Abstract
Background:
We used the Therapy Preference Scale, a 30-item questionnaire, to determine cancer treatment preferences of adults with cancer.
Methods:
We used Wilcoxon’s rank sum test and Fisher’s exact test to compare the preferences of younger (<60 years) versus older adults (≥60 years).
Results:
While 56% of patients would accept treatment offering increased life expectancy at an expense of short-term side effects, 75% preferred maintenance of cognition, functional ability and quality of life to quantity of days. Oral instead of intravenous treatment (p = 0.003), shorter hospital stay (p = 0.03), preservation of cognitive function (p = 0.01) and avoidance of pain (p = 0.02) were more important to older patients compared with younger patients.
Conclusion:
Many patients prioritized maintenance of cognition, functional ability and quality of life; older patients valued oral treatment, shorter hospital stay, preservation of cognitive function and avoidance of pain.
Keywords: : cancer, cognition, life expectancy, patients, preferences, quality of life, questionnaire, therapy, treatment
Lay abstract
Understanding the preferences of adults with cancer is important for physicians to develop personalized cancer treatment plans. We used a self-reported 30-item questionnaire, the Therapy Preference Scale, to help patients express their preferences with regard to safety, efficacy and other aspects of therapy. While 56% of the patients in our study would accept treatment offering increased life expectancy at an expense of short-term side effects, 75% preferred maintenance of cognition, functional ability and quality of life to quantity of days. Compared with younger patients, older patients preferred oral instead of intravenous treatment, shorter hospital stay, preservation of cognitive function and avoidance of pain.
Tweetable abstract
We used a novel questionnaire, the Therapy Preference Scale, to help cancer patients their treatment preferences. Results show that older patients prefer oral treatment, shorter hospital stay, preservation of cognitive function and avoidance of pain.
Understanding the therapy preferences and priorities of a patient with cancer is critical for selection of an appropriate treatment. In an era of increasingly complex therapies, patients’ preferences can help physicians to formulate a personalized treatment plan [1,2]. Patients’ preferences may differ based on their perspectives on various aspects of treatment including intent of therapy, side effects or adverse events, treatment burden (including out-of-pocket expenses) and quality of life [3]. Many factors play a significant role in determining patients’ preferences for cancer treatment, which may include age, functional and cognitive status, social and financial support, accessibility to healthcare services, and opinion from the patient’s healthcare team and family [4].
Treatment decisions can be difficult and are often made after discussions between the oncologist and the patient. Cancer treatments are usually complex, and decisions often have to be made quickly. Feelings of fear and uncertainty are common with a new diagnosis of cancer, disease progression or relapse. These factors can potentially lead to hasty decisions which may not always reflect patients’ preferences [5,6]. In addition, a lack of clear communication regarding patients’ preferences may affect clinical decision-making and lead to treatment choices that do not match patients’ goals and expectations [5,7]. Mismatch between the informational needs and the type of information physicians provide, and variability between physicians’ comfort with discussing patients’ goals and preferences, have been reported [8,9].
We developed a self-reported 30-item questionnaire, the Therapy Preference Scale (TPS; Supplementary Material) to help patients contemplate and express their values and preferences with regard to the safety, efficacy and other aspects of therapy [1,10,11]. Here we report the responses of 100 patients who filled in the TPS in our study. We also analyzed responses of patients <60 years compared with those ≥60 years to determine whether age influenced treatment preferences.
Patients & methods
Therapy Preference Scale
We developed the TPS on the basis of a theoretical model incorporating three key domains of treatment – safety, efficacy and therapy characteristics – that can affect patients’ choice of cancer treatment [1,10]. As described in further detail in previous papers, the TPS captures patients’ rating of the importance of safety, efficacy and other characteristics of systemic cancer treatment (19 questions, scored on a scale of 1–10) and importance of one aspect of treatment over others (eight questions; a four-item Likert scale with choices of ‘strongly disagree’, ‘disagree’, ‘agree’ and ‘strongly agree’) [1,10]. In addition, patients’ choices on the intent of therapy (cure, extend life or relieve symptoms), maximum acceptable out-of-pocket expenses (US$1000, US$5000, US$10,000, US$15,000 or US$20,000) and minimum life expectancy gain to accept cancer treatment (3, 6, 9, 12 or 15 months) are gathered through the three other questions [10,12]. The cost range in out-of-pocket expenses was based on our observations and initial discussions with the patients and providers who helped us finalize the questionnaire. Many of the insurance plans in the USA have deductibles and co-payments that amount to a few thousand dollars. The price ranges do not reflect the idea of cost–effectiveness of a drug. While some patients may be willing to pay more than $20,000 a year, given the median per capita income of about $34,000 the price range was limited to $20,000 [13].
Study design & participants
Patients with any cancer type, regardless of the stage, treatment type, duration of diagnosis and current state of cancer were recruited from the University of Nebraska Medical Center between July 2019 and December 2019. Patients were approached for enrollment during a scheduled outpatient visit or when hospitalized at the Fred and Pamela Buffett Cancer Center. Eligible patients were identified with the help of their oncologists. Patients were asked to complete the TPS based on their current treatment preferences. Our study was approved by the institutional review board at University of Nebraska Medical Center. All patients signed written informed consent.
