Abstract
Mechanical forces transmitted at the junction between two neighboring cells and at the junction between cells and the extracellular matrix are critical for regulating many processes ranging from development to immunology. Therefore, developing the tools to study these forces at the molecular scale is critical. Our group developed a suite of molecular tension sensors to quantify and visualize the forces generated by cells and transmitted to specific ligands. The most sensitive class of molecular tension sensors are comprised of nucleic acid stem-loop hairpins. These sensors use fluorophore-quencher pairs to report on the mechanical extension and unfolding of DNA hairpins under force. One challenge with DNA hairpin tension sensors is that they are reversible with rapid hairpin refolding upon termination of the tension and thus transient forces are difficult to record. In this article, we describe the protocols for preparing DNA tension sensors that can be “locked” and prevented from refolding to enable “storing” of mechanical information. This allows for the recording of highly transient piconewton forces, which can be subsequently “erased” by the addition of complementary nucleic acids that remove the lock. This ability to toggle between real-time tension mapping and mechanical information storing reveals weak, short-lived, and less abundant forces, that are commonly employed by T cells as part of their immune functions.
Keywords: DNA tension probes, mechanobiology, receptor force
SUMMARY:
This paper describes a detailed protocol for using DNA-based tension probes to image the receptor forces applied by immune cells. This approach can map receptor forces >4.7pN in real-time and can integrate forces over time.
INTRODUCTION:
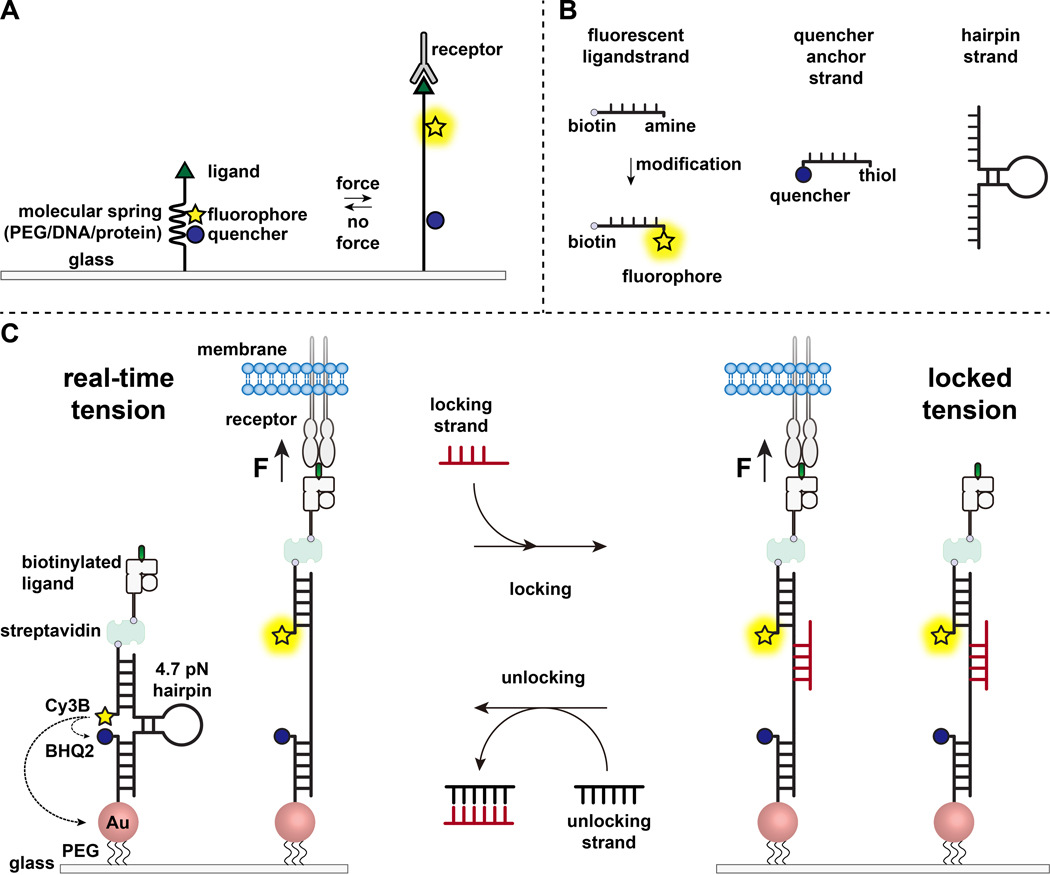
Immune cells defend against pathogens and cancer cells by continuously crawling and scanning the surfaces of target cells for antigens, studding their surface1,2. Antigen recognition is initiated upon binding between the T cell receptor (TCR) and the peptide-major histocompatibility complex MHC (pMHC) complex expressed on the surface of target cells. Because TCR-pMHC recognition occurs at the junction between two mobile cells, it has long been suspected of experiencing mechanical forces. Moreover, this led to the mechanosensor model of TCR activation, which suggests that TCR forces contribute to its function3,4. To understand when, where, and how mechanical forces contribute to T cell function, it is imperative to develop tools to visualize the molecular forces transmitted by T cells. Traditionally, methods such as traction force microscopy (TFM) and micropillar arrays are used to investigate cellular forces5,6. However, the force sensitivity of TFM and micropillar arrays is at the nanonewton (nN) scale and thus is often insufficient to study the molecular piconewton (pN) forces transmitted by cell receptors7. To improve the force and spatial resolution for detection, our lab pioneered the development of molecular tension probes, which were initially synthesized using polyethylene glycol (PEG) polymers7. Molecular tension probes are comprised of an extendible molecular “spring” (PEG, protein, DNA) flanked by a fluorophore and quencher and are anchored on a surface. Forces applied to the terminus of the probe lead to its extension, separating the fluorophore and quencher, and thus generating a strong fluorescence signal (Scheme 1A)8–10.
Scheme 1.
(A) General design of real-time molecular tension probe. (B) DNA strands for the tension probe construct. (C) Schematic of the engineered DNA-based tension probes and their toggling between real-time state and locked state.
