Abstract
Limonoids, as the vital bioactive chemical compounds in genus Melia plants, have attracted significant attention owing to their exclusive structural characteristics and remarkable biological activity. These compounds can be usually classified into two categories, including the ring-intact group and the ring-C-seco group. Benefiting from the development of separation and analysis technology, more than 200 limonoids have been isolated and identified from this genus. There is growing evidence that limonoids from genus Melia possess diverse pharmacological activities, especially anti-cancer effects, insecticidal activities, and anti-botulism effects. Toosendanin, one of the paramount limonoids, was considered as the pivotal bioactive marker in two medicinal herbs, including Melia toosendan Sieb. et Zucc and Melia azedarach L. In particular, limonoids are found to exhibit non-negligible toxic effects, a finding which needs further research. Besides this, the lack of clinical research data seriously hinders its further development and utilization, and necessary clinical trials should be taken into consideration. In this review, we systematically summarized the phytochemical compounds and their synthesis methods, pharmacological activities, and the structure–activity relationship, pharmacokinetics, and toxicology of genus Melia-derived limonoids. We believe that this up-to-date review could provide scientific evidence for the application of limonoids as agents beneficial to health in future clinical practice.
Keywords: limonoids, genus Melia , toosendanin, anti-tumor, insecticide, toxicology
Introduction
Genus Melia, a model genus of Meliaceae, has about 20 species in the world and is widely distributed in tropical and subtropical regions of the Eastern Hemisphere. Among them, Melia toosendan Sieb. et Zucc., Melia azedarach L., Melia azedarach var. japonica, and Melia volkensii Gürke have received a lot of attention (Liu et al., 2010). It is worth noting that Azadirachta indica A. Juss., a plant of the genus Azadirachta, is often wrongly recognized as M. azedarach L. for their similar morphological characteristics and botanical name (Saleem et al., 2018). Owing to their multiple bioactivities, the Melia plants have been used as folk herbs in treating leprosy, eczema, asthma, malaria, fever, and pain (Xie et al., 2008). M. toosendan Sieb. et Zucc and M. azedarach L., two common medicinal plants in China, have been used to treat diseases for thousands of years. The first traditional usage can be traced back to Shen Nong Ben Cao Jing, which is the earliest medical monograph in China that was written during the Eastern Han Dynasty (AD 25–220). In this monograph, the two herbs functioned as treatment for anxiety, destroying parasites, and promoting diuresis. According to Ben Cao Gang Mu (AD 1578), which is another famous medical classic, M. toosendan and M. azedarach could treat stomach ache and hernia. In addition, the ability to clear heat and promote diuresis was reported in Ben Cao Jing Shu (AD 1625). According to Chinese Pharmacopoeia, M. toosendan and M. azedarach have been recorded as insecticide and painkiller (Chinese Pharmacopoeia Commission, 2020). Various types of chemical compounds have been isolated and identified from different parts of genus Melia plants, including limonoids (triterpenoids), steroids, alkaloids, flavonoids, anthraquinones, etc. (Zhao et al., 2010). Modern pharmacological research demonstrated that limonoids, which are abundant in Melia species, exhibit a potential activity (Taylor, 1984). The word “limonoids” originated from the bitterness of lemon or other citrus fruits. Early chemical research of such compounds in Meliaceae started in 1960. The first limonoid compound named gedunin was isolated from wood of the West African plant Entandrophragma angolense, and its chemical structure was identified by comparison with limonin (Goerlich, 1960). The fundamental structure of limonoids is formed by the loss of four terminal carbons of the side chain in the apotirucallane or apoeuphane skeleton and then cyclized to form the 17β-furan ring, and thus limonoids are also known as tetranortriterpenoids (Tan and Luo, 2011). In this paper, a literature survey was carried out by searching the keywords including “limonoids”, “Melia”, “Melia toosendan Sieb. et Zucc”, and “Melia azedarach L.” from Pubmed, SciFinder, Science Direct, Scopus, the Web of Science, Google Scholar, China National Knowledge Infrastructure, and classic books of herbal medicine. All data were searched up to May 2021 to identify eligible studies. Some previous reviews have summarized the limonoids-related research progresses—for instance, the chemical compounds of Melia species and their bioactivities were summarized in 2010 (Zhao et al., 2010). In addition, a fantastic review has comprehensively covered the limonoids from Meliaceae, and their pharmacological effects were also concluded (Tan and Luo, 2011). Nevertheless, our work attempts to offer some constructive information that is favorable to the development of genus Melia-derived limonoids that originated from traditional medicinal herbs. Herein we systematically summarize the phytochemistry, synthesis, pharmacological activities, structure–activity relationships, pharmacokinetics, and safety aspects of limonoids, hoping that these could propel forward the exploration for this kind of valuable compound. Furthermore, the future research perspectives and difficulties are discussed as well.
Chemical Compounds
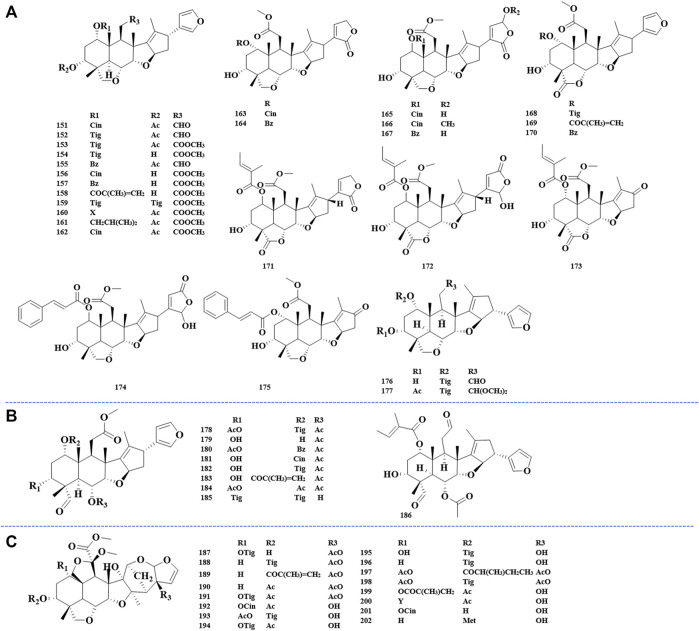
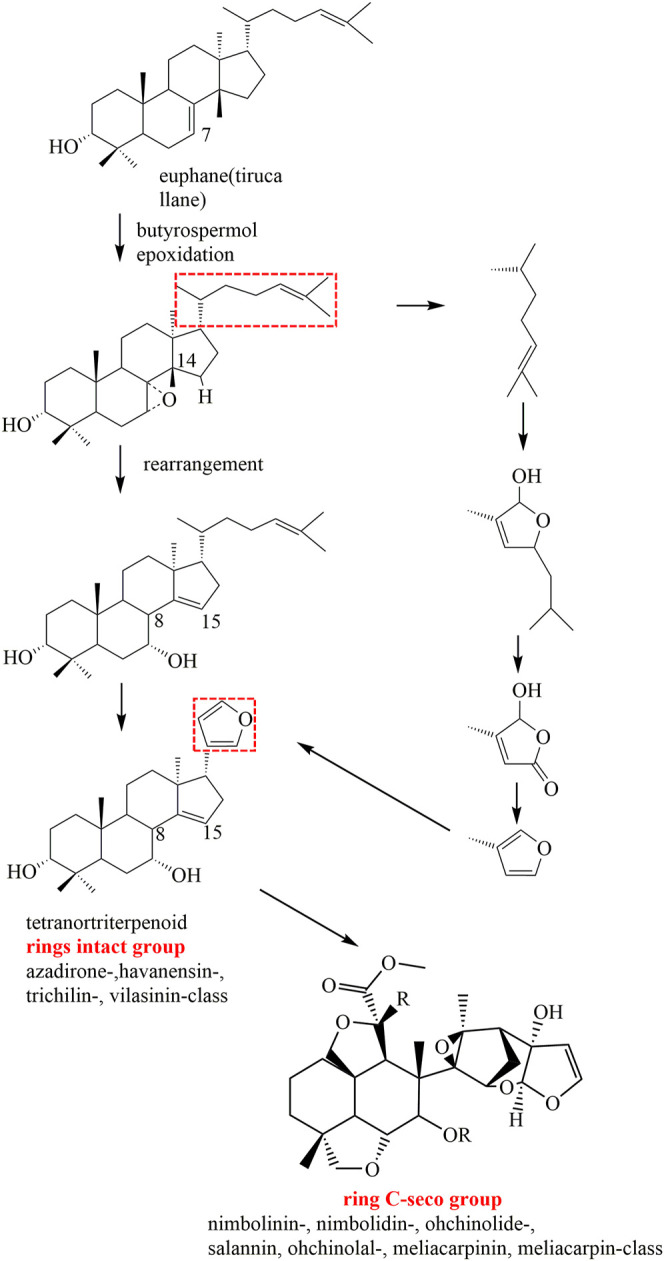
After years of phytochemical research, more than 200 limonoids have been isolated and identified from genus Melia plants. As expected, the majority of these compounds originated from M. toosendan and M. azedarach because these two species are commonly used as medicinal herbs. In addition, the distribution of these compounds also varied in different parts of the plants, of which the fruit, bark, and root bark possessed higher content. Interestingly, those parts mentioned above are consistent with the medicinal parts of M. toosendan and M. azedarach. Therefore, isolation of bioactive compounds from medicinal herbs is a promising strategy for discovering lead compounds in drug development. At present, it is generally acknowledged that the precursors for the biogenic synthesis of limonoids are two types of tetracyclic triterpenoids, including tirucallane and euphane. The biosynthesis pathways of limonoids are shown in Figure1. Firstly, the Δ7-double bond is oxidized to 7-epoxy, which was subsequently opened to cause a Wagner–Meerwein shift of Me-14 to C-8, and finally leads to the formation of OH-7 and the introduction of a double bond at C-14/15. On this basis, four carbons are lost from the rear side chain to form a 17-β-furan ring, and the last step is finished after the formation of the 4,4,8-trimethyl-steroid skeleton, which was regarded as the basic limonoid skeleton (Tan and Luo, 2011). The limonoids in genus Melia are mainly classified into two classes, including the ring-intact limonoids and the ring-C-seco limonoids, and the detailed chemical information will be discussed in this review (Table 1).
FIGURE 1.
Proposed major biosynthesis pathways of limonoids in genus Melia.
TABLE 1.
Classifications and sources of limonoids isolated from genus Melia.
| No | Compounds | Part of plant | Sources | Reference |
|---|---|---|---|---|
| Trichilin class | ||||
| 1 | Azedarachin A | Stem barks | M. toosendan | Zhou et al. (1996) |
| 2 | 12-O-Acetylazedarachin A | Root barks | M. toosendan | Nakatani (1999) |
| 3 | 12-O-Acetylazedarachin B | Stem barks | M. toosendan | Zhou et al. (1996) |
| 4 | Azedarachin C | Root barks | M. azedarach | D'Ambrosio and Guerriero (2002) |
| 5 | Azedarachin B | Root barks | M. toosendan | Nakatani (1999) |
| 6 | Sendanin | Stem and root barks | M. toosendan | Nakatani (1999) |
| 7 | 12-Hydroxyamoorastatin | Root barks | M. toosendan | Nakatani (1999) |
| 8 | Toosendanin | Stem barks | M. toosendan | Zhang et al. (2010c) |
| 9 | 12-O-Deacetyltrichilin H | Fruits | M. azedarach | Zhou et al. (2005) |
| 10 | 12-Acetyltrichilin B | Root barks | M. azedarach | Nakatani et al. (1994) |
| 11 | 7,12-Diacetyltrichilin B | Root barks | M. azedarach | Nakatani et al. (1994) |
| 12 | Trichilin H | Stem barks | M. toosendan | Zhou et al. (1996) |
| 13 | Trichilin B | Root barks | M. toosendan | Nakatani (1999) |
| 14 | Trichilin D | Root barks | M. azedarach | Nakatani et al. (1994) |
| 15 | Meliatoxin A2 | Root barks | M. azedarach | Nakatani et al. (1994) |
| 16 | Trichilin K | Stem barks | M. toosendan | Zhou et al. (1996) |
| 17 | Trichilin L | Stem barks | M. toosendan | Zhou et al. (1996) |
| 18 | Trichilin I | Stem barks | M. toosendan | Zhou et al. (1996) |
| 19 | Trichilin J | Stem barks | M. toosendan | Zhou et al. (1996) |
| 20 | 12-Deacetyltrichilin I | Barks | M. azedarach | Takeya et al. (1996b) |
| 21 | 1-Acetyltrichilin H | Barks | M. azedarach | Takeya et al. (1996b) |
| 22 | 3-Deacetyltrichilin H | Barks | M. azedarach | Takeya et al. (1996b) |
| 23 | 1-Acetyl-3-deacetyltrichilin H | Barks | M. azedarach | Takeya et al. (1996b) |
| 24 | 1-Acetyl-2-deacetyltrichilin H | Barks | M. azedarach | Takeya et al. (1996b) |
| 25 | 1,12-Diacetyltrichilin B | Barks | M. azedarach | Takeya et al. (1996b) |
| 26 | Meliatoosenin C | Stem barks | M. toosendan | Zhang et al. (2010c) |
| 27 | 24-Norchola-20,22-diene-4-carboralde-hyde-14,15:21,23-diepoxy-1,3,7,12,19-pentachydroxy-4,8-dimethyl-11-oxo-cyclic-4,19-hemiacetal[C(S),1α,3α,4β,5α,7α,12α,13α,14β,15β,17α]-(9Cl) | Stem barks | M. toosendan | Zhang et al. (2010c) |
| 28 | Meliatoosenin D | Stem barks | M. toosendan | Zhang et al. (2010c) |
| 29 | 1-O-Acetyltrichilin H | Root barks | M. toosendan | Zhou et al. (1998) |
| 30 | 12-Acetoxyamoorastatin | Barks | M. azedarach | Ahn et al. (1994) |
| 31 | 7-Benzoyltoosendanin | Seeds | M. azedarach | Liu et al. (2011) |
| 32 | 7-Cinnamoyltoosendanin | Seeds | M. azedarach | Liu et al. (2011) |
| 33 | Meliarachin B | Twigs and leaves | M. azedarach | Su et al. (2011) |
| 34 | Meliarachin C | Twigs and leaves | M. azedarach | Su et al. (2011) |
| 35 | Meliatoxin B1 | Fruits | M. toosendan | Tada et al. (1999) |
| 36 | 12α-Hydroxymeliatoxin B1 | Fruits | M. toosendan | Zhu et al. (2014) |
| 37 | 12α-Acetoxylmeliatoxin B2 | Fruits | M. toosendan | Zhu et al. (2014) |
| 38 | Meliatoosenin G | Fruits | M. toosendan | Zhang et al. (2012) |
| 39 | Meliatoosenin H | Fruits | M. toosendan | Zhang et al. (2012) |
| 40 | 29-[(2-Methylbutanoyl)oxy]-2α-hydroxyamoorastatone | Root barks | M. toosendan | Zhou et al. (2009) |
| 41 | 1,3-epi-29-[(2-Methylpropanoyl)oxy]-2α-hydroxyamoorastatone | Root barks | M. toosendan | Zhou et al. (2009) |
| 42 | Isotoosendanin | Fruits | M. toosendan | Xie et al. (2008) |
| 43 | Meliarachin L | Fruits | M. toosendan | Yan et al. (2020) |
| 44 | Neoazedarachin A | Root barks | M. toosendan | Zhou et al. (1998) |
| 45 | Neoazedarachin B | Root barks | M. toosendan | Zhou et al. (1998) |
| 46 | Neoazedarachin D | Root barks | M. toosendan | Zhou et al. (1998) |
| 47 | 12α-Hydroxyamoorastatone | Root barks | M. toosendan | Zhou et al. (1998) |
| 48 | Amoorastatone | Fruits | M. toosendan | Wang et al. (2020a) |
| 49 | 12-Dehydroneoazedarachin D | Fruits | M. azedarach | Akihisa et al. (2013) |
| 50 | Meliatoxin B2 | Barks | M. azedarach | Huang et al. (1994) |
| 51 | Meliarachin G | Twigs and leaves | M. azedarach | Su et al. (2011) |
| 52 | Meliarachin H | Twigs and leaves | M. azedarach | Su et al. (2011) |
| 53 | Meliarachin I | Twigs and leaves | M. azedarach | Su et al. (2011) |
| 54 | Meliarachin J | Twigs and leaves | M. azedarach | Su et al. (2011) |
| 55 | Meliarachin K | Twigs and leaves | M. azedarach | Su et al. (2011) |
| 56 | 12α-Hydroxymeliatoosenin I | Fruits | M. toosendan | Zhu et al. (2014) |
| 57 | Meliatoosenin I | Fruits | M. toosendan | Zhang et al. (2012) |
| 58 | Meliatoosenin J | Fruits | M. toosendan | Zhang et al. (2012) |
| 59 | Mesendanin H | Twigs and leaves | M. toosendan | Dong et al. (2010) |
| 60 | Meliarachin D | Twigs and leaves | M. azedarach | Su et al. (2011) |
| 61 | Meliarachin E | Twigs and leaves | M. azedarach | Su et al. (2011) |
| 62 | Meliazedalides B | Fruits | M. azedarach | Qiu et al. (2019) |
| 63 | Toosendalactonins | Fruits | M. azedarach | Park et al. (2020) |
| 64 | Meliatoosenin E | Fruits | M. toosendan | Zhang et al. (2012) |
| 65 | Mesendanin G | Twigs and leaves | M. toosendan | Dong et al. (2010) |
| 66 | Meliartenin | Fruits | M. azedarach | Carpinella et al. (2003) |
| 67 | Toosendanal | Fruits | M. toosendan | Tada et al. (1999) |
| Vilasinin class | ||||
| 68 | Meliatoosenin K | Fruits | M. toosendan | Zhang et al. (2012) |
| 69 | Trichilinin D | Root barks | M. toosendan | Nakatani (1999) |
| 70 | Trichilinin E | Root barks | M. toosendan | Nakatani (1999) |
| 71 | Toosendansin H | Fruits | M. toosendan | Li et al. (2020b) |
| 72 | 1-O-cinnamoyltrichilinin | Fruits | M. toosendan | Nakatani et al. (2000) |
| 73 | Trichilinin B | Root barks | M. toosendan | Nakatani (1999) |
| 74 | Trichilinin C | Root barks | M. toosendan | Nakatani (1999) |
| 75 | 24, 25,26, 27-Tetranorapotirucalla-(apoeupha)-1α-tigloyloxy-3α, 7α-dihydroxyl-12α-acetoxyl-14, 20, 22-trien-21, 23-epoxy-6,28-epoxy | Fruits | M. toosendan | Zhang et al. (2010d) |
| 76 | Meliavolkin | Barks | M. volkensii | Zeng et al. (1995a) |
| 77 | Meliavolkinin | Barks | M. volkensii | Rogers et al. (1998a) |
| 78 | 1,3-Diacetylvilasinin | Barks | M. volkensii | Rogers et al. (1998a) |
| 79 | 1-Acetyltrichilinin | Fruits | M. volkensii | Jaoko et al. (2020) |
| 80 | 1-Tigloyltrichilinin | Fruits | M. volkensii | Jaoko et al. (2020) |
| 81 | 11,15-Dioxotrichilinin | Fruits | M. toosendan | Zhu et al. (2014) |
| 82 | Toosendansin I | Fruits | M. toosendan | Li et al. (2020b) |
| Havanensin class | ||||
| 83 | Mesendanin C | Twigs and leaves | M. toosendan | Dong et al. (2010) |
| 84 | Mesendanin D | Twigs and leaves | M. toosendan | Dong et al. (2010) |
| 85 | Sendanal B | Fruits | M. toosendan | Yan et al. (2020) |
| 86 | 24,25,26,27-Tetra-norapotirucalla-(apoeupha)-1α,6α,12α-triacetoxyl-3α,7α-dihydroxyl-28-aldehyde-14,20,22-trien-21,23-epoxy | Fruits | M. toosendan | Zhang et al. (2013) |
| 87 | 14,15-Deoxy-11-oxohavanensin 3,12-diacetate | Fruits | M. toosendan | Zhu et al. (2014) |
| 88 | Mesendanin A | Twigs and leaves | M. toosendan | Dong et al. (2010) |
| 89 | Mesendanin B | Twigs and leaves | M. toosendan | Dong et al. (2010) |
| 90 | Mesendanin J | Twigs and leaves | M. toosendan | Dong et al. (2010) |
| 91 | Toosendone | Fruits | M. toosendan | Zhang et al. (2007) |
| 92 | Butenolide | Root barks | M. toosendan | Nakatani (1999) |
| 93 | Meliarachin A | Twigs and leaves | M. azedarach | Su et al. (2011) |
| 94 | Melianin C | Barks | M. volkensii | Rogers et al. (1998a) |
| 95 | Meliatoosenin F | Fruits | M. toosendan | Zhang et al. (2012) |
| 96 | Mesendanin I | Twigs and leaves | M. toosendan | Dong et al. (2010) |
| Azadirone class | ||||
| 97 | Meliatoosenin A | Stem barks | M. toosendan | Zhang et al. (2010c) |
| 98 | Azadirone | Root barks | M. toosendan | Nakatani (1999) |
| 99 | Acetyltrichilenone | Root barks | M. toosendan | Nakatani (1999) |
| Nimbolinin class | ||||
| 100 | 12-O-Methylvolkensin | Fruits | M. toosendan | Tada et al. (1999) |
| 101 | 1-Deacetylnimbolinin A | Root barks | M. toosendan | Nakatani et al. (1999) |
| 102 | Nimbolinin A | Fruits | M. toosendan | Su et al. (2013) |
| 103 | Nimbolinin C | Fruits | M. toosendan | Nakatani et al. (2000) |
| 104 | Nimbolinin D | Fruits | M. toosendan | Nakatani et al. (2000) |
| 105 | Nimbolinin B | Fruits | M. toosendan | Su et al. (2013) |
| 106 | 1-Deacetylnimbolinin B | Fruits | M. toosendan | Su et al. (2013) |
| 107 | 12-Ethoxynimbolinins A | Fruits | M. toosendan | Zhang et al. (2007) |
| 108 | 12-Ethoxynimbolinins B | Fruits | M. toosendan | Zhang et al. (2007) |
| 109 | 12-Ethoxynimbolinins C | Fruits | M. toosendan | Zhang et al. (2007) |
| 110 | 12-Ethoxynimbolinins D | Fruits | M. toosendan | Zhang et al. (2007) |
| 111 | 12-Ethoxynimbolinins H | Barks | M. toosendan | Zhang et al. (2018) |
| 112 | Nimbolin B | Root barks | M. volkensii | Zeng et al. (1995b) |
| 113 | 1-Decinnamoyl-1-(2′-methylacryloyl) nimbolinin C | Fruits | M. toosendan | Zhu et al. (2014) |
| 114 | 1-Decinnamoylnimbolinin C | Fruits | M. toosendan | Zhu et al. (2014) |
| 115 | 12-O-Methyl-1-O-deacetylnimbolinin B | Fruits | M. toosendan | Hu et al. (2011) |
| 116 | 12-O-Methyl-1-O-tigloyl-1-O-deacetylnimbolinin B | Fruits | M. toosendan | Hu et al. (2011) |
| 117 | 12-O-Ethylnimbolinin B | Fruits | M. toosendan | Hu et al. (2011) |
| 118 | 1α, 7α-Dihydroxyl-3α-acetoxyl-12α-ethoxylnimbolinin | Fruits | M. toosendan | Zhang et al. (2016) |
| 119 | 1α-Tigloyloxy-3α-acetoxyl-7α-hydroxyl-12β-ethoxylnimbolinin | Fruits | M. toosendan | Zhang et al. (2016) |
| 120 | 1α, 3α-Dihydroxyl-7α-tigloyloxy-12α-ethoxylnimbolinin | Fruits | M. toosendan | Zhang et al. (2016) |