Eligibility criteria
Eligible patients were adults aged ≥19 years with a diagnosis of cancer, able to speak and understand English, able to provide informed consent and sufficiently stable from a clinical perspective to complete the survey. We excluded patients with any condition (e.g., schizophrenia, major depression, other mental illness) that could potentially interfere with survey completion. We did not enroll any critically ill patients such as those in intensive care unit.
Data collection & analysis
Demographic, cancer-specific and therapy-related data were collected by a review of medical records and patient interviews. Data were descriptively summarized using frequencies and percentages. Median and interquartile range (IQR) were used to present the relevant data. We also performed comparisons of answers between younger and older adults (<60 years vs ≥60 years) on a per question basis. We chose the 60-year cut-off that is usually utilized in hematological malignancies to define the older patient population [14–17]. Questions measuring safety and quality of life, effectiveness and treatment characteristics on the 10-point Likert scale (Table 2) were compared using Wilcoxon’s rank sum test for independent samples [18]. Questions measuring treatment preferences in the four-item Likert scale were compared using Fisher’s exact test [19]. Responses of ‘strongly disagree’ and ‘disagree’ were categorized into ‘disagree’, while responses of ‘strongly agree’ and ‘agree’ were categorized into ‘agree’ for the purposes of this analysis (Table 3 & Supplementary Table 1). A significance level of p < 0.05 was used to determine statistical significance for each comparison; p-values were unadjusted, and the overall study-wide error rate is >0.05.
Table 2. . Patient responses to questions in the scale of 1–10.
| Question (n) | Questions | Total patients, median (IQR) | Patients aged <60 years, median (IQR) | Patients aged ≥60 years, median (IQR) |
|---|---|---|---|---|
| Safety and quality of life | ||||
| 1 | Maintains your appearance | 5 (3–8) | 5 (2.25–7) | 6 (4–8) |
| 2 | Maintains your sex life | 5 (2–8) | 5 (2.25–7) | 5 (2–8) |
| 3 | Avoids serious side effects | 9 (8–10) | 9 (8–10) | 9 (8–10) |
| 4 | Avoids short-term damage to your ability to think, remember things and make decisions | 7 (5–9.25) | 6.5 (5–8) | 8 (5–10) |
| 5 | Avoids long-term damage to your ability to think, remember things and make decisions | 10 (8–10) | 10 (8–10) | 10 (9–10) |
| 6 | Avoids short-term damage to your ability to do daily activities such as grooming, eating, or self-care | 8 (5–9) | 7 (5–8) | 8 (5–9) |
| 7 | Avoids long-term damage to your ability to do daily activities such as grooming, eating, or self-care | 9 (8–10) | 9 (8–10) | 9 (8–10) |
| 8 | Maintains your ability to have a child | 2 (1–8) | – | – |
| 9 | Maintains your ability to remain employed while undergoing cancer treatment (select ‘not applicable’ if you are unemployed or retired) | 7 (4–9) | – | – |
| Effectiveness of cancer treatment | ||||
| 10 | Helps you live longer without necessarily achieving a cure | 9.5 (7.75–10) | 9 (7–10) | 10 (8–10) |
| 11 | Offers the chance of a cure† | 10 (9–10) | 10 (10–10) | 10 (9–10) |
| 12 | Relieves your symptoms such as fatigue, pain or shortness of breath without necessarily increasing your life expectancy or achieving a cure | 8 (5–10) | 7 (5–10) | 8.5 (7–10) |
| Treatment characteristics | ||||
| 13 | Cancer medicine is given as a pill rather than by vein | 5 (3.75–8.25) | 5 (2–8) | 7.5 (5–9) |
| 14 | Treatment is available in a clinic close to your home (for example, within 2 h) | 8 (7–10) | 8 (6–9.75) | 9 (8–10) |
| 15 | Treatment is associated with a short or no hospital stay (for example, less than a week) | 8 (5–10) | 8 (5–9) | 9 (7.25–10) |
| 16 | Treatment limits the number of invasive procedures (for example, biopsies) necessary for making treatment decisions | 8 (6–9) | 8 (6–9) | 8 (6–9) |
| 17 | Treatment does not significantly disrupt your lifestyle (for example, due to frequent doctor visits or blood tests) | 7 (5–8) | 7 (5–8) | 8 (5.25–9) |
| 18 | Cost you pay for treatment, such as out-of-pocket expenses, is affordable | 9 (7–10) | 8.5 (7–10) | 9.5 (7.25–10) |
| 19 | Treatment does not result in a significant burden to your family or friends (for example, due to care necessary at home or for getting you to doctor visits) | 8 (7–10) | 7.5 (7–10) | 9 (7–10) |
Range is 3–10; for all other questions, range is 1–10.
IQR: Interquartile range.
