Video
EUS-guided microwave ablation of an unresectable pancreatic mass using a novel generator platform and a specialized 19.5-gauge needle antenna.
Abbreviations: MWA, microwave ablation; RFA, radiofrequency ablation
Introduction
Pancreatic neuroendocrine tumors have an incidence of ≤1 case per 100,000 individuals, accounting for up to 2% of all pancreatic neoplasms in the United States. The 5-year overall survival rate ranges from 37.6% to 50%.1 Curative surgical interventions are not feasible for most patients because most cases are detected in advanced unresectable stages, mainly in elderly patients with several comorbidities.2 Therefore, developing safe and effective alternatives for patients unfit for surgery is imperative for clinical practice.3, 4, 5
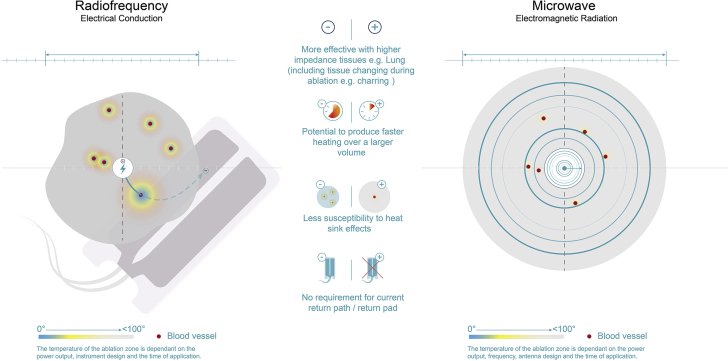
Radiofrequency ablation (RFA) is a popular ablative method for surgically unfit patients. It is based on a high-frequency alternating current of 450 to 500 kHz.6 With the changes in the alternating current, resistive heating energy accumulates in the tissue after a rapid rise in temperature beyond 100°C.6 Thus, RFA can be limited by the risk of causing unpredictable thermal injury.3,7
Microwave ablation (MWA) is based on frictional heating produced through the oscillation of dipole molecules, inducing a controlled temperature rise up to 90°C. This approach provides deep, homogenous, and consistent energy delivery without damage to peripheral tissues. Although RFA and MWA analogously achieve coagulative necrosis, the latter has several advantages: a minimal cooling time and a more effective energy transfer with less susceptibility to heat sinks. In addition, MWA lacks reliance on tissue impedance, is not limited by water vaporization and charring,7 does not need an electrical circuit, and has a lower cost than RFA.8
Case presentation
We report a case on a 72-year-old woman with an unresectable pancreatic tumor. The patient had hypertension, diabetes mellitus, and chronic kidney disease and had undergone a right nephrectomy 10 years prior for clear renal cell carcinoma. EUS-guided MWA was favored over observation because of the patient’s refusal of surgical intervention and high preoperative risk index. In addition, the lesion was deemed inoperable because of its size and location (invading the splenic artery) (Video 1; available online at www.giejournal.org).
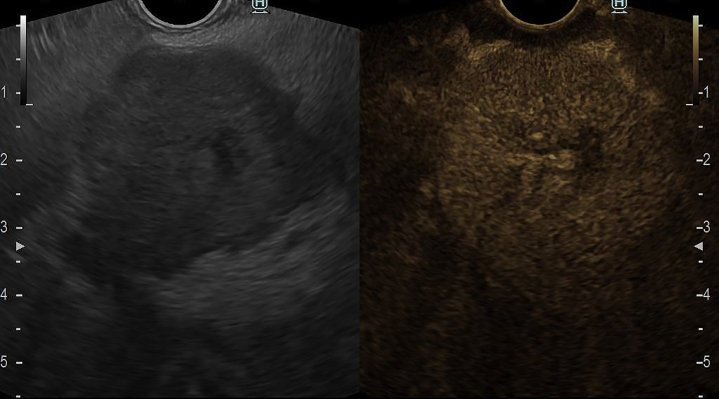
Our patient presented with a 3-month history of unspecific abdominal pain, unintentional weight loss, nausea, and malaise. EUS revealed a 35- × 32-mm hypoechoic pancreatic neck lesion and hyperenhancement on contrast-enhanced EUS (Fig. 1). EUS-guided fine-needle biopsy confirmed a pancreatic neuroendocrine tumor.
Figure 1.
