Abstract
Purpose:
To assess post-release outcomes associated with continuation of methadone treatment in correctional centers.
Methods:
This case-control study of the post-incarceration impact of pilot methadone programs operating in jails in New Haven and Bridgeport, Connecticut, USA was conducted in 2014–18. The study compared non-fatal overdose, fatal overdose, reincarceration, and resumption of methadone in the community experienced by 1564 eligible men, 660 (42.2%) of whom continued treatment while incarcerated.
Results:
Continuation of methadone was associated with a significant decrease in non-fatal overdose (OR:0.55; 95% CI: 0.36, 0.85) and a greater likelihood of resuming methadone treatment in the community post-release (OR:2.56; 95% CI: 2.07, 3.16). Time to resumption of methadone was shortened by treatment while time to non-fatal overdose was increased. Treatment while incarcerated resulted in a modest but not significant decrease in fatal overdoses and no difference in reincarceration between those who did and did not receive methadone. However, resumption of methadone after release did significantly reduce fatal overdoses (OR = 0.26, 95% CI: 0.11, 0.62, p = 0.002).
Conclusion and Relevance:
Improvements in post-release outcomes of non-fatal overdose and treatment reengagement emphasize the benefits of continuing medication-based treatment for opioid use disorder within the criminal justice system for those receiving it prior to being incarcerated.
Keywords: Opioid use disorder, Methadone, Correctional settings, Opioid overdose, Reincarceration
1. Introduction
The United States continues to have the highest rate of incarceration in the world, with almost 2.2 million people held in federal, state, or local correctional facilities(Bureau of Justice Statistics (BJS), 2017). The increase from 40,000 in 1980 to over 450,000 in 2017 is, in large part, due incarceration for drug-related offenses (The Sentencing Project, 2020). Incarcerated populations have high rates of substance use disorder compared to the general U. S. population. According to the U. S. Justice Department Bureau of Justice Statistics, 63% of people sentenced to jail meet DSM-IV criteria for drug or alcohol dependence compared to 4% in the general population, and 28.2% of people sentenced to prison or jail report ever having used opioids illicitly compared to 2.8% in the general population (Bronson et al., 2017; Jones et al., 2015). Of the 2.2 million Americans with opioid use disorder (OUD), one in three is arrested each year (Winkelman et al., 2018). Upon release, many relapse, and one-third is reincarcerated within three years (de Andrade et al., 2018; Langan and Levin, 2002).
The recognized gold-standard, evidence-based treatment for OUD (MOUD) employs long-acting opioid agonists, either methadone or buprenorphine (Committee on Medication-Assisted Treatment for Opioid Use Disorder, 2019; Mattick et al., 2014). Despite the high prevalence of OUD in carceral populations, less than 1% of jailed individuals with OUD receive MOUD while in custody(Sharma et al., 2016). Lack of MOUD while incarcerated heightens risk for fatal opioid overdoses in the few weeks following release (Binswanger et al., 2013; Bukten et al., 2017; Chang et al., 2015; Rosen et al., 2007).
Several studies have demonstrated the benefits of MOUD for incarcerated individuals with OUD. A pilot study of initiating methadone treatment for individuals incarcerated in the largest prison in Puerto Rico found a 78% reduction in past-week (22.5% to 5%) and past 30-month (37.5% to 5%) heroin use among inmates who received methadone as part of the study (Heimer et al., 2006). Provision of methadone and buprenorphine at the Riker’s Island jail in New York City has been studied, but the post-release benefits appear to be modest (Magura et al., 2009; Magura et al., 1993). By contrast, larger trials conducted in jails in Baltimore, Rhode Island, and New Mexico resulted in better post-release outcomes. In Baltimore, methadone treatment while incarcerated facilitated entry into community treatment, reduced self-reported opioid use, and reduced self-reported criminal activity (Gordon et al., 2008; Kinlock et al., 2008). In Rhode Island, provision of agonist treatment in the state’s correctional institution demonstrated reduced rates of fatal and non-fatal overdose following release, greater engagement with treatment in the community post-release, and lower self-reports of injection drug use (Brinkley-Rubinstein et al., 2018; Green et al., 2018; Rich et al., 2015). In New Mexico, continuation of methadone while jailed was associated with a lower rate of post-incarceration re-arrest and a higher rate of resumption of community methadone treatment (Westerberg et al., 2016).
Despite the evidence of demonstrated benefit during and after incarceration., barriers to MOUD in correctional centers remain. Most notably, these include security concerns, diversion, lack of knowledge of the benefits of MOUD, and preferences for abstinence-based approaches (Friedmann et al., 2012; McKenzie et al., 2009). Further evidence of the benefits of MOUD for incarcerated people, coming from an expanding number of corrections-based MOUD programs, may help reduce these barriers.