Over the past decade we have developed a library of different classes of molecular tension probes with spring elements made from nucleic acids11, proteins10, and polymers8. Among these, the DNA-based tension probes provide the highest signal to noise ratio and the greatest force sensitivity, which is easily tuned from a few pN up to ~20 pN11. We have used these real-time DNA tension probes to study the molecular forces generated by many diverse cell types, including fibroblasts, cancer cells, platelets, and immune cells11–13. This manuscript will describe protocols to synthesize and assemble DNA tension probes on a surface to map molecular receptor forces with pN force resolution using a conventional fluorescence microscope. While the current procedure includes chemical modifications to the nucleic acid to introduce the fluorescent reporter (Scheme 1B), it is important to note that many of the modification and purification steps can be outsourced to custom DNA synthesis companies. Therefore, DNA tension probes technology is facile, and accessible to the broader cell biology and mechanobiology communities.
Briefly, to assemble DNA tension sensors, a DNA hairpin is hybridized to a fluorescent ligand strand on one arm and a quencher anchor strand on the other arm and then immobilized on a glass substrate (Scheme 1C, real-time tension). In the absence of mechanical force, the hairpin is closed, and thus the fluorescence is quenched. However, when the applied mechanical force is greater than the F1/2 (the force at equilibrium that leads to a 50% probability of unfolding), the hairpin mechanically melts, and a fluorescent signal is generated.
Building on the real-time DNA tension sensor, we also describe protocols to map accumulated forces, which is particularly useful for studying interactions between receptors on immune cells and their natural ligand. This is because immune receptors often display short-lived bonds3,14. Accumulated forces are imaged using a “locking” strand that preferentially binds to open DNA hairpins and allows for storage of the fluorescence signals associated with mechanical pulling events (Scheme 1C, locked tension). The locking strand is designed to bind a cryptic binding site that is exposed upon mechanically induced melting of the hairpin and lock the hairpin in the open state by blocking hairpin refolding, thus storing the tension signal and generating an accumulated tension map. Moreover, the locking strand is designed with an eight-nucleotide toehold, which enables a toehold-mediated strand displacement reaction with its full complement, the “unlocking” strand. With the addition of the unlocking strand, the bound locking strand is stripped off the hairpin construct, erasing the stored tension signal and resetting the hairpin back to the real-time state.
The main protocol consists of four major sections: 1. oligonucleotide preparation, 2. surface preparation, 3. imaging, and 4. data analysis. This protocol has been successfully demonstrated by our lab and others in naïve and activated OT-1 CD8+ T cells, OT-II CD4+ cells, as well as hybridomas, and can be applied to interrogate different immune cell receptors including T cell receptor, programmed cell death receptor (PD1), and lymphocyte function-associated antigen 1 (LFA-1) forces. OT-1 CD8+ naïve T cells are used as an example cell line in this paper.
PROTOCOL:
Oligonucleotide preparation
Note that the DNA hairpin tension probes consist of 3 strands: a hairpin strand, a quencher anchor strand, and a fluorescent ligand strand (Scheme 1B). The hairpin strand is unmodified and can be directly custom synthesized. The anchor strand has a thiol anchoring group and a quencher BHQ2. The ligand strand also has a modification at each terminus, 5’ amine and 3’ biotin, to conjugate with the fluorophore and to present the biotinylated ligand. We use Cy3B dye due to its high brightness and photostability, but this dye is not generally offered commercially and requires in-house conjugation. Accordingly, the following section describes the preparation of a Cy3B conjugated ligand strand as well as an Atto647N locking strand. For end users that do not have access to facilities or resources for nucleic acid modification, modified nucleic acids can instead be purchased from custom DNA synthesis vendors that offer bright and photostable dyes, such as the Alexa and Atto family of dyes.
1.1 Dissolve the ligand strand DNA in water (18.2 MΩ resistivity, used throughout the whole protocol). Vortex and spin down the solution with a tabletop centrifuge. Tune the volume of water such that the final concentration is 1 mM. Validate the concentration by using a nanodrop spectrophotometer to measure the absorbance at 260 nm and determine the final concentration based on the extinction coefficient of the oligonucleotide.
1.2 Note the ligand strand is custom synthesized with a 5’ amine and a 3’ biotin group. First, the amine group in the ligand strand is conjugated with Cy3B NHS ester. Prepare 10× PBS and 1 M NaHCO3 solutions. Mix 10 μL of the 1 mM amine ligand strand solution (10 nmol) with 10 μL 10× PBS, 10 μL 1 M NaHCO3, and 60 μL H2O. Dissolve 50 μg Cy3B NHS ester in 10 μL DMSO immediately before use and add to the mixture for a total reaction volume of 100 μL. Note that the Cy3B NHS ester should be added last. Allow to react at room temperature for 1 h or 4 ºC overnight.
1.3 Prepare the Atto647N locking strand by conjugating the amine locking strand with Atto647N NHS ester. Prepare 10× PBS and 1 M NaHCO3 solution. Mix 10 μL of the 1 mM amine locking strand solution (10 nmol) with 10 μL 10× PBS, 10 μL 1 M NaHCO3, and 60 μL H2O. Dissolve 50 μg Atto647N NHS ester in 10 μL DMSO immediately before use and add to the mixture for a total reaction volume of 100 μL. Note that the Atto647N NHS ester should be added last. Allow to react at room temperature for 1 h or 4 ºC overnight.
1.4 After the reactions, remove by-products, excess dye, and salts by P2 desalting gel filtration. P2 gel should be hydrated at least 4 h before use with H2O. Dilute the reaction mixture with H2O to a total volume of 300 μL, which is appropriate for the subsequent HPLC purification step. Add 650 μL of hydrated P2 gel to a centrifugal device and spin down at 18000× g for 1 min. Remove the liquid at the bottom of the device and add the reaction mixture to the column containing P2 gel, and spin down at 18000× g for 1 min. Collect the reaction mixture at the bottom of the device.