Table 3. . Treatment preferences based on four-item Likert scale.
| Question (n) | Statements | Age <60 years | Age ≥60 years | Odds ratio (95% CI) age <60 vs age ≥60 |
p-value | ||
|---|---|---|---|---|---|---|---|
| Agree (%) | Disagree (%) | Agree (%) | Disagree (%) | ||||
| 21. | Living longer is important to me even if treatment will result in side effects such as life-threatening infection | 28 (67) | 14 (33) | 27 (54) | 23 (46) | 1.7 (0.7–4.4) | 0.3 |
| 22. | Living longer is important to me even if treatment will result in poor quality of life | 15 (36) | 27 (64) | 16 (32) | 34 (68) | 1.2 (0.4–3.1) | 0.8 |
| 23. | I would accept a treatment that is very effective but results in a financial burden including debt | 30 (71) | 12 (29) | 26 (52) | 24 (48) | 2.3 (0.9–6.1) | 0.08 |
| 24. | I would travel long distance (for example, 2 h or more) multiple times during treatment to receive care from cancer experts | 39 (93) | 3 (7) | 45 (90) | 5 (10) | 1.4 (0.3–9.9) | 0.7 |
| 25. | I would undergo a more effective treatment even if the treatment causes significant pain (for example, mouth sores or stomach cramps) | 39 (93) | 3 (7) | 37 (74) | 13 (26) | 4.5 (1.1–26.6) | 0.02† |
| 26. | I would undergo treatment that maintains or improves my quality of life but does not help me live longer | 30 (71) | 12 (29) | 42 (85) | 8 (16) | 0.5 (0.1–1.5) | 0.2 |
| 27. | I would rather live a shorter life than permanently lose my ability to think, remember things and make decisions | 27 (64) | 15 (36) | 44 (88) | 6 (12) | 0.2 (0.1–0.8) | 0.01† |
| 28. | I would rather live a shorter life than permanently lose my ability to do daily activities such as grooming, eating or self-care | 28 (67) | 14 (33) | 38 (76) | 12 (24) | 0.6 (0.2–1.7) | 0.3 |
p < 0.05.
Results
A total of 112 patients were approached. Of 100 patients who consented, 92 completed the entire survey and eight missed one or more questions. The median age of the study participants was 61 years (range: 23–89), 55% were aged ≥60 years, 56% were female and 63% had solid tumors (Table 1). At the time of enrollment, 52% of patients were receiving cancer treatment, 40% had completed treatment and 8% were newly diagnosed and had not yet started treatment. The median time to complete the survey was 10 min (IQR: 7–13).
Table 1. . Patient characteristics.
| Variable | Total patients | Age <60 years (n = 45) | Age ≥60 years (n = 55) |
|---|---|---|---|
| Current age (median, range) | 61 (23–89) | 51 (23–59) | 65 (60–89) |
| Age at diagnosis (median, range) | 59 (21–82) | 48 (21–59) | 64 (60–82) |
| Race (n, %) – White – Black – Others |
92 (92) 4 (4) 4 (4) |
39 (87) 2 (4) 4 (9) |
53 (96) 2 (4) |
| ECOG† (n, %) – 0 – 1 – ≥2 |
29 (29) 53 (53) 13 (13) |
11 (26) 24 (57) 7 (17) |
18 (34) 29 (55) 6 (11) |
| Type of malignancy (n, %) – Breast cancer – Lung cancer – GI/hepatobiliary/pancreatic cancers – Genitourinary cancer – Hematological malignancies – Others |
16 (16) 16 (16) 11 (11) 9 (9) 39 (39) 9 (9) |
8 (18) 4 (9) 5 (11) 3 (7) 21 (46) 4 (9) |
8 (14.5) 12 (22) 6 (11) 6 (11) 18 (32.5) 5 (9) |
| Currently on treatment (n, %) – Yes – No |
52 (52) 48 (48) |
24 (53) 21 (47) |
28 (51) 27 (49) |
| Intent of therapy (n, %) – Curative – Palliative – Others |
62 (62) 34 (34) 4 (4) |
27 (63) 16 (37) |
35 (66) 18 (34) |
| Current state of malignancy (n, %) – Remission – Not in remission/others‡ – Relapse/refractory/recurrent – New diagnosis |
46 (46) 24 (24) 22 (22) 8 (8) |
23 (51) 10 (22) 10 (22) 2 (5) |
23 (42) 14 (25) 12 (22) 6 (11) |
| Treatment change planned at the visit (n, %) – Yes – No |
12 (12) 88 (88) |
5 (11) 40 (89) |
7 (13) 48 (87) |
ECOG was not available in five female patients.
Chronic lymphocytic leukemia patient with no indication for treatment.
ECOG: Eastern Co-operative Oncology Group performance score; GI: Gastrointestinal.
Safety & quality of life
Avoiding long-term damage to one’s ability to think, remember things and make decisions (cognition) was the most important goal for the patients (median: 10; IQR: 8–10), followed by avoiding long-term damage to their ability to complete daily activities (median: 9; IQR: 8–10) and avoiding serious side effects (median: 9; IQR: 8–10; Table 2). Avoiding short-term side effects and the ability to maintain cognition or do daily activities had median responses of 7 (IQR: 5–9.25) and 8 (IQR: 5–9), respectively. Similarly, maintaining appearance, sex life and ability to have a child were scored 5 (IQR: 3–8), 5 (IQR: 2–8) and 2 (IQR: 1–8), respectively.