One such program has been the establishment of pilot methadone treatment programs in two Connecticut jails operated by the state’s Department of Correction (DoC) (Moore et al., 2018). In this report, we evaluate post-release outcomes of fatal and non-fatal overdose, reincarceration, and resumption of methadone treatment in the community comparing those whose methadone was discontinued to those continuing treatment while incarcerated. We hypothesized that those continuing treatment would be less likely to have a non-fatal or fatal overdose or be reincarcerated after release from jail and would be more likely to resume methadone treatment in the community after release.
2. Methods
2.1. Study Design and Population
This study was a retrospective observational case-control study covering the period from January 1, 2014 to December 30, 2018. The exposure is jail-based methadone treatment delivered as part of pilot programs that commenced in the New Haven Correctional Center in October 2013 and in Bridgeport Correctional Center in April 2014. Two local opioid treatment programs (OTP) – the APT Foundation in New Haven and the Recovery Network of Programs (RNP) in Bridgeport – provided staff and medication for daily dosing. Both jails are male high-security facilities and part of the unified State of Connecticut correctional system. Outcomes are events following release from custody: resumption of community-based methadone treatment, fatal and non-fatal opioid overdose, and reincarceration (Figure 1).
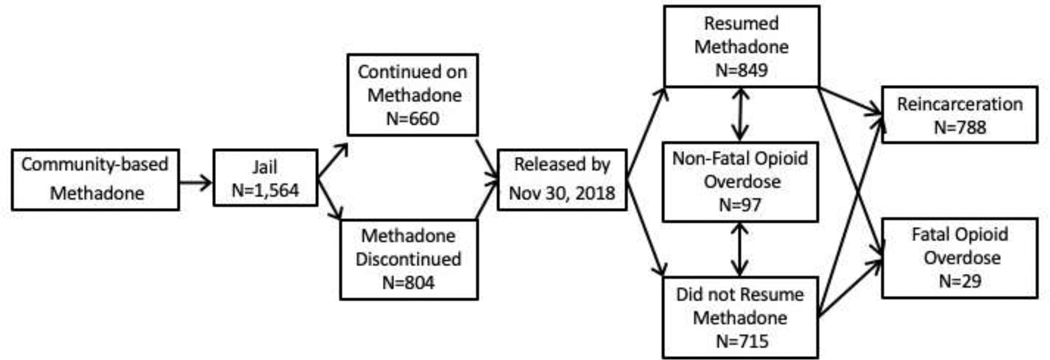
Figure 1.
Schematic of study design with possible outcomes for individuals released from jail.
DoC records identified men eligible to participate in the methadone treatment pilot. These men were being treated with methadone at an OTP in Connecticut immediately prior to entering one of the two jails, were sentenced for no more than 18 months, whose bail was less than $75,000, and who wished to continue treatment. Beginning with the initiation of the program in each of the jails, 1,927 eligible men were released by November 30, 2018. Excluded from this dataset were men who were released within three days of being jailed since their jail-based treatment had not yet begun (N=327), those reincarcerated within five days of release (N=8), and those whose DoC file was missing dates of birth (N=29) or DoC inmate numbers (N=7). The final population consisted of 1,564 men.
Continuation of methadone while in jail was predicated on availability of treatment slots in the pilot programs. Initially, the programs could treat a maximum of 30 men in the New Haven Correctional Center and 25 in the Bridgeport Correctional Center. Program capacity was incrementally expanded to 50 in each facility by the end of the study period. Given availability, which as first come, first served, men who reported treatment in the community with a last dose within five days were scheduled to start treatment in jail the day after the servicing OTP could confirm their medication dosage. Men transferred to other correctional facilities in Connecticut or elsewhere had their medication tapered and joined the unexposed cohort. Initially, treatment was considered a privilege and could be terminated, with those who were terminated moved into the unexposed cohort. The exposed cohort consisted of 660 men who received methadone throughout their period of incarceration; the unexposed cohort consisted of 904 men whose methadone treatment was terminated at the outset of or any time during their incarceration.
2.2. Data Sources for Post-release Outcomes
Information on reincarceration was contained in the dataset obtained from the DoC in a field that indicated the date when an individual was remanded to custody in any of the state’s thirteen correctional facilities. Data on drug-related overdose deaths were obtained from the Connecticut Office of the Chief Medical Examiner (OCME) and limited to opioid-involved deaths ruled accidental or of undetermined motive occurring between January 1, 2014 and December 31, 2018. The OCME dataset included the date of death and toxicology report for 3,997 individuals. Data on non-fatal overdoses were obtained from records of Emergency Medical Services (EMS) reported to the Connecticut Department of Public Health (DPH). The dataset of 15,777 incidents included non-fatal events in 2014–18 in which naloxone was administered or the complaint was listed as opioid-induced loss of consciousness. Information on the resumption of methadone treatment for OUD in the community following release from jail was available through the Connecticut Department of Mental Health and Addiction Services (DMHAS).