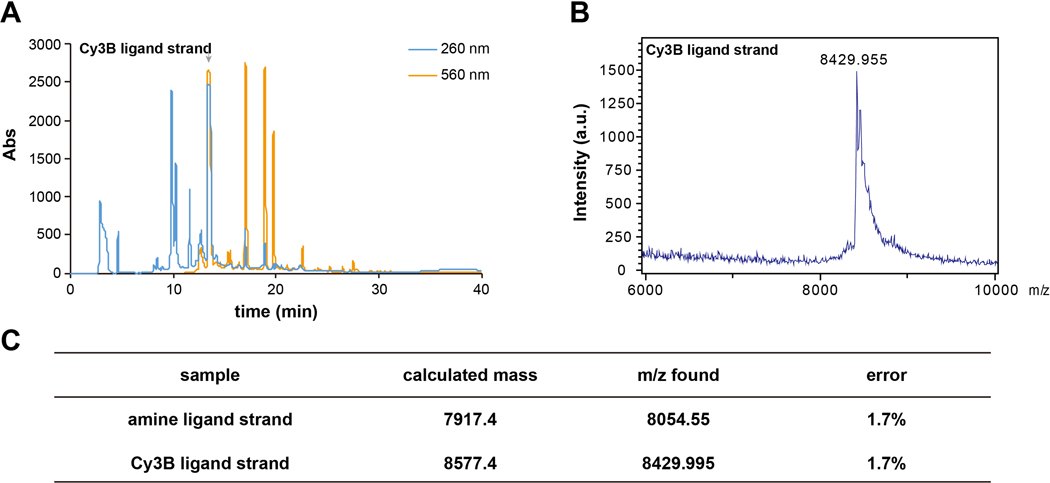
1.5 Purify the desalted reaction mixture with HPLC. We recommend using a C18 column designated for oligonucleotide purification, with solvent A: 0.1 M TEAA in H2O and B: ACN as the mobile phase for a linear gradient elution 10–100% B over 50 min at a flow rate of 0.5 mL/min. Inject the desalted reaction mixture in reverse-phase HPLC with a 500 μL injection loop for purification. Collect the product that has an absorbance peak for the DNA (260 nm) and an absorbance peak for the fluorophore (560 nm for Cy3B and 647 nm for Atto647N) and dry them in a vacuum centrifugal concentrator overnight. An example HPLC chromatogram can be found in Figure 1A.
1.6 Reconstitute the dried oligo-dye product in 100 μL water. Determine the concentration of the Cy3B ligand strand and Atto647N locking strand with the nanodrop spectrophotometer. The dye labeling ratio should be close to 1:1. Correct for the 260 nm absorbance of the dye if needed when determining the oligonucleotide concentration.
1.7 Validate the purified product with MALDI-TOF-MS using 3-HPA as the substrate in 50% ACN/H2O with 0.1% TFA and 5 mg/mL ammonium citrate. We recommend using 0.5 μL of the product at 1–5 μM for MALDI-TOF-MS sample preparation. An example mass spectrum can be found in Figure 1B.
1.8 Aliquot the oligonucleotide-dye product and freeze at −20 ºC for storage. Note that repeated freeze-thaw cycles are not problematic for oligonucleotides.
Figure 1.
Examples of (A) HPLC chromatogram of Cy3B ligand strand purification; (B) MALDI-TOF-MS spectra of the product; (C) Table of the calculated mass and found m/z peaks of the starting material and the labeled oligonucleotide.
2. Surface preparation
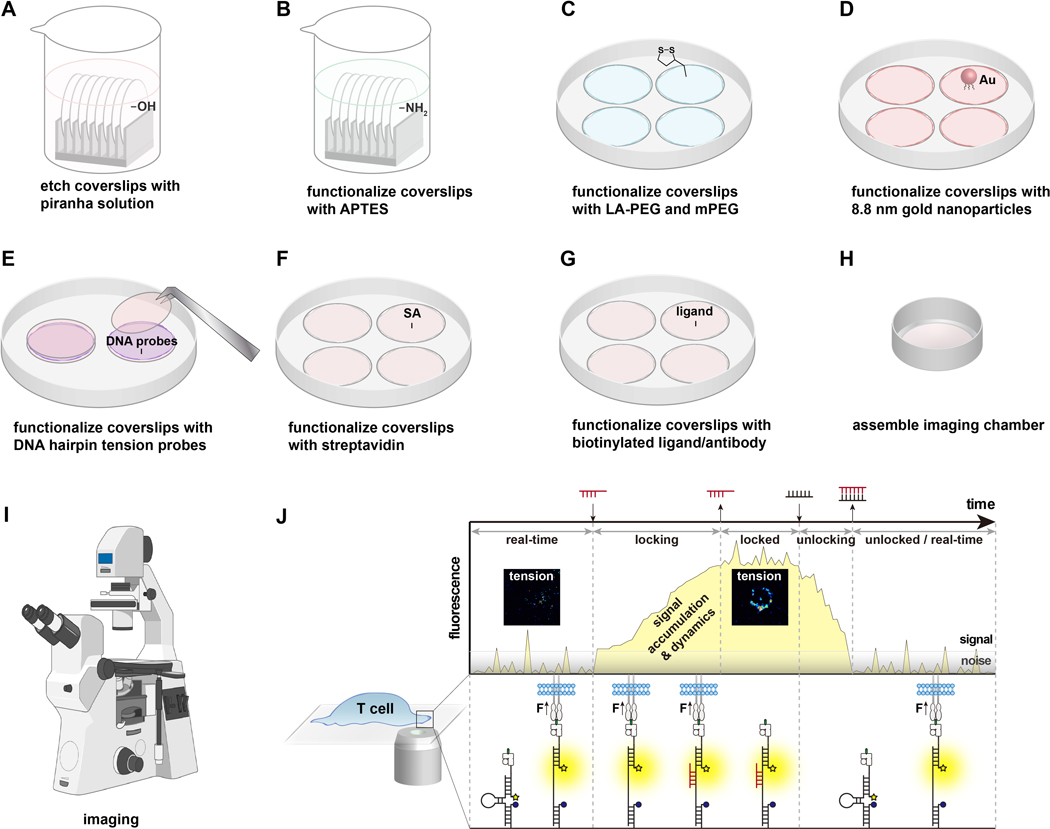
The preparation of DNA hairpin tension probe substrates takes two days. The DNA hairpin tension probe will be functionalized onto glass coverslips.
Day 1
2.1 Place the 25 mm coverslips on a Teflon rack in a 50 mL beaker. Each Teflon rack can hold up to 8 slides. Rinse the coverslips by submerging in water three times.
2.2 Add 40 mL of a 1:1 ratio (v:v) solution of ethanol mixed with water to the beaker containing the rack and coverslips, and seal the beaker using parafilm. Sonicate the beaker for 15 min in an ultrasonics cleaner (operating frequency 35 KHz) to clean the coverslips. After sonication, discard the liquid and rinse the beaker with the rack and coverslips in it with water at least 6 times to remove any remaining organic solvent.