| 121 | 1α, 7α-Ditigloyloxy-3α-acetoxyl-12α-ethoxylnimbolinin | Fruits | M. toosendan | Zhang et al. (2016) |
| 122 | 1α-Benzoyloxy-3α-acetoxyl-7α-hydroxyl-12β-ethoxylnimbolinin | Fruits | M. toosendan | Zhang et al. (2013) |
| 123 | 12α-1-O-Tigloyl-1-O-deacetyl-nimbolinin B | Fruits | M. toosendan | Su et al. (2013) |
| 124 | 1α-Benzoyloxy-3α-acetoxyl-7α-hydroxyl-12α-ethoxyl nimbolinin | Fruits | M. toosendan | Zhang et al. (2010b) |
| 125 | 1-O-Benzoyl-3-O-deactylnim-bolinin C | Fruits | M. azedarach | Park et al. (2020) |
| 126 | 1-Benzoylnimbolinin C | Seeds | M. azedarach | Liu et al. (2011) |
| 127 | Meliatoosenin L | Fruits | M. toosendan | Zhang et al. (2012) |
| 128 | Meliatoosenin N | Fruits | M. toosendan | Zhang et al. (2012) |
| 129 | Toosendansins B | Fruits | M. toosendan | Chen et al. (2014) |
| 130 | 3-Deacetyl-12-O-methylvolkensin | Fruits | M. toosendan | Zhu et al. (2014) |
| 131 | Meliatoosenin T | Fruits | M. toosendan | Yan et al. (2020) |
| 132 | Meliatoosenin U | Fruits | M. toosendan | Yan et al. (2020) |
| 133 | Meliazedalides A | Fruits | M. azedarach | Qiu et al. (2019) |
| Ohchinolide class | ||||
| 134 | Ohchinolide A | Fruits | M. azedarach | Ochi et al. (1979) |
| 135 | Ohchinolide B | Root barks | M. toosendan | Zhou et al. (1997) |
| 136 | Ohchinolide C | Barks | M. toosendan | Zhou et al. (1997) |
| 137 | 1-O-Deacetyl-1-O-tigloylohchinolide B | Fruits | M. azedarach | Zhou et al. (2004) |
| 138 | 1-O-Deacetyl-1-O-benzoylohchinolide B | Fruits | M. azedarach | Zhou et al. (2004) |
| 139 | 1-O-Deacetyl-1-O-tigloylohchinolide A | Fruits | M. azedarach | Zhou et al. (2004) |
| 140 | 1-O-Deacetylohchinolide B | Fruits | M. azedarach | Zhou et al. (2004) |
| 141 | 1-O-Deacetylohchinolide A | Fruits | M. azedarach | Zhou et al. (2004) |
| 142 | Azecin 2 | Roots | M. azedarach | Tan and Luo (2011) |
| Nimbolidin class | ||||
| 143 | Nimbolidin F | Barks | M. toosendan | Zhou et al. (1997) |
| 144 | Nimbolidin D | Barks | M. toosendan | Zhou et al. (1997) |
| 145 | Nimbolidin C | Root barks | M. toosendan | Nakatani et al. (1996) |
| 146 | Nimbolidin E | Root barks | M. toosendan | Nakatani et al. (1996) |
| 147 | Nimbolidin B | Root barks | M. toosendan | Nakatani et al. (1996) |
| 148 | 15-O-Deacetyl-15-O-methylnimbolidin A | Fruits | M. azedarach | Zhou et al. (2005) |
| 149 | 15-O-Deacetyl-15-O-methylnimbolidin B | Fruits | M. azedarach | Zhou et al. (2005) |
| 150 | 15-O-Deacetylnimbolidin B | Fruits | M. azedarach | Zhou et al. (2005) |
| Salannin class | ||||
| 151 | 1-O-Cinnamoyl-1-O-debenzoylohchinal | Fruits | M. toosendan | Hu et al. (2011) |
| 152 | 1-O-Tigloyl-1-O-debenzoylohchinal | Fruits | M. toosendan | Hu et al. (2011) |
| 153 | Salannin | Root barks | M. toosendan | Nakatani et al. (1996) |
| 154 | 3-O-Deacetylsalanni | Fruits | M. azedarach | Akihisa et al. (2013) |
| 155 | Ohchinal | Fruits | M. toosendan | Wang et al. (2020a) |
| 156 | Ohchinin | Leaves | M. azedarach | Pan et al. (2014b) |
| 157 | 1-O-Decinnamoyl-1-O-benzoylohchinin | Fruits | M. azedarach | Akihisa et al. (2013) |
| 158 | 3-Deacetyl-4′-demethylsalannin | Fruits | M. azedarach | Pan et al. (2014a) |
| 159 | 3-Deacetyl-3-tigloylsalannin | Fruits | M. azedarach | Akihisa et al. (2017) |
| 160 | 2′,3′-Dihydrosalannin | Fruits | M. volkensii | Jaoko et al. (2020) |
| 161 | 1-Detigloyl-1-isobutylsalannin | Fruits | M. volkensii | Jaoko et al. (2020) |
| 162 | Ohchinin-acetate | Fruits | M. volkensii | Jaoko et al. (2020) |
| 163 | Ohchininolide | Fruits | M. azedarach | Akihisa et al. (2013) |
| 164 | 1-O-Decinnamoyl-1-O-benzoylohchininolide | Fruits | M. azedarach | Akihisa et al. (2013) |
| 165 | 23-Hydroxyohchininolide | Leaves | M. azedarach | Pan et al. (2014b) |
| 166 | 23-Methoxyohchininolide A | Fruits | M. azedarach | Akihisa et al. (2013) |
| 167 | 1-O-Decinnamoyl-1-O-benzoyl-23-hydroxyohchininolide | Fruits | M. azedarach | Akihisa et al. (2013) |
| 168 | 3-Deacetyl-28-oxosalannin | Leaves | M. azedarach | Pan et al. (2014b) |
| 169 | 3-Deacetyl-4′-demethyl-28-oxosalannin | Leaves | M. azedarach | Pan et al. (2014b) |
| 170 | 1-O-Decinnamoyl-1-O-benzoyl-28-oxoohchinin | Fruits | M. azedarach | Akihisa et al. (2013) |
| 171 | 3-Deacetyl-28-oxosalannolactone | Leaves | M. azedarach | Pan et al. (2014b) |
| 172 | 3-Deacetyl-28-oxoisosalanninolide | Leaves | M. azedarach | Pan et al. (2014b) |
| 173 | 3-Deacetyl-17-defurano-17,28-dioxosalannin | Leaves | M. azedarach | Pan et al. (2014b) |
| 174 | 21-Hydroxyisoohchininolide | Leaves | M. azedarach | Akihisa et al. (2013) |
| 175 | 17-Defurano-17-oxoohchinin | Fruits | M. azedarach | Akihisa et al. (2013) |
| 176 | Meliatoosenin P | Fruits | M. toosendan | Zhang et al. (2012) |
| 177 | Meliatoosenin Q | Fruits | M. toosendan | Zhang et al. (2012) |
| Ohchinolal class | ||||
| 178 | 3-O-Acetylohchinolal | Barks | M. toosendan | Zhou et al. (1997) |
| 179 | 1-Detigloylohchinolal | Fruits | M. azedarach | Pan et al. (2014a) |
| 180 | 1-Benzoyl-1-detigloylohchinolal | Fruits | M. azedarach | Akihisa et al. (2017) |
| 181 | 1-Cinnamoyl-1-detigloylohchinolal | Fruits | M. azedarach | Akihisa et al. (2017) |
| 182 | Ohchinolal | Root barks | M. toosendan | Zhou et al. (1997) |
| 183 | Mesendanin E | Fruits | M. azedarach | (Dong et al., 2010; Pan et al., 2014a) |
| 184 | Mesendanin F | Twigs and leaves | M. toosendan | Dong et al. (2010) |
| 185 | Toosendansin G | Fruits | M. toosendan | Li et al. (2020b) |
| 186 | Meliatoosenin R | Fruits | M. toosendan | Zhang et al. (2012) |
| Meliacarpinin class | ||||
| 187 | Toosendane A | Barks | M. toosendan | Hu et al. (2018) |
| 188 | Toosendane B | Barks | M. toosendan | Hu et al. (2018) |
| 189 | Toosendane C | Barks | M. toosendan | Hu et al. (2018) |
| 190 | 3,20-Diacetyl-11-methoxymeliacarpinin | Barks | M. toosendan | Hu et al. (2018) |
| 191 | 3,20-Diacetyl-1-tigloyl-11-methoxymeliacarpinin | Barks | M. toosendan | Hu et al. (2018) |
| 192 | Meliacarpinin A | Root barks | M. toosendan | Nakatani (1999) |
| 193 | Meliacarpinin C | Root barks | M. toosendan | Nakatani (1999) |
| 194 | Meliacarpinin D | Root barks | M. toosendan | Nakatani (1999) |
| 195 | Meliacarpinin E | Root barks | M. azedarach | Huang et al. (1996) |
| 196 | 1-Deoxy-3-tigloyl-11-methoxymeliacarpinin | Barks | M. azedarach | Huang et al. (1994) |
| 197 | 3α-(2-Methylbutyryl)-1,20-diacetyl-11-methoxymeliacarpinin | Twigs and leaves | M. azedarach | Zhang et al. (2014) |
| 198 | 3-Tigloyl-1,20-diacetyl-11-methoxymeliacarpinin | Twigs and leaves | M. azedarach | Zhang et al. (2014) |
| 199 | 1-Methacrylyl-3-acetyl-11-methoxymeliacarpinin | Roots | M. azedarach | Nakatani (1999) |
| 200 | 1-(2-Methylpropanoyl)-3-acetyl-11-methoxymeliacarpinin | Roots | M. azedarach | Nakatani (1999) |
| 201 | 1-Cinnamoyl-3-hydroxy- 11-methoxymeliacarpinin | Barks | M. azedarach | Takeya et al. (1996a) |
| 202 | 1-Deoxy-3-methacrylyl-11-methoxymeliacarpinin | Barks | M. azedarach | Takeya et al. (1996a) |
| Meliacarpin class | ||||
| 203 | 1,3-Dicinnamoyl-11-hydroxymeliacarpin | Leaves | M. azedarach | Bohnenstengel et al. (1999) |
| 204 | 1-Cinnamoyl-3-methacrylyl-11-hydroxymeliacarpin | Leaves | M. azedarach | Bohnenstengel et al. (1999) |
| 205 | 1-Cinnamoyl-3-acetyl-11-hydroxymeliacarpin | Leaves | M. azedarach | Bohnenstengel et al. (1999) |
| 206 | 1-Cinnamoyl-3-feruloyl-11-hydroxymeliacarpin | Leaves | M. azedarach | Bohnenstengel et al. (1999) |
| 207 | Azadirachtin | Leaves | M. azedarach | Bohnenstengel et al. (1999) |
| 208 | 1-Cinnamoyl-3,11-dihydroxymeliacarpin | Leaves | M. azedarach | Barquero et al. (2006) |
| Others | ||||
| 209 | Meliatoosenin S | Fruits | M. toosendan | Zhang et al. (2012) |
| 210 | Spirosendan | Root barks | M. toosendan | Nakatani et al. (1999) |
| 211 | Volkensinin | Barks | M. volkensii | Rogers et al. (1998b) |
| 212 | Azecin 1 | Roots | M. azedarach | Tan and Luo (2011) |
| 213 | Azecin 3 | Roots | M. azedarach | Tan and Luo (2011) |
| 214 | Azecin 4 | Roots | M. azedarach | Tan and Luo (2011) |
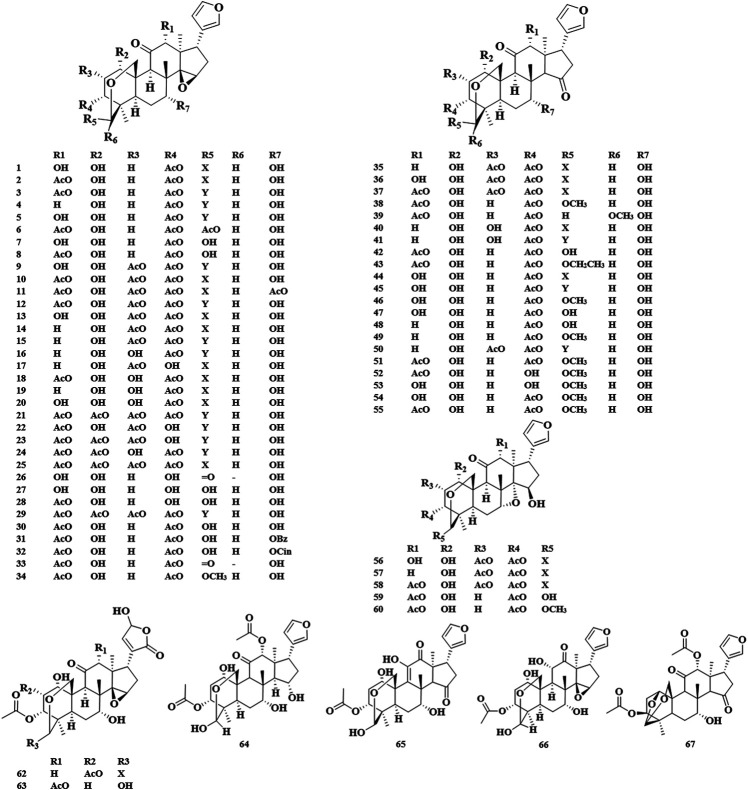
Trichilin Class (1–67)
The basic skeleton of the trichilin class limonoids contained the C-19/29 bridged acetal and the 14,15-epoxide moieties or C15 carbonyl. Toosendanin, as a characteristic compound in trichilin class, has been confirmed to be a potential bioactive component in the field of anti-tumor, insecticide, and anti-botulism. It should be noted that toosendanin can only be isolated from two plant species, M. toosendan and M. azedarach (Shi and Li, 2007). The first isolation and identification of toosendanin can be traced back to 1975. In 1980, Chinese researchers corrected its chemical structure due to the occurrence of two tautomers found by paper chromatography and silica gel plate method (Su and Liang, 1980). In 1984, iso-toosendanin, the isomer of toosendanin, was also isolated from these two plants. In 1998, Zhou et al. isolated four new trichilin class limonoids from the root barks of M. toosendan, of which neoazedarachin D was found to be the first natural 29-endo-derivative in C (19)/C (29) bridged acetal limonoids (Zhou et al., 1998). Apart from C-19/29 bridged acetal, toosendanal with C (1)/C (29) bridged acetal was also discovered in M. toosendan (Tada et al., 1999) (Figure 2).
FIGURE 2.
Structures of trichilin class limonoids 1–67.
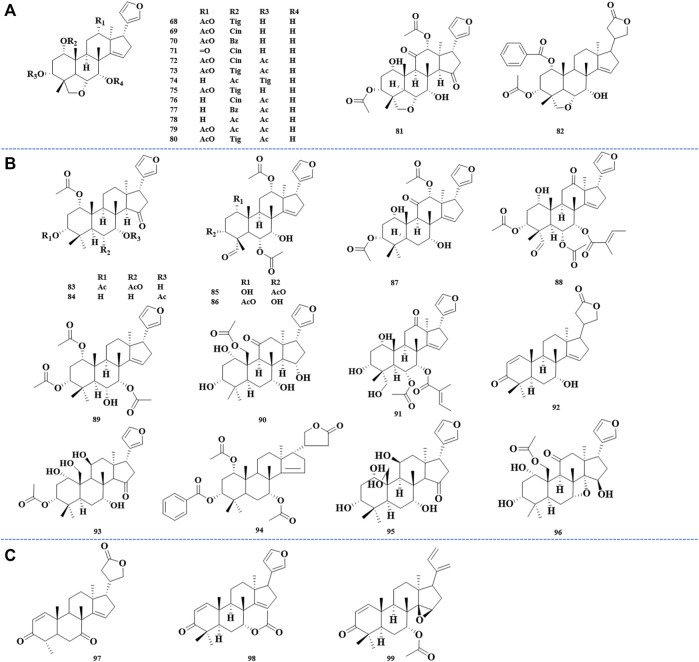
Vilasinin Class (68–82)
6α,28-ether bridge was supposed to be a typical skeleton of vilasinin class limonoids. Trichilinin B, C, D, and E, four new limonoids with 6α,28-ether bridge, were isolated and identified from the root barks of M. toosendan. It was found that the unique C-12 oxygen function in trichilinin B, C, and E seemed to be the biosynthetic precursors of ring-C-cleaved limonoids (Nakatani, 1999). Using activity-guided separation, meliavolkin was isolated from M. volkensii Gürke., and its structure was elucidated by MS, 1H and 13C NMR, COSY, NOESY, HETCOR, and COLOC spectra (Zeng et al., 1995a) (Figure 3A).
FIGURE 3.
Structures of vilasinin class limonoids 68–82 (A), structures of havanensin class limonoids 83–96 (B), and structures of azadirone class limonoids 97––99 (C).
Havanensin Class (83–96)
The havanensin class limonoids were characteristic of C-1, C-3, and C-7 oxygen substituents, and C-28 will be oxidized, which varies from methyl to carboxyl. In 1998, melianin C was isolated from M. volkensii Gürke., which was confirmed to have a consistent structure with the semisynthetic compound obtained by Jones oxidation of melianin A, and it was also the first report that melianin C appeared naturally (Rogers LL. et al., 1998). Sendanal was associated closely with a precursor of the 14,15-epoxy-12-hydroxymoiety, which could produce the ring-seco limonoids through a Grob fragmentation followed by the formation of an ether ring between the C-7 and C-15 hydroxyl groups via an SN1 mechanism (Tan and Luo, 2011) (Figure 3B).
Azadirone Class (97–99)
The 3-oxo-Δ1,2 and C-7 oxygenation was considered as a vital feature of azadirone-type limonoids. The first isolation of azadirone can be traced back to 1998, and it was firstly isolated from the root barks of M. toosendan, together with acetyltrichilenone (Nakatani, 1999) (Figure 3C).
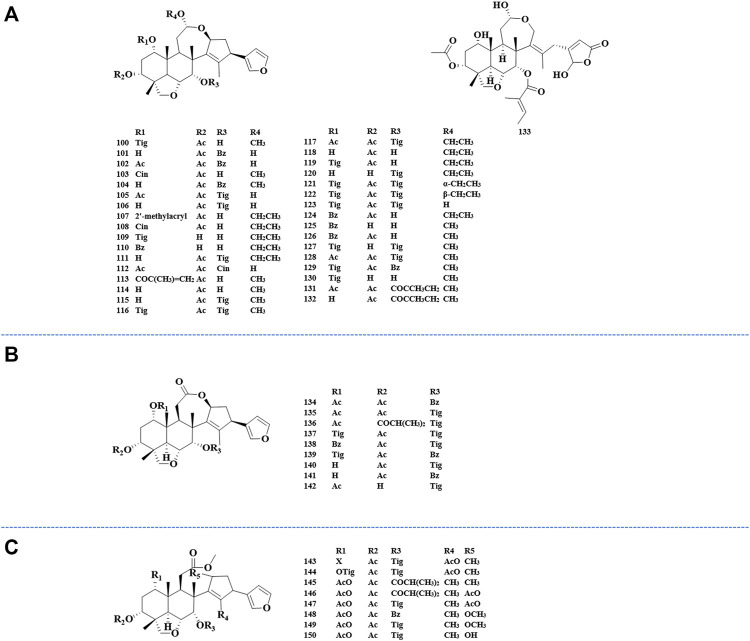
Ring-Seco Limonoids (100–208)
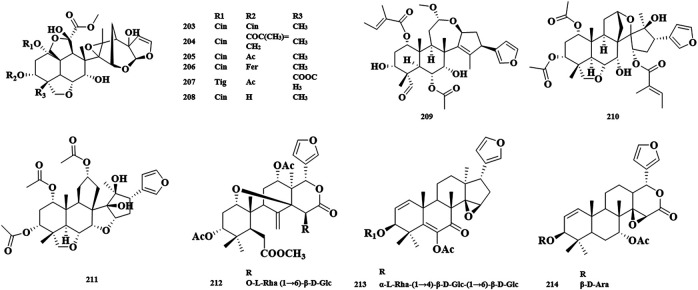
The ring-C-seco limonoids have the most typical chemical structure of all ring-seco limonoids. According to different structural characteristics, the ring C-seco limonoids can be classified into nimbolinin class, ohchinolide class, nimbolidin class, salannin class, ohchinolal class, meliacarpinin class, and meliacarpin class (Figures 4–6).
FIGURE 4.
Structures of nimbolinin class limonoids 100–133 (A), structures of ohchinolide class limonoids 134–142 (B), and structures of nimbolidin class limonoids 143–150 (C).
FIGURE 6.
Structures of meliacarpin class limonoids 203–208 (A) and structures of other-class limonoids 209–214 (B). Rha, rhamnose; Glc, clucose; Ara, arabinose.
FIGURE 5.
Structures of salannin class limonoids 151–177 (A), structures of ohchinolal class limonoids 178–186 (B), and structures of meliacarpinin class limonoids 187–202 (C).
FIGURE 7.
Abbreviation of structure.
Synthesis of Limonoids
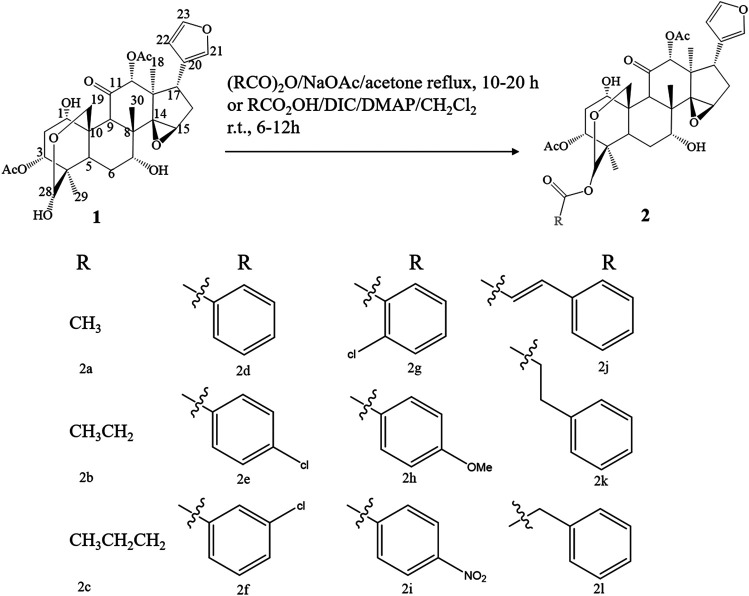
Due to the remarkable pharmacological activities and the unique structural features, many endeavors have been devoted to the synthesis of limonoids found in the genus Melia. Inspired by the anti-botulinum effect and structural diversity of toosendanin, researchers have conducted a synthesis or structural modification of this chemical structure. Excitedly, a similar framework can be found in all mammalian steroids and hormones. Although total synthesis seems like an ideal synthesis approach, the complexity of structure and uncertainty of total synthesis make researchers choose function-oriented synthesis (FOS) as an alternative. FOS aims to emulate a bioactive lead structure or possibly substitute for it with simpler scaffolds designed to contain the active chemical structure sites of target compounds (Wender, 2013). Considering that there were no previous reports on bioactive structural features, Nakai et al. cleaved toosendanin into AB and CD rings, and the biological activity of CD rings was probed by mouse lethality assay, which was deemed to be a “gold standard” in the validation of anti-botulinum activity. Using mesityl oxide and acetylacetone as synthetic substrates, a 4-acetoxy CD fragment analogue of toosendanin was synthesized in 14 steps. Unluckily, no significant anti-botulinum effect was found in the obtained 4-acetoxy CD fragment. Nevertheless, this synthetic method can provide certain inspiration for synthesizing the CD ring analogues (Nakai et al., 2009). Later, in 2010, Nakai et al. focused on AB ring synthesis, which contained a unique bridged hemiacetal, and the anti-botulinum effect was tested in a rat spinal cord cellular assay. The AB ring and epi-AB ring were successfully synthesized with a yield rate of 5.8 and 10% after 23 steps and 19 steps, respectively. Similar to the previously synthesized CD ring, the AB ring did not exhibit anti-botulism activity. All of these results indicated that the complete structure of the ABCD ring is indispensable in the treatment of botulism (Nakai et al., 2010). Apart from exploring the influence of ring structure on the bioactivity of toosendanin, the role of substituents at different positions was investigated as well. By modifying the C-28 position of toosendanin, 12 28-acyloxy derivatives were semi-synthesized, and the insecticidal activity against Mythimna separata Walker was evaluated. The outcomes revealed that the butanoyloxy and phenyl acryloyl oxymoiety at the 28-position plays a critical role in insecticidal activity (Xu and Zhang, 2011) (Figure8). Except for the organic synthesis mentioned above, the biosynthesis of toosendanin also aroused great attention. Since the cyclization of 2,3-oxidosqualene by oxidosqualene cyclase (OSC) is the first stage of triterpenoid biosynthesis to produce diverse triterpene frameworks, Lian et al. found potential OSC genes in M. toosendan by utilizing the triterpenoid profile, transcriptome data, and phylogenetic analysis. The following functional analysis discovered that MtOSC1 was a crucial enzyme in the formation of tirucalla-7,24-dien-3β-ol, which is a precursor for the biosynthesis of toosendanin (Lian et al., 2020). This study revealed that the practicable biosynthesis route of toosendanin may promote its application both in agricultural and medicinal use. Furthermore, salannin and 3-deacetylsalannin can be transformed into 3-deacetoxy-1-de[(E)-2-methylbut-2-enoloxy]salannin-1-en-3-one by Nocardia sp., and the transformed product was validated as a candidate bioactive compound with an α,β-unsaturated ketone moiety in ring A (Madyastha and Venkatakrishnan, 2000).
FIGURE 8.
The synthetic route of 28-acyloxy derivatives of toosendanin.
Biological Activities of Limonoids
Anti-tumor Effects
Accumulating studies have found that limonoids possessed broad-spectrum anti-tumor activities against multiple tumors, such as gastric tumor (Shao et al., 2020), lung tumor (Luo et al., 2018), colorectal tumor (Wang et al., 2015), glioblastoma (Cao et al., 2016), leukemia (Ju et al., 2012), ovarian tumor (Li et al., 2018b), hepatocellular carcinoma (He et al., 2010), breast tumor (Wang et al., 2018), Ewing’s sarcoma (Gao et al., 2019), neuroma (Tang et al., 2004), lymphoma (Zhang B. et al., 2005), and pancreatic tumor (Pei et al., 2017), which indicated that limonoids could be candidate anticancer drugs. Toosendanin (TSN), as a quality control marker in M. toosendan and M. azedarach, has been systematically investigated for its anti-tumor effects. Thus, this review primarily focused on summarizing the anti-tumor activity of TSN and its underlying mechanisms, including the inhibition of tumor cell proliferation, induction of apoptosis, suppression of migration, and invasion. Besides these, we also comprehensively reviewed the anti-tumor bioactivities of other limonoids in genus Melia plants.