Effectiveness of cancer treatment
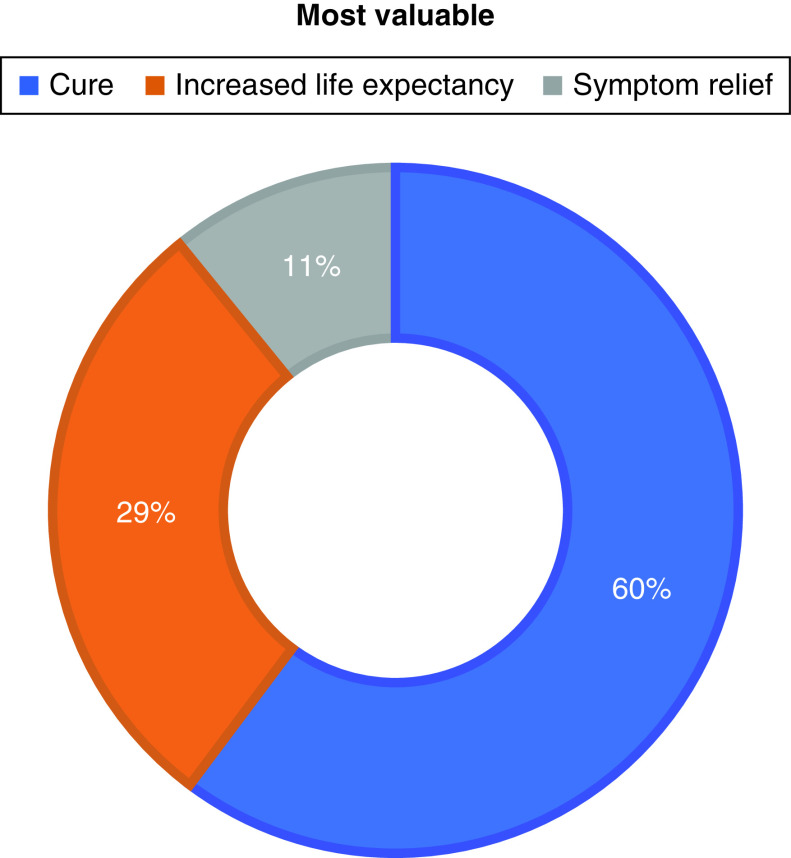
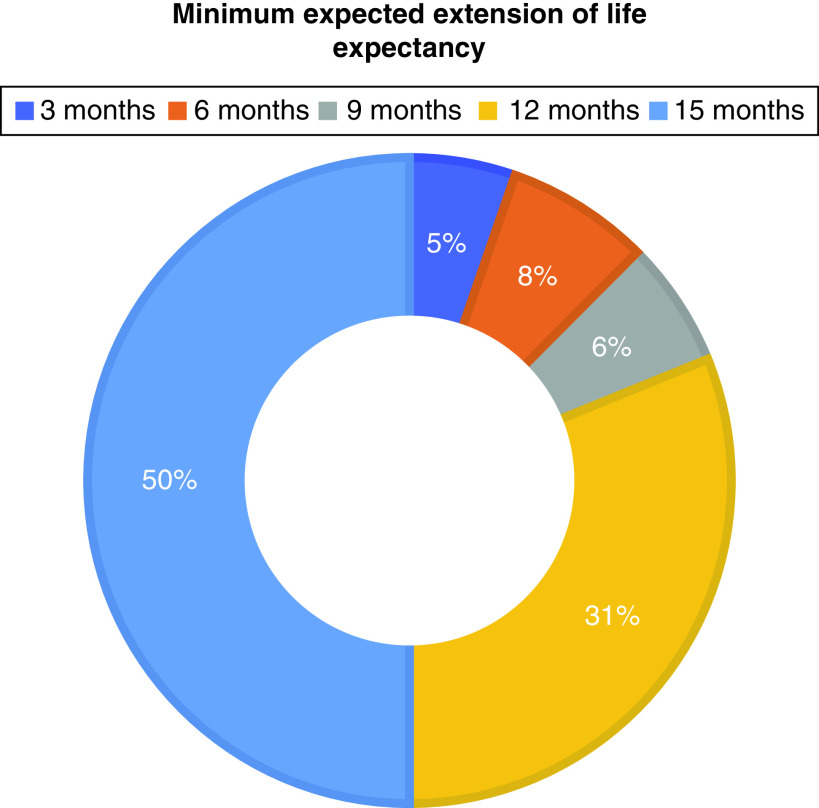
Treatments offering a chance of a cure (median: 9.5; IQR: 9–10) and helping patients live longer without cure (median: 10; IQR: 7.75–10) were rated as very important; responses were more varied for treatment which relieved symptoms without necessarily achieving a cure (median: 8; IQR: 5–10). When asked to rank priorities, cure (60%), increased life expectancy (29%) and symptom relief (11%) were prioritized. The majority of patients (88%) would reject a harsh treatment for improved life expectancy of ≤6 months (Figure 1).
Figure 1. . Patient preferences for intent of therapy.