-
2.3 Prepare fresh piranha solution by mixing sulfuric acid and hydrogen peroxide at a ratio of 3:1. To generate 40 mL of piranha solution, add 30 mL of sulfuric acid to a clean 50 mL beaker first and then slowly add 10 mL of H2O2. The piranha solution will rapidly heat and bubble upon addition of the H2O2. Gently mix the piranha using the end of a glass pipette. Next, transfer the rack that holds the coverslips to the beaker containing gently mixed piranha solution for etching (Figure 2A). Allow the piranha solution to hydroxylate and clean the coverslips for 30 min at room temperature. After piranha etching, transfer the rack with steel or Teflon tweezers to a clean 50 mL beaker with water and rinse again with water at least 6 times.
CAUTION: LARGE AMOUNTS OF ORGANIC SUBSTANCES COULD REACT VIGOROUSLY WITH PIRANHA SOLUTION AND MAY CAUSE EXPLOSION. BE CAREFUL AND ALWAYS WORK WITH PIRANHA SOLUTION IN A FUME HOOD. MAKE SURE TO WEAR A LABCOAT, GLOVES, AND SAFETY GOGGLES. NEVER STORE FRESH PIRANHA SOLUTION IN A SEALED CONTAINER.
THE HYDROGEN PEROXIDE TO SULFURIC ACID RATIO SHOULD BE KEPT UNDER 1:2 (v:v) AND SHOULD NEVER EXCEED 1:1. WHEN SUBMERGING THE RACK WITH SLIDES IN PIRANHA SOLUTION, PLACE THEM IN THE PIRANHA SOLUTION SLOWLY AND CAREFULLY. DO NOT DISCARD THE SOLUTION IMMEDIATELY AFTER ETCHING, AS IT IS STILL ACTIVE AND HOT. LEAVE IT IN THE BEAKER OVERNIGHT BEFORE POURING IT IN THE ACID WASTE CONTAINER.
2.4 Immerse the rack holding the coverslips in a 50 mL beaker with 40 mL ethanol to remove water. Discard the ethanol solution and repeat three times to ensure that the water has been removed. Then immerse the rack in 3% aminopropyl triethoxy silane (APTES) (v/v) in 40 mL of ethanol to react with the -OH on the coverslips for 1 h at room temperature (Figure 2B). After the reaction, rinse surfaces 6 times by submerging them into 40 mL ethanol, then dry in oven at 80 °C for 20 min. After cooling, the dried amine-modified slides can be stored at −20 °C for future use (up to 6 months).
2.5 Cover the bottom inner side of 10 cm diameter plastic petri dishes with parafilm. The parafilm prevents the coverslips from sliding inside the petri dish, and also helps keep the solution for the next steps of functionalization stay on the coverslips. Place the cooled-down amine-modified surfaces in the petri dishes. The side you wish to functionalize should be facing up. To modify the amine groups on the coverslips, add 300 μL of 0.5% w/v lipoic acid PEG NHS (LA-PEG-SC) and 2.5% w/v mPEG NHS (mPEG-SC) in 0.1 M NaHCO3 onto each surface and incubate for 1 h at room temperature (Figure 2C). (For each 25 mm surface, weigh 1.5 mg of LA-PEG-SC and 7.5 mg of mPEG-SC.) Note: dissolve the NHS reagents immediately before adding to the surfaces, as they have a short half-life (~10 min) in aqueous solution at room temperature. After the reaction, rinse surfaces three times with water.
2.6 Add 100 μL of 0.1 M NaHCO3 containing 1 mg/mL of sulfo-NHS acetate to a set of “sandwich” slides (two surfaces facing towards each other with reaction buffer in between). Allow passivation to occur for at least 30 min. To save reagent, this step could be done with 50 μL 1 mg/mL sulfo-NHS acetate. Rinse slides with water three times after passivation.
2.7 Add 0.5 mL gold nanoparticles (AuNP, 8.8 nm, tannic acid, 0.05 mg/mL) to each surface and incubate for 30 min at room temperature (Figure 2D). To save reagent, this step can be done by sandwiching two slides as well. Make sure no salts are present in the system from previous steps to avoid aggregation of gold nanoparticles. Do not leave the slides to dry after this step.
2.8 Meanwhile, 4.7 pN hairpin, Cy3B ligand strand, and BHQ2 anchor strand that form the molecular tension probes construct is pre-hybridized at a ratio of 1.1:1:1 in 1 M NaCl at 300 nM. Anneal the strands by heating the solution up to 95 °C for 5 min, then gradually cool down by decreasing the temperature to 20 °C over 30 min in a thermal cycler.
2.9 Rinse the slides with water three times after 30 min of incubation with gold nanoparticles. Add additional BHQ2 anchor strand (from 100 μM stock) to the annealed DNA solution to make the ratio between BHQ2 anchor strand and Cy3B ligand strand 10:1. At this point, the DNA solution should contain 300 nM of tension probe construct and 2.7 μM BHQ2 strand. Add 100 μL per two slides to make the “sandwich” (Figure 2E). Carefully place a wet lab tissue ball in the petri dish (away from slides) and seal the dish with parafilm to prevent the solution from drying up. Cover the dish with foil and incubate at 4 °C overnight.
Figure 2.
Functionalization of the DNA tension probe substrates and experiment procedures. Steps are described in the corresponding protocol.
DAY 2
2.10 Wash off the excess probes from coverslips with 1× PBS. Check for surface quality under an epifluorescent microscope (details in section 3.3.1).
2.11 Prepare 40 μg/mL streptavidin in 1× PBS and incubate on slides for 30 min at room temperature (Figure 2F). Usually 100 μL is sufficient for a 25 mm coverslip. Rinse with PBS three times after incubation to wash away the excess amount of streptavidin.
2.12 Prepare 40 μg/mL biotinylated antibody/ligand in 1× PBS. Add 50–100 μL per sandwich and incubate for 30 min at room temperature (Figure 2G). Rinse with PBS three times after incubation to wash away the excess amount of biotinylated antibody/ligand.
2.13 Assemble the clean imaging chambers with surfaces carefully. Surfaces can be easily cracked when tightening the chambers. Add 0.5–1 mL of Hank’s balanced salt solution (HBSS) to the imaging chambers, and they are ready for imaging with cells (Figure 2H).
Note there are alternative ways to perform certain steps, including:
In step 2.4, ethanol can be replaced by acetone.
In step 2.5, the NHS reaction can be performed at 4 °C overnight. NHS reagents have longer half-life before hydrolysis at 4 °C, which is around 4–6 hours. This will result in a three-day surface prep procedure.