Effect of Toosendanin on Tumor Cell Proliferation and Cell Cycle Arrest
Tumor cell proliferation is the basis of tumor growth and metastasis. Inhibition of tumor cell proliferation has been considered as one of the pivotal anti-tumor therapies (Whitfield et al., 2006). It was found that TSN could inhibit the proliferation of MKN-45 gastric cancer cells in a time-dependent and dose-dependent manner, and the IC50 value was 81.06 nM for 48 h (Shao et al., 2020). In addition, some other experiments showed that TSN was able to restrain the proliferation of various cancer cell lines, including SGC-7901 cells (Wang et al., 2017), MGC-803 cells, and HGC-27 cells with IC50 values of 0.11 μM (72 h), 20.30 nM (72 h), and 0.56 µM (48 h), respectively. Using the MTT method, Liu et al. reported that the proliferation of lung cancer A549 cells could be inhibited by TSN with an IC50 value of 40.206 μM for 48 h. Meanwhile, the same inhibitory effect was found in H1975 lung cancer cells. When treating colorectal cancer SW480 cells with TSN, it exhibited anti-proliferation effects with an IC50 value of 0.1059 µM (48 h) (Wang et al., 2015). In order to further explore the anti-tumor effect of TSN in colorectal cancer, a nude mouse xenograft model was established, which subcutaneously inoculated HCT116 cells into nude mice. The outcomes indicated that TSN significantly reduced the tumor volume and weight of the HCT116 xenografts (Wang et al., 2020b). Apart from cancer mentioned above, TSN was found to possess anti-proliferation effects on some other types of tumor, such as glioblastoma, leukemia, ovarian cancer, hepatocellular carcinoma, etc., with IC50 values ranging from 5.4 to 900 nM (Zhang B. et al., 2005; He et al., 2010; Cao et al., 2016; Gao et al., 2019).
The proliferation of tumor cells needs to go through four different stages: pre-DNA synthesis (G1), DNA synthesis (S), late DNA synthesis (G2), and mitosis (M). Since the aberrant activity of various cyclins can lead to the uncontrolled proliferation of tumor cells, treating aberrant cyclins was thus considered as an attractive target for tumor therapy (Otto and Sicinski, 2017). Shao et al. found that TSN-treated MKN-45 human gastric cancer cells markedly arrested at the G1 phase, which was regulated by the overexpression of mir-23a-3p (Shao et al., 2020). In another study, researchers showed that treatment with TSN at concentrations of 2 and 5 μM can cause G1/S arrest in AGS and HGC-27 gastric cancer cells. For a further evaluation of cell cycle arrest, the expression of G1 checkpoint-associated proteins, including cyclin D1 and p21, was assessed. It was observed that TSN treatment could decrease the expression of cyclin D1, while it could increase the expression of p21 (Zhou et al., 2018). Furthermore, in both 5-FU-sensitive and 5-FU-resistant colorectal cancer cells, G1 phase arrest could be induced by TSN. The results also found that, apart from decreased cyclin D1 and increased p21, the expression of p53, a G1-phase checkpoint-associated protein, increased as well (Wang et al., 2020b). In addition, cell cycle arrest also occurred in other tumor cells, such as glioblastoma (Wang Q. et al., 2020) and leukemia (Ju et al., 2013).
Effect of Toosendanin on Tumor Cell Apoptosis
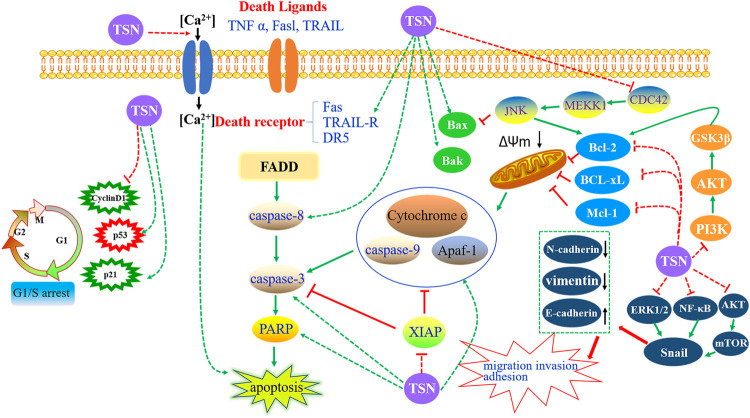
Concerning the anti-tumor effects of TSN, much work has focused on cell apoptosis. Inducing tumor cell apoptosis is now generally considered as the most important mechanism of anti-tumor drugs. Apoptosis, also known as programmed cell death, is a kind of cell-autonomous and orderly death mode, which is activated, expressed, and regulated by specific genes in cells (Li-Weber, 2013). TSN was recently regarded as a potential candidate that killed tumor cells through the induction of apoptosis in various cancer cells, including gastric cancer (Wang et al., 2017; Zhou et al., 2018; Shao et al., 2020), lung cancer (Wang et al., 2017), colorectal cancer (Wang et al., 2015; Wang et al., 2020b), glioblastoma (Cao et al., 2016; Wang Q. et al., 2020), leukemia (Ju et al., 2012; Ju et al., 2013), ovarian cancer (Li et al., 2018a; Li et al., 2019), hepatocellular carcinoma (He et al., 2010; Liu et al., 2016), breast cancer (Wang et al., 2018), Ewing’s sarcoma (Gao et al., 2019), neuroma (Tang et al., 2004), and lymphoma (Zhang B. et al., 2005). Herein our study summed up the detailed mechanisms of apoptosis induced by TSN (Figure 9).
FIGURE 9.
Targeting the apoptosis signaling pathways by toosendanin in cancers.
Targeting the Intrinsic Pathway
The intrinsic pathway is also known as cell mitochondrial apoptosis. When cells are exposed to various stimulating factors, such as hypoxia, activation of the oncogene, DNA damage, and cytotrophic factor deficiency, the steady state of mitochondrial membrane potential will be destroyed, and the permeability can increase. Subsequently, the pro-apoptotic factors in the mitochondrial membrane are released to the cytoplasm, which later activates the apoptosis pathway of the mitochondria and causes cell death (Estaquier et al., 2012). Cytochrome c (Cyt C), apoptosis-inducing factor, cysteinyl aspartate-specific proteinase (caspase) activator, and apoptotic protease-activating factor-1 (Apaf-1) are supposed to be pivotal pro-apoptotic factors. The apoptosome complex is formed by the combination of released Cyt C with Apaf-1 and caspase-9, which activates caspase-3 and eventually induces apoptosis. Importantly, regulating the B-cell lymphoma-2 (Bcl-2) protein family has been considered as an essential route to play a role in cell mitochondrial apoptosis signal pathway, the mechanism of which is to control the permeability of the mitochondrial membrane to regulate the release of Cyt C (Siddiqui et al., 2015).
Collectively, TSN-induced apoptosis of tumor cells is related to the activation of the intrinsic pathway. On the one hand, the capacity of cells to undergo mitochondrial apoptosis is governed by pro- and anti-apoptotic members of the Bcl-2 protein family. According to the difference of its functional domain, it can be divided into anti-apoptotic Bcl-2 subfamily (e.g., Bcl-2, Bcl-xL, and Mcl-1), pro-apoptotic Bax subfamily (e.g., Bax and Bak), and BH3-only pro-apoptotic family (e.g., Bid, Bim, Bik, and Noxa) (Campbell and Tait, 2018). On the other hand, the caspase family is known as the most well-studied apoptotic executive protein, which has the characteristics of post-aspartic protein hydrolysis. Based on their different roles in apoptosis, it can be classified into two types: the initiator (apical) caspases (e.g., caspase-2, -8, -9, and -10) and effector (executioner) caspases (e.g., caspase-3, -6, and -7) (Ola et al., 2011). The caspase family implements its pro-apoptotic effect mainly through the combination of apoptosis signal and death receptor to induce the activation process of initiator and effector caspase protein and the subsequent inactivation process of apoptosis-inhibitory protein and cause nucleic acid and cell structure cleavage and so on (Fiandalo and Kyprianou, 2012). When administrated with TSN, several typical characteristics of apoptosis occurred, including nuclei condensation, DNA fragmentation, reduction of mitochondrial membrane potential, and the upregulation of cytochrome c in cytosol (Tang et al., 2004; Shao et al., 2020). Subsequently, caspase 9 and caspase 3 were activated (Tang et al., 2004; He et al., 2010; Ju et al., 2013; Zhou et al., 2018; Gao et al., 2019; Wang et al., 2020b; Shao et al., 2020), and TSN promoted poly-ADP-ribose polymerase (a target protein of caspase 3) fragmentation (Tang et al., 2004; Ju et al., 2013; Zhou et al., 2018; Wang et al., 2020b), eventually leading to apoptosis. Some other researches showed that TSN treatment significantly increased the pro-apoptotic protein expression of Bax (He et al., 2010; Ju et al., 2013; Cao et al., 2016; Liu et al., 2016; Zhou et al., 2018; Gao et al., 2019; Shao et al., 2020), Bak (Cao et al., 2016), Bim, and Noxa Apaf-1 (Shao et al., 2020) and decreased the anti-apoptotic protein expression of Bcl-2 (He et al., 2010; Ju et al., 2013; Cao et al., 2016; Liu et al., 2016; Wang et al., 2017; Zhou et al., 2018; Gao et al., 2019; Wang et al., 2020b; Shao et al., 2020), Bcl-xL (Zhou et al., 2018; Wang et al., 2020b), Mcl-1 (Zhou et al., 2018; Wang et al., 2020b), and XIAP (Zhou et al., 2018; Wang et al., 2020b), thus inducing apoptosis-associated caspase activation.
Targeting the Extrinsic Pathway
The death receptor (DR)-mediated apoptosis signaling pathway is a kind of a signal pathway that interacts with proteins mediated by transmembrane receptors. Death receptors belong to the tumor necrosis factor receptor (TNFR) superfamily, including Fas (also known as CD95 or Apo-1), TNFR-1, TNFR-2, DR3, DR4, and DR5 (Tummers and Green, 2017). Up to now, the Fas/FasL signaling pathway is considered as the most crucial signaling pathway in death receptor-mediated apoptosis. The corresponding mechanism is as follows: FAS is activated after binding with FasL. The activated Fas receptor recruits Fas-associated death domain protein (FADD) through the interaction of death domain (DD). FADD can not only bind Fas through DD at the C-terminal but also recruit procaspase-8 through N-terminal death effector domain and finally form Fas receptor–FADD–procaspase-8 death-inducing signaling complex (DISC). With the increase of the local concentration of DISC, procaspase-8 can catalyze the formation of active caspase-8 in DISC and then activate the downstream caspase factor, thus initiating the apoptosis process dependent on caspase cascade reaction (Wajant, 2002; Singh et al., 2016). Integral in the regulation and initiation of death receptor-mediated activation of programmed cell death is caspase-8 (Tummers and Green, 2017). To escape exogenous apoptosis, cancer cells often downregulate the expression of death receptors by using antagonistic machinery (Friesen et al., 1997). Therefore, recovering Fas expression by drug intervention is anticipated to trigger cancer cell apoptosis.
It was observed that TSN could induce apoptosis by increasing the expression of Fas to inhibit hepatocellular carcinoma cells (He et al., 2010; Liu et al., 2016). In addition, for the ovarian cancer CAOV-3 and ES-2 cells, when treated with TSN, the expression of Fas and FasL increased firstly, subsequently followed by the activation of caspase-8 and caspase-3. After the administration of z-IETD-FMK and z-DEVE-FMK (the inhibitors of caspase-8 and caspase-3, respectively), the inhibitory effect of TSN on the proliferation of these cancer cells was reduced (Li et al., 2019). The increased expression of Fas, caspase-8, and caspase-3 was also exhibited in leukemia K562 cells after incubation with TSN. Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a promising anti-cancer agent that belongs to the TNF superfamily (Ashkenazi et al., 1999). Many cancer cells remain resistant to receptor-mediated apoptosis—for instance, the TRAIL-mediated apoptotic resistance appears in non-small-cell lung carcinoma, and the TSN showed a sensitivity-enhanced ability for cancer cell apoptosis. The underlying apoptotic mechanisms involved the upregulation of death receptor 5 (DR5) mediated by CCAAT/enhancer binding protein homologous protein (Li et al., 2017). Consistently, the upregulated expression of DR5 was found in TRAIL inhibiting hepatocellular carcinoma.
Other Mechanisms of Apoptosis
Recently, intracellular calcium ion (Ca2+) has been regarded to play a vital role in cell apoptosis, and Ca2+ overload was considered as the common final pathway of all types of cell death (Rizzuto et al., 2003). Ca2+ can induce apoptosis through regulating apoptosis-related molecules, producing reactive oxygen species (ROS), and changing cell membrane permeability (Orrenius et al., 2003). Shi et al. reported that TSN had an inhibitory effect on neuroblastoma glioma NG108-15 cells. Since TSN cannot penetrate the lipid bilayer, it was found that TSN exerted the anti-cancer role by facilitating the Ca2+ channel of the cell membrane (Hu et al., 1997). In order to further confirm which Ca2+ channel was regulated by TSN, a series of physiological experiments was carried out. These findings showed that TSN could facilitate the L-type Ca2+ channel in the cell membrane, thus promoting extracellular Ca2+ influx. In addition, intracellular Ca2+ overload can directly induce apoptosis (Li and Shi, 2004). It was reported that TSN, at different concentrations, could cause different changes to the PC12 cells, and these distinctions were closely related with the increase of [Ca2+]i, which indicated that Ca2+ played an essential role in cell apoptosis (Li and Shi, 2005).
Moreover, TSN can trigger some different pathways to induce cancer cell apoptosis. In one study, TSN was proven as a candidate of novel anti-cancer drugs for glioblastoma, and the underlying mechanism was inducing estrogen receptor β- and p53-mediated apoptosis (Cao et al., 2016). In addition, the c-Jun N-terminal kinase (JNK) signaling pathway was also involved in TSN-induced apoptosis. In a representative study, TSN was found to induce apoptosis by suppressing the CDC42/MEKK1/JNK pathway in human promyelocytic leukemia HL-60 cells (Ju et al., 2013). Furthermore, the PI3K–Akt signaling pathway acted a crucial role in apoptosis, and excessive activation of PI3K/Akt signaling in cancer cells was involved in the development of multidrug resistance (Burris, 2013). It was observed that TSN could reverse the resistance of human breast cancer cells 4T1 to adriamycin by inhibiting the PI3K/Akt signaling pathway (Wang et al., 2018). Recently, the role of microRNAs (miRNAs) in the treatment of cancer by TSN was explored. The outcomes showed that TSN could inhibit glioma progression property and induce apoptosis via upregulating the expression of miR-608 and downregulating the expression of miR-608 targets, Notch1 and Notch2, in glioma (Wang Q. et al., 2020).
Effect of Toosendanin on Tumor Invasion and Metastasis
About 90% of cancer-related deaths are associated with metastatic diseases rather than primary tumors (Jafri et al., 2017). Emerging evidence showed that TSN could inhibit cancer invasion and metastasis (Pei et al., 2017; Wang et al., 2017; Luo et al., 2018; Wang Q. et al., 2020). The migration and invasion of tumor cells is a complex process of multiple factors, stages, and steps. It involves the process of epithelial–mesenchymal transition (EMT), the degradation of basement membrane and extracellular matrix, the decrease of tumor cell adhesion, and angiogenesis. Among these pathological processes, EMT acts a pivotal role, and its function is transforming the polarized epithelial cell into the mesenchymal cell phenotype. E-cadherin is a marker of epithelial cells, which maintains the integrity of tissue structure by regulating the adhesion reaction between cells and matrix or between cells. Mesenchymal proteins such as N-cadherin and vimentin are highly expressed with the decrease of E-cadherin expression, which collaboratively induces the EMT process (Sachdeva and Mo, 2010). In the transforming growth factor-β1 (TGF-β1)-induced EMT cell model, the inhibitory effect of TSN on A549 and H1975 lung cancer cell migration, invasion, and adhesion was evaluated by wound healing, transwell, and adhesion assays. The outcomes indicated that TSN could weaken the abilities mentioned above, decrease the expression of N-cadherin, vimentin, and p-ERK1/2, and increase the expression of E-cadherin. Besides these, TGF-β1-induced EMT biomarkers can be reversed by silence Snail. These results demonstrated that TSN significantly inhibited TGF-β1-induced EMT and migration, invasion, and adhesion through the ERK/Snail pathway in lung cancer cells (Luo et al., 2018). Consistently, in CAVO-3 human ovarian cancer cells, invasion and migration could be inhibited by TSN, followed by increased NF-κB and E-cadherin and decreased N-cadherin, vimentin, and Snail. The underlying mechanism may be related with the NF-κB/Snail pathway (Li et al., 2018b). Recently, the β-catenin pathway has been considered as the crucial signaling pathway involved in EMT and plays an important role in metastasis. TSN was confirmed to inhibit migration, invasion, and TGF-β1-induced EMT via miR-200a-mediated downregulation of the β-catenin pathway in SGC-7901 human gastric carcinoma cells (Wang et al., 2017). Furthermore, TGF-β1-induced EMT and morphological change in pancreatic cancer cells can be reversed through upregulating E-cadherin expression and decreasing vimentin, ZEB1, and Snail levels. The corresponding mechanism was involved with blocking the Akt/mTOR signaling pathway (Pei et al., 2017).
Synergistic Effect of Toosendanin in Chemotherapy
Since the majority of chemotherapy drugs have serious side effects, it is still urgent to take adjuvant therapy to reduce the dosage of chemotherapy drugs and improve their anti-tumor activity. As a representative chemotherapy drug, cisplatin is used to treat non-small cell lung cancer (NSCLC), but due to the development of resistance, the benefit has been limited. It was found that TSN can enhance cisplatin sensitization against non-small cell lung cancer cells through targeting Anxa4 in NSCLC cells, decreasing the combination of Anxa4 with ATP7A, and decreasing the extracellular efflux of platinum. The results showed that TSN could be a potential candidate in treating drug-resistant NSCLC cells (Zheng et al., 2018). Adriamycin (ADM) is an anti-cancer drug with a high frequency of use in the treatment of breast cancer. Nevertheless, some kinds of breast cancer or breast cancers under repeated ADM exposure develop a strong resistance to ADM, which limits its clinical efficacy. Wang et al. found that TSN can attenuate the resistance of human breast cancer cells to ADM by inhibiting the expression of ABCB1 and PI3K/Akt signaling (Wang et al., 2018). Doxorubicin, another anti-cancer antibiotic, possesses a strong anti-tumor effect. However, the toxicity of high-dose doxorubicin is severe, especially to the heart. In MDA-MB-435 breast cancer cells, cotreatment of doxorubicin with TSN can significantly enhance the anti-breast cancer activity, and the mechanism may depend on the FoxO1-Bim/Noxa pathway.
Selective Inhibition Effect of Toosendanin on Tumor Cells
The specific inhibitory effect on tumors is crucial for the development of new anti-tumor drugs. In one study, Li et al. showed that TSN selectively increased the sensitivity of NSCLC cells, but not normal cells (293T, BEAS-2B, HBE, L02, and PBMC), to TRAIL-induced apoptosis (Li et al., 2017). For the toxicity of hepatocellular carcinoma, after treating with toosendanin (0.5 µM) for 72 h, the inhibition rate for SMMC-7721 and Hep3B cells was 56 and 42%, respectively, and for IAR-20 and L02 cells (normal liver cell lines), it was 21 and 20%, respectively (He et al., 2010). When treated with a lower dosage range (0.25–1 µM), toosendanin could selectively kill human gastric carcinoma cells (MGC-803, BGC-823, HGC-27, AGS, SGC-7901, and MKN-45) rather than normal human gastric epithelial cells (GES-1) (Wang et al., 2017). The selective inhibition effect of toosendanin against tumor cells was cross-validated in different research groups. According to the results of Yan and Zhang, the IC50 of toosendanin on normal primary hepatocyte cells was 30.65 and 14.94 μM, respectively, which was higher than its suppressing effect on hepatocellular carcinoma (0.5–0.9 μM) as reported by other teams (Zhang et al., 2008; He et al., 2010; Liu et al., 2016; Yan et al., 2019).
Anti-tumor Effects of Other Limonoids
Inspired by the remarkable bioactivity, numerous works have been conducted to isolate and identify the potential lead compounds from genus Melia plants for cancer treatment. Three limonoids, including trichilinin B, 3-deacetyl-4′-demethyl-28-oxosalannin, and 23-hydroxyohchininolide, were cytotoxic to gastric cancer cell line of AZ521 with IC50 values of 58.2, 3.2, and 78.5 µM, respectively (Pan et al., 2014b). In another study, 31 limonoids were isolated and identified from the fruits of M. azedarach; among them, several compounds including meliarachin C, 12-dehydroneoazedarachin D, salannin, 1-O-decinnamoyl-1-O-benzoylohchinin, ohchininolide, 1-O-decinnamoyl-1-O-benzoylohchininolide, 23-hydroxyohchininolide, 1-O-decinnamoyl-1-O-benzoyl-23-hydroxyohchininolide,1-O-decinnamoyl-1-O-benzoyl-28-oxoohchinin, mesendanin E, 1-benzoyl-1-detigloylohchinolal, and nimbolinin D possessed anti-tumor effect against AZ521 cells, and 12-dehydroneoazedarachin D showed the most potent cytotoxicity with an IC50 value of 11.8 µM (Akihisa et al., 2013). In addition, trichilinin E exhibited apparent cytotoxicity against the gastric cancer cell line SGC-7901 (Zhou et al., 2016).
For lung cancer treatment, 12-hydroxyamoorastatone, 12-hydroxyamoorastatin, 12-acetoxyamoorastatin, meliarachin C, 1-O-decinnamoyl-1-O-benzoylohchinin, 1-O-decinnamoyl-1-O-benzoyl-23-hydroxyohchininolide, and 1-benzoyl-1-detigloylohchinolal were reported to be toxic against A549 cell, and 1,12-diacetyltrichilin B showed significant cytotoxicity with an IC50 value of 0.93 µM (Tada et al., 1999; Zhao et al., 2012; Akihisa et al., 2013). The isolated limonoids can also inhibit colorectal cancers. Among them, 12-hydroxyamoorastatone, 12-hydroxyamoorastatin, and 12-acetoxyamoorastatin displayed obvious antitumor activity against HCT-15 cell. Notably, 12-acetoxyamoorastatin possessed stronger cytotoxicity than antimycin A (positive drug), which indicated that it could be a candidate in curing colorectal cancer (Ahn et al., 1994). The anti-tumor effect was also found in SW480 colorectal cancer cells by 1,12-diacetyltrichilin B (Yuan et al., 2013). It was reported that lots of limonoids have a cytotoxic effect on leukemia HL-60 cells. Among them, 3-deacetyl-4′-demethyl-28-oxosalannin, 1,12-diacetyltrichilin B, meliarachin C, 1-O-cinnamoyltrichilinin, 23-methoxyohchininolide A, 1-benzoyl-1-detigloylohchinolal, 1-cinnamoyl-1-detigloylohchinolal, and nimbolinin D were considered as the most potential compounds with an IC50 value lower than 10 µM (Zhao et al., 2012; Akihisa et al., 2013; Pan et al., 2014b). Simultaneously, 12 limonoids from the fruits of M. azedarach also possessed marked anti-tumor activity against breast cancer MCF-7 cell with IC50 values from 0.06 to 94.8 µM (Akihisa et al., 2013). Toosendansin H, meliatoxin B1, and 1,12-diacetyltrichilin B, which were isolated from the fruits and stem bark of M. toosendan, were toxic to MCF-7 cell (Zhao et al., 2012; Li et al., 2020b). Moreover, trichilinin B and 21-hydroxyisoohchininolide can inhibit the growth of SK-BR-3 cell, which is another type of breast cancer cell (Pan et al., 2014b).
MTT assay was applied to evaluate the cytotoxicity of nine limonoids against lymphoma P388 cell. The outcomes revealed that all the nine limonoids possessed apparent toxicity, of which 12-deacetyltrichilin I, 3-deacetyltrichilin H, and trichilin D exhibited significant bioactivity with IC50 values of 0.011, 0.045, and 0.055 μg/ml, respectively (Takeya et al., 1996b). In the treatment of oral epithelial carcinoma, trichilin H (IC50 = 0.11 μg/ml) and 12-O-methylvolkensin (IC50 = 8.72 μg/ml) were observed to be toxic to kB cells (Tada et al., 1999). Zhou et al. isolated three new C-seco limonoids and one new tetracyclic limonoid from the fruits of M. azedarach, and their cytotoxicity against cervical cancer HeLa S3 cells was assessed. The results showed that 15-O-deacetylnimbolidin B and 12-O-deacetyltrichilin H had a strong inhibitory effect, while 15-O-deacetyl-15-O-methylnimbolidin A and 15-O-deacetyl-15-O-methylnimbolidin B had weak cytotoxicity (Zhou et al., 2005). Furthermore, using bioassay-guided chemical investigation, 12-hydroxyamoorastatone, 12-hydroxyamoorastatin, and 12-acetoxyamoorastatin were isolated, and the anti-tumor effects were evaluated. Three compounds showed potent cytotoxicity against human malignant melanoma SK-MEL-2 and human CNS carcinoma XF498 (Ahn et al., 1994). Several limonoids also possessed noticeable hepatotoxicity. Among them, 12-ethoxynimbolinins G and 1,12-diacetyltrichilin B were toxic to SMMC-7721 cells, with IC50 values of 27.6 and 0.36 µM, respectively, and trichilinin E had cytotoxicity against HepG2 cell (Yuan et al., 2013; Zhou et al., 2016; Zhang et al., 2018).