EUS evaluation revealed a 35-mm × 32-mm hypoechoic pancreatic neck lesion with hyperenhancement on contrast-enhanced EUS.
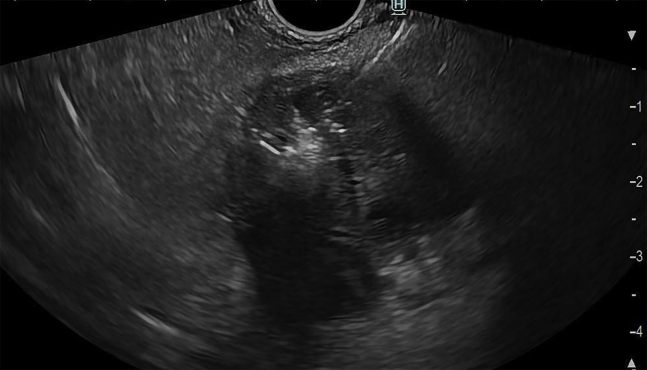
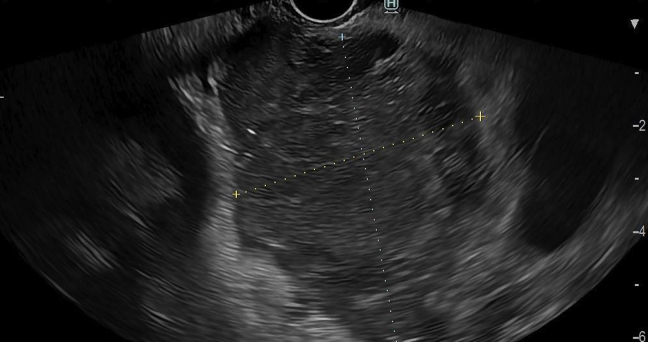
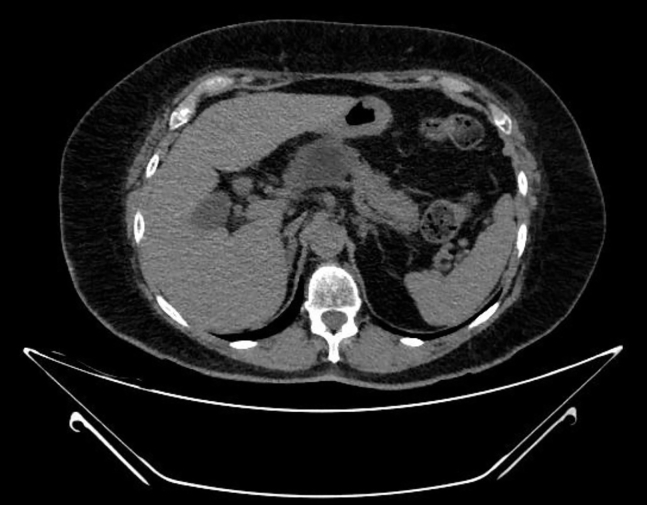
The local ethics committee and the patient consented to this ambulatory intervention. The endoscopic procedure was performed using a therapeutic linear echoendoscope (EG38-J10UT, Pentax Medical, Hamburg, Germany) attached to a dedicated generator platform (CROMA, Creo Medical, Chepstow, Wales, United Kingdom) and a 19.5-gauge needle antenna (MicroBlate fine, Creo Medical) to deliver 5.8 GHz continuously for 2 minutes, requiring 8 shots in total to cover the entire lesion area (Fig. 2). During ablation, white microbubbles that progressively burst were noted on EUS with the controlled rise of energy deployed in each shot. After complete ablation, previously dark areas turned white and a decrease in the size of the lesion was noted postintervention on EUS (Fig. 3) and on noncontrast CT performed 4 weeks after MWA (Fig. 4). No postprocedural abdominal pain or acute pancreatitis was reported in the short term or at the 4-month follow-up. Currently, the patient remains asymptomatic 8 months after treatment.
Figure 2.
EUS-guided microwave ablation with a 19.5-gauge needle delivering 5.8 GHz to the targeted pancreatic tumor.
Figure 3.
A decrease in the size of the pancreatic neck lesion and complete ablation was noted after intervention on EUS.
Figure 4.
Four-week follow-up CT scan on the cross-sectional plane showed good radiologic response with an avascular area in the head of the pancreas corresponding to the ablation zone.