3. Imaging cell receptor forces
The OT-1 transgenic mice are housed at the Division of Animal Resources Facility at Emory under the Institutional Animal Care and Use Committee.
-
3.1 OT-1 CD8+ naïve T cells
OT-1 CD8+ naïve T cells are isolated from the spleens of sacrificed mice and purified using the MACS mouse CD8+ T cell isolation kit with a MACS separator following manufacturer’s instruction. Purified OT-1 CD8+ naïve T cells are resuspended in HBSS at 2×106 cells/mL and kept on ice prior to use.
-
3.2 Plating cells
Plate around 4~10 ×104 cells onto each DNA tension probe functionalized coverslip and allow them to attach and spread for ~15 min at room temperature.
-
3.3 Fluorescence Imaging
-
3.3.1 Quality control of DNA hairpin tension probes substrates
Check the quality of DNA hairpin tension probe surface under a fluorescence microscope for quality control before adding ligands or plating cells. We recommend quantifying the number of DNA strands per gold nanoparticle and the number of gold nanoparticles per μm2 the first few times of surface preparation12. Meanwhile, use a fluorescence microscope to image and quantify the average background intensity in Cy3B channel of DNA hairpin tension probe surface from at least 5 different positions and 3 replicates. Keep the imaging acquisition conditions consistent so that this value can be used as a reliable marker of surface quality and probe density (Figure 3C).
-
3.3.2 Imaging real-time receptor forces with DNA hairpin tension probes
As cells are plated onto the DNA hairpin tension probes and start to spread, image the fluorescence signals that are generated in the Cy3B channel (Figure 2I).
-
3.3.3 Locking of tension signals with Atto647N labeled locking strand
After cells start to produce real-time tension signal on the DNA hairpin tension probe surface, acquire images in both Cy3B and Atto647N channels (TIRF microscopy gives better signal-to-noise ratio than epifluorescence). Subsequently, add Atto647N strand to the imaging chambers at a final concentration of 200 nM and allow for mechanically selective hybridization. After 10 min of incubation, quickly and gently remove the buffer containing the fluorescent Atto647N locking strand and replace with fresh Hank’s balanced salts. Image in both Cy3B and Atto647N channels again and determine the Pearson’s correlation coefficient with Fiji software15.
-
3.3.4 Locking of tension signals
At the timepoint of interest for the investigation, introduce non-fluorescent locking strand to the cells in the imaging chamber to store the tension signal. Prepare locking strand stock (100 μM in this paper) and add to the cells at a final concentration of 1 μM. Gently pipet to mix. The duration of locking can vary but we recommend using 10 min. Acquire time-lapse movies or end-point images in epifluorescence for both qualitative tension mapping and quantitative analysis as needed (Figure 2J and Figure 4).
-
3.3.5 Unlocking of tension signals
Unlocking of tension signals is not necessary if only one time point is of interest in the study. If tension measurement at multiple time points is desired, erasing of stored tension signals can be initiated by the addition of an unlocking strand. To avoid excess rinsing, a higher final concentration of unlocking strand at 2 μM is used to initiate a toehold-mediated strand displacement reaction with the locking strand for 3 min, which erases the stored signals (Figure 2J). Gently rinse the excess oligonucleotides off with HBSS. The DNA hairpin tension probe surface and the cells are ready for another round of tension storing and mapping.
-
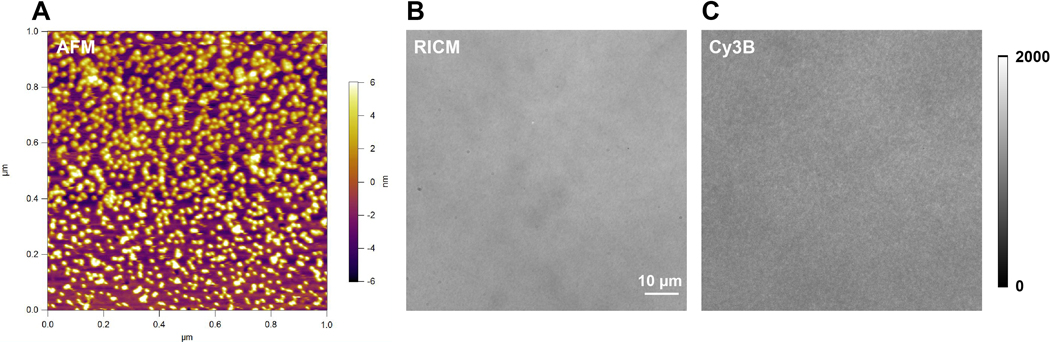
Figure 3.
Example of (A) Atomic force microscopy (AFM) characterization of the DNA tension probe surface; and raw microscopy images of a DNA tension probe surface with good quality in (B) RICM and (C) Cy3B channel.
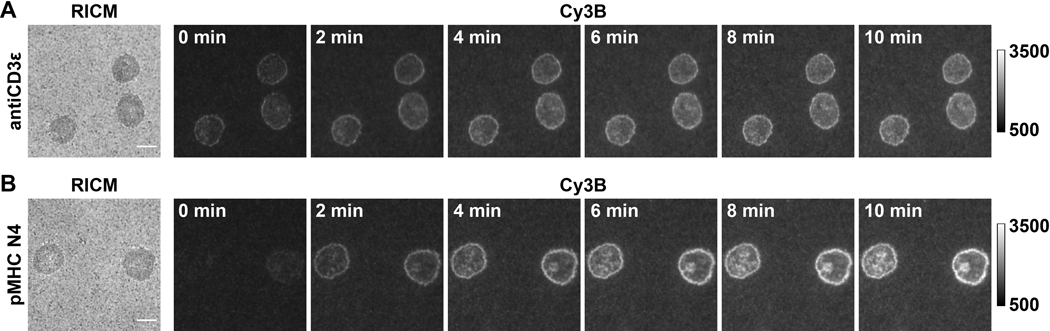
Figure 4.
Example of raw microscopy images of a successful experiment with OT-1 naïve CD8+ cells producing TCR forces against (A) antiCD3ε and (B) pMHC N4. Scale bar = 5 μm.
4. Data analysis
Image analysis is performed using Fiji software, and the quantitative analysis is performed using Excel or Prism software.
4.1 Correct any drift during image acquisition with “correct 3D drift” command in “Registration” under the “Plugins” menu.