Structure–Activity Relationships With Anti-tumor
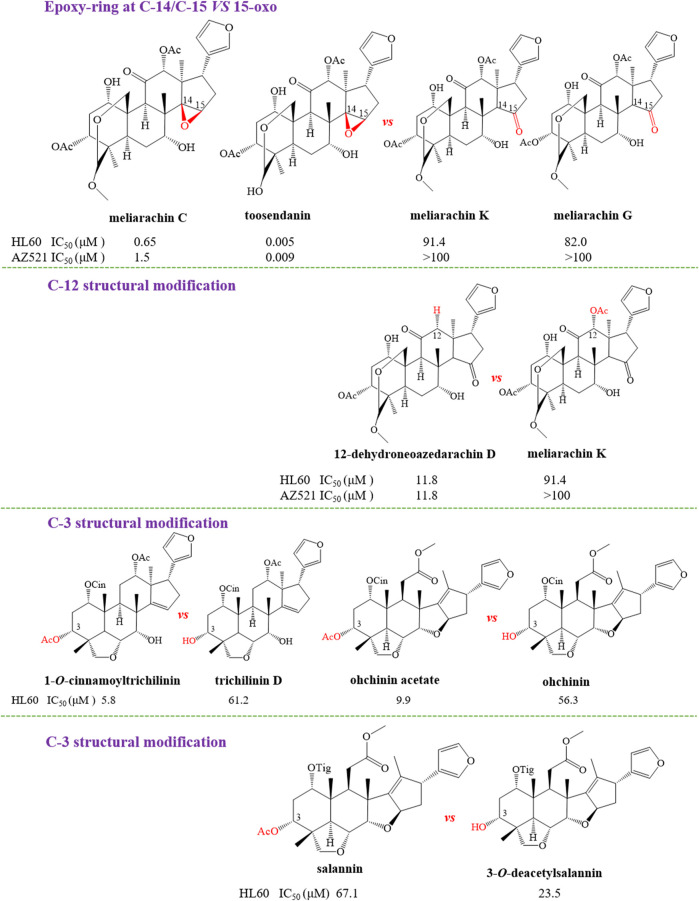
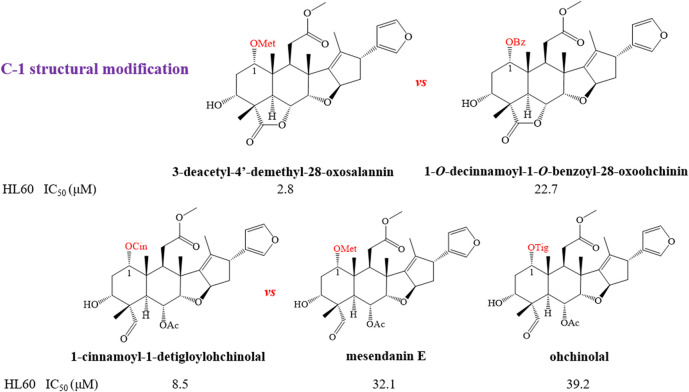
In order to further explain the effect of different skeletons and substituents on the anti-tumor activities of limonoids, the corresponding structure–activity relationships (SAR) were systematically summarized. For trichilin-type limonoids, it was found that meliarachin C and toosendanin with a β-oriented epoxy ring at C-14/C-15 possessed a significant bioactivity than those of their 15-oxo analogues (meliarachin K and meliarachin G) against HL60 and AZ521 cells. Meanwhile, the inhibitory effect of 15-oxo trichilins against HL60 and AZ521 cells could be enhanced by deacetoxylation at C-12 (12-dehydroneoazedarachin D vs. meliarachin K). For vilasanin-type limonoids, acetylation of the hydroxy group at C-3 was able to strengthen the anti-cancer effect against HL60 cells—for instance, 1-O-cinnamoyltrichilinin (IC50 = 5.8 μM) vs. trichilinin D (IC50 = 61.2 μM). This structural modification also enhanced the activity against HL60 cells in the salannin-type limonoids with a cinnamoyl group at C-1 (ohchinin acetate vs. ohchinin). By contrast, acetylation at C-3 could reduce the bioactivity of salannin-type limonoids, which bore a tigloyl group at C-1 (salannin vs. 3-O-deacetylsalannin). In addition, the substitution of a methacrylic group at C-1 with a benzoyl group reduced the activity against HL60 and AZ521 cells (3-deacetyl-4′-demethyl-28-oxosalannin vs. 1-O-decinnamoyl-1-O-benzoyl-28-oxoohchinin). The characteristic SAR was also found in ohchinolal-type limonoids. The study showed that the inhibitory properties against HL60 cells would be reduced when the cinnamoyl group at C-1 was substituted by a methacrylic or a tigloyl group (1-cinnamoyl-1-detigloylohchinolal vs. mesendanin E and ohchinolal) (Akihisa et al., 2013). Surprisingly, the demethacrylation of mesendanin E and the detigloylation of ohchinolal at C-1 were able to enhance the anti-cancer ability against HL60, A549, and AZ521 cells. This finding indicated that substitution of methacrylic or tigloyl group at C-1 was probably related with anti-cancer property (Pan et al., 2014a) (Figures 10).
FIGURE 10.
The anti-tumor structure–activity relationship of limonoids.
FIGURE 10.
(continue) The anti-tumor structure—activity relationship of limonoids.
Antifeeding and Insecticide Effects
Antifeeding and Insecticide Effects of Toosendanin
M. toosendan and M. azedarach, two common agricultural insecticidal and Ascaris-repellent Chinese herbs, have been recorded in ancient China, and TSN has been considered as the main active compound for insecticidal effect. The content of TSN in M. toosendan is high, and it is environmentally friendly due to its botanical property (Shi and Li, 2007). Recently, TSN showed distinguished potential in the field of agricultural anthelmintic. It was reported that TSN possessed remarkable repellent and insecticidal effects on Pieris rapae, and the underlying mechanism has been explored comprehensively. TSN also has obvious antifeedant activity. TSN at different dosages can affect the feeding reaction and feeding speed of P. rapae, which exhibited different poisoning symptoms. A high dose of TSN can induce the paralysis and coma of P. rapae and cause pathological changes of the midgut. Meanwhile, a low dose of TSN can transform the larvae into abnormal insects. One study was designed to evaluate the effects of TSN on the activities of several important enzymes in P. rapae. The results showed that TSN was able to reduce the activities of microsomal multifunctional oxidase, protease, and intestinal acetylase, which indicated that the interference of TSN on the detoxification enzymes of P. rapae may be responsible for the toxic reaction (Zhang and Zhao, 1992a). In addition, TSN was capable of causing severe damage to midgut tissue, resulting in abscission and autolysis (Zhang and Zhao, 1991). Furthermore, the physiological metabolism of P. rapae was disturbed by TSN, and abnormal biological oxidation was induced in vivo, which demonstrated that TSN could inhibit the central nervous system of the larva (Zhang et al., 1992b).
Apart from P. rapae, TSN possessed potent growth inhibition on other species of insects—for instance, TSN possessed strong stomach toxicity against Mythimna separate (LC50 = 252.23 μg/ml), and this process was located and targeted on the microvilli of the midgut cells. Subsequently, TSN induced the destruction of midgut epithelial cells, which caused the regurgitation, paralysis, and death of M. separate (Li H. et al., 2020). Another research reported that TSN could disrupt yolk deposition in oocytes, blood ingestion and digestion, and ovary ecdysteroid production in Aedes aegypti (Ma et al., 2013). Huang et al. found that the activities of pepsase and tryptase in Brachionus plicatilis can be suppressed by TSN, which may be responsible for the feeding deterrent property (Huang et al., 2017). In order to solve the problems of biological pollution in the cultivation scale of microalgae, TSN was selected to conduct an insecticidal test. The outcomes revealed that TSN possessed obvious toxicity to Stylonychia mytilus (a type of ciliate), while it is relatively safe to Chlorella pyrenoidosa (a type of microalgae) (Xu et al., 2019). Moreover, TSN was reported to inhibit the growth of Sirophilus oryzae and Cryptolestes ferrugineus, two common store-product insects (Xie et al., 1995). In addition to the control of agricultural pests, TSN has become a clinical anti-Ascaris drug in China since the 1950s (Shi and Li, 2007).
Antifeeding and Insecticide Effects of Other Limonoids
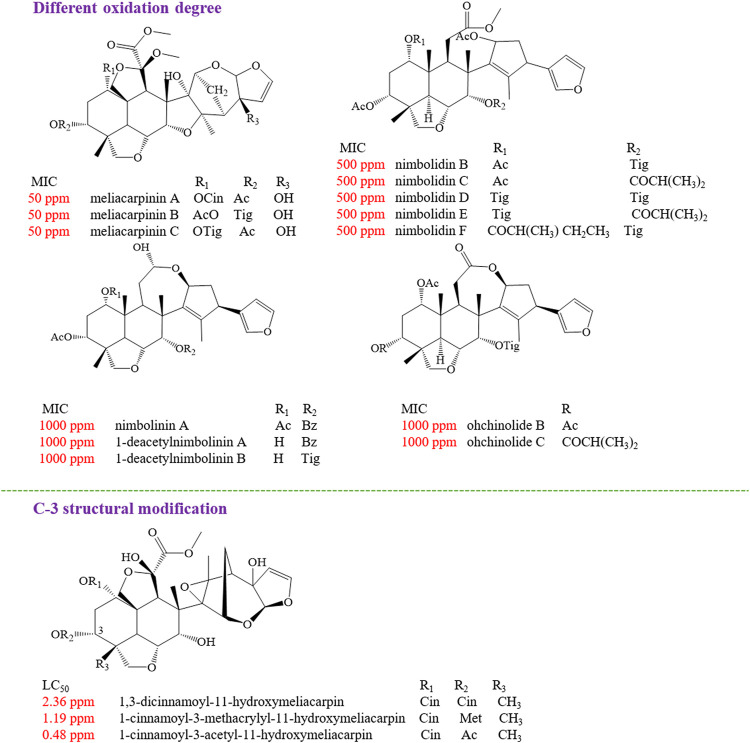
The insect antifeedant activities of other limonoids from genus Melia plants have been extensively evaluated, and the structure–activity relationships were also discussed. By using the conventional leaf disk method, the antifeedant activity against Spodoptera eridania was tested for ohchinolide C, salannin, nimbolidin B, nimbolidin C, nimbolidin D, nimbolidin E, and nimbolidin F. The outcomes showed that these compounds, at a concentration of 500 ppm, can significantly affect feeding (Nakatani et al., 1996; Zhou et al., 1997), while trichilinin B, trichilinin C, ohchinolide B, ohchinolide C, and 3-O-acetylohchinolal exhibited a weak antifeedant activity against S. eridania at a concentration of 1,000 ppm (Nakatani, 1999). At the concentration of 400 ppm, 12-O-acetylazedarachin A, 12-O-acetylazedarachin B, trichilin H, trichilin I, trichilin J, trichilin K, and trichilin L have an inhibitory effect on the feeding amounts of leaf compared to the controls (Nakatani, 1999). It was found that 12-hydroxyamoorastatone, azedarachin A, trichilin B, and 12-hydroxyamoorastatin displayed a potential antifeedant activity with MIC values of 250, 200, 200, and 150 ppm, respectively (Zhou et al., 1996; Nakatani, 1999). At the same time, the most potent antifeedant limonoids were found to be meliacarpinin A, meliacarpinin C, and meliacarpinin D, with a MIC value of 50 ppm (Nakatani, 1999). In addition, some studies focused on discovering the effective antifeedants against Spodoptera littoralis, another kind of plant pest. As a result, nimbolinin A, nimbolinin B, nimbolinin C, nimbolinin D, trichilinin D, trichilinin E, 1-O-cinnamoyltrichilinin, 1-deacetylnimbolinin A, spirosendan, and 2-deacetylnimbolinin B exhibited a weak activity at 1,000 ppm (Nakatani et al., 2000). The MIC value of neoazedarachin A, neoazedarachin B, neoazedarachin D, 13-O-acetylazedarachin A, and 1-acetyltrichilin H against S. littoralis was 400 ppm, and the MIC value for isotoosendanin, TSN, and azedarachin B was 300, 200, and 200 ppm, respectively (Nakatani, 1999; Nakatani et al., 2000). Furthermore, 1-cinnamoyl-3-acetyl-11-hydroxymeliacarpin was found to possess an attractive insecticidal activity with a LC50 value of 0.48 ppm and a EC50 value of 0.27 ppm (Bohnenstengel et al., 1999). LC–MS guided isolation method was applied to screen promising antifeedant compounds against the fifth-instar larvae of P. rapae, and TSN, isotoosendanin, 12α-hydroxyamoorastatone, mesendanin H, meliatoosenin E, 3-O-acetylohchinolal, salannin, and ohchinal were found to inhibit the feeding ability of P. rapae. Among these compounds, mesendanin H was proven to be the most active limonoid, with an AFC50 value of 0.11 mM (Wang et al., 2020a). The antifeedant activity against Spodoptera exigua of azedarachin A, 12-O-acetylazedarachin A, 12-O-acetylazedarachin B, and 1-deoxy-3-tigloyl-11-methoxymeliacarpinin was proven, and the MIC values ranged from 200 to 400 ppm (Huang et al., 1994). For the purpose of finding novel naturally occurring insecticides from plants, Carpinella et al. evaluated the antifeedant and insecticide properties of 12α-hydroxyamoorastatone and azadirachtin against Epilachna paenulata by choice and no-choice assays. In a choice-assay study, 12α-hydroxyamoorastatone and azadirachtin inhibited feeding with ED50 values of 0.80 and 0.72 ug/cm2. These two compounds both possessed a remarkable insecticide activity, which was evidenced by low food intake, weight loss, and even significant mortality (LD50 values of 0.76 and 1.24 μg/cm2) (Carpinella et al., 2003).
Structure–Activity Relationships With Insecticidal Effect
The structure–activity relationships of limonoids have attracted extensive interest for their remarkable insecticidal activity. It was found that the highly oxidized C-seco limonoids, such as meliacarpinin-type limonoids (meliacarpinin A, meliacarpinin C, and meliacarpinin D), exhibited the most potent antifeedant activity than the less oxidized types of C-seco limonoids, such as nimbolinins (nimbolinin A and 1-deacetylnimbolinin A-B), ohchinolides (ohchinolide B-C), nimbolidins (nimbolidin B-F), and salannin. Apart from meliacarpinins, the intact trichilin-type limonoids with C-19/C-29 bridged acetals were considered as the higher active compounds, of which 12-hydroxyamoorastatin showed the most potent activity (Nakatani, 1999). In another study, the most active compound against S. eridania was azedarachin A with a 12-OH function (Zhou et al., 1996). The insecticidal activity evaluation of three meliacarpins (1,3-dicinnamoyl-11-hydroxymeliacarpin, 1-cinnamoyl-3-methacrylyl-11-hydroxymeliacarpin, and 1-cinnamoyl-3-acetyl-11-hydroxymeliacarpin) suggested that the property of the ester substituent at C-3 acted a critical role for the insecticidal effect. The results showed that 1-cinnamoyl-3-acetyl-11-hydroxymeliacarpin that bore a small and relatively hydrophilic acetyl group at C-3 possessed a high activity, while 1,3-dicinnamoyl-11-hydroxymeliacarpin with a bulky and more lipophilic cinnamoyl group exhibited the lowest activity (Bohnenstengel et al., 1999) (Figure 11).
FIGURE 11.
The insecticidal structure–activity relationship of limonoids
Anti-botulinum Effect
Botulinum neurotoxin (BoNT), the strongest known biotoxin in the world, is a kind of toxin secreted by Clostridium botulinum, which includes seven distinct serotypes (identified as A to G). The nerve endings of a cholinergic neuromuscular junction are considered as its key targets. Botulinum neurotoxin can inhibit the release of acetylcholine through the enzymatic hydrolysis of SNARE protein, which plays a crucial role in neurotransmitter release. Then, the signal transmission of neuromuscular connection was further blocked to paralyze the muscle, which eventually led to the death of animals due to respiratory failure (Rossetto et al., 2014). The four-step action hypothesis, the proposed mechanism of botulinum toxin on target cells, demonstrated that botulinum toxin binds to receptors on the cell surface firstly and enters the nerve cell through receptor-mediated internalization. Subsequently, acidification of endocytic vesicles caused the translocation of light chains from the vesicles to the cytoplasm, and the light chains cleave the SNARE protein in the cytosol, thereby completing the last step of the intracellular action of the toxin (Humeau et al., 2000). TSN has been proven to block neuromuscular connection in the phrenic nerve–diaphragm specimens of rats, which was similar to that of BoNT in many aspects—for instance, the dosage–response relationship, the higher temperature coefficient, and the irreversibility of the blocking effect existed in both substances when blocking neuromuscular connections. Nevertheless, there are some differences between TSN and BoNT—for instance, TSN can cause submicroscopic changes of presynaptic motor nerve endings, which refer to the reduced number of synaptic vesicles and the widened synaptic cleft (Xiong, 1985). It was noteworthy that TSN has a facilitation phase before the final inhibition of neurotransmitter release. It means that the quantum release of acetylcholine by TSN is a biphasic regulation of facilitation followed by inhibition (Shi et al., 1982).
Interestingly, TSN, which has similar bioactive properties with BoNT, unexpectedly possesses a prominent anti-botulism effect. Early in the 1980s, TSN was proven to have an obvious therapeutic effect on BoNT-poisoned animals. In the isolated phrenic nerve and diaphragm muscle specimens of mice, both the TSN and BoNT administered simultaneously or TSN administered beforehand showed a significant antagonistic effect on BoNT (Li and Sun, 1983). The anti-botulism effect of TSN exhibited the properties of fast onset and long action time in the isolated neuromuscular specimens and rat models. In addition, TSN has a therapeutic effect on various types of botulism, such as A, B, and E, in mice and rhesus monkeys, which showed broad-spectrum anti-botulism effects (Janda, 2008). Furthermore, in a clinical study, oral administration of TSN can relieve symptoms of botulism, and the patients will recover when treated with the combination of TSN and anti-botulinum serum (Zou et al., 1985). Undoubtedly, structure–activity relationships attracted enough attention in drug research and development. Whether the unusual AB-ring of TSN is related with its anti-botulinum properties was tested. A synthetic strategy allowing access to the AB-fragment of TSN was achieved from a trans-decalin system. However, none of these synthetic structures exhibited antagonism against BoNT/A in the rat spinal cord cellular assay, which demonstrated that complete ABCD nucleus was essential for the anti-BoNT effect of TSN (Nakai et al., 2010).
Recently, the mechanism study revealed that TSN was capable of endowing neuromuscular junctions with a high tolerance to BoNT. Preincubation with TSN for 5 min can enhance the tolerance to BoNT/A in the isolated phrenic nerve and diaphragm muscle specimens of mice (Li and Sun, 1983). In another study, rat phrenic nerve–diaphragm specimens that were preincubated with TSN showed apparent antagonism to botulism, which was manifested as significantly delayed at the time of neuromuscular transmission inhibition. It was found that the anti-BoNT effect of TSN was closely related with the facilitation of neurotransmitter release induced by TSN in a time-dependent manner. In one research, rats injected with TSN (7 mg/kg, i.h.) or blank solvent were sacrificed at different time points (from 15 min to several days). The results showed that the rat phrenic nerve–diaphragm specimens exhibited high BoNT tolerance after injection with TSN, and the frequency of miniature end-plate potentials was higher than that of the control (Shih and Hsu, 1983).
In the light of the four-step action hypothesis, cleaving of SNARE protein by the light chains was considered as the final process to activate a toxic effect, therefore inhibiting the cleaving procedure emerges as a potential therapeutical strategy. Inspired by this tactic, Zhou et al. explored the anti-BoNT mechanism of TSN by Western blotting. Surprisingly, TSN treatment did not affect the expression of synaptosomal-associated protein of 25 kDa (SNAP-25), syntaxin, and synaptobrevin/vesicle-associated membrane protein in rat cerebral synaptosomes. However, it can completely antagonize the cleavage of SNAP-25 induced by BoNT/A. When BoNT acts directly on synaptosomes, TSN can still partially antagonize the cleavage of SNAP-25, but it cannot inhibit the cleavage of SNAP-25 on the synaptosome membrane by light-chain BoNT/A. Thus, the underlying mechanism of TSN against BoNT/A is to block the approach of BoNT to its enzyme substrate (Zhou et al., 2003).
As we know, cleaving of SNARE protein by light chains is a critical event in botulism, and the heavy-chain-formed channel has been confirmed to be indispensable in the translocation of the light chain into the cytosol (Blaustein et al., 1987). To verify the effect of TSN on the channel-forming activity of BoNT/A, the inside-out single-channel recording patch-clamp technique was applied to record the BoNT/A-induced currents in the presence and absence of TSN. The results showed that TSN administration could delay the channel formation and reduce the size of the channels in the PC12 cell membrane, which indicated that TSN could inhibit BoNT/A by interfering with the translocation of BoNT via inhibiting its pore-forming activity (Li and Shi, 2006).
Anti-inflammatory Effect
Limonoids from genus Melia were considered as an emerging source for discovering potential anti-inflammatory lead compounds. In a representative research, the anti-inflammatory effect of TSN was found in dextran sulfate sodium-induced colitis model. After treatment with TSN, the secretion of proinflammatory cytokines and oxidative stress were blocked, and M1 macrophage polarization and the activation of NLR family pyrin domain containing 3 inflammasome were restrained, accompanied with upregulation of HO-1/Nrf2 expression (Fan et al., 2019). In addition, acetic acid-induced vascular permeability, λ-carrageenan-induced hind paw edema tests, and acetic acid-induced writhing and hot-plate tests were applied to evaluate the anti-inflammatory effect of limonoids. As a result, two limonoids, iso-toosendanin and 1-O-tigloyl-1-O-debenzoylohchinal, isolated from the fruit of M. toosendan were proven to have anti-inflammatory and analgesic effects (Xie et al., 2008). The NF-κB pathway plays a critical role in inflammation, and one report demonstrated that 1-O-tigloyl-1-O-deacetyl-nimbolinin B (TNB) possessed the most obvious anti-inflammatory effect than the other two limonoids, including nimbolinin A and nimbolinin B, and the underlying mechanism was through suppressing NF-κB and JNK activation in microglia cells. In this study, administration with TNB can reduce the production of nitric oxide (NO) and TNF-α in lipopolysaccharide (LPS)-stimulated microglia cells (Tao et al., 2014). In another research, NF-κB luciferase assay was applied to rapidly screen potential NF-κB regulators from the fruits of M. toosendan. As a result, seven limonoids were found to inhibit NF-κB activation at the concentration of 10 μM (Zhu et al., 2014).
In the LPS-induced mouse macrophage RAW 264.7 cell inflammatory model, five limonoids, including trichilinin B, 3-deacetyl-28-oxosalannolactone, ohchinin, 23-hydroxyohchininolide, and 21-hydroxyisoohchininolide, exhibited a significant anti-inflammatory activity by inhibiting the production of NO with IC50 values from 28.7 to 87.3 μM. Meanwhile, all these five compounds displayed no or almost no toxicity to the cells (Pan et al., 2014b). Toosendane B and toosendane C, another two limonoids from the bark of M. toosendan, can also obviously reduce NO production in RAW 264.7 cells induced by LPS (Hu et al., 2018). Four NO-inhibited limonoids, including ohchinin, salannin, nimbolinin D, and mesendanin E, were isolated from M. azedarach, of which salannin was found to inhibit the expression levels of iNOS and COX-2 proteins in a concentration-dependent manner (Akihisa et al., 2017). In addition, meliazedalides B, a trichilin-class limonoid from the fruits of M. azedarach, also showed a weak anti-inflammatory effect (Qiu et al., 2019). In order to screen anti-neuroinflammatory compounds from M. azedarach, a LPS-induced microglia BV-2 cell model was established. Seven compounds, including 1-O-benzoyl-3-O-deactylnim-bolinin C, 3-deacetyl-12-O-methylvolkensin, 12-O-methyl-1-O-deacetyl-nimbolinin, trichilinin B, 1-O-cinnamoyltrichilinin, 1-acetyltrichilinin, and 12α-hydroxyamoorastatone, were isolated and confirmed as potential inhibitors of NO production, with IC50 values from 7.73 to 26.75 μM (Park et al., 2020).
Antibacterial and Antifungal Effects
Recently, several limonoids were found to possess a potential antibacterial activity. In the year 2007, 10 limonoids were isolated from the fruits of M. toosendan, among which three compounds, including 12-ethoxynimbolinins C, 1-O-cinnamoyltrichilinin, and trichilinin B, were validated to have an antibacterial activity against oral pathogen Porphyromonas gingivalis, with MIC values of 15.6, 31.3, and 31.5 μg/ml, respectively. Nevertheless, they exhibited no obvious bioactivity against Streptococcus mutans. The results indicated that these three limonoids might be specific inhibitors of P. gingivalis (Zhang et al., 2007). In 2016, this research group found that 1α,7α-ditigloyloxy-3α-acetoxyl-12α-ethoxylnimbolinin and 1α-tigloyloxy-3α-acetoxyl-7α-hydroxyl-12β-ethoxylnimbolinin, the other two new limonoids, possessed antibacterial activity against P. gingivalis, with MIC values of 15.2 and 31.25 μg/ml, respectively (Zhang et al., 2016). Unlike previously reported limonoids from fruits or bark, three limonoids were isolated from the seeds of M. azedarach, and 7-cinnamoyltoosendanin had significant inhibitory effects against Micrococcus luteus and Bacillus subtilis, with MIC values of 6.25 and 25 μg/ml, respectively (Liu et al., 2011). By using a microdilution assay, Su et al. determined the antibacterial activity of meliarachin D and meliarachin H. The results showed that meliarachin D can inhibit both Staphylococcus aureus (MIC = 50 μg/ml) and B. subtilis (MIC = 50 μg/ml); in contrast, meliarachin H can only suppress the growth of B. subtilis, with a MIC value of 25 μg/ml (Su et al., 2011).
Other Pharmacological Effects
Apart from the pharmacological effects mentioned above, limonoids also possessed some other bioactivities. In 2018, TSN was reported to exhibit anti-obesity property. By using Oil Red O staining assay, TSN could attenuate lipid accumulation in preadipocytes 3T3-L1. In addition, treatment with TSN can also decreased the mRNA and protein levels of adipocytokines (adiponectin and leptin), CCAAT/enhancer-binding proteins α, peroxisome proliferator-activated receptor γ, fatty acid synthase, and acetyl-CoA carboxylase in adipocytes. Moreover, the weight of gonadal white fat and the serum triacylglycerol content in high-fat-diet-fed mice were reduced by administration with TSN. The underlying mechanism is through activating the Wnt/β-catenin pathway (Chen et al., 2018). One study showed that, through altering polymerase acidic protein nuclear localization, TSN could inhibit early influenza A virus infection (Jin YH. et al., 2019). Similarly, hepatitis C virus infection was also inhibited by TSN. It can enhance the effect of alpha interferon (α-IFN), a classical drug in the treatment of HCV infection, and upregulate the expression of α-IFN-related protein (Watanabe et al., 2011). The pharmacological effects are summarized in Table 2.