Treatment characteristics
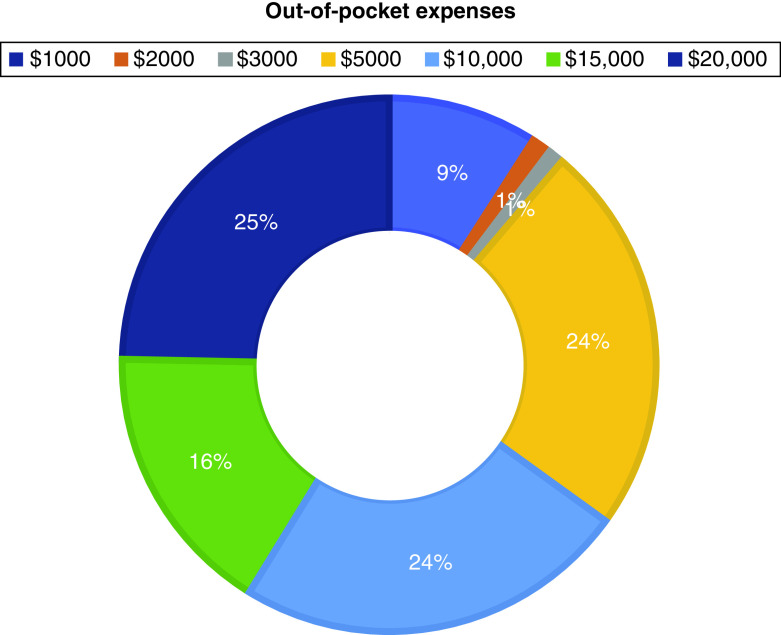
Treatment with affordable out-of-pocket expenses (median: 9; IQR: 7–10), treatment available in a clinic close to home (median: 8; IQR: 7–10) and treatment with less burden to family and friends (median: 8; IQR: 7–10) were important. Fewer invasive procedures and a reduced disruption of patients’ own lifestyles had median scores of 8 (IQR: 6–9) and 7 (IQR: 5–8), respectively (Figures 2 & 3).
Figure 2. . Patient preferences for out-of-pocket expenses.
Figure 3. . Patient preferences for minimum increase in life expectancy to make harsh treatment worthwhile.
Treatment preferences
Just over one-half (56%) of the patients were willing to accept short-term side effects for increased life expectancy; 31% agreed that living longer was more important even if treatment would result in poor quality of life (Table 3). The majority of patients (≥75%) would prefer treatment improving quality of life, maintaining cognition or maintaining functional ability over increasing life expectancy. Almost 60% would accept effective treatment despite financial burden; 60% were willing to pay US$10,000 or more out-of-pocket for their cancer treatment. The majority (80%) would travel a long distance multiple times to receive treatment from cancer experts.
Preferences based on age
When asked to rank priorities, patients aged ≥60 years, in comparison to patients aged <60 years, prioritized cure (67 vs 55%), increased life expectancy (28 vs 30%) and symptom relief (5 vs 15%). Four responses in the TPS reached statistical differences between younger and older patients. Patients aged ≥60 years, compared with those <60 years, preferred cancer medicine be given as a pill rather than intravenously (median score: 7.5 vs 5; p = 0.003) and preferred treatment with short or no hospital stay (median score: 9 vs 8; p = 0.03). Among younger patients, 93% would agree to a more effective treatment even if the treatment caused significant pain, compared with 74% of older patients (p = 0.02). Compared with 88% of younger patients, 67% of older patients would be willing to accept a shorter life expectancy rather than permanently losing the ability to think, remember things and make decisions (p = 0.01).
Discussion
In this study we utilized a novel self-reported questionnaire, the TPS, to explore patients’ preferences regarding their cancer treatment and compared the responses from younger and older adults. Regardless of age group, patients in general valued improved life expectancy, even when treatment would result in short-term serious toxicities, but not necessarily when the likely improvement in life expectancy was ≤6 months. Avoiding long-term impairments of cognition, functional ability and quality of life was of greater importance than improved life expectancy to many. Also, reducing treatment burden on family and friends was important to patients, even more so than avoiding disruption of patients’ own lifestyle; this is an important finding of this study.
Among the participants, 60% indicated cure as their priority rather than an increase in life expectancy or symptom relief. This preference differed with age, with about two-thirds of younger patients prioritizing cure compared with about one-half of older patients. Younger patients were more willing to undergo effective treatment in spite of a risk of significant pain. Older patients were more likely to prefer maintenance of cognitive ability and memory over prolongation of life. The use of oral chemotherapy and the likelihood of a shorter hospital stay were more important for older adults than for younger patients.
Prior studies have reported increased survival as a highly important outcome; however, studies focusing on patient preferences with exact delineations of the term ‘cure’ versus ‘increased life expectancy’ are sparse [20–22]. Prior studies also indicate that patients hope for durable responses and possible cure despite reports of modest survival gain and minimal chances of long-term survival [23,24]. However, it should be noted that patients’ expectations of survival benefit for curative-intent versus palliative-intent therapy may be different, leading to differences in treatment preferences. For patients with non-small-cell lung cancer with estimated survival of 3–5 years, adjuvant chemotherapy after curative resection was considered worthwhile if the survival increased by ≥9 months [25]. In another study of locally advanced or metastatic gastric cancer, patients expected a survival benefit of at least 6 months before undergoing palliative therapy with increased side effects. In palliative-intent therapy, preservation of cognitive ability and quality of life are considered to be equally, if not more, important compared with survival [20,26,27]. However, it should be noted that the treatment patterns differ significantly between solid and hematological malignancies; the difference between curative and palliative therapy may not always be discrete, especially in hematological malignancies, thus putting curative and palliative intent in the same continuum for patients’ goals and preferences. Similarly, patient preferences may differ based on the current status of the cancer and its treatment; for example, newly diagnosed patients may focus more on effectiveness and survival, while patients in remission may prioritize less toxicity and side effects. Notably, our patient cohort would avoid harsh treatment if the survival benefit was ≤6 months, which aligns well with existing literature [22,28]. This is an important factor to consider during drug development and approval, as patients may forgo treatment with short survival benefit but significant adverse effects [29,30].