2.3. Data Matching and Analysis
The study team identified men who were released by November 30, 2018 and matched the men in the DoC dataset to those in the OCME and DPH sets. Matching of men across data sources relied primarily on name and date of birth, supplemented with data on race/ethnicity when available. Names, dates, and available race/ethnicity data were shared with DMHAS, where a DMHAS employee matched them to their internal records and provided the study team with a dataset containing matches along with the date of treatment resumption.
Descriptive statistics for the full cohort were produced. Using Chi-square tests (and the Fisher-Freeman-Halton Exact Test, where relevant), bivariate analyses of methadone-exposed versus -unexposed men assessed differences in demographics, reincarceration, overdose, and resumption of methadone at OTPs in Connecticut. Sub-analyses were conducted for both the New Haven and Bridgeport Correctional Centers to determine if results were location-specific. Multivariable logistic regression analyses were performed using backward elimination procedures. A significance level for retaining variables was set at p<0.05 level. All statistical analyses were performed using SAS® 9.4 software.
Time-to-event analysis for each of the four outcomes following release from custody was conducted using Cox proportional hazards regression. Individuals were tracked from the time of release until the study end date (December 31, 2018), until they experienced a fatal overdose, or until they were reincarcerated. As reincarceration was considered a censoring event, any other outcomes of interest occurring after reincarceration were not included in analysis. We tested the proportional hazards assumption for each model using the supremum test, computed on 1000 simulated patterns. In those cases where the proportional hazards assumption was found to be violated, we used an accelerated failure time model instead, with a log-logistic, log-normal, or exponential distribution for log survival time.
3. Results
Of the 1,564 men in the sample (Table 1), 681 (43.5%) were incarcerated at the Bridgeport Correctional Center and 883 (56.5%) were incarcerated at the New Haven Correctional Center. Taken together, the mean age was 40.5 years (range 22–73). The majority identified as White (58.5%), followed by Hispanic (21.0%), and Black (10.4%). Among the full sample, 660 (42.2%) individuals received methadone throughout their period of incarceration and 904 (57.8%) did not. Those incarcerated in New Haven were significantly more likely to receive treatment than those incarcerated in Bridgeport (44.5% and 39.2%, respectively, p = 0.035). Receipt of treatment during incarceration varied by race/ethnicity (χ2 = 12.13, p < 0.001). According to post-hoc pairwise comparison, White men (43.3%) were more likely than Hispanic (32.9%) men (χ2 = 10.73, p = 0.001) but not Black (35.8%) men (χ2 = 3.15, p = 0.076) to receive methadone treatment during incarceration.
Table 1.
Bivariate associations between study variables and receiving methadone in jail (N = 1564)
| Characteristic | N* | N (%) receiving methadone | N (%) not receiving methadone | p† | OR (95% CI) |
|---|---|---|---|---|---|
|
| |||||
| Location | 0.035 | ||||
| New Haven | 883 | 393 (44.5) | 490 (55.5) | 1.24 (1.02, 1.52) | |
| Bridgeport | 681 | 267 (39.2) | 414 (60.8) | 1 | |
| Race/ethnicity | 0.006 | ||||
| White | 915 | 396 (43.3) | 519 (56.7) | 1 | |
| Black | 162 | 58 (35.8) | 104 (64.2) | 0.73 (0.52, 1.03) | |
| Hispanic | 328 | 108 (32.9) | 220 (67.1) | 0.64 (0.49, 0.84) | |
| Other | 10 | 4 (40.0) | 6 (60.0) | 0.87 (0.25, 3.12) | |
| Age (years) | 0.519 | ||||
| 20–29 | 185 | 69 (37.3) | 116 (62.7) | 1 | |
| 30–39 | 644 | 280 (43.5) | 364 (56.5) | 1.29 (0.92, 1.81) | |
| 40–49 | 410 | 174 (42.4) | 236 (57.6) | 1.24 (0.87, 1.77) | |
| 50+ | 325 | 137 (42.2) | 188 (57.9) | 1.23 (0.85, 1.77) | |
| Reincarcerated | 0.386 | ||||
| Yes | 788 | 341 (43.3) | 447 (56.7) | 1.09 (0.89, 1.34) | |
| No | 776 | 319 (41.1) | 457 (58.9) | 1 | |
| Fatal overdose | 0.107 | ||||
| Yes | 29 | 8 (27.6) | 21 (72.4) | 0.52 (0.23, 1.17) | |
| No | 1535 | 652 (42.5) | 883 (57.5) | 1 | |
| Non-fatal overdose | 0.011 | ||||
| Yes | 97 | 29 (29.9) | 68 (70.1) | 0.57 (0.36, 0.88) | |
| No | 1467 | 631 (43.0) | 836 (57.0) | 1 | |
| Resumption of community methadone | <0.001 | ||||
| Yes | 849 | 454 (53.5) | 395 (46.5) | 2.84 (2.30, 3.51) | |
| No | 715 | 206 (28.8) | 509 (71.2) | 1 | |
Odds ratios are for unadjusted logistic regression
Numbers may not sum to total due to missing data.