TABLE 2.
Pharmacological effects of limonoids.
| Compounds | Models | Treatment | In vitro/vivo | Effects | Reference | |
|---|---|---|---|---|---|---|
| Anti-tumor effect | — | |||||
| Gastric cancer | — | |||||
| Toosendanin | MKN-45 cells | 60–100 nM | In vitro | Inducing apoptosis | Shao et al. (2020) | |
| Toosendanin | AGS cells | 0.5–2 μM | In vitro | Inhibiting cell proliferation and inducing apoptosis | Zhou et al. (2018) | |
| Toosendanin | HGC-27 cells | 0.1–0.5 μM | In vitro | Inhibiting cell proliferation and inducing apoptosis | Zhou et al. (2018) | |
| Toosendanin | SGC-7901 cells | 0.25–4 µM | In vitro | Inhibiting proliferation, invasion, migration and inducing apoptosis | Wang et al. (2017) | |
| Toosendanin | MGC-803 cells | 30–70 nM | In vitro | Inhibiting cell proliferation | Liu et al. (2019) | |
| Toosendanin | HGC-27 cells | 0.6 µM | In vitro | Inhibiting cell proliferation and invasion | Liu et al. (2019) | |
| Trichilinin B | AZ521 cells | IC50 = 58.2 µM | In vitro | Cytotoxicity against AZ521 cells | Pan et al. (2014b) | |
| 3-Deacetyl-4′-demethyl-28-oxosalannin | AZ521 cells | IC50 = 3.2 µM | In vitro | Inducing apoptosis | Pan et al. (2014b) | |
| 23-Hydroxyohchininolide | AZ521 cells | IC50 = 78.5 µM | In vitro | Cytotoxicity against AZ521 cells | Pan et al. (2014b) | |
| Meliarachin C | AZ521 cells | IC50 = 1.5 µM | In vitro | Cytotoxicity against AZ521 cells | Akihisa et al. (2013) | |
| Toosendanin | AZ521 cells | IC50 = 0.009 µM | In vitro | Cytotoxicity against AZ521 cells | Akihisa et al. (2013) | |
| 12-Dehydroneoazedarachin D | AZ521 cells | IC50 = 11.8 µM | In vitro | Cytotoxicity against AZ521 cells | Akihisa et al. (2013) | |
| Salannin | AZ521 cells | IC50 = 55.4 µM | In vitro | Cytotoxicity against AZ521 cells | Akihisa et al. (2013) | |
| 1-O-Decinnamoyl-1-O-benzoylohchinin | AZ521 cells | IC50 = 82.3 µM | In vitro | Cytotoxicity against AZ521 cells | Akihisa et al. (2013) | |
| Ohchininolide | AZ521 cells | IC50 = 82.9 µM | In vitro | Cytotoxicity against AZ521 cells | Akihisa et al. (2013) | |
| 1-O-decinnamoyl-1-O-benzoylohchininolide | AZ521 cells | IC50 = 34.7 µM | In vitro | Cytotoxicity against AZ521 cells | Akihisa et al. (2013) | |
| 23-Hydroxyohchininolide | AZ521 cells | IC50 = 78.5 µM | In vitro | Cytotoxicity against AZ521 cells | Akihisa et al. (2013) | |
| 1-O-Decinnamoyl-1-O-benzoyl-23-hydroxyohchininolide | AZ521 cells | IC50 = 55.7 µM | In vitro | Cytotoxicity against AZ521 cells | Akihisa et al. (2013) | |
| 1-O-Decinnamoyl-1-O-benzoyl-28-oxoohchinin | AZ521 cells | IC50 = 61.7 µM | In vitro | Cytotoxicity against AZ521 cells | Akihisa et al. (2013) | |
| Mesendanin E | AZ521 cells | IC50 = 80 µM | In vitro | Cytotoxicity against AZ521 cells | Akihisa et al. (2013) | |
| 1-Benzoyl-1-detigloylohchinolal | AZ521 cells | IC50 = 81.6 µM | In vitro | Cytotoxicity against AZ521 cells | Akihisa et al. (2013) | |
| Nimbolinin D | AZ521 cells | IC50 = 96.8 µM | In vitro | Cytotoxicity against AZ521 cells | Akihisa et al. (2013) | |
| Trichilinin E | SGC-7901 cells | IC50 = 6.9 µM | In vitro | Cytotoxicity against SGC-7901 cells | Zhou et al. (2016) | |
| Lung cancer | ||||||
| Toosendanin | A549 cells, tumor xenograft model | 50–400 nM | In vitro and in vivo | Enhancing apoptosis and inducing autophagy | Li et al. (2017) | |
| Toosendanin | A549 and A549/DDP cells | 50 μg/ml | In vitro | Inducing cell growth inhibition and enhancing CDDP sensitization | Zheng et al. (2018) | |
| Toosendanin | A549 and H1975 cells | 2–10 nM | In vitro | Inhibiting TGF-β1-induced EMT, adhesion, invasion, and migration | Luo et al. (2018) | |
| Toosendanin | A549 cells | 20–60 μM | In vitro | Inducing apoptosis | ||
| 12-Hydroxyamoorastatone | A549 cells | ED50 = 0.92 μg/ml | In vitro | Cytotoxicity against A549 cells | Ahn et al. (1994) | |
| 12-Hydroxyamoorastatin | A549 cells | ED50 = 0.04 μg/ml | In vitro | Cytotoxicity against A549 cells | Ahn et al. (1994) | |
| 12-Acetoxyamoorastatin | A549 cells | ED50 = 0.01 μg/ml | In vitro | Cytotoxicity against A549 cells | Ahn et al. (1994) | |
| 1,12-Diacetyltrichilin B | A549 cells | IC50 = 0.93 µM | In vitro | Cytotoxicity against A549 cells | Yuan et al. (2013) | |
| Meliarachin C | A549 cells | IC50 = 63.6 µM | In vitro | Cytotoxicity against A549 cells | Akihisa et al. (2013) | |
| 1-O-Decinnamoyl-1-O-benzoylohchinin | A549 cells | IC50 = 82.3 µM | In vitro | Cytotoxicity against A549 cells | Akihisa et al. (2013) | |
| 1-O-Decinnamoyl-1-O-benzoyl-23-hydroxyohchininolide | A549 cells | IC50 = 90.1 µM | In vitro | Cytotoxicity against A549 cells | Akihisa et al. (2013) | |
| 1-Benzoyl-1-detigloylohchinolal | A549 cells | IC50 = 83.5 µM | In vitro | Cytotoxicity against A549 cells | Akihisa et al. (2013) | |
| Colorectal cancer | ||||||
| Toosendanin | CRC SW480 cells | 0.5 µM | In vitro | Inducing apoptosis | Wang et al. (2015) | |
| Toosendanin | HEK293T, SW620, HCT-15/5FU-R cells and BALB/c nude mice | 1–2 μM; 0.69 mg/kg for 22 days/i.p | In vitro and in vivo | Inhibiting cell proliferation and inducing apoptosis | Wang et al. (2020b) | |
| 12-Hydroxyamoorastatone | HCT-15 cells | ED50 = 1.6 μg/ml | In vitro | Cytotoxicity against HCT-15 cells | Ahn et al. (1994) | |
| 12-Hydroxyamoorastatin | HCT-15 cells | ED50 = 0.08 μg/ml | In vitro | Cytotoxicity against HCT-15 cells | Ahn et al. (1994) | |
| 12-Acetoxyamoorastatin | HCT-15 cells | ED50 = 0.04 μg/ml | In vitro | Cytotoxicity against HCT-15 cells | Ahn et al. (1994) | |
| 1,12-Diacetyltrichilin B | SW480 cells | IC50 = 0.04 µM | In vitro | Cytotoxicity against SW480 cells | Yuan et al. (2013) | |
| Glioblastoma | ||||||
| Toosendanin | GBM U87 and C6 cell lines | 10 nM | In vitro | Inhibiting cell proliferation and inducing apoptosis | Cao et al. (2016) | |
| Toosendanin | U-138MG | 50–150 nM | In vitro | Inducing apoptosis | Wang et al. (2020c) | |
| Leukemia | ||||||
| Toosendanin | HL-60 cells | 12.5–100 nM | In vitro | Inducing apoptosis | Ju et al. (2012) | |
| Toosendanin | K562 cells | 30, 50 nM | In vitro | Inhibiting cell proliferation and inducing apoptosis | ||
| Toosendanin | HL-60 cells | 20–80 ng/ml | In vitro | Inhibiting proliferation and cell cycle, inducing apoptosis | Ju et al. (2013) | |
| Trichilinin B | HL-60 cells | IC50 = 22.2 µM | In vitro | Cytotoxicity against HL-60 cells | Pan et al. (2014b) | |
| 3-Deacetyl-28-oxosalannin | HL-60 cells | IC50 = 39.1 µM | In vitro | Cytotoxicity against HL-60 cells | Pan et al. (2014b) | |
| 3-Deacetyl-4′-demethyl-28-oxosalannin | HL-60 cells | IC50 = 2.8 µM | In vitro | Cytotoxicity against HL-60 cells | Pan et al. (2014b) | |
| Ohchinin | HL-60 cells | IC50 = 56.3 µM | In vitro | Cytotoxicity against HL-60 cells | Pan et al. (2014b) | |
| 23-Hydroxyohchininolide | HL-60 cells | IC50 = 25.1 µM | In vitro | Cytotoxicity against HL-60 cells | Pan et al. (2014b) | |
| 21-Hydroxyisoohchininolide | HL-60 cells | IC50 = 22.7 µM | In vitro | Cytotoxicity against HL-60 cells | Pan et al. (2014b) | |
| 1,12-Diacetyltrichilin B | HL-60 cells | IC50 = 0.55 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| Meliarachin C | HL-60 cells | IC50 = 0.65 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| Toosendanin | HL-60 cells | IC50 = 0.005 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| Meliarachin K | HL-60 cells | IC50 = 91.4 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| Meliarachin G | HL-60 cells | IC50 = 82 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| 12-Dehydroneoazedarachin D | HL-60 cells | IC50 = 11.8 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| Trichilinin D | HL-60 cells | IC50 = 61.2 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| 1-O-Cinnamoyltrichilinin | HL-60 cells | IC50 = 5.8 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| Salannin | HL-60 cells | IC50 = 67.1 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| Ohchinin | HL-60 cells | IC50 = 56.3 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| Ohchinin–acetate | HL-60 cells | IC50 = 9.9 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| 1-O-Decinnamoyl-1-O-benzoylohchinin | HL-60 cells | IC50 = 54.8 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| Ohchininolide | HL-60 cells | IC50 = 31.7 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| 1-O-Decinnamoyl-1-O-benzoylohchininolide | HL-60 cells | IC50 = 14.1 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| 23-Methoxyohchininolide A | HL-60 cells | IC50 = 4.9 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| 23-Hydroxyohchininolide | HL-60 cells | IC50 = 25.1 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| 1-O-Decinnamoyl-1-O-benzoyl-23-hydroxyohchininolide | HL-60 cells | IC50 = 12.6 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| 21-Hydroxyisoohchininolide | HL-60 cells | IC50 = 22.7 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| 17-Defurano-17-oxoohchinin | HL-60 cells | IC50 = 50.4 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| 1-O-Decinnamoyl-1-O-benzoyl-28-oxoohchinin | HL-60 cells | IC50 = 22.7 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| Ohchinolal | HL-60 cells | IC50 = 39.2 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| Mesendanin E | HL-60 cells | IC50 = 32.1 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| 1-Benzoyl-1-detigloylohchinolal | HL-60 cells | IC50 = 5.1 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| 1-Cinnamoyl-1-detigloylohchinolal | HL-60 cells | IC50 = 8.5 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| Nimbolinin D | HL-60 cells | IC50 = 5.4 µM | In vitro | Cytotoxicity against HL-60 cells | Yuan et al. (2013) | |
| Ovarian cancer | ||||||
| Toosendanin | CAVO-3, SKOV-3 cells | 500 nM | In vitro | Inhibiting invasion and migration | Li et al. (2018a) | |
| Toosendanin | CAVO-3, ES-2 cells | 500 nM | In vitro | Inducing apoptosis | Li et al. (2019) | |
| Toosendanin | CAVO-3, A2870 cells | 500 nM | In vitro | Inducing apoptosis | Li et al. (2018b) | |
| 12-Hydroxyamoorastatone | SKOV-3 cells | ED50 = 13.7 μg/ml | In vitro | Cytotoxicity against SKOV-3cells | Ahn et al. (1994) | |
| 12-Hydroxyamoorastatin | SKOV-3 cells | ED50 = 0.25 μg/ml | In vitro | Cytotoxicity against SKOV-3cells | Ahn et al. (1994) | |
| 12-Acetoxyamoorastatin | SKOV-3 cells | ED50 = 0.02 μg/ml | In vitro | Cytotoxicity against SKOV-3cells | Ahn et al. (1994) | |
| Hepatocellular carcinoma | ||||||
| Toosendanin | SMMC-7721 and Hep3B cells; BALB/c mice | IC50 = 0.5 µM; IC50 = 0.9 µM, 0.69 mg/kg/day for 15 days/i.p | In vitro and in vivo | Inducing apoptosis | He et al. (2010) | |
| Toosendanin | SMMC-7721, Hep3B cells | 0.5 μM | In vitro | Inhibiting cell proliferation and inducing apoptosis | Liu et al. (2016) | |
| 12-Ethoxynimbolinins G | SMMC-7721 cells | IC50 = 27.6 µM | In vitro | Cytotoxicity against SMMC-7721 cells | Zhang et al. (2018) | |
| 1,12-Diacetyltrichilin B | SMMC-7721 cells | IC50 = 0.36 µM | In vitro | Cytotoxicity against SMMC-7721 cells | Yuan et al. (2013) | |
| Trichilinin E | HepG2 cells | IC50 = 6.9 µM | In vitro | Cytotoxicity against HepG2 cells | Zhou et al. (2016) | |
| Breast cancer | ||||||
| Toosendanin | MCF-7/ADM cells | 50 nM; 0.69 mg/kg/every 2 days for 15 days/i.p | In vitro and in vivo | Inducing apoptosis | Wang et al. (2018) | |
| Trichilinin B | SK-BR-3 cells | IC50 = 45.1 µM | In vitro | Cytotoxicity against SK-BR-3 cells | Pan et al. (2014b) | |
| 21-Hydroxyisoohchininolide | SK-BR-3 cells | IC50 = 91.5 µM | In vitro | Cytotoxicity against SK-BR-3 cells | Pan et al. (2014b) | |
| Toosendansin H | MCF-7 cells | 6.25 µM | In vitro | Cytotoxicity against MCF-7 cells | Li et al. (2020b) | |
| Meliatoxin B1 | MCF-7 cells | 6.25 µM | In vitro | Cytotoxicity against MCF-7 cells | Li et al. (2020b) | |
| 1,12-Diacetyltrichilin B | MCF-7 cells | IC50 = 0.06 µM | In vitro | Cytotoxicity against MCF-7 cells | Yuan et al. (2013) | |
| Meliarachin C | MCF-7 cells | IC50 = 90.4 µM | In vitro | Cytotoxicity against MCF-7 cells | Akihisa et al. (2013) | |
| Trichilinin D | MCF-7 cells | IC50 = 41.5 µM | In vitro | Cytotoxicity against MCF-7 cells | Akihisa et al. (2013) | |
| Salannin | MCF-7 cells | IC50 = 88.1 µM | In vitro | Cytotoxicity against MCF-7 cells | Akihisa et al. (2013) | |
| 1-O-Decinnamoyl-1-O-benzoylohchinin | MCF-7 cells | IC50 = 14.9 µM | In vitro | Cytotoxicity against MCF-7 cells | Akihisa et al. (2013) | |
| 1-O-Decinnamoyl-1-O-benzoylohchininolide | MCF-7 cells | IC50 = 54.5 µM | In vitro | Cytotoxicity against MCF-7 cells | Akihisa et al. (2013) | |
| 1-O-Decinnamoyl-1-O-benzoyl-23-hydroxyohchininolide | MCF-7 cells | IC50 = 4.3 µM | In vitro | Cytotoxicity against MCF-7 cells | Akihisa et al. (2013) | |
| 21-Hydroxyisoohchininolide | MCF-7 cells | IC50 = 91.5 µM | In vitro | Cytotoxicity against MCF-7 cells | Akihisa et al. (2013) | |
| Ohchinolal | MCF-7 cells | IC50 = 94.4 µM | In vitro | Cytotoxicity against MCF-7 cells | Akihisa et al. (2013) | |
| Mesendanin E | MCF-7 cells | IC50 = 70.5 µM | In vitro | Cytotoxicity against MCF-7 cells | Akihisa et al. (2013) | |
| 1-Benzoyl-1-detigloylohchinolal | MCF-7 cells | IC50 = 85.0 µM | In vitro | Cytotoxicity against MCF-7 cells | Akihisa et al. (2013) | |
| 1-Cinnamoyl-1-detigloylohchinolal | MCF-7 cells | IC50 = 94.8 µM | In vitro | Cytotoxicity against MCF-7 cells | Akihisa et al. (2013) | |
| Nimbolinin D | MCF-7 cells | IC50 = 60.8 µM | In vitro | Cytotoxicity against MCF-7 cells | Akihisa et al. (2013) | |
| Ewing’s sarcoma | ||||||
| Toosendanin | SK-ES-1 cells | 25, 50 µM | In vitro | Inducing apoptosis | Gao et al. (2019) | |
| Neuroma | ||||||
| Toosendanin | PC12 cells | 0.87 µM | In vitro | Inducing apoptosis | Tang et al. (2004) | |
| Lymphoma | ||||||
| Toosendanin | U937 cells | 0.87 µM | In vitro | Suppressing the cell cycle progression and inducing apoptosis | Zhang et al. (2005a) | |
| 12-Deacetyltrichilin I | P388 cells | IC50 = 0.011 μg/ml | In vitro | Cytotoxicity against P388 cells | Takeya et al. (1996b) | |
| 1-Acetyltrichilin H | P388 cells | IC50 = 0.47 µg/ml | In vitro | Cytotoxicity against P388 cells | Takeya et al. (1996b) | |
| 3-Deacetyltrichilin H | P388 cells | IC50 = 0.045 µg/ml | In vitro | Cytotoxicity against P388 cells | Takeya et al. (1996b) | |
| 1-Acetyl-3-deacetyltrichilin H | P388 cells | IC50 = 0.4 µg/ml | In vitro | Cytotoxicity against P388 cells | Takeya et al. (1996b) | |
| 1-Acetyl-2-deacetyltrichilin H | P388 cells | IC50 = 0.66 µg/ml | In vitro | Cytotoxicity against P388 cells | Takeya et al. (1996b) | |
| Meliatoxin B1 | P388 cells | IC50 = 5.4 µg/ml | In vitro | Cytotoxicity against P388 cells | Takeya et al. (1996b) | |
| Trichilin H | P388 cells | IC50 = 0.16 µg/ml | In vitro | Cytotoxicity against P388 cells | Takeya et al. (1996b) | |
| Trichilin D | P388 cells | IC50 = 0.055 µg/ml | In vitro | Cytotoxicity against P388 cells | Takeya et al. (1996b) | |
| 1,12-Diacetyltrichilin B | P388 cells | IC50 = 0.46 µg/ml | In vitro | Cytotoxicity against P388 cells | Takeya et al. (1996b) | |
| Pancreatic cancer | ||||||
| Toosendanin | PANC-1, AsPC-1 cells; BALB/c mice | 200 nM; 0.2 mg/kg/day for 28 days/i.p | In vitro and in vivo | Inhibiting tumorous growth, inhibit migration and invasion, reversing EMT | Pei et al. (2017) | |
| Oral epithelial carcinoma | ||||||
| Trichilin H | KB cells | IC50 = 0.11 μg/ml | In vitro | Cytotoxicity against P388 cells | Tada et al. (1999) | |
| Toosendanin | KB cells | IC50 = 3.82 μg/ml | In vitro | Cytotoxicity against P388 cells | Tada et al. (1999) | |
| 12-O-Methylvolkensin | KB cells | IC50 = 8.72 μg/ml | In vitro | Cytotoxicity against P388 cells | Tada et al. (1999) | |
| Cervical carcinoma | ||||||
| 15-O-Deacetylnimbolidin B | HeLa S3 cells | IC50 = 0.1 µM | In vitro | Cytotoxicity against Hela S3 cells | Zhou et al. (2005) | |
| 12-O-Deacetyltrichilin H | HeLa S3 cells | IC50 = 0.48 µM | In vitro | Cytotoxicity against Hela S3 cells | Zhou et al. (2005) | |
| 15-O-Deacetyl-15-O-methylnimbolidin A | HeLa S3 cells | IC50 = 37.4 µM | In vitro | Cytotoxicity against Hela S3 cells | Zhou et al. (2005) | |
| 15-O-Deacetyl-15-O-methylnimbolidin B | HeLa S3 cells | IC50 = 28.3 µM | In vitro | Cytotoxicity against Hela S3 cells | Zhou et al. (2005) | |
| Melanoma | ||||||
| 12-Hydroxyamoorastatone | SK-MEL-2 cells | IC50 = 0.38 μg/ml | In vitro | Cytotoxicity against SK-MEL-2 cells | Ahn et al. (1994) | |
| 12-Hydroxyamoorastatin | SK-MEL-2 cells | IC50 = 0.01 μg/ml | In vitro | Cytotoxicity against SK-MEL-2 cells | Ahn et al. (1994) | |
| 12-Acetoxyamoorastatin | SK-MEL-2 cells | IC50 = 0.0007 μg/ml | In vitro | Cytotoxicity against SK-MEL-2 cells | Ahn et al. (1994) | |
| Central nervous system tumors | ||||||
| 12-Hydroxyamoorastatone | XF498 cells | IC50 = 2 μg/ml | In vitro | Cytotoxicity against XF498 cells | Ahn et al. (1994) | |
| 12-Hydroxyamoorastatin | XF498 cells | IC50 = 0.02 μg/ml | In vitro | Cytotoxicity against XF498 cells | Ahn et al. (1994) | |
| 12-Acetoxyamoorastatin | XF498 cells | IC50 = 0.007 μg/ml | In vitro | Cytotoxicity against XF498 cells | Ahn et al. (1994) | |
| Antifeeding and insecticide effects | ||||||
| Toosendanin | M. separate | LC50 = 252.23 μg/ml | In vitro | Causing the destruction of midgut epithelial cells, leading to the regurgitation, paralysis | Li et al. (2020a) | |
| Toosendanin | A. aegypti | LC50 = 60.8 μg/ml | In vitro | Disrupting yolk deposition in oocytes, blood ingestion and digestion, and ovary ecdysteroid production | Ma et al. (2013) | |
| Toosendanin | B. plicatilis | 1.76–2.59 mg m−3 | In vitro | Inhibiting digestive enzymes including pepsase and tryptase | Huang et al. (2017) | |
| Toosendanin | S. oryzae, C. ferrugineus | LC50 = 675 and 875 ppm, respectively | In vitro | Reducing fecundity and expelling parasite | Xie et al. (1995) | |
| Toosendanin | S. mytilus | LC50 = 6.4 μg/L | In vitro | Reducing the population density and fecundity | Xu et al. (2019) | |
| Toosendanin | P. rapae | 100–1,000 μg/ml | In vitro | Inducing antifeeding | ||
| Toosendanin | P. rapae | 3 μg/piece | In vitro | Reducing activity of microsomal multifunctional oxidase, protease, intestinal acetylase | Zhang and Zhao (1992a) | |
| Toosendanin | P. rapae | 400 ppm | In vitro | Destructing midgut tissue | Zhang and Zhao (1991) | |
| Toosendanin | P. rapae | 2 μg/piece | In vitro | Interfering physiological metabolism and inhibiting respiratory center | Zhang et al. (1992b) | |
| Azedarachin A | S. eridania | MIC = 200 ppm | In vitro | Inducing antifeeding | Zhou et al. (1996) | |
| Nimbolidin F | S. eridania | MIC = 500 ppm | In vitro | Inducing antifeeding | Zhou et al. (1997) | |
| Ohchinolide C | S. eridania | MIC = 500 ppm | In vitro | Inducing antifeeding | Zhou et al. (1997) | |
| Salannin | S. eridania | MIC = 500 ppm | In vitro | Inducing antifeeding | Zhou et al. (1997) | |
| Nimbolidin C | S. eridania | MIC = 500 ppm | In vitro | Inducing antifeeding | Nakatani et al. (1996) | |
| Nimbolidin D | S. eridania | MIC = 500 ppm | In vitro | Inducing antifeeding | Nakatani et al. (1996) | |
| Nimbolidin E | S. eridania | MIC = 500 ppm | In vitro | Inducing antifeeding | Nakatani et al. (1996) | |
| Nimbolidin B | S. eridania | MIC = 500 ppm | In vitro | Inducing antifeeding | Nakatani et al. (1996) | |
| Toosendanin | S. littoralis | MIC = 200 ppm | In vitro | Inducing antifeeding | Nakatani et al. (2000) | |
| Nimbolinin A | S. littoralis | MIC = 1,000 ppm | In vitro | Inducing antifeeding | Nakatani et al. (2000) | |
| Nimbolinin C | S. littoralis | MIC = 1,000 ppm | In vitro | Inducing antifeeding | Nakatani et al. (2000) | |
| Nimbolinin D | S. littoralis | MIC = 1,000 ppm | In vitro | Inducing antifeeding | Nakatani et al. (2000) | |
| Nimbolinin B | S. littoralis | MIC = 1,000 ppm | In vitro | Inducing antifeeding | Nakatani et al. (2000) | |
| Trichilinin D | S. littoralis | MIC = 1,000 ppm | In vitro | Inducing antifeeding | Nakatani et al. (2000) | |
| Trichilinin E | S. littoralis | MIC = 1,000 ppm | In vitro | Inducing antifeeding | Nakatani et al. (2000) | |
| 1-O-Cinnamoyltrichilinin | S. littoralis | MIC = 1,000 ppm | In vitro | Inducing antifeeding | Nakatani et al. (2000) | |
| 1-Deoxy-3-tigloyl-11-methoxymeliacarpinin | S. exigua | 3 μg/cm2 | In vitro | Inducing antifeeding | Nakatani et al. (1993) | |
| Trichilinin B | S. eridania | MIC = 1,000 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| Trichilinin C | S. eridania | MIC = 1,000 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| 12-Hydroxyamoorastatin | S. eridania | MIC = 150 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| Toosendanin | S. eridania | MIC = 300 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| 12-O-Acetylazedarachin A | S. eridania | MIC = 400 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| 13-O-Acetylazedarachin A | S. littoralis | MIC = 400 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| Azedarachin B | S. littoralis | MIC = 200 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| 12-O-Acetylazedarachin B | S. eridania | MIC = 400 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| Trichilin B | S. eridania | MIC = 200 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| Trichilin H | S. eridania | MIC = 400 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| Trichilin I | S. eridania | MIC = 400 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| Trichilin J | S. eridania | MIC = 400 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| Trichilin K | S. eridania | MIC = 400 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| Trichilin L | S. eridania | MIC = 400 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| 1-Acetyltrichilin H | S. littoralis | MIC = 400 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| 12-Hydroxyamoorastatone | S. eridania | MIC = 250 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| Isotoosendanin | S. littoralis | MIC = 300 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| Neoazedarachin A | S. littoralis | MIC = 400 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| Neoazedarachin B | S. littoralis | MIC = 400 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| Neoazedarachin D | S. littoralis | MIC = 400 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| 1-Deacetylnimbolinin A | S. littoralis | MIC = 1,000 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| 2-Deacetylnimbolinin B | S. littoralis | MIC = 1,000 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| Ohchinolide B | S. eridania | MIC = 1,000 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| Ohchinolide C | S. eridania | MIC = 1,000 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| 3-O-Acetylohchinolal | S. eridania | MIC = 1,000 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| Meliacarpinin A | S. eridania | MIC = 50 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| Meliacarpinin C | S. eridania | MIC = 50 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| Meliacarpinin D | S. eridania | MIC = 50 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| Spirosendan | S. littoralis | MIC = 1,000 ppm | In vitro | Inducing antifeeding | Nakatani (1999) | |