Literature directly comparing differences in treatment preferences among older and younger patients is limited. While prior studies have demonstrated that older patients regard functional ability and cognitive function highly, our results demonstrate that older patients do value survival as a significant factor. Similarly, functional and cognitive abilities are important factors when considering treatment preferences in younger patients. Oral instead of intravenous treatment, shorter hospital stay, preservation of cognitive ability and freedom from pain were more valuable to older patients than younger patients. In a study of patients with breast cancer, older patients more commonly reported a wish to maintain their current quality of life and independence, rather than taking chemotherapy with significant side effects [31]. Similarly, older adults are known to value health outcomes, such as maintaining cognitive ability and functional status and freedom from pain and other symptoms, more than survival [26,28,32]. Older patients preferred low burden of treatment, including shorter hospital stay, which aligns well with the preferences of older adults in our study [32]. Thus factors such as treatment being available closer to home or a shorter commute to the treatment center, short or no hospital stay and oral instead of intravenous chemotherapy do affect patient preferences, even though they may not be high on the priority list [20,33–36].
Cancer costs are an important patient concern, with skyrocketing costs of several newer therapies and the risk of serious financial burden. Financial burden is known to affect patients’ compliance with cancer treatment, health disparities and decreased quality of life [37–39]. However, out-of-pocket costs are not frequently addressed and discussed in detail by the patients and the physicians when making important treatment decisions [38]. In our study, more than one-half of the patients were willing to accept financial burden and pay ≥US$10,000 for effective treatment; however, the affordability of cancer treatment was considered highly important. One study of patients with chronic lymphocytic leukemia observed that a modest increase in out-of-pocket cost significantly affected patients’ treatment preferences [40]. Most patients are not able to afford huge out-of-pocket expenses such as US$100,000; however, many insurance plans have out-of-pocket expenses of a few thousand dollars. A report by the American Cancer Society Cancer Action Network indicated that patients spent US$5.6bn in out-of-pocket costs for cancer treatment in 2015 [41]. Patients paid around US$5000–$13,000 out of pocket with a comprehensive insurance plan, compared with more than US$50,000 with a short-term limited-duration insurance [41]. Comprehensive insurance plans usually require higher premiums than short-term limited-duration plans and may not be affordable for some patients. In contrast, short-term limited-duration plans may have lower premiums but do not cover essential health care costs, including prescription medications. Thus elucidating patients’ ‘financial’ treatment preferences and affordability has an important and practical clinical implication.
Our study has potential limitations because of a small sample size which prohibited subgroup analyses. The single-center study design may limit generalizability. Many patients enrolled in the study had already received treatment; the study findings may be different for newly diagnosed patients. Newly diagnosed patients may focus more on treatment aggressiveness and prolonging life, in contrast to patients in remission who may focus on avoiding toxicity. Treatment preferences may also change over time and may need to be recaptured with changes in disease status or patients’ health. We excluded patients who were admitted to the intensive care unit with serious medical illness, who may have different preferences than patients recruited at clinics or those admitted in a hospital with less severe illness. Nonetheless, overall, more than 80% of the patients approached were willing to complete the survey, which indicates both their willingness to participate in a study regarding their preferences and the feasibility of the study. Patients, on average, took 10 min to complete the TPS, indicating that the TPS can be filled out during clinic visits despite the time constraints.
Our study has important strengths, and the results have clinical implications for therapy selection. We utilized a novel scale and gathered patient perspectives on a number of factors relevant to treatment preferences including patients’ preferences to trade off between safety and efficacy, out-of-pocket costs, an increase in life expectancy that is meaningful for a patient, and several other treatment characteristics. Discussion of such factors can engage patients in shared decision-making and may lead to selection of treatment that is felt to be the most appropriate for a patient [3].
Conclusion
Our study results demonstrate that many patients are willing to accept short-term side effects for improved life expectancy but prefer maintenance of cognition, functional ability and quality of life. Harsh treatment offering ≤6 months’ improvement of life expectancy was not valued. Oral instead of intravenous treatment, shorter hospital stay, preservation of cognitive function and avoidance of adverse effects resulting in significant pain are more important to older patients than younger patients. Our study highlights the utility of discussing patients’ priorities and preferences in clinical practice; the TPS can help physicians develop in-depth understanding of patient preferences and guide selection of treatment that will meet patients’ goals of care.
Summary points.
In an era of increasingly complex therapies, understanding the therapy preferences of a patient with cancer can help physicians to formulate a personalized treatment plan.
We utilized a novel self-reported 30-item questionnaire, the Therapy Preference Scale, to determine patients’ preferences with regard to safety, efficacy and other aspects of therapy.
We recruited 100 patients with any cancer type, regardless of the stage, treatment type, duration of diagnosis or current state of the cancer.
56% of patients were willing to accept treatment offering an increase in life expectancy or a cure at an expense of short-term side effects.
≥75% of patients would prefer treatment improving quality of life, maintaining cognition or maintaining functional ability over increasing life expectancy.