P-value for χ2 test, except for race/ethnicity which required the Fisher-Freeman-Halton Exact Test due to small sample size for Other category.
After release, men in the sample were tracked for an average of 496.9 days, with the observation time ranging from 5 days to 1,908 days (median = 345.5, IQR 637). Observation time did not differ significantly by receipt of methadone while incarcerated (484.8 vs. 513.4 days of follow-up; p = 0.216). In the full sample, 849 men (54.3%) resumed treatment with methadone after being released from jail prior to reincarceration.
3.1. Resumption of Methadone after Release from Custody
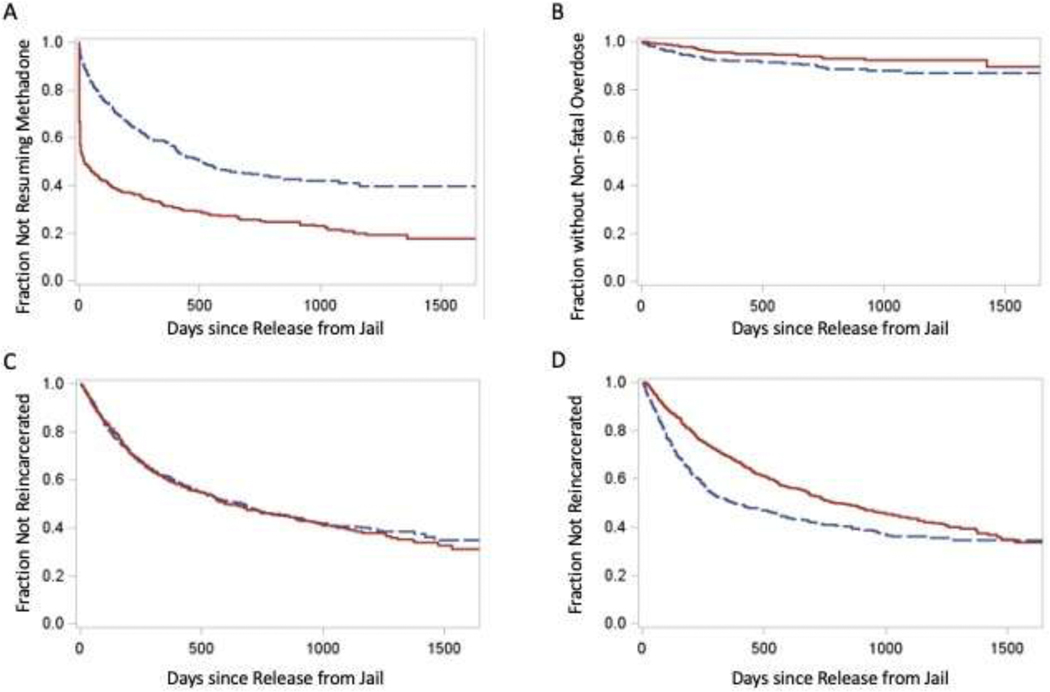
The men who received methadone in jail had higher odds of resuming methadone treatment in the community (OR: 2.84; 95% CI: 2.30, 3.51; p <0.001). This difference remained significant after adjusting for location of incarceration, reincarceration, and non-fatal overdose (aOR: 3.00, 95% CI: 2.39, 3.78, p <0.001). (Table 2B) Among those in the sample who resumed methadone, the mean number of days from release to resumption was 88.1 days (SD= 214.7, range = 0.5–1,360) for those who had received methadone while incarcerated and 202.3 days (SD = 236.6, range = 0.5–1,650) for those who had not. For the association of the continuation of methadone treatment during incarceration with time to resumption of methadone treatment after release, we found that the proportional hazards assumption was violated (maximum absolute value = 6.26, p<0.0001). A more detailed investigation revealed that the difference attenuated over time. Therefore, we explored this association using an accelerated failure time model, with the exponential distribution yielding the most conservative effect size estimate. The time to community resumption of methadone after release among those who continued methadone treatment during incarceration was about a third (0.373; 95% CI: 0.325; 0.429, p<0.001) of what it was for those who did not continue receiving methadone during incarceration. Prompt resumption of treatment after release appears to account for much of the difference (Figure 2A).
Table 2.
