine. Am J Med 1989;87(3A):14S–20S. [DOI] [PubMed] [Google Scholar]
- [13].Van Der Meeren O, Peterson JT, Dionne M, Beasley R, Ebeling PR, Ferguson M, et al. Prospective clinical trial of hepatitis B vaccination in adults with and without type-2 diabetes mellitus. Hum Vaccin Immunother 2016;12(8):2197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bruce MG, Bruden D, Hurlburt D, Zanis C, Thompson G, Rea L, et al. Antibody levels and protection after hepatitis B vaccine: results of a 30-year follow-up study and response to a booster dose. J Infect Dis 2016;214(1):16–22. [DOI] [PubMed] [Google Scholar]
- [15].Williams WW, Lu PJ, O’Halloran A, Kim DK, Grohskopf LA, Pilishvili T, et al. Surveillance of vaccination coverage among adult populations—United States, 2015. MMWR Surveill Summ 2017;66(11):1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Community Preventive Services Task Force. Vaccination. The Community Guide website. https://www.thecommunityguide.org/topic/vaccination [accessed July 2, 2018].
- [17].National Association of County and City Health Officials. Executive summary of the final report: formative evaluation of methods to improve the CDC Adult Hepatitis B Vaccination Initiative. http://archived.naccho.org/.
- [18].Jacobson Vann JC, Jacobson RM, CoyneBeasley T, AsafuAdjei JK, Szilagyi PG. Patient reminder and recall interventions to improve immunization rates. Cochrane Database Syst Rev 2018;1:CD003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nelson JC, Bittner RC, Bounds L, Zhao S, Baggs J, Donahue JG, et al. Compliance with multiple-dose vaccine schedules among older children, adolescents, and adults: results from a vaccine safety datalink study. Am J Public Health 2009;99(Suppl 2):S389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gunn RA, Lee MA, Murray PJ, Gilchick RA, Margolis HS. Hepatitis B vaccination of men who have sex with men attendng an urban STD clinic: impact of an ongoing vaccination program, 1998–2003. Sex Transm Dis 2007;34(9):663–8. [DOI] [PubMed] [Google Scholar]
- [21].Jackson S, Lentino J, Kopp J, Murray L, Ellison W, Rhee M, et al. Immunogenicity of a two-dose investigational hepatitis B vaccine, HBsAg-1018, using a toll-like receptor 9 agonist adjuvant compared with a licensed hepatitis B vaccine in adults. Vaccine 2018;36(5):668–74. [DOI] [PubMed] [Google Scholar]
- [22].Halperin SA, Ward B, Cooper C, Predy G, Diaz-Mitoma F, Dionne M, et al. Comparison of safety and immunogenicity of two doses of investigational hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligodeoxyribonucleotide and three doses of a licensed hepatitis B vaccine in healthy adults 18–55 years of age. Vaccine 2012;30(15):2556–63. [DOI] [PubMed] [Google Scholar]
- [23].Janssen RS, MangooKarim R, Pergola PE, Girndt M, Namini H, Rahman S, et al. Immunogenicity and safety of an investigational hepatitis B vaccine with a toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared with a licensed hepatitis B vaccine in patients with chronic kidney disease. Vaccine 2013;31(46):5306–13. [DOI] [PubMed] [Google Scholar]
- [24].Janssen JM, Jackson S, Heyward WL, Janssen RS. Immunogenicity of an investigational hepatitis B vaccine with a toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared with a licensed hepatitis B vaccine in subpopulations of healthy adults 18–70 years of age. Vaccine 2015;33(31):3614–8. [DOI] [PubMed] [Google Scholar]
- [25].Bridges CB, Hurley LP, Williams WW, Ramakrishnan A, Dean AK, Groom AV. Meeting the challenges of immunizing adults. Vaccine 2015;33(Suppl 4): D114–20. [DOI] [PubMed] [Google Scholar]
- [26].Glazner JE, Beaty B, Berman S. Cost of vaccine administration among pediatric practices. Pediatrics 2009;124(Suppl 5):S492–8. [DOI] [PubMed] [Google Scholar]