Discussion
EUS guidance provides real-time high-quality imaging, enabling precise localization of therapy. MWA offers an effective ablative therapy strategy while improving the safety profile by increasing control and homogeneity of the ablation zone and reducing the heating time and rise in temperature.
Several adverse events have been reported with EUS-RFA, including asymptomatic increases in serum lipase levels, acute pancreatitis, pancreatic ductal stenosis, and small-bowel perforation.9,10 As seen in our case, the application of EUS-guided MWA is a safer alternative based on its technical benefits (Fig. 5), namely its homogenous and consistent energy deployment without damage to surrounding structures or the pancreas itself. Additionally, MWA can induce cytoreduction, providing symptomatic relief with minimal to nonexistent postprocedural adverse events. Furthermore, MWA may serve as a bridge therapy to other therapeutic methods, such as chemotherapy, radiotherapy, or even surgery.
Figure 5.
Schematic comparison between radiofrequency and microwave ablation.
In conclusion, EUS-guided MWA was a feasible, effective, and safe alternative for the management of an unresectable pancreatic neuroendocrine tumor. Large case series and prospective cohorts are required to evaluate this technique in clinical practice.
Disclosure
Dr Robles-Medranda is a consultant and key opinion leader for Pentax Medical, Boston Scientific, Steris, Medtronic, Motus, Micro-tech, G-Tech Medical Supply, CREO Medical, and Mdconsgroup. All other authors disclosed no financial relationships.
Supplementary data
EUS-guided microwave ablation of an unresectable pancreatic mass using a novel generator platform and a specialized 19.5-gauge needle antenna.
References
- 1.Das S., Dasari A. Epidemiology, incidence, and prevalence of neuroendocrine neoplasms: are there global differences? Curr Oncol Rep. 2021;23:43. doi: 10.1007/s11912-021-01029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kircher S.M., Krantz S.B., Nimeiri H.S., et al. Therapy of locally advanced pancreatic adenocarcinoma: unresectable and borderline patients. Expert Rev Anticancer Ther. 2011;11:1555–1565. doi: 10.1586/era.11.125. [DOI] [PubMed] [Google Scholar]
- 3.Rossi M., Orgera G., Hatzidakis A., et al. Minimally invasive ablation treatment for locally advanced pancreatic adenocarcinoma. Cardiovasc Intervent Radiol. 2014;37:586–591. doi: 10.1007/s00270-013-0724-x. [DOI] [PubMed] [Google Scholar]
- 4.Ierardi A.M., Lucchina N., Petrillo M., et al. Systematic review of minimally invasive ablation treatment for locally advanced pancreatic cancer. Radiol Med. 2014;119:483–498. doi: 10.1007/s11547-014-0417-9. [DOI] [PubMed] [Google Scholar]
- 5.Carrafiello G., Ierardi A.M., Fontana F., et al. Microwave ablation of pancreatic head cancer: safety and efficacy. J Vasc Interv Radiol. 2013;24:1513–1520. doi: 10.1016/j.jvir.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhry M., McGinty K.A., Mervak B., et al. The LI-RADS version 2018 MRI treatment response algorithm: evaluation of ablated hepatocellular carcinoma. Radiology. 2020;294:320–326. doi: 10.1148/radiol.2019191581. [DOI] [PubMed] [Google Scholar]
- 7.Rossi G., Petrone M.C., Capurso G., et al. Standardization of a radiofrequency ablation tool in an ex-vivo porcine liver model. Gastrointest Disord. 2020;2:300–309. [Google Scholar]
- 8.Egorov A.V., Vasilyev I.A., Musayev G.H., et al. The role of microwave ablation in management of functioning pancreatic neuroendocrine tumors. Gland Surg. 2019;8:766–772. doi: 10.21037/gs.2019.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rustagi T., Chhoda A. Endoscopic radiofrequency ablation of the pancreas. Dig Dis Sci. 2017;62:843–850. doi: 10.1007/s10620-017-4452-y. [DOI] [PubMed] [Google Scholar]
- 10.Barthet M., Giovannini M., Lesavre N., et al. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: a prospective multicenter study. Endoscopy. 2019;51:836–842. doi: 10.1055/a-0824-7067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
EUS-guided microwave ablation of an unresectable pancreatic mass using a novel generator platform and a specialized 19.5-gauge needle antenna.
EUS-guided microwave ablation of an unresectable pancreatic mass using a novel generator platform and a specialized 19.5-gauge needle antenna.