Multivariable logistic regression model of factors associated with resumption of methadone and reincarceration after release from custody (N = 1415)
| Variable | Adjusted OR (95% CI) |
|---|---|
|
| |
| A. Resumption of Methadone | |
| Methadone while incarcerated | |
| No | ref |
| Yes | 3.00 (2.39, 3.78) |
| Race | |
| White | ref |
| Black | 0.72 (0.49, 1.04) |
| Hispanic | 0.91 (0.69, 1.18) |
| Other (incl. Asian) | 0.73 (0.20, 2.71) |
| Age | 1.00 (0.99, 1.01) |
| Location | |
| Bridgeport | ref |
| New Haven | 0.95 (0.76, 1.20) |
| Reincarcerated | |
| No | ref |
| Yes | 0.79 (0.64, 0.99) |
| Experienced non-fatal overdose | |
| No | ref |
| Yes | 1.28 (0.83, 1.99) |
| B. Reincarceration | |
| Methadon while incarcerated | |
| No | ref |
| Yes | 1.37 (1.10, 1.72) |
| Race | |
| White | ref |
| Black | 1.64 (1.13, 2.37) |
| Hispanic | 1.05 (0.81, 1.36) |
| Other (incl. Asian) | 0.98 (0.28, 3.43) |
| Age | 0.98 (0.97, 0.99) |
| Location | |
| Bridgeport | ref |
| New Haven | 1.25 (1.00, 1.55) |
| Resumed MOUD | |
| No | ref |
| Yes | 0.79 (0.64, 0.99) |
| Experienced nonfatal overdose | |
| No | ref |
| Yes | 1.17 (0.76, 1.79) |
Figure 2.
Time to event for the major outcomes following release from jail for men whose methadone treatment was continued or terminated while in jail. (A) Time to resumption of methadone treatment in the community (B) Time to non-fatal overdose. (C) Time to reincarceration. (D) For those who did or did not resume community methadone, time to reincarceration. For panels A, B, C, solid line is for individuals who continued treatment and dashed line is for those whose treatment was terminated. For panel D, solid line is for individuals who resumed treatment after release from jail and dashed line is for those who did not.
3.2. Overdose Events after Release from Custody
Non-fatal opioid overdose events in the DPH database were matched to 97 men in the DoC dataset. Those who had received methadone experienced significantly lower odds of experiencing a non-fatal overdose post-release (OR: 0.57; 95% CI: 0.36; 0.88, p = 0.012). (Table 2). Participants were followed on average for 265.0 days (range: 0.5, 1,638 days). The mean number of days from the date of release to the first non-fatal overdose was 404.4 days (SD = 443.4, range = 11 – 1638) for those who had received methadone during incarceration and 201.1 days (SD = 244.5, range = 0.5, 1071) for those who had not (Figure 2B). Individuals who continued methadone while incarcerated had a longer time to non-fatal overdose post-incarceration compared to those who did not continue methadone during incarceration (HR = 1.78, 95% CI:1.15, 2.75).
There were 29 fatal overdoses identified with no significant difference in the number of fatal overdoses between those who received methadone in jail versus those who did not (OR = 0.52, 95% CI: 0.23, 1.17, p = 0.114). Cox proportional hazards regression did not reveal a significant difference in time to fatal overdose when comparing those who continued methadone treatment during incarceration to those who did not (HR = 1.94, 95% CI: 0.86, 4.38). Resuming methadone in the community was protective against fatal opioid overdoses. Seven men who resumed community methadone treatment experienced a fatal overdose; the remaining 22 overdose deaths occurred among men who had not resumed methadone treatment in the community (OR = 0.26, 95% CI: 0.11, 0.62, p = 0.002).
3.3. Reincarceration
Half the men from the cohort were reincarcerated (n = 788, 50.4%). Among those reincarcerated, the average time to reincarceration was 287.9 days (range: 5 – 1532). The proportion reincarcerated did not differ by receipt of methadone while incarcerated (OR = 1.09; 95% CI: 0.89, 1.34; p = 0.386). (Table 1) Among those who were reincarcerated, the mean number of days from release to reincarceration was 287.9 (range: 5, 1,532), with no significant difference (p = 0.083) between those who had received methadone while initially in jail (308.8 days; SD = 312.8) and those who had not (271.9 days; SD = 312.8) (Figure 2C). After adjusting for age, race/ethnicity, location of incarceration, resumption of methadone in the community, and non-fatal overdose, those who had received methadone (aOR = 1.37; 95% CI: 1.09, 1.71; p = 0.031) and Black men compared to White men (aOR:1.64; 95% CI: 1.14, 2.38; p = 0.008) were significantly more likely to be reincarcerated. (Table 2B) In terms of time to reincarceration, there was no significant difference based on receipt of methadone treatment during incarceration (HR = 0.98, 95% CI: 0.85, 1.13).