4.2 Remove the camera background of the image with “subtract” command under the “Process” menu.
4.3 Determine the Pearson’s correlation coefficient with the “colocalization” function under “analyze” menu.
4.4 Average and subtract the fluorescence background produced by the unopened probes from three different local background regions. Draw ROIs of cells on either background-subtracted images or RICM (reflection interference contrast microscopy) images with Image J “freehand selections” tool. Measure any metric of interest of the ROIs, e.g., integrated fluorescence intensity and tension occupancy using “measure” tool under “analyze” menu (Figure 5).
4.5 Export the measurements for quantitative analysis with Excel or Prism software.
4.6 Plot the data with Excel or Prism software.
Figure. 5.
Examples of data processing and quantitative analysis.
REPRESENTATIVE RESULTS:
Here we show representative surface quality control images (Figure 3). A high-quality surface should have a clean background in RICM channel (Figure 3B), and uniform fluorescence intensity in Cy3B channel (Figure 3C). With the same imaging equipment and identical fluorescence imaging acquisition conditions, the background fluorescence intensity should be consistent and reproducible each time when conducting experiments with DNA probes. We recommend using it as a marker for surface quality control before each set of individual experiments.
In this paper we show some representative data (Figure 4) with OT-1 naïve CD8+ cells as a model cell type, which specifically recognize chicken ovalbumin. The CD3ε antibody is included as a positive control for the system and the cognate antigen pMHC N4 (SIINFEKL) is used as an example. Cells were plated on substrates that were functionalized with 4.7 pN DNA hairpin probes presenting either antiCD3ε or pMHC N4. After 30 min, cell spreading in RICM and the real-time tension in Cy3B channel were captured, and the locking strand was introduced at a final concentration of 1 μM. The time-lapse of tension locking showed that both TCR-antiCD3ε and TCR-pMHC tension signals were amplified as a result of hairpin locking and signal accumulation. Furthermore, TCR-pMHC N4 interaction showed weak force signal in real-time (0 min), likely due to its short bond lifetimes under 1 s. The locking strand recorded these short-lived mechanical pulling events, which facilitates quantitative analysis. Additionally, locking revealed the pattern of the TCR-pMHC force as it accumulates, which was quite distinct from the antiCD3ε control and resembled the “bull’s-eye” pattern of immune synapses. The quantification of the fluorescence signal is described in step 4.4. We typically use the tension occupancy (defined as the percentage of area that has tension signal over the contact area) and integrated fluorescence intensity of each cell as a metric to evaluate accumulated tension that is > 4.7 pN.
DISCUSSION:
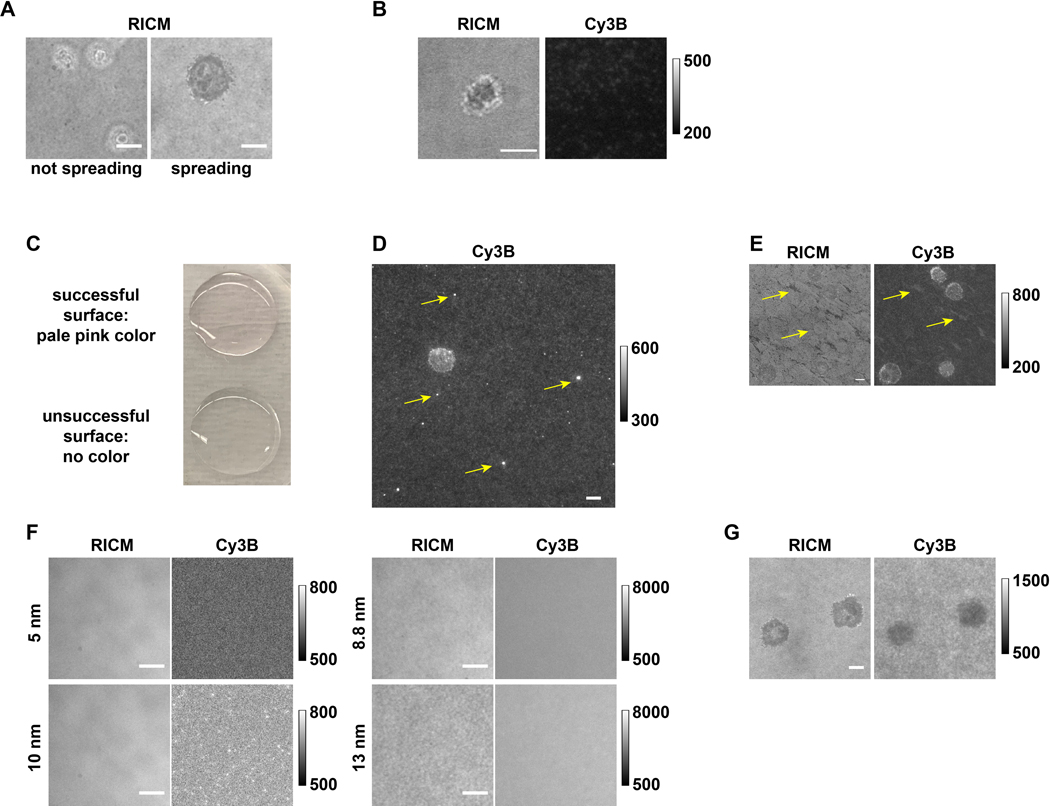
With the detailed procedures provided here, one is able to prepare DNA hairpin tension probe substrates to map and quantify the receptor tension produced by immune cells. When cells are plated onto the DNA hairpin tension probe substrate, they land, attach, and spread as the receptors sense the ligands both chemically and mechanically, the latter of which is detected by our probes. However, in some cases cells may fail to spread (Figure 6A) or fail to produce tension signal. This is often a consequence of surface chemistry issues and requires troubleshooting (Table 1). Low ligand density is one of the most common issues that results in a failure of cell spreading. This could be a result of multiple factors, such as degraded ligand, degraded DNA strands, hydrolyzed reagents like APTES and/or LA-PEG-NHS, aggregated gold nanoparticles, or insufficient glass coverslip cleaning. By comparing substrate background fluorescence intensity over successful experiments and failed ones (Figure 6B), one can quickly assess the source of the problem. For example, surfaces with strong fluorescence signal from the background measurements indicate that the immobilization of DNA probes was successful. Low background intensity suggests issues in the preparation steps prior to the addition of the streptavidin and the biotinylated ligand. If the issue is caused by the ligand, its quality can be checked by coating it on a glass slide or wells in a 96-well plate at 10 μg/mL to test for cell adhesion. This is a useful functional test of ligand activity for pMHC ligands and anti-CD3 antibodies. By observing the color change (pale pink) of the functionalized coverslips after the Au NP incubation step, one can assess whether the problem is caused by DNA degradation or the steps preceding incubation with DNA (Figure 6C). The DNA strand quality can be validated with HPLC and MALDI-TOF-MS, in which a single DNA sample will show multiple peaks if degraded. The quality of Au NPs can be confirmed by comparing the UV-Vis spectra and TEM images with the characterization provided by manufacturer. If the quality of the ligand, DNA strands, and Au NP is validated and these reagents do not cause the surface issue, we recommend replacing reagents including APTES, LA-PEG-NHS, sulfuric acid, and H2O2 for future surface preparation instead of spending time to precisely locate the cause of the surface chemistry issue. Except for the low-density problem that will cause a major failure of the DNA probe substrate, there are a few minor issues that could affect data quality. Since the surface preparation has multiple incubation steps, buffers used during surface preparation should be made fresh, filtered, and kept at 4 ºC for storage to avoid contamination. If a surface is contaminated, it will be visible in the RICM channel and may result in a strange fluorescence background. Other than contamination, aggregates can form in the DNA stock solution over time, which may result in bright dots in Cy3B channel (Figure 6D). Usually a few aggregates will not affect the data quality; however, if a cell lands on regions with such defects, data analysis is less