When asked to rank priorities, patients aged ≥60 years, in comparison to patients aged <60 years, prioritized cure (67 vs 55%), increased life expectancy (28 vs 30%) and symptom relief (5 vs 15%).
Oral instead of intravenous treatment, shorter hospital stay, preservation of cognitive function and avoidance of pain were more important to older patients than younger patients.
Our study highlights the utility of discussing patients’ priorities and preferences in clinical practice.
The Therapy Preference Scale can help physicians develop in-depth understanding of patient preferences and guide selection of treatment that will meet patients’ goals of care.
Supplementary Material
Acknowledgments
An abstract of this study was selected for online publication at the American Society of Clinical Oncology Annual Meeting held on May 2020.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/fon-2021-0260
Author contributions
P Dhakal, C Wichman and V Bhatt conceived and designed the study; P Dhakal and V Bhatt consented the patients to the study; P Dhakal collected the data from the medical records; C Wichman performed the data analysis; all authors interpreted the results. P Dhakal wrote an initial draft of the manuscript and critically revised the draft; all authors reviewed and edited the manuscript. All authors approved the final version of the manuscript. M Weaver contributed to this paper in a private capacity; no official support or endorsement by the US Department of Veterans Affairs is intended, nor should be inferred.
Financial & competing interests disclosure
This work was supported in part by the National Institute of General Medical Sciences, 1 U54 GM115458, which funds the Great Plains Institutional Development Award (IDeA) Clinical Translational Research (CTR) Network. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. V Bhatt reports receiving consulting fees from Genentech, Rigel, Agios, Incyte, Omeros, Takeda, Partnership for health analytic research, LLC (which in turn, receives funds from Jazz Pharmaceuticals) and Abbvie; research funding (institutional) from Abbvie, Pfizer, Incyte, Jazz, Tolero Pharmaceuticals, Inc. and National Marrow Donor Program; and drug support (institutional) from Oncoceutics for a trial. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Bhatt VR. Understanding patients’ values and priorities in selecting cancer treatments: developing a therapy preference scale. J. Geriatr. Oncol. 10(5), 677–679 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt VR. Personalizing therapy for older adults with acute myeloid leukemia: role of geriatric assessment and genetic profiling. Cancer Treat. Rev. 75, 52–61 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong Y-N, Roach N, Meropol NJ. Addressing patients’ priorities as a strategy to improve value. Oncologist 21(11), 1279–1282 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pant M, Bhatt VR. Early mortality and survival in older adults with acute myeloid leukemia. Int. J. Hematol. Oncol. 6(3), 61–63 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeBlanc TW, Fish LJ, Bloom CT et al. Patient experiences of acute myeloid leukemia (AML): a qualitative study about diagnosis, illness understanding, and treatment decision-making. Blood 126(23), 2119 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Denberg TD, Melhado TV, Steiner JF. Patient treatment preferences in localized prostate carcinoma: the influence of emotion, misconception, and anecdote. Cancer 107(3), 620–630 (2006). [DOI] [PubMed] [Google Scholar]
- 7.El-Jawahri A, Abel GA, Greer J et al. Perceptions of prognosis and treatment risk in older patients with acute myeloid leukemia. Blood 130(Suppl. 1), 349 (2017). [Google Scholar]
- 8.LeBlanc TW, Fish LJ, Bloom CT et al. Patient experiences of acute myeloid leukemia: a qualitative study about diagnosis, illness understanding, and treatment decision-making. Psychooncology 26(12), 2063–2068 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Liu P-H, Landrum MB, Weeks JC et al. Physicians’ propensity to discuss prognosis is associated with patients’ awareness of prognosis for metastatic cancers. J. Palliat. Med. 17(6), 673–682 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatt VR, Dhakal P, Wichman CS, Pozehl BJ. Therapy preference scale: a novel questionnaire to understand patients’ preferences of cancer treatment. J. Clin. Oncol. 37(Suppl. 15), e23179 (2019). [Google Scholar]
- 11.Bhatt VR, Dhakal P, Wichman CS, Pozehl B. Development and validation of the Therapy Preference Scale to understand patients’ systemic cancer treatment preferences. Future Oncol. 17(1), 37–44 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes how the Therapy Preference Scale was developed, revised and validated before finalizing the current version.
- 12.Ayre C, Scally AJ. Critical values for Lawshe’s content validity ratio: revisiting the original methods of calculation. Meas. Eval. Couns. Dev. 47(1), 79–86 (2013). [Google Scholar]
- 13.Quick facts United States: Income and Poverty (2019). www.census.gov/quickfacts/fact/table/US/SEX255219
- 14.International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N. Engl. J. Med. 329(14), 987–994 (1993). [DOI] [PubMed] [Google Scholar]
- 15.Baer MR, George SL, Dodge RK et al. Phase 3 study of the multidrug resistance modulator PSC-833 in previously untreated patients 60 years of age and older with acute myeloid leukemia: Cancer and Leukemia Group B Study 9720. Blood 100(4), 1224–1232 (2002). [PubMed] [Google Scholar]
- 16.Stone RM, Berg DT, George SL et al. Postremission therapy in older patients with de novo acute myeloid leukemia: a randomized trial comparing mitoxantrone and intermediate-dose cytarabine with standard-dose cytarabine. Blood 98, 548–553 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Klepin HD, Geiger AM, Tooze JA et al. The feasibility of inpatient geriatric assessment for older adults receiving induction chemotherapy for acute myelogenous leukemia. J. Am. Geriatr. Soc. 59(10), 1837–1846 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilcoxon F. Individual comparisons by ranking methods. Biometrics Bulletin. 1(6), 80–83 (1945). [Google Scholar]
- 19.Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of p. J. R. Stat. Soc. 85(1), 87–94 (1922). [Google Scholar]
- 20.Hofheinz R, Clouth J, Borchardt-Wagner J et al. Patient preferences for palliative treatment of locally advanced or metastatic gastric cancer and adenocarcinoma of the gastroesophageal junction: a choice-based conjoint analysis study from Germany. BMC Cancer 16(1), 937 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A study evaluating patients’ preferences for a hypothetical chemotherapy regimen to treat gastric cancer.