On the other hand, those resuming methadone after release were less likely to be incarcerated (aOR = 0.79; 95% CI: 0.64, 0.99; p = 0.038). In examining the relationship between time to reincarceration and resumption of methadone treatment in the community, we found that the proportional hazards assumption was violated (maximum absolute value = 3.00, p<0.0001). A more detailed investigation revealed that the difference attenuated over time (Figure 2D). As such, we explored this association using an accelerated failure time model, with the exponential distribution yielding the most conservative effect size estimate. The time to reincarceration among those who resumed methadone treatment in the community was 59% longer (1.590; 95% CI: 1.074, 1.828) compared to those who did not.
4. Discussion
We were able to evaluate the impact of continuing methadone treatment on multiple key outcomes by working with state agencies to obtain and match data across multiple administrative databases, which affords a more comprehensive examination of outcomes compared to prior studies. The proportion of men resuming community-based methadone treatment was higher for those who received methadone while in jail, and they resumed treatment more promptly after their release. Rates of non-fatal overdose were significantly reduced and time to non-fatal overdose was significantly longer for those who received methadone treatment while in jail. These findings add to the growing evidence that continuing MOUD, whether methadone or buprenorphine, during periods of incarceration is a successful strategy in reducing opioid-involved morbidity (McKenzie et al., 2012; Rich et al., 2015).
We did not detect a significant difference in opioid-involved overdose deaths by receipt of methadone treatment during incarceration. However, those who resumed methadone in the community after release experienced lower odds of overdose death compared to those who did not. This is consistent with prior studies showing that resumption of community-based treatment following release was protective against opioid-involved fatalities (Dolan et al., 2006; Marzo et al., 2009). One study from Rhode Island found a significant decline in deaths when MOUD was offered throughout the correctional system. Studies of prisons in England found reductions in fatal overdose in the first month after release, when former inmates have the highest risk of death (Farrell and Marsden, 2008; Marsden et al., 2017).
We found no significant difference in reincarceration between the two groups. However, resumption of methadone in the community post-release seemed to be protective against reincarceration. Given the relationship between continued treatment with methadone during incarceration and subsequent resumption of methadone treatment, this suggests that receipt of methadone treatment is indirectly protective against reincarceration through a mediated pathway, as it is with fatal overdose. Prior studies are contradictory on the impact of methadone during incarceration on subsequent reincarceration (Brinkley-Rubinstein et al., 2018; Kinlock et al., 2008; McMillan et al., 2008; Westerberg et al., 2016). All of these studies had far shorter follow-up periods and most did not compare groups in which methadone treatment was the base condition prior to incarceration. Our findings are in line with an earlier study that found that one third of prisoners with OUD are reincarcerated within three years after release (Langan and Levin, 2002). We are continuing to explore how the types of arrest charges (i.e., newly committed crimes, old crimes with outstanding warrants, technical violations of community supervision) led to reincarceration, which may clarify our findings.
A strength of our study is that all men followed were being treated with methadone when they entered jail. This allowed direct comparison between those who continued treatment while incarcerated and those who did not because differences between the treated and untreated groups can be attributed to programmatic issues, such as the capacity of the pilot programs and the different race/ethnicity profiles of the populations remanded to jail in the two cities. The New Haven Correctional Center had a smaller number of men initially eligible to continue treatment. Hence, a higher proportion of participants received treatment in New Haven, with the racial/ethnic differences in our sample skewed to reflect the incarcerated population in New Haven.
There are several limitations to this analysis. The data provided by various agencies were not originally collected for research purposes. This limited consistent matching to names and dates of birth, introducing the potential for loss of matches. This problem was especially acute for the non-fatal overdoses. The DPH system failed to capture an estimated 5–7% of non-fatal overdose events when the data collection system was being upgraded to a newer version in 2017 and the existing data may contain missing or incorrect information entered hurriedly at the overdose scene. There are also many instances of non-fatal overdose that occur without an emergency medical response and that would not be captured in the DPH dataset. Because it is likely that the dataset does not include all non-fatal overdoses that occurred during our study period, the number of events we report should be considered as the lower bound experienced by the study population. The number of individuals resuming methadone in the community may also be an undercount if OTP providers failed to report treatment to DMHAS. Additionally, those released towards the end of the study period experienced shorter observation time compared to those released earlier in the study period.
The absence of randomization into treatment while in custody creates a potential limitation related to unequal selection into the treated and untreated groups. The possibility exists that unmeasured characteristics could be confounding our results. The administrative databases contain far less information on individuals than would a standard research database, so there is little opportunity to identify and adjust for potential confounders. However, the main factor in assigning the men to one or the other of the two groups was the treatment capacity of the programs, which would reduce the likelihood that individual characteristics of the men influenced their group assignment.