| 1-Cinnamoyl-3-acetyl-11-hydroxymeliacarpin | S. littoralis | EC50 = 0.27 ppm | In vitro | Inducing antifeeding | Bohnenstengel et al. (1999) | |
| Toosendanin | P. rapae | AFC50 = 0.21 mM | In vitro | Inducing antifeeding | Wang et al. (2020a) | |
| Isotoosendanin | P. rapae | AFC50 = 0.46 mM | In vitro | Inducing antifeeding | Wang et al. (2020a) | |
| 12α-Hydroxyamoorastatone | P. rapae | AFC50 = 0.64 mM | In vitro | Inducing antifeeding | Wang et al. (2020a) | |
| Mesendanin H | P. rapae | AFC50 = 0.11 mM | In vitro | Inducing antifeeding | Wang et al. (2020a) | |
| Meliatoosenin E | P. rapae | AFC50 = 1.03 mM | In vitro | Inducing antifeeding | Wang et al. (2020a) | |
| 3-O-Acetylohchinolal | P. rapae | AFC50 = 0.89 mM | In vitro | Inducing antifeeding | Wang et al. (2020a) | |
| Salannin | P. rapae | AFC50 = 1.35 mM | In vitro | Inducing antifeeding | Wang et al. (2020a) | |
| Ohchinal | P. rapae | AFC50 = 1.79 mM | In vitro | Inducing antifeeding | Wang et al. (2020a) | |
| 12α-Hydroxyamoorastatone | E. paenulata | LD50 = 0.76 μg/cm2 | In vitro | Inducing antifeeding and death | Carpinella et al. (2003) | |
| Azadirachtin | E. paenulata | LD50 = 1.24 μg/cm2 | In vitro | Inducing antifeeding and death | Carpinella et al. (2003) | |
| Toosendanin | E. paenulata | ED50 = 3.69 μg/cm2 | In vitro | Inducing antifeeding | Carpinella et al. (2003) | |
| Azedarachin A | S. exigua | MIC = 200 ppm | In vitro | Inducing antifeeding | Huang et al. (1994) | |
| 12-O-Acetylazedarachin A | S. exigua | MIC = 400 ppm | In vitro | Inducing antifeeding | Huang et al. (1994) | |
| Anti-botulinum effect | ||||||
| Toosendanin | mice | 9 mg/kg i.p | In vivo | Causing submicroscopic changes of presynaptic motor nerve endings | Xiong (1985) | |
| Toosendanin | KM mice | 8 mg/kg i.p | In vivo | Making the synaptosomes completely resistant to botulinum neurotoxin A-mediated cleavage of SNAP-25 | Zhou et al. (2003) | |
| Toosendanin | PC12 cells | 35 μM | In vitro | Delaying channel formation and reducing the sizes of the channels | Li and Shi (2006) | |
| Toosendanin | mice | 10–5 g/ml | In vivo | Prolonging the paralysis time of botulism specimens | Li and Sun (1983) | |
| Toosendanin | rats | 8 mg/kg i.p | In vivo | Prolonging the paralysis time of botulism specimens | Li and Sun (1983) | |
| Toosendanin | rats | 7 mg/kg i.h | In vivo | Increasing the frequency of miniature end-plate potentials | Shih and Hsu (1983) | |
| Anti-inflammatory effects | ||||||
| 1-O-Tigloyl-1-O-deacetyl-nimbolinin B | BV-2 cells | IC50 = 7.892 μM | In vitro | Inhibiting LPS-stimulated inflammatory via blocking the activation of NF-κB and JNK signaling pathways | Tao et al. (2014) | |
| Trichilinin B | RAW 264.7 cells | IC50 = 29.2 μM | In vitro | Inhibiting LPS-stimulated inflammatory | Pan et al. (2014b) | |
| 3-Deacetyl-28-oxosalannolactone | RAW 264.7 cells | IC50 = 86 μM | In vitro | Inhibiting LPS-stimulated inflammatory | Pan et al. (2014b) | |
| Ohchinin | RAW 264.7 cells | IC50 = 28.7 μM | In vitro | Inhibiting LPS-stimulated inflammatory | Pan et al. (2014b) | |
| 23-Hydroxyohchininolide | RAW 264.7 cells | IC50 = 58.6 μM | In vitro | Inhibiting LPS-stimulated inflammatory | Pan et al. (2014b) | |
| 21-Hydroxyisoohchininolide | RAW 264.7 cells | IC50 = 87.3 μM | In vitro | Inhibiting LPS-stimulated inflammatory | Pan et al. (2014b) | |
| Toosendane B | RAW 264.7 cells | IC50 = 21.3 μM | In vitro | Inhibiting LPS-stimulated inflammatory | Hu et al. (2018) | |
| Toosendane C | RAW 264.7 cells | IC50 = 20.7 μM | In vitro | Inhibiting LPS-stimulated inflammatory | Hu et al. (2018) | |
| Ohchinin | RAW 264.7 cells | IC50 = 28.7 μM | In vitro | Inhibiting LPS-stimulated inflammatory | Akihisa et al. (2017) | |
| Salannin | RAW 264.7 cells | IC50 = 27.9 μM | In vitro | Inhibiting LPS-stimulated inflammatory via reducing the expression of iNOS and COX-2 | Akihisa et al. (2017) | |
| Nimbolinin D | RAW 264.7 cells | IC50 = 24.4 μM | In vitro | Inhibiting LPS-stimulated inflammatory | Akihisa et al. (2017) | |
| Mesendanin E | RAW 264.7 cells | IC50 = 23.8 μM | In vitro | Inhibiting LPS-stimulated inflammatory | Akihisa et al. (2017) | |
| 12-Ethoxynimbolinin A | HepG2 cells | 10 μM | In vitro | Suppressing NF-κB activation | Zhu et al. (2014) | |
| Nimbolinin C | HepG2 cells | 10 μM | In vitro | Suppressing NF-κB activation | Zhu et al. (2014) | |
| 1-O-Tigloyl-1-O-debenzoylohchinal | HepG2 cells | 10 μM | In vitro | Suppressing NF-κB activation | Zhu et al. (2014) | |
| 1-Acetyltrichilinin | HepG2 cells | 10 μM | In vitro | Suppressing NF-κB activation | Zhu et al. (2014) | |
| Trichilinin B | HepG2 cells | 10 μM | In vitro | Suppressing NF-κB activation | Zhu et al. (2014) | |
| 1-O-Cinnamoyltrichilinin | HepG2 cells | 10 μM | In vitro | Suppressing NF-κB activation | Zhu et al. (2014) | |
| Trichilinin D | HepG2 cells | 10 μM | In vitro | Suppressing NF-κB activation | Zhu et al. (2014) | |
| Isotoosendanin | Mice | 100 mg/kg | In vivo | Inhibiting acetic acid-induced vascular permeability and λ-carrageenan induced hind paw edema | Xie et al. (2008) | |
| 1-O-Tigloyl-1-O-debenzoylohchinal | Mice | 100 mg/kg | In vivo | Inhibiting acetic acid-induced vascular permeability and λ-carrageenan induced hind paw edema | Xie et al. (2008) | |
| Meliazedalides B | RAW 264.7 | 37.41 μM | In vitro | Inhibiting LPS-stimulated inflammatory | Qiu et al. (2019) | |
| 1-O-benzoyl-3-O-deactylnim-bolinin C | BV-2 cells | 21.95 μM | In vitro | Inhibiting LPS-stimulated inflammatory | Park et al. (2020) | |
| 3-Deacetyl-12-O-methylvolkensin | BV-2 cells | 21.37 μM | In vitro | Inhibiting LPS-stimulated inflammatory | Park et al. (2020) | |
| 12-O-Methyl-1-O-deacetyl-nimbolinin | BV-2 cells | 23.16 μM | In vitro | Inhibiting LPS-stimulated inflammatory | Park et al. (2020) | |
| Trichilinin B | BV-2 cells | 15.28 μM | In vitro | Inhibiting LPS-stimulated inflammatory | Park et al. (2020) | |
| 1-O-Cinnamoyltrichilinin | BV-2 cells | 7.73 μM | In vitro | Inhibiting LPS-stimulated inflammatory | Park et al. (2020) | |
| 1-Acetyltrichilinin | BV-2 cells | 20.61 μM | In vitro | Inhibiting LPS-stimulated inflammatory | Park et al. (2020) | |
| 12α-Hydroxyamoorastatone | BV-2 cells | 26.75 μM | In vitro | Inhibiting LPS-stimulated inflammatory | Park et al. (2020) | |
| Toosendanin | Mice | 0.5, 1.0 mg/kg i.p. for 7 days | In vivo | Alleviating DSS-induced colitis via inhibiting M1 macrophage polarization and regulating NLRP3 inflammasome and Nrf2/HO-1 signaling | Fan et al. (2019) | |
| Antibacterial and antifungal effects | ||||||
| 1α,7α-Ditigloyloxy-3α-acetoxyl-12α-ethoxylnimbolinin | P. gingivalis | MIC = 15.2 μg/ml | In vitro | Inhibitory effects against P. gingivalis | Zhang et al. (2016) | |
| 1α-Tigloyloxy-3α-acetoxyl-7α-hydroxyl-12β-ethoxylnimbolinin | P. gingivalis | MIC = 31.25 μg/ml | In vitro | Inhibitory effects against P. gingivalis | Zhang et al. (2016) | |
| 12-Ethoxynimbolinins C | P. gingivalis | MIC = 15.6 μg/ml | In vitro | Inhibitory effects against P. gingivalis | Zhang et al. (2007) | |
| 1-O-Cinnamoyltrichilinin | P. gingivalis | MIC = 31.3 μg/ml | In vitro | Inhibitory effects against P. gingivalis | Zhang et al. (2007) | |
| Trichilinin B | P. gingivalis | MIC = 31.5 μg/ml | In vitro | Inhibitory effects against P. gingivalis | Zhang et al. (2007) | |
| 7-Cinnamoyltoosendanin | M. luteus | MIC = 6.25 μg/ml | In vitro | Inhibitory effects against M. luteus | Liu et al. (2011) | |
| 7-Cinnamoyltoosendanin | B. subtilis | MIC = 25 μg/ml | In vitro | Inhibitory effects against B. subtilis | Liu et al. (2011) | |
| Meliarachin D | S. aureus | MIC = 50 μg/ml | In vitro | Inhibitory effects against S. aureus | Su et al. (2011) | |
| Meliarachin D | B. subtilis | MIC = 50 μg/ml | In vitro | Inhibitory effects against B. subtilis | Su et al. (2011) | |
| Meliarachin H | B. subtilis | MIC = 25 μg/ml | In vitro | Inhibitory effects against B. subtilis | Su et al. (2011) | |
Pharmacokinetics of Limonoids
There are few investigations on the pharmacokinetics of limonoids, and only several studies focus on the pharmacokinetics of TSN for elucidating the bioactivity and toxicity mechanism. In 2012, the first UPLC–MS/MS method was established for the pharmacokinetic determination of TSN in the rat plasma sample. The results showed that TSN possessed a fast absorption rate with a T max (h) value of 0.63 h after oral administration. In terms of V d level, oral administration (444,380.3 ± 204,747.7 ml/kg) had a wider distribution than intravenous administration (32,062.4 ± 18,562.8 ml/kg). Moreover, there was a significant variation of clearance between the two types of administration, and the results showed that TSN had a quick elimination rate for oral administration. However, the C max values of TSN were found to be at a low level despite a high oral administration dose of 60 mg/kg body weight in rat. Furthermore, this study also found a low bioavailability of TSN (9.9%), which suggested that the bioactivity and toxicity of TSN may be related with its metabolites (Wang et al., 2013). In order to analyze metabolites, the human liver microsomes were incubated with TSN, and these metabolites of TSN were identified by ultra-high performance liquid chromatography–quadrupole-time of flight mass spectrometry. Six metabolites (M1–M6) were tentatively deduced by MS spectra information. Among them, M1, M2, and M3 were produced by oxidation of TSN, and M6 was produced by dehydrogenation of TSN, while M4 and M5 can be obtained by oxidation and dehydrogenation. It is worth noting that the stability of metabolites varied in terms of time, and M1–M5 possessed strong stability, which can last for 120 min. By contrast, M6 reached a maximum value at 20 min and then decreased gradually. These evidence provide powerful founding for unraveling the metabolic fate of TSN (Wu et al., 2013).
The reactive metabolites, which were formed by the bioactivation of drugs, can partially induce liver injury by causing protein dysfunction and DNA damage (Holt and Ju, 2006). According to reports, several compounds possessed apparent toxicity, which can be ascribed to the bioactivated furan ring (Druckova et al., 2007). Therefore, the TSN that contains a structural alert of the furan ring needs to be explored comprehensively for its metabolites and the bioactivation mechanism. One research showed that esterolysis and conjugation with amino acids were proved as the principal metabolic pathways of TSN. Further experiments found that CYP3A4 could bioactivate the furan ring and produce a cis-butene-1,4-dial intermediate, and the products possibly interacted with peptides or proteins, which might account for the hepatotoxicity caused by TSN. In addition, N-conjugation of the furan ring from diverse amino acids and glutathione (GSH), rather than the widely accepted S-conjugation, acted a key role in the elimination of reactive metabolites of TSN (Yu et al., 2014). Some studies also discussed the potential detoxification mechanism from the aspect of pharmacokinetics. After the combination use of trans-anethole, the C max (294.8 ± 92.01 ng/L), AUC(0–t) (347.2 ± 128.7 ng/Lh), AUC(0–∞) (475.6 ± 210.8 ng/Lh), MRT(0–t) (2.667 ± 0.989 h), and MRT(0–∞) (5.386 ± 2.886 h) of TSN were decreased significantly, while the values of V z/F (8.110 ± 5.502 L/kg) were notably increased. These outcomes indicated that co-administration of trans-anethole can decrease the absorption and bioavailability of TSN and shorten the elimination process of TSN, which reduced the risk of toxicity accumulation (Yu et al., 2020).
Toxicity of Limonoids
Although limonoids, especially TSN, possess multiple biological activities, such as anti-tumor effect, insecticidal effect, anti-botulinum effect, anti-inflammation effect, etc., a few toxic activities have also been discovered and gradually received attention (Table 3). M. toosendan and M. azedarach were recorded with mild toxicity in Chinese Pharmacopoeia. A large body of evidence found TSN as the main bioactive compound of these two herbs and is also responsible for their toxicity. In vivo and in vitro experiments have proven that hepatotoxicity, pregnancy-toxicity, and embryotoxicity were the primary toxic effects of TSN. In order to verify whether serum miRNAs can be used as potential indicators of hepatotoxicity induced by TSN, miRNA chip analysis was applied to detect the target miRNAs in serum. The experiment results showed that administration with TSN (10 mg/kg) could induce severe liver injury, which manifested as increased alanine aminotransferase/aspartate aminotransferase (ALT/AST) activity and typical pathological changes. Compared to the variation of ALT/AST activity, 22 potential miRNAs can better reflect the characteristics of liver toxicity induced by TSN. According to this research, the increased expression of miRNA-122-3p and mcmv-miRNA-m01-4-3p both existed in the TSN group and other hepatotoxicant groups, including acetaminophen, monocrotaline, and diosbuibin B. Interestingly, increased miRNA-367-3p was only found in the TSN group, which indicated that it could be used as a characteristic biomarker for discovering and discriminating TSN-induced liver injury (Yang et al., 2019).
TABLE 3.
Toxicities and side effects of limonoids.
| Compounds | Toxic type | Model | LD50/toxic dose range | Toxic reactions | Reference |
|---|---|---|---|---|---|
| Toosendanin | Pregnancy toxicity | Mice | 0.2–0.8 mg/kg (i.p. for 7 days) | Increasing the level of IFN-γ, TNF-α, CD4+, and CD8+ T lymphocytes | Zhang et al. (2010a) |
| Toosendanin | Hepatotoxicity | Mice | 10 mg/kg (i.p. for 6, 12, 24 h) | Increasing the level of serum ALT and AST and decreasing the expression of mir-367-3p | Yang et al. (2019) |
| Toosendanin | Hepatotoxicity | Mice | 80 mg/kg (i.p. for 9 days) | Decreasing the body weight, increasing the level of serum ALT and AST, and enhancing the expression of Fmo3 | Lu et al. (2016) |
| Toosendanin | Hepatotoxicity | Mice | 3.75–15 mg/kg (i.p. for 24 h) | Decreasing the body weight, increasing the level of serum ALT and AST, and inducing energy metabolism disorder and hepatic steatosis | Yan et al. (2019) |
| Toosendanin | Hepatotoxicity | Primary hepatocyte cells | IC50 = 30.65 μM | Promoting Na+ influx and K+ efflux, decreasing the size of the cell membrane, and increasing the cell membrane permeability | Yan et al. (2019) |
| Toosendanin | Hepatotoxicity | Mice | 10 mg/kg (i.p. for 6, 12, 24 h) | Increasing the level of serum ALT, AST, ALP, TBIL, ROS, and MDA and decreasing GSH content and Nrf2 expression | Jin et al. (2019b) |
| Toosendanin | Hepatotoxicity | L-02 cells | 2–10 μM | Reducing GCL activity, GSH content, and expression of GCLC/GCLM and Nrf2; increasing ROS and Keap1 expression | Jin et al. (2019b) |
| Toosendanin | Hepatotoxicity | Primary hepatocyte cells | IC50 = 14.94 μM | Inducing mitochondrial dysfunction and caspase activation | Zhang et al. (2008) |
| Toosendanin | Embryotoxicity | Mice | 0.46 mg/kg (i.p. for 3 days) | Inducing abortion | Zhang et al. (2005b) |
Aside from investigating the role of miRNA expression alone, Lu et al. integrated the expression of microRNA with mRNA to explore the intricate and dynamic behavior of liver injury induced by TSN. An apparent liver injury was found 9 days after administration with TSN (80 mg/kg). Unexpectedly, the damage will be restored after 21 days of administration with TSN (80 mg/kg), which may account for liver regeneration. In addition, there existed dose- and time-specific alterations in global miRNA and mRNA expressions after being exposed to TSN. It can be inferred that the liver injury started with glutathione depletion, accompanied by mitochondrial dysfunction and lipid dysmetabolism, finally leading to hepatocyte necrosis in the liver from functional analyses of the miRNA–mRNA intersection dataset (Lu et al., 2016).
The application of multi-omics strategy, including proteomics and metabolomics, was considered as an advanced approach to explain the toxicity mechanism of TSN. Yan et al. found that triosephosphate isomerase 1 (TPI1) and α-enolase (ENOA), two glycolytic enzymes responsible for hepatotoxicity, were covalently modified by reactive metabolites of TSN. In vitro and in vivo experiments indicated that modifications would reduce the activity of TPI1 while inducing the activity of ENOA. Alterations of metabolites were also monitored and analyzed by metabolomics, and the outcomes showed a downward trend in tricarboxylic acid cycle, fatty acid β-oxidation, and amino acid metabolism, which revealed that the hepatocyte energy metabolism disorder could be induced by TSN (Yan et al., 2019). In another study, the role of Nrf2/GCL/GSH antioxidant signaling pathway in preventing hepatotoxicity from TSN was elucidated. It was found that TSN can decrease GSH content and Nrf2 expression, increase the expression of the Nrf2 inhibitor protein Kelch-like ECH-associated protein-1, and finally block the antioxidant signaling pathway to induce liver oxidative injury. Quercetin, a natural flavonoid with marked antioxidant ability, was validated to alleviate liver injury caused by TSN, which provided a new insight into compatibility detoxification of drugs (Jin Y. et al., 2019). In addition, fructus foeniculi, a typical detoxification drug for toosendan fructus, was reported to eliminate the toxicity of TSN by decreasing the absorption and bioavailability and accelerating the elimination process (Yu et al., 2020).
Since apoptosis plays an important role in inducing cell death, and TSN can exert its anti-tumor effect through the apoptotic pathway, whether apoptosis also acts as a critical regulator in liver damage caused by TSN needs further exploration. One representative study showed that TSN could inhibit primary rat hepatocyte growth with an IC50 value of 14.94 μM for 24 h. The underlying mechanism can be illustrated as TSN caused a decrease of mitochondrial membrane potential and intracellular ATP level and released cytochrome c to the cytoplasm, ultimately activating caspase-8, -9, and -3 to induce cell death. In the meantime, the activation of ROS and MAP kinases was also involved in this process (Zhang et al., 2008).
In recent years, with the increasing adverse effects of plant and vegetable pesticide residues on pregnant female individuals, the reproductive toxicity of TSN was also explored. The first study of reproductive toxicity caused by TSN was conducted by the team of Zhang, and they found that an intraperitoneal injection of TSN (0.46 mg/kg) could cause specific embryotoxicity to pregnant mice (Zhang X. F. et al., 2005). Later, in another study, the authors found that TSN-induced abortion was related with the imbalance of Th1/Th2 type cytokines, which manifested as increased levels of TNF-α and IFN-γ and CD4+/CD8+ ratio. These results partially proved that the immune imbalance is an important event in the reproductive toxicity induced by TSN (Zhang JL. et al., 2010).
The potential toxicity of limonoids has been noticed and emphasized in clinical application. Furthermore, the two herbs were commonly processed prior to clinical use, which can considerably reduce the toxicity. In addition, the compatibility of multiple herbs is another feasible method for toxicity reduction. In fact, countless innovative drugs, including atropine, scopolamine, and anisodamine, from Chinese medicinal herbs are toxic, but they are widely used for clinical purposes after an in-depth study. Therefore, the toxic limonoids (mainly focused on hepatotoxicity) could be candidate drugs via structural modification or targeted drug delivery.
Conclusion
Taken together, the limonoids isolated from genus Melia possess certain similar skeleton structures and can be classified into diverse types, including trichilin class, vilasinin class, havanensin class, azadirone class, nimbolinin class, nimbolidin class, salannin class, ohchinolal class, meliacarpinin class, meliacarpin class, and others. Although enormous attempts have been devoted to synthesize, semi-synthesize, and biosynthesize the momentous TSN and other limonoids, no ideal tactic was established for the complicated synthesis process yet. Limonoids, which possess promising bioactivities, such as anti-tumor activity, antifeeding activity, anti-botulism effect, anti-inflammatory effect, and antibacterial effect, have obtained global acceptance in agricultural applications and contemporary medicine. In the meantime, the structure-related activity, toxic effects, and pharmacokinetics have also been summarized in this review. Notwithstanding that great breakthroughs have been made in the comprehensive exploration and application of limonoids, some in-depth works are still extremely urgent to be conducted in the future.
Firstly, as a marker of bioactivity and toxicity of M. toosendan and M. azedarach, TSN has a rigorous criterion for its content limit. Owing to the presence of a hemiacetal structure in the molecular structure, tautomerism will occur, which implies that there are two chromatographic peaks when detecting the content of TSN. In addition, the tautomerism also existed in 1-deacetylnimbolinin B, nimbolinin B, nimbolinin A, and 1-O-tigloyl-1-O-deacetyl-nimbolinin B (Shen et al., 2014). Even though the content of these compounds can be measured by calculating the sum of the areas of the two peaks, it is important to find another strategy to address this dilemma. Confused by this phenomenon, some researchers tried to utilize acetylation reactions to make 28-OH become a single compound. Nevertheless, this approach was difficult to achieve completely due to the small amount of acetic anhydride and pyridine and was not suitable for extracts of toosendan fructus and meliae cortex (Zhong et al., 1975). Since a tautomer may exert different roles in the treatment of diseases, even the opposite effect, it is therefore crucial to figure out whether there is any difference in bioactivity between the two tautomers.