reliable. Occasionally, irregular patterns that affect tension mapping can be observed in both the RICM and Cy3B channels, which could come from insufficient washing after the APTES step, or accidental drying of the surface during the steps after gold particles are functionalized onto the surface (Figure 6E). These patterns might affect the tension mapping depending on how severe it is. To save relatively precious reagents such as antibodies or pMHCs, we recommend always checking the surface quality (see 2.10 and 3.3.1) before functionalization of DNA hairpin tension probes with antibody/ligand. Though this surface preparation protocol has been robust and reliable in our hands, note that it does not always tolerate alterations at certain steps very well. For example, when base and plasma etching are used instead of piranha etching, it is very likely that the probe density decreases and more surface defects are present. Alterations of Au NP size and capping reagents will likely dramatically affect the surface functionalization results (Figure 6F). For example, we have found that the 5 and 10 nm Au NPs do not bind to the lipoic acid modified surfaces very well, which results in minimal DNA after the surface preparation. However, 8.8 nm and 13 nm Au NPs can bind to the lipoic acid surfaces at a much high density, which has been observed in RICM (roughness), and Cy3B channel (fluorescence intensity).
Figure 6.
Examples of successful and unsuccessful surfaces and tension mapping. (A) Cells that do not spread compared to cells that spread on the pMHC N4 surfaces in RICM. (B) OT-1 cell that spread but did not generate any tension signal on an antiCD3ε surface with low DNA tension probe density. (C) Picture showing that the successful surface is pale pink and the unsuccessful surface is colorless. (D) Image in Cy3B channel showing the fluorescent aggregates from an old DNA stock. (E) The pattern in RICM channel and Cy3B channel that is likely due to either insufficient washing or accidental surface drying between steps. (F) Images in RICM and Cy3B channels showing the effect of using Au NPs of different sizes. (G) RICM and Cy3B images of OT-1 cells that did not exert tension signals but instead depleted DNA. Scale bar = 5 μm. Note the raw data shown here was collected by different group members with different image acquisition settings from 3 microscopes, thus having different fluorescence background levels.
Table 1.
Troubleshooting with the DNA-based tension probe system.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 2.7 | Surfaces are not pink after AuNP incubation | Insufficient etching | Include a base-etching step after piranha etching. Etch coverslips in 0.5 M KOH and etch in 0.5M KOH in ice-bath with sonication. |
| APTES is hydrolyzed | Use new APTES | ||
| NHS reagents are hydrolyzed | Use new PEG-NHS reagents | ||
| PEG-NHS reagents are hydrolyzed before being added to the surface | Work faster | ||
| AuNP aggregated | Use fresh AuNP | ||
| Residue water on the coverslips diluted the AuNP | Make sure there is no/little water residue before adding AuNP | ||
| 3.3.2 | Cells don’t spread on the surface | Low DNA probe density | If the surfaces are not as pink, troubleshoot as described in discussion; If the AuNP functionalization is normal, synthesize and use new DNA |
| Low ligand density | Use fresh streptavidin and ligand reagents | ||
| Cells donť spread on the ligand | Check cell spreading on a ligand-coated surface, if the cells donť spread still, include adhering molecules as described in ref12. | ||
| Cells spread well but no tension signal is observed | The receptor-ligand interaction does not have force transmission | NA | |
| Force is short-lived or force signals are sparse | Use locking oligonucleotide | ||
| 3.3.3 | No locking signal is observed in Atto647N | The oligonucleotide has degraded | Check with MALDI-TOF-MS |
| Locking signal is antilocalized with real-time probe | The receptor force is higher than the working range of locking nucleotide | Use 19 pN real-time hairpin probe from ref. 12 as described. |
Once DNA hairpin tension probe surfaces of good quality are prepared, one can conduct experiments with cells of interest and acquire data with a fluorescence microscope. The real-time tension can be captured with an ordinary fluorescence microscope equipped with a high NA (numerical aperture) objective and EMCCD. Do note however that we have observed that primary T cells occasionally display artifacts on the DNA tension probe surfaces due to that particular mouse’s conditions. Anecdotally, such artifacts are more likely to happen with older mice. For example, we have observed negative depletion of the fluorescence instead of turn-on fluorescence signals (Figure 6G). In this case, terminate the experiment and redo with a new batch of cells. Before using non-fluorescent locking strand for quantitative analysis of receptor forces, the tension signal storage and erasing need to be confirmed with a fluorescent Atto647N locking strand. After initial locking for 10 min, the Atto647N signal should be strongly co-localized with Cy3B signal, as it reflects tension history during this incubation. As Cy3B signal reflects not only the tension history but also real-time forces after removing locking strand, it is possible that a small subset of Cy3B signal is not accompanied by Atto647N signal (Figure 2J). For a more quantitative analysis of tension, we recommend using a non-fluorescent locking strand which would eliminate the potential energy transfer between the Cy3B and Atto647N, as well as avoiding excess washes in between image acquisitions. Note that the addition of locking strand will inevitably cause a slight fluorescence background increase, as the thermodynamics will drive some minor hybridization between the hairpin and the locking strand, which can be subtracted before quantification. One can quantify the integrated intensity of each cell after locking procedure to study the force generated by a certain receptor. Additionally, the kinetics of locking likely reflects the 2D kon and koff of a receptor to its ligand.