Another limitation is that we considered reincarceration as a censoring event. It is possible that subsequent to that reincarceration an individual might continue on methadone if he had resumed it following his first release. It is also possible that after release from reincarceration an individual might experience an overdose. But determining these downstream outcomes would require a much more complex analytical strategy that would yield only a few cases that would be unlikely to significantly change the results obtained with the simpler strategy.
5. Conclusion
The implications of this study for policy makers, corrections administrators, and drug treatment providers are that there are significant health and safety benefits subsequent to release from custody when agonist-based MOUD is provided in correctional settings.
Highlights.
A pilot program provided methadone to jailed individual who had been receiving methadone to treat their opioid use disorder, allowing 42% to continue treatment throughout their period of incarceration.
Treatment while in jail significantly increased the likelihood and rate of resumption of treatment post-release and
Treatment while in jail significantly decreased the likelihood of non-fatal opioid overdoses.
Treatment while in jail had no discernable effect on the likelihood or rate of reincarceration and resulted in a modest but non-significant decrease in fatal overdoses.
Acknowledgements
The authors wish to thank Dr. Kathleen Maurer and Colleen Gallagher at the Connecticut Department of Correction for their efforts in finding resources to conduct the evaluation, supplying a unified dataset and resolving inconsistencies, and securing interagency memoranda of understanding to gain access to the relevant data to assess outcomes. We also wish to thank Nancy Navaretta and Hsiuju Lin at the Connecticut Department of Mental Health and Addiction Services for matching data on resumption of methadone treatment following release and Ann Kloter at Connecticut Department of Public Health for creating the non-fatal overdose dataset.
Funding
This project is supported by an. evaluation grant from the Centers for Disease Control and Prevention (CDC), Cooperative Agreement number CDC-RFA-OT18-1804: Technical Assistance for Response to Public Health or Healthcare Crises. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention (CDC) or the Department of Health and Human Services or the CT state agencies that supplied the data.
Robert Heimer has received funding from the Gilead Corporation for a research study to develop an intervention to prevent hepatitis C virus reinfection among people successfully treated.
Footnotes
Author Disclosures
None of the other authors have anything to disclose.
Conflicts of Interest
None of the authors have any conflicts to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Binswanger IA, Blatchford PJ, Mueller S, Stern MF, 2013. Mortality after prison release: Opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Annals of Internal Medicine 159, 592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley-Rubinstein L, McKenzie M, Macmadu A, Larney S, Zaller N, Dauria S, Rich J, 2018. A randomized, open label trial of methadone continuation versus forced withdrawal in a combined US prison and jail: Findings at 12 months post-release. Drug and Alcohol Dependence 184, 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson J, Stroop J, Zimmer S, Berzofsky M, 2017. Drug Use, Dependence, and Abuse Among State Prisoners and Jail Inmates, 2007–2009. US Department of Justice, Washington, DC. [Google Scholar]
- Bukten A, Stavseth MR, Skurtveit S, Tverdal A, Strang J, Clausen T, 2017. High risk of overdose death following release from prison: variations in mortality during a 15-year observation period. Addiction 112, 1432–1439. [DOI] [PubMed] [Google Scholar]
- Bureau of Justice Statistics (BJS), 2017. Key Statistic: Total correctional population. US Department of Justice, Washington, DC. [Google Scholar]
- Chang Z, Lichtenstein P, Larsson H, Fazel S, 2015. Substance use disorders, psychiatric disorders, and mortality after release from prison: a nationwide longitudinal cohort study. Lancet Psychiatry 2, 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Medication-Assisted Treatment for Opioid Use Disorder, 2019. Medications for Opioid Use Disorder Save Lives. National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- de Andrade D, Ritchie J, Rowlands M, Mann E, Hides L, 2018. Substance use and recidivism outcomes for prison-based drug and alcohol interventions Epidemiologic Reviews 40, 121–133. [DOI] [PubMed] [Google Scholar]
- Dolan KA, Shearer J, White B, Zhou J, Kaldor J, Wodak AD, 2006. Four year follow-up of imprisoned male heroin users and methadone treatment: mortality, re incarceration and hepatitis C infection. Addiction 100, 820–828. [DOI] [PubMed] [Google Scholar]
- Farrell M, Marsden J, 2008. Acute risk of drug-related death among newly released prisoners in England and Wales. Addiction 103, 251–255. [DOI] [PubMed] [Google Scholar]