- 21.PEBC's Ovarian Oncology Guidelines Group. A systematic review of patient values, preferences and expectations for the treatment of recurrent ovarian cancer. Gynecol. Oncol. 146(2), 392–398 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Herzog TJ, Armstrong DK, Brady MF et al. Ovarian cancer clinical trial endpoints: Society of Gynecologic Oncology white paper. Gynecol. Oncol. 132(1), 8–17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lakdawalla DN, Romley JA, Sanchez Y, Maclean JR, Penrod JR, Philipson T. How cancer patients value hope and the implications for cost-effectiveness assessments of high-cost cancer therapies. Health Aff. (Millwood) 31(4), 676–682 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Shafrin J, Schwartz TT, Okoro T, Romley JA. Patient versus physician valuation of durable survival gains: implications for value framework assessments. Value Health 20(2), 217–223 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Blinman P, Hughes B, Crombie C et al. Patients’ and doctors’ preferences for adjuvant chemotherapy in resected non-small-cell lung cancer: what makes it worthwhile? Eur. J. Cancer 51(12), 1529–1537 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Soto Perez De Celis E, Li D, Sun C-L et al. Patient-defined goals and preferences among older adults with cancer starting chemotherapy (CT). J. Clin. Oncol. 36(Suppl. 15), 10009 (2018). [Google Scholar]
- 27.Kuchuk I, Bouganim N, Beusterien K et al. Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res. Treat. 142(1), 101–107 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Havrilesky LJ, Alvarez Secord A, Ehrisman JA et al. Patient preferences in advanced or recurrent ovarian cancer. Cancer 120(23), 3651–3659 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatt VR, Shostrom V, Armitage JO, Gundabolu K. Treatment patterns and outcomes of octogenarian patients with acute myeloid leukemia. Am. J. Hematol. 94(6), E169–e172 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhatt VR, Shostrom V, Gundabolu K, Armitage JO. Utilization of initial chemotherapy for newly diagnosed acute myeloid leukemia in the United States. Blood Adv. 2(11), 1277–1282 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamelinck VC, Bastiaannet E, Pieterse AH et al. A prospective comparison of younger and older patients’ preferences for adjuvant chemotherapy and hormonal therapy in early breast cancer. Clin. Breast Cancer 16(5), 379–388 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N. Engl. J. Med. 346(14), 1061–1066 (2002). [DOI] [PubMed] [Google Scholar]; •• This study used a questionnaire to evaluate the therapy preferences of seriously ill patients with regard to life-sustaining treatment.
- 33.Eek D, Krohe M, Mazar I et al. Patient-reported preferences for oral versus intravenous administration for the treatment of cancer: a review of the literature. Patient Prefer. Adherence 10, 1609–1621 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spaich S, Kinder J, Hetjens S, Fuxius S, Gerhardt A, Sütterlin M. Patient preferences regarding chemotherapy in metastatic breast cancer – a conjoint analysis for common taxanes. Front Oncol. 8, 535 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.da Costa DiBonaventura M, Copher R, Basurto E, Faria C, Lorenzo R. Patient preferences and treatment adherence among women diagnosed with metastatic breast cancer. Am. Health Drug Benefits 7(7), 386–396 (2014). [PMC free article] [PubMed] [Google Scholar]
- 36.Bridges JF, Mohamed AF, Finnern HW, Woehl A, Hauber AB. Patients’ preferences for treatment outcomes for advanced non-small cell lung cancer: a conjoint analysis. Lung Cancer 77(1), 224–231 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Dusetzina SB, Winn AN, Abel GA, Huskamp HA, Keating NL. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J. Clin. Oncol. 32(4), 306–311 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Zafar SY, Peppercorn JM, Schrag D et al. The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist 18(4), 381–390 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neugut AI, Subar M, Wilde ET et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J. Clin. Oncol. 29(18), 2534–2542 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mansfield C, Masaquel A, Sutphin J et al. Patients’ priorities in selecting chronic lymphocytic leukemia treatments. Blood Adv. 1(24), 2176–2185 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; ••Surveyed patients’ preferences for treatment attributes and impact of out-of-pocket cost on patients’ choices.
- 41.The costs of cancer. The American Cancer Society Cancer Action Network; (2020). www.fightcancer.org/sites/default/files/National%20Documents/Costs-of-Cancer-2020-10222020.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.