The application of mechanical information storage is particularly useful for mapping receptor forces generated by immune cells, which are often transient and less abundant. As determined by single-molecule spectroscopy measurements, the peak bond lifetime is observed with the application of ~10 pN of force with a lifetime ranging from less than 100 milliseconds to several seconds long. Such short duration bonds are difficult to capture with conventional DNA hairpin tension probe imaging15. Another use of this mechanical storage system is when the density of the receptor of interest is relatively low on the cell surface16, which results in a sparse signal that is often difficult to distinguish from the background with conventional DNA hairpin tension probes. Such low-density receptor forces can be revealed by accumulated tension mapping with this strategy. Note that we specifically limit the application of this strategy to immune cells, as the forces present in immune cells are known to be in the lower regime (TCRs < 19 pN) comparing to integrins in fibroblasts or platelets (up to or more than 56 pN)12,13,15. The reason is that the hybridization rate constant khyb is not impaired when the load is less than 20 pN according to optical tweezers measurements, which is in the range of 106 M−1S−1. Moreover, the khyb under forces < 20 pN is predicted to be slightly higher than at khyb at zero force, as the weak force helps aligns the strand for the complementary strand to hybridize with17. On the other hand, larger forces are expected to hinder the hybridization, as the koff increases and creates a barrier for hybridization to happen18. For a lock that is 15–17 nucleotide long, we estimate that a force around 27.8 to 30.8 pN will hinder the hybridization. Given these limitations, we suggest to exclusively apply this method in investigations of immune cell receptor forces. Additionally, the presence of the locking strand is likely to tilt the energy landscape for hairpin unfolding, which might slightly decrease the effective F1/2.
ACKNOWLEDGMENTS:
This work was supported by NIH Grants R01GM131099, NIH R01GM124472, and NSF CAREER 1350829. We thank the NIH Tetramer Facility for pMHC ligands. This study was supported, in part, by the Emory Comprehensive Glycomics Core.
Footnotes
A complete version of this article that includes the video component is available at http://dx.doi.org/10.3791/62348.
DISCLOSURES:
The authors declare no conflict of interest.
REFERENCES:
- 1.Dustin ML T-cell activation through immunological synapses and kinapses. Immunological Reviews. 221 (1), 77–89, (2008). [DOI] [PubMed] [Google Scholar]
- 2.Spillane KM,Tolar P. B cell antigen extraction is regulated by physical properties of antigen-presenting cells. Journal of Cell Biology. 216 (1), 217–230, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng Y, Brazin KN, Kobayashi E, Mallis RJ, Reinherz EL,Lang MJ Mechanosensing drives acuity of αβ T-cell recognition. Proceedings of the National Academy of Sciences. 114 (39), E8204–E8213, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong J, Ge C, Jothikumar P, Yuan Z, Liu B, Bai K, Li K, Rittase W, Shinzawa M,Zhang Y. A TCR mechanotransduction signaling loop induces negative selection in the thymus. Nature Immunology. 19 (12), 1379–1390, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basu R, Whitlock BM, Husson J, Le Floc’h A, Jin W, Oyler-Yaniv A, Dotiwala F, Giannone G, Hivroz C,Biais N. Cytotoxic T cells use mechanical force to potentiate target cell killing. Cell. 165 (1), 100–110, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bashour KT, Gondarenko A, Chen H, Shen K, Liu X, Huse M, Hone JC,Kam LC CD28 and CD3 have complementary roles in T-cell traction forces. Proceedings of the National Academy of Sciences. 111 (6), 2241–2246, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma VPY,Salaita K. DNA nanotechnology as an emerging tool to study mechanotransduction in living systems. Small. 15 (26), 1900961, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Yehl K, Narui Y,Salaita K. Tension sensing nanoparticles for mechano-imaging at the living/nonliving interface. Journal of the American Chemical Society. 135 (14), 5320–5323, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glazier R, Brockman JM, Bartle E, Mattheyses AL, Destaing O,Salaita K. DNA mechanotechnology reveals that integrin receptors apply pN forces in podosomes on fluid substrates. Nature communications. 10 (1), 1–13, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galior K, Liu Y, Yehl K, Vivek S,Salaita K. Titin-based nanoparticle tension sensors map high-magnitude integrin forces within focal adhesions. Nano Letters. 16 (1), 341–348, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Ge C, Zhu C,Salaita K. DNA-based digital tension probes reveal integrin forces during early cell adhesion. Nature communications. 5 5167, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Blanchfield L, Ma VP-Y, Andargachew R, Galior K, Liu Z, Evavold B,Salaita K. DNA-based nanoparticle tension sensors reveal that T-cell receptors transmit defined pN forces to their antigens for enhanced fidelity. Proceedings of the National Academy of Sciences. 113 (20), 5610–5615, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Qiu Y, Blanchard AT, Chang Y, Brockman JM, Ma VP-Y, Lam WA,Salaita K. Platelet integrins exhibit anisotropic mechanosensing and harness piconewton forces to mediate platelet aggregation. Proceedings of the National Academy of Sciences. 115 (2), 325–330, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J, Zarnitsyna VI, Liu B, Edwards LJ, Jiang N, Evavold BD,Zhu C. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature. 464 (7290), 932–936, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma R, Kellner AV, Ma VP-Y, Su H, Deal BR, Brockman JM,Salaita K. DNA probes that store mechanical information reveal transient piconewton forces applied by T cells. Proceedings of the National Academy of Sciences. 116 (34), 16949–16954, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, Sasmal DK, Huang J, Kim JM,Mellman I. T cell costimulatory receptor CD28 is a primary target for PD-1–mediated inhibition. Science. 355 (6332), 1428–1433, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitley KD, Comstock MJ,Chemla YR Elasticity of the transition state for oligonucleotide hybridization. Nucleic Acids Research. 45 (2), 547–555, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brockman JM, Su H, Blanchard AT, Duan Y, Meyer T, Quach ME, Glazier R, Bazrafshan A, Bender RL,Kellner AV Live-cell super-resolved PAINT imaging of piconewton cellular traction forces. Nature Methods. 17 (10), 1018–1024, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]