Secondly, although diverse pharmacological activities of limonoids have been confirmed, their definite molecular targets are still unclear. Given the promising anti-tumor bioactivity against various types of cancers, more studies should be devoted to molecular and cellular mechanism research through the combination of metabolomics and proteomics. To date, there is a large body of research focusing on the pharmacological activities of limonoids by animal or in vitro cell models. However, few clinical trials have been exerted, which restricted its application. We have searched clinical trials of limonoids in “ClinicalTrials.gov” and “trialsearch.who.int”, but only one study was conducted to investigate the anti-hypercholesterolemic effect of a limonoid-rich beverage, and no results were posted. We acknowledge that it is a long journey from the discovery of natural bioactive compounds to the development of new drugs. Due to the reported side effect, the potential clinical trials of limonoids were limited. Nevertheless, cautious clinical trials are necessary after reasonable structural modification and toxicity evaluation.
Thirdly, an increasing number of studies reported the toxic effects of limonoids, and TSN has received the most attention. It was reported that hepatotoxicity was considered as the predominant toxicity, which is of great concern worldwide. Nevertheless, few studies focused on the toxicity of other limonoids that have a skeleton structure similar to that of TSN. Previous reports showed that the content of 1-deacetylnimbolinin B was higher than TSN in fructus toosendan, and the contents of nimbolinin B, nimbolinin A, and 1-O-tigloyl-1-O-deacetyl-nimbolinin B exhibited no noticeable difference with TSN (Shen et al., 2014). Thus, it is indispensable to carry out extensive toxicity screening of other limonoids for the comprehensive quality control of fructus toosendan and meliae cortex. Moreover, the lack of a clear toxicity mechanism seriously restricts its clinical application. Hence, the toxic molecular target and the metabolism changes in vivo, accompanied with potential detoxification and/or a toxicity enhancement metabolism pathway, need to be further explored. Interestingly, processing can reduce the toxicity of fructus toosendan, which suggests that heating, acidification, and other physical processes may transform toxic limonoids into less toxic compounds. Although the synthesis of toosendanin is challenging yet, a breakthrough has been made in the synthesis of azadirachtin (one limonoid of genus Azadirachta), which points out the direction of future study. The successful total synthesis was achieved by the strategy called “relay route” or “relay synthesis”, which attempted to degrade azadirachtin to a specific potential synthetic intermediate and then transform this back into the natural product. Therefore, a similar strategy is expected to be applied in the synthesis of toosendanin.
Author Contributions
WF contributed to writing—original draft. LF contributed to resources. ZW contributed to conceptualization and supervision. LY contributed to conceptualization, writing—review and editing, and supervision.
Funding
This work is financially supported by the Program of Shanghai Municipal Commission of Health and Family Planning (ZY (2021-2023)-0215) and Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine. (ZYYCXTD-D-202004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Glossary
- Cyt C
Cytochrome c
- AIF
Apoptosis-inducing factor
- Caspase
Cysteinyl aspartate-specific proteinase
- Apaf-1
Apoptotic protease-activating factor-1
- Bcl-2
B-cell lymphoma-2
- PARP
Poly-ADP-ribose polymerase
- TNFR
Tumor necrosis factor receptor
- DR
Death receptor
- FADD
Fas-associated death domain protein
- DD
Death domain
- DED
Death effector domain
- DISC
Death-inducing signaling complex
- TRAIL
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand
- DR5
Death receptor 5
- CHOP
CCAAT/enhancer-binding protein homologous protein
- ROS
Reactive oxygen species
- JNK
c-Jun N-terminal kinase
- miRNAs
MicroRNAs
- EMT
Epithelial–mesenchymal transition
- ECM
Extracellular matrix
- TGF-β1
Transforming growth factor-β1
- NSCLC
Non-small cell lung cancer
- ADM
Adriamycin
- SAR
Structure–activity relationships
- DDS
Dextran sulfate sodium
- NLRP3
NLR family pyrin domain containing 3
- NO
Nitric oxide
- TNF
Tumor necrosis factor
- iNOS
Inducible nitric oxide synthase
- COX-2
Cyclooxygenase
- IL
Interleukin
- LPS
Lipopolysaccharide
- C/EBP-α
CCAAT/enhancer-binding proteins α
- PPAR-γ
Peroxisome proliferator-activated receptor γ
- HFD
High-fat diet
- α-IFN
Alpha interferon
- GSH
Glutathione
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- TPI1
Triosephosphate isomerase 1
- ENOA
α-Enolase
- Keap1
Kelch-like ECH-associated protein-1
References
- Ahn J.-W., Choi S.-U., Lee C.-O. (1994). Cytotoxic Limonoids from Melia Azedarach Var. Japonica. Phytochemistry 36, 1493–1496. 10.1016/s0031-9422(00)89749-6 [DOI] [Google Scholar]
- Akihisa T., Nishimoto Y., Ogihara E., Matsumoto M., Zhang J., Abe M. (2017). Nitric Oxide Production-Inhibitory Activity of Limonoids from Azadirachta indica and Melia Azedarach. Chem. Biodivers. 14. 10.1002/cbdv.201600468 [DOI] [PubMed] [Google Scholar]
- Akihisa T., Pan X., Nakamura Y., Kikuchi T., Takahashi N., Matsumoto M., et al. (2013). Limonoids from the Fruits of Melia Azedarach and Their Cytotoxic Activities. Phytochemistry 89, 59–70. 10.1016/j.phytochem.2013.01.015 [DOI] [PubMed] [Google Scholar]
- Ashkenazi A., Pai R. C., Fong S., Leung S., Lawrence D. A., Marsters S. A., et al. (1999). Safety and Antitumor Activity of Recombinant Soluble Apo2 Ligand. J. Clin. Invest. 104, 155–162. 10.1172/jci6926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barquero A. A., Michelini F. M., Alché L. E. (2006). 1-Cinnamoyl-3,11-dihydroxymeliacarpin Is a Natural Bioactive Compound with Antiviral and Nuclear Factor-kappaB Modulating Properties. Biochem. Biophys. Res. Commun. 344, 955–962. 10.1016/j.bbrc.2006.03.226 [DOI] [PubMed] [Google Scholar]
- Blaustein R. O., Germann W. J., Finkelstein A., DasGupta B. R. (1987). The N-Terminal Half of the Heavy Chain of Botulinum Type A Neurotoxin Forms Channels in Planar Phospholipid Bilayers. FEBS Lett. 226, 115–120. 10.1016/0014-5793(87)80562-8 [DOI] [PubMed] [Google Scholar]
- Bohnenstengel F. I., Wray V., Witte L., Srivastava R. P., Proksch P. (1999). Insecticidal Meliacarpins (C-Seco Limonoids) from Melia Azedarach. Phytochemistry 50, 977–982. 10.1016/s0031-9422(98)00644-x [DOI] [Google Scholar]
- Burris H. A. (2013). Overcoming Acquired Resistance to Anticancer Therapy: Focus on the PI3K/AKT/mTOR Pathway. Cancer Chemother. Pharmacol. 71, 829–842. 10.1007/s00280-012-2043-3 [DOI] [PubMed] [Google Scholar]
- Campbell K. J., Tait S. W. G. (2018). Targeting BCL-2 Regulated Apoptosis in Cancer. Open Biol. 8, 11. 10.1098/rsob.180002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L., Qu D., Wang H., Zhang S., Jia C., Shi Z., et al. (2016). Toosendanin Exerts an Anti-cancer Effect in Glioblastoma by Inducing Estrogen Receptor β- and P53-Mediated Apoptosis. Int. J. Mol. Sci. 17. 10.3390/ijms17111928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpinella M. C., Defago M. T., Valladares G., Palacios S. M. (2003). Antifeedant and Insecticide Properties of a Limonoid from Melia Azedarach (Meliaceae) with Potential Use for Pest Management. J. Agric. Food Chem. 51, 369–374. 10.1021/jf025811w [DOI] [PubMed] [Google Scholar]
- Chen L., Zhang J. X., Wang B., Mu S. Z., Hao X. J. (2014). Triterpenoids with Anti-tobacco Mosaic Virus Activities from Melia Toosendan. Fitoterapia 97, 204–210. 10.1016/j.fitote.2014.06.010 [DOI] [PubMed] [Google Scholar]
- Chen T. X., Cheng X. Y., Wang Y., Yin W. (2018). Toosendanin Inhibits Adipogenesis by Activating Wnt/β-Catenin Signaling. Sci. Rep. 8, 4626. 10.1038/s41598-018-22873-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Pharmacopoeia Commission (2020). Pharmacopoeia of the People’s republic of China[M]. Beijing, China: China Medical Science and Technology Press, p44–44. [Google Scholar]
- D'Ambrosio M., Guerriero A. (2002). Degraded Limonoids from Melia Azedarach and Biogenetic Implications. Phytochemistry 60, 419–424. 10.1016/s0031-9422(02)00107-3 [DOI] [PubMed] [Google Scholar]
- Dong S. H., Zhang C. R., He X. F., Liu H. B., Wu Y., Yue J. M. (2010). Mesendanins A-J, Limonoids from the Leaves and Twigs of Melia Toosendan. J. Nat. Prod. 73, 1344–1349. 10.1021/np100150n [DOI] [PubMed] [Google Scholar]
- Druckova A., Mernaugh R. L., Ham A. J., Marnett L. J. (2007). Identification of the Protein Targets of the Reactive Metabolite of Teucrin A In Vivo in the Rat. Chem. Res. Toxicol. 20, 1393–1408. 10.1021/tx7001405 [DOI] [PubMed] [Google Scholar]
- Estaquier J., Vallette F., Vayssiere J.-L., Mignotte B. (2012). “The Mitochondrial Pathways of Apoptosis,” in Advances in Mitochondrial Medicine. Editors Scatena R., Bottoni P., Giardina B. (Berlin: Springer-Verlag Berlin; ), 157–183. 10.1007/978-94-007-2869-1_7 [DOI] [PubMed] [Google Scholar]
- Fan H., Chen W., Zhu J., Zhang J., Peng S. (2019). Toosendanin Alleviates Dextran Sulfate Sodium-Induced Colitis by Inhibiting M1 Macrophage Polarization and Regulating NLRP3 Inflammasome and Nrf2/HO-1 Signaling. Int. Immunopharmacol. 76, 105909. 10.1016/j.intimp.2019.105909 [DOI] [PubMed] [Google Scholar]
- Fiandalo M. V., Kyprianou N. (2012). Caspase Control: Protagonists of Cancer Cell Apoptosis. Exp. Oncol. 34, 165–175. [PMC free article] [PubMed] [Google Scholar]
- Friesen C., Fulda S., Debatin K. M. (1997). Deficient Activation of the CD95 (APO-1/Fas) System in Drug-Resistant Cells. Leukemia 11, 1833–1841. 10.1038/sj.leu.2400827 [DOI] [PubMed] [Google Scholar]
- Gao T., Xie A., Liu X., Zhan H., Zeng J., Dai M., et al. (2019). Toosendanin Induces the Apoptosis of Human Ewing's Sarcoma Cells via the Mitochondrial Apoptotic Pathway. Mol. Med. Rep. 20, 135–140. 10.3892/mmr.2019.10224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerlich B. (1960). Chemical Assay of the Glycosides with Cardiac Effect in Scilla Maritima l., Var. alba. Arzneimittelforschung 10, 770–774. [PubMed] [Google Scholar]
- He Y., Wang J., Liu X., Zhang L., Yi G., Li C., et al. (2010). Toosendanin Inhibits Hepatocellular Carcinoma Cells by Inducing Mitochondria-dependent Apoptosis. Planta Med. 76, 1447–1453. 10.1055/s-0029-1240902 [DOI] [PubMed] [Google Scholar]
- Holt M. P., Ju C. (2006). Mechanisms of Drug-Induced Liver Injury. AAPS J. 8, E48–E54. 10.1208/aapsj080106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.-F., Fan H., Wang L.-J., Wu S.-B., Zhao Y. (2011). Limonoids from the Fruits of Melia Toosendan. Phytochemistry Lett. 4, 292–297. 10.1016/j.phytol.2011.05.003 [DOI] [Google Scholar]
- Hu Q., Huang F., Shi Y. (1997). Inhibition of Toosendanin on the Delayed Rectifier Potassium Current in Neuroblastoma X Glioma NG108-15 Cells. Brain Res. 751, 47–53. 10.1016/s0006-8993(96)01389-3 [DOI] [PubMed] [Google Scholar]
- Hu Y., Heng L., Xu R., Li J., Wei S., Xu D., et al. (2018). Meliacarpinin-Type Limonoids from the Bark of Melia Toosendan. Molecules 23. 10.3390/molecules23102590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R. C., Okamura H., Iwagawa T., Nakatani M. (1994). The Structures of Azedarachins, Limonoid Antifeedants from ChineseMelia azedarachLinn. Bcsj 67, 2468–2472. 10.1246/bcsj.67.2468 [DOI] [Google Scholar]
- Huang R. C., Tadera K., Yagi F., Minami Y., Okamura H., Iwagawa T., et al. (1996). Limonoids from Melia Azedarach. Phytochemistry 43, 581–583. 10.1016/0031-9422(96)00353-6 [DOI] [PubMed] [Google Scholar]
- Huang Y., Liu J., Pang T., Li L. (2017). Growth Inhibitory and Antifeedant Effects of Sublethal Concentrations of Toosendanin on the Rotifer Brachionus plicatilis . Biomass and Bioenergy 99, 31–37. 10.1016/j.biombioe.2017.02.013 [DOI] [Google Scholar]
- Humeau Y., Doussau F., Grant N. J., Poulain B. (2000). How Botulinum and Tetanus Neurotoxins Block Neurotransmitter Release. Biochimie 82, 427–446. 10.1016/s0300-9084(00)00216-9 [DOI] [PubMed] [Google Scholar]
- Jafri M. A., Al-Qahtani M. H., Shay J. W. (2017). Role of miRNAs in Human Cancer Metastasis: Implications for Therapeutic Intervention. Semin. Cancer Biol. 44, 117–131. 10.1016/j.semcancer.2017.02.004 [DOI] [PubMed] [Google Scholar]
- Janda K. D. (2008). Small Molecule Therapeutic Approaches for the Treatment of Botulinum Neurotoxins A and B Intoxication. Toxicon 51, 13. 10.1016/j.toxicon.2008.04.040 [DOI] [Google Scholar]
- Jaoko V., Nji Tizi Taning C., Backx S., Mulatya J., Van den Abeele J., Magomere T., et al. (2020). The Phytochemical Composition of Melia Volkensii and its Potential for Insect Pest Management. Plants (Basel) 9. 10.3390/plants9020143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Huang Z. L., Li L., Yang Y., Wang C. H., Wang Z. T., et al. (2019b). Quercetin Attenuates Toosendanin-Induced Hepatotoxicity through Inducing the Nrf2/GCL/GSH Antioxidant Signaling Pathway. Acta Pharmacol. Sin. 40, 75–85. 10.1038/s41401-018-0024-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y. H., Kwon S., Choi J. G., Cho W. K., Lee B., Ma J. Y. (2019a). Toosendanin from Melia Fructus Suppresses Influenza A Virus Infection by Altering Nuclear Localization of Viral Polymerase PA Protein. Front. Pharmacol. 10, 1025. 10.3389/fphar.2019.01025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju J., Qi Z., Cai X., Cao P., Huang Y., Wang S., et al. (2012). The Apoptotic Effects of Toosendanin Are Partially Mediated by Activation of Deoxycytidine Kinase in HL-60 Cells. Plos One 7, e52536. 10.1371/journal.pone.0052536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju J., Qi Z., Cai X., Cao P., Liu N., Wang S., et al. (2013). Toosendanin Induces Apoptosis through Suppression of JNK Signaling Pathway in HL-60 Cells. Toxicol. Vitro 27, 232–238. 10.1016/j.tiv.2012.09.013 [DOI] [PubMed] [Google Scholar]
- Kai W., Yating S., Lin M., Kaiyong Y., Baojin H., Wu Y., et al. (2018). Natural Product Toosendanin Reverses the Resistance of Human Breast Cancer Cells to Adriamycin as a Novel PI3K Inhibitor. Biochem. Pharmacol. 152, 153–164. 10.1016/j.bcp.2018.03.022 [DOI] [PubMed] [Google Scholar]
- Li H., Zhang J., Ma T., Li C., Ma Z., Zhang X. (2020a). Acting Target of Toosendanin Locates in the Midgut Epithelium Cells of Mythimna Separate Walker Larvae (Lepidoptera: Noctuidae). Ecotoxicol. Environ. Saf. 201, 110828. 10.1016/j.ecoenv.2020.110828 [DOI] [PubMed] [Google Scholar]
- Li M. F., Shi Y. L. (2005). The Long-Term Effect of Toosendanin on Current through Nifedipine-Sensitive Ca2+ Channels in NG108-15 Cells. Toxicon 45, 53–60. 10.1016/j.toxicon.2004.09.010 [DOI] [PubMed] [Google Scholar]
- Li M. F., Shi Y. L. (2006). Toosendanin Interferes with Pore Formation of Botulinum Toxin Type A in PC12 Cell Membrane. Acta Pharmacol. Sin. 27, 66–70. 10.1111/j.1745-7254.2006.00236.x [DOI] [PubMed] [Google Scholar]
- Li M. F., Shi Y. L. (2004). Toosendanin, a Triterpenoid Derivative, Acts as a Novel Agonist of L-type Ca2+ Channels in Neonatal Rat Ventricular Cells. Eur. J. Pharmacol. 501, 71–78. 10.1016/j.ejphar.2004.08.027 [DOI] [PubMed] [Google Scholar]
- Li P. Z., Sun G. Z. (1983). Antagonistic Effect of Toosendanin to Botulinum Toxin on Neuromuscular Preparations of Mice. Acta Physiol. Sinica. 4, 480–483. [Google Scholar]
- Li S., Li Y., Xu R., Kong L. Y., Luo J. (2020b). New Meliacarpin-type (C-Seco) and C-Ring Intact Limonoids from the Fruits of Melia Toosendan. Fitoterapia 144, 104605. 10.1016/j.fitote.2020.104605 [DOI] [PubMed] [Google Scholar]
- Li S., Li Y., Xu R., Kong L. Y., Luo J., Zhu X. M., et al. (2020). New Meliacarpin-type (C-Seco) and C-Ring Intact Limonoids from the Fruits of Melia Toosendan. Fitoterapia 144, 104605–104622. 10.1016/j.fitote.2020.104605 [DOI] [PubMed] [Google Scholar]
- Li X., You M., Liu Y. J., Ma L., Jin P. P., Zhou R., et al. (2017). Reversal of the Apoptotic Resistance of Non-small-cell Lung Carcinoma towards TRAIL by Natural Product Toosendanin. Sci. Rep. 7, 42748. 10.1038/srep42748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. Y., Jin L. T., Lin J. T., Ding Y. M., Yao S. H. (2018a). Effect of Toosendanin on the Apoptosis of Human Ovarian Cancer Cells through Mitochondrial Pathway. Chin. Pharm. J. 2, 109–113. 10.11669/cpj.2018.02.007 [DOI] [Google Scholar]
- Li Y. Y., Shao X. Y., Jin L. T., Li Q. F., Chen X. M. (2019). Apoptosis of Ovarian Cancer Cells Induced by Toosendanin through Fas/FasL Signaling Pathway. Chin. J. Integr. Trad. West. Med. 9, 1089–1094. 10.7661/j.cjim.20190729.089 [DOI] [Google Scholar]
- Li Y. Y., Zhang K. N., Cai J. W., Lin J. T., Shao X. Y. (2018b). Effect of Toosendanin on Invasion and Migration of Human Ovarian Cancer Cells. Chin. J. Pathophysiol. 1, 70–74. 10.3969/j.issn.1000-4718.2018.01.012 [DOI] [Google Scholar]
- Li-Weber M. (2013). Targeting Apoptosis Pathways in Cancer by Chinese Medicine. Cancer Lett. 332, 304–312. 10.1016/j.canlet.2010.07.015 [DOI] [PubMed] [Google Scholar]
- Lian X., Zhang X., Wang F., Wang X., Xue Z., Qi X. (2020). Characterization of a 2,3-oxidosqualene Cyclase in the Toosendanin Biosynthetic Pathway of Melia Toosendan. Physiol. Plant 170, 528–536. 10.1111/ppl.13189 [DOI] [PubMed] [Google Scholar]
- Liu H., Liu Y., Bian Z., Zhang J., Zhang R., Chen X., et al. (2019). Correction to: Circular RNA YAP1 Inhibits the Proliferation and Invasion of Gastric Cancer Cells by Regulating the miR-367-5p/p27 Kip1 axis. Mol. Cancer 18, 117. 10.1186/s12943-019-1045-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. B., Zhang C. R., Dong S. H., Dong L., Wu Y., Yue J. M. (2011). Limonoids and Triterpenoids from the Seeds of Melia Azedarach. Chem. Pharm. Bull. (Tokyo) 59, 1003–1007. 10.1248/cpb.59.1003 [DOI] [PubMed] [Google Scholar]
- Liu S. C., Tang W. Z., Ma T., Yao Q. Q. (2010). Review on Studies of the Chemical Constituents and Pharmcological Activities of Melia L. Qilu Pharm. Aff. 5, 290–293. 10.3969/j.issn.1672-7738.2010.05.017 [DOI] [Google Scholar]
- Liu X. L., Wang H., Zhang L., Wang Y. L., Wang J., Wang P., et al. (2016). Anticancer effects of crude extract from Melia toosendan Sieb. et Zucc on hepatocellular carcinoma In Vitro and In Vivo . Chin. J. Integr. Med. 22, 362–369. 10.1007/s11655-015-2084-7 [DOI] [PubMed] [Google Scholar]
- Lu X., Ji C., Tong W., Lian X., Wu Y., Fan X., et al. (2016). Integrated Analysis of microRNA and mRNA Expression Profiles Highlights the Complex and Dynamic Behavior of Toosendanin-Induced Liver Injury in Mice. Sci. Rep. 6, 34225. 10.1038/srep34225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Liu X., Sun W., Lu J. J., Wang Y., Chen X. (2018). Toosendanin, a Natural Product, Inhibited TGF-Β1-Induced Epithelial-Mesenchymal Transition through ERK/Snail Pathway. Phytother. Res. 32, 2009–2020. 10.1002/ptr.6132 [DOI] [PubMed] [Google Scholar]
- Ma Z., Gulia-Nuss M., Zhang X., Brown M. R. (2013). Effects of the Botanical Insecticide, Toosendanin, on Blood Digestion and Egg Production by Female Aedes aegypti (Diptera: Culicidae): Topical Application and Ingestion. J. Med. Entomol. 50, 112–121. 10.1603/me12119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madyastha K. M., Venkatakrishnan K. (2000). Structural Flexibility in the Biocatalyst-Mediated Functionalization of Ring 'A' in Salannin, a Tetranortriterpene from Azadirachta indica. J. Chem. Soc. Perkin Trans. 1 118, 3055–3062. 10.1039/b004260i [DOI] [Google Scholar]
- Nakai Y., Pellett S., Tepp W. H., Johnson E. A., Janda K. D. (2010). Toosendanin: Synthesis of the AB-Ring and Investigations of its Anti-botulinum Properties (Part II). Bioorg. Med. Chem. 18, 1280–1287. 10.1016/j.bmc.2009.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai Y., Tepp W. H., Dickerson T. J., Johnson E. A., Janda K. D. (2009). Function-oriented Synthesis Applied to the Anti-botulinum Natural Product Toosendanin. Bioorg. Med. Chem. 17, 1152–1157. 10.1016/j.bmc.2008.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani M., Chun Huang R., Okamura H., Naoki H., Iwagawa T. (1994). Limonoid Antifeedants from Chinese Melia Azedarach. Phytochemistry 36, 39–41. 10.1016/s0031-9422(00)97008-0 [DOI] [Google Scholar]
- Nakatani M., Fukuman Y., Sakumoto T., Yamashita N., Okamura H., Iwagawa T. (2000). Nimbolinins, C-Seco Limonoids from the Fruits of Melia Toosendan. Heterocycles 53, 689. 10.3987/com-99-8768 [DOI] [Google Scholar]
- Nakatani M., Huang R. C., Okamura H., Iwagawa T. (1993). The Structure of a New Antifeeding Meliacarpinin from ChinesMelia azedarachL. Chem. Lett. 22, 2125–2128. 10.1246/cl.1993.2125 [DOI] [Google Scholar]