- Friedmann PD, Hoskinson R, Gordon M, Schwartz R, Kinlock T, Knight K, Flynn PM, Welsh WN, Stein LAR, Sacks S, O’Connell DJ, Knudsen HK, Shafer MS, Hall E, Frisman LK, 2012. Medication-assisted treatment in criminal justice agencies affiliated with the criminal justice-drug abuse treatment studies (CJ-DATS): availability, barriers & intentions. Substance Abuse 33, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MS, Kinlock TW, Schwartz RP, O’Grady KE, 2008. A randomized clinical trial of methadone maintenance for prisoners: findings at 6 months post-release. Addiction 103, 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TC, Clarke J, Brinkley-Rubinstein L, et al. , 2018. Postincarceration fatal overdoses after implementing medications for addiction treatment in a statewide correctional system. JAMA Psychiatry 75, 405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer R, Catania H, Newman RG, Zambrano J, Brunet A, Ortiz AM, 2006. Methadone maintenance in prison: Evaluation of a pilot program in Puerto Rico. Drug and Alcohol Dependence 83, 122–129. [DOI] [PubMed] [Google Scholar]
- Jones CM, Logan J, Gladden M, Bohm MK, 2015. Vital Signs: Demographic and Substance Use Trends Among Heroin Users — United States, 2002–2013. Morbidity and Mortality Weekly Report (MMWR) 64, 719–725. [PMC free article] [PubMed] [Google Scholar]
- Kinlock TW, Gordon MS, Schwartz RP, O’Grady KE, 2008. A study of methadone maintenance for male prisoners. Criminal Justice and Behavior 35, 34–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan PA, Levin DJ, 2002. Recidivism of Prisoners Released in 1994. Office of Justice Programs, US Department of Justice, Washington, DC. [Google Scholar]
- Magura S, Lee JD, Hershberger J, Joseph H, Marsch L, Shropshire C, Rosenblum A, 2009. Buprenorphine and methadone maintenance in jail and post-release: A randomized clinical trial. Drug and Alcohol Dependence 99, 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magura S, Rosenblum A, C L, Joseph H, 1993. The effectiveness of in-jail methadone maintenance. Journal of Drug Issues 23, 75–99. [Google Scholar]
- Marsden J, Stillwell G, Jones H, Cooper A, Eastwood B, Farrell M, Lowden T, Maddalena N, Metcalfe C, Shaw J, Hickman M, 2017. Does exposure to opioid substitution treatment in prison reduce the risk of death after release? A national prospective observational study in England. Addiction 112, 1408–1418. [DOI] [PubMed] [Google Scholar]
- Marzo JN, Roti M, Meroueh F, Varastet M, Hunault C, Obradovic I, Zin A, 2009. Maintenance therapy and 3-year outcome of opioid-dependent prisoners: a prospective study in France (2003–06). Addiction 104, 1233–1240. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M, 2014. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Reviews 10.1002/14651858.CD002207.pub4. [DOI] [Google Scholar]
- McKenzie M, Nunn A, Zaller ND, Bazazi AR, Rich JD, 2009. Overcoming obstacles to implementing methadone maintenance therapy for prisoners: Implications for policy and practice. Journal of Opioid Management 5, 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie M, Zaller N, Dickman SL, Green TC, Parikh A, Friedmann PD, Rich JD, 2012. A randomized trial of methadone initiation prior to release from incarceration. Substance Abuse 33, 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan GP, Lapham S, Lackey M, 2008. The effect of a jail methadone maintenance therapy (MMT) program on inmate recidivism. Addiction 103, 2017–2023. [DOI] [PubMed] [Google Scholar]
- Moore KE, Oberleitner L, Smith KMZ, Maurer K, McKee SA, 2018. Feasibility and effectiveness of continuing methadone maintenance treatment during incarceration compared with forced withdrawal. . Journal of Addiction Medicine 12, 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich JD, McKenzie M, Larney S, Wong JB, Tran L, Clarke J, Noska A, Reddy M, Zaller N, 2015. Methadone continuation versus forced withdrawal on incarceration in a combined US prison and jail: a randomised, open-label trial. Lancet 386, 350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DL, Schoenbach VJ, Wohl DA, 2007. All-cause and cause-specific mortality among men released from state prison, 1980–2005. American Journal of Public Health 98, 2278–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, O’Grady KE, Kelly SM, Gryczynski J, Mitchell SG, Schwartz RP, 2016. Pharmacotherapy for opioid dependence in jails and prisons: research review update and future directions. Substance Abuse and Rehabilitation 7, 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Sentencing Project, 2020. Criminal Justice Facts. https://www.sentencingproject.org/criminal-justice-facts/. Last accessed, October 7, 2020).
- Westerberg VS, McCrady BS, Owens M, Guerin P, 2016. Community-based methadone maintenance in a large detention center is associated with decreases in inmate recidivism. Journal of Substance Abuse Treatment 70, 1–6. [DOI] [PubMed] [Google Scholar]
- Winkelman TNA, Chang VW, Binswanger IA, 2018. Health, polysubstance use, and criminal justice involvement among adults with varying levels of opioid use. JAMA Network Open 1(3). [DOI] [PMC free article] [PubMed] [Google Scholar]