- Nakatani M. (1999). Limonoids from Melia Toosendan (Meliaceae) and Their Antifeedant Activity. Heterocycles 50, 595–609. 10.3987/rev-98-sr(h)5 [DOI] [Google Scholar]
- Nakatani M., Shimokoro M., Zhou J.-B., Okamura H., Iwagawa T., Tadera K., et al. (1999). Limonoids from Melia Toosendan. Phytochemistry 52, 709–714. 10.1016/s0031-9422(99)00332-5 [DOI] [Google Scholar]
- Nakatani M., Zhou J.-B., Nakayama N., Okamura H., Iwagawa T. (1996). Nimbolidins C-E, Limonoid Antifeedants from Melia Toosendan. Phytochemistry 41, 739–743. 10.1016/0031-9422(95)00696-6 [DOI] [Google Scholar]
- Ochi M., Kotsuki H., Ido M., Nakai H., Shiro M., Tokoroyama T. (1979). The Structures of Ohchinolide a and B, Two New Limonoids Frommelia Azedarachlinn.Var. Japonicamakino. Chem. Lett. 8, 1137–1140. 10.1246/cl.1979.1137 [DOI] [Google Scholar]
- Ola M. S., Nawaz M., Ahsan H. (2011). Role of Bcl-2 Family Proteins and Caspases in the Regulation of Apoptosis. Mol. Cell. Biochem. 351, 41–58. 10.1007/s11010-010-0709-x [DOI] [PubMed] [Google Scholar]
- Orrenius S., Zhivotovsky B., Nicotera P. (2003). Regulation of Cell Death: the Calcium-Apoptosis Link. Nat. Rev. Mol. Cel Biol 4, 552–565. 10.1038/nrm1150 [DOI] [PubMed] [Google Scholar]
- Otto T., Sicinski P. (2017). Cell Cycle Proteins as Promising Targets in Cancer Therapy. Nat. Rev. Cancer 17, 93–115. 10.1038/nrc.2016.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Matsumoto M., Nakamura Y., Kikuchi T., Zhang J., Ukiya M., et al. (2014a). Three New and Other Limonoids from the Hexane Extract of Melia Azedarach Fruits and Their Cytotoxic Activities. Chem. Biodivers. 11, 987–1000. 10.1002/cbdv.201400052 [DOI] [PubMed] [Google Scholar]
- Pan X., Matsumoto M., Nishimoto Y., Ogihara E., Zhang J., Ukiya M., et al. (2014b). Cytotoxic and Nitric Oxide Production-Inhibitory Activities of Limonoids and Other Compounds from the Leaves and Bark of Melia Azedarach. Chem. Biodivers. 11, 1121–1139. 10.1002/cbdv.201400190 [DOI] [PubMed] [Google Scholar]
- Park S., Nhiem N. X., Subedi L., Oh I., Kim J. Y., Kim S. Y., et al. (2020). Isolation of Bioactive Limonoids from the Fruits of Melia Azedarach. J. Asian Nat. Prod. Res. 22, 830–838. 10.1080/10286020.2019.1666826 [DOI] [PubMed] [Google Scholar]
- Pei Z., Fu W., Wang G. (2017). A Natural Product Toosendanin Inhibits Epithelial-Mesenchymal Transition and Tumor Growth in Pancreatic Cancer via Deactivating Akt/mTOR Signaling. Biochem. Biophys. Res. Commun. 493, 455–460. 10.1016/j.bbrc.2017.08.170 [DOI] [PubMed] [Google Scholar]
- Qiu L., Heng L., Xu R., Luo J., Li Y. (2019). Two New Nimbolinin- and Trichilin-Class Limonoids Isolated from the Fruits of Melia Azedarach. Chin. J. Nat. Med. 17, 227–230. 10.1016/s1875-5364(19)30025-1 [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Pinton P., Ferrari D., Chami M., Szabadkai G., Magalhães P. J., et al. (2003). Calcium and Apoptosis: Facts and Hypotheses. Oncogene 22, 8619–8627. 10.1038/sj.onc.1207105 [DOI] [PubMed] [Google Scholar]
- Rogers L. L., Zeng L., Kozlowski J. F., Shimada H., Alali F. Q., Johnson H. A., et al. (1998a). New Bioactive Triterpenoids from Melia Volkensii. J. Nat. Prod. 61, 64–70. 10.1021/np9704009 [DOI] [PubMed] [Google Scholar]
- Rogers L. L., Zeng L., McLaughlin J. L. (1998b). Volkensinin: A New Limonoid from Melia Volkensii. Tetrahedron Lett. 39, 4623–4626. 10.1016/s0040-4039(98)00854-5 [DOI] [Google Scholar]
- Rossetto O., Pirazzini M., Montecucco C. (2014). Botulinum Neurotoxins: Genetic, Structural and Mechanistic Insights. Nat. Rev. Microbiol. 12, 535–549. 10.1038/nrmicro3295 [DOI] [PubMed] [Google Scholar]
- Sachdeva M., Mo Y. Y. (2010). MicroRNA-145 Suppresses Cell Invasion and Metastasis by Directly Targeting Mucin 1. Cancer Res. 70, 378–387. 10.1158/0008-5472.can-09-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem S., Muhammad G., Hussain M. A., Bukhari S. N. A. (2018). A Comprehensive Review of Phytochemical Profile, Bioactives for Pharmaceuticals, and Pharmacological Attributes of Azadirachta indica. Phytother. Res. 32, 1241–1272. 10.1002/ptr.6076 [DOI] [PubMed] [Google Scholar]
- Shao S., Li S., Liu C., Zhang W., Zhang Z., Zhu S., et al. (2020). Toosendanin Induces Apoptosis of MKN45 Human Gastric Cancer Cells Partly through miR23a3pmediated Downregulation of BCL2. Mol. Med. Rep. 22, 1793–1802. 10.3892/mmr.2020.11263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L. Q., Peng F., Cai J. N., Liu J. Y., Hao L. L., Feng Y. L., et al. (2014). Fingerprint Analysis of Limonoids in Fructus Toosendan by HPLC-ELSD. Chin. J. Pharm. Anal. 8, 1431–1434. 10.16155/j.0254-1793.2014.08.007 [DOI] [Google Scholar]
- Shi Y. L., Li M. F. (2007). Biological Effects of Toosendanin, a Triterpenoid Extracted from Chinese Traditional Medicine. Prog. Neurobiol. 82, 1–10. 10.1016/j.pneurobio.2007.02.002 [DOI] [PubMed] [Google Scholar]
- Shi Y. L., Wang W. P., Yan S. C., Xu K. (1982). Effects of Calcium Ions and Nerve Impulses on Changes in Miniature End-Plate Potential Frequency Produced by Toosendanin. Acta Physiol. Sinica. 3, 304–309. [Google Scholar]
- Shih Y. L., Hsu K. (1983). Anti-botulismic Effect of Toosendanin and its Facilitatory Action on Miniature End-Plate Potentials. Jpn. J. Physiol. 33, 677–680. 10.2170/jjphysiol.33.677 [DOI] [PubMed] [Google Scholar]
- Siddiqui W. A., Ahad A., Ahsan H. (2015). The Mystery of BCL2 Family: Bcl-2 Proteins and Apoptosis: an Update. Arch. Toxicol. 89, 289–317. 10.1007/s00204-014-1448-7 [DOI] [PubMed] [Google Scholar]
- Singh N., Hassan A., Bose K. (2016). Molecular Basis of Death Effector Domain Chain Assembly and its Role in Caspase-8 Activation. FASEB J. 30, 186–200. 10.1096/fj.15-272997 [DOI] [PubMed] [Google Scholar]
- Su G. X., Liang X. T. (1980). A Correction of the Structure of Chuanliansu. Acta Chim. Sinica. 2, 196–198. [Google Scholar]
- Su S., Shen L., Zhang Y., Liu J., Cai J., Hao L., et al. (2013). Characterization of Tautomeric Limonoids from the Fruits of Melia Toosendan. Phytochemistry Lett. 6, 418–424. 10.1016/j.phytol.2013.05.006 [DOI] [Google Scholar]
- Su Z.-S., Yang S.-P., Zhang S., Dong L., Yue J.-M. (2011). Meliarachins A-K: Eleven Limonoids from the Twigs and Leaves of Melia Azedarach. Hca 94, 1515–1526. 10.1002/hlca.201000444 [DOI] [Google Scholar]
- Tada K., Takido M., Kitanaka S. (1999). Limonoids from Fruit of Melia Toosendan and Their Cytotoxic Activity. Phytochemistry 51, 787–791. 10.1016/s0031-9422(99)00115-6 [DOI] [PubMed] [Google Scholar]
- Takeya K., Qiao Z. S., Hirobe C., Itokawa H. (1996a). Cytotoxic Azadirachtin-type Limonoids from Melia Azedarach. Phytochemistry 42, 709–712. 10.1016/0031-9422(96)00044-1 [DOI] [PubMed] [Google Scholar]
- Takeya K., Quio Z. S., Hirobe C., Itokawa H. (1996b). Cytotoxic Trichilin-type Limonoids from Melia Azedarach. Bioorg. Med. Chem. 4, 1355–1359. 10.1016/0968-0896(96)00128-9 [DOI] [PubMed] [Google Scholar]
- Tan Q. G., Luo X. D. (2011). Meliaceous Limonoids: Chemistry and Biological Activities. Chem. Rev. 111, 7437–7522. 10.1021/cr9004023 [DOI] [PubMed] [Google Scholar]
- Tang M. Z., Wang Z. F., Shi Y. L. (2004). Involvement of Cytochrome C Release and Caspase Activation in Toosendanin-Induced PC12 Cell Apoptosis. Toxicology 201, 31–38. 10.1016/j.tox.2004.03.023 [DOI] [PubMed] [Google Scholar]
- Tao L., Zhang F., Hao L., Wu J., Jia J., Liu J. Y., et al. (2014). 1-O-tigloyl-1-O-deacetyl-nimbolinin B Inhibits LPS-Stimulated Inflammatory Responses by Suppressing NF-κB and JNK Activation in Microglia Cells. J. Pharmacol. Sci. 125, 364–374. 10.1254/jphs.14025FP [DOI] [PubMed] [Google Scholar]
- Taylor D. A. H. (1984). The Chemistry of the Limonoids from Meliaceae. Prog. Chem. Org. Nat. Prod. 45, 1–102. 10.1007/978-3-7091-8717-3_1 [DOI] [Google Scholar]
- Tummers B., Green D. R. (2017). Caspase-8: Regulating Life and Death. Immunol. Rev. 277 (1), 76–89. 10.1111/imr.12541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajant H. (2002). The Fas Signaling Pathway: More Than a Paradigm. Science 296, 1635–1636. 10.1126/science.1071553 [DOI] [PubMed] [Google Scholar]
- Wang G., Feng C. C., Chu S. J., Zhang R., Lu Y. M., Zhu J. S., et al. (2015). Toosendanin Inhibits Growth and Induces Apoptosis in Colorectal Cancer Cells through Suppression of AKT/GSK-3β/β-catenin Pathway. Int. J. Oncol. 47, 1767–1774. 10.3892/ijo.2015.3157 [DOI] [PubMed] [Google Scholar]
- Wang G., Huang Y.-X., Zhang R., Hou L.-D., Liu H., Chen X.-Y., et al. (2017). Toosendanin Suppresses Oncogenic Phenotypes of Human Gastric Carcinoma SGC-7901 Cells Partly via miR-200a-Mediated Downregulation of β-catenin Pathway. Int. J. Oncol. 51, 1563–1573. 10.3892/ijo.2017.4139 [DOI] [PubMed] [Google Scholar]
- Wang H., Dong H. Y., He Q. M., Liang J. L., Zhao T., Zhou L. (2020a). Characterization of Limonoids Isolated from the Fruits of Melia Toosendan and Their Antifeedant Activity against Pieris Rapae. Chem. Biodivers. 17, e1900674. 10.1002/cbdv.201900674 [DOI] [PubMed] [Google Scholar]
- Wang H., Wen C., Chen S., Wang F., He L., Li W., et al. (2020b). Toosendanin-induced Apoptosis in Colorectal Cancer Cells Is Associated with the κ-opioid Receptor/β-Catenin Signaling axis. Biochem. Pharmacol. 177, 114014. 10.1016/j.bcp.2020.114014 [DOI] [PubMed] [Google Scholar]
- Wang Q., Wang Z., Hou G., Huang P. (2020c). Toosendanin Suppresses Glioma Progression Property and Induces Apoptosis by Regulating miR-608/Notch Axis. Cancer Manag. Res. 12, 3419–3431. 10.2147/cmar.s240268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wang C., Wang Z. (2013). Determination of Toosendanin in Rat Plasma by Ultra-performance Liquid Chromatography-Electrospray Ionization-Mass Spectrometry and its Application in a Pharmacokinetic Study. Biomed. Chromatogr. 27, 222–227. 10.1002/bmc.2779 [DOI] [PubMed] [Google Scholar]
- Watanabe T., Sakamoto N., Nakagawa M., Kakinuma S., Itsui Y., Nishimura-Sakurai Y., et al. (2011). Inhibitory Effect of a Triterpenoid Compound, with or without Alpha Interferon, on Hepatitis C Virus Infection. Antimicrob. Agents Chemother. 55, 2537–2545. 10.1128/aac.01780-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wender P. A. (2013). Toward the Ideal Synthesis and Transformative Therapies: the Roles of Step Economy and Function Oriented Synthesis. Tetrahedron 69 (36), 7529–7550. 10.1016/j.tet.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield M. L., George L. K., Grant G. D., Perou C. M. (2006). Common Markers of Proliferation. Nat. Rev. Cancer 6, 99–106. 10.1038/nrc1802 [DOI] [PubMed] [Google Scholar]
- Wu J. L., Leung E. L., Zhou H., Liu L., Li N. (2013). Metabolite Analysis of Toosendanin by an Ultra-high Performance Liquid Chromatography-Quadrupole-Time of Flight Mass Spectrometry Technique. Molecules 18, 12144–12153. 10.3390/molecules181012144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F., Zhang M., Zhang C. F., Wang Z. T., Yu B. Y., Kou J. P. (2008). Anti-inflammatory and Analgesic Activities of Ethanolic Extract and Two Limonoids from Melia Toosendan Fruit. J. Ethnopharmacol. 117, 463–466. 10.1016/j.jep.2008.02.025 [DOI] [PubMed] [Google Scholar]
- Xie Y. S., Fields P. G., Isman M. B., Chen W. K., Zhang X. (1995). Insecticidal Activity of Melia Toosendan Extracts and Toosendanin against Three Stored-Product Insects. J. Stored Prod. Res. 31, 259–265. 10.1016/0022-474x(95)00003-p [DOI] [Google Scholar]
- Xiong C. S. (1985). The Interaction between Toosendanin and Botulinum Toxin at the Neuromuscular junction, an Ultrastructure Observation. Acta Pharm. Sin. 7, 495–499. [PubMed] [Google Scholar]
- Xu H., Zhang J. L. (2011). Natural Products-Based Insecticidal Agents 9. Design, Semisynthesis and Insecticidal Activity of 28-acyloxy Derivatives of Toosendanin against Mythimna Separata Walker In Vivo . Bioorg. Med. Chem. Lett. 21, 1974–1977. 10.1016/j.bmcl.2011.02.031 [DOI] [PubMed] [Google Scholar]
- Xu R., Zhang L., Liu J. (2019). The Natural Triterpenoid Toosendanin as a Potential Control Agent of the Ciliate Stylonychia mytilus in Microalgal Cultures. J. Appl. Phycol. 31, 41–48. 10.1007/s10811-018-1522-2 [DOI] [Google Scholar]
- Yan X., Zhuo Y., Bian X., Li J., Zhang Y., Ma L., et al. (2019). Integrated Proteomics, Biological Functional Assessments, and Metabolomics Reveal Toosendanin-Induced Hepatic Energy Metabolic Disorders. Chem. Res. Toxicol. 32, 668–680. 10.1021/acs.chemrestox.8b00350 [DOI] [PubMed] [Google Scholar]
- Yang F., Li L., Yang R., Wei M., Sheng Y., Ji L. (2019). Identification of Serum microRNAs as Potential Toxicological Biomarkers for Toosendanin-Induced Liver Injury in Mice. Phytomedicine 58, 152867. 10.1016/j.phymed.2019.152867 [DOI] [PubMed] [Google Scholar]
- Yu J., Deng P., Zhong D., Chen X. (2014). Identification of Amino Acid and Glutathione N-Conjugates of Toosendanin: Bioactivation of the Furan Ring Mediated by CYP3A4. Chem. Res. Toxicol. 27, 1598–1609. 10.1021/tx5002145 [DOI] [PubMed] [Google Scholar]
- Yu J., Zhang R., Zhang T., Zhao J., Zhang Y., Wang Q., et al. (2020). Determination of Toosendanin and Trans-anethole in Fructus Meliae Toosendan and Fructus Foeniculi by HPLC-MS/MS and GC-MS/MS in Rat Plasma and Their Potential Herb-Herb Interactions. Biomed. Chromatogr. 34, e4837. 10.1002/bmc.4837 [DOI] [PubMed] [Google Scholar]
- Yuan C. M., Zhang Y., Tang G. H., Li Y., He H. P., Li S. F., et al. (2013). Cytotoxic Limonoids from Melia Azedarach. Planta Med. 79, 163–168. 10.1055/s-0032-1328069 [DOI] [PubMed] [Google Scholar]
- Zeng L., Gu Z.-m., Fang X.-p., Fanwick P. E., Chang C.-j., Smith D. L., et al. (1995a). Two New Bioactive Triterpenoids from Melia Volkensii (Meliaceae). Tetrahedron 51, 2477–2488. 10.1016/0040-4020(95)00018-4 [DOI] [Google Scholar]
- Zeng L., Gu Z. M., Fanwick P. E., Chang C. J., Smith D. L., McLaughlin J. L. (1995b). Additional Bioactive Triterpenoids from Melia-Volkensii (Meliaceae). Heterocycles 41, 741–752. [Google Scholar]
- Zhang B., Wang Z. F., Tang M. Z., Shi Y. L. (2005a). Growth Inhibition and Apoptosis-Induced Effect on Human Cancer Cells of Toosendanin, a Triterpenoid Derivative from Chinese Traditional Medicine. Invest. New Drugs 23, 547–553. 10.1007/s10637-005-0909-5 [DOI] [PubMed] [Google Scholar]
- Zhang J. L., Shi W. Y., Zhong W., Ma A. T., Wang X. D., Zhao Y. T., et al. (2010a). Effects of Toosendanin on Pregnancy and Uterine Immunity Alterations in Mice. Am. J. Chin. Med. 38, 319–328. 10.1142/s0192415x10007877 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Li J. K., Ge R., Liang J. Y., Li Q. S., Min Z. D. (2013). Novel NGF-Potentiating Limonoids from the Fruits of Melia Toosendan. Fitoterapia 90, 192–198. 10.1016/j.fitote.2013.07.019 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Li Q. S., Liang J. Y., Min Z. D. (2010d). Limonoids from Fruits of Melia Toosendan. Yao Xue Xue Bao 45, 475–478. 10.16438/j.0513-4870.2010.04.006 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Shi Y., Liu X. T., Liang J. Y., Ip N. Y., Min Z. D. (2007). Minor Limonoids from Melia Toosendan and Their Antibacterial Activity. Planta Med. 73 (12), 1298–1303. 10.1055/s-2007-981618 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Zheng Q. H., Liang J. Y., Li Q. S., Min Z. D. (2016). Two New Limonoids Isolated from the Fuits of Melia Toosendan. Chin. J. Nat. Med. 14, 692–696. 10.1016/s1875-5364(16)30082-6 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Zheng Q. H., Sang Y. S., Sung H. H., Min Z. D. (2018). New Limonoids Isolated from the Bark of Melia Toosendan. Chin. J. Nat. Med. 16, 946–950. 10.1016/S1875-5364(18)30136-5 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Liang J. Y., Li Q. S., Da Min Z. (2010b). New Limonoids from the Fruits of Melia Toosendan. Chin. Chem. Lett. 21, 838–841. 10.1016/j.cclet.2010.02.018 [DOI] [Google Scholar]
- Zhang W. M., Liu J. Q., Peng X. R., Wan L. S., Zhang Z. R., Li Z. R., et al. (2014). Triterpenoids and Sterols from the Leaves and Twigs of Melia Azedarach. Nat. Prod. Bioprospect. 4, 157–162. 10.1007/s13659-014-0019-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. F., Wang J. H., Zhang S. F., Liu Y., Fan L. C., Shi X. Y. (2005b). Research of Embryotoxicity of Toosendanin in Kunming Mice. Acta Vet. Zootechnica Sinica 3, 301–305. 10.3321/j.issn:0366-6964.2005.03.020 [DOI] [Google Scholar]
- Zhang X., Zhao S. H. (1992a). Effect of Toosendanin on Several Enzyme Systems of the Cabbage Worm Pieris Rapae L. Acta Entomol. Sinica. 2, 171–177. [Google Scholar]
- Zhang X., Zhao S. H. (1992b). Effect of Toosendanin on the Respiration and Other Physiological Parameters of the Imported Cabbage Worm (Pieris Rapae L). J. South. China Agr. Univ. 2, 5–11. [Google Scholar]
- Zhang X., Zhao S. H. (1991). Studies on the Histopathology of the Midgut of Cabbageworm Pieris Rapae L. Caused by Toosendanin. Acta Entomol. Sinica. 4, 501–502. [Google Scholar]
- Zhang Y., Qi X., Gong L., Li Y., Liu L., Xue X., et al. (2008). Roles of Reactive Oxygen Species and MAP Kinases in the Primary Rat Hepatocytes Death Induced by Toosendanin. Toxicology 249, 62–68. 10.1016/j.tox.2008.04.005 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Tang C. P., Ke C. Q., Li X. Q., Xie H., Ye Y. (2012). Limonoids from the Fruits of Melia Toosendan. Phytochemistry 73, 106–113. 10.1016/j.phytochem.2011.10.001 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Tang C. P., Ke C. Q., Yao S., Ye Y. (2010c). Limonoids and Triterpenoids from the Stem Bark of Melia Toosendan. J. Nat. Prod. 73, 664–668. 10.1021/np900835k [DOI] [PubMed] [Google Scholar]
- Zhao L., Huo C. H., Shen L. R., Yang Y., Zhang Q., Shi Q. W. (2010). Chemical Constituents of Plants from the Genus Melia. Chem. Biodivers. 7, 839–859. 10.1002/cbdv.200900043 [DOI] [PubMed] [Google Scholar]
- Zhao Q., Song Y., Feng C., Chen H. (2012). Cytotoxic Tirucallane Triterpenoids from the Stem Bark of Melia Toosendan. Arch. Pharm. Res. 35, 1903–1907. 10.1007/s12272-012-1106-7 [DOI] [PubMed] [Google Scholar]
- Zheng M. D., Wang N. D., Li X. L., Yan J., Tang J. H., Zhao X. H., et al. (2018). Toosendanin Mediates Cisplatin Sensitization through Targeting Annexin A4/ATP7A in Non-small Cell Lung Cancer Cells. J. Nat. Med. 72, 724–733. 10.1007/s11418-018-1211-0 [DOI] [PubMed] [Google Scholar]
- Zhong C. C., Xie J. X., Chen S. F., Liang X. T. (1975). The Structure of Chuanliansu. Acta Chim. Sinica. 1, 35–47. [Google Scholar]
- Zhou C.-Y., Tang H.-Y., Li X., Sang Y.-S. (2009). Three New Limonoids fromMelia Toosendan. Hca 92, 1191–1197. 10.1002/hlca.200800428 [DOI] [Google Scholar]
- Zhou F., Ma X. H., Li Z. J., Li W., Zheng W. M., Wang Z. B., et al. (2016). Four New Tirucallane Triterpenoids from the Fruits of Melia Azedarach and Their Cytotoxic Activities. Chem. Biodivers. 13, 1738–1746. 10.1002/cbdv.201600149 [DOI] [PubMed] [Google Scholar]
- Zhou H., Hamazaki A., Fontana J. D., Takahashi H., Esumi T., Wandscheer C. B., et al. (2004). New Ring C-Seco Limonoids from Brazilian Melia Azedarach and Their Cytotoxic Activity. J. Nat. Prod. 67, 1544–1547. 10.1021/np040077r [DOI] [PubMed] [Google Scholar]
- Zhou H., Hamazaki A., Fontana J. D., Takahashi H., Wandscheer C. B., Fukuyama Y. (2005). Cytotoxic Limonoids from Brazilian Melia Azedarach. Chem. Pharm. Bull. (Tokyo) 53, 1362–1365. 10.1248/cpb.53.1362 [DOI] [PubMed] [Google Scholar]
- Zhou J.-B., Minami Y., Yagi F., Tadera K., Nakatani M. (1997). Ring C-Seco Limonoids from Melia Toosendan. Phytochemistry 46, 911–914. 10.1016/s0031-9422(97)00378-6 [DOI] [Google Scholar]
- Zhou J.-B., Okamura H., Iwagawa T., Nakatani M. (1996). Limonoid Antifeedants from Melia Toosendan. Phytochemistry 41, 117–120. 10.1016/0031-9422(95)00558-7 [DOI] [Google Scholar]
- Zhou J. B., Tadera K., Minami Y., Yagi F., Kurawaki J., Takezaki K., et al. (1998). New Limonoids from Melia Toosendan. Biosci. Biotechnol. Biochem. 62, 496–500. 10.1271/bbb.62.496 [DOI] [PubMed] [Google Scholar]
- Zhou J. Y., Wang Z. F., Ren X. M., Tang M. Z., Shi Y. L. (2003). Antagonism of Botulinum Toxin Type A-Induced Cleavage of SNAP-25 in Rat Cerebral Synaptosome by Toosendanin. FEBS Lett. 555, 375–379. 10.1016/s0014-5793(03)01291-2 [DOI] [PubMed] [Google Scholar]
- Zhou Q., Wu X., Wen C., Wang H., Wang H., Liu H., et al. (2018). Toosendanin Induces Caspase-dependent Apoptosis through the P38 MAPK Pathway in Human Gastric Cancer Cells. Biochem. Biophysical Res. Commun. 505, 261–266. 10.1016/j.bbrc.2018.09.093 [DOI] [PubMed] [Google Scholar]
- Zhu G. Y., Bai L. P., Liu L., Jiang Z. H. (2014). Limonoids from the Fruits of Melia Toosendan and Their NF-κB Modulating Activities. Phytochemistry 107, 175–181. 10.1016/j.phytochem.2014.08.009 [DOI] [PubMed] [Google Scholar]
- Zou J., Ye H. J., He X. Y., Yu Y. X., XiaZhang H. Q. L. S., Zhang A. P., et al. (1985). The Therapeutic Effect of Orally Administrated Toosendanin on Human Botulism. Mil. Med. Sci. 37, 307–310. [Google Scholar]